The Effects of Light Environment on Adult Odonate Communities in Disturbed and Intact Forest: The Importance of Small-Scale Effects
Abstract
1. Introduction
2. Methods
3. Results
3.1. Overview of Odonate Communities
3.2. Light Environments
3.3. Analyses of Odonate Communities on Streams
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carvalho, F.G.; Pint, N.S.; Oliveira-Júnior, J.M.B.; Juen, L. Effects of marginal vegetation removal on Odonata communities. Acta Limnol. Bras. 2013, 25, 1018. [Google Scholar] [CrossRef]
- Monteiro-Júnior, C.S.; Couceiro, S.R.M.; Hamada, N.; Juen, L. Effect of vegetation removal for road building on richness and composition of Odonata communities in Amazonia, Brazil. Int. J. Odonatol. 2013, 16, 135–144. [Google Scholar] [CrossRef]
- Monteiro-Júnior, C.S.; Juen, L.; Hamada, N. Effects of urbanization on stream habitats and associated adult dragonfly and damselfly communities in central Brazilian Amazonia. Landsc. Urban Plan. 2014, 127, 28–40. [Google Scholar] [CrossRef]
- Brasil, L.S.; Batista, J.D.; Giehl, N.F.S.; Valadão, M.B.X.; Santos, J.O.; Dias-Silva, K. Environmental integrity and damselfly species composition in Amazonian streams at the “arc of deforestation” region, Mato Grosso, Brazil. Acta Limnol. Bras. 2014, 26, 278–287. [Google Scholar] [CrossRef]
- Renner, S.; Sahlén, G.; Périco, E. Testing dragonflies as species richness indicators in a fragmented subtropical Atlantic forest environment. Neotrop. Entomol. 2016, 45, 231–239. [Google Scholar] [CrossRef]
- Oliveira-Júnior, J.M.B.; Dias-Silva, K.; Teodósio, M.A.; Juen, L. The Response of Neotropical dragonflies (Insecta: Odonata) to local and regional abiotic factors in small streams of the Amazon. Insects 2019, 10, 446. [Google Scholar] [CrossRef]
- Silva, L.F.R.; Castro, D.M.P.; Juen, L.; Castillo, M.; Hughes, R.M.; Hermes, M.G.-M. Functional responses of Odonata larvae to human disturbances in neotropical savannah headwater streams. Ecol. Indic. 2021, 133, 108376. [Google Scholar] [CrossRef]
- Silva, L.F.R.; Castro, D.M.P.; Juen, L.; Castillo, M.; Hughes, R.M.; Hermes, M.G.-M. A matter of suborder: Are Zygoptera and Anisoptera larvae influenced by riparian vegetation in Neotropical Savannah streams? Hydrobiologia 2021, 848, 4433–4443. [Google Scholar] [CrossRef]
- Silva, L.F.R.; Castro, D.M.P.; Juen, L.; Castillo, M.; Hughes, R.M.; Hermes, M.G.-M. Ecological thresholds of Odonata larvae to anthropogenic disturbances in neotropical savannah headwater streams. Hydrobiologica 2024, 851, 313–326. [Google Scholar] [CrossRef]
- Kutcher, T.E.; Bried, J.T. Adult Odonata conservatism as an indicator of freshwater wetland condition. Ecol. Indic. 2014, 38, 31–39. [Google Scholar] [CrossRef]
- Oliveira-Júnior, J.M.B.; Shimano, Y.; Gardner, T.A.; Hughes, R.M.; De Marco Júnior, P.; Juen, L. Neotropical dragonflies (Insecta: Odonata) as indicators of ecological condition of small streams in the eastern Amazon. Austral. Ecol. 2015, 40, 733–774. [Google Scholar] [CrossRef]
- Manu, M.K.; Ashiagbor, G.; Seidu, I.; Groen, T.; Gyimah, T.; Toxopeus, B. Odonata as bioindicator for monitoring anthropogenic disturbance of Owabi wetland sanctuary, Ghana. Aquat. Insects 2022, 44, 151–169. [Google Scholar] [CrossRef]
- Šigutová, H.; Dolný, A.; Samways, M.J.; Sönke, H.; Oliveira-Júnior, J.M.; Juen, L.; Van Dinh, K.; Bried, J.T. Odonata as indicators of pollution, habitat quality, and landscape disturbance. In Dragonflies and Damselflies: Model Organisms for Ecological and Evolutionary Research, 2nd ed.; Cordoba-Aguilar, A., Beatty, C., Bried, J.T., Eds.; Oxford University Press: Oxford, UK, 2022. [Google Scholar]
- Da Silva Junior, W.F.; Calvão, L.B.; Carvalho, F.G.; Medina-Espinoza, F.; Brasil, L.S. Use of the Zygoptera/Anisoptera ratio (Insecta: Odonata) for habitat alteration assessment in Cerrado streams. Int. J. Odonatol. 2023, 26, 124–131. [Google Scholar] [CrossRef]
