A Preliminary Assessment of Lichens in Different Landscapes of Hanoi, Vietnam
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Lichen Sampling and Identification
3. Results
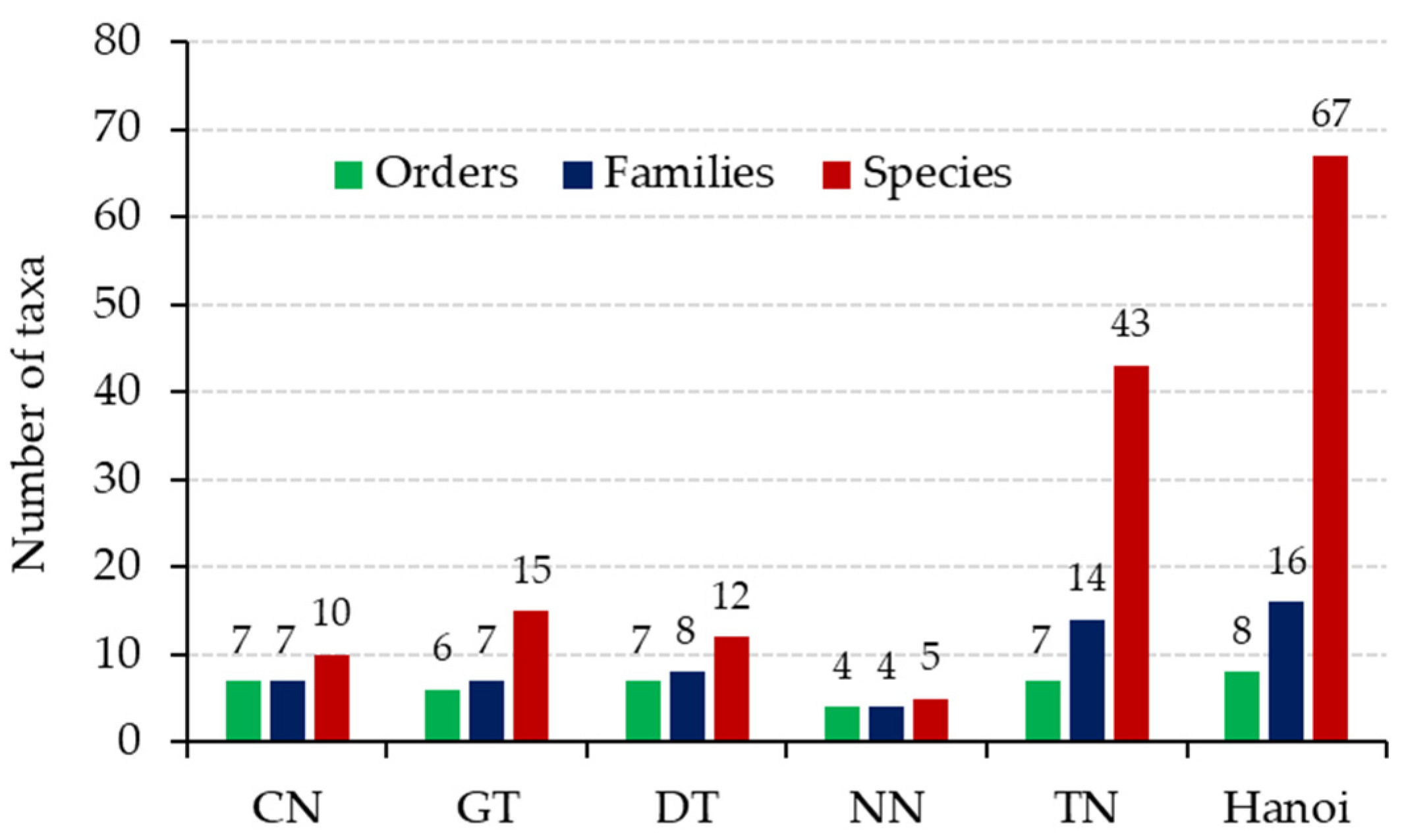
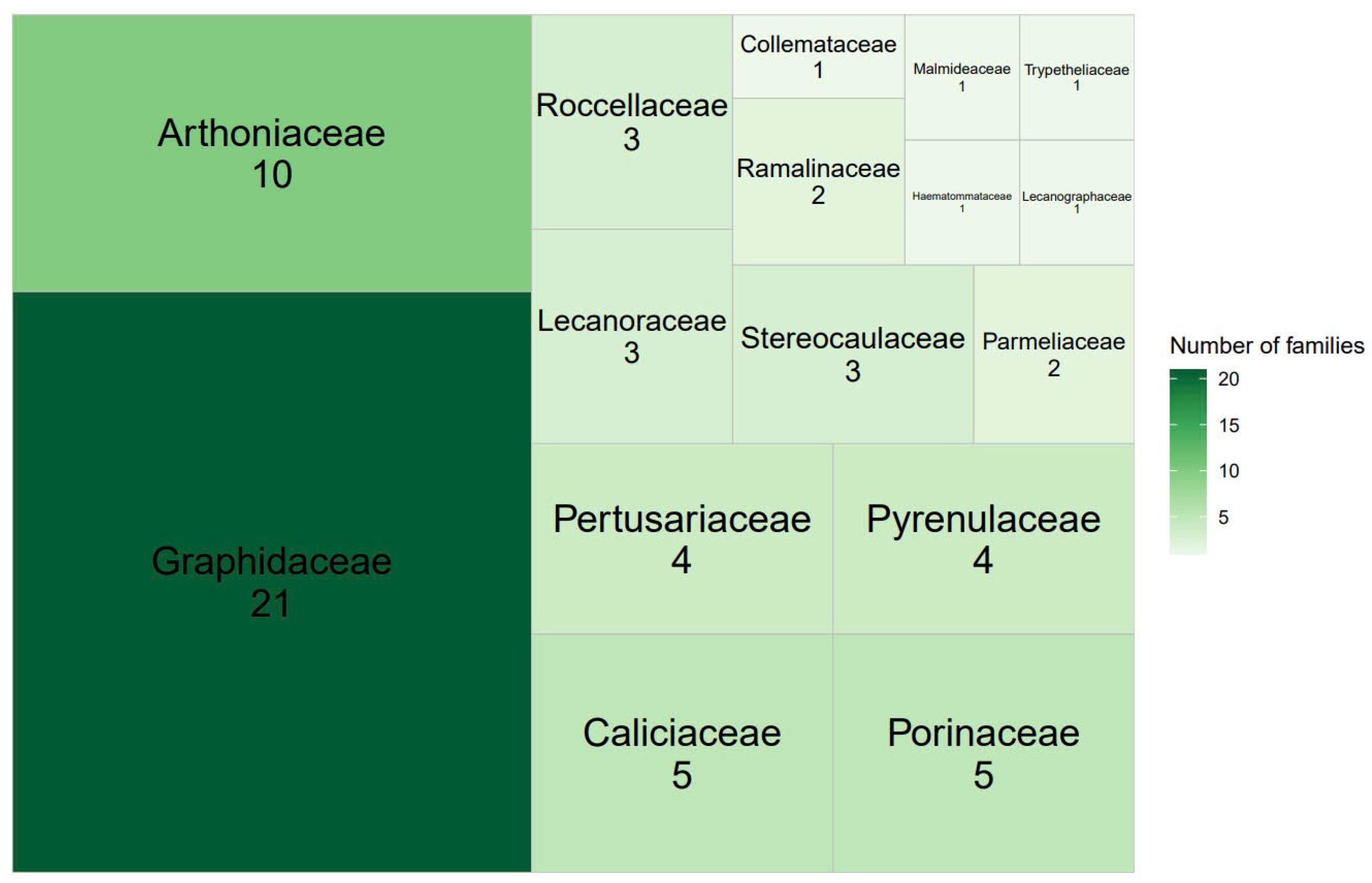
3.1. Lichen Diversity of Hanoi
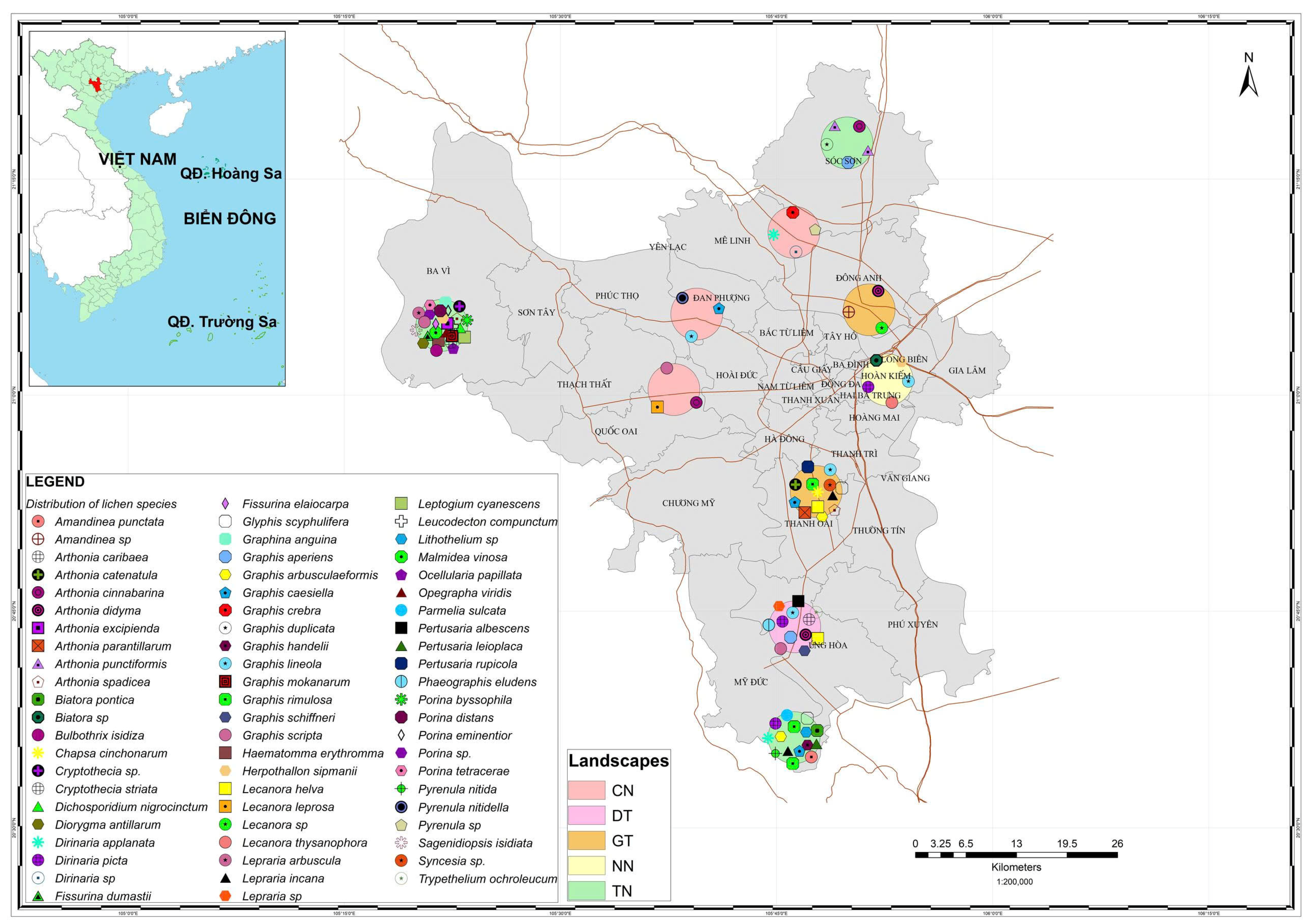
3.2. Lichen Distribution in Hanoi
3.3. The Similarity Index Among Sampling Sites in Hanoi
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Spribille, T.; Tuovinen, V.; Resl, P.; Vanderpool, D.; Wolinski, H.; Aime, M.C.; McCutcheon, J.P. Basidiomycete yeasts in the cortex of ascomycete macrolichens. Science 2016, 353, 488–492. [Google Scholar] [CrossRef] [PubMed]
- McKane, L.; Kandel, J. Microbiology: Essentials and Applications, 2nd ed.; McGraw-Hill: New York, USA, 1996. [Google Scholar]
- Dobson, F.S. Lichens: An Illustrated Guide to the British and Irish Species; Richmond: Slough, UK, 2011. [Google Scholar]
- Dahlman, L.; Palmqvist, K. Growth in two foliose tripartite lichens, Nephroma arcticum and Peltigera aphthosa: Empirical modelling of external vs. internal factors. Funct. Ecol. 2003, 17, 821–831. [Google Scholar] [CrossRef]
- Hale, M.E. The Biology of Lichens; Edward Arnold: London, UK, 1983. [Google Scholar]
- Alexander, K.E. Morphological Changes and Damages of Indicator Lichens from Sakhalin Island. Mod. Phytomorphol. 2013, 4, 215–216. [Google Scholar] [CrossRef]
- Conti, M.E.; Cecchetti, G. Biological monitoring: Lichens as bioindicators of air pollution assessment—A review. Environ. Pollut. 2001, 114, 471–492. [Google Scholar] [CrossRef]
- Rope, S.; Pearson, L. Lichens as air pollution biomonitors in a semiarid environment in Idaho. [Lecanora melanophthalma (Ram.); Artemisia tridentata]. Bryologist 2009, 93, 50–61. [Google Scholar] [CrossRef]
- Richardson, D.H.S. Pollution Monitoring with Lichens; Richmond Publishing Company: London, UK, 1992. [Google Scholar]
