Abstract
Bacterial microbiomes influence global carbon and nutrient cycling as the environment changes. Rain-fed rock basins are ephemeral aquatic systems, potentially subject to extreme environmental stress, that can host a wide variety of biological communities, including bacteria. However, bacterial communities are barely described in these habitats. Here we provide a detailed description on the occurrence, diversity and distribution patterns of the bacterial communities within and between rain-fed granite mountain rock basins located in the Sierra de Guadarrama National Park, Spain, using high-throughput sequencing of 16S RNA. We recovered a highly diverse community consisting of 3174 operational taxonomic units (OTUs) belonging to 32 phyla. In total, 50% of OTUs were shared among basins and 6–10% were basin-exclusive OTUs, suggesting a robust global bacterial metacommunity colonizes the basins. The existence of 6% replicate-exclusive OTUs and the fact that at least four replicates were required to catalogue 90% of the basin bacterial community emphasized the heterogeneity of these habitats. Both environmental filtering and random dispersal are likely to be involved in the arrangement of the bacterial communities. The taxa identified in this study are versatile in metabolism, and some have biotechnological potential. The taxonomic affiliation of many of the OTUs found suggests that rain-fed rock basins could be a resource for mining novel bacterial biocompounds.
1. Introduction
Rock basins are depressions excavated in solid bedrock [1] with occasional inflows of water and formation of sediments at their bottom [2]. These geological landforms develop from shallow hollows which tend to enlarge over the time because of the weathering of the rock and the removal of the waste products caused by the interrelationships between the host rock, their biological communities and water [3,4,5,6,7].
Rock basins are a normal feature of rock outcrops (e.g., granite, limestone, sandstone) in all major biomes throughout the world, from inland areas (freshwater pools) to coasts (brackish and sea pools) [8]. They are of variable dimensions, from centimetres to metres deep, rather flat or concave, rarely hemispherical in shape and frequently occurring in groups [5]. In addition to “rock basins”, there is a large range of names for these landforms, including rock pools, gnammas, weathering pits, panholes, potholes, cupular pools, “mares cupulaires”, ephemeral pools, cisterns, tanks, caldrons, tinajas and kamenitzas (a term explicitly used for those found in limestone) [5]. The geomorphologically unspecific term “rock pools” is probably the most frequently used, specifically for those pools generated in rocky coasts under tidal influence [5].
There is particular interest in granitic rock basins since granite is the main bedrock of the continental mass. These formations are found worldwide, from Europe to South Africa, Australia, eastern and central Asia, parts of South America and western North America [9]. From an ecological perspective, granitic rock basins represent ephemeral aquatic habitats. The granitic basins studied herein are considered ombrotrophic basins, because they solely depend on precipitation as a source of water [2]. These rain-fed basins collect rainwater, giving rise to small seasonal wetland-like habitats. A variable amount of sediment can develop at the bottom due to the dissolution and mechanical erosion of the rock itself and the accumulation of sedimentary substrates [5]. Rock basins experience hydration cycles which occur only during precipitation, followed by periods of desiccation during dry seasons, where high temperatures cause water evaporation, and the basin dries [10]. Since granitic rocks are impermeable, water does not percolate and simply evaporates [11]. These habitats can experience periods of inundation–desiccation through changes in the weather conditions, both daily and seasonally. The length and recurrence of the periods of desiccation often make rock basins extreme environments. The strong variability of their environment means that the biological communities that inhabit them are adapted to changing conditions and have a high plasticity and wide tolerance to stress [2,12].
As temporary aquatic systems, rock basins can host a wide variety of biological communities. These habitats (particularly the closely located rain-fed basins) have been proposed as “model habitats” to test ecological concepts—such as community assembly, spatial population dynamics and local extinction—given that they are small and easily delimited ecosystems, and their food webs should be relatively short [13,14,15]. Community composition has been reported to differ spatially between pools and temporarily within the same pools, revealing the great variability and biodiversity these habitats can harbour. These communities can vary depending, among other factors, on biotic interactions among different community levels, nutrient content, water availability, temperature, pH or the chemical composition of the rock itself, and these levels can be altered periodically [16]. Several works have been carried out in biological communities colonising granitic basins, and they focused extensively on the diversity of invertebrates [2,7,8,17,18,19,20] and plants [5,21].
By contrast, the literature on microbial communities from granite rock pool habitats is sparse. There is some research on phytoplankton [22,23] and on microparasites of invertebrates from coastal pools [24]. This is surprising: as drivers of Earth’s biogeochemical cycles, microbiomes can influence global carbon and nutrient cycling as the environment changes [25]. We have previously described the microbial eukaryotic community in rain-fed granite rock basins [26,27,28] and revealed a large heterogeneity of metabolically diverse organisms that may coexist due to the different temporal patterns of their active populations. There are some studies of bacterial communities in intertidal igneous rocks [29] and in rock basins of a sedimentary origin, specifically in sandstones [30]. However, studies that focus on bacteria in rain-fed granitic rock basins are to our knowledge absent, and, therefore, the meaning of their function in these habitats is still largely unknown.
Bacterial communities are one of the most abundant and species-rich groups of microorganisms [31], mediating many of the environmental processes that support life on the planet, such as the decomposition of organic matter [32] or their roles in the nitrogen cycle [33]. Bacteria–granite interactions can play a crucial role in the biogeochemical cycling of bioessential elements [34,35,36]. Some authors have experimentally tested the important role bacteria can play in the weathering of granite [37]. It has been suggested that the production of “hygroscopic extracellular polymeric substances” by terrestrial bacteria can intensify the weathering effects of freeze–thaw cycles [38], and that microbial weathering of the rock could be in part due to the need of obtaining micronutrients by the bacteria [39].
To further explore the role of bacteria in mediating geomorphological processes within ombrotrophic rock basins, it is first necessary to decipher the structure and composition of bacterial communities, as species patterns can pave the way to understanding their relation to ecosystem functioning.
Therefore, the aim of the present study is to provide, by high-throughput sequencing, a detailed description on the occurrence, diversity and distribution patterns of the bacterial communities within and between rain-fed granite mountain rock basins located in a national park.
In the absence of connectivity among the basins, the occurrence in a basin of passive species—as microbial species fundamentally are—will very likely depend on their stochastic dispersal by wind or by animals. The long-term establishment of populations is however a more complex process that will involve physical–chemical and biotic interactions on and among the communities that will conduct species sorting and patchy distributions [16,40]. The basins under study here are not interconnected spatially nor through underground streams or rivulets, so we hypothesise that random dispersal is likely to be a much less significant factor than species sorting (environmental filtering) for the differences observed in the bacterial communities between basins. However, we predict that dispersal-related mechanisms and species sorting will both be strong drivers of the variability of intra-basin bacterial assemblages and that random dispersal may lessen bacterial intra-basin differences.
2. Materials and Methods
2.1. Study Area and Sample Collection
The study area is in La Pedriza de Manzanares (UTM: 30N 425279 4511417, DATUM: ETRS89) within the Sierra de Guadarrama National Park in Madrid, Spain. Due to its location in the centre of the Iberian Peninsula, the climate of the Sierra de Guadarrama National Park has a marked continental character, with cold winters and hot summers and important interannual variations. The sampled location (La Pedriza) has clear mountain features with altitudes ranging between 800 and 1200 m above sea level. The origin of the precipitation is predominantly Atlantic and mainly concentrated in a rainy season from October to February and again in April and May. The driest months are July and August with rainfall usually below 30 mm. On the summits of the national park and main mountain ranges, the number of below-zero days is around 150 days per year. Precipitation in the form of snow is permanent on the ground for usually less than 10 days a year [41].
Three basins (A, B and C) located within an area of 4354 m2 at altitudes of, respectively, 1086 m, 1139 m and 1110 m above sea level, were selected in October 2019 (Figure 1). Basin A is located in an isolated monolith, further away from the other two basins that are closer together (138 m from basin A to B, 214 m from basin A to C and 91 m from basin B to C). Basin B is the largest of the three with over 39,000 cm3 and has the most diverse ecosystem with a large presence of plants. Basin A is still large with over 28,500 cm3, while basin C is the smallest one with around 8500 cm3.

Figure 1.
Rock basins sampled in the Sierra de Guadarrama National Park (La Pedriza). (A): Basin A; (B): Basin B; (C): Basin C.