- Bota-Sierra, C.A.; Flórez, V.C.; Escobar, F.; Sandoval, H.J.; Novelo-Gutiérrez, R.; Londoño, G.A.; Cordero-Rivera, A. The importance of tropical mountain forests for the conservation of dragonfly biodiversity: A case from the Colombian Western Andes. Int. J. Odonatol. 2021, 24, 233–247. [Google Scholar] [CrossRef] [PubMed]
- May, M. Thermoregulation and adaptation to temperature in dragonflies (Odonata: Anisoptera). Ecol. Monogr. 1976, 46, 1–32. [Google Scholar] [CrossRef]
- Fererras-Romero, M.; Márquez-Rodríguez, J.; Ruiz-García, A. Implications of anthropogenic disturbance factors on the Odonata assemblage in a Mediterranean fluvial system. Int. J. Odonatol. 2009, 12, 413–428. [Google Scholar] [CrossRef]
- De Marco Júnior, P.; Batista, J.D.; Cabette, H.S.R. Community assembly of adult odonates in tropical streams: An ecophysiological hypothesis. PLoS ONE 2015, 10, e0123023. [Google Scholar] [CrossRef]
- Seidu, I.; Danquah, E.; Ayine, C.; Amaning, D.; Lancaster, T. Odonata community structure and patterns of land use in the Atewa Range Forest Reserve, Eastern Region (Ghana). Int. J. Odonatol. 2017, 20, 173–189. [Google Scholar] [CrossRef]
- Calvão, L.B.; Brito, J.D.S.; Ferreira, D.; Cunha, E.J.; Oliveira-Júnior, J.M.B.; Juen, L. Effects of the loss of forest cover on odonate communities in eastern Amazonia. J. Insect Conserv. 2022, 27, 205–218. [Google Scholar] [CrossRef]
- Badu, I.K.; Combey, R.; Abraham, J. Response of Odonata assemblages to disturbance in urban freshwater habitats. Afr. J. Ecol. 2024, 62, e13258. [Google Scholar] [CrossRef]
- Calvão, L.B.; Juen, L.; Oliveir-Júnior, J.M.B.; Batista, J.D.; De Marco Junior, P. Land use modifies Odonata diversity in streams in the Brazilian cerrado. J. Insect Conserv. 2018, 22, 675–685. [Google Scholar] [CrossRef]
- Von Ellenrieder, N. Odonata of the Argentine Yungas cloud forest: Distribution patterns and conservation status. Odonatologica 2009, 38, 39–53. Available online: https://ri.conicet.gov.ar/handle/11336/53901 (accessed on 15 June 2024).
- García-García, P.L.; Vasquez, G.; Novelo-Gutiérrez, R.; Favila, M.E. Effects of land use on larval Odonata assemblages in cloud forest streams in central Veracruz, Mexico. Hydrobiologia 2007, 785, 19–33. [Google Scholar] [CrossRef]
- Gómez-Anaya, J.A.; Novelo-Gutiérrez, R. Richness and structure of an Odonata larval assemblage of a cloud forest stream in western Mexico. Odonatologica 2022, 51, 225–246. [Google Scholar] [CrossRef]
- Miller, K.; Chang, E.; Johnson, N. Defining Common Ground for the Mesoamerican Biological Corridor; World Resource Institute: Washington, DC, USA, 2001; Available online: https://files.wri.org/d8/s3fs-public/pdf/mesoamerica_english.pdf (accessed on 2 June 2024).
- Independent Evaluation Group. The Mesoamerican Biological Corridor. Regional Program Review. 2011. Available online: https://ieg.worldbankgroup.org/sites/default/files/Data/reports/mbc_rpr.pdf (accessed on 2 June 2024).
- Allen, K.E.; Vaśquez, S.P. Forest cover, development, and sustainability in Costa Rica: Can one policy fit all? Land Use Policy 2017, 67, 212–221. [Google Scholar] [CrossRef]
- Moran, M.D.; Monroe, A.; Stallcup, L. A proposal for practical and effective biological corridors to connect protected areas in northwest Costa Rica. Nat. Conserv. 2019, 36, 113–137. [Google Scholar] [CrossRef]
- Morera-Beita, C.; Murillo, L.F.S.; Alvarado, L.D.A. Ecological corridors in Costa Rica: An evaluation applying landscape structure, fragmentation-connectivity process, and climate adaptation. Conserv. Sci. Pract. 2021, 3, e475. [Google Scholar] [CrossRef]
- Ramírez, A.; Paulson, D.R.; Esquivel, C. Odonates of Costa Rica: Diversity and checklist of species. Rev. Biol. Trop. 2000, 48, 247–254. Available online: https://tropicalstudies.org/rbt/attachments/volumes/vol48-1/28_Ramirez_Odonata.pdf (accessed on 3 June 2024).
