Abstract
A study of diatoms in eight watercourses (four spawning rivers and four streams) in the area of the Ozernovsky Mining and Metallurgical Complex (MMC) on the Kamchatka Peninsula was carried out for the first time. A total of 174 taxa were identified, and a comparative analysis of periphyton species diversity at the sampling stations was carried out. A new species for science was proposed: Gomphonema anissimovae Glushchenko, Kezlya & Kulikovskiy sp. nov. The composition and quantitative characteristics of plankton were analysed only in samples collected from rivers. It was shown that all rivers are oligotrophic in terms of phytoplankton biomass. The work includes lists of taxa with indication of their abundance, as well as illustrative material of found diatom taxa, which will provide an opportunity to monitor changes in planktonic and periphyton microalgae communities in the studied watercourses in the context of potentially high anthropogenic load from industry.
Keywords:
algae; Bacillariophyceae; diatoms; plankton; freshwater; microalgal diversity; periphyton; voucher specimens 1. Introduction
The Kamchatka Peninsula is located in the north-eastern part of Russia. It is bordered by the Sea of Okhotsk to the west and the Bering Sea and the Pacific Ocean to the east.
More than 140,000 large and small rivers flow through the peninsula, of which 95.3% are small watercourses up to 10 km long, and the density of the river network is 0.78 km/km2 [1]. Kamchatka is a unique region where the natural spawning of six species of Pacific salmon still remains [2]. Each water body and watercourse is a spawning and rearing ground for these, where they go through all stages of early ontogenesis from the development of eggs to the formation of smolts [3].
The diversity of freshwater microalgae of the Kamchatka Peninsula has been studied very unevenly. Most of the existing works are devoted to the study of phytoplankton of lakes. The phytoplankton of Lake Kurilskoye has been studied most extensively. A large array of data on phytoplankton (collections for the period from 1980 to 2000) was processed and summarised in the dissertation work of E.V. Lepskaya [4] and the article by Lepskaya and Bonk [5]. Large-scale work was carried out to study the spatial distribution and the dynamics of quantitative characteristics of phytoplankton in Lake Dalneye [6]. A bloom of the diatoms of the genus Stephanodiscus Ehrenberg was noted in the upper water layer in early summer, after the ice melting. Lake Kronotskoye is the second largest by catchment area and the third deepest among the water bodies of the Kamchatka Peninsula. A review of literature data on microalgae and the results of our own research are given in the work of S.I. Genkal and E.V. Lepskaya [7]. The authors note that in the early studies the information on microalgae is fragmentary and incomplete. It was shown that diatoms, together with representatives of green algae and cyanobacteria, have a major role in the phytoplankton of Lake Kronotskoye.
Long-term studies of phytoplankton (from 1999 to 2013) were conducted in the Tolmachevskoye Reservoir in connection with a large-scale experiment on the introduction of the freshwater form of sockeye salmon—kokanee salmon (Oncorhynchus nerka kennerlyi)—from the native population of Lake Kronotskoye [8]. Long-term changes in quantitative and structural characteristics of phytoplankton biomass were studied under the conditions of transforming the lake into a reservoir, changes in temperature regime, and biogenic conditions. The species composition includes 77 taxa from 5 phyla, with diatoms having a major role in the plankton.
In 2019, the first data on the study of planktonic microalgae of the inaccessible Lake Talovskoye and some water bodies of its basin (peat lakes, the old river Maelgovayam) were published [9]. Fifty-four taxa from six phyla were found. The most diversely represented groups were diatoms (21 taxa) and cyanobacteria (10 taxa). The publication contains illustrative tables with images of the found algae.
In the works devoted to the study of centric diatoms of the Verkhneavachinskiye lakes (Demidovskoye, Medvezhye, Verkhneavachinskoye, Vysokoye, Maloye, and several unnamed lakes) located in the spurs of the Bakening volcano [10,11], it is mentioned that data on the physical, chemical, and biological characteristics of these reservoirs are limited because the region is difficult to access, and many data have not been published and are available only in archival materials. It is noted that diatoms were dominant in all lakes.
A list of 275 diatom taxa is presented in the paper devoted to the study of diatom flora in the lakes of the Koryak Mountains (Vatyt-Gytkhyn, Ilir-Gytkhyn, and Potat-Gytkhyn) [12]. It is shown that each lake is characterised by a distinctive community of diatom algae. The article contains brief diagnoses, data on distribution and ecology, and original illustrations of species new to the flora of Russia (31), as well as 50 forms defined only to the genus level.
A number of studies are aimed at detailed investigations of individual taxa. Morphology and seasonal abundance dynamics of the permanently dominant phytoplankton species Aulacoseira subarctica (O. Müller) E.Y. Haworth was studied [13]. The morphological variability of Gomphonema ventricosum Gregory was studied on the example of materials from Lake Kurilskoye, Lake Baikal, and Lake Khövsgöl (Mongolia) [14]. Genkal et al. [15] studied the morphological variability of the centric diatom Cyclotella tripartita Håkansson in Holocene sediments of Lake Dalneye, Upper Pleistocene sediments of spring Burlyashchyj and stream Komarinyj, and in the plankton of spawning and feeding lakes Vatyt-Gytkhyn, Listvennichnoye, and Kurilskoye. The diagnosis of the species was expanded; in all studied lakes the species developed abundantly and was a part of the dominant complex.
A paleoecological analysis of the diatom community was carried out at Lake Spasatelnyj Krug [16]. Here, 83 sediment samples were studied, and 163 species and subspecies of diatoms were identified. As a result of a consideration of 6500 years of ecological history, it was noted that between 6300 and 3900 years ago there was an event of natural eutrophication of the water body. It was shown that diatoms and chironomids demonstrated almost simultaneous changes in composition.
A number of studies involve the examination of soil microalgae and cyanobacteria of Kamchatka: the investigations have included volcanic substrates [14,15,16,17,18,19,20], lava tubes [21], and diatom communities in ecotones [18].
A high diversity of diatoms was found in various water bodies on Bering Island, the largest of the Commander Islands [22]. Diatom samples were collected in summer 2008 from 85 sites, mainly from ponds, small lakes, rivers, and streams. A total of 313 diatom taxa were found, most of which are illustrated (the publication contains 401 images). It was noted that the diatom flora of the island consisted mainly of species characteristic of northern and highland regions of Eurasia and North America. Five new species from the genera Eunotia, Diadesmis, Psammothidium, and Pinnularia were described. The author concludes that Aulacoseira nivalis, Stauroforma exiguiformis, and various species of Frustulia, Eunotia, and Pinnularia are the most common in weakly acidic and soft-water tundra ponds. The rivers have predominantly slightly alkaline water and the dominant species are Planothidium lanceolatum, P. haynaldii, Hannaea arcus, and Diatoma mesodon. In a few places that were obviously influenced by the inflow of marine waters the dominant species were Melosira moniliformis and Mastogloia elliptica. The most abundant species were Ctenophora pulchella and Pseudostaurosira subsalina.
Thus, the diversity of freshwater microalgae in Kamchatka is still insufficiently studied. Most of the works are devoted to the study of lake phytoplankton. A significant part of them is carried out by single samples due to inaccessibility of the water bodies. Long-term studies were conducted only on Lake Kurilskoye and the Tolmachevskoye Reservoir. The main contribution to the formation of phytoplankton biomass and zooplankton food base in Kamchatka lakes is often made by diatoms. So far, the Kamchatka watercourses remain practically unstudied in terms of microalgae research.
The creation of taxonomic reference voucher floras of local regions is currently fundamentally important due to the rapidly developing taxonomy of microalgae, revision of species boundaries, active description of new taxa, and application of next generation sequencing approaches to studies of protist communities and ecological monitoring. Well-documented biodiversity and community structure provide a basis for verifying species richness, assessing changes in the communities due to anthropogenic pressure and climate change, harmonising existing and future monitoring data, and comparing data from modern metabarcoding approaches [23,24,25,26,27,28]. In this respect, information on microalgae and cyanobacteria of Kamchatka is mostly published in the form of taxonomic lists, and microphotographs are presented only in a few works: [5] (54 taxa), [7] (15 taxa), [12] (31 taxa, new for Russia), [29] (16 taxa of centric diatoms), [22] (238 taxa), [10,14] (1 taxon each).
The present study provides the first information on the diversity of diatom periphyton of four streams (Etalonnyj-1, Etalonnyj-2, Nezhnyj, Homut, unnamed stream (PC 547-20)) and four rivers (Ozernaya, Levaya Ozernaya, Pravaya Ozernaya, Perevalnaya) located in the area of the Ozernovsky Mining and Metallurgical Complex (OMMC). The first information on the composition and quantitative development of phytoplankton of the rivers is also given. The streams Etalonnyj-1, Etalonnyj-2, and Homut are tributaries of the Levaya Ozernaya River, the stream Nezhnyj is a tributary of the Perevalnaya River; they flow through the territory of the OMMC and experience a strong anthropogenic impact from the industry. The prospects of significant production expansion and the proximity of large spawning rivers to the enterprise make it necessary to control the ecological condition of watercourses. After all, the contamination by production wastes (such as acidification (acid drainage), uncontrolled discharge of water polluted with heavy metals from the tailings dump and cesspools into the underground horizons, possible accidents or seismic events, etc.) will potentially cause degradation of the food chain starting from microalgae and micro–macrozoobenthos and ending with disruption of the development of fish eggs and juvenile fish and, as a consequence, destruction of the food base of the Kamchatka brown bear, not to mention the reduction of commercial and recreational fishing in these rivers.
The results obtained in the present work can be used as a reference for monitoring changes in species composition and ecological status of these watercourses on the basis of diatoms under the conditions of expanding the production capacity of the enterprise.
2. Materials and Methods
2.1. Study Area and Environmental Conditions
The studied watercourses are located on the eastern macroslope of the Kamchatka Sredinny Range, which is the main water divide structure of the peninsula, in the zone of anthropogenic impact of the Ozernovsky MMC (Figure 1). The watercourses belong to the Anadyro-Kolyma basin district, water management area 19.06.00.003, and are part of the basins of the large highly productive salmon rivers Uka (flows into the Karaginsky Bay of the Bering Sea) and Ozernaya (flows into the Ozernovsky Bay of the Bering Sea—Commander Basin). According to open source data, the Ozernovsky MMC project implementation started in 2010, with the first stage of production introduced in June 2018 (accessed 8 August 2024 from https://xn--b1agjasmlcka4m.xn--p1ai/event/otkrylsya-ozernovskiy-gorno-metallurgicheskiy-kombinat). The Ozernovsky MMC is the fourth major gold mining operation in Kamchatka, with total reserves of 80.2 tonnes of gold and 79.9 tonnes of silver (accessed 8 August 2024 from https://dprom.online/metalls/ozernovskij-gmk-v-2-raza-uvelichit-zolotuyu-moshhnost). According to expert assessments, the production capacity is estimated at 140 thousand tonnes of ore per year. In the coming years it is planned to introduce the second stage of production and increase the capacity of the plant to 700 thousand tonnes of gold ore per year (accessed 8 August 2024 from https://zolotodb.ru/article/11806).

Figure 1.
Scheme of watercourses and sample collection point locations (blue marks represent background stations without influence of the OMMC, red marks—stations under direct influence of effluents from OMMC, yellow marks—control stations, downstream from OMMC discharge area).
The largest watercourses draining the ore localisation zone (Ozernovskoye ore field) include the Levaya Ozernaya and Perevalnaya rivers. The rivers have their sources in the eastern spurs of the Sredinny Range at altitudes of 1000–1300 m, while their tributaries within the ore field originate in the water-divide-adjacent areas at altitudes of 700–800 m. Descriptions of the watercourses are given from the 2013 report of the Kamchatka Branch of the Russian Federal Research Institute of Fisheries and Oceanography (KamchatNIRO) [30].
2.2. Description of Studied Watercourses
The Ozernaya River is one of the largest rivers in Kamchatka, formed by the confluence of the Levaya Ozernaya and Pravaya Ozernaya rivers. It flows into the Ozernaya Bay of the Bering Sea and is 199 km long. The catchment area is 8480 km2. The river basin has 173 tributaries less than 10 km long (accessed 8 August 2024 from https://textual.ru/gvr/index.php?card=278859). Almost all species of Pacific salmon (pink salmon, chum salmon, sockeye salmon, coho salmon, chinook salmon, as well as rainbow trout and char) are present in the Ozernaya River basin. The population density and biomass of grayling in the Ozernaya River is one of the highest not only in Kamchatka but also in the world. This river is a popular object of sport and amateur fishing (collection points O-16, O-L).
The Levaya Ozernaya River flows into the Ozernaya River 145 km from its mouth. The source is located in the water-divide-adjacent zone of the Sredinny Range, 2–3 km north-east of Lake Bolshoye, draining the crater part of the Bolshoy palaeovolcano. The river is 45 km long, and the basin includes 49 tributaries less than 10 km long. The floodplain in the area of the MMC is waterlogged. The channel is meandering, unstable, with a gravel–pebble–sand bottom. The river valley has a wide floodplain, pools, old riverbeds, and a meandering channel. The channel is 20–30 m wide, up to 40 m in high water. The average annual flow rate is 1.8 m3/s (Figure 2A; Figure 1, collection points O-5, O-13, O-14).

Figure 2.
Studied watercourses. (A) Levaya Ozernaya River, (B) Perevalnaya River. The inset photos in white frames show the appearance of periphyton at the sampling sites.
The Perevalnaya River belongs to the Uka River basin, is 30 kilometres long, has 21 tributaries less than 10 kilometres long, and flows into the Uka River 116 kilometres from its mouth. In turn, the Uka River flows into the Uka Bay of Karaginsky Bay (Figure 2B, Figure 1, collection points O-3, O-10, O-11). The Perevalnaya River is a spawning watercourse for pink salmon, chum salmon, and coho salmon.
Limnokren is a unique watercourse, a large channel backwater with a moderate current, light siltation, and numerous groundwater outlets. It flows into the Levaya Ozernaya River from the right bank, below the confluence of the channels between the mouths of the streams Konglomeratovyj and Homut. The water discharge at the mouth of the Limnokren is more than 9 m3/s. The middle and lower reaches of the Limnokren are home to the largest spawning ground for chum and sockeye salmon in the Ozernaya River basin (Figure 3; Figure 1, collection point O-1).

Figure 3.
Sampling site. Limnokren.
Pravaya Ozernaya River originates in the foothills of the Elovsky volcano, is approximately 40 km long, has a meandering channel, and the floodplain is waterlogged. Precise hydrological information is not available (Figure 1, collection point O-15).
Homut Stream is a left tributary of the Levaya Ozernaya River with its source at the foot of a basalt plateau at an altitude of 700 metres. Its catchment area is 14.4 km2 and its length is 6.0 km. The stream valley is V-shaped, asymmetrical, canyon-like in some places; the depth of channel incision is 20–180 metres. The width of the stream channel varies from 0.6 to 3.2 m in the upper reaches, increasing to 7–8 m in the lower reaches. Water discharge in the creek during the warm season ranges from 0.17 to 2.50 m3/s (Figure 1, collection point O-4).
Etalonnyj-1 Stream. The source of the stream is 100–200 m east-southeast of the Ozernovsky MMC. The width of the stream is about 1.0 m. The average depth is 0.04 m and the maximum depth is 0.08 m. The current is swift, with a velocity of up to 1.1 m/s. The bottom of the stream is stony. The right bank is high, steep, 10–12 m high, well sodded. The left bank is gently sloping, about 6–7 m high to the bedrock bank. The stream is crossed by a road and passes through a culvert. The slope above the culvert below the road is steep, stone-lined, and landslide-prone (Figure 1 (collection point O-7-1), Figure 4A).

Figure 4.
Sampling sites. (A) Etalonnyj-1 Stream, (B) Nezhnyj Stream. The inset photos in white frames show the appearance of periphyton at the sampling sites.
Etalonnyj-2 Stream originates on a watershed plateau made of glacial deposits and peat swamped massifs between the Levaya Ozernaya and Perevalnaya rivers, in the area of the existing MMC tailings dump. In the middle reaches the channel is stony, boulder–pebble, and the stream valley is trough-shaped and vaguely pronounced with a flat bed up to 10–20 m wide, with flat sides up to 1–2 m. The current is fast. At the outlet to the waterlogged floodplain of the Levaya Ozernaya River, the stream meanders strongly. At the mouth, the flow is weak, 2–5 m wide, with a swampy silty bottom. In the floodplain of Levaya Ozernaya river it merges with Etalonnyj-1 Stream to form Etalonnyj Stream (Figure 1, collection point O-7-2).
Nezhnyj Stream is a right tributary of the Perevalnaya River. The catchment area of the stream contains the tailings dump site for semi-dry storage of cyanidation waste (cakes). The stream receives groundwater runoff from the base of the tailings dump. The bed of the channel is made up of boulders with large pebbles (Figure 1 (collection point O-9), Figure 4B).
Watercourse feeding is mixed. The share of snow and rain feeding is respectively 30–35% and 5–10% of the total runoff volume. The remaining part is groundwater feeding. Ice formation starts from November–December, ice reaches its greatest thickness in March, and rivers open in the second half of April–early May. Formation of ice patches is common from December–January. As a rule, streams are covered with ice in wide areas, while narrow areas are covered with snow. Ice drifting on streams is not observed. River water temperature in the warm season varies from 6 to 14 °C, in winter the water temperature under the ice usually does not exceed +0.2–1.0 °C.
All rivers are natural spawning grounds for salmonid fish species.
2.3. Sample Collection
Phytoperiphyton and phytoplankton sampling was conducted in five streams (Etalonnyj-1, Etalonnyj-2, Nezhnyj, Homut, unnamed stream (PC 547-20)) and four rivers (Ozernaya, Levaya Ozernaya, Pravaya Ozernaya, Perevalnaya) from 19 to 24 September 2023 (Figure 1, Table 1). The sampling plan included baseline points (no MMC influence, O-1, O-3, O-12), “discharge” points (watercourses that flow through MMC territory—O-4, O-7-1, O-7-2, O-9), and control points (channel sections downstream of the “discharge” points—O-5, O-10, O-11, O-13, O-14, O-15, O-L, O-16).

