Non-Indigenous Species (NIS) Know No Geopolitical Borders—An Update of NIS in the Aegean Sea
Abstract
1. Introduction
2. Methodology
3. Results
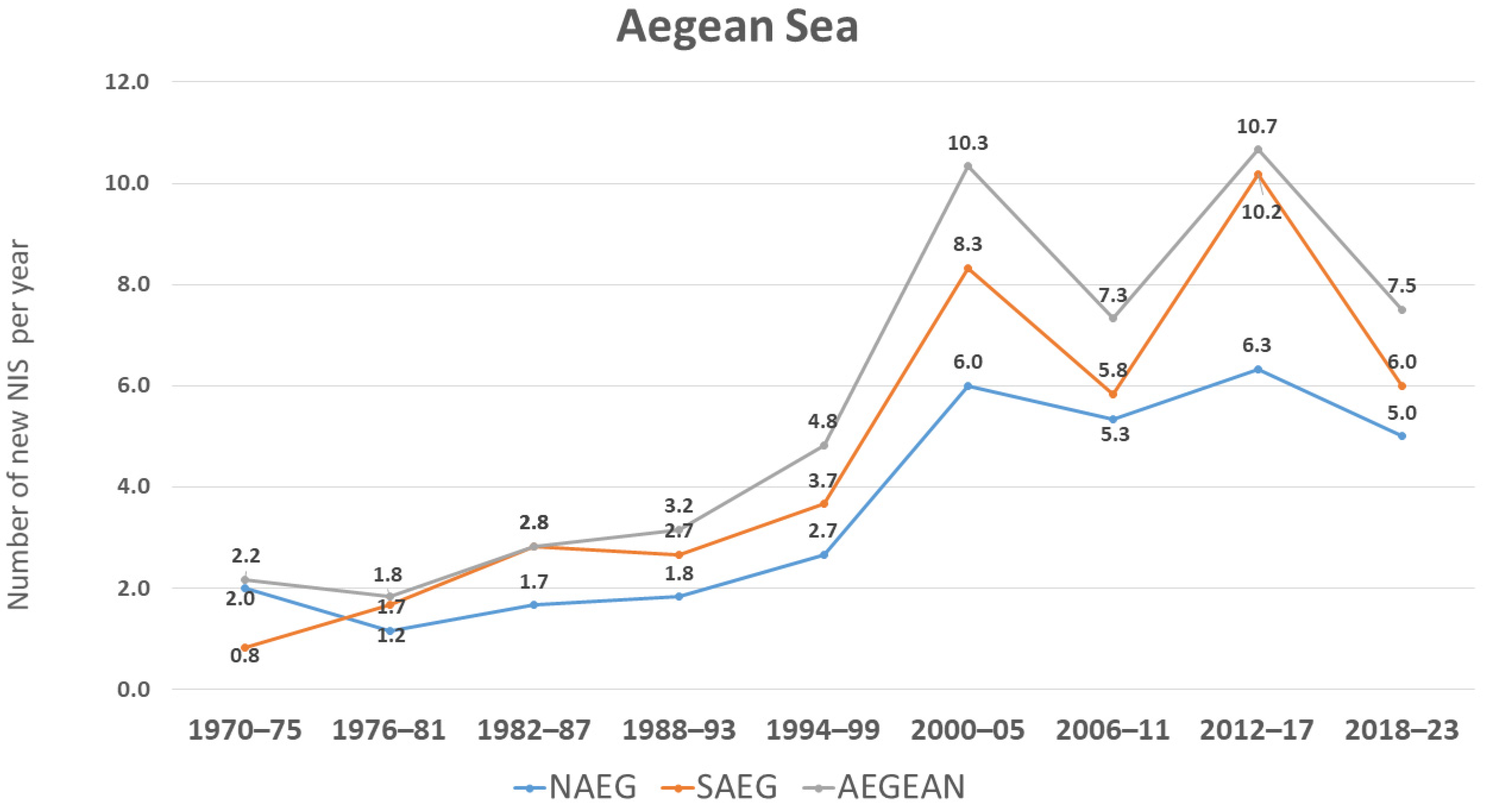
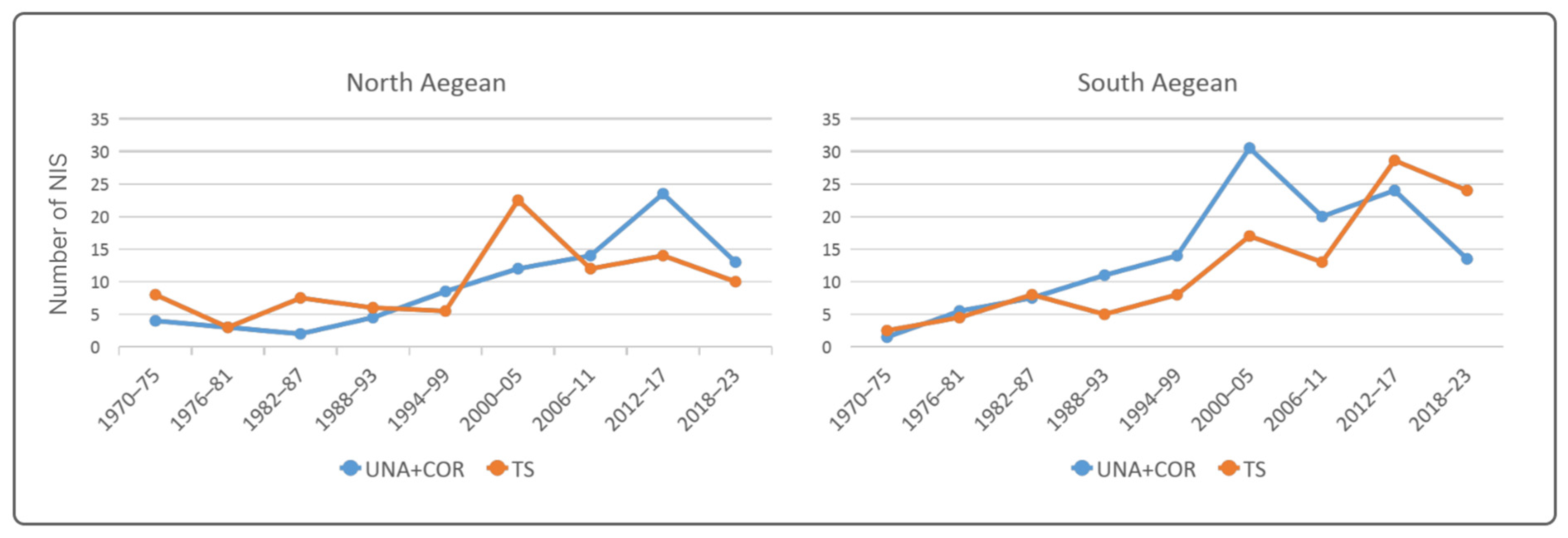
3.1. Trends in NIS Introduction
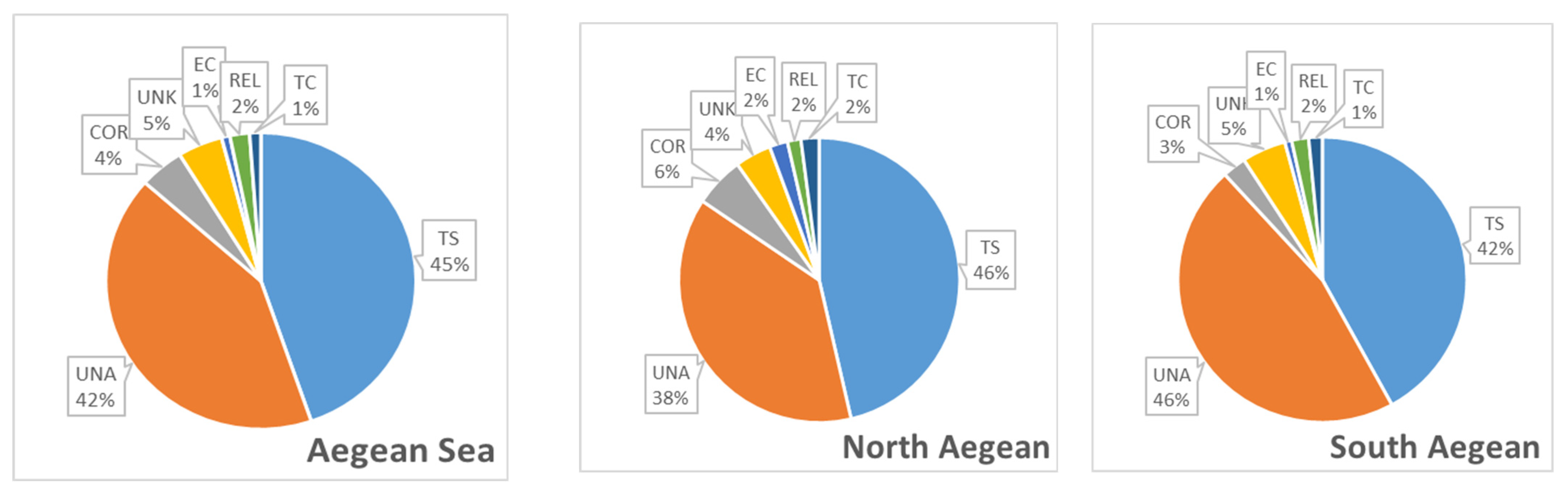
3.2. Trends in Pathway
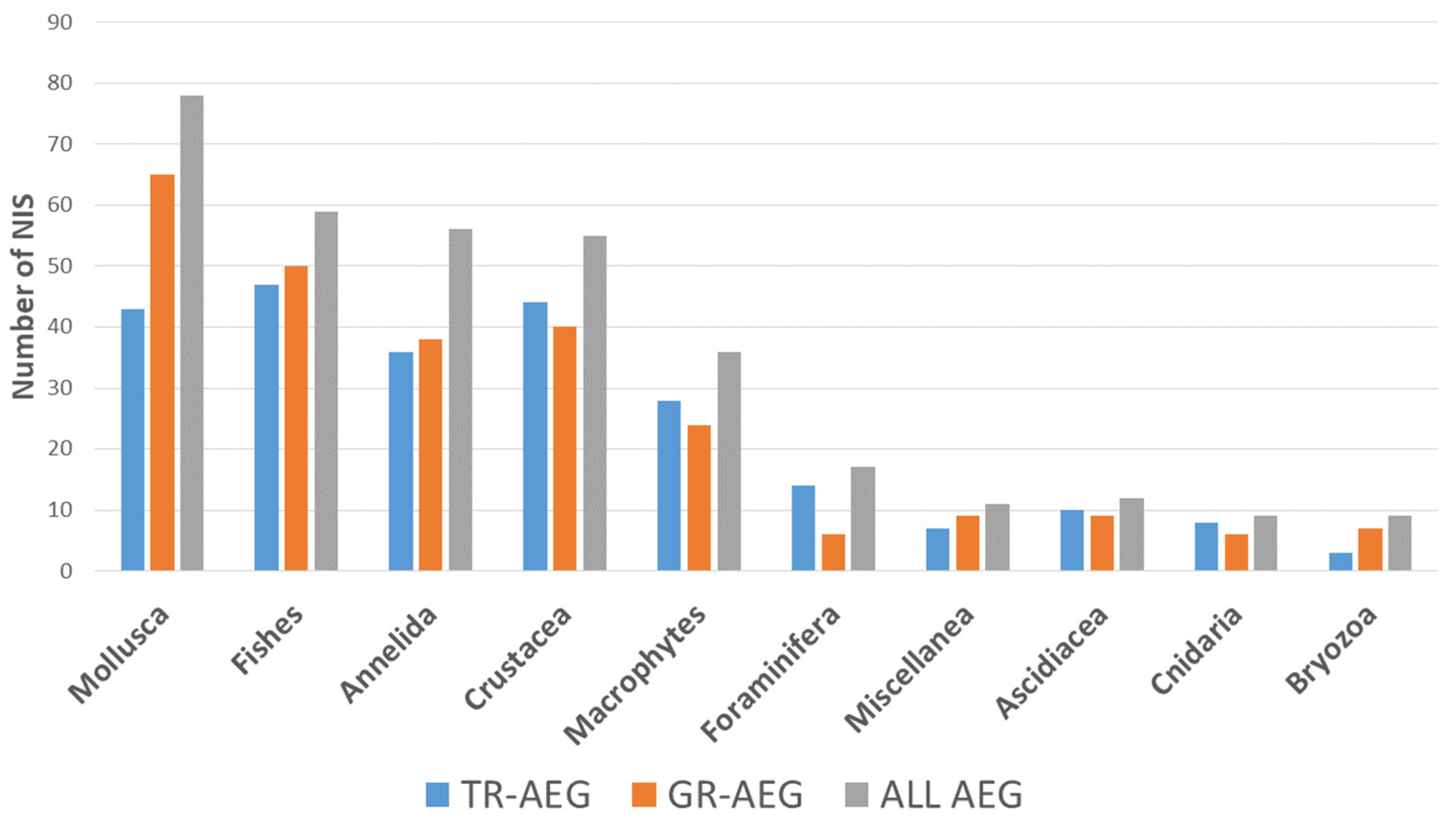
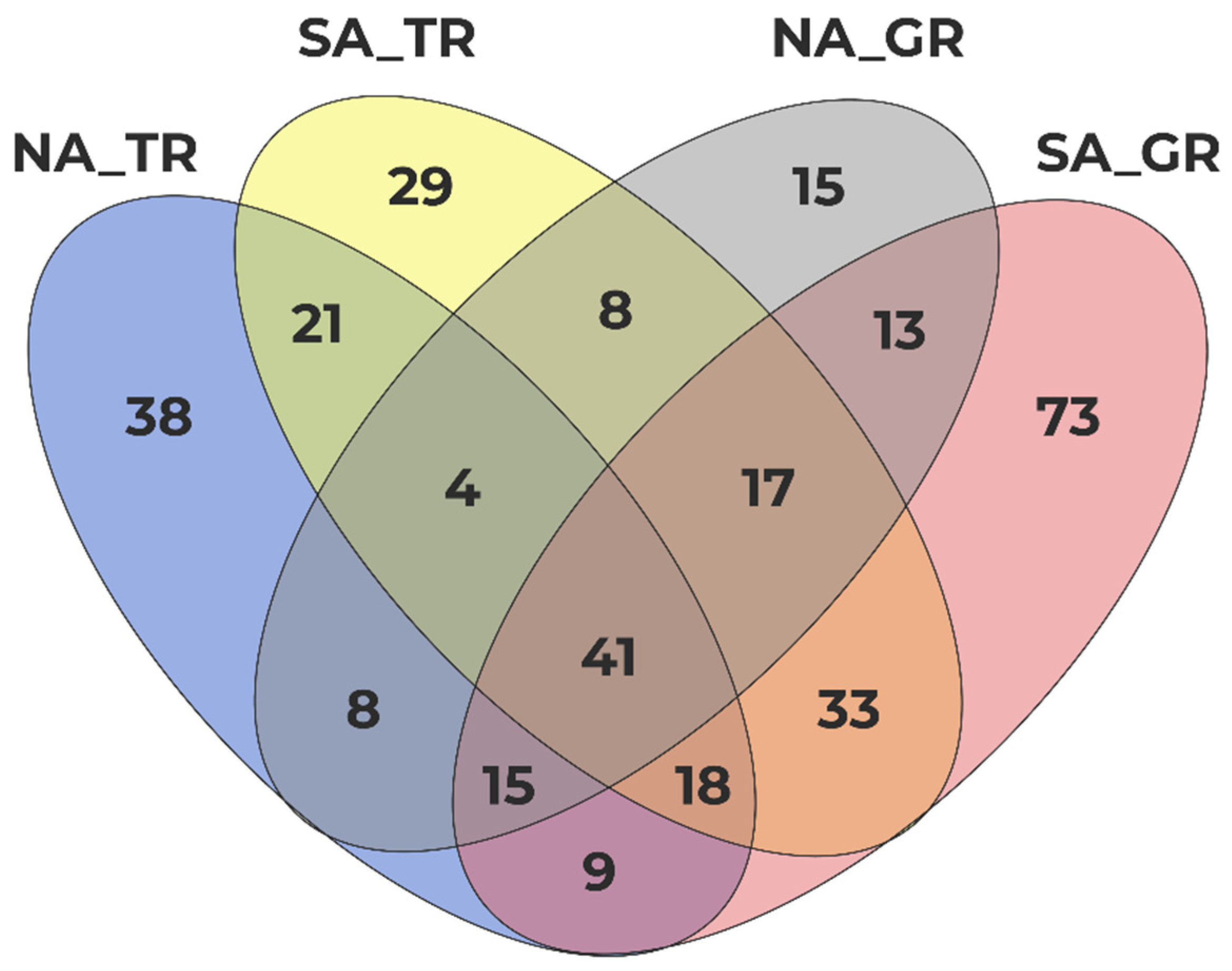
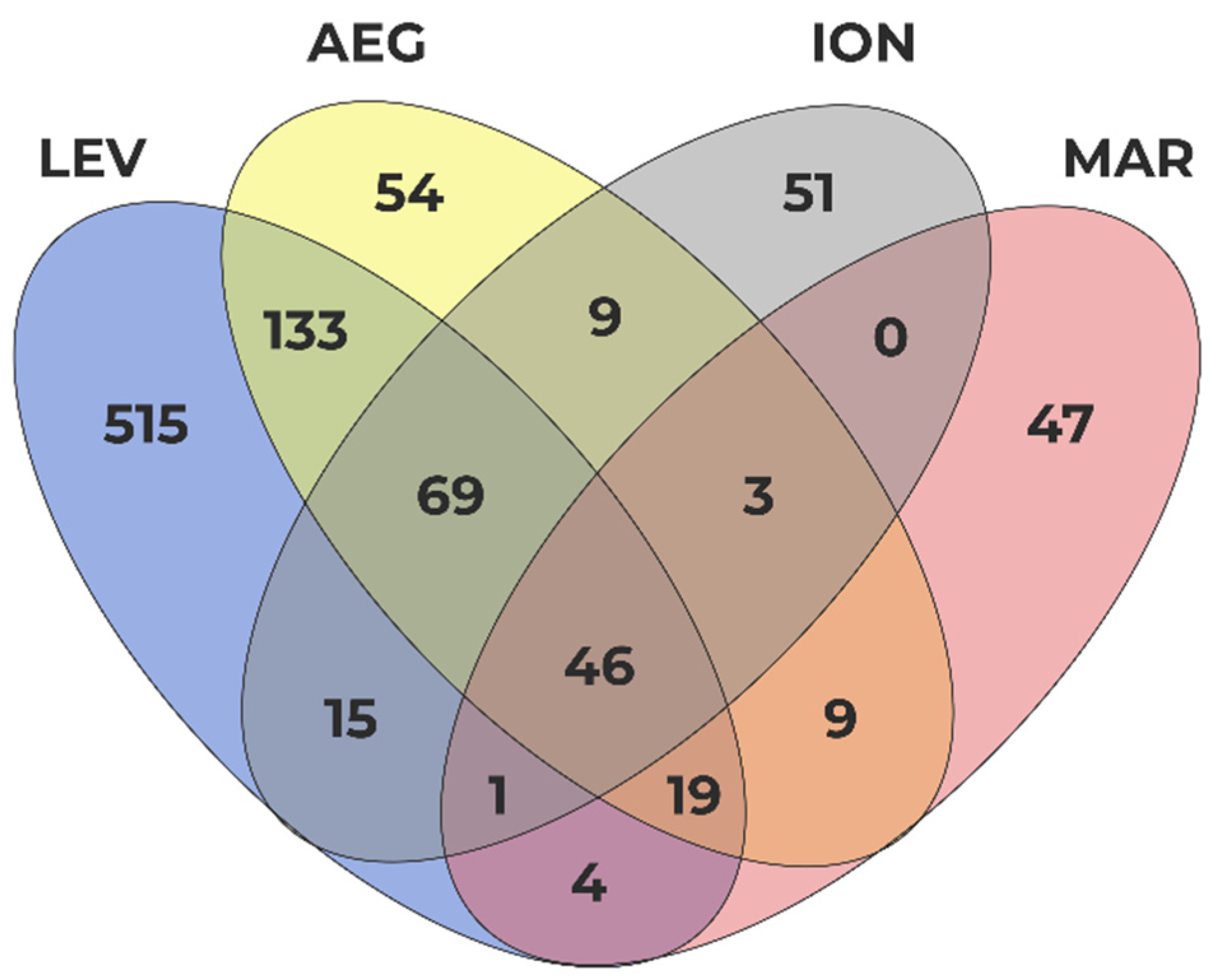
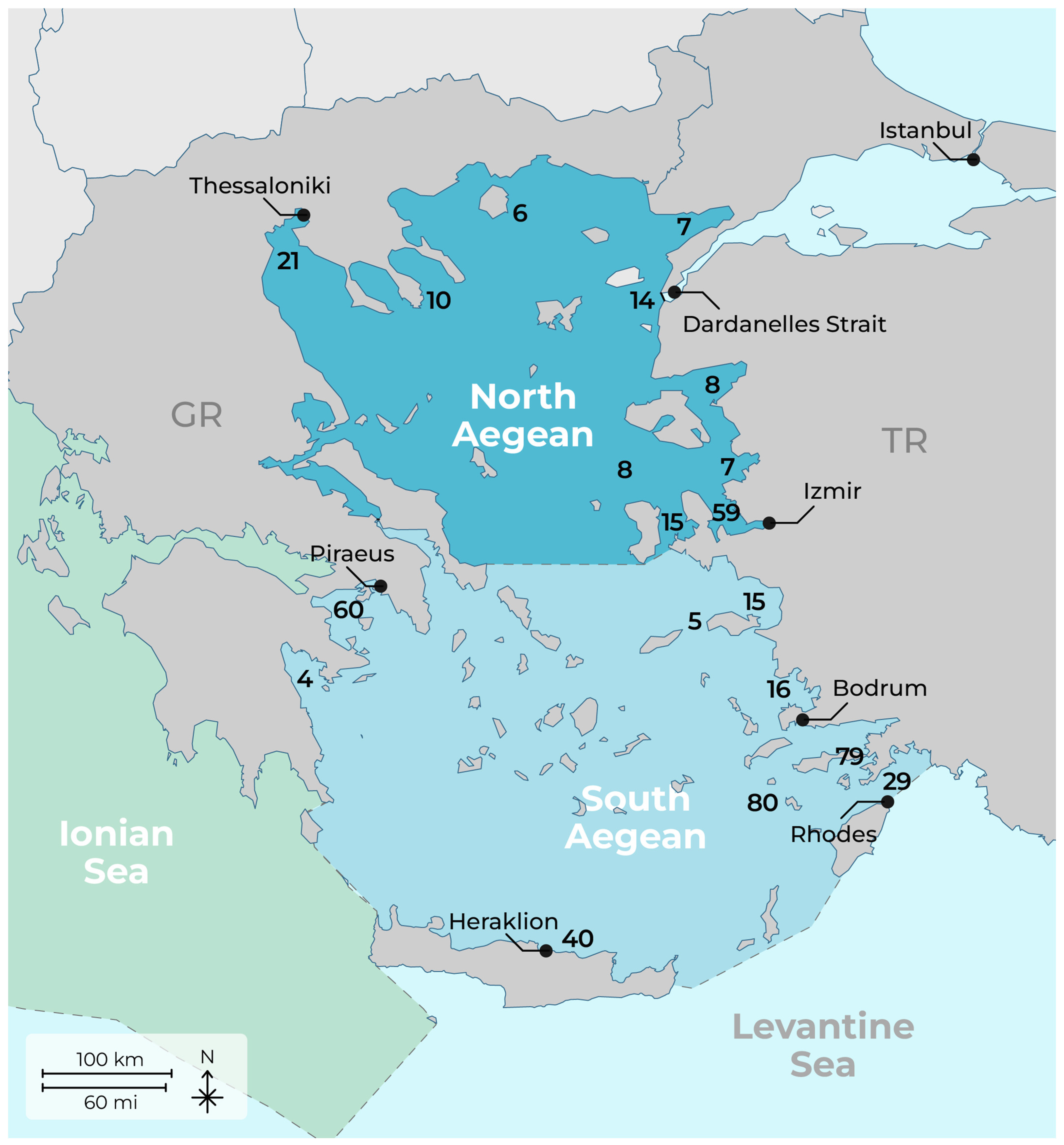
3.3. Hot Spot Areas
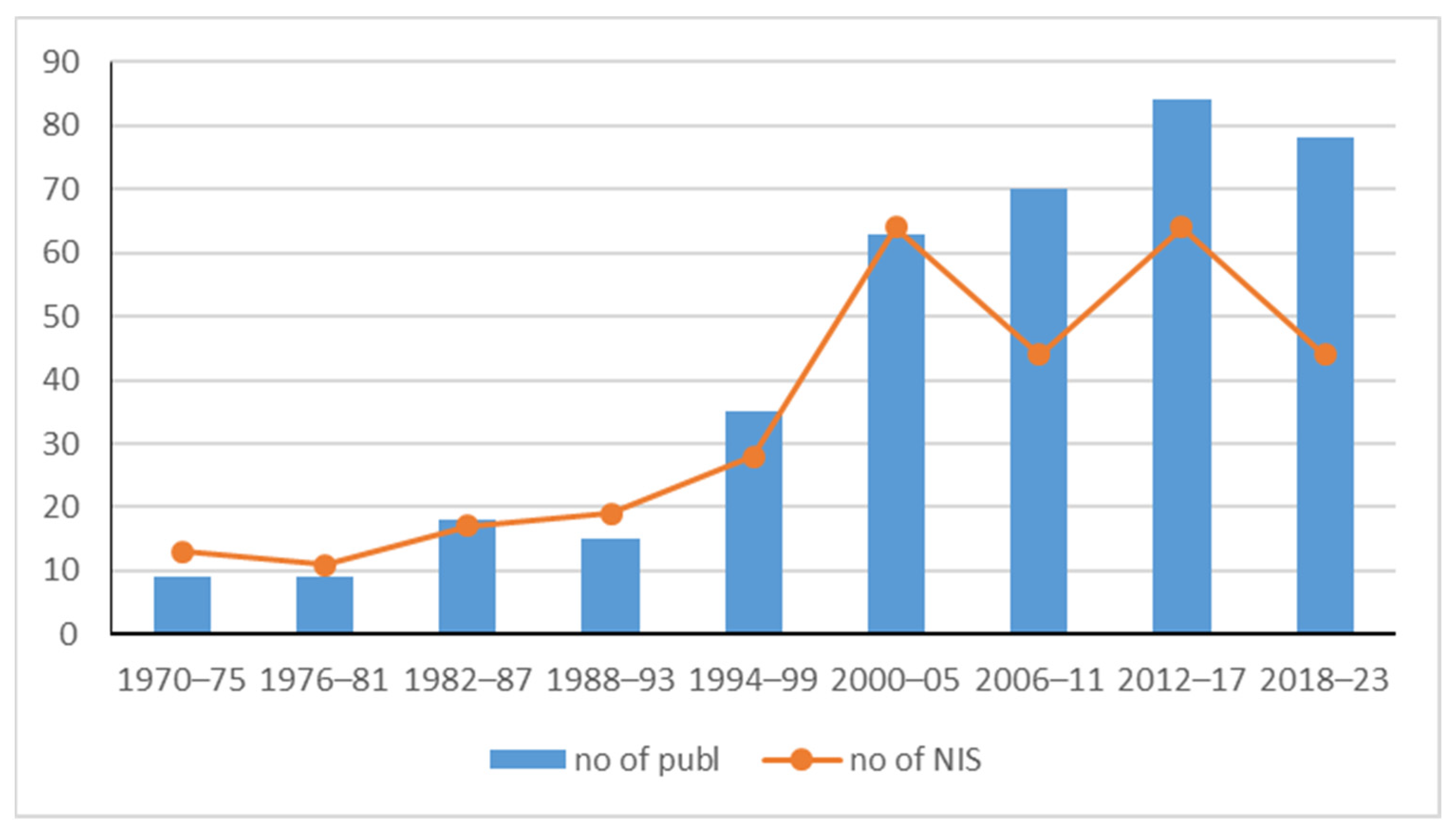
3.4. Research Effort
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Katağan, T.; Tokaç, A.; Beşiktepe, Ş.; Öztürk, B. (Eds.) The Aegean Sea Marine Biodiversity, Fisheries, Conservation and Governance; Publication No 41; Turkish Marine Research Foundation (TUDAV): Istanbul, Turkey, 2015. [Google Scholar]
- Anagnostou, C.; Katsanevakis, S.; Kastanidi, E.; Streftaris, N.; Pagou, K.; Papathanassiou, E. Integrated Planning for the Adaptive Management of Human Activities and Supporting Marine Conservation in the Aegean Sea. In The Handbook of Environmental Chemistry; Springer: Berlin/Heidelberg, Germany, 2023. [Google Scholar] [CrossRef]
- REMPEC. Study on Trends and Outlook of Marine Pollution from Ships and Activities and of Maritime Traffic and Offshore Activities in the Mediterranean; Report by the Regional Marine Pollution Emergency Response Centre for the Mediterranean Sea; REMPEC: Floriana, Malta, 2021; p. 181. ISBN 978-9918-0-0322-8. [Google Scholar]
- Sini, M.; Katsanevakis, S.; Koukourouvli, N.; Gerovasileiou, V.; Dailianis, T.; Buhl-Mortensen, L.; Damalas, D.; Dendrinos, P.; Dimas, X.; Frantzis, A.; et al. Assembling Ecological Pieces to Reconstruct the Conservation Puzzle of the Aegean Sea. Front. Mar. Sci. 2017, 4, 347. [Google Scholar] [CrossRef]
- Giakoumi, S.; Sini, M.; Gerovasileiou, V.; Mazor, T.; Beher, J.; Possingham, H.P.; Abdulla, A.; Çinar, M.E.; Dendrinos, P.; Gucu, A.C.; et al. Ecoregion-Based Conservation Planning in the Mediterranean: Dealing with Large-Scale Heterogeneity. PLoS ONE 2013, 8, e76449. [Google Scholar] [CrossRef] [PubMed]
- Panayotidis, P.; Orfanidis, S.; Tsiamis, K. Cystoseira crinita community in the Aegean Sea. Rapp. Comm. Int. Pour Explor. Sci. Mer Mediterr. 2007, 38, 570. [Google Scholar]
- Güçlüsoy, H. Marine and coastal protected areas of Turkish Aegean coasts. In The Aegean Sea Marine Biodiversity, Fisheries, Conservation and Governance; Katağan, T., Tokaç, A., Beşiktepe, Ş., Öztürk, B., Eds.; Publication No. 41; Turkish Marine Research Foundation: Istanbul, Turkey, 2015; pp. 669–684. [Google Scholar]