Five replicates of sediment from each basin were collected with sterile spatulas and stored in sterile polypropylene containers. In the lab, the sediments were spread on sterile Petri dishes, protected from light and left to fully dry at ambient temperature (21 °C ± 0.2) until DNA extraction and sequencing. Values for chemical variables were obtained from the remaining sediment of each of the replicates. Before the analyses, sediment was homogenised by pulverisation until a final granulometry < 125 µm was reached. Carbon, hydrogen, nitrogen and sulphur (CHNS) were determined with elemental microanalysis by combustion using microanalyser LECO CHNS-932 (LECO Corporation, St Joseph, MI, USA). The analysis was performed three times per replicate. Sulphur was not retrieved in two replicates of basin A due to combustion problems. Therefore, it was excluded from the analyses.
2.2. DNA Extraction, Sequencing and Bioinformatics
Aliquots of approximately 1 g of dry sediment from each basin were sent to Novogene Europe (Novogene Co., Ltd. Cambridge Sequencing Center, Cambridge, UK) for DNA extraction and high-throughput sequencing. In brief, amplicons were obtained using the V4 16S rRNA primers Forward 515F 5′ GTGCCAGCMGCCGCGGTAA 3′ and Reverse 806R 5′ GGACTACHVGGGTWTCTAAT 3′ and sequenced on Illumina paired-end platform (PE250. Illumina UK, Cambridge, UK). Data split and read merging were performed with FLASH software (V1.2.7), and the quality control (data filtration and chimera removal) with QIIME software (V1.7.0). Sequence analyses were performed by Uparse software (Uparse v7.0.1090) [42]. Sequences with 97% similarity were assigned to the same operational taxonomic unit (OTU). OTUs were classified according to their relative abundances as rare (≤0.001%), intermediate (>0.001% and <0.1%) or abundant (≥0.1%).
2.3. Statistical Analysis
2.3.1. Global Community Structure: OTU Richness and Abundance
Venn diagrams (R package: VennDiagram; version: 1.7.3) [43] were used to relate both the common and the unique OTUs. The number of OTUs detected were plotted in species accumulation curves using the exact method (R library specaccum; R package: vegan; version: 2.6-4) [44], and rarefaction curves were normalised by both the total number of reads and the total number of OTUs (R package: iNEXT; version: 3.0.0) [45]. These analyses were performed between basins and between replicates.
2.3.2. Diversity Analysis
Alpha diversity richness and Shannon–Weaver and inverse Simpson diversity indices were calculated based on raw reads, and beta diversity was represented using a Bray–Curtis dissimilarity heatmap for abundance data (R package: vegan; version: 2.6-4) [44].
2.3.3. Multivariate Analysis
The PERmutational Multivariate Analysis of Variance (PERMANOVA) was used to assess the difference in the composition of OTUs and phyla in the basins. Non-metric multidimensional scaling (NMDS) was applied to visualise comparatively the OTUs and phyla abundances (R package: vegan; version: 2.6-4) [44]. Indicator species analysis was used to determine the species that best indicate the difference in the basins (R package: indicspecies, version: 1.7.13) [46]. Redundance analysis (RDA) was performed to relate the abundance of the OTUs selected as indicator species to the matrix of chemical (explanatory) variables. OTU data was first transformed using Hellinger transformation method. Significance was obtained by permutation tests (number of permutations: 999) for the whole model, the RDA axes and the explanatory variables. The significance of the RDA axes and explanatory variables was examined further only if the overall RDA model was found to be significant after permutation. Linear dependencies within explanatory variables were verified using variance inflation factor, and forward selection was used in case of collinearity (R package: vegan 2.6-4) [44].
All analyses were performed with RStudio software (V4.0.3) [47,48].
3. Results
3.1. Global Community Structure: OTU Richness and Abundance
A total of 2,037,651 reads of the V4 regions of the 16S RNA were detected, which grouped in 3174 OTUs belonging to 32 phyla.
Basin C had the largest number of OTUs (2553) and basin A the lowest (2097). However, basins A and B had a larger number of reads than basin C (687,687; 714,354 and 635,610 reads, respectively). Almost half of the OTUs identified (1575; 49.6%) were shared in the three rock basins (Figure 2a). The percentage occupied by the OTUs which were exclusive to just one basin was 6% for basin A, 9.5% for basin B and 10% for basin C. Basins B and C shared many more OTUs with each other than with basin A, which had more OTUs in common with basin C than with basin B (Figure 2a).
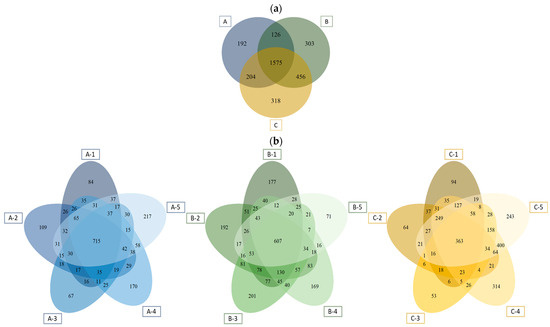
Figure 2.
Venn diagrams representing the exclusive and shared OTUs among the three basins (a) and within the replicates of each basin (b). A-1 to A-5: Represent replicates of basin A; B-1 to B-5 represent replicates of Basin B; C-1 to C-5 represent replicates of basin C.
Of the total number of OTUs present in basins A and B, 34.1% and 24.7%, respectively, were present in all five replicates, and far less, 14.2% of OTUs, in the case of basin C (Figure 2b). However, the exclusive OTUs when pooling together the five replicates of each basin represented a similar percentage in the three basins studied: 30.8% in basin A; 32.9% in basin B and 30.1% in basin C, that is, an average of around 6.0% of exclusive species per replicate (Figure 2b).
The Venn diagrams of basins A and C show that two replicates accumulated more exclusive OTUs than the rest of the replicates in each of the basins, whereas in basin B one of the replicates had far fewer exclusive OTUs than the other four (Figure 2b).
The mean number of reads per replicate was similar in basins A and B (137,205 and 142,871, respectively) and a bit higher than that of basin C (127,122). In addition, the variance coefficient was much higher in basin C (0.15) compared to basins A and B (0.06 and 0.05).
The species accumulation curve for the basins all together shows that it takes over two-thirds of the replicates (10 replicates) to obtain over 90% of the total OTUs found (Figure 3a). The species accumulation curves per basin show that four of the five replicates taken are needed to detect over 90% of the OTUs of each basin (Figure 3b).
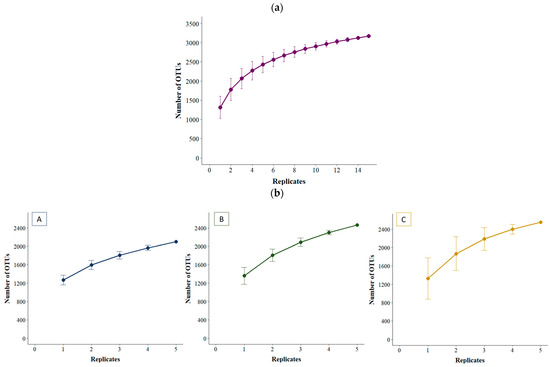
Figure 3.
Species accumulation curves for the three basins combined (a) and within each basin (b). A, B and C represent the three basins sampled.
The OTUs’ normalised rarefaction curves for the three basins show that the curves for all basins are very similar and reach the asymptote at around 80% of the overall number of reads (Figure 4). These curves show that with 10% of the reads over 50% of the OTUs would be detected. In the rarefaction curves of each basin, most replicates also have a very similar tendency, although there was one replicate in basin A and, especially, one in basin C that fluctuated slightly from the other replicates of the basins. These two replicates are also the ones with the lowest number of exclusive OTUs within their basin (Figure S1).
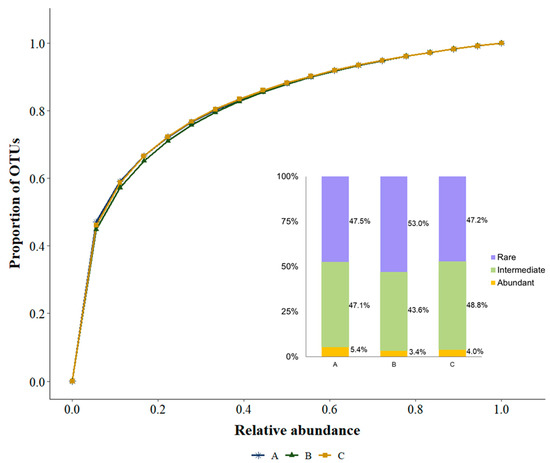
Figure 4.