Table 1.
List of sample collection points and physico-chemical parameters of water.
Samples were collected at 16 stations. For periphyton sampling, 5–7 stones were collected along the shore in an area of 10–20 metres. The stones were washed in a clean plastic container using a sterile toothbrush and 50 mL of distilled water was added. The sample was then shaken and a portion was transferred to a 15 mL test tube and fixed with 40% formaldehyde solution to a final concentration of 4%.
Plankton samples were collected from 5 watercourses: Perevalnaya River (collection points O-3, O-11, O-10), Stream PC 547-20 (point O-12), Levaya Ozernaya River (O-13, O-14), Pravaya Ozernaya River (O-15), Ozernaya River (O-L, O-16, O-1). Sampling was carried out using an Apstein plankton net. First, 100 litres of river water was passed through the net, then the concentrate (50 mL) was transferred to a test tube and fixed with 40% formaldehyde solution to a final concentration of 4%.
Water temperature and pH were measured using the OHAUS Starter ST300 portable device, specific conductivity was measured using the HANNA HI 98312 Dist6 portable analyser, redox potential was measured using the HANNA ORP HI 98201 portable device, and turbidity was measured using the HANNA HI 98703 portable device. The physico-chemical parameters of water are given in Table 1.
2.4. Preparation of Slides and Microscope Investigation
For the identification of diatoms, two permanent slides were prepared for each sample according to the standard procedure: part of the sample was treated with concentrated hydrogen peroxide (≈37%) to remove organic matter and boiled for 5 h, covering the beakers with a watch glass. After the beakers cooled, the material was washed with distilled water (5 times at 12 h intervals). The suspension was then spread on coverslips and dried at room temperature. Permanent diatom preparations were mounted in Naphrax® (Chippenham, UK).
The composition of microalgae was studied using a Carl Zeiss Axioscope A1 microscope equipped with an immersion objective (×100, n.a. 1.4, Nomarski differential interference contrast (DIC)) and an Axio Cam ERc 5s camera in the Laboratory of Molecular Systematics of Aquatic Plants, K.A. Timiryazev Institute of Plant Physiology, Russian Academy of Sciences. Ultrastructure of the valves was examined with the scanning electron microscope TESCAN Vega III (TESCAN, Brno, Czech Republic).
Identification was carried out using identification books and scientific articles, taking into account modern transformations in the systematics for each group. The names of taxa are given according to the AlgaeBase database [31].
The map of the study area was created using the Google Earth programme. Tables and images of microalgae were created using Adobe Photoshop CC ver. 19.0 software (Adobe, San Jose, CA, USA).
2.5. Data Analysis
Periphyton. A minimum of 500 diatom valves were counted in each permanent preparation to determine the relative abundance of species. In the cases where diatoms were scarce, fewer valves were counted. The valves were identified to the level of species or subspecies. The frequency of species occurrence was then calculated as a percentage. A list of detected taxa and their frequency of species occurrence in samples is presented in Table S1 (Supplementary Material).
The Shannon diversity index, the Pielou index of species participation (a measure of alignment), and the Simpson dominance index [32] were used to assess the periphyton community structure.
The Shannon species diversity index was calculated using the following formula:
where H is diversity in bits, pi is the proportion of species in the community, which is calculated using the following formula: pi = number of individuals of species i/number of counted individuals in the sample.
H = −∑pi ln pi,
Pielou index of alignment was calculated based on the Shannon index (H):
where H is the Shannon index, S is the number of species.
The Simpson index was calculated using the following formula:
where ni is the number of organisms belonging to species i, N is the total number of organisms.
The similarity of species composition between composition collection points was calculated using the Sørensen coefficient (Ks) [32,33]:
where c is the number of species common for the two samples being compared; a is the number of species in the first sample; b is the number of species in the second sample.
To analyse the commonality of species composition in watercourses, cluster analysis based on the Sørensen coefficient (Ks) was carried out. The association distance was calculated using the following formula: X = (1 − Ks). The dendrogram of hierarchical clustering was constructed using the average connectivity method [34] using RStudio software v. 2024.04.1+748.
Phytoplankton. For quantitative processing of phytoplankton, a 0.001 cm3 Goryaev counting chamber was used. A cell was taken as a counting unit. Recalculation of abundance was performed using the following formula:
where N is the number of cells in 1 cm3 of water, n is the number of cells in a 0.001 cm3 chamber, V1 is the volume of sample concentrate, V2 is the volume of the chamber, w is the volume of filtered water [35].
To determine the biomass of phytoplankton, the biomass of individual species was calculated. For this purpose, the average cell volume of each species in each sample was determined by measuring 20–30 cells and calculating the average size. Each cell encountered and identified in the plankton was measured using an ocular micrometer. Cell density was assumed to be equal to one, and cell shape was equated to the shape of a geometric body. Biomass was converted using the following formula:
where B is the biomass of algae (mg/L), N is the number of cells of a given species per 1 L, u is the volume (μL3) of the geometric body to which the species is equated. Biomass is expressed in g/m3 with an accuracy of 0.1–0.01 g/m3 [35]. The resulting abundance and biovolume data for species, as well as phytoplankton biomass values at the collection points, is given in Table S2 (Supplementary Material).
Phytoplankton biomass was used to determine the trophicity of the water body, using the classification of I.S. Trifonova [36]: biomass < 1 g/m3—oligotrophic type of water body; 1–5 g/m3—mesotrophic; 5–10 g/m3—eutrophic; > 10 g/m3—highly eutrophic water body.
Diagrams and graphs were made using Microsoft Excel software v16.0.
3. Results
A total of 174 species of diatoms were found in the studied periphyton samples, belonging to 62 genera, 32 families, 16 orders, and 6 subclasses of the classes Bacillariophyceae, Coscinodiscophyceae, and Mediophyceae. Representatives of Bacillariophyceae predominate (167 species out of 174). Among the genera the most diverse are Pinnularia (15 species), Navicula (14 species), Eunotia (12) Nitzschia (8), and Gomphonema (6). The remaining genera have no more than five species. From 37 to 58 species were found in each sample. The highest species diversity was observed at stations O-9 Nezhnyj Stream (58 taxa) and O-11 Perevalnaya River (57 taxa). The list of detected taxa and their abundance in samples (values above 1% are marked in bold) are given in Table S1 (Supplementary Material). Common taxa (those occurring in 60–100% of the studied samples) are highlighted in grey.
Microalgae were not found at the collection points of Etalonnyj-1 (O-7-1) and Etalonnyj-2 (O-7-2) streams flowing through the territory of the MMC. These watercourses differ from the others by increased values of specific conductivity, 520 and 220 μS/cm, respectively, and in other watercourses the values of specific conductivity do not exceed 130 μS/cm. At the point O-7-2 the water has a weakly acidic reaction, and the pH value is 5.67 (Table 1). Point O-4 (the mouth of Homut Stream) ought to be highlighted. Here high metrics are noted: optical turbidity of water (39.6 NTU) and specific conductivity (350 µS/cm). For comparison, turbidity values in the Ozernaya, Levaya Ozernaya, and Pravaya Ozernaya rivers (at all sampling points) do not exceed 1.3 NTU, and turbidity values in the Perevalnaya River do not exceed 20.4 NTU. The periphyton sample from point O-4 was characterised by poor presence of microalgae, and cells were found only sporadically in the preparation. However, high species diversity should be noted (48 taxa were identified).
Below, the main quantitative and qualitative characteristics of the found species are given. Species are given in the order of numbering of the tables. Data on the frequency of occurrence of a species (%) in samples are given in Table S1 (Supplementary Material).
Aulacoseira italica (Ehrenberg) Simonsen 1979 (Figure 5A–C).

Figure 5.
(A–AH). Centric and araphid diatoms. LM, DIC. (A–C). Aulacoseira italica. (D–F). A. alpigena. (G). Ellerbeckia arenaria. (H,I). Melosira varians. (J,K). Stephanodiscus hantzschii. (L,L’). S. medius. (M). Stephanocyclus meneghinianus. (N–Q). Fragilaria vaucheriae. (R,R’). Fragilariforma horstii. (S). Staurosira sviridae. (T). S. construens. (U,V). Fragilariforma virescens. (W–Z). Staurosira venter. (AA,AB). Fragilariforma bicapitata. (AC–AE). Tabellaria flocculosa. (AF–AH). T. fenestrata. Scale bar = 10 μm.
Remarks. Frustules in the colonies were 11–13 μm long, 5–10 μm wide and had 20–23 striae in 10 μm.
This species was found at stations no. O-11, O-12, O-13, O-L, O-4, O-9 (pH 6.4–8.7, conductivity 70–350 μS/cm).
Aulacoseira alpigena (Grunow) Krammer 1991 (Figure 5D–F).
Remarks. The specimens in our samples were 3–5 μm long, 5–9 μm wide and had 15–16 striae in 10 μm.
This species was found at stations no. O-4, O-9 (pH 6.4, conductivity 130–350 μS/cm).
Ellerbeckia arenaria (D. Moore ex Ralfs) Dorofeyuk & Kulikovskiy 2012 (Figure 5G).
Remarks. The specimen in our sample was 57 μm in diameter and had 20 striae in 10 μm.
This species was found at station no. O-14 (pH 7.77, conductivity 90 μS/cm).
Melosira varians C. Agardh 1827 (Figure 5H,I).
Remarks. The specimens in our samples were 13.2 μm long, 14.7–25.4 μm in diameter.
This species was found at stations no. O-3, O-11, O-L (pH 6.82–8.7, conductivity 60–90 μS/cm).
Stephanodiscus hantzschii Grunow in P.T. Cleve & Grunow 1880 (Figure 5J,K).
Remarks. The specimens in our samples were 6.5–7.7 μm in diameter and had 6–9 fascicles based on circumferential count.
This species was found at stations no. O-L, O-16 (pH 8.2–8.7, conductivity 70–90 μS/cm).
Stephanodiscus medius Håkansson 1986 (Figure 5L,L’).
Remarks. The specimen in our sample was 15.7 μm in diameter and had 8–13 fascicles based on circumferential density.
This species was found at station no. O-L (pH 8.7, conductivity 90 μS/cm).
Stephanocyclus meneghinianus (Kützing) Kulikovskiy, Genkal & Kociolek in Kulikovskiy et al. 2022 (Figure 5M).
Remarks. The specimen in our samples was 14.6 μm in diameter and had 7–8 chambered striae by circumference.
This species was found at station no. O-L (pH 8.7, conductivity 90 μS/cm).
Fragilaria vaucheriae (Kützing) Petersen 1938 (Figure 5N–Q).
Remarks. The specimens in our samples were 19.4–23.6 μm long, 3.5–4.3 μm wide and had 15–17 striae in 10 μm.
This species was found at all stations (pH 6.4–8.7, conductivity 50–350 μS/cm).
Fragilariforma horstii Morales, Manoylov & Bahls 2012 (Figure 5R,R’).
Remarks. The specimen in our sample was 14.4 μm long, 11 μm wide and had 33 striae in 10 μm.
This species was found at station no. O-14 (pH 7.77, conductivity 90 μS/cm).
Staurosira sviridae Kulikovskiy, Genkal & Mikheeva 2011 (Figure 5S).
Remarks. The specimen in our sample was 13 μm long, 5.7 μm wide and had 16 striae in 10 μm.
This species was found at station no. O-15 (pH 7.79, conductivity 50 μS/cm).
Staurosira construens Ehrenberg 1843 (Figure 5T).
Remarks. The specimen in our sample was 11.2 μm long, 5.9 μm wide and had 14 striae in 10 μm.
This species was found at station no. O-14 (pH 7.77, conductivity 90 μS/cm).
Fragilariforma virescens (Ralfs) D.M. Williams & Round 1988 (Figure 5U,V).
Remarks. The specimens in our samples were 29.2–40.5 μm long, 7.6–8.6 μm wide and had 18 striae in 10 μm.
This species was found at stations no. O-11, O-14 (pH 6.82–7.77, conductivity 70–90 μS/cm).
Staurosira venter (Ehrenberg) P.T. Cleve & J.D. Möller 1879 (Figure 5W–Z).
Remarks. The specimens in our samples were 7.9–13.7 μm long, 3.6–7.8 μm wide and had 13 striae in 10 μm.
This species was found at stations no. O-3, O-13, O-14, O-15, O-L. O-1, O-5 (pH 7.18–8.7, conductivity 60–90 μS/cm).
Fragilariforma bicapitata (A. Mayer) D.M. Williams & Round 1988 (Figure 5AA,AB).
Remarks. The specimens in our samples were 16.0–20.3 μm long, 4.0–4.9 μm wide and had 8–9 striae in 10 μm.
This species was found at stations no. O-9, O-14 (pH 6.4–7.77, conductivity 90–130 μS/cm).
Tabellaria flocculosa (Roth) Kützing 1844 (Figure 5AC–AE).
Remarks. The specimens in our samples were 23.8–24.4 μm long, 6.8–7.2 μm wide at the central part and had 18–19 striae in 10 μm.
This species was found at stations no. O-11, O-12, O-13, O-14 (pH 6.82–7.77, conductivity 70–140 μS/cm).
Tabellaria fenestrata (Lyngbye) Kützing 1844 (Figure 5AF–AH).
Remarks. The specimens in our samples were 57.1–75.1 μm long, 6.8–7.4 μm wide at the central part and had 18–19 striae in 10 μm.
This species was found at stations no. O-12, O-13, O-14, O-4 (pH 7.6–7.77, conductivity 80–350 μS/cm).
Ulnaria ulna (Nitzsch) Compère 2001 (Figure 6A–D).

Figure 6.
(A–AD). Araphid diatoms. LM, DIC. (A–D). Ulnaria ulna. (E–G). Hannaea inaequidentata. (H–J). Fragilaria gracilis. (K–N). Meridion circulare. (O–Q). Meridion constrictum. (R). Pseudostaurosira pseudoconstruens. (S–U). Staurosirella leptostauron. (V–Y). Pseudostaurosira robusta. (Z–AD). Ulnaria goulardii. Scale bars = 10 μm.
Remarks. The specimens in our samples were 135.9–189.1 μm long, 8.3–8.8 μm wide and had 9–10 striae in 10 μm.
This species was found at all stations except O-1, O-5 (pH 6.4–8.7, conductivity 50–350 μS/cm).
Hannaea inaequidentata (Lagerstedt) Genkal & Kharitonov 2008 (Figure 6E–G).
Remarks. The specimens in our samples were 35.5–63.5 μm long, 5.8–6.1 μm wide and had 16–17 striae in 10 μm.
This species was found at all stations (pH 6.4–8.7, conductivity 50–350 μS/cm).
Fragilaria gracilis Østrup 1910 (Figure 6H–J).
Remarks. The specimens in our samples were 21.5–36.4 μm long, 2.2–2.5 μm wide and had 20–22 striae in 10 μm.
This species was found at stations no. O-3, O-10, O-11, O-12, O-13, O-15, O-L, O-9 (pH 6.4–8.7, conductivity 50–140 μS/cm).
Meridion circulare (Greville) C. Agardh 1831 (Figure 6K–N).
Remarks. The specimens in our samples were 19.0–45.9 μm long, 4.3–5.7 μm wide, had 5–6 costae in 10 μm and 20–22 striae in 10 μm.
This species was found at all stations except O-10, O-16, O-1 (pH 6.4–8.7, conductivity 50–350 μS/cm).
Meridion constrictum Ralfs 1843 (Figure 6O–Q).
Remarks. The specimens in our samples were 31.6–22.2 μm long, 5.4–6.2 μm wide, had 4–5 costae in 10 μm and 20 striae in 10 μm.
This species was found at stations no. O-10, O-11, O-12, O-9 (pH 6.4–7.6, conductivity 70–140 μS/cm).
Pseudostaurosira pseudoconstruens (Marciniak) D.M. Williams & Round 1987 (Figure 6R).
Remarks. The specimen in our sample was 12.4 μm long, 7.2 μm wide and had 15 striae in 10 μm.
This species was found at stations no. O-1, O-5 (pH n/d, conductivity 60–70 μS/cm).
Staurosirella leptostauron (Ehrenberg) D.M. Williams & Round 1988 (Figure 6S–U).
Remarks. The specimens in our samples were 16.5–19.8 μm long, 11.3–12.3 μm wide and had 9 striae in 10 μm.
This species was found at stations no. O-1, O-5, O-9, O-15 (pH 6.4–7.79, conductivity 50–130 μS/cm).
Pseudostaurosira robusta (Fusey) D.M. Williams & Round 1988 (Figure 6V–Y).
Remarks. The specimens in our samples were 11.3–14.1 μm long, 5.3–5.6 μm wide and had 15 striae in 10 μm.
This species was found at stations no. O-1, O-5, O-13 (pH 7.6, conductivity 60–80 μS/cm).
Ulnaria goulardii (Brébisson ex P.T. Cleve & Grunow) D.M. Williams, Potapova & C.E. Wetzel 2022 (Figure 6Z–AD).
Remarks. The specimens in our samples were 43.8–63.9 μm long, 8.2–9.4 μm wide and had 13–14 striae in 10 μm.
This species was found at stations no. O-14, O-L, O-16 (pH 7.77–8.7, conductivity 70–90 μS/cm).
Hannaea arcus (Ehrenberg) R.M. Patrick 1966 (Figure 7A–I).

Figure 7.
(A–O). Araphid diatoms. Hannaea spp. LM, DIC. (A–I). Hannaea arcus. (J–O). Hannaea mongolica. Scale bar = 10 μm.
Remarks. The specimens in our samples were 35.4–108.8 μm long, 5.0–6.9 μm wide and had 17–18 striae in 10 μm.
This species was found at all stations except O-9 (pH 6.82–8.7, conductivity 50–350 μS/cm).
Hannaea mongolica Glushchenko, Kulikovskiy, Q. Liu & Kociolek in Liu et al. 2019 (Figure 7J–O).
Remarks. The specimens in our samples were 43.1–95.9 μm long, 6.5–7.7 μm wide and had 16–17 striae in 10 μm.
This species was found at stations no. O-3, O-10, O-11, O-12, O-13, O-14, O-L, O-16, O-5 (pH 6.82–8.7, conductivity 60–140 μS/cm).
Odontidium neolongissimum Jüttner, D.M. Williams, Z. Levkov, E. Falasco, M. Battegazzore, M. Cantonati, B. Van de Vijver, C. Angele & Ector 2015 (Figure 8A,B).