- Çınar, M.E.; Bilecenoğlu, M. Alien species invading the Aegean Sea habitats—An eastern synthesis. In The Aegean Sea Marine Biodiversity, Fisheries, Conservation and Governance; Katağan, T., Tokaç, A., Beşiktepe, Ş., Öztürk, B., Eds.; Publication No. 41; Turkish Marine Research Foundation: Istanbul, Turkey, 2015; pp. 636–653. [Google Scholar]
- Katsanevakis, S.; Zenetos, A.; Corsini-Foka, M.; Tsiamis, K. Biological Invasions in the Aegean Sea: Temporal Trends, Pathways, and Impacts. In The Handbook of Environmental Chemistry; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar] [CrossRef]
- Katsanevakis, S.; Tsirintanis, K.; Sini, M.; Gerovasileiou, V.; Koukourouvli, N. Aliens in the Aegean—A sea under siege (ALAS). Res. Ideas Outcomes 2020, 6, e53057. [Google Scholar] [CrossRef]
- Evangelopoulos, A.; Karampetsis, D.; Christidis, A.; Gubili, C.; Sapounidis, A.; Adamidou, A.; Kamidis, N.; Koutrakis, E. Non-native fish species in the North Aegean Sea: A review of their distributions integrating unpublished fisheries data. Front. Mar. Sci. 2024, 11, 1398037. [Google Scholar] [CrossRef]
- Çinar, M.E.; Bilecenoglu, M.; Ozturk, B.; Katagan, T.; Aysel, V. Alien species on the coasts of Turkey. Mediterr. Mar. Sci. 2005, 6, 119–146. [Google Scholar] [CrossRef][Green Version]
- Çinar, M.E.; Bilecenoglu, M.; Ozturk, B.; Katagan, T.; Yokeş, M.B.; Aysel, V.; Dağlı, E.; Açik, S.; Ozcan, T.; Erdogan, H. An updated review of alien species on the coasts of Turkey. Mediterr. Mar. Sci. 2011, 12, 257–315. [Google Scholar] [CrossRef]
- Çinar, M.E.; Bilecenoğlu, M.; Yokeş, M.B.; Öztürk, B.; Taşkin, E.; Bakir, K.; Doğan, A.; Açik, Ş. Current status (as end of 2020) of marine alien species in Turkey. PLoS ONE 2021, 16, e0251086. [Google Scholar] [CrossRef]
- Tsiamis, K.; Palialexis, A.; Stefanova, K.; Gladan, N.; Skejić, S.; Despalatović, M.; Cvitković, I.; Dragičević, B.; Dulčić, J.; Vidjak, O.; et al. Non-indigenous species refined national baseline inventories: A synthesis in the context of the European Union’s Marine Strategy Framework Directive. Mar. Pollut. Bull. 2019, 145, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Tsiamis, K.; Palialexis, A.; Connor, D.; Antoniadis, S.; Bartilotti, C.; Bartolo, G.A.; Berggreen, U.C.; Boschetti, S.; Buschbaum, C.; Canning-Clode, J.; et al. Marine Strategy Framework Directive, Descriptor 2, Non-Indigenous Species: Delivering Solid Recommendations for Setting Threshold Values for Non-Indigenous Species Pressure on European Seas; Publications Office of the European Union: Luxembourg, 2021; p. 36. [Google Scholar] [CrossRef]