Normalised rarefaction curves of the number of OTUs for the three basins. A, B and C represent the three basins sampled.
3.2. Taxonomical Description
The most diverse phyla were Chloroflexota, Pseudomonadota and Planctomycetota with over 500 OTUs each, i.e., 566 (17.8%), 588 (18.5%) and 588 (18.5%) OTUs, respectively (Table S1). Chloroflexota and Pseudomonadota were evenly present in all basins, while Planctomycetota was twice more abundant in basins B and C than in basin A. Other phyla had over 100 OTUs identified (Bacillota: 346 OTUs; Actinobacteriota: 286 OTUs; Acidobacteriota 157 OTUs; Myxococcota: 148 OTUs). Overall, replicates from basin A had a similar number of OTUs per phylum, although some of them had a higher number of OTUs from Planctomycetota. In basin B the number of OTUs of different phyla was also consistent per replicate, although one replicate had fewer OTUs, particularly for Planctomycetota and Pseudomonadota. In basin C, two replicates differed from the rest with more OTUs in most phyla. Conversely, thirteen basin C phyla had less than 10 OTUs overall.
The most abundant phyla overall were Bacillota (816,194 reads, 40.1%) and Pseudomonadota (641,517 reads, 31.5%). Only seven out of the 32 bacterial phyla had an abundance higher than 1.0% (Figure 5), and nine phyla had less than 50 reads. The same four phyla (Bacillota, Pseudomonadota, Actinobacteriota and Bacteroidota) were the most abundant phyla within the three basins, taking in more than 85% of the abundance of each basin.
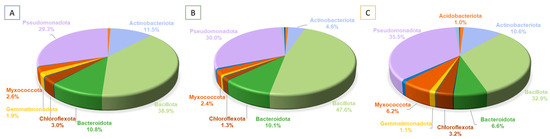
Figure 5.
Relative abundances (%) of the phyla at each basin. Only groups with relative abundances ≥1% are depicted. (A–C) represent the three basins sampled.
Most phyla had a higher relative abundance in basin C than in the other basins. However, Abditibacteriota, Deinococcota, Gemmatimonadota and Patescibacteria were mostly present in basin A and Dependentiae, Elusimicrobiota, GAL-15, RCP2-54 and WPS-2 in basin B. Latescibacterota was only present in basin C (Figure 6).
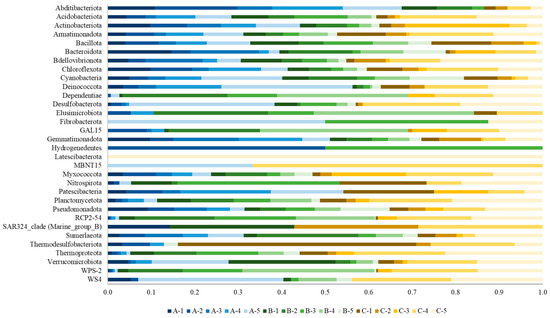
Figure 6.
Relative abundances (in a scale of 0 to 1) of phyla in each of the 15 replicates collected from the sediments of the three basins (A, B, C).
Only one phylum of the 32 phyla retrieved in the analysis included Archaea (Thermoproteota), and it was mainly present in basin C.
3.3. Diversity Analysis
Mean OTU richness was highest in basin B followed by basins C and A. Basin C had the largest deviation in richness among replicates. The Shannon’s diversity index was higher for basin A than for basins B and C. The inverse Simpson index was also higher for basin A and lowest for basin C (Figure 7a). Similar results were obtained for phyla (Figure 7b).
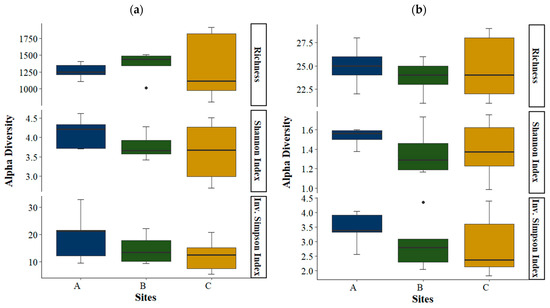
Figure 7.
Box plots for OTU richness and Shannon and inverse Simpson diversity indices in each basin (N = 5), by OTU (a) and by phyla (b). Boxes represent the interquartile range and thick lines are the median. Whiskers indicate the highest and lowest values. Outliers are presented as dots. A, B and C represent the three basins sampled.
The Bray–Curtis dissimilarity heatmap shows that overall replicates from basins A and B were more similar to replicates from their own basin. Most replicates from basin C were particularly different to all the other replicates, irrespective of the basin. Furthermore, two replicates from basin C were more similar to replicates from basins A and B than to other basin C replicates (Figure 8).
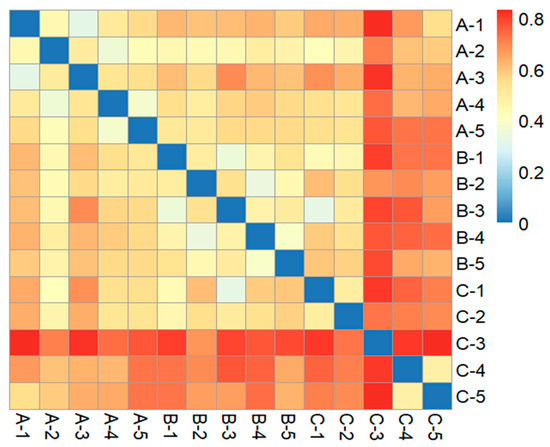
Figure 8.
Bray–Curtis dissimilarity heatmap between replicates of the basins. A, B and C represent the three basins sampled.
3.4. Chemical Analysis
There were also differences between basins in the mean percentage of the chemical variables analysed. Basin B had higher C, H and N values than the other basins (Figure 9). Chemical proportions also varied among replicates, excepting for N content in basin A, where all the replicates had similar values (Figure S2).
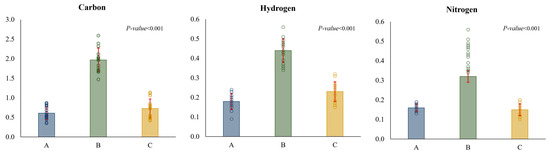
Figure 9.
Mean values of the chemical variables in each basin (A, B and C) (N = 15). Standard deviation is shown in red. Open circles represent the individual data points (dot plots). The p-values represent differences between basins and were computed using one-way ANOVA.
3.5. Multivariate Analysis
PERMANOVA showed that there was a difference in the composition of basins according to OTUs but not for phyla (F-statistic: 2.162; p-value: 0.001; F-statistic: 0.9421; p-value: 0.44, respectively).
The NMDS analysis by OTUs showed that only replicates from basin A were clearly clustered together. Replicates from basins B and C were more distributed along both axes. In the case of basin C, one replicate was separated from all the others while the other four were grouped by pairs. Due to the high number of OTUs, it is difficult to establish any relationship between the OTUs and the basins in the NMDS graph (Figure 10a). When conducting the NMDS analysis by phyla, the replicates of each basin appeared closer to each other, but no clear pattern of replicate grouping by basin was observed (Figure 10b).
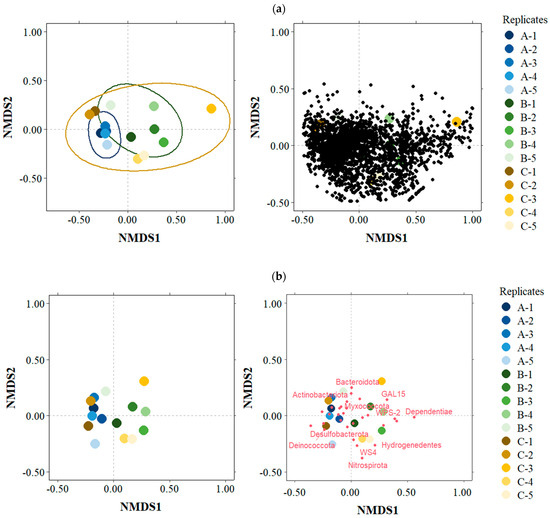
Figure 10.
Bray–Curtis based non-metric multidimensional scaling (NMDS) plot for the total OTUs (a) and phyla (b). A, B and C represent the three basins sampled.