- Esquivel, C. Dragonflies and Damselflies of Middle America and the Caribbean; InBio: Santo Domingo de Heredia, CR, USA, 2006. [Google Scholar]
- Paulson, D.; Haber, W. Dragonflies and Damselflies of Costa Rica; Comstock Publishing Associates: Ithaca, NY, USA, 2023; p. 400. [Google Scholar]
- Worthen, W.B. Perch selection in a guild of tropical dragonflies (Odonata: Libellulidae): Relationships with body size and thermal ecology. Int. J. Odonatol. 2017, 20, 63–78. [Google Scholar] [CrossRef]
- Worthen, W.B. Confirming the relationship between body size and perch height in tropical odonates (Odonata: Libellulidae): Wet-season contrasts and experimental tests. Int. J. Odonatol. 2018, 21, 229–239. [Google Scholar] [CrossRef]
- Hofhansl, F.P.; Schneeweihs, S. Banderillas: Effects of deforestation on dragonflies (Insects, Odonata) in the Pacific lowland of Costa Rica. Stapfia 2008, 88, 237–247. Available online: https://archive.org/details/stapfia-88-237-247 (accessed on 4 June 2024).
- Foster, P. The potential negative impacts of global climate change on tropical montane cloud forests. Earth-Sci. Rev. 2001, 55, 73–106. [Google Scholar] [CrossRef]
- Roucourt-Cezário, R.R.; Firme, P.P.; Pestana, G.C.; Vilela, D.S.; Juen, L.; Cordero-Rivera, A.; Guillermo, R. Sampling methods for dragonflies and damselflies. In Measuring Arthropod Biodiversity; Santos, J.C., Fernandes, G.W., Eds.; Springer: Cham, Switzerland, 2020; pp. 223–240. [Google Scholar] [CrossRef]
- Chiu, C.H.; Wang, Y.T.; Walther, B.A.; Chao, A. An improved nonparametric lower bound of species richness via a modified Good–Turing frequency formula. Biometrics 2014, 70, 671–682. [Google Scholar] [CrossRef]
- Chao, A.; Wang, Y.T.; Jost, L. Entropy and the species accumulation curve: A novel entropy estimator via discovery rates of new species. Methods Ecol. Evol. 2013, 4, 1091–1100. [Google Scholar] [CrossRef]
- Chao, A.; Ma, K.H.; Hsieh, T.C.; Chiu, C.H. Online program SpadeR (species-richness prediction and diversity estimation in R). In Program and User’s Guide; National Tsing Hua University: Hsinchu, Taiwan, 2019; Available online: http://chao.stat.nthu.edu.tw/wordpress/software_download/ (accessed on 2 February 2024).
- IBM Corp. IBM SPSS Statistics for Windows, Version 28.0; IBM Corp.: Armonk, NY, USA, 2021.
- R Core Team. R: A Language and Environment for Statistical Computing, Version 4.4.1; R Foundation for Statistical Computing: Vienna, Austria, 2021. Available online: https://www.R-project.org/ (accessed on 14 July 2024).