Figure 8.
(A–AI). Araphid diatoms. LM, DIC. (A,B). Odontidium neolongissimum. (C–E). O. hyemale. (F–N). Odontidium mesodon. (O–S). Diatoma problematica. (T–AB). Diatoma moniliformis. (AC–AI). Odontidium anceps. Scale bar = 10 μm.
Remarks. The specimens in our samples were 63.6–75.2 μm long, 9.3–9.6 μm wide, had four transapical ribs in 10 μm and there were 4–6 rows of striae between adjacent ribs.
This species was found at stations no. O-10, O-11, O-13 (pH 6.82–7.6, conductivity 70–80 μS/cm).
Odontidium hyemale (Roth) Kützing 1844 (Figure 8C–E).
Remarks. The specimens in our samples were 40.3–66.6 μm long, 10.4–11.0 μm wide, had 3–4 transapical ribs in 10 μm and there were 6–8 rows of striae between adjacent ribs.
This species was found at station no. O-14 (pH 7.77, conductivity 90 μS/cm).
Odontidium mesodon (Ehrenberg) Kützing 1844 (Figure 8F–N).
Remarks. The specimens in our samples were 10.9–19.6 μm long, 5.6–7.6 μm wide, had 7–8 transapical ribs in 10 μm and there were 6–8 rows of striae between adjacent ribs.
This species was found at stations no. O-12, O-13, O-15 (pH 7.6–7.79, conductivity 50–140 μS/cm).
Diatoma problematica Lange-Bertalot 1993 (Figure 8O–S).
Remarks. The specimens in our samples were 22.4–34.1 μm long, 5.3–5.9 μm wide, had 2–4 transapical ribs in 10 μm.
This species was found at station no. O-16 (pH 8.2, conductivity 70 μS/cm).
Diatoma moniliformis (Kützing) D.M. Williams 2012 (Figure 8T–AB).
Remarks. The specimens in our samples were 21.1–63.9 μm long, 2.5–4.0 μm wide, had 6–7 transapical ribs in 10 μm.
This species was found at stations no. O-11, O-13, O-14, O-15, O-L (pH 6.82–8.7, conductivity 50–90 μS/cm).
Odontidium anceps (Ehrenberg) Ralfs in Pritchard 1861 (Figure 8AC–AI).
Remarks. The specimens in our samples were 27.6–42.4 μm long, 5.8–6.3 μm wide, had 5–6 transapical ribs in 10 μm and there were 4–7 rows of striae between adjacent ribs.
This species was found at stations no. O-3, O-10, O-12, O-14, O-15, O-L, O-5, O-4, O-9 (pH 6.4–8.7, conductivity 50–350 μS/cm).
Eunotia major (W. Smith) Rabenhorst 1864 sensu auct. (Figure 9A).

Figure 9.
(A–AA). Eunotioid diatoms. LM, DIC. (A). Eunotia maior sensu auct. (B). E. praerupta. (C). E. lapponica. (D). E. sarek. (E–G). E. parapraerupta. (H). E. islandica. (I). E. cf. nymmaniana. (J,K). E. praerupta. (L–O). E. botuliformis. (P–S). E. perpusilla. (T–X). E. minor. (Y–AA). E. bilunaris. Scale bar = 10 μm.
Remarks. The specimen in our sample was 99.2 μm long, 10.9 μm wide, had nine striae in 10 μm and 25 areolae in 10 μm.
This species was found at station no. O-9 (pH 6.4, conductivity 130 μS/cm).
Eunotia praerupta Ehrenberg 1843 (Figure 9B,J,K).
Remarks. The specimens in our samples were 32.1–67.2 μm long, 11.1–14.7 μm wide and had 7–9 striae in 10 μm.
This species was found at stations no. O-12, O-15, O-4, O-9 (pH 6.4–7.79, conductivity 50–350 μS/cm).
Eunotia lapponica Grunow ex A. Cleve 1895 (Figure 9C).
Remarks. The specimen in our sample was 65.6 μm long, 10.4 μm wide, had 23 striae in 10 μm and 20 spines in 10 μm.
This species was found at stations no. O-9, O-10, O-11 (pH 6.4–7.33, conductivity 70–130 μS/cm).
Eunotia sarek Å. Berg 1939 (Figure 9D).
Remarks. The specimens in our sample were 44.2 μm long, 15.3 μm wide and had 14 striae in 10 μm.
This species was found at station no. O-11 (pH 6.82, conductivity 70 μS/cm).
Eunotia parapraerupta Lange-Bertalot & Metzeltin in Metzeltin et al. 2009 (Figure 9E–G).
Remarks. The specimens in our samples were 26.1–39.5 μm long, 8.5–9.1 μm wide and had 9–11 striae in 10 μm.
This species was found at station no. O-4 (pH n/d, conductivity 350 μS/cm).
Eunotia islandica Østrup 1918 (Figure 9H).
Remarks. The specimen in our sample was 44.6 μm long, 10.1 μm wide and had 14 striae in 10 μm.
This species was found at station no. O-L (pH 8.7, conductivity 90 μS/cm).
Remarks. The specimen in our sample was 42.8 μm long, 2.2 μm wide and had 21 striae in 10 μm.
This species was found at station no. O-9 (pH 6.4, conductivity 130 μS/cm).
Eunotia botuliformis F. Wild, Nörpel-Schempp & Lange-Bertalot in Lange-Bertalot 1993 (Figure 9L–O).
Remarks. The specimens in our samples were 19.6–31.7 μm long, 2.5–2.9 μm wide and had 17–18 striae in 10 μm.
This species was found at stations no. O-4, O-9 (pH 6.4, conductivity 130–350 μS/cm).
Eunotia perpusilla (Grunow) Å. Berg 1939 (Figure 9P–S).
Remarks. The specimens in our samples were 11.4–17.7 μm long, 3.5–3.8 μm wide and had 16 striae in 10 μm.
This species was found at stations no. O-4, O-9 (pH 6.4, conductivity 130–350 μS/cm).
Eunotia minor (Kützing) Grunow 1881 (Figure 9T–X).
Remarks. The specimens in our samples were 21.9–32.0 μm long, 4.5–5.6 μm wide and had 13–15 striae in 10 μm.
This species was found at station no. O-9 (pH 6.4, conductivity 130 μS/cm).
Eunotia bilunaris (Ehrenberg) Schaarschmidt 1880 (Figure 9Y–AA).
Remarks. The specimens in our samples were 30.9–37.7 μm long, 4.0–4.6 μm wide and had 14–17 striae in 10 μm.
This species was found at station no. O-9 (pH 6.4, conductivity 130 μS/cm).
Gliwiczia calcar (P.T. Cleve) Kulikovskiy, Lange-Bertalot & Witkowski 2013 (Figure 10A–D).

Figure 10.
(A–AZ). Monoraphid and amphoroid diatoms. LM, DIC. (A–D). Gliwiczia calcar. (E–G). Skabitschewskia peragalloi. (H,I). Cocconeis euglypta sensu lato. (J–R). Karayevia laterostrata. (S–U). K. suchlandtii. (V,V’). Platessa montana. (W–Z). Planothidium reichardtii. (AA–AB’). P. lanceolatum. (AC,AD). Achnanthidium latecephalum. (AE–AG). Eucocconeis laevis. (AH–AJ’). Psammothidium acidoclinatum. (AK–AO). Rossithidium kriegeri. (AP–AR). Achnanthidium minutissimum. (AS–AT). Amphora cf. copulata. (AU). Amphora inariensis. (AV–AZ). A. pediculus. Scale bar = 10 μm.
Remarks. The specimens in our samples were 14.0–15.4 μm long, 8.7–9.9 μm wide and had 32 striae in 10 μm.
This species was found at station no. O-5 (pH n/d, conductivity 70 μS/cm).
Skabitschewskia peragalloi (Brun & Héribaud) Kulikovskiy & Lange-Bertalot in Kulikovskiy et al. 2015 (Figure 10E–G).
Remarks. The specimens in our samples were 13.2–15.0 μm long, 7.3–7.6 μm wide and had 22 striae in 10 μm on the raphe valve and 20 in 10 μm on the rapheless valve.
This species was found at stations no. O-1, O-5 (pH n/d, conductivity 60–70 μS/cm).
Cocconeis euglypta Ehrenberg 1854 sensu lato (Figure 10H,I).
Remarks. The specimens in our samples were 19.5–22.4 μm long, 10.8–11.2 μm wide and had 21 striae in 10 μm on the raphe valve and 22 in 10 μm on the rapheless valve.
This species was found at all stations except O-4 (pH 6.4–8.7, conductivity 50–140 μS/cm).
Karayevia laterostrata (Hustedt) Bukhtiyarova 1999 (Figure 10J–R).
Remarks. The specimens in our samples were 10.9–13.8 μm long, 5.3–6.3 μm wide and had 16–18 striae in 10 μm.
This species was found at stations no. O-1, O-5, O-16 (pH 8.2, conductivity 60–70 μS/cm).
Karayevia suchlandtii (Hustedt) Bukhtiyarova 1999 (Figure 10S–U).
Remarks. The specimens in our samples were 9.7–11.6 μm long, 4.5–5.0 μm wide and had 18–19 striae in 10 μm.
This species was found at station no. O-14 (pH 7.77, conductivity 90 μS/cm).
Platessa montana (Krasske) Lange-Bertalot in Krammer & Lange-Bertalot 2004 (Figure 10V,V’).
Remarks. The specimen in our sample was 10.3 μm long, 4.9 μm wide and had 24 striae in 10 μm.
This species was found at station no. O-3. (pH 7.18, conductivity 60 μS/cm).
Planothidium reichardtii Lange-Bertalot & Werum 2004 in Werum & Lange-Bertalot 2004 (Figure 10W–Z).
Remarks. The specimens in our samples were 14.0–17.3 μm long, 6.9–7.8 μm wide and had 16–17 striae in 10 μm.
This species was found at stations no. O-1, O-5 (pH n/d, conductivity 60–70 μS/cm).
Planothidium lanceolatum (Brébisson ex Kützing) Bukhtiyarova 1999 (Figure 10AA–AB’).
Remarks. The specimens in our samples were 15.5–17.3 μm long, 5.9 μm wide and had 16–17 striae in 10 μm.
This species was found at all stations except no. O-12 (pH 7.6, conductivity 140 μS/cm).
Achnanthidium latecephalum H. Kobayasi 1997 (Figure 10AC,AD).
Remarks. The specimens in our samples were 14.9–17.2 μm long, 4.2–4.5 μm wide and had 21–23 striae in 10 μm.
This species was found at station no. O-11 (pH 6.82, conductivity 70 μS/cm).
Eucocconeis laevis (Østrup) Lange-Bertalot in Lange-Bertalot & Genkal 1999 (Figure 10AE–AG).
Remarks. The specimens in our samples were 15.5–19.5 μm long, 6.9–7.1 μm wide and had 30 striae in 10 μm.
This species was found at all stations except no. O-4, O-16, O-L (pH 6.4, conductivity 50–140 μS/cm).
Psammothidium acidoclinatum (Lange-Bertalot) Lange-Bertalot in Lange-Bertalot & Genkal 1999 (Figure 10AH–AJ).
Remarks. The specimens in our samples were 10.3–11.3 μm long, 4.5–5.1 μm wide and had 28 striae in 10 μm.
This species was found at stations no. O-1, O-3, O-9, O-10, O-11, O-13, O-14, O-15 (pH 6.4–7.79, conductivity 50–130 μS/cm).
Rossithidium kriegeri (Krasske) Bahls 2019 (Figure 10AK–AO).
Remarks. The specimens in our samples were 9.2–11.8 μm long, 2.7–3.1 μm wide and had 20–21 striae in 10 μm.
This species was found at station no. O-3 (pH 7.18, conductivity 60 μS/cm).
Achnanthidium minutissimum (Kützing) Czarnecki 1994 (Figure 10AP–AR).
Remarks. The specimens in our samples were 11.9–12.6 μm long, 2.6–3.0 μm wide and had 30 striae in 10 μm.
This species was found at all stations except no. O-12, O-13, O-L (pH 6.4–8.2, conductivity 50–350 μS/cm).
Remarks. The specimens in our samples were 37.4–32.8 μm long, 7.8–8.2 μm wide and had 15–16 striae in 10 μm.
This species was found at station no. O-1 (pH n/d, conductivity 60 μS/cm).
Amphora inariensis Krammer 1980 (Figure 10AU).
Remarks. The specimens in our sample were 27.4 μm long, 5.1 μm wide and had 16 striae in 10 μm.
This species was found at station no. O-14 (pH 7.77, conductivity 90 μS/cm).
Amphora pediculus (Kützing) Grunow in A. Schmidt 1875 (Figure 10AV–AZ).
Remarks. The specimens in our samples were 13.4–21.0 μm long, 2.9–4.4 μm wide and had 18–19 striae in 10 μm.
This species was found at stations no. O-1, O-5, O-16 (pH 8.2, conductivity 60–70 μS/cm).
Navicula radiosa Kützing 1844 (Figure 11A).
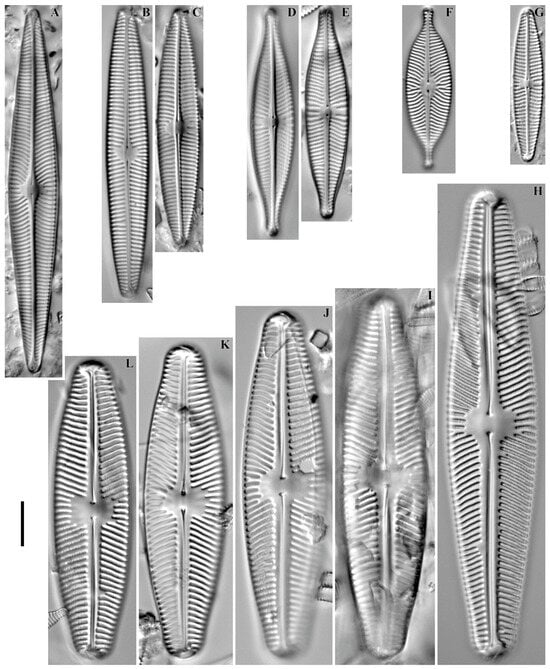
Figure 11.
(A–L). Navicula spp. LM, DIC. (A). Navicula radiosa. (B,C). N. avenacea. (D,E). N. rhynchotella. (F). N. salinarum. (G). N. libonensis. (H–L). N. aurora. Scale bar = 10 μm.
Remarks. The specimen in our sample was 81.2 μm long, 11.2 μm wide and had 12 striae in 10 μm.
This species was found at station no. O-11 (pH 6.82, conductivity 70 μS/cm).
Navicula avenacea (Rabenhorst) Brébisson ex Grunow in Schneider 1878 (Figure 11B,C).
Remarks. The specimens in our samples were 53.1–64.2 μm long, 9.4–9.5 μm wide and had 11–12 striae in 10 μm.
This species was found at station no. O-5 (pH n/d, conductivity 70 μS/cm).
Navicula rhynchotella Lange-Bertalot 1993 (Figure 11D,E).
Remarks. The specimens in our samples were 41.6–51.0 μm long, 9.6–9.8 μm wide and had 11–12 striae in 10 μm.
This species was found at station no. O-11 (pH 6.82, conductivity 70 μS/cm).
Navicula salinarum Grunow in P.T. Cleve & Grunow 1880 (Figure 11F).
Remarks. The specimen in our sample was 36.2 μm long, 10.0 μm wide and had 13 striae in 10 μm.
This species was found at station no. O-11 (pH 6.82, conductivity 70 μS/cm).
Navicula libonensis Schoeman 1970 (Figure 11G).
Remarks. The specimen in our sample was 34.5 μm long, 6.6 μm wide and had 13 striae in 10 μm.
This species was found at station no. O-9 (pH 6.4, conductivity 130 μS/cm).
Navicula aurora Sovereign 1958 (Figure 11H–L).
Remarks. The specimens in our samples were 66.6–104.6 μm long, 16.6–19.3 μm wide and had 7–8 striae in 10 μm.
This species was found at stations no. O-12, O-15, O-L, O-1 (pH 7.6–8.7, conductivity 50–140 μS/cm).
Navicula schweigeri Bahls 2012 (Figure 12A–E).