- Zenetos, A.; Karachle, P.K.; Corsini-Foka, M.; Gerovasileiou, V.; Simboura, N.; Xentidis, N.J.; Tsiamis, K. Is the trend in new introductions of marine non-indigenous species a reliable criterion for assessing good environmental status? The case study of Greece. Mediterr. Mar. Sci. 2020, 21, 775–793. [Google Scholar] [CrossRef]
- Agamennone, F.; Sbrana, C.; Nardi, N.; Siragusa, F.; Germanà, A. Dikoleps micalii n. sp.(Gastropoda: Skeneidae) from the Eastern Aegean Sea. Boll. Malacol. 2020, 56, 91–95. [Google Scholar]
- Agamennone, F.; Micali, P.; Siragusa, F. Melanella orientalis n. sp. (Gastropoda: Eulimidae) from the Eastern Mediterranean. Boll. Malacol. 2020, 56, 172–175. [Google Scholar]
- Albano, P.G.; Steger, J.; Bakker, P.A.; Bogi, C.; Bošnjak, M.; Guy-Haim, T.; Huseyinoglu, M.F.; LaFollette, P.I.; Lubinevsky, H.; Mulas, M.; et al. Numerous new records of tropical non-indigenous species in the Eastern Mediterranean highlight the challenges of their recognition and identification. ZooKeys 2021, 1010, 1–95. [Google Scholar] [CrossRef] [PubMed]
- Crocetta, F.; Al Mabruk, S.A.A.; Azzurro, E.; Bakiu, R.; Bariche, M.; Batjakas, I.E.; Bejaoui, T.; Ben Souissi, J.; Cauchi, J.; Corsini-Foka, M.; et al. New alien Mediterranean biodiversity records (November 2021). Mediterr. Mar. Sci. 2021, 22, 724–746. [Google Scholar] [CrossRef]
- Digenis, M.; Ragkousis, M.; Vasileiadou, K.; Gerovasileiou, V.; Katsanevakis, S. New records of the Indo-Pacific shrimp Urocaridella pulchella Yokeş and Galil, 2006 from the Eastern Mediterranean Sea. BioInvasions Rec. 2021, 10, 295–303. [Google Scholar] [CrossRef]
- Kampouris, T.E.; Economidis, P.S.; Batjakas, I.E. First record of Pagrus major (Temminck & Schlegel, 1843) (Perciformes: Sparidae) from east Mediterranean Sea and the northernmost Mediterranean record of Por’s goatfish Upeneus pori Ben-Tuvia & Golani, 1989 (Perciformes: Mullidae) from Thermaikos Gulf, North-West Aegean Sea, Greece. Cah. Biol. Mar. 2020, 61, 253–258. [Google Scholar] [CrossRef]
- Kampouris, T.E.; Gkafas, G.A.; Sarantopoulou, J.; Exadactylos, A.; Batjakas, I.E. An American in the Aegean: First Record of the American Lobster Homarus americanus, H. Milne Edwards, 1837 from the Eastern Mediterranean Sea. BioInvasions Rec. 2021, 10, 170–180. [Google Scholar] [CrossRef]
- Kontadakis, C.; Mbazios, G.; Manousis, T.; Galinous-Mitsoudi, S. Records of the Indo-pacific species Heliacus implexus (Mighels, 1845)(Gastropoda, Architectonicidae) established in the Mediterranean Sea. Xenophora Taxon. 2021, 32, 18–22. [Google Scholar]
- Manousis, T. Hellenic Conches; Conchbooks: Harxheim, Germany, 2021; 609p, ISBN 978-3-948603-17-5.