- Nash, T.H.; Gries, C. Lichens as Indicators of Air Pollution. In Air Pollution; Springer: Berlin/Heidelberg, Germany, 1991; pp. 1–29. [Google Scholar] [CrossRef]
- Pinho, P.; Augusto, S.; Branquinho, C.; Bio, A.; Pereira, M.J.; Soares, A.; Catarino, F. Mapping Lichen Diversity as a First Step for Air Quality Assessment. J. Atmos. Chem. 2004, 49, 377–389. [Google Scholar] [CrossRef]
- Jovan, S. Lichen Bioindication of Biodiversity, Air Quality, and Climate: Baseline Results from Monitoring in Washington, Oregon, and California; U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station: Corvallis, OR, USA, 2008. [Google Scholar]
- Will-Wolf, S. Analyzing Lichen Indicator Data in the Forest Inventory and Analysis Program; U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station: Corvallis, OR, USA, 2010. [Google Scholar]
- Gadsdon, S.R.; Dagley, J.R.; Wolseley, P.A.; Power, S.A. Relationships between lichen community composition and concentrations of NO2 and NH3. Environ. Pollut. 2010, 158, 2553–2560. [Google Scholar] [CrossRef]
- Müller-Argoviensis, L. Lichenes tonkinensis, a Cl. Balansa lecti. Hedwigia 1891, 30, 181–189. [Google Scholar]
- Hue, A. Lichenes Extra-Europaei a pluribus collectoribus ad Museum Parisiense missi. Nouv. Arch. Mus. D’histoire Nat. 1901, 3, 21–146. [Google Scholar]
- Schmid, M. Vegetation du Viet-Nam: Le Massif Sud-Annamitique et les Regions Limitrophes; ORSTOM: Paris, France, 1974. [Google Scholar]
- Tixier, P. Flore et Végétation Orophiles de l’Asie Tropicale: Les Epiphytes du Flanc Méridional du Massif Sud Annamitique; Société d’édition d’enseignement Supérieur: Paris, France, 1966. [Google Scholar]
- Pham, H.H. An Illustrated Flora of Vietnam (Part I); NXB Tre: Hanoi, Vietnam, 1999. [Google Scholar]