Figure 12.
(A–L). Navicula spp. LM, DIC. (A–E). Navicula schweigeri. (F). N. subalpina. (G,H). N. ceciliae. (I–M). N. slesvicensis. (N–T). N. arctotenelloides. (U–Y). N. gregaria. (Z–AB). N. exilis. (AC–AE). N. radiosafallax. Scale bar = 10 μm.
Remarks. The specimens in our samples were 41.8–56.1 μm long, 9.0–10.0 μm wide and had 14–15 striae in 10 μm.
This species was found at all stations except no. O-1, O-9, O-13 (pH 6.82–8.7, conductivity 50–350 μS/cm).
Navicula subalpina Reichardt 1988 (Figure 12F).
Remarks. The specimens in our samples were 42.0 μm long, 8.7 μm wide and had 11 striae in 10 μm.
This species was found at stations no. O-3, O-11, O-12, O-L (pH 6.82–8.7, conductivity 60–140 μS/cm).
Navicula ceciliae Van de Vijver, Jarlman & Lange-Bertalot 2010 (Figure 12G,H).
Remarks. The specimens in our sample were 29.0–34.7 μm long, 6.8 μm wide and had 16–17 striae in 10 μm.
This species was found at stations no. O-11, O-9 (pH 6.4–6.82, conductivity 70–130 μS/cm).
Navicula slesvicensis Grunow in Van Heurck 1880 (Figure 12I–M).
Remarks. The specimens in our samples were 21.3–25.6 μm long, 4.1–4.6 μm wide and had 11 striae in 10 μm.
This species was found at station O-14, O-15 (pH 7.77–7.79, conductivity 50–90 μS/cm).
Navicula arctotenelloides Lange-Bertalot & Metzeltin in Lange-Bertalot et al. 1996 (Figure 12N–T).
Remarks. The specimens in our samples were 17.3–20.3 μm long, 8.5–8.8 μm wide and had 17–18 striae in 10 μm.
This species was found at all stations except O-4, O-9, O-12, O-L (pH 6.82–8.7, conductivity 50–90 μS/cm).
Navicula gregaria Donkin 1861 (Figure 12U–Y).
Remarks. The specimens in our samples were 23.4–29.5 μm long, 6.1–6.5 μm wide and had 19–20 striae in 10 μm.
This species was found at stations no. O-14, O-15 (pH 7.77–7.79, conductivity 50–90 μS/cm).
Navicula exilis Kützing 1844 (Figure 12Z–AB).
Remarks. The specimens in our samples were 23.8–28.5 μm long, 5.3–5.7 μm wide and had 16–18 striae in 10 μm.
This species was found at stations no. O-9, O-10, O-13 (pH 6.4–7.6, conductivity 70–130 μS/cm).
Navicula radiosafallax Lange-Bertalot 1993 (Figure 12AC–AE).
Remarks. The specimens in our samples were 19.2–25.8 μm long, 5.5–5.9 μm wide and had 16–17 striae in 10 μm.
This species was found at station no. O-9 (pH 6.4, conductivity 130 μS/cm).
Decussiphycus placenta (Ehrenberg) M.D. Guiry & K. Gandhi 2019 (Figure 13A).

Figure 13.
(A–AP). Naviculoid diatoms. LM, DIC. (A). Decussiphycus placenta. (B–D). Boreozonacola natchikae. (E,H). Boreozonacola hustedtii. (I). Cavinula lapidosa. (J). C. pseudoscutiformis. (K–O). Prestauroneis protractoides. (P). Adlafia detenta. (Q–T). A. bryophyla. (U,V). Adlafia belyakovae. (W–Y). Diatomella balfouriana. (Z). Humidophila perpusilla. (AA–AD). H. contenta. (AE). Luticola acidoclinata. (AF–AL). Cavinula variostriata. (AM). Brachysira sp. 1. (AN–AP). Brachysira sp. 2. Scale bar = 10 μm.
Remarks. The specimen in our sample was 39.7 μm long, 17.0 μm wide and had 23 transapical striae in 10 μm.
This species was found at station no. O-4 (pH n/d, conductivity 350 μS/cm).
Boreozonacola natchikae (J.B. Petersen) Lange-Bertalot, Kulikovskiy & Witkowski in Kulikovskiy et al. 2010 (Figure 13B–D).
Remarks. The specimens in our samples were 31.0–33.3 μm long, 7.4–8.1 μm wide and had 19–20 striae in 10 μm.
This species was found at station no. O-4 (pH n/d, conductivity 350 μS/cm).
Boreozonacola hustedtii Lange-Bertalot, Kulikovskiy & Witkowski in Kulikovskiy et al. 2010 (Figure 13E–G).
Remarks. The specimens in our samples were 19.0–25.5 μm long, 5.5–6.6 μm wide and had 20–21 striae in 10 μm.
This species was found at stations no. O-4, O-11 (pH 6.82, conductivity 70–350 μS/cm).
Cavinula lapidosa (Krasske) Lange-Bertalot in Lange-Bertalot & Metzeltin 1996 (Figure 13I).
Remarks. The specimen in our sample was 16.2 μm long, 7.3 μm wide and had 27 striae in 10 μm.
This species was found at station no. O-14 (pH 7.77, conductivity 90 μS/cm).
Cavinula pseudoscutiformis (Hustedt) D.G. Mann & Stickle in Round et al. 1990 (Figure 13J).
Remarks. The specimen in our sample was 13.7 μm long, 11.2 μm wide and had 23 striae in 10 μm.
This species was found at station no. O-11 (pH 6.82, conductivity 70 μS/cm).
Prestauroneis protractoides (Hustedt) Q. Liu & Kociolek in Q. Liu et al. 2015 (Figure 13K–O).
Remarks. The specimens in our samples were 22.2–24.9 μm long, 5.6–6.1 μm wide and had 17–19 striae in 10 μm.
This species was found at station no. O-15 (pH 7.79, conductivity 50 μS/cm).
Adlafia detenta (Hustedt) Heudre, C.E. Wetzel & Ector in Heudre et al. 2018 (Figure 13P).
Remarks. The specimens in our sample were 16.0 μm long, 5.9 μm wide and had 30 striae in 10 μm.
This species was found at station no. O-13, O-15 (pH 7.6–7.79, conductivity 50–80 μS/cm).
Adlafia bryophila (J.B. Petersen) Lange-Bertalot in Moser et al. 1998 (Figure 13Q–T).
Remarks. The specimens in our samples were 12.2–16.5 μm long, 2.9–3.3 μm wide and had 26 striae in 10 μm.
This species was found at station no. O-10, O-11, O-13, O-14, O-1 (pH 6.82–7.77, conductivity 60–90 μS/cm).
Adlafia belyakovae Chudaev & Levkov 2023 (Figure 13U,V).
Remarks. The specimens in our samples were 12.0–12.2 μm long, 3.7–3.8 μm wide.
This species was found at stations no. O-3, O-10 (pH 7.18–7.33, conductivity 60–70 μS/cm).
Diatomella balfouriana Greville 1855 (Figure 13W–Y).
Remarks. The specimens in our samples were 13.2–22.5 μm long, 4.2–4.6 μm wide and had 19–21 striae in 10 μm.
This species was found at station no. O-4 (pH n/d, conductivity 350 μS/cm).
Humidophila perpusilla (Grunow) R.L. Lowe, Kociolek, J.R. Johansen, Van de Vijver, Lange-Bertalot & Kopalová 2014 (Figure 13Z).
Remarks. The specimen in our sample was 11.7 μm long, 4.7 μm wide.
This species was found at station no. O-5 (pH n/d, conductivity 70 μS/cm).
Humidophila contenta (Grunow) Lowe, Kociolek, Johansen, Van de Vijver, Lange-Bertalot & Kopalová 2014 (Figure 13AA–AD).
Remarks. The specimens in our samples were 11.4–14.9 μm long, 2.9–3.5 μm wide.
This species was found at stations no. O-10, O-11, O-1, O-5, O-9 (pH 6.4–7.33, conductivity 60–130 μS/cm).
Luticola acidoclinata Lange-Bertalot in Lange-Bertalot & Metzeltin 1996 (Figure 13AE).
Remarks. The specimen in our sample was 18.6 μm long, 6.8 μm wide and had 22 striae in 10 μm.
This species was found at stations no. O-4, O-9, O-11 (pH 6.4–6.82, conductivity 70–350 μS/cm).
Cavinula variostriata (Krasske) D.G. Mann & Stickle in Round et al. 1990 (Figure 13AF–AL).
Remarks. The specimens in our samples were 19.8–31.5 μm long, 6.0–8.1 μm wide and had 26–28 striae in 10 μm.
This species was found at station no. O-4 (pH n/d, conductivity 350 μS/cm).
Brachysira sp. 1. (Figure 13AM).
Remarks. The specimen in our sample was 22.2 μm long, 5.5 μm wide and had 33 striae in 10 μm.
This species was found at station no. O-4 (pH n/d, conductivity 350 μS/cm).
Brachysira sp. 2. (Figure 13AN–AP).
Remarks. The specimens in our samples were 19.5–29.2 μm long, 5.5–5.7 μm wide and had 28 striae in 10 μm.
This species was found at station no. O-14 (pH 7.77, conductivity 90 μS/cm).
Caloneis tenuis (W. Gregory) Krammer 1985 (Figure 14A–F).

Figure 14.
(A–AB). Naviculoid diatoms. LM, DIC. (A–F). Caloneis tenuis. (G). Chamaepinnularia krookiformis. (H–J). Sellaphora seminulum. (K). Sellaphora simillima. (L). Stauroneis amphicephala. (M). Caloneis silicula. (N). Nupela impexiformis. (O–Q). Stauroneis kriegeri. (R). Stauroneis borrichii. (S). Stauroneis thermicola. (T–W). Sellaphora pseudobacillum. (X–Z). Diploneis tundra. (AA,AB). D. mollenhaueri. Scale bar = 10 μm.
Remarks. The specimens in our samples were 23.5–33.8 μm long, 4.6–4.9 μm wide and had 21–23 striae in 10 μm.
This species was found at station no. O-4 (pH n/d, conductivity 350 μS/cm).
Chamaepinnularia krookiformis (Krammer) Lange-Bertalot & Krammer in Lange-Bertalot & Genkal 1999 (Figure 14G).
Remarks. The specimen in our sample was 15.9 μm long, 4.2 μm wide and had 20 striae in 10 μm.
This species was found at station no. O-15 (pH 7.79, conductivity 50 μS/cm).
Sellaphora seminulum (Grunow) D.G. Mann 1989 (Figure 14H–J).
Remarks. The specimens in our samples were 10.4–12.2 μm long, 3.3–3.7 μm wide and had 20 striae in 10 μm.
This species was found at stations no. O-10, O-11, O-12, O-13, O-15, O-1 (pH 6.82–7.79, conductivity 50–140 μS/cm).
Sellaphora simillima Metzeltin, Lange-Bertalot & Soninkhishig 2009 (Figure 14K).
Remarks. The specimen in our sample was 45.1 μm long, 11.3 μm wide and had 21 striae in 10 μm.
This species was found at stations no. O-4, O-9, O-15 (pH 6.4–7.79, conductivity 50–350 μS/cm).
Stauroneis amphicephala Kützing 1844 (Figure 14L).
Remarks. The specimen in our sample was 48.5 μm long, 11.7 μm wide and had 25 striae in 10 μm.
This species was found at station no. O-10 (pH 7.33, conductivity 70 μS/cm).
Caloneis silicula (Ehrenberg) P.T. Cleve 1894 (Figure 14M).
Remarks. The specimen in our sample was 54.1 μm long, 12.9 μm wide and had 18 striae in 10 μm.
This species was found at station no. O-1 (pH n/d, conductivity 60 μS/cm).
Nupela impexiformis (Lange-Bertalot in Lange-Bertalot & Krammer) Lange-Bertalot 1999 (Figure 14N).
Remarks. The specimen in our sample was 24.1 μm long, 5.5 μm wide.
This species was found at station no. O-11 (pH 6.82, conductivity 70 μS/cm).
Stauroneis kriegeri R.M. Patrick 1945 (Figure 14O–Q).
Remarks. The specimens in our samples were 21.8–23.1 μm long, 4.8–5.1 μm wide and had 27–28 striae in 10 μm.
This species was found at station no. O-3 (pH 7.18, conductivity 60 μS/cm).
Stauroneis borrichii (J.B. Petersen) J.W.G. Lund 1946 (Figure 14R).
Remarks. The specimen in our sample was 21.8 μm long, 5.5 μm wide and had 19 striae in 10 μm.
This species was found at station no. O-11 (pH 6.82, conductivity 70 μS/cm).
Stauroneis thermicola (J.B. Petersen) J.W.G. Lund 1946 (Figure 14S).
Remarks. The specimen in our sample was 15.7 μm long, 3.1 μm wide and had 22 striae in 10 μm.
This species was found at station no. O-5 (pH n/d, conductivity 70 μS/cm).
Sellaphora pseudobacillum (Grunow) Lange-Bertalot & Metzeltin in Metzeltin et al. 2009 (Figure 14T–W).
Remarks. The specimens in our samples were 30.3–43.6 μm long, 10.4–11.8 μm wide and had 18–20 striae in 10 μm.
This species was found at station no. O-15 (pH 7.79, conductivity 50 μS/cm).
Diploneis tundra Lange-Bertalot & Fuhrmann 2016 (Figure 14X–Z).
Remarks. The specimens in our samples were 28.0–33.7 μm long, 15.5–17.2 μm wide and had 12–13 striae in 10 μm.
This species was found at stations no. O-1, O-5 (pH n/d, conductivity 60–70 μS/cm).
Diploneis mollenhaueri Lange-Bertalot & Fuhrmann 2020 (Figure 14AA,AB).
Remarks. The specimens in our samples were 31.1–32.5 μm long, 16.1–17.4 μm wide and had 12–13 striae in 10 μm.
This species was found at stations no. O-10, O-11, O-4 (pH 6.82–7.33, conductivity 70–350 μS/cm).

Figure 15.
(A–P). Pinnularia spp. LM, DIC. (A). Pinnularia cf. viridis. (B). P. stomatophora. (C). P. viridiformis. (D). P. cf. esoxiformis. (E). P. parvulissima. (F,G). P. cf. marchica. (H,I). P. sp. 1. (J–L). P. borealis s.l. (M). P. nodosa. (N). P. cf. minigibba. (O). P. sp. 2. (P). P. joculata. Scale bar = 10 μm.
Remarks. The specimen in our sample was 134.2 μm long, 24.1 μm wide and had 7 striae in 10 μm.
This species was found at station no. O-1 (pH n/d, conductivity 60 μS/cm).
Pinnularia stomatophora (Grunow) P.T. Cleve 1895 (Figure 15B).
Remarks. The specimen in our sample was 94.4 μm long, 12.3 μm wide and had 13 striae in 10 μm.
This species was found at station no. O-9 (pH 6.4, conductivity 130 μS/cm).
Pinnularia viridiformis Krammer 2000 (Figure 15C).
Remarks. The specimens in our sample were 73.8 μm long, 17.5 μm wide and had 10 striae in 10 μm.
This species was found at station no. O-11 (pH 6.82, conductivity 70 μS/cm).
Remarks. The specimen in our sample was 69.3 μm long, 11.1 μm wide and had 8 striae in 10 μm.
This species was found at station no. O-11 (pH 6.82, conductivity 70 μS/cm).
Pinnularia parvulissima Krammer 2000 (Figure 15E).
Remarks. The specimen in our sample was 60.0 μm long, 9.3 μm wide and had 10 striae in 10 μm.
This species was found at station no. O-9 (pH 6.4, conductivity 130 μS/cm).
Remarks. The specimens in our samples were 28.7–32.0 μm long, 4.9–5.0 μm wide and had 8 striae in 10 μm.
This species was found at station no. O-9 (pH 6.4, conductivity 130 μS/cm).
Pinnularia sp. 1. (Figure 15H,I).
Remarks. The specimens in our samples were 27.7–28.8 μm long, 4.2–4.5 μm wide and had 12 striae in 10 μm.
This species was found at station no. O-9 (pH 6.4, conductivity 130 μS/cm).
Pinnularia borealis Ehrenberg 1843 s.l. (Figure 15J–L).
Remarks. The specimens in our samples were 27–46 μm long, 8.0–10.2 μm wide and had 5–6 striae in 10 μm.
This species was found at stations no. O-3, O-10, O-11, O-12, O-L, O-4 (pH 6.82–8.7, conductivity 60–350 μS/cm).
Pinnularia nodosa (Ehrenberg) W. Smith 1856 (Figure 15M).
Remarks. The specimen in our sample was 50.5 μm long, 7.9 μm wide and had 10 striae in 10 μm.
This species was found at station no. O-12 (pH 7.6, conductivity 140 μS/cm).
Remarks. The specimens in our samples were 38.5 μm long, 5.3 μm wide and had 11 striae in 10 μm.
This species was found at station no. O-9 (pH 6.4, conductivity 130 μS/cm).
Pinnularia sp. 2. (Figure 15O).
Remarks. The specimen in our sample was 27.9 μm long, 4.3 μm wide and had 13 striae in 10 μm.
This species was found at station no. O-12 (pH 7.6, conductivity 140 μS/cm).
Pinnularia joculata (Manguin) Krammer 2000 (Figure 15P).
Remarks. The specimen in our sample was 22.6 μm long, 4.3 μm wide and had 11 striae in 10 μm.
This species was found at station no. O-4 (pH n/d, conductivity 350 μS/cm).
Amphipleura pellucida (Kützing) Kützing 1844 (Figure 16A).