- Orfanidis, S.; Alvito, A.; Azzurro, E.; Badreddine, A.; Ben Souissi, J.; Chamorro, C.; Crocetta, F.; Dalyan, C.; Fortič, A.; Galanti, L.; et al. New Alien Mediterranean Biodiversity Records (March 2021). Mediterr. Mar. Sci. 2021, 22, 180–198. [Google Scholar] [CrossRef]
- Agamennone, F.; Micali, P. First Mediterranean record of Atys ehrenbergi (A. Issel, 1869) with notes on some species of the genus Retusa (T. Brown, 1827) (Gastropoda: Cephalaspidea). Boll Malacol. 2022, 58, 87–93. [Google Scholar] [CrossRef]
- Kolátková, V.; Smulders, F.O.; Ward, E.A.; Vohník, M. Range expansion of Marinomyxa marina, a phytomyxid parasite of the invasive seagrass Halophila stipulacea, to the Caribbean. Aquat. Bot. 2022, 182, 103554. [Google Scholar] [CrossRef]
- Kourkoutmani, P.; Michaloudi, E. First record of the calanoid copepod Pseudodiaptomus marinus Sato, 1913 in the North Aegean Sea, in Thessaloniki Bay, Greece. BioInvasions Rec. 2022, 11, 738–746. [Google Scholar] [CrossRef]
- Kytinou, E.; Zotou, M.; Virgili, R.; Crocetta, F.; Katsanevakis, S. The Indo-Pacific nudibranch Baeolidia moebii Bergh, 1888 in Greece, with the first documented spawning aggregation in the Mediterranean Sea. BioInvasions Rec. 2022, 11, 461–472. [Google Scholar] [CrossRef]
- Montesanto, F.; Chimienti, G.; Gissi, C.; Mastrototaro, F. Polyclinum constellatum (Tunicata, Ascidiacea), an emerging non-indigenous species of the Mediterranean Sea: Integrated taxonomy and the importance of reliable DNA barcode data. Mediterr. Mar. Sci. 2022, 23, 69–83. [Google Scholar] [CrossRef]
- Uttieri, M.; Anadoli, O.; Banchi, E.; Battuello, M.; Beşiktepe, Ş.; Carotenuto, Y.; Cotrim Marques, S.; de Olazabal, A.; Di Capua, I.; Engell-Sørensen, K.; et al. The distribution of Pseudodiaptomus marinus in European and neighbouring waters-A rolling review. J. Mar. Sci. Eng. 2023, 11, 1238. [Google Scholar] [CrossRef]
- Tiralongo, F.; Akyol, O.; Al Mabruk, S.A.; Battaglia, P.; Beton, D.; Bitlis, B.; Borg, J.A.; Bouchoucha, M.; Çinar, M.E.; Crocetta, F.; et al. New Alien Mediterranean Biodiversity Records (August 2022). Mediterr. Mar. Sci. 2022, 23, 725–747. [Google Scholar] [CrossRef]
- Kondylatos, G.; Mavrouleas, D.; Gratsia, E.; Kasapidis, P.; Corsini-Foka, M.; Klaoudatos, D. First record of Arcania brevifrons Chen, 1989 (Decapoda; Leucosiidae) and further record of Macrophthalmus (Macrophthalmus) indicus Davie, 2012 (Decapoda; Macrophthalmidae) in Hellenic waters. BioInvasions Rec. 2023, 12, 234–244. [Google Scholar] [CrossRef]
- Kondylatos, G.; Kalaentzis, K.; Gratsia, E.; Mavrouleas, D.; Kasapidis, P.; Tsiamis, K.; Klaoudatos, D. Halimeda incrassata (Bryopsidales, Chlorophyta) in Rhodes, Greece, Eastern Mediterranean. Mediterr. Mar. Sci. 2023, 24, 633–638. [Google Scholar] [CrossRef]
- Martaeng, R.; Obst, M.; Kuklinski, P. Phylogeographic study using autonomous reef monitoring structures indicates fast range expansion of the invasive bryozoan Juxtacribrilina mutabilis. Hydrobiologia 2023, 850, 4115–4126. [Google Scholar] [CrossRef]
- Guerra-García, J.M.; Revanales, T.; Saenz-Arias, P.; Navarro-Barranco, C.; Ruiz-Velasco, S.; Pastor-Montero, M.; Sempere-Valverde, J.; Chebaane, S.; Vélez-Ruiz, A.; Martínez-Laiz, G. Quick spreading of the exotic amphipod Laticorophium baconi (Shoemaker, 1934): Another small stowaway overlooked? Meditter. Mar. Sci. 2023, 24, 644–655. [Google Scholar] [CrossRef]
- Ragkousis, M.; Zenetos, A.; Ben Souissi, J.; Hoffman, R.; Ghanem, R.; Taşkin, E.; Muresan, M.; Karpova, E.; Slynko, E.; Dağlı, E.; et al. Unpublished Mediterranean and Black Sea records of marine alien, cryptogenic, and neonative species. Bioinvasions Rec. 2023, 12, 339–369. [Google Scholar] [CrossRef]
- Alvanou, M.V.; Feidantsis, K.; Papadopoulos, D.K.; Lattos, A.; Theodorou, J.A.; Michaelidis, B.; Giantsis, I.A. Major ascidian species with negative impacts on bivalve aquaculture: Current knowledge and future research aims. Open Geosci. 2024, 16, 20220660. [Google Scholar] [CrossRef]
- Ovalis, P.; Zenetos, A. Two more alien micromolluscs in the Greek Seas: New records of Stosicia lineata and Finella pupoides from the Saronikos Gulf. Cah. Biol. Mar. 2024, 65, 169–172. [Google Scholar] [CrossRef]
- Christidis, G.; Ammar, I.A.; Antit, M.; Barhoum, Y.M.; Brundu, G.; Colletti, A.; Crocetta, F.; Desiderato, A.; Digenis, M.; Gökoğlu, M.; et al. New records of introduced species in the Mediterranean (August 2024). Mediterr. Mar. Sci. 2024, 25, 453–479. [Google Scholar] [CrossRef]
- Mutlu, E. Ecological gradients of epimegafaunal distribution along the sectors of Gulf of İzmir, Aegean Sea. COMU J. Mar. Sci. Fish. 2021, 4, 130–158. [Google Scholar] [CrossRef]
- Besiktepe, S.; Terbıyık Kurt, T.; Gubanova, A. Mesozooplankton composition and distribution in İzmir Bay, Aegean Sea: With special emphasis on copepods. Reg. Stud. Mar. Sci. 2022, 55, 102567. [Google Scholar] [CrossRef]
- Cerim, H.; Yapıcı, S.; Gül¸sahin, A.; Soykan, O.; Bilge, G. The first record of the red Cornetfısh (Fistularia petimba Lacepède, 1803) in the Aegean Sea. Düzce Üniv. Bilim Teknol. Derg. 2021, 9, 607–615. [Google Scholar] [CrossRef]
- Taşkın, E.; Çakır, M. Marine macroalgal flora on the Aegean and the Levantine coasts of Turkey. Bot. Mar. 2022, 65, 231–241. [Google Scholar] [CrossRef]
- Fortič, A.; Al-Sheikh, R.R.; Almajid, Z.; Badreddine, A.; Baez, J.C.; Belmonte-Gallegos, A.; Bettoso, N.; Borme, D.; Camisa, F.; Caracciolo, D.; et al. New records of introduced species in the Mediterranean Sea (April 2023). Mediterr. Mar. Sci. 2023, 24, 182–202. [Google Scholar] [CrossRef]
- Langeneck, J.; Bakiu, R.; Chalari, N.; Chatzigeorgiou, G.; Crocetta, F.; Doğdu, S.A.; Durmishaj, S.; García-Charton, J.A.; Gül¸sahin, A.; Hoffman, R.; et al. New records of introduced species in the Mediterranean Sea (November 2023). Mediterr. Mar. Sci. 2023, 24, 610–632. [Google Scholar] [CrossRef]
- Çinar, M.E.; Özgül, A. Clogging nets-Didemnum vexillum (Tunicata: Ascidiacea) is in action in the eastern Mediterranean. J. Mar. Biol. Assoc. United Kingd. 2023, 103, e89. [Google Scholar] [CrossRef]
- Öztürk, B.; Türkçü, N.; Bitlis, B. New records of gastropods (Caenogastropoda and Heterobranchia) from the Turkish coasts with observations on some poorly known species. Turk. J. Zool. 2023, 47, 135–146. [Google Scholar] [CrossRef]
- Dağli, E.; Bakir, K.; Gündeğer, G.; Nerlovic, V.; Doğan, A. Review of marine alien isopods in Türkiye with two new records: Of Paracerceis sculpta and Paranthura japonica. Mediterr. Mar. Sci. 2024, 25, 204–212. [Google Scholar] [CrossRef]
- Katsanevakis, S.; Poursanidis, D.; Hoffman, R.; Rizgalla, J.; Rothman, S.B.-S.; Levitt-Barmats, Y.; Hadjioannou, L.; Trkov, D.; Garmendia, J.M.; Rizzo, M.; et al. Unpublished Mediterranean records of marine alien and cryptogenic species. Bioinvasions Rec. 2020, 9, 165–182. [Google Scholar] [CrossRef]
- Ragkousis, M.; Sini, M.; Koukourouvli, N.; Zenetos, A.; Katsanevakis, S. Invading the Greek Seas: Spatiotemporal Patterns of Marine Impactful Alien and Cryptogenic Species. Diversity 2023, 15, 353. [Google Scholar] [CrossRef]
- Vagenas, G.; Karachle, P.K.; Oikonomou, A.; Stoumboudi, M.T.; Zenetos, A. Decoding the spread of non-indigenous fishes in the Mediterranean Sea. Sci. Rep. 2024, 14, 6669. [Google Scholar] [CrossRef]
- Zenetos, A.; Cinar, M.E.; Pancucci-Papadopoulou, M.A.; Harmelin, J.G.; Furnari, G.; Andaloro, F.; Bellou, N.; Streftaris, N.; Zibrowius, H. Annotated list of marine alien species in the Mediterranean with records of the worst invasive species. Mediterr. Mar. Sci. 2005, 6, 63–118. [Google Scholar] [CrossRef]
- Zenetos, A.; Meriç, E.; Verlaque, M.; Galli, P.; Boudouresque, C.F.; Giangrande, A.; Çinar, M.E.; Bilecenoglu, M. Additions to the annotated list of marine alien biota in the Mediterranean with special emphasis on Foraminifera and Parasites. Mediterr. Mar. Sci. 2008, 9, 119–166. [Google Scholar] [CrossRef]
- Zenetos, A.; Gofas, S.; Verlaque, M.; Cinar, M.E.; Raso, J.E.G.; Bianchi, C.N.; Morri, C.; Azzurro, E.; Bilecenoglu, M.; Froglia, C.; et al. Alien species in the Mediterranean Sea by 2010. A contribution to the application of European Union’s Marine Strategy Framework Directive (MSFD). Part, I. Spatial distribution. Mediterr. Mar. Sci. 2010, 11, 381–493. [Google Scholar] [CrossRef]
- Zenetos, A.; Gofas, S.; Morri, C.; Rosso, A.; Violanti, D.; Garcia Raso, J.E.; Cinar, M.E.; Almogi-Labin, A.; Ates, A.S.; Azzurro, E.; et al. Alien species in the Mediterranean Sea by 2012. A contribution to the application of European Union’s Marine Strategy Framework Directive (MSFD). Part 2. Introduction trends and pathways. Mediterr. Mar. Sci. 2012, 13, 328–352. [Google Scholar] [CrossRef]