- Vo, T.P.G. New records of lichens from Vietnam. Sci. Technol. Dev. 2009, 12, 54–60. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Joshi, Y.; Dzung, N.A.; Hur, J.-S. First report of a fertile specimen of Coenogonium disciforme: A species new to the Vietnamese lichen flora. Lichenologist 2011, 43, 184–186. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Joshi, Y.; Lücking, R.; Nguyen, A.D.; Wang, X.Y.; Jin Koh, Y.; Hur, J.-S. Seven new records of foliicolous lichens from Vietnam. Mycotaxon 2011, 117, 93–99. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Joshi, Y.; Lücking, R.; Wang, X.-Y.; Dzung, N.A.; Koh, Y.-J.; Hur, J.-S. Notes on Some New Records of Foliicolous Lichens from Vietnam. Taiwania 2010, 55, 402–406. [Google Scholar]
- Jayalal, U.; Aptroot, A.; Nguyen, T.T.; Dzung, N.A.; Joshi, S.; Oh, S.-O.; Hur, J.-S. Further additions to the macrolichen mycota of Vietnam. Mycotaxon 2013, 124, 51–59. [Google Scholar] [CrossRef]
- Joshi, S.; Jayalal, U.; Oh, S.-O.; Nguyen, T.T.; Dzung, N.A.; Hur, J.-S. The lichen genus Graphis from Vietnam. Mycotaxon 2013, 125, 69–80. [Google Scholar] [CrossRef]
- Joshi, S.; Nguyen, T.T.; Dzung, N.A.; Jayalal, U.; Oh, S.-O.; Hur, J.-S. New records of corticolous lichens from Vietnam. Mycotaxon 2013, 123, 479–489. [Google Scholar] [CrossRef]
- Joshi, S.; Nguyen, T.T.; Dzung, N.A.; Jayalal, U.; Oh, S.-O.; Hur, J.-S. The lichen genus Fissurina (Graphidaceae) in Vietnam. Mycotaxon 2013, 124, 309–321. [Google Scholar] [CrossRef]
- Dung, N.T.M. Nghiên cứu Thành Phần hoá học của hai loài địa y Lobaria Orientalis và Dendriscosticta Platyphylloides (Lobariaceae); University of Science, VNU HCMC: Ho Chi Minh City, Vietnam, 2013. [Google Scholar]
- Thai, K.D.; Hoang, T.H.T. Study on the accumulation of metals in bioindicator by neutron activation analysis. Ho Chi Minh City Univ. Educ. J. Sci. 2008, 14, 104–110. [Google Scholar]
- Nguyen, T.L. Địa y và đài thực vật phụ sinh trên thân cây với thực trạng không khí ở TP. HCM.; Ho Chi Minh City Department of Science and Technology: Ho Chi Minh City, Vietnam, 2020. [Google Scholar]