- Bota-Sierra, C.A.; García-Robledo, C.; Escobar, F.; Novelo-Gutiérrez, R.; Londoño, G.A. Environment, taxonomy and morphology constrain insect thermal physiology along tropical mountains. Funct. Ecol. 2022, 36, 1924–1935. [Google Scholar] [CrossRef]
- Carvalho, F.G.; Duarte, L.; Nakamura, G.; dos Santos-Seger, G.D.; Juen, L. Changes of phylogenetic and taxonomic diversity of Odonata (Insecta) in response to land use in Amazonia. Forests 2021, 12, 1061. [Google Scholar] [CrossRef]
- Pereira-Moura, L.; Veras, D.S.; de Carvalho, F.G.; Juen, L.; Couceiro, S.R.M. Habitat specificity and morphology-main filters for the distribution of Odonata in the Cerrado Maranhense, Brazil. Aquat. Ecol. 2023, 57, 443–458. [Google Scholar] [CrossRef]
- Oliveira-Júnior, J.M.B.; De Marco, P.; Dias-Silva, K.; Pereira-Leitão, R.; Gontijo-Leal, C.; Santos Pompeu, P.; Gardner, T.A.; Hughes, R.M.; Juen, L. Effects of human disturbance and riparian conditions on Odonata (Insecta) assemblages in eastern Amazon basin streams. Limnologica 2017, 66, 31–39. [Google Scholar] [CrossRef]
- Grof-Tisza, P.; LoPresti, E.; Heath, S.K.; Karban, R. Plant structural complexity and mechanical defenses mediate predator-prey interactions in an odonate-bird system. Ecol. Evol. 2017, 7, 1650–1659. [Google Scholar] [CrossRef]
- Mendes, T.P.; Oliveira-Júnior, J.M.B.; Cabette, H.S.R.; Batista, J.D.; Juen, L. Congruence and the biomonitoring of aquatic ecosystems: Are odonate larvae or adults the most effective for the evaluation of impacts. Neotrop. Entomol. 2017, 46, 631–641. [Google Scholar] [CrossRef]
- Worthen, W.B.; Fravel, R.K.; Horne, C.P. Downstream changes in odonate (Insecta: Odonata) communities along a suburban to urban gradient; Untangling natural and anthropogenic effects. Insects 2021, 12, 201. [Google Scholar] [CrossRef] [PubMed]