- Zenetos, A.; Çinar, M.E.; Crocetta, F.; Golani, D.; Rosso, A.; Servello, G.; Shenkar, N.; Turon, X.; Verlaque, M. Uncertainties and validation of alien species catalogues: The Mediterranean as an example. Estuar. Coast. Shelf Sci. 2017, 191, 171–187. [Google Scholar] [CrossRef]
- Galanidi, M.; Aissi, M.; Ali, M.; Bakalem, A.; Bariche, M.; Bartolo, A.G.; Bazairi, H.; Beqiraj, S.; Bilecenoglu, M.; Bitar, G.; et al. Validated Inventories of Non-Indigenous Species (NIS) for the Mediterranean Sea as Tools for Regional Policy and Patterns of NIS Spread. Diversity 2023, 15, 962. [Google Scholar] [CrossRef]
- Çevik, C.; Dogan, A.; Önen, M.; Zenetos, A. First record of the Indo-Pacific species Electroma vexillum (Mollusca: Bivalvia: Pterioida) in the eastern Mediterranean. Mar. Biodivers. Rec. 2008, 1, e1. [Google Scholar] [CrossRef]
- Kapiris, K.; Katağan, T.; Ateş, S.A.; Conides, A. Review of alien decapods (Crustacea) in the Aegean Sea. J. Black Sea/Mediterr. Environ. 2012, 18, 177–187. [Google Scholar]
- Yokeş, M.; Andreou, V.; Bakiu, R.; Bonanomi, S.; Camps, J.; Christidis, G.; Crocetta, F.; Giovos, I.; Gori, A.; Jureti’c, T.; et al. New Mediterranean Biodiversity Records (November 2018). Mediterr. Mar. Sci. 2018, 19, 673–689. [Google Scholar] [CrossRef]
- IHO (International Hydrographic Organization). Limits of Oceans and Seas, 3rd ed.; Special Publication 1953, No. 23 (S-23); International Hydrographic Organization: Monte Carlo, Monaco, 1953. [Google Scholar]
- Spalding, M.D.; Fox, H.E.; Allen, G.R.; Davidson, N.; Ferdaña, A.Z.; Finlayson, M.; Halpern, B.S.; Jorge, M.A.; Lombana, A.; Lourie, S.A. Marine ecoregions of the world: A bioregionalization of coastal and shelf areas. BioScience 2007, 57, 573–583. [Google Scholar] [CrossRef]
- Jensen, H.M.; Panagiotidis, P. Technical Document on the Delineation of MSFD Article 4 Marine Regions and Subregions 4/10/2017; Version 2.0; European Environment Agency: København, Denmark, 2017; 21p. [Google Scholar]
- Fourcy, D.; Lorvelec, O. A new digital map of limits of oceans and seas consistent with high-resolution global shorelines. J. Coast. Res. 2013, 29, 471–477. [Google Scholar] [CrossRef]
- IHO (International Hydrographic Organization). Report of the International Hydrographic Organisation. Working Paper No. 57 (WP 57). In Proceedings of the 20th Session of the United Nations Group of Experts on Geographical Names, New York, NY, USA, 17–28 January 2000. [Google Scholar]
- Ministry of Environment and Energy (MinEnv), Greece. Technical Report for the Preparation Stage of Action Plan for Marine Strategies in Greece, for the Implementation of Marine Strategy Framework Directive 2008/56/EC; APC Advanced planning-consulting SA; University of the Aegean: Lesvos, Greece, 2012.
- Zenetos, A.; Albano, P.G.; Garcia, E.L.; Stern, N.; Tsiamis, K.; Galanidi, M. Established non-indigenous species increased by 40% in 11 years in the Mediterranean Sea. Meditter. Mar. Sci. 2022, 23, 196–212. [Google Scholar] [CrossRef]
- Zenetos, A.; Albano, P.G.; Garcia, E.L.; Stern, N.; Tsiamis, K.; Galanidi, M. Corrigendum to the Review Article. Meditter. Mar. Sci. 2022, 23, 196–212, Erratum in Meditter. Mar. Sci. 2022, 23, 876–878. [Google Scholar] [CrossRef]
- Langeneck, J.; Lezzi, M.; Del Pasqua, M.; Musco, L.; Gambi, M.C.; Castelli, A.; Giangrande, A. Non-indigenous polychaetes along the coasts of Italy: A critical review. Mediterr. Mar. Sci. 2020, 21, 238–275. [Google Scholar] [CrossRef]
- UNEP/MAP. Baseline for the IMAP Common Indicator 6 related to Non-Indigenous Species. In Proceedings of the 9th Meeting of the Ecosystem Approach Coordination Group, Online, 5 July 2022. UNEP/MED WG.521/Inf.8. [Google Scholar]
- WoRMS Editorial Board. World Register of Marine Species. Available online: https://www.marinespecies.org at VLIZ (accessed on 2 February 2024).
- CBD. Pathways of Introduction of Invasive Species, Their Prioritization and Management. UNEP/CBD/SBSTTA/18/9/Add.1. Secretariat of the Convention on Biological Diversity, Montréal. 2014; p. 18. Available online: https://www.cbd.int/doc/meetings/sbstta/sbstta-18/official/sbstta-18-09-add1-en.pdf (accessed on 10 June 2024).
- Pergl, J.; Brundu, G.; Harrower, C.A.; Cardoso, A.C.; Genovesi, P.; Katsanevakis, S.; Lozano, V.; Perglová, I.; Rabitsch, W.; Richards, G.; et al. Applying the Convention on Biological Diversity Pathway Classification to alien species in Europe. Neobiota 2020, 62, 333–363. [Google Scholar] [CrossRef]
- Yan, L. ggvenn: Draw Venn Diagram by ‘ggplot2’, R Package Version 0.1.9; 2021. Available online: https://CRAN.R-project.org/package=ggvenn (accessed on 5 May 2023).
- Okuş, E.; Yüksek, A.; Yokeş, B.; Yılmaz, I.N.; Aslan-Yılmaz, A.; Karhan, Ü.; Demirel, N.; Demir, V.; Zeki, S.; Taş, S.; et al. The Final Report on Determination of the Coastal and Marine Areas Biodiversity of Gökova Special Environment Protection Area; Turkish Ministry of Environment and Forestry Environment Protection Agency for Special Areas: Ankara, Turkey, 2006; 504p, ISBN 975-8273-91-4. (In Turkish) [Google Scholar]
- Manousis, T.; Kontadakis, C.; Zaminos, G.; Zeimbekis, C.; Mbazios, G.; Galinou-Mitsoudi, S. New records of Lower Heterobranchia (Mollusca: Gastropoda) for the Mediterranean and the Hellenic Seas. Xenophora Taxon. 2020, 30, 22–39. [Google Scholar]
- Okuş, E.; Sur, H.İ.; Yüksek, A.; Yılmaz, İ.N.; Aslan-Yılmaz, A.; Karhan, S.Ü.; Öz, M.İ.; Demirel, N.; Taş, S.; Altıok, A.; et al. Datça-Bozburun Özel Çevre Koruma Bölgesinin Denizsel ve Kıyısal Alanlarının Biyolojik Çeşitliliğinin Tespiti Projesi; Final Raporu, İstanbul Üniversitesi Deniz Bilimleri ve İşletmeciliği Enstitüsü (Sunulan Kuruluş, T.C. Çevre ve Orman Bakanlığı Özel Çevre Koruma Kurumu Başkanlığı); İstanbul Üniversitesi: Ankara, Turkey, 2004; 698p. (In Turkish) [Google Scholar]
- Lipej, L.; Acevedo, I.; Akel, E.H.K.; Anastasopoulou, A.; Angelidis, A.; Azzurro, E.; Castriota, M.; Çelik, L.; Cilenti, F.; Crocetta, A.; et al. New Mediterranean Biodiversity Records (March 2017). Mediterr. Mar. Sci. 2017, 18, 179. [Google Scholar] [CrossRef]
- Öztürk, B.; Bitlis-Bakır, B.; Micali, P. Heterostropha species of the Turkish coasts: Odostomiinae Pelseneer, 1928 (Gastropoda, Heterobranchia, Pyramidellidae). Turk. J. Fish. Aquat. Sci. 2013, 13, 139–157. [Google Scholar]
- Zaminos, G.; Apostolou, C.; Porfyris, A.; Manousis, T.; Zeimbekis, C.; Tsiaras, S.; Galinou-Mitsoudi, S. New records of extant and fossil Mollusca for the Hellenic Seas. Xenophora Taxon. 2021, 34, 16–25. [Google Scholar]
- Crocetta, F.; Tringali, L.P. Mapping alien mollusca distribution in the Mediterranean Sea: The Lessepsian immigrant Retusa desgenettii (Audouin, 1826) reaches Turkey. Quat. Int. 2015, 390, 15–20. [Google Scholar] [CrossRef]
- Manousis, T. The Marine Mollusca of Greece (by January 2023): An updated, systematic catalogue, documented with bibliographic and pictorial references. Xenophora Taxon. 2023, 41, 25–63. [Google Scholar]
- Koçak, C.; Katağan, T. A comparative study of the impacts of three fish farms on the macrofauna in Izmir Bay (Aegean Sea, Turkey). Ege J. Fish. Aquat. Sci. 2005, 22, 287–296. [Google Scholar]
- Reuben Shipway, J.; Borges, L.M.; Müller, J.; Cragg, S.M. The broadcast spawning Caribbean shipworm, Teredothyra dominicensis (Bivalvia, Teredinidae), has invaded and become established in the eastern Mediterranean Sea. Biol. Invasions 2014, 16, 2037–2048. [Google Scholar] [CrossRef][Green Version]
- Ovalis, P.; Mifsud, C. A new species of Turbonilla (Risso, 1826) from SE Turkey (Pyramidellidae: Turbonillinae). Triton 2017, 35, 14. [Google Scholar]
- Ulman, A.; Ferrario, J.; Occhipinti-Ambrogi, A.; Arvanitidis, C.; Bandi, A.; Bertolino, M.; Bogi, C.; Chatzigeorgiou, G.; Çiçek, B.A.; Deidun, A.; et al. A massive update of non-indigenous species records in Mediterranean marinas. PeerJ 2017, 5, e3954. [Google Scholar] [CrossRef]
- Chatzigeorgiou, G.; Androulakis, D.; Skouradakis, G.; Rallis, I.; Gratsia, E. Polychaetes and Amphipodes from Major Ports in Greece in 2020–2021. v1.12. Hellenic Center for Marine Research. Dataset/Samplingevent. 2022. Available online: http://ipt.medobis.eu/resource?r=alienports_polychaetes&v=1.12 (accessed on 10 January 2024).
- Chatzigeorgiou, G.; Faulwetter, S.; Arvanitidis, C. Polychaetes from Two Subtidal Rocky Shores of the North Coast of Crete, Collected for the NaGISA Project 2007–2008. V1.2. Hellenic Centre for Marine Research. 2016. Available online: http://ipt.medobis.eu/resource?r=nagisa_species_2007_2008 (accessed on 7 April 2016).