- Giordani, P.; Brunialti, G. Sampling and Interpreting Lichen Diversity Data for Biomonitoring Purposes. In Recent Advances in Lichenology: Modern Methods and Approaches in Biomonitoring and Bioprospection; Upreti, D.K., Divakar, P.K., Shukla, V., Bajpai, R., Eds.; Springer: New Delhi, India, 2015; Volume 1, pp. 19–46. [Google Scholar] [CrossRef]
- Gradstein, S.R.; Nadkarni, N.M.; Krömer, T.; Holz, I.; Nöske, N. A protocol for rapid and representative sampling of vascular and non-vascular epiphyte diversity of tropical rain forests. Selbyana 2003, 24, 105–111. [Google Scholar]
- Khastini, R.; Sari, I.; Herysca, Y.; Sulasanah, S. Lichen diversity as indicators for monitoring ecosystem health in Rawa Danau Nature Reserve, Banten, Indonesia. Biodiversitas 2019, 20, 489–496. [Google Scholar] [CrossRef][Green Version]
- LÜCking, R.; Archer, A.W.; Aptroot, A. A world-wide key to the genus Graphis (Ostropales: Graphidaceae). Lichenologist 2009, 41, 363–452. [Google Scholar] [CrossRef]
- Orange, A.; James, P.W.; White, F.J. Microchemical Methods for the Identification of Lichens; British Lichen Society: London, UK, 2010. [Google Scholar]
- Bungartz, F.; Nash Iii, T.; Rosentreter, R. Field Guide to Common Epiphytic Macrolichens in Arizona—An identification Manual for the USDA Forest Inventory and Analysis Program; USDA Forest Service & Arizona State University Lichen Herbarium: Tempe, AZ, USA, 2002. [Google Scholar]
- Kelly, A.M. Guide to Common Macrolichens and Bryophytes of the Umatilla National Forest; United States Department of Agriculture: Washington, DC, USA, 2006. [Google Scholar]
- Sorensen, T. A Method of Establishing Groups of Equal Amplitude in Plant Sociology Based on Similarity of Species Content and Its Application to Analyses of the Vegetation on Danish Commons. Biol. Skr./K. Dan. Vidensk. Selsk. 1948, 5, 1–34. [Google Scholar]