Figure 16.
(A–P). Naviculoid diatoms. LM, DIC. (A). Amphipleura pellucida. (B). Frustulia krammeri. (C). F. lange-bertalotii. (D–F). F. vulgaris. (G,H). F. crassinervia. (I,J). Pinnularia septentrionalis. (K). P. esoxiformis. (L–P). Neidium bisulcatum. Scale bar = 10 μm.
Remarks. The specimen in our sample was 98.0 μm long, 8.5 μm wide.
This species was found at station O-11 (pH 6.82, conductivity 70 μS/cm).
Frustulia krammeri Lange-Bertalot & Metzeltin in Metzeltin & Lange-Bertalot 1998 (Figure 16B).
Remarks. The specimen in our sample was 92.8 μm long, 19.4 μm wide and had 24 striae in 10 μm and 25 areolae in 10 μm.
This species was found at station O-16 (pH 8.2, conductivity 70 μS/cm).
Frustulia lange-bertalotii Metzeltin in Lange-Bertalot & Genkal 1999 (Figure 16C).
Remarks. The specimen in our sample was 66.0 μm long, 9.9 μm wide and had 30 striae in 10 μm.
This species was found at station O-13, O-16 (pH 7.6–8.2, conductivity 70–80 μS/cm).
Frustulia vulgaris (Thwaites) De Toni 1891 (Figure 16D–F).
Remarks. The specimens in our samples were 44.4–50.9 μm long, 10.2–11.3 μm wide and had 29–30 striae in 10 μm.
This species was found at stations O-4, O-16 (pH 8.2, conductivity 70–350 μS/cm).
Frustulia crassinervia (Brébisson ex W. Smith) Lange-Bertalot & Krammer in Lange-Bertalot & Metzeltin 1996 (Figure 16G,H).
Remarks. The specimens in our samples were 37.7–42.9 μm long, 11.2–11.6 μm wide and had 35 striae in 10 μm.
This species was found at station O-4 (pH n/d, conductivity 350 μS/cm).
Pinnularia septentrionalis Krammer 2000 (Figure 16I,J).
Remarks. The specimens in our samples were 62.5–68.0 μm long, 11.0–11.5 μm wide at the central part, 12.0–12.1 μm wide at the widest part and had 10–11 striae in 10 μm.
This species was found at stations O-1, O-3 (pH 7.18, conductivity 60 μS/cm).
Pinnularia esoxiformis Fusey 1951 (Figure 16K).
Remarks. The specimen in our sample was 65.2 μm long, 10.5 μm wide and had 10 striae in 10 μm.
This species was found at station O-L (pH 8.7, conductivity 90 μS/cm).
Neidium bisulcatum (Lagerstedt) P.T. Cleve 1894 (Figure 16L–P).
Remarks. The specimens in our samples were 30.7–70.8 μm long, 6.3–8.3 μm wide and had 29–33 striae in 10 μm.
This species was found at stations no. O-4, O-9 (pH 6.4, conductivity 130–350 μS/cm).
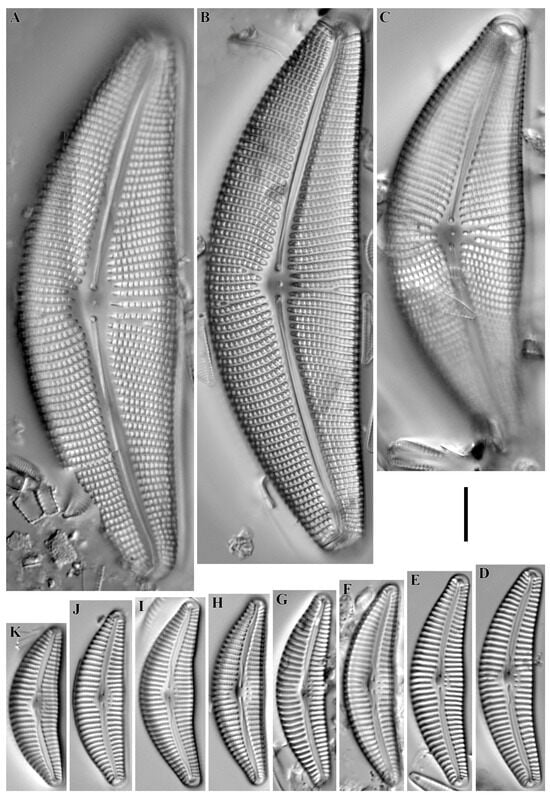
Figure 17.
(A–K). Cymbella spp. LM, DIC. (A–C). Cymbella mexicana var. kamtschatica. (D–K). C. diversistigmata. Scale bar = 10 μm.
Remarks. The specimens in our samples were 82.1–102.4 μm long, 25.7–29.1 μm wide and had 7–8 striae and 10–11 areolae in 10 μm.
This species was found at stations no. O-16, O-5 (pH 8.2, conductivity 70 μS/cm).
Cymbella diversistigmata Krammer 2002 (Figure 17D–K).
Remarks. The specimens in our samples were 29.4–40.5 μm long, 8.8–10.2 μm wide and had 11–13 striae in 10 μm.
This species was found at stations no O-1, O-12 (pH 7.6, conductivity 60–140 μS/cm).
Cymbella nepalensis (Jüttner & Van de Vijver) Vishnjakov 2015 (Figure 18A–F).

Figure 18.
(A–J). Cymbella spp. LM, DIC. (A–F). Cymbella nepalensis. (G–J). C. amplificata. Scale bar = 10 μm.
Remarks. The specimens in our samples were 49.5–74.4 μm long, 14.0–16.5 μm wide and had 11–12 striae in 10 μm.
This species was found at stations no. O-12, O-13, O-14, O-15 (pH 7.6–7.79, conductivity 50–140 μS/cm).
Cymbella amplificata Krammer 2002 (Figure 18G–J).
Remarks. The specimens in our samples were 83.0–97.2 μm long, 20.5–23.5 μm wide and had 9 striae in 10 μm.
This species was found at stations no. O-1, O-3, O-5, O-12, O-14, O-15, O-16 (pH 7.18–8.2, conductivity 50–140 μS/cm).
Cymbopleura acuta (A. Schmidt) Krammer 2003 (Figure 19A,B).

Figure 19.
(A–Y). Cymbelloid diatoms. LM, DIC. (A,B). Cymbopleura acuta. (C). C. tynnii. (D–F). C. anglica. (G–I). C. pernaviculiformis. (J). C. hybrida. (K–P). Encyonema minutum. (Q–U). E. silesiacum. (V–Y). E. ventricosum. Scale bar = 10 μm.
Remarks. The specimens in our samples were 60.9–63.7 μm long, 18.8–19.9 μm wide and had 10 striae in 10 μm.
This species was found at station no. O-1 (pH n/d, conductivity 60 μS/cm).
Cymbopleura tynnii (Krammer) Krammer 2003 (Figure 19C).
Remarks. The specimen in our sample was 49.1 μm long, 16.4 μm wide and had 10 striae in 10 μm.
This species was found at stations no. O-5, O-9 (pH 6.4, conductivity 70–130 μS/cm).
Cymbopleura anglica (Lagerstedt) Krammer 2003 (Figure 19D–F).
Remarks. The specimens in our samples were 35.6–43.1 μm long, 13.5–13.8 μm wide and had 11–13 striae in 10 μm.
This species was found at station no. O-1 (pH n/d, conductivity 60 μS/cm).
Cymbopleura pernaviculiformis Kulikovskiy, Lange-Bertalot & Dorofeyuk in Kulikovskiy et al. 2009 (Figure 19G–I).
Remarks. The specimens in our samples were 29.9–41.0 μm long, 10.0–10.2 μm wide and had 15–16 striae in 10 μm.
This species was found at stations no. O- 9, O-12, O-14 (pH 6.4–7.77, conductivity 90–140 μS/cm).
Cymbopleura hybrida (Grunow) Krammer 2003 (Figure 19J).
Remarks. The specimen in our sample was 34.7 μm long, 6.8 μm wide and had 16 striae in 10 μm.
This species was found at station no. O-14 (pH 7.77, conductivity 90 μS/cm).
Encyonema minutum (Hilse) D.G. Mann in Round et al. 1990 (Figure 19K–P).
Remarks. The specimens in our samples were 10.7–17.7 μm long, 4.1–5.1 μm wide and had 17–19 striae in 10 μm.
This species was found at all stations except O-4 (pH 6.4–8.7, conductivity 50–140 μS/cm).
Encyonema silesiacum (Bleisch in Rabenhorst) D.G. Mann in Round et al. 1990 (Figure 19Q–U).
Remarks. The specimens in our samples were 28.0–41.4 μm long, 8.0–9.5 μm wide and had 13–14 striae in 10 μm.
This species was found at all stations except O-9 (pH 6.82–8.7, conductivity 50–350 μS/cm).
Encyonema ventricosum (C. Agardh) Grunow in A. Schmidt 1875 (Figure 19V–Y).
Remarks. The specimens in our samples were 18.6–28.4 μm long, 6.3–7.9 μm wide and had 14–15 striae in 10 μm.
This species was found at all stations except O-4, O-9 (pH 6.82–8.7, conductivity 50–140 μS/cm).
Didymosphenia geminata (Lyngby) M. Schmidt 1899 (Figure 20A–C).
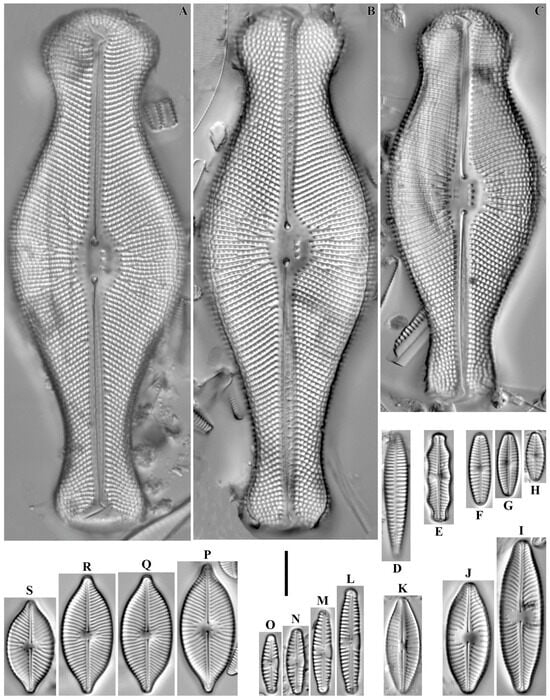
Figure 20.
(A–S). Cymbelloid diatoms. LM, DIC. (A–C). Didymosphenia geminata. (D). Rhoicosphenia abbreviata. (E). Geissleria ignota. (F–H). G. acceptata. (I,J). Witkowskia cuneata. (K). W. hambergii. (L–O). Reimeria sinuata. (P–S). Witkowskia clementispronina. Scale bar = 10 μm.
Remarks. The specimens in our samples were 88.9–117.1 μm long, 33.2–39.3 μm wide and had 8–9 striae in 10 μm.
This species was found at stations no. O-13, O-14, O-L, O-16, O-5 (pH 7.6–8.7, conductivity 70–90 μS/cm).
Rhoicosphenia abbreviata (C. Agardh) Lange-Bertalot 1980 (Figure 20D).
Remarks. The specimen in our sample was 27.8 μm long, 5.0 μm wide and had 13 striae in 10 μm.
This species was found at station no. O-14 (pH 7.77, conductivity 90 μS/cm).
Geissleria ignota (Krasske) Lange-Bertalot & Metzeltin 1996 (Figure 20E).
Remarks. The specimen in our sample was 19.8 μm long, 5.3 μm wide and had 16 striae in 10 μm.
This species was found at station no. O-L (pH 8.7, conductivity 90 μS/cm).
Geissleria acceptata (Hustedt) Lange-Bertalot & Metzeltin 1996 (Figure 20F–H).
Remarks. The specimens in our samples were 10.8–16.0 μm long, 4.1–4.8 μm wide and had 16–17 striae in 10 μm.
This species was found at station no. O-5, O-14 (pH 7.77, conductivity 70–90 μS/cm).
Witkowskia cuneata (M. Möller ex Foged) Kulikovskiy, Glushchenko, Mironov & Kociolek in Mironov et al. 2024 (Figure 20I,J).
Remarks. The specimens in our samples were 24.8–34.0 μm long, 9.8 μm wide and had 12–13 striae in 10 μm.
This species was found at station no. O-11 (pH 6.82, conductivity 70 μS/cm).
Witkowskia hambergii (Hustedt) Kulikovskiy, Glushchenko, Mironov & Kociolek in Mironov et al. 2024 (Figure 20K).
Remarks. The specimen in our sample was 21.3 μm long, 7.7 μm wide and had 14 striae in 10 μm.
This species was found at station no. O-4 (pH n/d, conductivity 350 μS/cm).
Reimeria sinuata (W. Gregory) Kociolek & Stoermer 1987 (Figure 20L–O).
Remarks. The specimens in our samples were 13.2–23.5 μm long, 3.8–4.5 μm wide and had 13–14 striae in 10 μm.
This species was found at stations no. O-3, O-13, O-14, O-15, O-L, O-16 (pH 7.18–8.7, conductivity 60–90 μS/cm).
Witkowskia clementispronina (Lange-Bertalot & Wojtal) Kulikovskiy, Glushchenko, Mironov & Kociolek in Mironov et al. 2024 (Figure 20P–S).
Remarks. The specimens in our samples were 20.9–28.8 μm long, 10.7–12.4 μm wide and had 17 striae in 10 μm.
This species was found at station no. O-1 (pH n/d, conductivity 60 μS/cm).
Gomphadelpha eriensis (Grunow) Jahn & Abarca in Abarca et al. 2023 (Figure 21A–G).
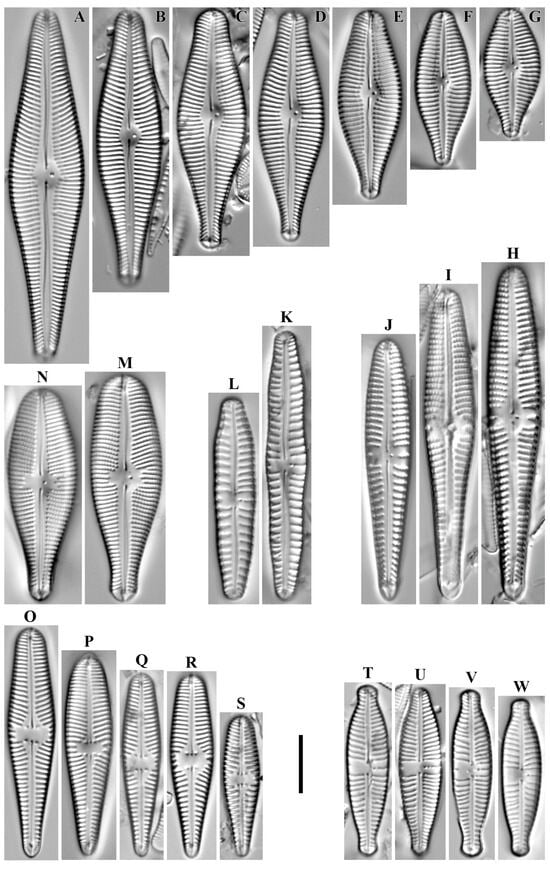
Figure 21.
(A–W). Gomphonemoid diatoms. LM, DIC. (A–G). Gomphadelpha eriensis. (H–J). Gomphonema popovae. (K,L). G. distans. (M,N). G. cf. ventricosum. (O–S). Gomphonella olivaceoides. (T–W). Gomphonema micropus. Scale bar = 10 μm.
Remarks. The specimens in our samples were 22.4–60.9 μm long, 9.7–13.0 μm wide and had 13–15 striae in 10 μm.
This species was found found at stations no. O-1, O-5, O-11, O-14, O-15, O-L, O-16 (pH 6.82–8.7, conductivity 50–90 μS/cm).
Gomphonema popovae Levadnaja 1973 (Figure 21H–J).
Remarks. The specimens in our samples were 43.3–57.8 μm long, 7.9–9.3 μm wide and had 10–11 striae in 10 μm.
This species was found at station no. O-1 (pH n/d, conductivity 60 μS/cm).
Gomphonema distans (A. Cleve) Lange-Bertalot & Reichardt in Lange-Bertalot & Genkal 1999 (Figure 21K,L).
Remarks. The specimens in our samples were 35.1–46.5 μm long, 6.9–7.2 μm wide and had 8 striae in 10 μm.
This species was found at station no. O-12 (pH 7.6, conductivity 140 μS/cm).
Remarks. The specimens in our samples were 36.8–39.4 μm long, 11.8–12.3 μm wide and had 15 striae in 10 μm.
This species was found at station no. O-11, O-L (pH 6.82–8.7, conductivity 70–90 μS/cm).
Gomphonella olivaceoides (Hustedt) Tuji 2020 (Figure 21O–S).
Remarks. The specimens in our samples were 24.0–39.6 μm long, 6.2–8.0 μm wide and had 14–15 striae in 10 μm.
This species was found at all stations except O-4, O-9 (pH 6.82–8.7, conductivity 50–140 μS/cm).
Gomphonema micropus Kützing 1844 (Figure 21T–W).
Remarks. The specimens in our samples were 27.1–29.3 μm long, 6.7–7.4 μm wide and had 11–12 striae in 10 μm.
This species was found at stations no. O-3, O-4, O-5, O-10, O-11, O-12, O-13, O-15, O-L (pH 6.4–8.7, conductivity 50–350 μS/cm).
Epithemia gibba (Ehrenberg) Kützing 1844 (Figure 22A).