Indicator Species Analysis
There were 39 OTUs associated with the basins: 18 associated with basin A, 13 associated with basin B and 8 associated with basin C. Most of the OTUs associated with basin A belonged to the phylum Chloroflexota (44.4%). Other indicator OTUs with high abundance in this basin were the OTUs SC-1-84 and Rhizorhapis (Pseudomonadota), a representative of the family Chitinophagaceae (Bacteriodota) and a representative of the family Sporomusaceae and Thermobacillus (both Bacillota). In basin B, most of the indicator OTUs belonged to the phylum Planctomycetota (53.8% of the OTUs in this basin), mainly to Aquisphaera sp. and other representatives of the family Isosphaeraceae. Other indicator OTUs in this basin were the genera grouping Craurococcus-Caldovatus and Acidibacter (Pseudomonadota) and Paenibacillus (Bacillota). There was no dominant phylum for the indicator OTUs associated with basin C. The most representative indicator OTUs in this basin were AKIW781 (Chloroflexota), Gemmata (Planctomycetota) and a representative OTU from the phylum Armatimonadota (Figure 11 and Table S2).
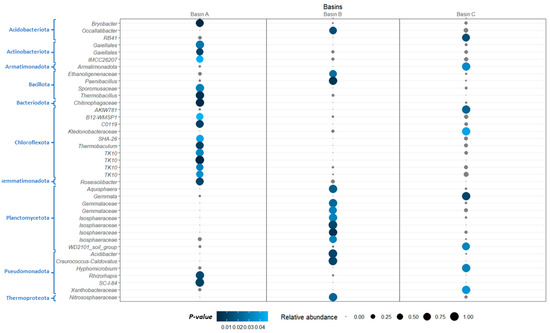
Figure 11.
Abundance and statistical significance of the OTUs associated with each of the three basins (revealed by ISA, Indicator Species Analysis; see M and M for details on the analysis). Size of the circles represents the mean relative abundance of the OTU in that basin, and colour of the circles represents p-values. Circles coloured in grey represent insignificant taxa.
The RDA analysis was performed on the 39 OTUs selected as indicator species for basins A, B or C, using C and N as explanatory variables. The explanatory (environmental) variables explained 24.8% of the variance. The first and second axes explained 25.1% and 10.5% of the model. The variance partitioning analysis showed that C (6.8%) accounted for a little more of the variation than N (5.7%), and shared variance was 12.3%. The permutation test proved the model to be significant (F = 3.307; p-value = 0.002). Both C and N were statistically significant and had a strong effect especially on replicates from basin B and the OTUs Aquisphaera (Planctomycetota), Acidibacter (Pseudomonadota) and Occallatibacter (Acidobacteriota) (Figure 12).
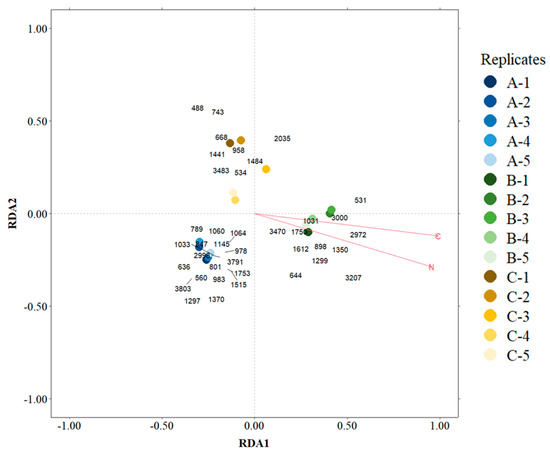
Figure 12.
Redundance analysis (RDA) for the OTUs selected in the indicator species analysis. 488—Xanthobacteraceae; 531—Isosphaeraceae; 534—WD2101_soil_group; 560—Bryobacter; 636—Thermobaculum; 644—Gemmataceae; 668—Gemmata; 743—RB41; 789—SHA-26; 801—IMCC26207; 847—C0119; 898—Isosphaeraceae; 958—AKIW781; 978—SC-I-84; 983—Gaiellales; 1031—Isosphaeraceae; 1033—Chitinophagaceae; 1060—Gaiellales; 1064—TK10; 1145—TK10; 1297—TK10; 1299—Ethanoligenenaceae; 1350—Acidibacter; 1370—Thermobacillus; 1441—Hyphomicrobium; 1484—Ktedonobacteraceae; 1515—Roseisolibacter; 1612—Gemmataceae; 1750—Isosphaeraceae; 1753—Rhizorhapis; 2035—Nitrososphaeraceae; 2972—Occallatibacter; 2998—Sporomusaceae; 3000—Paenibacillus; 3207—Aquisphaera; 3470—Craurococcus-Caldovatus; 3483—Armatimonadota; 3791—B12-WMSP1; 3803—TK10. A, B and C represent the three basins sampled.
4. Discussion
Our results on ombrotrophic mountain granitic basins show that these habitats host bacterial populations with a heterogeneous and largely complex distribution. Although the global partitioning of bacteria abundance into OTUs was rather similar in the basins studied (i.e., similar rarefaction and accumulation curves), large differences in alpha and beta diversity indicators, both among and within basins, were observed. To our knowledge, the present study is the first that focuses on the study of the structure, distribution patterns and metabarcoding identification of bacterial populations in ombrotrophic granitic basins.
Almost half of the total OTUs retrieved were shared among the basins studied, and only 6 to 10% of the OTUs were basin-exclusive OTUs. This suggests the existence of a robust global bacterial metacommunity in the basins. However, when the scale was reduced to replicate size, a similar average of 6% of replicate-exclusive OTUs was also found. This emphasizes the heterogeneity of these habitats and reveals an interesting pattern in the distribution of bacteria which seems to be independent of sample size. It was necessary to analyse almost all replicates collected from each basin to identify the majority of the OTU richness present in the basin, which clearly indicates that a single replicate is not enough to disclose the potential bacterial community these habitats can harbour; it is possible that in some cases a replicate may even constitute a tailored microecosystem for the bacteria. Our study underlines the need to standardise barcoding and metataxonomic studies with inclusive experimental sampling designs involving the collection of several replicates from each basin in order to unfold a representative and stable portion of the microbial community.
4.1. Community Analysis and Bacterial Taxonomy
We found OTUs belonging to 32 bacterial phyla in the rock basins studied. The most diversified but low abundant phylum was Chloroflexota, which are anoxygenic phototrophs (green non-sulphur bacteria). The most abundant phyla were Bacillota and Pseudomonadota. These phyla are known to be cosmopolitan and represent an important component in the soil and in ecosystems considered as hostile to colonization [49], including acidic environments, those with high salt content [50] and those with both high temperatures [51] and low, even at freezing, values [52]. The resistance of these phyla to desiccation, starvation and extreme temperatures is due to their versatile metabolic capacities and notable adaptation strategies, such as the formation of endospores [52,53,54].
One of the general ecological principles that can be tested in temporary discrete habitats such as granitic basins is the species–area relationship [12,15]. Our results on the bacterial community in these basins do not show a correlation between OTU richness (species richness) and size of the basin, as the smallest basin (C) is in fact the richest in OTUs. These results are similar to those observed in eukaryotic microbial communities by previous authors (12, 28) and support the idea that a reservoir of dormant microbial OTUs may allow the coexistence of similar physiological types through a different temporal excystation of the organisms, even in the smaller basins.
There are some previous studies reporting bacterial community composition in intertidal igneous rock basins, although to our knowledge no taxonomic report was included [29], and in semi-arid basins of sedimentary origin, specifically in sandstones [6,30,51]. A higher number of phyla has been retrieved in our ombrotrophic rock basins than in those other studies featuring basins of different composition, climate and environmental conditions. Chan et al. [6] analysed the black coating biofilms lining the bottom of sandstone basins (potholes) and found they contained cyanobacteria and also heterotrophic bacteria. Madsen (2020) [30] found 19 phyla in their study of semi-arid sandstone rock basins in the Colorado Plateau, and most of them belonged to Pseudomonadota. However, Madsen’s rock basins had a high proportion of Chloroflexota and Cyanobacteriota, while the rock basins studied here do not sustain a permanent photosynthetic primary producing bacterial community, as both phyla were found in rather low proportion in our basins. These granitic rock basin sediments are mostly colonised by heterotrophic and chemoautotrophic bacteria. These types of bacteria may predominate in stone sediments as they can occupy deeper, aphotic, niches where phototrophs cannot thrive [51]. Hughes et al. [51] also found that more than 80% of the bacteria in semi-desert Colorado basins were Pseudomonadota, while Bacillota, which were very abundant in our granitic rock pools, accounted for less than 10%. This may be explained by several factors including the clearly different abiotic conditions as well as the different nature of the rocks, sandstone versus granite. Despite not studying rock basins directly, but rather the soil–rock interface at the base of basin-bearing granitic outcrops of a national park, Olsson-Francis et al. (2016) [35] revealed that the bacteria colonizing this habitat were primarily phyla Pseudomonadota and Bacillota too, although in this case the most dominant were Pseudomonadota bacteria.