- Sipman, H.J.M. Tropical urban lichens: Observations from Singapore. Blumea Biodivers. Evol. Biogeogr. Plants 2009, 54, 297–299. [Google Scholar] [CrossRef]
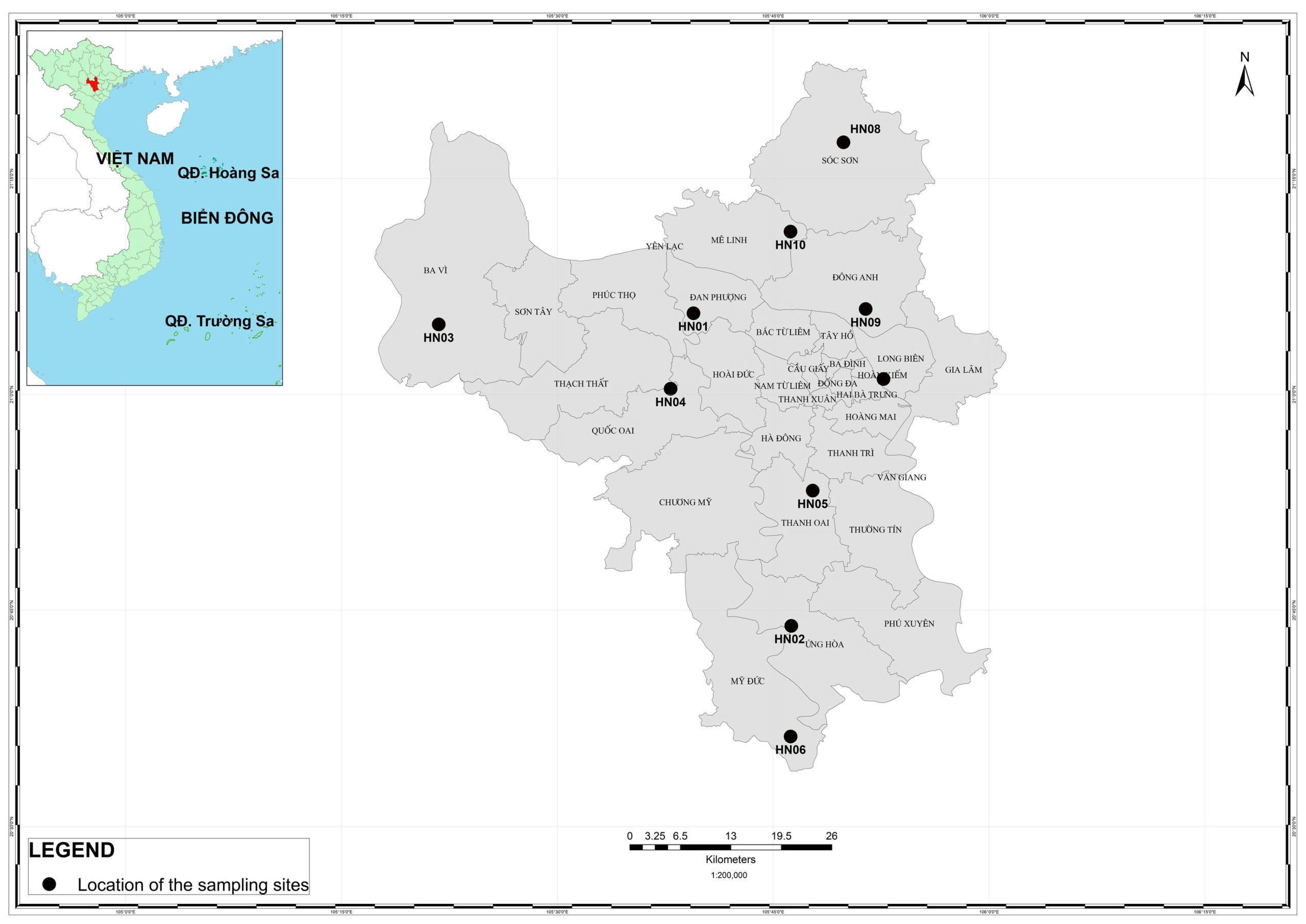
| No. | Sampling Sites | Landscapes | Latitude (°N) | Longitude (°E) | Temperature (°C) | Relative Humidity (%) | PM2.5 (µg/m3) | NO2 (µg/m3) | SO2 (µg/m3) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Cầu Gáo industrial park | CN | 21.093637 | 105.656982 | 32 | 60 | 84 | 35.595 | 46.238 |
| 2 | Van Dinh town | DT | 21.106552 | 105.538488 | 30 | 62 | 40 | 20.051 | 39.285 |
| 3 | Ba Vi national park | TN | 21.083683 | 105.371891 | 25 | 88 | 2 | 2.176 | 14.949 |
| 4 | Thach That industrial park | CN | 21.006415 | 105.631266 | 33 | 58 | 42 | 15.388 | 28.856 |
| 5 | Southern traffic axis | GT | 21.098805 | 105.857090 | 32 | 55 | 48 | 13.057 | 33.723 |
| 6 | Chua Huong heritage | TN | 20.604024 | 105.770221 | 28 | 82 | 13 | 9.948 | 27.465 |
| 7 | Long Bien rural area | NN | 20.998762 | 105.944736 | 31 | 62 | 30 | 15.388 | 28.856 |
| 8 | Den Giong temple | TN | 21.291945 | 105.831473 | 28 | 70 | 34 | 11.502 | 21.207 |
| 9 | Hoang Sa road | GT | 21.098805 | 105.857090 | 33 | 54 | 47 | 37.149 | 43.457 |
| 10 | Quang Minh industrial park | CN | 21.188471 | 105.770051 | 30 | 62 | 70 | 44.921 | 61.535 |
| No. | Species | Families | Orders | Landscapes |
|---|---|---|---|---|
| 1 | Arthonia caribaea (Ach.) A. Massal. | Arthoniaceae | Arthoniales | DT |
| 2 | Arthonia catenatula Nyl. | Arthoniaceae | Arthoniales | GT |
| 3 | Arthonia cinnabarina (DC.) Wallr | Arthoniaceae | Arthoniales | CN, TN |
| 4 | Arthonia didyma Körber | Arthoniaceae | Arthoniales | DT, GT |
| 5 | Arthonia excipienda (Nyl.) Nyl. | Arthoniaceae | Arthoniales | TN |
| 6 | Arthonia parantillarum Aptroot | Arthoniaceae | Arthoniales | GT |
| 7 | Arthonia punctiformis Ach. | Arthoniaceae | Arthoniales | TN |
| 8 | Arthonia spadicea Leight. | Arthoniaceae | Arthoniales | GT, TN |
| 9 | Cryptothecia sp. | Arthoniaceae | Arthoniales | TN |