Figure 22.
(A–P). Gomphonemoid, epithemioid, and surirelloid diatoms. LM, DIC. (A). Epithemia gibba. (B,C). E. turgida. (D). E. adnata. (E). E. gibberula. (F,G). Surirella sp. (H–P). Gomphonema demersum. Scale bar = 10 μm.
Remarks. The specimen in our sample was 82.9 μm long, 9.4 μm wide and had 15 striae in 10 μm.
This species was found at station no. O-9 (pH 6.4, conductivity 130 μS/cm).
Epithemia turgida (Ehrenberg) Kützing 1844 (Figure 22B,C).
Remarks. The specimens in our samples were 72.1–77.1 μm long, 15.2–15.8 μm wide and had 9–10 striae in 10 μm and 5 costae in 10 μm.
This species was found at station no. O-1, O-5, O-16, O-4 (pH 6.4–8.2, conductivity 60–350 μS/cm).
Epithemia adnata (Kützing) Brébisson 1838 (Figure 22D).
Remarks. The specimen in our sample was 70.8 μm long, 9.5 μm wide and had 13 striae in 10 μm and 4 costae in 10 μm.
This species was found at station O-4 (pH n/d, conductivity 350 μS/cm).
Epithemia gibberula (Ehrenberg) Kützing 1844 (Figure 22E).
Remarks. The specimens in our samples were 55.5 μm long, 7.8 μm wide and had 22 striae in 10 μm and 4 costae in 10 μm.
This species was found at station O-9 (pH 6.4, conductivity 130 μS/cm).
Surirella sp. (Figure 22F,G).
Remarks. The specimens in our samples were 32.0–67.8 μm long, 10.0–11.4 μm wide and had 22 striae in 10 μm.
This species was found at station no. O-11, O-15, O-L (pH 6.82–8.7, conductivity 50–90 μS/cm).
Gomphonema demersum Reichardt 2009 (Figure 22H–P).
Remarks. The specimens in our samples were 20.2–35.3 μm long, 4.2–5.0 μm wide and had 11–13 striae in 10 μm.
This species was found at stations O-1, O-12, O-14, O-L (pH 7.6–8.7, conductivity 60–140 μS/cm).
Nitzschia palea (Kützing) W. Smith 1856 (Figure 23A–D).
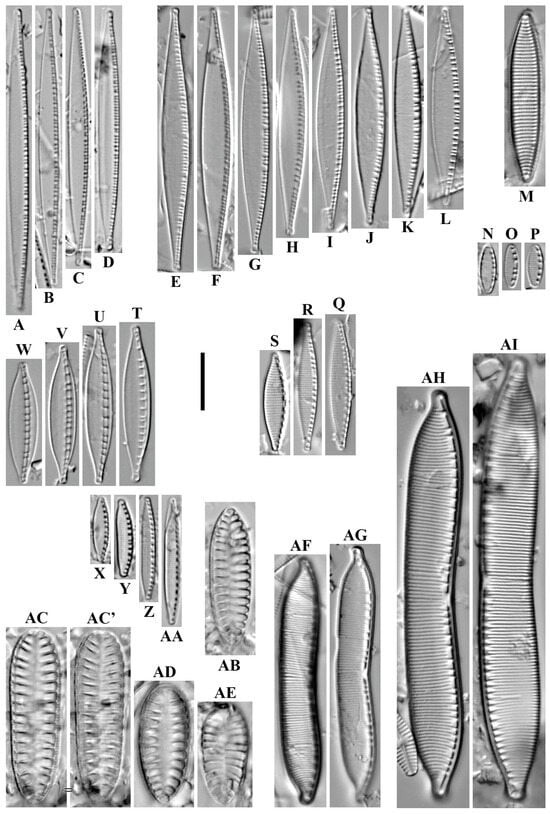
Figure 23.
(A–AI). Nitzschioid and surirelloid diatoms. LM, DIC. (A–D). Nitzschia palea. (E–L). N. tubicola. (M). N. angustata. (N–P). N. iconspicua. (Q–S). N. fonticola. (T–W). N. dissipata. (X–AA). N. acidoclinata. (AB). Surirella cf. angusta. (AC–AE). S. pinnata. (AF–AG). Hantzschia calcifuga. (AH,AI). H. abundans. Scale bar = 10 μm.
Remarks. The specimens in our samples were 41.8–52.3 μm long, 4.9–5.1 μm wide and had 15–19 fibulae in 10 μm.
This species was found at all stations except O-5, O-16 (pH 6.4–8.7, conductivity 50–350 μS/cm).
Nitzschia tubicola Grunow in P.T. Cleve & Grunow 1880 (Figure 23E–L).
Remarks. The specimens in our samples were 34.3–45.6 μm long, 4.5–4.9 μm wide and had 15 striae in 10 μm.
This species was found at all stations except O-L (pH 6.4–8.2, conductivity 50–350 μS/cm).
Nitzschia angustata W. Smith 1853 (Figure 23M).
Remarks. The specimen in our sample was 29.6 μm long, 5.0 μm wide and had 17 striae in 10 μm.
This species was found at station no. O-5 (pH n/d, conductivity 70 μS/cm).
Nitzschia inconspicua Grunow 1860 (Figure 23N–P).
Remarks. The specimens in our samples were 7.4–8.4 μm long, 2.5–2.8 μm wide and had 30 striae in 10 μm.
This species was found at stations no. O-1, O-5, O-14 (pH 7.77, conductivity 60–90 μS/cm).
Nitzschia fonticola (Grunow) Grunow in Van Heurck 1881 (Figure 23Q–S).
Remarks. The specimens in our samples were 17.1–22.5 μm long, 3.5–4.1 μm wide and had 24–27 striae in 10 μm.
This species was found at stations no. O-3, O-14, O-L, O-16, O-1, O-5, O-9 (pH 6.4–8.7, conductivity 60–130 μS/cm).
Nitzschia dissipata (Kützing) Rabenhorst 1860 (Figure 23T–W).
Remarks. The specimens in our samples were 21.0–27.2 μm long, 4.5–4.9 μm wide and had 8–10 fibulae in 10 μm.
This species was found at stations no. O-10, O-13, O-15, O-L, O-16, O-1 (pH 7.33–8.7, conductivity 50–90 μS/cm).
Nitzschia acidoclinata Lange-Bertalot 1977 (Figure 23X–AA).
Remarks. The specimens in our samples were 11.3–17.7 μm long, 2.5–2.7 μm wide and had 32–33 striae in 10 μm.
This species was found at all stations (pH 6.4–8.7, conductivity 50–350 μS/cm).
Remarks. The specimen in our sample was 24.5 μm long, 7.3 μm wide and had eight costae in 10 μm.
This species was found at station no. O-13 (pH 7.6, conductivity 80 μS/cm).
Surirella pinnata W. Smith 1853 (Figure 23AC–AE).
Remarks. The specimens in our samples were 17.7–30.5 μm long, 8.2–9.0 μm wide and had 16 striae in 10 μm.
This species was found at stations O-11, O-15, O-L (pH 6.82–8.7, conductivity 50–90 μS/cm).
Hantzschia calcifuga Reichardt & Lange-Bertalot in Werum & Lange-Bertalot 2004 (Figure 23AF–AG).
Remarks. The specimens in our samples were 42.8–44.7 μm long, 6.5–6.7 μm wide and had 22–23 striae in 10 μm.
This species was found at station no. O-4 (pH n/d, conductivity 350 μS/cm).
Hantzschia abundans Lange-Bertalot 1993 (Figure 23AH,AI).
Remarks. The specimens in our samples were 71.1–77.1 μm long, 9.3–9.7 μm wide and had 15–18 striae in 10 μm.
This species was found at stations O-11, O-4 (pH 6.82, conductivity 70–350 μS/cm).
We also propose a new species for science:
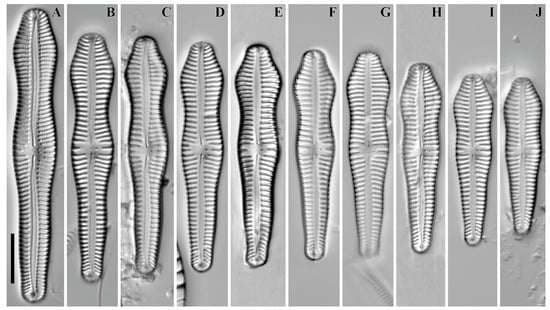
Figure 24.
(A–J). Gomphonema anissimovae Glushchenko, Kezlya & Kulikovskiy sp. nov. LM, DIC. Sample no. O-L. Slide no. 08744. Size diminution series. (B). Holotype. Scale bar = 10 μm.

Figure 25.
(A–H). Gomphonema anissimovae Glushchenko, Kezlya & Kulikovskiy sp. nov. SEM, internal views. Sample no. O-L. Stub no. 08742. Scale bars (A–C) = 10 μm; (D) = 5 μm; (E,F,H) = 2 μm; (G) = 1 μm.
Holotype here designated. Deposited in the herbarium of MHA, Main Botanical Garden, Russian Academy of Science, Moscow, Russia, the holotype here designated, slide no. 08742 (Figure 24B).
Isotype. Collection of Maxim Kulikovskiy at the Herbarium of the Institute of Plant Physiology, Russian Academy of Science, Moscow, Russia, slide no. 08742a.
Type locality. Stream PC 547-20, Kamchatka, Russia. Periphyton on stones on the bottom, 57.547333333, 161.029805556, leg. E.M. Kezlya on 19 September 2023 (Table 1).
Representative specimens. Slides no. 08742m, 08743, 08748, 08750 in the Collection of Maxim Kulikovskiy at the Herbarium of the Institute of Plant Physiology, Russian Academy of Science, Moscow, Russia.
Etymology. Species is dedicated with gratitude to the Russian phycologist Dr. Olga Viktorovna Anissimova, a specialist on desmids and the first phycology teacher of E.M. Kezlya.
Distribution. The new species was found in the periphyton of Levaya Ozernaya River (station O-13), Stream PC 547-20 (station O-12), Limnocren (station O-1), Hamut Stream (station O-4), Nezhnyj Stream (station O-9) (Table 1 and Table S1).
Description. LM (Figure 23A–J). Valves heteropolar, narrowly clavate. Headpole rhomboid and obtusely rounded. Footpole widely rounded, sometimes slightly deviated from the apical axis. Constriction visible between headpole and central part of the valve. Length 31.9–59.2 μm, breadth 7.2–9.0 μm in the central part, 7.6–9.3 μm in the headpole. Axial area narrowly lanceolate. Central area irregular, bordered by 1–2 shortened striae on each side. Raphe distinctly lateral with slightly expanded proximal raphe endings. Distal raphe ends deflected onto the valve mantle. One (rarely two) isolated pore present near the central area at the end of shortened stria. Striae weakly radial at the central part of the valve, becoming more radial at the ends, 10.5–12.5 in 10 μm. Areolae visible in LM.
SEM, internal views (Figure 24A–H). Proximal raphe ends hooked, located on raised central nodule. Isolated pore with slit-like opening. Small pseudosepta are present, visible at footpole and headpole. The helictoglossae are slightly offset from the raphe branch, in the opposite direction from the proximal raphe ends. Transapical striae continue on valve margin. The areolae are located in narrow foraminal rows between strongly silificed vimines, 30–35 in 10 μm. Siliceous flaps covering most of the opening on the areolas are present. The apical pore fields project slightly from behind the pseudoseptum.
Differential diagnosis
Gomphonema anissimovae sp. nov. belongs to the G. acuminatum-brebissonii species group. These species are distinguished by an expanded headpole and central part of the valves, which unites them with the G. acuminatum species group [37,38]. Literature data indicate a rather large variety of species within this group, including species close to G. brebissonii. For the comparative analysis, some characteristic species were selected, also in the understanding of different authors, presented in Table 2.
Morphologically, the closest species to G. anissimovae sp. nov. is G. brebissonii Kützing (Table 2). The species are similar in the width of the valves in the central part (7.2–9.0 μm in G. anissimovae sp. nov. vs. 7.5–9.5 μm in G. brebissonii) and in the width of the headpole (7.6–9.3 μm in G. anissimovae sp. nov. vs. 7–9 μm in G. brebissonii). They have a similar striae density: 10.5–12.5 in 10 μm in G. anissimovae sp. nov. vs. 9–11 in 10 μm in G. brebissonii, and also are similar in the shape of the axial and central areas. The main difference in these species is the shape of the headpole of the valves. In G. brebissonii, the headpole is clearly wedge-shaped, only the large valves have a blunted apex ([39], p. 151, Figure 1). The footpoles of the valves also differ between species, in G. anissimovae sp. nov. the edges of the valves narrow gradually towards the basal end, and there is a slight widening of the valves between their central part and basal ends, whereas in G. brebissonii the edges of the valves are more narrowed and more pointed, not widened in the area between the central part of the valves and their basal ends ([39], p. 151, Figure 2, Figure 3, Figure 4, Figure 5, Figure 6, Figure 7, Figure 8, Figure 9, Figure 10, Figure 11, Figure 12, Figure 13 and Figure 14).
G. anissimovae sp. nov. is close to the species identified by some authors as G. montanum (Schumann) Grunow. The type material of G. acuminatum var. montanum Schumann was not found, and the author’s drawings are rather schematic ([39], p. 151, Figure 16 and Figure 17). The understanding of the morphology of the taxon G. acuminatum var. montanum Schumann is rather diverse. For example, the material shown by Lange-Bertalot ([40], p. 821, Pl. 96, Figure 6, Figure 7, Figure 8, Figure 9 and Figure 10) has the greatest similarity in valve outline to the Schumann drawings. The material of G. anissimovae sp. nov. has similar values of valve length and width and striae density, however, it differs from the material of G. montanum (Schumann) Grunow sensu Cantonati et al., 2017 [40], primarily in the shape of the headpole of the valves. In G. anissimovae sp. nov. the ends of the valves are rhombic in general outline and bluntly wedge-shaped at the apexes, while in G. montanum (Schumann) Grunow sensu Cantonati et al., 2017 [40] they have no obvious rhombic end and only one specimen has a rhombically elongated end ([40], p. 821, Pl. 96, Figure 9).
G. montanum sensu Reichardt, illustrated from Austria ([41], p. 178, Taf. 58, Figure 9), with similar quantitative characteristics (see Table 2), differs from G. anissimovae sp. nov. by the shape of the headpole of the valve. In G. montanum sensu Reichardt, the headpole is wedge-shaped with a bluntly rounded apex and, in general, looks simpler than the headpole of G. anissimovae sp. nov. which has a stronger inflation.
The species identified as G. montanum and illustrated by Rumrich et al. from Chile ([42], p. 496, Taf. 128, Figure 1 and Figure 2) differs fundamentally in valve outline both from Schumann’s images and from other valves identified as G. montanum by different authors (see Table 2). The main difference is that the valves of G. montanum sensu Rumrich et al. do not have the expanded headpole characteristic of both Schumann’s taxon and G. montanum as understood by some authors (see Table 2). Also, in G. montanum sensu Rumrich et al. there is no slightly expanded area between the central part of the valve and its basal end, which is present in our species.
The species identified as G. montanum and shown from Mongolia ([43], p. 271, Pl. 98, Figure 6, Figure 7, Figure 8 and Figure 9) is close to G. anissimovae sp. nov. both in valve outline and in the main quantitative characters (see Table 2). In our opinion, G. montanum sensu Kulikovskiy et al. is in fact G. anissimovae sp. nov.
An interesting find is a valve identified by Potapova as G. interpositum Reichardt ([22], p. 96, Figure 336). The main quantitative characteristics of this specimen are similar to those of our species (Table 2). The general valve shape and the shape of the inflations of the headpole and footpole also have general similarity to our material. At the same time, G. interpositum sensu Potapova has more pronounced constrictions on both sides of the central part of the valve ([22], p. 96, Figure 336), whereas in specimens of G. anissimovae sp. nov. the constrictions are less pronounced. Thus, G. interpositum sensu Potapova requires additional comparison with G. anissimovae sp. nov.
The species G. montanum sensu Lange-Bertalot & Genkal ([44], p. 252, Taf. 68, Figure 11, Figure 12 and Figure 13) also has a general similarity to G. anissimovae sp. nov., in particular in the valve outline. At the same time, G. anissimovae sp. nov. has less constricted valves in the region of the headpole and footpole and generally has larger valve width (given equal length of the studied specimens of both species): in the central part of the valve 7.2–9.0 µm in G. anissimovae sp. nov. vs. 7.0–7.5 µm in G. montanum sensu Lange-Bertalot & Genkal; in the region of the headpole 7.6–9.3 in G. anissimovae sp. nov. vs. 7.5–8.0 in G. montanum sensu Lange-Bertalot & Genkal).