- Ergen, Z.; Çınar, M.E.; Dağlı, E.; Kurt, G. Lessepsian polychaete species from the Turkish coasts. In Proceedings of Workshop on Lessepsian Migration; Öztürk, B., Basusta, N., Eds.; Turkish Marine Research Foundation: Istanbul, Turkey, 2002; Volume 9, pp. 50–55. [Google Scholar]
- Pérès, J.M. Contribution a la connaisance des Polychetes benthiques des profondeurs moyennes de la Mediterranee. Trav. Stn. Mar. Endoume 1959, 25, 103–135. [Google Scholar]
- Simboura, N.; Nicolaidou, A. The Polychaetes (Annelida, Polychaeta) of Greece: Checklist, Distribution and Ecological Characteristics; Monographs on Marine Sciences; Series no 4; NCMR: Athens, Greece, 2001; 115p. [Google Scholar]
- Yokoyama, H.; Dağlı, E.; Çinar, M.E. First record of Paraprionospio coora Wilson, 1990 (Polychaeta: Spionidae) from the Mediterranean Sea. Mediterr. Mar. Sci. 2010, 11, 133–142. [Google Scholar] [CrossRef][Green Version]
- Simboura, N.; Kurt Sahin, G.; Panagoulia, A.; Katsiaras, N. Four new alien species on the coasts of Greece (Eastern Mediterranean). Mediterr. Mar. Sci. 2010, 11, 341–352. [Google Scholar] [CrossRef][Green Version]
- Arvanitidis, C. Systematic and Bionomic Study of the Macrobenthic Polychaeta of the Northern Aegean. Ph.D. Thesis, Aristotelian University of Thessaloniki, Thessaloniki, Greece, 1994; 512p. (In Greek). [Google Scholar]
- Aker, H.V. Seasonal Distribution of Planktonıc Copepods in the Turkish Coastal Waters of the Middle Aegean Sea. Ph.D. Thesis, Ege University, İzmir, Turkey, 2002. (In Turkish). [Google Scholar]
- ELNAIS. Ellenic Network on Aquatic Invasive Species. Available online: https://elnais.hcmr.gr/elnais-species/ (accessed on 10 May 2023).
- Geldiay, R.; Kocataş, A. A preliminary research on the benthos of Izmir Bay. Ege Univ. Fac. Sci. Monograph. Ser. 1972, 12, 1–34. (In Turkish) [Google Scholar]
- Bilecenoğlu, M.; Çınar, M.E. Alien Species Threat across Marine Protected Areas of Turkey—An Updated Inventory. J. Mar. Sci. Eng. 2021, 9, 1077. [Google Scholar] [CrossRef]
- Soykan, O.; Bakır, K.; Kınacıgil, H.T. Demersal trawl discards with spatial and bathymetric emphasis in the Turkish coast of the Aegean Sea. Mar. Biol. Res. 2019, 15, 113–123. [Google Scholar] [CrossRef]
- Gerovasileiou, V.; Chintiroglou, C.C.; Vafidis, D.; Koutsoubas, D.; Sini, M.; Dailianis, T.; Issaris, Y.; Akritopoulou, E.; Dimarchopoulou, D.; Voultsiadou, E. Census of biodiversity in marine caves of the eastern Mediterranean Sea. Meditter. Mar. Sci. 2015, 16, 245–265. [Google Scholar] [CrossRef]
- Corsini-Foka, M.; Kondylatos, G.; Pancucci-Papadopoulou, M.A. A new alien crab for the Mediterranean Sea: Xanthias lamarckii (H. Milne Edwards, 1834) (Crustacea: Decapoda: Brachyura: Xanthidae). Mediterr. Mar. Sci. 2013, 14, 295–297. [Google Scholar] [CrossRef]
- Brunetti, R.; Griggio, F.; Mastrototaro, F.; Gasparini, F.; Gissi, C. Toward a resolution of the cosmopolitan Botryllus schlosseri species complex (Ascidiacea, Styelidae): Mitogenomics and morphology of clade E (Botryllus gaiae). Zool. J. Lin. Soc. 2020, 190, 1175–1192. [Google Scholar] [CrossRef]
- Frantzis, A. A Long and Deep Step in Range Expansion of an Alien Marine Mammal in the Mediterranean: First Record of the Indian Ocean Humpback Dolphin Sousa plumbea (G. Cuvier, 1829) in the Greek Seas. BioInvasions Rec. 2018, 7, 83–87. [Google Scholar] [CrossRef]
- Vohník, M.; Borovec, O.; Ozbek, E.O.; Okudan Aslan, E.S. Rare phytomyxid infection on the alien seagrass Halophila stipulacea in the southeast Aegean Sea. Mediterr. Mar. Sci. 2017, 18, 433–442. [Google Scholar] [CrossRef]
- García-Escudero, C.A.; Tsigenopoulos, C.S.; Gerakaris, V.; Tsakogiannis, A.; Apostolaki, E.T. Its DNA Barcoding Reveals That Halophila stipulacea Still Remains the Only Non-Indigenous Seagrass of the Mediterranean Sea. Diversity 2022, 14, 76. [Google Scholar] [CrossRef]
- Chaibi, M.; Azzouna, A.; Martín, D. First record of Lepidonotus tenuisetosus (Annelida: Polynoidae) from Tunisia with distributional notes. Mediterr. Mar. Sci. 2023, 24, 7–18. [Google Scholar] [CrossRef]
- Karachle, P.K.; Corsini Foka, M.; Crocetta, F.; Dulčić, J.; Dzhembekova, N.; Galanidi, M.; Ivanova, P.; Shenkar, N.; Skolka, M.; Stefanova, E.; et al. Setting-up a billboard of marine invasive species in the ESENIAS area: Current situation and future expectancies. Acta Adriat. 2017, 58, 429–458. [Google Scholar] [CrossRef][Green Version]
- Zenetos, A.; Corsini-Foka, M.; Crocetta, F.; Gerovasileiou, V.; Karachle, P.K.; Simboura, N.; Tsiamis, K.; Pancucci-Papadopoulou, M.-A. Deep cleaning of alien and cryptogenic species records in the Greek Seas (2018 update). Manag. Biol. Invasions 2018, 9, 209–226. [Google Scholar] [CrossRef]
- Albano, P.G.; Sabbatini, A.; Lattanzio, J.; Päßler, J.F.; Steger, J.; Hua, Q.; Kaufman, D.K.; Szidat, S.; Zuschin, M.; Negri, A. Alleged Lessepsian foraminifera prove native and suggest Pleistocene range expansions into the Mediterranean Sea. Mar. Ecol. Prog. Ser. 2022, 700, 65–78. [Google Scholar] [CrossRef]
- Nascimento, K.B.; Migotto, A.E.; Fehlauer-Ale, K.H. Molecular data suggest the worldwide introduction of the bryozoan Amathia verticillata (Ctenostomata, Vesiculariidae). Mar. Biol. 2021, 168, 33. [Google Scholar] [CrossRef]
- Karachle, P.K.; Zenetos, A.; Xentidis, N.J. The ESENIAS countries’ Marine alien species experts: An updated inventory. Acta Zool. Bulg. 2017, 9, 261–282. [Google Scholar]
- Zenetos, A.; Ovalis, P.; Giakoumi, S.; Kontadakis, C.; Lefkaditou, E.; Mpazios, G.; Simboura, N.; Tsiamis, K. Saronikos Gulf: A hotspot area for alien species in the Mediterranean Sea. BioInvasions Rec. 2020, 9, 873–889. [Google Scholar] [CrossRef]
- Zenetos, A.; Delongueville, C.; Scaillet, R. An Overlooked Group of Citizen Scientists in NonIndigenous Species (NIS) Information: Shell Collectors and Their Contribution to Molluscan NIS Xenodiversity. Diversity 2024, 16, 299. [Google Scholar] [CrossRef]
- Ros, M.; Navarro-Barranco, C.; González-Sánchez, M.; Ostalé-Valriberas, E.; Cervera-Currado, L.; Guerra-García, J.M. Starting the stowaway pathway: The role of dispersal behavior in the invasion success of low-mobile marine species. Biol. Invasions 2020, 22, 2797–2812. [Google Scholar] [CrossRef]
- Fryganiotis, K.; Chintiroglou, C.C. First record of the isopod Paracerceis sculpta in the Aegean Sea: Established populations in north-Aegean marinas. In Katsanevakis, S.; Acar, Ü.; Ammar, I.; Balci, B.A.; Bekas, P.; Belmonte, M.; Chintiroglou, C.C.; Consoli, P.; Dimiza, M.; Fryganiotis, K.; et al. New Mediterranean Biodiversity Records (October, 2014). Mediterr. Mar. Sci. 2014, 15, 675–695. [Google Scholar]
- Bakır, K.; Katağan, T. On the occurrence of Caprella scaura Templeton, 1836 (Crustacea: Amphipoda) in Turkish waters. Zool. Middle East 2011, 52, 125–126. [Google Scholar] [CrossRef]
- Ros, M.; Guerra-García, J.M.; Navarro-Barranco, C.; Cabezas, M.P.; Vázquez-Luis, M. The spreading of the non-native caprellid (Crustacea: Amphipoda) Caprella scaura Templeton, 1836 into southern Europe and northern Africa: A complicated taxonomic history. Mediterr. Mar. Sci. 2014, 15, 145–155. [Google Scholar] [CrossRef]
- Taşkın, E.; Evcen, A.; Bilgiç, F. Further expansion of the alien marine green macroalga Caulerpa taxifolia var. distichophylla (Sonder) Verlaque, Huisman & Procacini in Türkiye. J. Black Sea/Mediterr. Environ. 2023, 29, 121–126. [Google Scholar]
- Zenetos, A.; Tsiamis, K.; Galanidi, M.; Carvalho, N.; Bartilotti, C.; Canning-Clode, J.; Castriota, L.; Chainho, P.; Comas-González, R.; Costa, A.C.; et al. Status and trends in the rate of introduction of marine non-indigenous species in European seas. Diversity 2022, 14, 1077. [Google Scholar] [CrossRef]
- European Commission. European Commission Decision (EC2017/848 of 17 May 2017 laying down criteria and methodological standards on good environmental status of marine waters and specifications and standardized methods for monitoring and assessment, and repealing Decision 2010/477/EU. Off. J. Eur. Union 2017, L125, 43–74. [Google Scholar]
- Zenetos, A.; Gratsia, E.; Cardoso, A.; Tsiamis, K. Time lags in reporting of biological invasions: The case of Mediterranean Sea. Meditterr. Mar. Sci. 2019, 20, 469–475. [Google Scholar] [CrossRef]
- Kondylatos, G.; Vagenas, G.; Kalaentzis, K.; Mavrouleas, D.; Conides, A.; Karachle, P.K.; Corsini-Foka, M.; Klaoudatos, D. Exploring the Structure of Static Net Fisheries in a Highly Invaded Region: The Case of Rhodes Island (Eastern Mediterranean). Sustainability 2023, 15, 14976. [Google Scholar] [CrossRef]
- Androulidakis, Y.; Krestenitis, Y.; Kourafalou, V. Marine heatwaves over different coastal environments: Findings from the NE Mediterranean Sea to south Florida In Marine Heatwaves in the Mediterranean Sea and Beyond; Briand, F., Ed.; CIESM Monograph 51; CIESM Publisher: Paris, France, 2024; 174p. [Google Scholar]
- Zervoudaki, S.; Siokou, I.; Krasakopoulou, E.; Kontoyiannis, H.; Pavlidou, A.; Assimakopoulou, G.; Katsiaras, N.; Reizopoulou, S.; Karageorgis, A.P.; Kaberi, H.; et al. Biogeochemical Characteristics in the Saronikos Gulf (Aegean Sea, Eastern Mediterranean). In The Handbook of Environmental Chemistry; Springer: Berlin/Heidelberg, Germany, 2022. [Google Scholar] [CrossRef]