| 10 | Cryptothecia striata Thor | Arthoniaceae | Arthoniales | TN |
| 11 | Amandinea punctata (Hoffm.) Coppins & Scheid. | Caliciaceae | Caliciales | TN |
| 12 | Amandinea sp. | Caliciaceae | Caliciales | GT |
| 13 | Dirinaria applanata (Fée) D.D.Awasthi | Caliciaceae | Caliciales | CN, TN |
| 14 | Dirinaria picta (Sw.) Clem. & Schear | Caliciaceae | Caliciales | DT, NN, TN |
| 15 | Dirinaria sp. | Caliciaceae | Caliciales | CN |
| 16 | Leptogium cyanescens (Ach.) Körb. | Collemataceae | Peltigerales | TN |
| 17 | Chapsa cinchonarum (Fée) Frisch | Graphidaceae | Ostropales | GT |
| 18 | Diorygma antillarum (Vain.) Nelsen, Lücking & Rivas Plata | Graphidaceae | Ostropales | TN |
| 19 | Fissurina dumastii Fée | Graphidaceae | Ostropales | TN |
| 20 | Fissurina elaiocarpa (A.W. Archer) A.W. Archer | Graphidaceae | Ostropales | TN |
| 21 | Glyphis scyphulifera (Ach.) Staiger | Graphidaceae | Ostropales | GT, TN |
| 22 | Graphina anguina (Mont.) Müll.Arg. | Graphidaceae | Ostropales | TN |
| 23 | Graphis aperiens Müll. Arg | Graphidaceae | Ostropales | DT, TN |
| 24 | Graphis arbusculaeformis (Vain.) Lücking | Graphidaceae | Ostropales | GT, TN |
| 25 | Graphis caesiella Vain. | Graphidaceae | Ostropales | CN, GT, TN |
| 26 | Graphis crebra Vain | Graphidaceae | Ostropales | CN |
| 27 | Graphis duplicata Ach. | Graphidaceae | Ostropales | TN |
| 28 | Graphis handelii Zahlbr | Graphidaceae | Ostropales | TN |
| 29 | Graphis lineola Ach | Graphidaceae | Ostropales | CN, DT, GT, NN |
| 30 | Graphis mokanarum Lücking, Moncada & M.C. Martínez | Graphidaceae | Ostropales | TN |
| 31 | Graphis rimulosa (Mont.) Trevis. | Graphidaceae | Ostropales | GT, TN |
| 32 | Graphis schiffneri (Zahlbr) | Graphidaceae | Ostropales | DT |
| 33 | Graphis scripta (L.) Ach. | Graphidaceae | Ostropales | CN, DT, TN |
| 34 | Herpothallon sipmanii Aptroot, Lücking & Rivas Plata | Graphidaceae | Ostropales | NN, TN |