The species-level study of the bacteria colonising granitic rock basins is of pivotal interest. The adverse and varying conditions often occurring in these ephemeral habitats contribute to rock erosion and leaching, which causes the creation of sediment that accumulates in the basins. In addition to purely chemical erosion, there is also the effect of the bacteria that inhabit the basins, which can effectively contribute to, and even increase, mineral erosion [55]. Bacterial species can accelerate the elemental release of minerals by increasing the dissolution rate of granite, either directly by acquiring limiting nutrients or indirectly by producing metabolic byproducts that reduce the pH or change the saturation state of the mineral [35].
Aerobic heterotrophic bacteria, as retrieved in our study, can increase the erosion of silicates through acidification [35]. Among the granite-degrading bacteria that are effective at mineral weathering are those belonging to the genera Paenibacillus and Sphingomonas [35,55] which are abundant OTUs in our basins. Additionally, bacteria can retain water, which undergoes large changes in volume due to drying and rehydration of the basins, magnifying the effects of weathering due to freeze–thaw cycles [6,38].
In our study, 31 bacterial OTUs with more than 10,000 reads were recovered in the basins (Table S1). Some of these OTUs are important in a variety of applied fields. Microvirga, which had the highest relative abundance by far in the ombrotrophic rock basins studied here, is a metabolically versatile and widely distributed genus. Microvirga spp. are reportedly beneficial microbes in the soil–plant ecosystem, i.e., by improving soil nutrients, promoting plant growth and controlling soil-borne diseases [56]. However, our knowledge is still limited on the ecological traits of Microvirga species. Brevibacillus is one of the most widespread genera of Gram-positive bacteria and is widespread in diverse environmental habitats. It is recognised as a source of various enzymes of great biotechnological interest due to their ability to bioremediate compounds and to eliminate toxic heavy metals from soils, water and the atmosphere [57,58]. Azospirillum, a free-living nitrogen-fixing bacterium, was also found with high abundance in the rock basins [59]. Another abundant OTU, Solirubrobacter, a genus within the phylum Actinobacteriota, is commonly found in soils and rhizospheres yet remains rather unexplored as revealed through metagenomic studies [60]. Conexibacter members present a rich pool of hydrolytic enzymes, and their isolates may be of great interest for industry [61]. Aneurinibacillus species were also found in the rock basins in high abundance. Strains of this genus continue to be discovered and are known to possess plant growth-promoting and environmentally beneficial traits. There is some interest in exploring Aneurinibacillus as bioinoculants for sustainable crop production in the future [62]. Sphingomonads, an important bacterial group with metabolic versatility, are ubiquitous in polluted environments and show significant potential in biodegradation and bioremediation [63]. The genus Phenylobacterium was also abundant in the rock basins studied. It comprises a single species, P. immobile, which possesses extremely restrictive nutritional requirements [64]. As for the genus Lysobacter, several studies show that bacteria belonging to this genus may be used for the bioremediation of contaminated soils [65]. Anoxybacillus, a speciose genus of thermophilic and alkaliphilic bacteria, is also an abundant representative of these granitic rock basins. Many diverse thermostable enzymes with biotechnological application have been identified in this genus, and promising results have been obtained in bioremediation. Moreover, some recently discovered metabolites in Anoxybacillus species are shown to have potential in biomedicine [66].
4.2. Indicator Species
Indicator species analysis revealed 39 OTUs whose occurrence and abundance were associated to one of the three basins. This analysis showed a clear contrasting pattern in the bacterial metabolism in the basins. Basin A was dominated by anoxygenic phototroph (Chloroflexota) indicator OTUs, while basin B was dominated by chemotroph (Planctomycetota) indicator OTUs. In basin C there was not a clear pattern to dominant phyla or metabolisms. Some indicator OTUs (e.g., OTU TK 10 or OTU SC-1-84) appear in the databases only as non-published bacteria, while some others have been recently described and only one or few species are reported. For many, ecological and physiological information is lacking.
As the most abundant indicator OTUs in basin A, it is worth noticing the genus Rhizorhapis (Pseudomonadota), rhizosphere bacteria including strains that cause corky root of lettuce and also non-pathogenic strains used in biocontrol of plant diseases [67]; Chitinophagaceae (Bacteriodota), bacteria that can degrade chitin [68]; and Thermobacillus (Bacillota), a thermophilic genus that can grow at temperatures up to 63 °C [69]. In basin B, the most abundant indicator OTUs were Aquisphaera sp. and other representatives of the family Isosphaeraceae (Planctomycetota). Aquisphaera is a chemoheterotrophic, strictly aerobic genus of bacteria with only two known species (A. giovannonii, [70], and A. insulae, [71]) isolated, respectively, from sediments in a freshwater aquarium at Portugal and an Indian freshwater lake. Other indicator OTUs in basin B are the genera grouping Craurococcus-Caldovatus (Pseudomonadota), which has also been reported in previous metagenomic studies [72]; Acidibacter (Pseudomonadota), an acid-loving genus with only one described species [73]; and Paenibacillus (Bacillota), a genus that contains many plant growth-promoting bacteria [74] and, as mentioned previously, is effective in granite weathering. In basin 3, the most abundant indicators were the OTU AKIW781 (Chloroflexota), an uncultured bacterial clone previously found in biodeteriorated sandstone sculptures [75]; an OTU from the Armatimonadota, a phylum spread in many different types of habitats from hot springs to freshwater or marine sediments [76]; and the genus Gemmata (Planctomycetota). This latter genus is mainly found in aquatic and soil environments but also in hospital water networks, therefore attention should be paid to opportunistic pathogens within this genus [77].
RDA analysis related some OTUs with high content of C and N in the basins: the above-mentioned Aquisphaera (Planctomycetota) and Acidibacter (Pseudomonadota) and also Occallatibacter (Acidobacteriota). The genus Occallatibacter currently comprises the two species O. riparius and O. savannae that are strictly aerobic, moderately acidophilic and chemoorganotrophic mesophiles [78]. Interestingly, this genus has been previously reported to have a strong positive relationship with nitrate reductase-encoding (nir) genes, used as markers to detect the denitrification potential in soil [79]. The association we found in the present study between N and Occallatibacter may mean that most of the N content in basin B is in the form of nitrate.
4.3. Bacterial Turnover and Drivers of Beta Diversity
It has been suggested that a differential spatial distribution and rate of replacement (beta-diversity) of bacterial communities is determined by the combination of mainly environmental filtering but also dispersal-related mechanisms [29,80]. In our study, the environmental conditions analysed (CHN) were statistically different among basins; basin B was the most different. Basin B is a distinctive basin, which contains a representative portion of vegetation and whose sediment has become of a granular structure and consistence comparable to a soil sample. The presence of plants and shrubs in this basin is probably the cause of an elevated productivity and therefore of its higher C and N content. Within each basin, environmental indicators were also different (i.e., significant differences in the CHN values among replicates of the same basin, excepting the N content in basin A replicates). The differences in the bacterial communities observed among basins in this study should, in theory, be mainly attributed to environmental filtering because random dispersal should not act among basins. However, we propose that random dispersal does indeed contribute to the distribution of bacteria in replicates from the same basin, lessening the effect of environmental filtering, as the sediments and therefore their populations can be mixed together in many different ways: when precipitation causes basin overflow and sediment is washed out of the bedrock, during freeze–melt cycles, through invertebrates colonizing the basin (“hitch-hiking” the invertebrates), via plant roots penetrating the soils and/or at periods of high pore-water connectivity [80]. For two basins (A and B), more similarities within replicates of the same basin were observed than to the replicates of other basins. This might indicate that random dispersal (that is, absence of dispersal limitation) can also drive the distribution of the bacteria, making the replicates more similar within basins than they are among basins. It seems that random dispersal contributes towards balancing the differences among the replicates of each basin. Basin C, however, seems to display a more complex and heterogeneous structure despite being the smallest in area and volume. Three of its replicates showed large differences with the other replicates of their same basin. These differences were equal to those shown with replicates of the other two basins. This means that random dispersal may not be acting with the same force as it is acting in the other basins. This result may be explained by the fact that this basin is an almost perfect sphere, rather deep, probably the most static and difficult to access basin, with harder to mix sediment than in the other basins. Therefore, dispersal might not be rapid enough to allow homogenization of the rock basin bacterial communities into a single metacommunity but instead be sufficiently restricted to allow a fine-scale spatial structure to become noticeable [29]. It should be pointed out that there will be other environmental factors and biotic conditioning factors (temperature, pH, frequency of water in the basins, chemical rock composition, morphology and size of the basins, anthropogenic influence, presence of animal faeces, etc.) that we have not addressed in this study and that may affect the structure of the bacterial community, being additional drivers of the community assemblages.