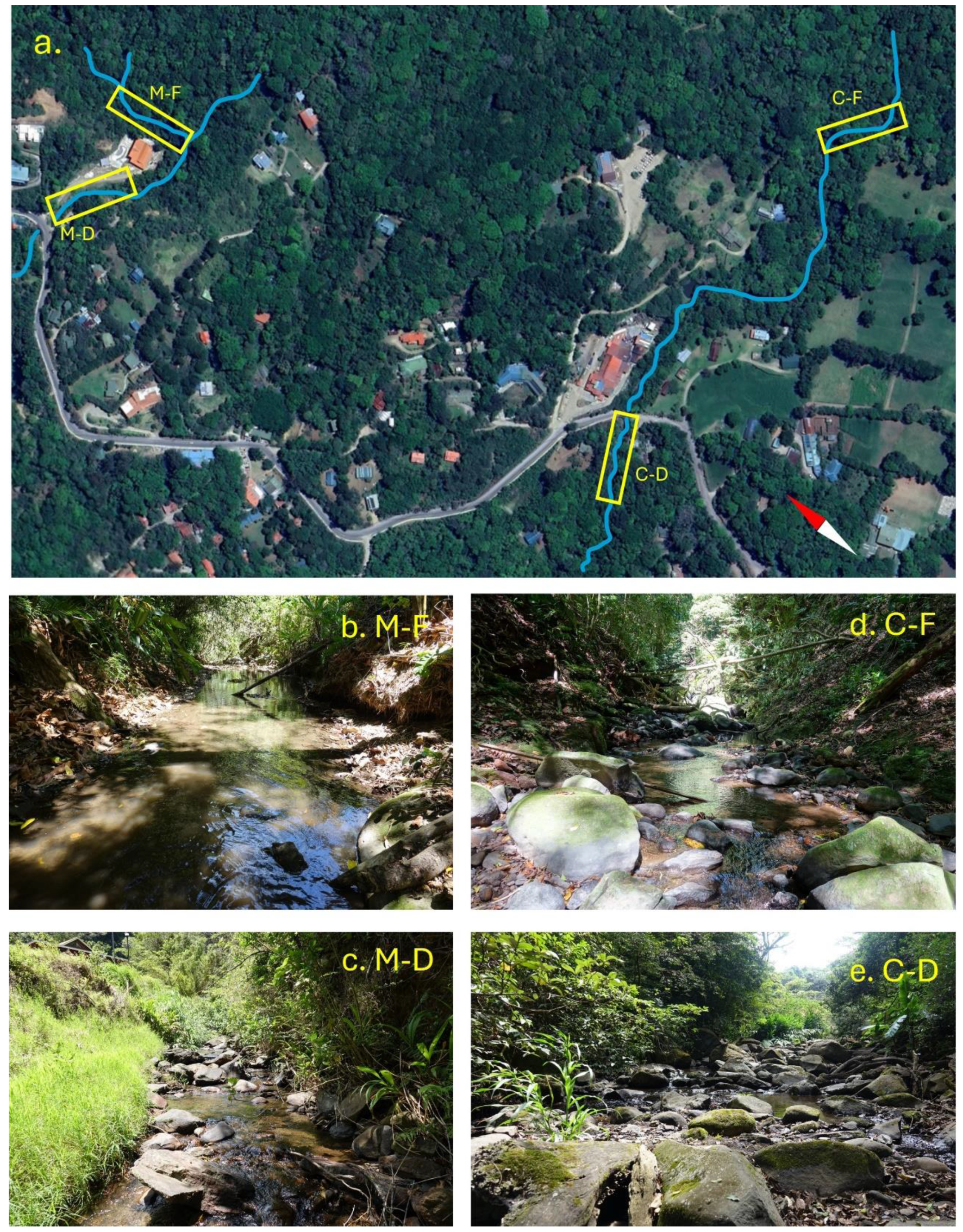
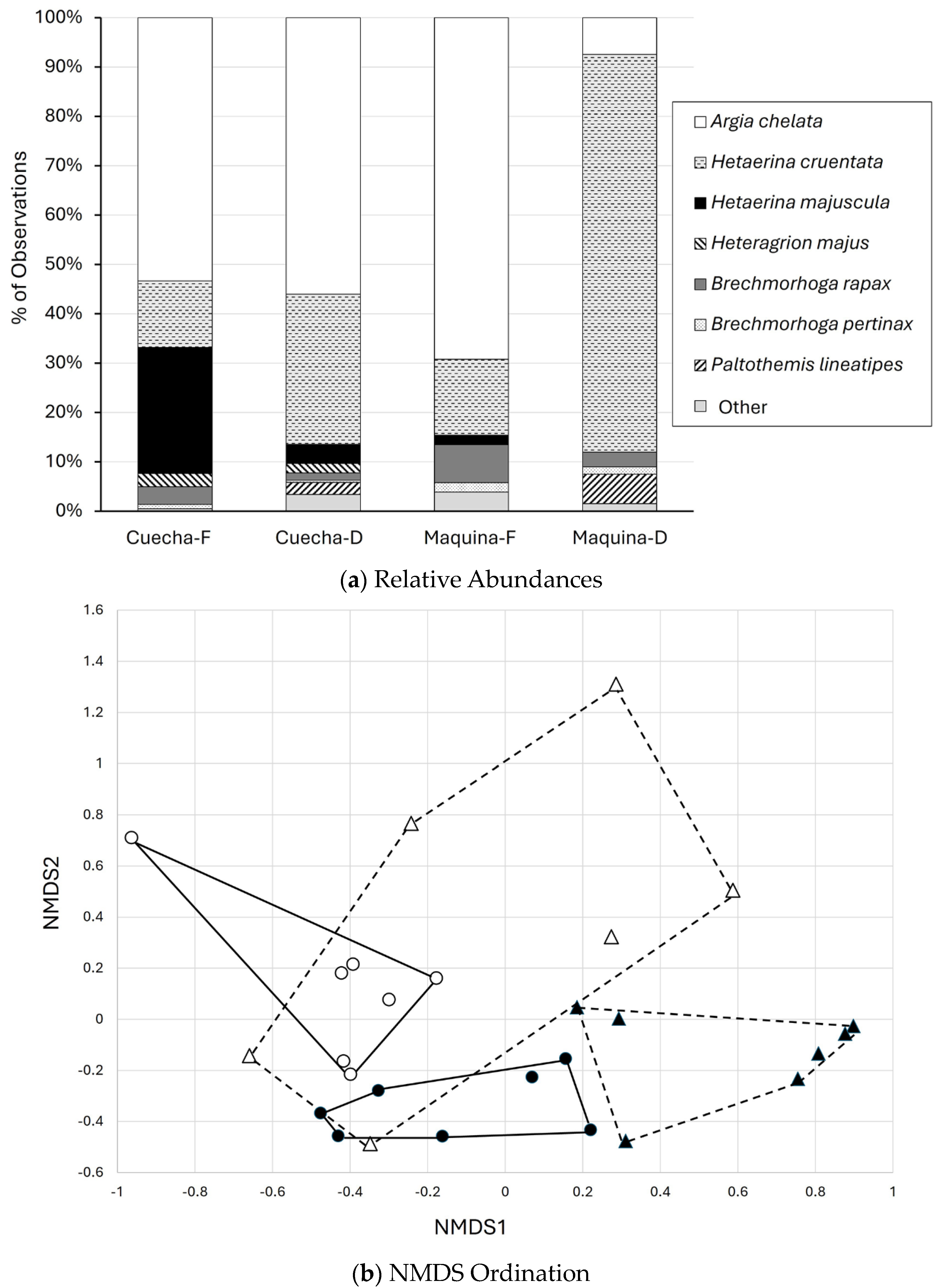
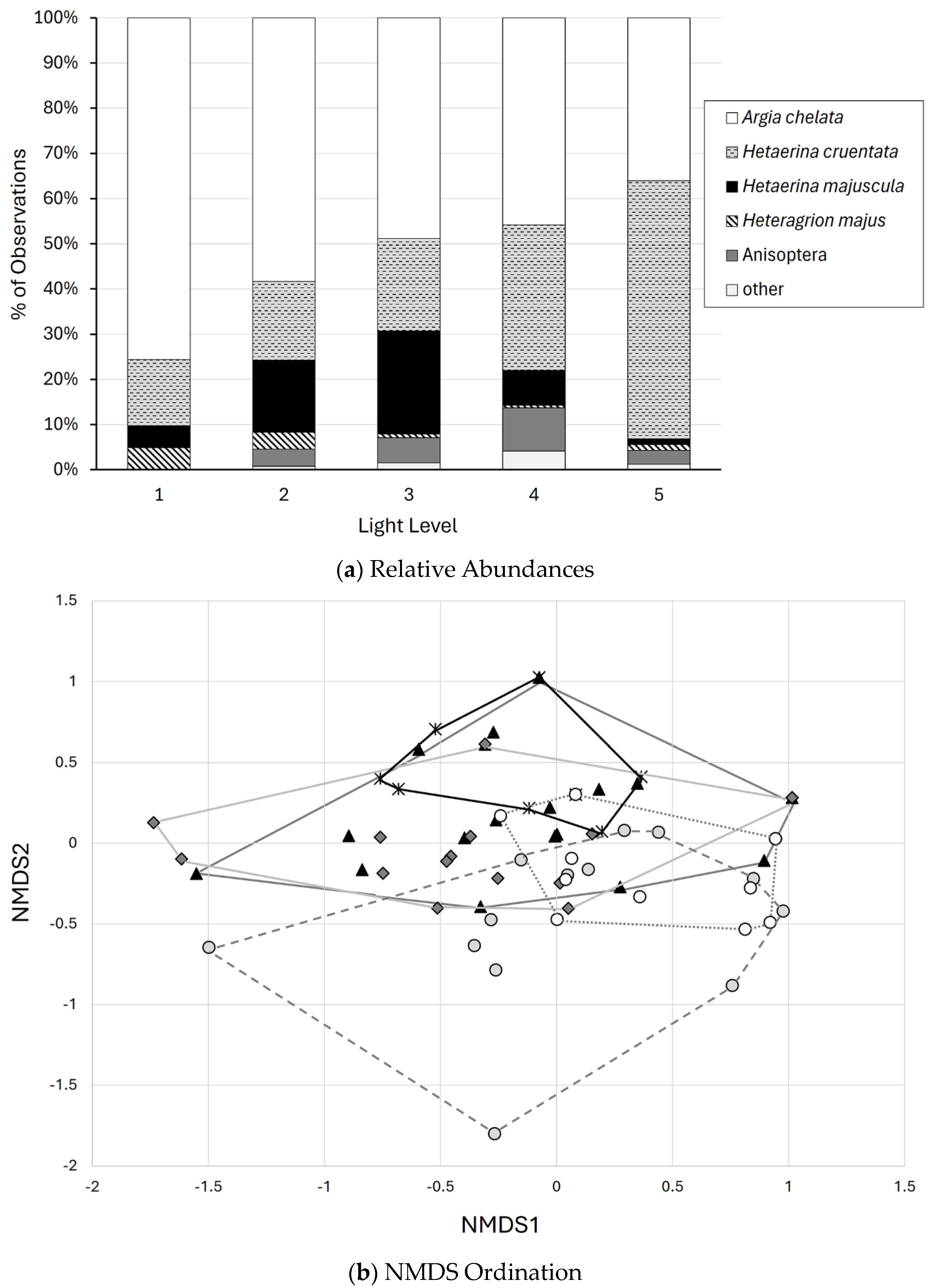
| Cuecha | Maquina | |||
|---|---|---|---|---|
| Species | Forest | Disturb | Forest | Disturb |
| Zygoptera | ||||
| Argia anceps Garrison | 0 | 4 | 0 | 1 |
| Argia chelata Calvert | 119 | 116 | 36 | 5 |
| Argia elongata Garrison & Von Ellenrieder | 0 | 0 | 1 | 0 |
| Argia underwoodi Calvert | 0 | 0 | 1 | 0 |
| Hetaerina cruentata (Rambur) | 30 | 63 | 8 | 54 |
| Hetaerina majuscula Selys | 57 | 8 | 1 | 0 |
| Heteragrion majus Selys | 6 | 4 | 0 | 0 |
| Lestes henshawi Calvert | 0 | 3 | 0 | 0 |
| Philogenia peacocki Brooks | 1 | 0 | 0 | 0 |
| Anisoptera | ||||
| Brechmorhoga pertinax (Hagen) | 2 | 1 | 1 | 1 |
| Brechmorhoga rapax Calvert | 8 | 3 | 4 | 2 |
| Paltothemis lineatipes Karsch | 0 | 5 | 0 | 4 |
| Total | 223 | 207 | 52 | 67 |
| (a) Summary of GLM | |||||
| Light Level | % Canopy Cover | ||||
| Variable | df | X2 | p | X2 | p |
| River | 1 | 0.484 | ns | 27.130 | 0.001 |
| Habitat | 1 | 15.022 | 0.001 | 58.671 | 0.001 |
| River × Habitat | 1 | 7.200 | 0.007 | 15.865 | 0.001 |
| (b) Comparison of Estimated Means | |||||
| Light Level | % Canopy Cover | ||||
| Plot | N | X ± 1 se | X ± 1 se | ||
| Maquina-disturbed | 20 | 3.80 ± 0.25 a | 35.75 ± 3.59 a | ||
| Cuecha-disturbed | 20 | 2.95 ± 0.25 ab | 68.75 ± 3.59 b | ||
| Maquina-forested | 20 | 2.15 ± 0.25 b | 77.55 ± 3.59 bc | ||
| Cuecha-forested | 20 | 2.65 ± 0.25 b | 81.95 ± 3.59 c | ||
| (a) Summary of GLM | |||||||||||||
| Total Odonate Abundance | Zygopteran Abundance | Anisopteran Abundance | Species Richness | Species Diversity | An./Zyg. Ratio | ||||||||