| 35 | Leucodecton compunctum (Ach.) A. Massal. | Graphidaceae | Ostropales | TN |
| 36 | Ocellularia papillata (Leight.) Zahlbr. | Graphidaceae | Ostropales | TN |
| 37 | Phaeographis eludens (Stirt.) Shirley | Graphidaceae | Ostropales | DT |
| 38 | Haematomma erythromma (Nyl.) Zahlbr. | Haematommataceae | Lecanorales | TN |
| 39 | Opegrapha viridis (Ach.) Behlen & Desberger | Lecanographaceae | Arthoniales | TN |
| 40 | Lecanora helva Stizenb. | Lecanoraceae | Lecanorales | DT, GT |
| 41 | Lecanora leprosa (Fée) | Lecanoraceae | Lecanorales | CN |
| 42 | Lecanora sp. | Lecanoraceae | Lecanorales | GT |
| 43 | Malmidea vinosa (Eschw.) Kalb, Rivas Plata & Lumbsch | Malmideaceae | Lecanorales | TN |
| 44 | Bulbothrix isidiza (Nyl.) Hale | Parmeliaceae | Lecanorales | TN |
| 45 | Parmelia sulcata Taylor | Parmeliaceae | Lecanorales | TN |
| 46 | Verseghya thysanophora Harris | Pertusariaceae | Pertusariales | NN |
| 47 | Pertusaria albescens (Huds.) M.Choisy & Werner | Pertusariaceae | Pertusariales | DT |
| 48 | Pertusaria leioplaca (Ach.) DC. | Pertusariaceae | Pertusariales | TN |
| 49 | Pertusaria rupicola (Fr.) Harm. | Pertusariaceae | Pertusariales | GT |
| 50 | Porina byssophila (Hepp) Zahlbr. | Porinaceae | Ostropales | TN |
| 51 | Porina distans Vĕzda & Vivant | Porinaceae | Ostropales | TN |
| 52 | Porina eminentior (Nyl.) P.M. McCarthy | Porinaceae | Ostropales | TN |
| 53 | Porina sp. | Porinaceae | Ostropales | TN |
| 54 | Porina tetracerae (Ach.) Müll.Arg. | Porinaceae | Ostropales | TN |
| 55 | Lithothelium sp. | Pyrenulaceae | Pyrenulales | TN |
| 56 | Pyrenula nitida (Weigel) Ach. | Pyrenulaceae | Pyrenulales | TN |
| 57 | Pyrenula nitidella (Flörke) Müll. Arg. | Pyrenulaceae | Pyrenulales | CN |
| 58 | Pyrenula sp. | Pyrenulaceae | Pyrenulales | CN |
| 59 | Biatora pontica Printzen & Tønsberg | Ramalinaceae | Lecanorales | TN |
| 60 | Biatora sp. | Ramalinaceae | Lecanorales | NN |