Granitic basins are habitats subject to very variable, often extreme conditions, i.e., occasional exit and entry of water, seasonal cycles of freezing and thawing, drought, effect of wind, in addition to the influences of the flora and fauna that live inside or near the basins [2,12]. These events create habitats with highly variable physical–chemical and biotic conditions, even at the scale of a few centimetres of distance, conditions that will affect the bacterial populations that establish in these habitats. Altogether, our results suggest that each ombrotrophic granitic basin is a distinctive conjunction of habitats with a high diversity of both aerobic and anaerobic chemoautotrophic and heterotrophic bacteria which manifest a wide range of metabolic strategies, many of which could be the source of yet unexplored and novel ecological and biotechnological applications.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d16090544/s1, Figure S1: Normalized rarefaction curves of the number of OTUs for each basin; Figure S2: Mean values of the chemical variables in each replicate. The p-values represent differences between replicates of the same basin and were computed using one-way ANOVA; Table S1: Abundance per basin and richness of OTUs by phyla; Table S2: Indicator species analysis of OTUs between basins.
Author Contributions
Conceptualisation, M.M.-C., A.S.-J. and A.d.C.-G.; Methodology, A.d.C.-G., A.S.-J., B.P.-U. and M.M.-C.; Formal Analysis, A.d.C.-G., M.M.-C. and A.S.-J.; Investigation: M.M.-C., A.d.C.-G. and A.S.-J.; Resources: M.M.-C., A.S.-B., R.A.J.W. and M.M.-D.; Data Curation: A.d.C.-G., A.S.-B. and M.M.-D.; Writing—Original Draft Preparation: A.d.C.-G. and M.M.-C.; Writing—Review and Editing: R.A.J.W., B.P.-U. and A.S.-J.; Supervision: M.M.-C. and A.S.-J.; Project Administration: M.M.-C. and B.P.-U.; Funding Acquisition: M.M.-C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Project Santander-UCM Ref: PR44/21-29928 to PI M.M.-C. A.S.-B., A.C.-G., B.P.-U. and M.M.-C. are funded by the research group “971006 - MADMIB - Modelización, Análisis de Datos y Métodos Informáticos en Biología”. A.S.-B. was funded by the “Programa Investigo-MITES 2023-2024”.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
V4-rDNA sequences will be made available upon acceptance of the manuscript. Other data are available from the authors upon reasonable request.
Acknowledgments
Permits to collect samples and facilities provided by The Parque Nacional Sierra de Guadarrama are gratefully acknowledged. The authors are grateful to Unidad de Microanálisis Elemental UCM for their contribution to chemical analyses.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Twidale, C.R. Granite Landforms; Elsevier Scientific Publishing Company: Amsterdam, The Netherlands, 1982; p. 359. ISBN 0444597646. [Google Scholar]
- Jocque, M.; Vanschoenwinkel, B.; Brendonck, L. Freshwater Rock Pools: A Review of Habitat Characteristics, Faunal Diversity and Conservation Value. Freshw. Biol. 2010, 55, 1587–1602. [Google Scholar] [CrossRef]
- Sel, A.; Binal, A. Does Bacterial Weathering Play a Significant Role in Rock Weathering? Environ. Earth Sci. 2021, 80, 778. [Google Scholar] [CrossRef]
- Wang, Q.; Cheng, C.; He, L.; Huang, Z.; Sheng, X. Characterization of Depth-Related Changes in Bacterial Communities Involved in Mineral Weathering along a Mineral-Rich Soil Profile. Geomicrobiol. J. 2014, 31, 431–444. [Google Scholar] [CrossRef]
- Baonza Díaz, J. Vegetación de Las Pilas o Pilancones de La Sierra de Guadarrama y La Serena (España). An. Jard. Bot. Madrid 2009, 66, 109–129. [Google Scholar] [CrossRef]
- Chan, M.A.; Moser, K.; Davis, J.M.; Southam, G.; Hughes, K.; Graham, T. Desert Potholes: Ephemeral Aquatic Microsystems. Aquat. Geochem. 2005, 11, 279–302. [Google Scholar] [CrossRef]
- Timms, B.V. Community Ecology of Aquatic Invertebrates in Gnammas (Rock-Holes) of North-Western Eyre Peninsula, South Australia. Trans. R. Soc. South Aust. 2014, 138, 147–160. [Google Scholar] [CrossRef]
- Troell, S.; Jönsson, K.I. Occurrence of Tardigrades and Morphometric and Chemical Conditions in Rock Pools by the Baltic Sea. Sci. Rep. 2023, 13, 19776. [Google Scholar] [CrossRef]
- Williams, W.D. Biotic Adaptations in Temporary Lentic Waters, with Special Reference to Those in Semi-Arid and Arid Regions. Hydrobiologia 1985, 125, 85–110. [Google Scholar] [CrossRef]
- Jocque, M.; Martens, K.; Riddoch, B.; Brendonck, L. Faunistics of Ephemeral Rock Pools in Southeastern Botswana. Arch. Hydrobiol. 2006, 165, 415–431. [Google Scholar] [CrossRef]
- Heilmeier, H.; Durka, W.; Woitke, M.; Hartung, W. Ephemeral Pools as Stressful and Isolated Habitats for the Endemic Aquatic Resurrection Plant Chamaegigas Intrepidus. Phytocoenologia 2005, 35, 449–468. [Google Scholar] [CrossRef]
- Brendonck, L.; Jocque, M.; Hulsmans, A.; Vanschoenwinkel, B. Pools “on the Rocks”: Freshwater Rock Pools as Model System in Ecological and Evolutionary Research. Limnetica 2010, 29, 25–40. [Google Scholar] [CrossRef]
- Srivastava, D.S.; Kolasa, J.; Bengtsson, J.; Gonzalez, A.; Lawler, S.P.; Miller, T.E.; Munguia, P.; Romanuk, T.; Schneider, D.C.; Trzcinski, M.K. Are Natural Microcosms Useful Model Systems for Ecology? Trends Ecol. Evol. 2004, 19, 379–384. [Google Scholar] [CrossRef] [PubMed]
- De Meester, L.; Declerck, S.; Stoks, R.; Louette, G.; Van De Meutter, F.; De Bie, T.; Michels, E.; Brendonck, L. Ponds and Pools as Model Systems in Conservation Biology, Ecology and Evolutionary Biology. Aquat. Conserv. Mar. Freshw. Ecosyst. 2005, 15, 715–725. [Google Scholar] [CrossRef]
- Soininen, J.; Meier, S. Phytoplankton Richness Is Related to Nutrient Availability, Not to Pool Size, in a Subarctic Rock Pool System. Hydrobiologia 2014, 740, 137–145. [Google Scholar] [CrossRef]
- Usman Gabi, A.; Matias Peralta, H.M. Plankton Diversity, Physico-Chemical Parameters and Conservation Value of Temporary Freshwater Rock Pools. Int. J. Res. Rev. 2015, 2, 562–573. [Google Scholar]
- Pajunen, V.I.; Pajunen, I. Habitat Characteristics Contributing to Local Occupancy and Habitat Use in Rock Pool Daphnia Metapopulations. Hydrobiologia 2007, 592, 291–302. [Google Scholar] [CrossRef]
- Rosenfeld, S.; Blaustein, L.; Kneitel, J.; Duchet, C.; Horwitz, R.; Rybak, O.; Polevikov, A.; Rahav, E. The Abundance and Larval Performance of Aedes phoeniciae in Supralittoral Rock-Pools. Hydrobiologia 2019, 846, 181–192. [Google Scholar] [CrossRef]
- Brendonck, L.; Lanfranco, S.; Timms, B.; Vanschoenwinkel, B. Invertebrates in Rock Pools BT—Invertebrates in Freshwater Wetlands: An International Perspective on Their Ecology; Batzer, D., Boix, D., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 25–53. ISBN 978-3-319-24978-0. [Google Scholar]