- Kalyvioti, G.; Galanidi, M.; Zenetos, A. Risk assessment to identify high-risk voyage origin ports and a watch list for NIS introduction in the Mediterranean with vessels: The case of Saronikos Gulf, Greece. Manag. Biol. Invasions 2024, 15, 337–370. [Google Scholar] [CrossRef]
- Galanidi, M.; Zenetos, A. Data-Driven Recommendations for Establishing Threshold Values for the NIS Trend Indicator in the Mediterranean Sea. Diversity 2022, 14, 57. [Google Scholar] [CrossRef]
- Corsini Foka, M.; Zenetos, A.; Crocetta, F.; Cinar, M.E.; Kocak, F.; Golani, D.; Katsanevakis, S.; Tsiamis, K.; Cook, E.; Froglia, C.; et al. Inventory of alien and cryptogenic species of the Dodecanese (Aegean Sea, Greece): Collaboration through COST action training school. Manag. Biol. Invasions 2015, 6, 351–366. [Google Scholar] [CrossRef]
- Bizsel, K.C.; Kozludere, S.; Beşiktepe, Ş.; Bizsel, N.; Sayın, E.; Yüksek, A.; Kaboğlu, G.; Akçalı, B.; Can Yılmaz, E.; Kavcıoğlı, R. The Final Report on Determination of Marine and Coastal Biodiversity of Köyceğiz-Dalyan Special Environmental Protection Area Project; Environment Protection Agency for Special Areas: Ankara, Turkey, 2010. (In Turkish) [Google Scholar]
- Kıraç, C.O.; Ünal, V.; Veryeri, N.O.; Güçlüsoy, H.; Yalçıner, A.C. The inventory of the coastal zone management based projects in Gökova and effectiveness in conservation. In Proceedings of the Coastal and Marine Areas of Turkey IX. National Congress, Antakya, Hatay, 14–17 November 2012; pp. 241–252. (In Turkish). [Google Scholar]
- Okus, E.; Yüksek, A.; Yilmaz, I.N.; Yilmaz, A.A.; Karhan, S.Ü.; Öz, M.İ.; Demirel, N.; Tas, S.; Demir, V.; Zeki, S.; et al. Marine biodiversity of Datça-Bozburun specially protected area (Southeastern Aegean Sea, Turkey). J. Black Sea/Mediterr. Environ. 2007, 13, 39–49. [Google Scholar]

| Group | Species | Türkiye | Greece | Source |
|---|---|---|---|---|
| MOL | Acteocina mucronata (Philippi, 1849) | 2017 | Bitlis and Özturk in [34] | |
| MOL | Atys ehrenbergi (Issel, 1869) | 2016 | Agamennone and Micali [28] | |
| MOL | Baeolidia moebii Bergh, 1888 | 2021 | Kytinou et al. [31] | |
| MOL | Biuve fulvipunctata (Baba, 1938) | 2004 | 2020 | TR [78]; GR [79] |
| MOL | Cingulina isseli (Tryon, 1886) | 2021 | Ovalis and Zenetos in [21] | |
| MOL | Cycloscala hyalina (G.B. Sowerby II, 1844) | 2017–2018 | Manousis [26] | |
| MOL | Dikoleps micalii Agamennone, Sbrana, Nardi, Siragusa and Germanà, 2020 | 2016 | Agamennone et al. [18] | |
| MOL | Elysia nealae Ostergaard, 1955 | 2019 | Manousis et al. [79] | |
| MOL | Elysia tomentosa K. R. Jensen, 1997 | 2002–2004 | Okuş et al. [80] | |
| MOL | Finella pupoides A. Adams, 1860 | 2012 | Manousis [26] | |
| MOL | Haloa japonica (Pilsbry, 1895) | 2020 | Manousis et al. [79] | |
| MOL | Heliacus implexus (Mighels, 1845) | 2019 | Kontadakis et al. [25] | |
| MOL | Isognomon bicolor (C. B. Adams, 1845) | 2016 | Angelidis in [81] as M. regula | |
| MOL | Juxtacribrilina mutabilis (Ito, Onishi & Dick, 2015) | 2019–2020 | Martaeng et al. [37] | |
| MOL | Megastomia lorioli (Hornung & Mermod, 1924) | 2015 | 2010 | TR [82]; GR [26] |
| MOL | Melanella orientalis Agamennone, Micali & Siragusa, 2020 | 2016 | Agamennone et al. [19] | |
| MOL | Nudiscintilla cf. glabra Lützen & C. Nielsen, 2005 | 2019 | Geirun and Zenetos in [27] | |
| MOL | Odostomia sp. | 2020–2021 | Zaminos et al. [83] | |
| MOL | Retusa desgenettii (Audouin, 1826) | 2002 | 2014 | TR [84]; GR [85] |
| MOL | Rissoina bertholleti Issel, 1869 | 2005 | Koçak and Katağan [86] | |
| MOL | Spondylus cf. spinosus Schreibers, 1793 | 2002–2004 | Okuş et al. [80] | |
| MOL | Stosicia lineata (Dunker, 1860) | 2023 | Ovalis and Zenetos [41] | |
| MOL | Teredothyra dominicensis (Bartsch, 1921) | 2011 | Reuben Shipway et al. [87] | |
| MOL | Turbonilla edgarii (Melvill, 1896) | 2020 | Manousis [26] | |
| MOL | Turbonilla cangeyrani Ovalis Mifsud, 2017 | 2016 | Ovalis and Mifsud [88] | |
| RHO | Colaconema codicola (Børgesen) Stegenga, J.J.Bolton R.J.Anderson | 2019 | Tsioli and Orfanidis in [27] | |
| RHO | Womersleyella setacea (Hollenberg) R.E.Norris | 2022 | Taşkin and Minareci in [48] | |
| OCHR | Colpomenia peregrina Sauvageau | 2022 | This study (Tübitak Project 121Y215) | |
| CHLO | Udotea flabellum (J. Ellis Solander) M. Howe | 2022 | Okudan and Tuney Kizilkaya in [47] | |
| POL | Branchiomma bairdi (McIntosh, 1885) | 2015 | 2015 | GR: Ulman et al. [89] |
| POL | Branchiosyllis maculata (Imajima, 1966) | 2020–2021 | Chatzigeorgiou et al. [90] | |
| POL | Caulleriella fragilis (Leidy, 1855) | 2020–2021 | Chatzigeorgiou et al. [90] | |
| POL | Dodecaceria sextentaculata (Delle Chiaje, 1822–1826) | 2020–2021 | Chatzigeorgiou et al. [90] | |
| POL | Eurythoe complanata (Pallas, 1766) | 2008 | Chatzigeorgiou et al. [91] | |
| POL | Hydroides amri Sun, Wong, ten Hove, Hutchings, Williamson Kupriyanova, 2015 | 2020–2021 | Chatzigeorgiou et al. [90] | |
| POL | Hydroides operculata (Treadwell, 1929) | 2020–2021 | Chatzigeorgiou et al. [90] | |
| POL | Metasychis gotoi (Izuka, 1902) | 1996 | 1955 | TR [92]; GR [93] |
| POL | Neopseudocapitella brasiliensis Rullier Amoureux, 1979 | 1991 | GR: Simboura and Nicolaidou [94] | |
| POL | Notomastus mossambicus (Thomassin, 1970) | 2018 | Katsanevakis et al. [52] | |
| POL | Paraprionospio cooraWilson, 1990 | 1999 | 1983 | TR [95]; GR. [96] |
| POL | Prionospio japonica Okuda, 1935 | 2020–2021 | Chatzigeorgiou et al. [90] | |
| POL | Syllis crassicirrata (Treadwell, 1925) | 2020–2021 | Chatzigeorgiou et al. [90] | |
| POL | Terebella ehrenbergiGrube, 1869 | 1970 | Arvanitidis [97] | |
| CRU/AMP | Laticorophium baconi (Shoemaker, 1934) | 2022 | Guerra-García et al. [38] | |
| CRU/COP | Calanopia elliptica(Dana, 1849) | 1999 | 2018 | TR: Aker [98]; GR [99] |
| CRU/COP | Labidocera pavoGiesbrecht, 1889 | 1999 | Aker [98] | |
| CRU/COP | Pseudodiaptomus marinus Sato, 1913 | 2015 | 2016 | TR [44]; GR [33] |
| CRU/COP | Parvocalanus crassirostris (Dahl F., 1894) | 2016 | Besiktepe et al. [44] | |
| CRU/CIR | Megabalanus tintinnabulum(Linnaeus, 1758) | 1969 | Geldiay and Kocataş [100] | |
| CRU/ISO | Paracerceis sculpta (Holmes, 1904) | 2022 | Dağlı et al. [51] | |
| CRU/DEC | Gonioinfradens giardi (Nobili, 1905) | 2015 | Katsanevakis et al. [52] | |
| CRU/DEC | Homarus americanus H. Milne Edwards, 1837 | 2019 | Kampouris et al. [24] | |
| CRU/DEC | Matuta victor (J.C. Fabricius, 1781) | 2022 | Karachle and Martinez in [47] | |
| CRU/DEC | Metapenaeus monoceros (Fabricius, 1798) | 2021 | Bilecenoğlu and Çınar [101] | |
| CRU/DEC | Penaeus semisulcatusDe Haan, 1844 | 2012 | Soykan et al. [102] | |
| CRU/DEC | Pilumnus minutusDe Haan, 1835 | 2010 | Gerovasileiou et al. [103] | |
| CRU/DEC | Urocaridella pulchella Yokeş Galil, 2006 | 2020 | Digenis et al. [22] | |
| CRU/DEC | Xanthias lamarckii(H. Milne Edwards, 1834) | 2013 | Corsini-Foka et al. [104] | |
| ASC | Botryllus gaiae Brunetti, 2020 | 2000 | Brunetti et al. [105] | |
| ASC | Clavelina oblonga Herdman, 1880 | 2020 | Alvanou et al. [40] | |
| ASC | Didemnum vexillum Kott, 2002 | 2022 | Çinar and Özgül [49] | |
| ASC | Microcosmus squamiger Michaelsen, 1927 | 2019 | Montesanto and Mastrototaro in [34] | |
| ASC | Polyclinum constellatum Savigny, 1816 | 2019 | Montesanto et al. [32] | |
| BRY | Celleporaria brunnea (Hincks, 1884) | 2020 | Georgiadis and Evangelopoulos in [34] | |
| CNI | Phyllorhiza punctata von Lendenfeld, 1884 | 2018 | This study: ELNAIS [99] | |
| FISH | Cheilodipterus novemstriatus (Rüppell, 1838) | 2023 | 2022 | TR [48]; GR [42] |
| FISH | Fistularia petimba Lacepède, 1803 | 2019 | 2020 | TR [45]; GR [11] |
| FISH | Oncorhynchus kisutch (Walbaum, 1792) | 2021 | Kampouris and Batjakas in [21] | |
| FISH | Pagrus major (Temminck Schlegel, 1843) | 2019 | Kampouris et al. [23] | |
| FISH | Oxyurichthys petersii (Klunzinger, 1871) | 2018 | Evangelopoulos et al. [11] | |
| FISH | Scarus ghobban Forsskål in Niebuhr, 1775 | 2021 | Akyol and Unal in [34] | |
| MAM | Sousa plumbea(G. Cuvier, 1829) | 2018 | Frantzis [106] | |
| PAR | Marinomyxa marina Kolátková, Cepicka, Hoffman et Vohník | 2015 | 2018 | TR [107]; GR [29] |
| Group | Species | GR 2020 | TR 2021 | Comments for the Mediterranean Sea |
|---|---|---|---|---|
| RHO | Ganonema farinosum (Lamouroux) Fan and Wang | no | yes | Status unresolved (debatable species) |
| RHO | Acanthophora nayadiformis (Delile) Papenfuss | no | yes | Status unresolved (debatable species) |
| RHO | Ceramium bisporum D.L.Ballantine | yes | no | Cryptogenic |
| RHO | Polysiphonia kampsaxii Boergesen | no | yes | Questionable record |
| RHO | Vertebrata fucoides (Hudson) Kuntze | no | yes | NIS in TR—cryptogenic elsewhere |
| TRA | Halophila decipiens Ostenfeld | yes | no | Misidentification ([108]) |
| POL | Caulleriella viridis (Langerhans, 1881) | yes | no | Probably cryptogenic ([70]) |
| POL | Lepidonotus tenuisetosus (Gravier, 1902) | yes | no | Misidentification ([109]) |
| POL | Neanthes agulhana (Day, 1963) | yes | no | Likely cryptogenic ([70]) |
| POL | Sigambra parva (Day, 1963) | yes | no | Probably cryptogenic ([70]) |
| ASC | Diplosoma listerianum (Milne Edwards, 1841) | yes | yes | Status unresolved (debatable species) |
| ASC | Ascidiella aspersa (Müller, 1776) | yes | yes | Cryptogenic |