| Variable | df | X2 | p | X2 | p | X2 | p | X2 | p | X2 | p | X2 | p |
| River | 1 | 153.59 | 0.001 | 153.44 | 0.001 | 1.61 | ns | 0.29 | ns | 0.26 | ns | 2.34 | ns |
| Habitat | 1 | 0.74 | ns | 0.61 | ns | 0.96 | ns | 1.13 | ns | 0.04 | ns | 1.57 | ns |
| River × Habitat | 1 | 2.47 | ns | 2.11 | ns | 0.35 | ns | 0.21 | ns | 2.01 | ns | 3.13 | ns |
| (b) Comparisons of Estimated Means | |||||||||||||
| Total Odonate Abundance | Zygopteran Abundance | Anisopteran Abundance | Species Richness | Species Diversity | An./Zyg. Ratio | ||||||||
| River | N | X ± 1 se | X ± 1 se | X ± 1 se | X ± 1 se | X ± 1 se | X ± 1 se | ||||||
| Cuecha | 14 | 30.69 ± 1.48 a | 29.34 ± 1.44 a | 1.36 ± 0.31 a | 4.63 ± 0.73 a | 3.09 ± 0.30 a | 0.08 ± 0.04 a | ||||||
| Maquina | 14 | 8.43 ± 0.78 b | 7.59 ± 0.74 b | 0.84 ± 0.25 a | 4.05 ± 0.80 a | 2.82 ± 0.44 a | 0.16 ± 0.04 a | ||||||
| (a) Summary of GLM | |||||||||||
| Total Odonate Abundance | Zygopteran Abundance | Anisopteran Abundance | Species Richness | Species Diversity | |||||||
| Variable | df | X2 | p | X2 | p | X2 | p | X2 | p | X2 | p |
| Light | 1 | 36.767 | 0.001 | 23.202 | 0.001 | 12.751 | 0.005 | 6.61 | ns | 4.434 | ns |
| River | 4 | 57.936 | 0.001 | 62.518 | 0.001 | 0.400 | ns | 3.695 | ns | 0.004 | ns |
| Habitat | 1 | 0.163 | ns | 0.095 | ns | 2.466 | ns | 1.898 | ns | 0.037 | ns |
| Light × River | 4 | 4.603 | ns | 4.678 | ns | 0.470 | ns | 1.447 | ns | 3.142 | ns |
| Light × Habitat | 4 | 3.746 | ns | 3.309 | ns | 3.396 | ns | 2.886 | ns | 0.187 | ns |
| River × Habitat | 1 | 0.25 | ns | 0.014 | ns | 1.347 | ns | 0.447 | ns | 0.180 | ns |
| (b) Comparison of Estimated Means | |||||||||||
| Total Odonate | Zygopteran | Anisopteran | Species | Species | |||||||
| Abundance | Abundance | Abundance | Richness | Diversity | |||||||