- Timms, B.V. A Study on the Pools of a Granitic Mountain Top at Moonbi, New South Wales. Proc. Linn. Soc. New South Wales 2016, 138, 61–68. [Google Scholar]
- Cross, A.T.; Turner, S.R.; Merritt, D.J.; Van Niekerk, A.; Renton, M.; Dixon, K.W.; Mucina, L. Vegetation Patterns and Hydro-Geological Drivers of Freshwater Rock Pool Communities in the Monsoon-Tropical Kimberley Region, Western Australia. J. Veg. Sci. 2015, 26, 1184–1197. [Google Scholar] [CrossRef]
- Anusa, A.; Ndagurwa, H.G.T.; Magadza, C.H.D. The Influence of Pool Size on Species Diversity and Water Chemistry in Temporary Rock Pools on Domboshawa Mountain, Northern Zimbabwe. African J. Aquat. Sci. 2012, 37, 89–99. [Google Scholar] [CrossRef]
- Meier, S.; Soininen, J. Phytoplankton Metacommunity Structure in Subarctic Rock Pools. Aquat. Microb. Ecol. 2014, 73, 81–91. [Google Scholar] [CrossRef]
- Bengtsson, J.; Ebert, D. Distributions and Impacts of Microparasites on Daphnia in a Rockpool Metapopulation. Oecologia 1998, 115, 213–221. [Google Scholar] [CrossRef]
- Allison, S.D. Microbial Drought Resistance May Destabilize Soil Carbon. Trends Microbiol. 2023, 31, 780–787. [Google Scholar] [CrossRef]
- Pérez-Uz, B.; Velasco-González, I.; Murciano, A.; Sanchez-Jimenez, A.; García-Rodríguez, M.; Centeno, J.D.; Montero, E.; Muñoz, B.; Olmedo, C.; Quintela-Alonso, P.; et al. Rain-Fed Granite Rock Pools in a National Park: Extreme Niches for Protists. Limnetica 2021, 40, 1–18. [Google Scholar] [CrossRef]
- Velasco-González, I.; Lara, E.; Singer, D.; de Cos-Gandoy, A.; García-Rodríguez, M.; Murciano, A.; Pérez-Uz, B.; Williams, R.; Sanchez-Jimenez, A.; Martín-Cereceda, M. Diversity of DNA Sequences from Pathogenic and Potentially Pathogenic Eukaryotic Microorganisms in Protected Granite Mountain Rocks. Diversity 2023, 15, 594. [Google Scholar] [CrossRef]
- Velasco-González, I.; Sanchez-Jimenez, A.; Singer, D.; Murciano, A.; Díez-Hermano, S.; Lara, E.; Martín-Cereceda, M. Rain-Fed Granite Rock Basins Accumulate a High Diversity of Dormant Microbial Eukaryotes. Microb. Ecol. 2020, 79, 882–897. [Google Scholar] [CrossRef] [PubMed]
- Langenheder, S.; Ragnarsson, H. The Role of Environmental and Spatial Factors for the Composition of Aquatic Bacterial Communities. Ecology 2007, 88, 2154–2161. [Google Scholar] [CrossRef]
- Madsen, M. Patterns of Microbial Diversity and Community Composition in Slot Canyons, Rock Pools, and Other Ephemeral and Perennial Aquatic Habitats. Ph.D. Thesis, Utah State University, Logan, UT, USA, 2020. [Google Scholar]
- Horner-Devine, M.C.; Carney, K.M.; Bohannan, B.J.M. An Ecological Perspective on Bacterial Biodiversity. Proc. R. Soc. B Biol. Sci. 2004, 271, 113–122. [Google Scholar] [CrossRef]
- Hayer, M.; Wymore, A.S.; Hungate, B.A.; Schwartz, E.; Koch, B.J.; Marks, J.C. Microbes on Decomposing Litter in Streams: Entering on the Leaf or Colonizing in the Water? ISME J. 2022, 16, 717–725. [Google Scholar] [CrossRef]
- Hayatsu, M.; Katsuyama, C.; Tago, K. Overview of Recent Researches on Nitrifying Microorganisms in Soil. Soil Sci. Plant Nutr. 2021, 67, 619–632. [Google Scholar] [CrossRef]
- Song, W.; Ogawa, N.; Oguchi, C.T.; Hatta, T.; Matsukura, Y. Effect of Bacillus subtilis on Granite Weathering: A Laboratory Experiment. Catena 2007, 70, 275–281. [Google Scholar] [CrossRef]
- Olsson-Francis, K.; Pearson, V.K.; Schofield, P.F.; Oliver, A.; Summers, S. A Study of the Microbial Community at the Interface between Granite Bedrock and Soil Using a Culture-Independent and Culture-Dependent Approach. Adv. Microbiol. 2016, 06, 233–245. [Google Scholar] [CrossRef]
- Kumar, A.; Ng, D.H.P.; Wu, Y.; Cao, B. Microbial Community Composition and Putative Biogeochemical Functions in the Sediment and Water of Tropical Granite Quarry Lakes. Microb. Ecol. 2019, 77, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Ogawa, N.; Oguchi, C.T.; Hatta, T.; Matsukura, Y. Expérimentation En Laboratoire de La Météorisation Du Granite et de Ses Minéraux Constituants. Geomorphol. Reli. Process. Environ. 2010, 327–336. [Google Scholar] [CrossRef]
- Ortega-Calvo, J.J.; Ariño, X.; Hernandez-Marine, M.; Saiz-Jimenez, C. Factors Affecting the Weathering and Colonization of Monuments by Phototrophic Microorganisms. Sci. Total Environ. 1995, 167, 329–341. [Google Scholar] [CrossRef]
- Bennett, P.C.; Rogers, J.R.; Choi, W.J.; Hiebert, F.K. Silicates, Silicate Weathering, and Microbial Ecology. Geomicrobiol. J. 2001, 18, 3–19. [Google Scholar] [CrossRef]
- Székely, A.J.; Langenheder, S. The Importance of Species Sorting Differs between Habitat Generalists and Specialists in Bacterial Communities. FEMS Microbiol. Ecol. 2014, 87, 102–112. [Google Scholar] [CrossRef]
- Domínguez Villar, D. Análisis Morfométrico de Pilancones: Consideraciones Genéticas, Evolutivas y Paleoambientales; UCM: Madrid, Spain, 2007. [Google Scholar]
- Edgar, R.C. UPARSE: Highly Accurate OTU Sequences from Microbial Amplicon Reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Chen, H.; Boutros, P.C. VennDiagram: A Package for the Generation of Highly-Customizable Venn and Euler Diagrams in R. BMC Bioinform. 2011, 12, 35. [Google Scholar] [CrossRef]
- Oksanen, J.; Simpson, G.; Blanchet, F.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Solymos, P.; Stevens, M.H.; Szoecs, E.; et al. Vegan: Community Ecology Package; R Package Version 2.6-4; R Foundation of the Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Hsieh, T.C.; Ma, K.H.; Chao, A. NEXT: INterpolation and EXTrapolation for Species Diversity; R Package Version 3.0.1; R Foundation of the Statistical Computing: Vienna, Austria, 2024. [Google Scholar]
- De Cáceres, M.; Legendre, P. Associations between Species and Groups of Sites: Indices and Statistical Inference. Ecology 2009, 90, 3566–3574. [Google Scholar] [CrossRef]
- R Core. Team R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024. [Google Scholar]
- RStudio Team. RStudio: Integrated Development for R; RStudio, PBC: Boston, MA, USA, 2024; Available online: http://www.rstudio.com/ (accessed on 7 August 2024).
- Filippidou, S.; Price, A.; Spencer-Jones, C.; Scales, A.; Macey, M.C.; Franchi, F.; Lebogang, L.; Cavalazzi, B.; Schwenzer, S.P.; Olsson-Francis, K. Diversity of Microbial Mats in the Makgadikgadi Salt Pans, Botswana. Microorganisms 2024, 12, 147. [Google Scholar] [CrossRef] [PubMed]
- Santini, T.C.; Gramenz, L.; Southam, G.; Zammit, C. Microbial Community Structure Is Most Strongly Associated with Geographical Distance and PH in Salt Lake Sediments. Front. Microbiol. 2022, 13, 56. [Google Scholar] [CrossRef] [PubMed]
- Hughes, K.A. Bacterial Communities and Their Influence on the Formation and Development of Potholes in Sandstone Surfaces of the Semi-Arid Colorado Plateau. Electron. Thesis Diss. Repos. 2012, 543, 215. Available online: https://ir.lib.uwo.ca/etd/543 (accessed on 7 August 2024).