| BRY | Amathia verticillata (delle Chiaje 1822) | yes | yes | Status unresolved (debatable species) |
| CNI | Filellum serratum (Clarke, 1879) | no | yes | Status unresolved (debatable species) |
| CRU/DEC | Calappa pelii Herklots, 1851 | yes | no | Status unresolved (debatable species) |
| CRU/CIR | Amphibalanus improvisus (Darwin, 1854) | no | yes | Status unresolved (debatable species) |
| CRU/CIR | Coleusia signata (Paulson, 1875) | yes | no | Absent. Present only in the Levantine |
| CRU/TAN | Paradoxapseudes intermedius (Hansen, 1895) | no | yes | Native |
| CRU/ISO | Mesanthura cf. romulea Poore and Lew-Ton, 1986 | yes | no | In the Levantine only |
| FOR | Adelosina colomii (Le Calvez Le Calvez, 1958) | no | yes | Native |
| FOR | Amphisorus hemprichii Ehrenberg, 1840 | no | yes | Status unresolved (debatable species) |
| FOR | Articulina alticostata Cushman, 1944 | no | yes | Questionable record |
| FOR | Articulina carinata Wiesner, 1923 | no | yes | Native |
| FOR | Astacolus insolitus (Schwager, 1866) | no | yes | Questionable record |
| FOR | Bolivina arta MacFadyen, 1931 | no | yes | Native/range expanding |
| FOR | Bolivina striatula Cushman, 1922 | no | yes | Native/range expanding |
| FOR | Coscinospira acicularis (Batsch, 1791) | no | yes | Native/range expanding |
| FOR | Cushmanina striatopunctata (Parker and Jones, 1865) | no | yes | Cryptogenic |
| FOR | Cymbaloporetta plana (Cushman, 1915) | no | yes | Status unresolved (debatable species) |
| FOR | Cymbaloporetta squammosa (d’Orbigny, 1839) | no | yes | Status unresolved (debatable species) |
| FOR | Dentalina albatrossi (Cushman, 1923) | no | yes | Questionable record |
| FOR | Euthymonacha polita (Chapman, 1904) | no | yes | Status unresolved (debatable species) |
| FOR | Globobulimina auriculata (Bailey, 1894) | no | yes | Native/range expanding |
| FOR | Iridia diaphana Heron-Allen and Earland, 1914 | no | yes | Native/range expanding |
| FOR | Melonis affinis (Reuss, 1851) | no | yes | Circumglobal distribution |
| FOR | Nodophthalmidium antillarum (Cushman, 1922) | no | yes | Native/range expanding |
| FOR | Peneroplis arietinus (Batsch, 1791) | no | yes | Native/range expanding |
| FOR | Peneroplis pertusus (Forsskål in Niebuhr, 1775) | no | yes | Status unresolved (debatable species) |
| FOR | Peneroplis planatus (Fichtel and Moll, 1798) | no | yes | Native/range expanding |
| FOR | Planogypsina squamiformis (Chapman, 1901) | no | yes | Native/range expanding |
| FOR | Polymorphina fistulosa Williamson, 1858 | no | yes | Status unresolved (debatable species) |
| FOR | Pseudonodosaria brevis (d’Orbigny, 1846) | no | Yes | Questionable record |
| FOR | Pulleniatina obliquiloculata (Parker Jones, 1862) | no | yes | Native/range expanding |
| FOR | Pyramidulina catesbyi (d’Orbigny, 1839) | no | yes | Native/range expanding |
| FOR | Pyramidulina perversa (Schwager, 1866) | no | yes | Questionable record |
| FOR | Quinqueloculina carinatastriata (Wiesner, 1923) | no | yes | Native |
| FOR | Quinqueloculina sp. C d’Orbigny, 1826 | no | yes | Questionable record |
| FOR | Recurvoidella bradyi (Robertson, 1891) | no | yes | Native/range expanding |
| FOR | Sorites orbiculus Ehrenberg, 1839 | no | yes | Native/range expanding |
| FOR | Triloculina affinis d’Orbigny, 1852 | no | yes | Synonym of Triloculina trigonula (Lamarck, 1804), which is native |
| FOR | Triloculina cf. fichteliana d’Orbigny, 1839 | no | yes | Native/range expanding |
| FOR | Triloculina sp. A d’Orbigny, 1826 | no | yes | Questionable record |
| FOR | Vaginulinopsis sublegumen Parr, 1950 | no | yes | Questionable record |
| FOR | Veleroninoides scitulus (Brady, 1881) | no | yes | Cryptogenic, Circumglobal distribution |
| FISH | Abudefduf cf. saxatilis (Linnaeus, 1758) | yes | no | Native, crytogenic, misidentification Merged with A. cf. vaigiensis reported as A. saxatilis/vaigiensis/troschelii |
| FISH | Tylosurus crocodilus (Péron Lesueur, 1821) | yes | no | Misidentification |
| Group | Species | |
|---|---|---|
| MOL | Bursatella leachii Blainville, 1817 | Cryptogenic in IL, IT, MT—NIS elsewhere |
| ECH | Ophiactis savignyi (Müller Troschel, 1842) | Cryptogenic in IT—NIS elsewhere |
| POL | Hydroides dirampha Mörch, 1863 | Cryptogenic in ES—NIS elsewhere |
| POL | Hydroides elegans (Haswell, 1883) | cryptogenic in ES—NIS elsewhere |
| POL | Eurythoe complanata (Pallas, 1766) | Likely alien polychaete |
| POL | Metasychis gotoi (Izuka, 1902) | Likely alien polychaete |
| POL | Neopseudocapitella brasiliensis Rullier Amoureux, 1979 | Likely alien polychaete |
| POL | Pista unibranchia Day, 1963 | Likely alien polychaete |
| POL | Ficopomatus enigmaticus (Fauvel, 1923) | NIS questioned as cryptogenic |
| PATH WAY | Levantine Sea | Aegean Sea | Marmara Sea | Ionian Sea | |
|---|---|---|---|---|---|
| Amphistegina lobifera Larsen, 1976 | UNA/TS | 1950 | 1967 | 1955–1961 | |
| Asparagopsis taxiformis (Delile) Trevisan de Saint-Léon | UNA | 2006 | 1992 | 1984 | 1992 |
| Brachidontes pharaonis (P. Fischer, 1870) | UNA/TS | 1876 | 1975 | 2004 | 1969 |
| Bregmaceros nectabanus Whitley, 1941 | UNA | 2002 | 2005 | - | 2016 |
| Bursatella leachii Blainville, 1817 | UNA | 1940 | 1975 | 2020 | 1973 |
| Callinectes sapidus Rathbun, 1896 | TS | <1941 | 1947 | 2001 | 1999 |
| Callionymus filamentosus Valenciennes, 1837 | UNA | 1953 | 2003 | - | 2007 |
| Caulerpa cylindracea Sonder | TS | 1991 | 1996 | 2020 | 1993 |
| Celleporaria brunnea (Hincks, 1884) | TS | 2003 | 2004 | - | 2013 |
| Chaetozone corona Berkeley and Berkeley, 1941 | TS | 2005 | 1980 | 2008 | 1982 |
| Champsodon nudivittis (Ogilby, 1895) | UNA | 2008 | 2010 | - | - |
| Ciona robusta Hoshino and Tokioka, 1967 | TS | 1816 | 1901 | - | 2021 |
| Codium fragile (Suringar) Hariot | TS | 1998 | 1983 | 1998 | ≤1974 |
| Cutleria multifida (Turner) Greville | TS | 1997 | 1932 | 1984 | 1904 |
| Diadema setosum (Leske, 1778) | UNA | 2009 | 2014 | - | 2019 |
| Etrumeus golanii DiBattista, Randall and Bowen, 2012 | UNA | 1931 | 1999 | - | - |
| Fistularia commersonii Rüppell, 1838 | UNA | 1975 | 2001 | - | 2007 |
| Halophila stipulacea (Forsskål) Ascherson | UNA/TS | 1894 | 1923 | - | 1955 |
| Hydroides elegans (Haswell, 1883) | TS | 1904 | 1972 | 2012 | 1964 |
| Lagocephalus guentheri Miranda Ribeiro, 1915 | UNA | 1949 | 1952 | 2007 | 2005 |
| Lagocephalus sceleratus (Gmelin, 1789) | UNA | 2004 | 2003 | 2008 | 2009 |
| Lagocephalus suezensis Clark and Gohar, 1953 | UNA | 1975 | 2000 | - | - |
| Lophocladia trichoclados (C.Agardh) F.Schmitz | TS | 1953 | 1908 | - | 1969 |
| Lysidice collaris Grube, 1870 | TS | 1968 | 1975 | - | <1961 |
| Magallana/Crassostrea sp./spp. | REL | 1992 | 2006 | 2004 | 1966 |
| Metasychis gotoi (Izuka, 1902) | TS | 1957 | 1955 | 2008 | 1984 |
| Mnemiopsis leidyi A. Agassiz, 1865 | TS | 1992 | 1990 | 1991 | 2009 |
| Notomastus aberans Day, 1957 | TS | 1997 | 1964 | 2013 | 1990 |
| Penaeus aztecus Ives, 1891 | EC | 2009 | 2012 | - | 2013 |
| Pinctada radiata (Leach, 1814) | UNA/REL | 1874 | 1961 | - | 1995 |
| Pterois miles (Bennett, 1828) | UNA | 1991 | 2009 | - | 2016 |
| Saurida lessepsianus Russell, Golani and Tikochinski, 2015 | UNA | 1951 | 1960 | - | 1990 |
| Scomberomorus commerson (Lacepède, 1800) | UNA | 1935 | 1994 | - | 2017 |
| Siganus luridus (Rüppell, 1829) | UNA | 1930 | 1964 | - | 1973 |
| Siganus rivulatus Forsskål and Niebuhr, 1775 | UNA | 1924 | 1925 | 2019 | 2009 |
| Sphyraena chrysotaenia Klunzinger, 1884 | UNA | 1931 | 1966 | - | 2011 |
| Stephanolepis diaspros Fraser-Brunner, 1940 | UNA | 1924 | 1943 | 2011 | 1967 |
| Styela plicata (Lesueur, 1823) | TS | 1927 | 1968 | - | 1948 |
| Stypopodium schimperi (Kützing) M.Verlaque and Boudouresque | UNA | 1973 | 1989 | - | 2008 |
| Synaptula reciprocans (Forsskål, 1775) | UNA | 1967 | 1986 | 2014 | - |
| Upeneus moluccensis (Bleeker, 1855) | UNA | 1930 | 1947 | 2017 | 1976 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zenetos, A.; Doğan, A.; Bakir, A.K.; Chatzigeorgiou, G.; Corsini-Foka, M.; Dağli, E.; Evangelopoulos, A.; Meriç, E.; Stoumboudi, M.; Taşkin, E.; et al. Non-Indigenous Species (NIS) Know No Geopolitical Borders—An Update of NIS in the Aegean Sea. Diversity 2025, 17, 12. https://doi.org/10.3390/d17010012
Zenetos A, Doğan A, Bakir AK, Chatzigeorgiou G, Corsini-Foka M, Dağli E, Evangelopoulos A, Meriç E, Stoumboudi M, Taşkin E, et al. Non-Indigenous Species (NIS) Know No Geopolitical Borders—An Update of NIS in the Aegean Sea. Diversity. 2025; 17(1):12. https://doi.org/10.3390/d17010012
Chicago/Turabian StyleZenetos, Argyro, Alper Doğan, Ahmet Kerem Bakir, Georgios Chatzigeorgiou, Maria Corsini-Foka, Ertan Dağli, Athanasios Evangelopoulos, Engin Meriç, Maria Stoumboudi, Ergun Taşkin, and et al. 2025. "Non-Indigenous Species (NIS) Know No Geopolitical Borders—An Update of NIS in the Aegean Sea" Diversity 17, no. 1: 12. https://doi.org/10.3390/d17010012
APA StyleZenetos, A., Doğan, A., Bakir, A. K., Chatzigeorgiou, G., Corsini-Foka, M., Dağli, E., Evangelopoulos, A., Meriç, E., Stoumboudi, M., Taşkin, E., Yokeş, M. B., & Galanidi, M. (2025). Non-Indigenous Species (NIS) Know No Geopolitical Borders—An Update of NIS in the Aegean Sea. Diversity, 17(1), 12. https://doi.org/10.3390/d17010012