| Light Level | N | X ± 1 se | X ± 1 se | X ± 1 se | X ± 1 se | X ± 1 se | |||||
| 1 | 11 | 2.94 ± 0.62 a | 3.02 ± 0.64 a | 0.00 ± 0.00 a | 1.96 ± 0.79 a | 2.23 ± 0.71 a | |||||
| 2 | 25 | 4.49 ± 0.53 a | 4.38 ± 0.52 a | 0.12 ± 0.09 a | 2.06 ± 0.41 a | 2.02 ± 0.61 a | |||||
| 3 | 18 | 5.42 ± 0.71 a | 4.92 ± 0.69 a | 0.32 ± 0.18 ab | 2.70 ± 0.45 a | 3.55 ± 0.68 a | |||||
| 4 | 14 | 9.50 ± 0.98 b | 8.02 ± 0.92 b | 1.11 ± 0.30 b | 3.59 ± 0.47 a | 2.59 ± 0.34 a | |||||
| 5 | 12 | 6.76 ± 1.82 ab | 6.87 ± 1.86 ab | 0.00 ± 0.00 a | 2.46 ± 0.87 a | 2.31 ± 0.68 a | |||||
| Source | Df | SS | R2 | F | p |
|---|---|---|---|---|---|
| River | 1 | 2.7161 | 0.1555 | 18.2778 | 0.0001 |
| Habitat | 1 | 2.1531 | 0.1233 | 14.4891 | 0.0001 |
| Light | 1 | 1.2071 | 0.0691 | 8.1229 | 0.0001 |
| River × Habitat | 1 | 1.6729 | 0.0958 | 11.2579 | 0.0001 |
| River × Light | 1 | 0.0759 | 0.0043 | 0.5106 | 0.7535 |
| Habitat × Light | 1 | 0.1930 | 0.0111 | 1.2986 | 0.2618 |
| River × Habitat × Light | 1 | 0.2349 | 0.0135 | 1.5808 | 0.1634 |
| Residual | 62 | 9.2133 | 0.5275 |
| (a) Plot level | ||||
| (1) Complete data set (N = 27) | ||||
| Light Level | Canopy Cover | NMDS1 | NMDS2 | |
| Light Level | −0.822 *** | 0.481 ** | −0.423 * | |
| Canopy Cover | −0.663 *** | 0.404 * | ||
| An./Zyg. Ratio-Abundance | −0.230 ns | 0.163 ns | 0.171 ns | 0.282 + |
| An./Zyg. Ratio-Richness | −0.177 ns | 0.111 ns | 0.171 ns | 0.196 ns |
| (2) Reduced data set: Anisopterans present (N = 16) | ||||
| Light Level | Canopy Cover | NMDS1 | NMDS2 | |
| Light Level | −0.683 *** | 0.032 ns | −0.805 *** | |
| Canopy Cover | −0.502 * | 0.459 * | ||
| An./Zyg. Ratio-Abundance | −0.160 ns | −0.318 ns | 0.836 *** | 0.337 ns |