Table 2.
Comparison of morphological features of Gomphonema anissimovae sp. nov. and related species.
Table 2.
Comparison of morphological features of Gomphonema anissimovae sp. nov. and related species.
| G. anissimovae sp. nov. | G. interpositum Reichardt 1999 | G. brebissonii Kützing 1849 | G. montanum sensu Reichardt 1999 | G. spec. cf. montanum sensu Lange-Bertalot & Genkal 1999 | G. montanum (Schumann) Grunow sensu Cantonati et al. 2017 | G. montanum sensu Kulikovskiy et al. 2010 | G. montanum sensu Rumrich et al. 2000 | G. interpositum Reichardt sensu Potapova 2014 | |
|---|---|---|---|---|---|---|---|---|---|
| Outline | heteropolar, narrowly clavate | heteropolar, narrowly clavate | heteropolar, narrowly clavate to linear with slightly undulate margins | heteropolar, narrowly clavate to linear with slightly undulate margins | heteropolar, narrowly clavate | weakly club-shaped with triundulate margins | heteropolar, narrowly clavate * | heteropolar, narrowly clavate * | heteropolar, narrowly clavate * |
| Headpole shape | rhomboid and obtusely rounded | bluntly wedge-shaped with a broadly truncated to rounded end | narrowly rhomboid and obtusely rounded | wedge-shaped with a bluntly rounded apex * | narrowly rhomboid and obtusely rounded | rather broad, almost flat rounded | rhomboid and obtusely rounded * | obtusely rounded * | obtusely rounded * |
| Axial area | narrowly lanceolate | moderately narrow | narrowly lanceolate | narrowly lanceolate | narrowly lanceolate | usually moderately narrow | narrowly lanceolate * | narrowly lanceolate * | narrowly lanceolate * |
| Central area | irregular, bordered by 1–2 shortened stria on each side | irregular | irregular, bordered by 1 shortened stria on each side | irregular, bordered by 1 shortened stria on each side * | irregular, bordered by 1 shortened stria on each side * | almost roundish to rhombic, rather small | irregular, bordered by 1–2 shortened striae on each side * | irregular, bordered by 1 shortened stria on each side * | irregular, bordered by 1 shortened stria on each side * |
| Valve length (μm) | 31.9–59.2 | 32–55 | 31–61 | 46 * | 50–52 | 30–73 | 39–55 * | 52–68 * | 46 * |
| Valve width at the central part (μm) | 7.2–9.0 | 7.3–8.5 | 7.5–9.5 | 8.5 * | 7.0–7.5 | 8.5–11.0 | 8.0–8.5 * | 8–9 * | 8 * |
| Valve width at the headpole (μm) | 7.6–9.3 | 8–10 * | 7–9 | 8 | 7.5–8.0 | 8.5 | 8–10 * | n.d. * | 8 * |
| Striae in 10 μm | 10–12 | 10–11 | 9–11 | 12 | 10–12 | 11 | 11–12 * | 9–10 * | 12 * |
| Areolae in 10 μm | 30–35 | n.d. | 22–25 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Isolated pores | 1, rarely 2 | 1 | 1 | 1 | 1 | 1 | 1 * | 1 * | 1 * |
| Distribution | Kamchatka | Europe, Austria | Holarctic | Europe, Austria | Finland and Siberia | Central Europe | Mongolia | Chile | Kamchatka |
| References | This study | [41] | [39,45] | [41] | [44] | [40] | [43] | [42] | [22] |
* Counted by us from published data.
4. Discussion
4.1. Distribution of Common Taxa
In the studied samples the most widespread species were representatives of the genus Encyonema. Encyonema silesiacum (Bleisch) D.G. Mann reached mass or high development practically in all collection points. The abundance of this species in the samples ranges from 7.1 to 45% (Table S1); it is relatively lower at collection point O-5 (Ozernaya River near the confluence of Homut Stream), at only 4.1%. This species was not found in the Nezhnyj Stream. Encyonema minutum (Hilse) D.G. Mann in Round et al. and Encyonema ventricosum (C. Agardh) Grunow are distributed with lower abundance values (from 1.5 to 14.5% and from 0.4 to 9.2%, respectively) (Table S1).
Among the common taxa reaching high abundance values, species of the genus Hannaea characteristic for the Arctic region should be noted. Species of this genus were found in all samples with abundances ranging from 0.2% (singular finds) to 14.8% (Hannaea arcus (Ehrenberg) R.M. Patrick at point O-3 (Perevalnaiya River, background point) and up to 22% (Hannaea inaequidentata (Lagerstedt) Genkal & Kharitonov at point O-11 Perevalnaiya River) (Table S1).
Among the widespread taxa we should mention cosmopolitan species: Achnanthidium minutissimum (Kützing) Czarnecki (found mostly sporadically, maximum abundance of 5.5% was recorded at point O-9 Nezhnyj Stream); Cocconeis euglypta Ehrenberg—found sporadically in all samples (abundance of 0.2–2.4%), except for point O-4 (Homut Stream); Diatoma cf. rostrata (Levkov & Jüttner) Glushchenko & Kulikoviskiy—found in all samples, no more than 5–7 valves recorded in most slides, most frequently found in samples O-16 (Ozernaya River, 8.3%) and O-5 (Ozernaya River near the confluence of Homut Stream, 14.5%) (Table S1); Eucocconeis laevis (Østrup) Lange-Bertalot—found sporadically in 10 samples; Fragilaria vaucheriae (Kützing) Petersen—found in all samples, maximum abundance observed at points O-12 (Stream PC 547-20, 19%), O-13 (Levaya Ozernaya River, 19%), O-15 (Pravaya Ozernaya River, 13.6%); Gomphoneis olivaceoides Hustedt—was sporadically found in all samples except for points O-4 (Homut Stream) and O-9 (Nezhnyj Stream); Meridion circulare (Greville) C. Agardh—maximum abundance was recorded in Nezhnyj Stream (16.3%), in other samples it occurred sporadically (Table S1); Nitzschia acidoclinata Lange-Bertalot—occurred sporadically in all samples, abundance of 5.1% was recorded only at point O-16 (Ozernaya River), 3.3% at point O-9 (Nezhnyj Stream); Nitzschia palea (Kützing) W. Smith—occurred sporadically in all samples; Nitzschia tubicola Grunow—abundance of 8.5–9.7% was recorded in Levaya Ozernaya River (points O-13, O14), 14–14.6% in Pravaya Ozernaya River (O-15) and Ozernaya River (O-16); Odontidium anceps (Ehrenberg) Ralfs—high abundance (25%) of this species was recorded in Nezhnyj Stream, at other points the species was rare. Planothidium cf. haynaldii (Schaarschmidt) Lange-Bertalot and Planothidium lanceolatum (Brébisson ex Kützing) Lange-Bertalot were recorded sporadically in all samples, but according to [22] in the rivers on Bering Island these species were dominant. Ulnaria ulna (Nitzsch) Compère occurs sporadically in most samples, with an abundance of 3.4–4.5% only in the Perevalnaiya River.
The composition of diatoms at stations O-16 and O-L (Ozernaya River) is characterised by significant development of Ulnaria goulardii (Brébisson ex Cleve & Grunow) D.M. Williams, Potapova & C.E. Wetzel (abundance 15.5 and 6.6%, respectively). This species was found only in these samples.
4.2. Comparative Analysis of Species Diversity
An important indicator of the state of aquatic ecosystems is the complexity of the structure of hydrobiont communities: the more complex the structure, the more stable the community ([46], p. 231). To assess the complexity of the algocenosis structure, we used the Shannon diversity index. Based on the representation of species in the community, it varies from 0 (the community consists of a single species) to 5 (characterises high species diversity). In the studied watercourses, Shannon index values ranged from 2.13 bits/ex (station O-3, background point on the Perevalnaiya River) to 2.85 bits/ex (station O-15, control station on the Pravaya Ozernaya River), indicating medium complexity of the community structure (Figure 26).

Figure 26.
The value of species diversity indices at stations of the studied watercourses.
The maximum index value (3.8 bits/ex) at the station located at the mouth of Homut Stream (O-4, “discharge from MMC”) should be discussed separately. As written above, physico-chemical properties of water at this station differ significantly from the values at background and control stations (turbidity and specific conductivity were several times higher) (Table 1). Diatom slides were poor, despite a relatively high species diversity (49 taxa), and diatom valves were sporadic (50–55 valves per slide). When viewing fixed samples, without peroxide treatment, we did not find valves with chloroplasts, nor did we find other groups of microalgae or cyanobacteria. All this may indicate the oppression and degradation of the microalgae community under the influence of the MMC. The found valves apparently represent the community that existed prior to the commissioning of the plant. As for the high value of the Shannon index at this point, in this case it apparently does not correctly reflect information on the organisation of the ecosystem. As is well known, the Simpson, Pielou, and Shannon indices, based on the dispersion or entropy of taxa abundance, assess only the level of evenness of the distribution of relative fractions, do not depend on absolute values of abundance N, and have little sensitivity to changes in species richness ([47], p. 106). “Indeed, Shannon, Simpson and other indices deliver maximum biodiversity in the case of equal abundance of all species” ([47], p. 110). In the case of station O-4, the Shannon index reflects high alignment and diversity of the diatom community (almost every species is found in the sample once). At the same time, the Pielou alignment index takes the maximum value (0.99), and the Simpson index is 0, which indicates the complete absence of dominant taxa. Thus, in our opinion, at point O-4 the calculated Pielou and Shannon indices based on diatoms do not reflect the actual state of algocenosis. Based on the small number of valves in the slides and the absence of living cells in the samples, the state of the latter can be characterised as severe degradation.
Simpson’s index indicates the dominance of certain species in a community. An increase in Simpson’s index means a decrease in diversity and an increase in the degree of dominance of one species. In the studied watercourses, Simpson’s index values ranged from 0 (station O-4, Homut Stream (“OMMC discharge”)) to 0.24 (station O-L, Ozernaya River (“control”)). In general, the calculated values of the Simpson index are low, indicating an even distribution of species without predominance of one of them. The index values at stations O-3 (Perevalnaiya River, “background”) and O-L (Ozernaya River, “control”) are relatively higher than the others, which corresponds to the high abundance of Encyonema silesiacum (Bleisch) D.G. Mann in Round et al. in these samples (41 and 45%, respectively). At station O-5 (Levaya Ozernaya River, “control”) the index value of 0.18 reflects high development of Staurosirella sp. 1 (37% abundance); similar index values at stations O-11 (Perevalnaiya River, “control”) and O-14 (Levaya Ozernaya River, “control”) of 0.16 and 0.15, respectively, reflect relatively high abundance of Encyonema silesiacum (31.2 and 33.9%, respectively). The maximum values of taxa abundance at other stations do not exceed 25.3% (Table S1).
Pielou’s evenness index (E) reflects the evenness of taxa abundance in the biocenosis and varies from 0 to 1, with E = 1 when all taxa are equally abundant ([47], p. 100). In the studied watercourses, the values of the Pielou index are rather high, ranging from 0.59 at station O-L (Ozernaya River, “control”) to 0.78 at station O-12 (Stream PC 547-20, “background”). The maximum index value at station O-4 (Homut Stream, “discharge”) is discussed above. In general, the dynamics of Pielou index values correspond to the dynamics of Simpson dominance index values: minimum values of the latter correspond to maximum values of the former (Figure 26).
Comparisons of species similarity between stations were made using the Sørensen–Chekanovsky index and cluster analysis with the unweighted pair-group method using arithmetic averages (UPGMA) (Table 3, Figure 27). As can be seen from Table 3, the watercourses flowing through the territory of the OMMC—Nezhnyj Stream (O-9) and Homut Stream (O-4)—are the most peculiar. The coefficient values for pairwise comparison of species compositions at all stations vary between 0.29 and 0.44, and cluster analysis shows significant differences in species composition between stations (Sørensen–Chekanovsky index value 0.44) and from the other studied watercourses, forming a separate cluster (Figure 27).

Table 3.
Values of the Sørensen–Czekanovsky similarity coefficient. Maximum coefficient values are highlighted in bold.

Figure 27.
Dendrogram of the similarity of the species composition of periphyton diatoms at the sampling stations of the studied watercourses. The ordinate axis is the merging distance (1–Ks), the abscissa axis is the sampling station.
The vast majority of values of the pairwise comparison coefficient for stations of other watercourses are above 0.5, indicating a high degree of commonality of species composition.
Based on the cluster analysis using the Sørensen index (Ks), we can see that the Perevalnaiya River, Levaya Ozernaya River, Pravaya Ozernaya River, Ozernaya River, and Stream PC 547-20 form a single cluster, which is divided into two clades (Figure 27). We conducted cluster analysis based on the species composition of individual stations (not combined species lists by watercourse, as initially the number of sampling stations in watercourses differed significantly (e.g., Perevalnaya River and Levaya Ozernaya River have three stations, Pravaya Ozernaya River and Stream PC 547-20 both have one station, the different sample sizes are not comparable)). Nevertheless, we expected to obtain several clusters corresponding to watercourses. However, the results were somewhat different: maximum similarity of diatom species composition was observed for stations O-3 (Perevalnaiya River, “background”) and O-15 (Pravaya Ozernaya River), and the branch is complemented by stations O-14 (Levaya Ozernaya River) and O-16 (Ozernaya River). A branch with high similarity of species composition is formed by closely located stations O-1 (Limnokren, a tributary of the Levaya Ozernaya River) and O-5 (Levaya Ozernaya River, downstream of the Limnokren confluence) (Figure 27). The species composition of the above stations forms a separate clade (Figure 27). High similarity of species composition was noted between stations O-10 and O-11 on the Perevalnaiya River and between stations O-13 (Levaya Ozernaya River), O-L (Ozernaya River), and O-12 (Stream PC 547-20). These two branches form another clade.
Thus, the analysis of similarity degree comparison of diatom species diversity at the sampling stations of the studied watercourses using the Sørensen–Chekanovsky index and cluster analysis on its basis revealed significant differences in the composition of species in Homut Stream (O-4) and Nezhnyj Stream (O-9), which flow through the territory of the OMMC and differ from other watercourses in terms of physical and chemical parameters of water: lower pH value (6.4 vs. 6.8–8.82), higher values of specific conductivity (350 μS/cm at station O-4, 130 μS/cm at station O-9 vs. 50–90 μS/cm at other stations). In rivers at different sampling stations differences in species composition and indices of quantitative development of taxa were noted. At the same time, the results of pairwise comparison of species composition indicate high commonality of species composition (the vast majority of Sørensen–Chekanovsky index values are above 0.5).
4.3. Analysis of Phytoplankton Taxonomic Composition and Quantitative Indicators
As a result of phytoplankton sampling, a total of 39 taxa of microalgae and two species of cyanobacteria (Leptolyngbia sp., Oscillatoria sp.) were identified. The composition is dominated by diatoms of the class Bacillariophyceae; three species from the class Coscinodisciscophyceae were found, as well as one representative of green filamentous algae from the classes Zygnematophyceae (Spirogyra sp.) and Ulvophyceae (Ulotrix sp.). Phytoplankton composition is relatively poor, with 6 to 16 species found in samples (Figure 28, Table S2).