| 61 | Dichosporidium nigrocinctum (Ehrenb.) G.Thor | Roccellaceae | Arthoniales | TN |
| 62 | Sagenidiopsis isidiata G. Thor, Elix, Lücking & Sipman | Roccellaceae | Arthoniales | TN |
| 63 | Syncesia sp. | Roccellaceae | Arthoniales | GT |
| 64 | Lepraria arbuscula (Nyl.) Lendemer & Hodk. | Stereocaulaceae | Lecanorales | TN |
| 65 | Lepraria incana (L.) Ach. | Stereocaulaceae | Lecanorales | GT, TN |
| 66 | Lepraria sp. | Stereocaulaceae | Lecanorales | DT |
| 67 | Trypethelium ochroleucum (Eschw.) Nyl. | Trypetheliaceae | Trypetheliales | DT |
| CN | GT | DT | NN | TN | |
|---|---|---|---|---|---|
| CN | 0.1429 | 0.1333 | 0.0909 | 0.1905 | |
| GT | 0.1429 | 0.2000 | 0.0909 | 0.2857 | |
| DT | 0.1333 | 0.2000 | 0.1667 | 0.1364 | |
| NN | 0.0909 | 0.0909 | 0.1667 | 0.1111 | |
| TN | 0.1905 | 0.2857 | 0.1364 | 0.1111 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khac, H.N.; Truong, L.D.; Hanh, N.T.H.; Lien, N.T.H.; Binh, N.Q.; Giao, V.T.P.; Tinh, P.H.; Thu, B.T. A Preliminary Assessment of Lichens in Different Landscapes of Hanoi, Vietnam. Diversity 2025, 17, 27. https://doi.org/10.3390/d17010027
Khac HN, Truong LD, Hanh NTH, Lien NTH, Binh NQ, Giao VTP, Tinh PH, Thu BT. A Preliminary Assessment of Lichens in Different Landscapes of Hanoi, Vietnam. Diversity. 2025; 17(1):27. https://doi.org/10.3390/d17010027
Chicago/Turabian StyleKhac, Hoang Ngoc, Le Dac Truong, Nguyen Thi Hong Hanh, Nguyen Thi Hong Lien, Nguyen Quoc Binh, Vo Thi Phi Giao, Pham Hong Tinh, and Bui Thi Thu. 2025. "A Preliminary Assessment of Lichens in Different Landscapes of Hanoi, Vietnam" Diversity 17, no. 1: 27. https://doi.org/10.3390/d17010027
APA StyleKhac, H. N., Truong, L. D., Hanh, N. T. H., Lien, N. T. H., Binh, N. Q., Giao, V. T. P., Tinh, P. H., & Thu, B. T. (2025). A Preliminary Assessment of Lichens in Different Landscapes of Hanoi, Vietnam. Diversity, 17(1), 27. https://doi.org/10.3390/d17010027