| An./Zyg. Ratio-Richness | −0.016 ns | −0.460 * | 0.856 *** | 0.131 ns |
| (b) Subplot Level | ||||
| (1) Complete data Set (N = 70) | ||||
| Light Level | Canopy Cover | NMDS1 | NMDS2 | |
| Light Level | −0.634 *** | 0.314 * | −0.603 ** | |
| Canopy Cover | −0.595 ** | 0.395 * | ||
| An./Zyg. Ratio-Abundance | 0.239 * | −0.158 + | 0.008 ns | −0.544 ** |
| An./Zyg. Ratio-Richness | 0.258 * | −0.174 + | 0.010 ns | −0.560 ** |
| (2) Reduced data set: Anisopterans present (N = 16) | ||||
| Light Level | Canopy Cover | NMDS1 | NMDS2 | |
| Light Level | −0.697 *** | 0.314 * | −0.603 ** | |
| Canopy Cover | −0.595 ** | 0.395 * | ||
| An./Zyg. Ratio-Abundance | 0.039 ns | −0.354 + | 0.008 ns | −0.544 ** |
| An./Zyg. Ratio-Richness | 0.381 + | −0.462 * | 0.010 ns | −0.560 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Worthen, W.B.; Guevara-Mora, M. The Effects of Light Environment on Adult Odonate Communities in Disturbed and Intact Forest: The Importance of Small-Scale Effects. Diversity 2024, 16, 557. https://doi.org/10.3390/d16090557
Worthen WB, Guevara-Mora M. The Effects of Light Environment on Adult Odonate Communities in Disturbed and Intact Forest: The Importance of Small-Scale Effects. Diversity. 2024; 16(9):557. https://doi.org/10.3390/d16090557
Chicago/Turabian StyleWorthen, Wade B., and Meyer Guevara-Mora. 2024. "The Effects of Light Environment on Adult Odonate Communities in Disturbed and Intact Forest: The Importance of Small-Scale Effects" Diversity 16, no. 9: 557. https://doi.org/10.3390/d16090557
APA StyleWorthen, W. B., & Guevara-Mora, M. (2024). The Effects of Light Environment on Adult Odonate Communities in Disturbed and Intact Forest: The Importance of Small-Scale Effects. Diversity, 16(9), 557. https://doi.org/10.3390/d16090557