Figure 28.
Taxonomic composition of phytoplankton at sampling stations of the studied watercourses.
The largest number of taxa was recorded at collection points O-12 (Stream PC 547-20, 16 species) and O-1 (Limnokren, Ozernaya River, 15 species); the smallest number at points O-11 (Perevalnaiya River) and O-L (Ozernaya River), only six species each. At each of the other stations, 9–10 taxa were found. Figure 28 shows that at all stations the species composition is dominated by diatoms of the class Bacillariophyceae, other classes are represented by no more than three species. Representatives of cyanobacteria were found at points O-12 (Oscillatoria sp.) and O-16 (Leptolyngbya sp.). Fragments of Ulotrix sp. (class Ulvophyceae) were found in phytoplankton of three watercourses at stations O-3 (Perevalnaiya River), O-12 (Stream PK 547-20), O-14 (Levaya Ozernaya River), and O-16 (Ozernaya River).
The list of common phytoplancton taxa includes six diatom species belonging to the class Bacillariophyceae (five taxa) and Coscinodiscophyceae (Melosira varians). All of them (with the exception of Melosira varians) were recorded as common in periphyton samples. Diatoma cf. rostrata occurred sporadically in most periphyton samples, and low abundance values were also recorded in phytoplankton—from 312.5 cells/L (point O-10, Perevalnaiya River) to 6500 cells/L (point O-13, Levaya Ozernaya River). Encyonema silesiacum belongs to benthic species, is a mass background species of periphyton, and was found in phytoplankton in eight samples with low abundance values (from 250 to 2000 cells/L). Hannaea inaequidentata was highly abundant in periphyton in Perevalnaiya River (relative abundance of the species in samples from 6.9% to 22.5%), while in other watercourses the species was found sporadically. In phytoplankton, relatively high abundance values of this species (from 53,500 to 124,000 cells/L) were recorded in Perevalnaiya and Pravaya Ozernaya rivers. Low abundance of this species was recorded in the plankton of Levaya Ozernaya River (point O-14) and Ozernaya (station O-16) (3500–7000 cells/L). Hannaea mongolica was recorded in phytoplankton of all watercourses at six stations with low abundance values (from 250 to 6500 cells/L). Interestingly, this is the first finding of the species after its description from Lake Khövsgöl (Mongolia). Evidently this species has a wider distribution. Ulnaria ulna is a widespread planktonic species; in periphyton it was recorded in almost all samples with low abundance values (from 0.2 to 4.5% (Perevalnaiya River, Stream PC 547-20)). The maximum abundance of this species in phytoplankton was recorded in the Perevalnaiya River (from 3438 to 10,500 cells/L), while in other watercourses the abundance did not exceed 2000 cells/L. A representative of centric diatoms, Melosira varians was found in phytoplankton of all watercourses except for Ozernaya River with low abundance values (from 500 to 23,750 cells/L).
Figure 29 presents a graphical representation of the values of phytoplankton abundance and biomass in the studied watercourses. It should be noted that the total values of phytoplankton abundance per sample are relatively small. The minimum values were recorded at points O-L (Ozernaya River) and O-12 (Stream PK 547-20) (3750 and 15,175 cells/L, respectively). Maximum abundance was observed at point O-1 (Limnokren) due to high development of small-celled representatives of the genus Staurosirella (1,655,750 cells/L). For comparison, for example, in the Tolmachevskoye Reservoir, phytoplankton abundance since 2004 has fluctuated around 2 million cells/L [8].
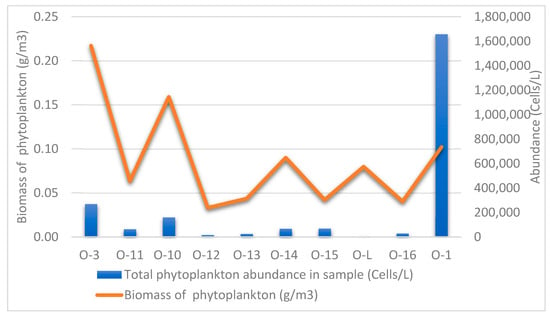
Figure 29.
Abundance and biomass of phytoplankton at sampling stations in the studied rivers.
The biomass of microalgae and cyanobacteria at almost all collection points did not exceed 0.1 g/m3; only at points O-3 and O-10 (Perevalnaiya River) were values of 0.22 g/m3 and 0.16 g/m3, respectively, recorded (Figure 29, Table S2) due to high abundance of large-celled species Ulnaria ulna and Melosira varians (the share of these species in the biomass is 77% (O-3) and 55% (O-10)). Thus, the trophic status of the watercourses determined by phytoplankton biomass corresponds to oligotrophic at all collection points.
5. Conclusions
This study examined microalgae species diversity in periphyton and phytoplankton samples collected from eight watercourses in the north-east of the Kamchatka Peninsula in the area of the Ozernovsky Mining and Metallurgical Complex. As a result, no microalgae were found in two streams flowing through the territory of the Ozernovsky Mining and Metallurgical Complex (Etalonnyj-1 and Etalonnyj-2), which apparently indicates complete degradation of the community. Analysis of physico-chemical parameters of water in these streams revealed increased values of specific conductivity (550 and 220 μS/cm, respectively) and low pH values corresponding to acidic reaction of water (pH 5.67). In a sample taken at the mouth of the Homut Stream, which also flows through the Ozernovsky MMC, microalgae cells were sporadic and the sample was poor. Physico-chemical parameters indicate increased optical turbidity of water (39.6 NTU), high values of specific conductivity (350 µS/cm), and weakly acidic reaction of water (pH 6.6). The microalgae community of the Homut Stream also appears to be degrading.
All streams flow into the Ozernaya River, which is a feeding and spawning watercourse for a number of salmonid fish species. The absence of microalgae in the streams and high physico-chemical parameters of water indicate a significant influence of the OMMC on microflora.
The remaining samples taken from rivers at background and control stations revealed rich microalgal species composition, with 37 to 58 taxa detected in the samples. Background taxa that occurred in almost all samples with different frequency of occurrence were identified: Encyonema minutum (Hilse) D.G. Mann in Round et al. (1.5–15.4%), Encyonema silesiacum (Bleisch) D.G. Mann (4.1–45%), Encyonema ventricosum (C. Agardh) Grunow (0.4–9.2%), Fragilaria vaucheriae (Kützing) Petersen (0.4–27%), Hannaea arcus (Ehrenberg) R.M. Patrick (0.2–14.8%), Hannaea inaequidentata (Lagerstedt) Genkal & Kharitonov (0.2–22.2%), Nitzschia acidoclinata Lange-Bertalot (0.2–3.3%), Nitzschia tubicola Grunow (0.2–14.6%), Ulnaria ulna (Nitzsch) Compère (0.2–4.5%).
The Nezhnyj Stream, in the catchment area in which the tailings dump site of semi-dry cyanidation waste storage is located, differs significantly in composition and species development from the algoflora of the studied rivers. It lacks the background species of these watercourses from the genera Encyonema and Hannaea. At the same time, species that occurred only sporadically in the rivers reach high abundance values in the stream, e.g., Odontidium anceps (Ehrenberg) Ralfs (25%), Meridion circulare (Greville) C. Agardh (16.3%). An acidophilic species Eunotia minor (Kützing) Grunow was found only in this stream with an abundance of 3.3%. In general, the microalgae community of the Nezhnyj Stream is diverse, differences in composition are related to local physical and chemical conditions, and no degradation of the community was observed.
The trophic status of the rivers, as determined by phytoplankton biomass, corresponds to oligotrophic at all collection points.
This work, including exhaustive illustrative material, will provide an opportunity to track changes in planktonic and periphyton microalgal communities in watercourses with intensive anthropogenic load due to the proximity of the Ozernovsky MMC.
The studied watercourses are located in inaccessible sites far from populated areas, which limits the possibility of regular (year-round) monitoring. Nevertheless, in the future it is necessary to monitor the ecological state in order to assess the impact of the OMMC on the biota of watercourses and to take timely measures to protect the unique spawning rivers of Kamchatka.
Our study identified some taxa that are shown for Kamchatka for the first time. The species Hannaea mongolica was originally described from Terkhiin Tsagaan Lake, Mongolia [48]. We found it in almost all studied watercourses (Perevalnaiya, Levaya Ozernaya, Ozernaya Rivers, Stream PC 547-20) with an abundance of 0.2–9.5% (Table S1). Thus, we extend the distribution of this species. Another interesting species shown by us is Fragilarioforma horstii. This species was originally described from Alaska [49], later shown for western Montana, and is considered a North American endemic [50]. We found this species in the Levaya Ozernaya River (station O-14). The finding of this species is the first for Russia. In all probability, the species has a wider distribution.
The newly described species G. anissimovae sp. nov. indicates a great taxonomic potential within the G. brebissonii species group. Further comprehensive studies of the diatom flora of this interesting and unique region are needed.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d16090592/s1, Table S1. List of detected taxa and their frequency of species occurrence in samples (%), Table S2. List of phytoplankton taxa and their abundance in the studied watercourses of the Kamchatka Peninsula.
Author Contributions
Conceptualisation, E.M.K.; investigation, methodology, E.M.K. and A.M.G.; sampling, E.M.K.; morphology investigation, E.M.K., A.M.G. and M.S.K.; funding acquisition, M.S.K.; writing—original draft preparation, E.M.K., A.M.G. and M.S.K.; and writing—review and editing, A.M.G. and M.S.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work is based on research carried out with financial support by the Russian Science Foundation (24-14-00165, https://rscf.ru/project/24-14-00165/ accessed on 8 August 2024) for LM and SEM and by the framework of state assignment of the Ministry of Science and Higher Education of the Russian Federation (theme 122042700045-3) for finishing the manuscript.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data is contained within the article or supplementary material.
Acknowledgments
The authors express their gratitude to senior researcher R.A. Rakitov (instrument analytics room of the Borissiak Paleontological Institute of the Russian Academy of Science, PIN RAS) for assistance in working with a scanning electron microscope. The authors express their gratitude to D.A. Chudaev for assistance in identifying some species of Navicula. The authors express their gratitude to the staff of the Kamchatka branch of the Pacific Institute of Geography, Far Eastern Branch of the Russian Academy of Sciences, who took part in the expedition.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Vvedenskaya, T.L. Fishery importance of watercourses of Petropavlovsk-Kamchatsky. Res. Aquat. Biol. Resour. Kamchatka North-West Part Pac. Ocean 2011, 23, 88–101. [Google Scholar]
- Zaporozhets, G.V.; Zaporozhets, O.M. Kamchatka fish hatcheries: Some consequences of Pacific salmon reproduction. In Proceedings of the Preservation of Biodiversity of Kamchatka and Adjacent Seas, XI International Scientific Conference Dedicated to the 100th Anniversary of the Birth of Outstanding Russian Ichthyologists A.P. Andryashev and A.Ya. Taranets, Petropavlovsk-Kamchatsky, Russian, 24–25 November 2010; pp. 186–189. [Google Scholar]
- Vvedenskaya, T.L.; Ulatov, A.V. Results of monitoring of small rives withing the basin of Avacha River, situated in the area of anthropogenic influence. Res. Aquat. Biol. Resour. Kamchatka North-West Part Pac. Ocean 2012, 26-1, 124–136. [Google Scholar]
- Lepskaya, E.V. Phytoplankton in the Ecosystem of Lake Kurilskoye. Ph.D. Thesis, Institute of Marine Biology, Far Eastern Branch of the RAS, Vladivostok, Russia, 2004. [Google Scholar]
- Lepskaya, E.V.; Bonk, T.V. Specifics of pelagic feeding by Daphnia longiremis Sars in the Kurile Lake in terms of structural transformation of phytoplankton. Res. Aquat. Biol. Resour. Kamchatka North-West Part Pac. Ocean 2021, 63, 50–58. [Google Scholar] [CrossRef]
- Sorokin, Y.I.; Paveljeva, E.B. On the quantitative characteristics of the pelagic ecosystem of Dalnee Lake (Kamchatka). Hydrobiologia 1972, 40, 519–552. [Google Scholar] [CrossRef]
- Genkal, S.I.; Lepskaya, E.V. Materials to the Flora of Bacillariophyta of Lake Kronotskoye (the Kamchatka Peninsula, Russia). Int. J. Algae 2015, 17, 14–22. [Google Scholar] [CrossRef]
- Lepskaya, E.V.; Koval, M.V.; Bazarkina, L.A.; Bonk, T.V.; Bochkova, E.V.; Bugaev, V.F.; Vinogradova, D.S.; Losenkova, K.V.; Gavruseva, T.V.; Sviridenko, V.D.; et al. Formation and modern state of ecosystem in Tolmachevskoye reservoir (Kamchatka) and the acclimatized there population of kokanee (Oncorhynchus nerka kennerlyi). Izv. TINRO 2014, 178, 95–115. [Google Scholar] [CrossRef]
- Lepskaya, E.V.; Bonk, T.V.; Bekker, E.I. Freshwater microalgae and invertebrates in the basin of Talovskoye Lake (Koryak Reserve, Kamchatka). Res. Aquat. Biol. Resour. Kamchatka North-West Part Pac. Ocean 2019, 52, 108–119. [Google Scholar] [CrossRef]
- Genkal, S.I.; Lepskaya, E.V. Stephanodiscus popovskayae, a new species from the volcanic lakes of Kamchatka in East Asia, Russia. Diatom Res. 2013, 28, 365–372. [Google Scholar] [CrossRef]
- Genkal, S.I.; Lepskaya, E.V. Centric diatom algae of volcanic Verkhneavachinsk lakes (Kamchatka). Inland Water Biol. 2014, 7, 1–9. [Google Scholar] [CrossRef]
- Genkal, S.I.; Lepskaya, E.V. Diatom flora of salmon lakes of the Koryak Highlands, Kamchatka. Res. Aquat. Biol. Resour. Kamchatka North-West Part Pac. Ocean 2014, 35, 31–47. [Google Scholar] [CrossRef]
- Lepskaya, E.V.; Jewson, D.H.; Usoltseva, M.V. Aulacoseira subarctica in Kurilskoye Lake, Kamchatka: A deep, oligotrophic lake and important Pacific salmon nursery. Diatom Res. 2010, 25, 323–335. [Google Scholar] [CrossRef]
- Yoshitake, S.; Fukushima, H.; Kimura, T.; Lepskaya, E.V.; Ko-Bayashi, T. Variability of the pennatae diatom Gomphonema ventricosum Gregory from far eastern lakes. Acta Bot. Croat. 2009, 68, 421–430. [Google Scholar]
- Genkal, S.I.; Lupikina, E.G.; Lepskaya, E. V Cyclotella tripartita Håkansson from the lakes in Kamchatka, Russia. Bot. Zhurnal 2004, 89, 426–435. (In Russian) [Google Scholar]
- Solovieva, N.; Klimaschewski, A.; Self, A.E.; Jones, V.J.; Andrén, E.; Andreev, A.A.; Hammarlund, D.; Lepskaya, E.V.; Nazarova, L. The Holocene environmental history of a small coastal lake on the north-eastern Kamchatka Peninsula. Glob. Planet. Change 2015, 134, 55–66. [Google Scholar] [CrossRef]
- Kuzyakina, T.I. Transformation of volcanic ash by microorganisms. In Volcanism and Associated Processes; Petropavlovsk-Kamchatski: Dalnauka, Russia, 1985; pp. 232–234. [Google Scholar]
- Fazlutdinova, A.I.; Allaguvatova, R.Z.; Gaysina, L.A. Ecotonic Communities of Diatoms in the Southeastern Part of the Kamchatka Peninsula. Earth 2023, 4, 209–222. [Google Scholar] [CrossRef]
- Allaguvatova, R.Z.; Nikulin, A.Y.; Nikulin, V.Y.; Bagmet, V.B.; Gaysina, L.A. Study of Biodiversity of Algae and Cyanobacteria of Mutnovsky and Gorely Volcanoes Soils (Kamchatka Peninsula) Using a Polyphasic Approach. Diversity 2022, 14, 375. [Google Scholar] [CrossRef]
- Abdullin, S.R.; Bagmet, V.B.; Nikulin, A.Y.; Nikulin, V.Y.; Gorpenchenko, T.Y.; Grishin, S.Y.; Allaguvatova, R.Z.; Gontcharov, A.A. Emended description of the genus Eremochloris (Trebouxiophyceae, Chlorophyta), with Eremochloris kamchatica sp. nov. from Kamchatka, Russia. Phycologia 2022, 61, 175–183. [Google Scholar] [CrossRef]
- Abdullin, S. Cyanobacteriae and algae of lava tubes in Kamchatka, Russia. Cave Karst Sci. 2013, 40, 141–144. [Google Scholar]
- Potapova, M. Diatoms of Bering Island, Kamchatka, Russia. Nova Hedwigia 2014, 143, 63–102. [Google Scholar]
- Bishop, I.W.; Esposito, R.M.; Tyree, M.; Spaulding, S.A. A diatom voucher flora from selected southeast rivers (USA). Phytotaxa 2017, 332, 101–140. [Google Scholar] [CrossRef]
- Alers-García, J.; Lee, S.S.; Spaulding, S.A. Resources and practices to improve diatom data quality. Limnol. Oceanogr. Bull. 2021, 30, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Noble, P.J.; Seitz, C.; Lee, S.S.; Manoylov, K.M.; Chandra, S. Characterization of algal community composition and structure from the nearshore environment, Lake Tahoe (United States). Front. Ecol. Evol. 2023, 10, 1053499. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, V.A.; Lee, S.S.; Rober, A.R.; Furey, P.C.; Manoylov, K.M.; Wyatt, K.H. A Voucher Flora of Diatoms from Fens in the Tanana River Floodplain, Alaska. Water 2023, 15, 2803. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.M.; Manoylov, K.M. Living Algal Community Voucher and Taxonomy in Dickinson County Iowa: A Case for Collection Based Research. Diversity 2024, 16, 21. [Google Scholar] [CrossRef]
- Potapova, M.G.; Lee, S.S.; Spaulding, S.A.; Schulte, N.O. A harmonized dataset of sediment diatoms from hundreds of lakes in the northeastern United States. Sci. Data 2022, 9, 540. [Google Scholar] [CrossRef]
- Genkal, S.I.; Lepskaya, E.V. Diatoms (Bacillariophyta) of the Tolmachev reservoir (Kamchatka, Russia). Bot. Pacifica 2023, 12, 133–139. [Google Scholar] [CrossRef]
- EIA Report of FSUE KamchatNIRO. Identification and Assessment of Impact Factors, Calculation of Possible Unavoidable Damage to Aquatic Bioresources, Development of Recommendations on Reduction of Impact on Aquatic Biocenoses and Implementation of Compensatory Measures during Construction and Operation of Experimental Industrial Production for Mining and Processing of Gold-Bearing Ores of BAM and Khomut Sections of Ozernovsky Deposit of Kamchatka Krai; EIA Report of FSUE KamchatNIRO: Petropavlovsk-Kamchatsky, Russia, 2013; 57p. (In Russian) [Google Scholar]
- Guiry, M.D.; Guiry, G.M. AlgaeBase; World-Wide Electronic Publication; National University of Ireland: Galway, Ireland, 2021; Available online: http://www.algaebase.org (accessed on 8 August 2024).
- Magurran, E. Ecological Diversity and Its Measurement; Princeton University Press: London/Croom Helm, UK, 1988. [Google Scholar]
- Shitikov, V.K.; Rosenberg, G.S.; Zinchenko, T.D. Quantitative Hydroecology: Methods of System Identification; IEVB RAS: Tolyatti, Russia, 2003; 463p. (In Russian) [Google Scholar]
- Yakimov, B.N.; Shurganova, G.V.; Cherepennikov, V.V.; Kudrin, I.A.; Il’in, M.Y. Methods for comparative assessment of the results of cluster analysis of hydrobiocenoses structure (by the example of zooplankton communities of the Linda River, Nizhny Novgorod region). Inland Water Biol. 2016, 9, 200–208. [Google Scholar] [CrossRef]
- Abakumov, V.A.; Bubnova, N.P.; Kholikova, N.I.; Goridchenko, T.P.; Liepa, R.A.; Svirskaya, N.L.; Gan’shina, L.A.; Semin, V.A.; Hromov, V.M.; Nikitin, D.I.; et al. Guide to Methods of Hydrobiological Analysis of Surface Waters and Bottom Sediments; Gidrometeoizdat: Leningrad, Russia, 1983; 239p. (In Russian) [Google Scholar]
- Trifonova, I.S. Ecology and Succession of Lake Phytoplankton; Nikolaev, I.N., Ed.; Nauka: Leningrad, Russia, 1990; 184p. (In Russian) [Google Scholar]
- Chudaev, D.A.; Kociolek, J.P.; Gololobova, M.A. Gomphonema megalobrebissonii sp. nov.: A new large-celled taxon in species complex around G. acuminatum from the sediments of Lake Glubokoe (European Russia). Beih. Zur Nova Hedwig. 2014, 143, 255–269. [Google Scholar] [CrossRef]
- Kulikovskiy, M.S.; Kociolek, J.P.; Solak, C.N.; Kuznetsova, I. The diatom genus Gomphonema Ehrenberg in Lake Baikal. II. Revision of taxa from Gomphonema acuminatum and Gomphonema truncatum-capitatum complexes. Phytotaxa 2015, 233, 251–272. [Google Scholar] [CrossRef]
- Mitić-Kopanja, D.; Wetzel, C.E.; Ector, E.; Levkov, Z. Two new Gomphonema Ehrenberg (Bacillariophyceae) species from Macedonia and comparison with type material of G. brebissonii Kützing. Fottea 2014, 14, 149–160. [Google Scholar] [CrossRef]
- Cantonati, M.; Kelly, M.G.; Lange-Bertalot, H. Freshwater Benthic Diatoms of Central Europe: Over 800 Common Species Used in Ecological Assessment; Koeltz Botanical Books: Schmitten-Oberreifenberg, Germany, 2017; 942p. [Google Scholar]
- Reichardt, E. Zur Revision der Gattung Gomphonema. Die Arten um G. affine/insigne, G. angustatum/micropus, G. acuminatum sowie gomphonemoide Diatomeen aus dem Oberoligozän in Böhmen. Iconogr. Diatomol. 1999, 8, 1–203. [Google Scholar]
- Rumrich, U.; Lange-Bertalot, H.; Rumrich, M. Diatoms of the Andes. From Venezuela to Patagonia/Tierra del Fuego and two additional contributions. Iconogr. Diatomol. 2000, 9, 1–673. [Google Scholar]
- Kulikovskiy, M.S.; Lange-Bertalot, H.; Witkowski, A.; Dorofeyuk, N.I.; Genkal, S.I. Diatom assemblages from Sphagnum bogs of the world. I. Nur bog in northern Mongolia. Bibl. Diatomol. 2010, 55, 1–326. [Google Scholar]
- Lange-Bertalot, H.; Genkal, S.I. Diatoms from Siberia, I. Islands in the Arctic Ocean (Yugorsky-Shar Strait). Iconogr. Diatomol. 1999, 6, 1–292. [Google Scholar]
- Levkov, Z.; Mitić-Kopanja, D.; Reichardt, E. The diatom genus Gomphonema from the Republic of Macedonia. Diatoms Eur. 2016, 8, 1–552. [Google Scholar]
- Barinova, S.S.; Bilous, O.P.; Tsarenko, P.M. Algal Indication of Water Bodies in Ukraine: Methods and Perspectives; Haifa, Kiev, University of Haifa Publisher: Haifa, Israel, 2019; 367p. [Google Scholar]
- Shitikov, V.K.; Rosenberg, G.S. Biodiversity assessment: An attempt at a formal treatment. In Quantitative Methods of Ecology and Hydrobiology (Collection of Scientific Papers Dedicated to the Memory of A.I. Bakanov); Rosenberg, G.S., Ed.; SamNC RAS: Tolyatti, Russia, 2005; pp. 91–129. (In Russian) [Google Scholar]
- Liu, Q.; Glushchenko, A.; Kulikovskiy, M.; Maltsev, Y.; Kociolek, J.P. New Hannaea Patrick (Fragilariaceae, Bacillariophyta) species from Asia, with comments on the biogeograhy of the genus. Cryptogam. Algol. 2019, 40, 41–61. [Google Scholar] [CrossRef]
- Morales, E.A.; Manoylov, K.M.; Bahls, L.L. Fragilariforma horstii sp. nov. (Bacillariophyceae) a new araphid species from the northern United States of America. Nova Hedwig. Beih. 2012, 141, 141–154. [Google Scholar]
- Kociolek, J.P.; Theriot, E.C.; Williams, D.M.; Julius, M.; Stoermer, E.F.; Kingston, J.C. Centric and Araphid Diatoms. In Freshwater Algae of North America; Wehr, J.D., Sheath, R.G., Kociolek, J.P., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 653–708. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).