- Savaglia, V.; Lambrechts, S.; Tytgat, B.; Vanhellemont, Q.; Elster, J.; Willems, A.; Wilmotte, A.; Verleyen, E.; Vyverman, W. Geology Defines Microbiome Structure and Composition in Nunataks and Valleys of the Sør Rondane Mountains, East Antarctica. Front. Microbiol. 2024, 15, 6633. [Google Scholar] [CrossRef]
- Soina, V.S.; Mulyukin, A.L.; Demkina, E.V.; Vorobyova, E.A.; El-Registan, G.I. The Structure of Resting Bacterial Populations in Soil and Subsoil Permafrost. Astrobiology 2004, 4, 345–358. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-H.; Gao, L.; Jiang, H.-C.; Fang, B.-Z.; Huang, Y.; Li, L.; Li, S.; Abdugheni, R.; Lian, W.-H.; Zhang, J.-Y.; et al. Response of Microbial Diversity and Function to the Degradation of Barkol Saline Lake. Front. Microbiol. 2024, 15, 1358222. [Google Scholar] [CrossRef]
- Uroz, S.; Picard, L.; Turpault, M.-P. Recent Progress in Understanding the Ecology and Molecular Genetics of Soil Mineral Weathering Bacteria. Trends Microbiol. 2022, 30, 882–897. [Google Scholar] [CrossRef]
- Yang, J.; Wang, Y.; Cui, X.; Xue, K.; Zhang, Y.; Yu, Z. Habitat Filtering Shapes the Differential Structure of Microbial Communities in the Xilingol Grassland. Sci. Rep. 2019, 9, 19326. [Google Scholar] [CrossRef]
- Panda, A.K.; Bisht, S.S.; DeMondal, S.; Senthil Kumar, N.; Gurusubramanian, G.; Panigrahi, A.K. Brevibacillus as a Biological Tool: A Short Review. Antonie Van Leeuwenhoek 2014, 105, 623–639. [Google Scholar] [CrossRef]
- Bigurra-Quintero, U.; Mendoza-Villarreal, R.; Robledo-Torres, V.; Paredes-Jácome, J.R. Cinética de Crecimiento in Vitro de Brevibacillus Brevisen Diferentes Medios de Cultivo Kinetics of in Vitro Growth of Brevibacillus Brevisin Different Culture Media. Ecosistemas Recur. Agropecu. 2020, 7, 2310. [Google Scholar] [CrossRef]
- Steenhoudt, O.; Vanderleyden, J. Azospirillum, a Free-Living Nitrogen-Fixing Bacterium Closely Associated with Grasses: Genetic, Biochemical and Ecological Aspects. FEMS Microbiol. Rev. 2000, 24, 487–506. [Google Scholar] [CrossRef] [PubMed]
- Jara-Servin, A.; Mejia, G.; Romero, M.F.; Peimbert, M.; Alcaraz, L.D. Unraveling the Genomic and Environmental Diversity of the Ubiquitous Solirubrobacter. bioRxiv 2023, 44. [Google Scholar] [CrossRef]
- Miralles, I.; Ortega, R.; del Carmen Montero-Calasanz, M. Functional and Biotechnological Potential of Microbiome Associated with Soils Colonised by Cyanobacteria in Drylands. Appl. Soil Ecol. 2023, 192, 105076. [Google Scholar] [CrossRef]
- Kaur, C.; Selvakumar, G.; Ganeshamurthy, A.N. Exploring the Utility of Aneurinibacillus as a Bioinoculant for Sustainable Crop Production and Environmental Applications BT—Bacilli and Agrobiotechnology: Phytostimulation and Biocontrol; Islam, M.T., Rahman, M.M., Pandey, P., Boehme, M.H., Haesaert, G., Eds.; Springer International Publishing: Cham, Switzerland, 2019; Volume 2, pp. 135–141. ISBN 978-3-030-15175-1. [Google Scholar]
- Song, D.; Chen, X.; Xu, M. Characteristics and Functional Analysis of the Secondary Chromosome and Plasmids in Sphingomonad. Int. Biodeterior. Biodegrad. 2022, 171, 105402. [Google Scholar] [CrossRef]
- Eberspächer, J.; Lingens, F. The Genus Phenylobacterium BT—The Prokaryotes: Proteobacteria: Alpha and Beta Subclasses; Dworkin, M., Falkow, S., Rosenberg, E., Schleifer, K.-H., Stackebrandt, E., Eds.; Springer: New York, NY, USA, 2006; Volume 5, pp. 250–256. ISBN 978-0-387-30745-9. [Google Scholar]
- Brescia, F.; Pertot, I.; Puopolo, G. Beneficial Microbes in Agro-Ecology: Bacteria and Fungi; Amaresan, N., Kumar, M.S., Annapurna, K., Kumar, K., Sankaranarayanan, A., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 313–338. ISBN 0128235586. [Google Scholar]
- da Rosa, D.F.; Macedo, A.J. The Genus Anoxybacillus: An Emerging and Versatile Source of Valuable Biotechnological Products. Extremophiles 2023, 27, 22. [Google Scholar] [CrossRef]
- van Bruggen, A.H.C.; Francis, I.M.; Jochimsen, K.N. Non-Pathogenic Rhizosphere Bacteria Belonging to the Genera Rhizorhapis and Sphingobium Provide Specific Control of Lettuce Corky Root Disease Caused by Species of the Same Bacterial Genera. Plant Pathol. 2014, 63, 1384–1394. [Google Scholar] [CrossRef]
- Rosenberg, E. The Family Chitinophagaceae BT—The Prokaryotes: Other Major Lineages of Bacteria and The Archaea; Rosenberg, E., DeLong, E.F., Lory, S., Stackebrandt, E., Thompson, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 493–495. ISBN 978-3-642-38954-2. [Google Scholar]
- Touzel, J.P.; O’Donohue, M.; Debeire, P.; Samain, E.; Breton, C. Thermobacillus Xylanilyticus Gen. Nov., Sp. Nov., a New Aerobic Thermophilic Xylan-Degrading Bacterium Isolated from Farm Soil. Int. J. Syst. Evol. Microbiol. 2000, 50 Pt 1, 315–320. [Google Scholar] [CrossRef]
- Bondoso, J.; Albuquerque, L.; Nobre, M.F.; Lobo-da-Cunha, A.; da Costa, M.S.; Lage, O.M. Aquisphaera Giovannonii Gen. Nov., Sp. Nov., a Planctomycete Isolated from a Freshwater Aquarium. Int. J. Syst. Evol. Microbiol. 2011, 61, 2844–2850. [Google Scholar] [CrossRef]
- Kumar, G.; Lhingjakim, K.L.; Uppada, J.; Ahamad, S.; Kumar, D.; Kashif, G.M.; Sasikala, C.; Ramana, C.V. Aquisphaera insulae sp. nov., a New Member in the Family Isosphaeraceae, Isolated from the Floating Island of Loktak Lake and Emended Description of the Genus Aquisphaera. Antonie Van Leeuwenhoek 2021, 114, 1465–1477. [Google Scholar] [CrossRef]
- Moura, J.B.; Delforno, T.P.; Do Prado, P.F.; Duarte, I.C. Extremophilic Taxa Predominate in a Microbial Community of Photovoltaic Panels in a Tropical Region. FEMS Microbiol. Lett. 2021, 368, fnab105. [Google Scholar] [CrossRef]
- Falagán, C.; Johnson, D.B. Acidibacter ferrireducens Gen. Nov., Sp. Nov.: An Acidophilic Ferric Iron-Reducing Gammaproteobacterium. Extremophiles 2014, 18, 1067–1073. [Google Scholar] [CrossRef]
- Grady, E.N.; MacDonald, J.; Liu, L.; Richman, A.; Yuan, Z.-C. Current Knowledge and Perspectives of Paenibacillus: A Review. Microb. Cell Fact. 2016, 15, 203. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Zhang, Y.; He, D.; Gu, J.D.; Guo, Q.; Liu, X.; Duan, Y.; Zhao, J.; Wang, W.; Feng, H. Community Structures of Bacteria and Archaea Associated with the Biodeterioration of Sandstone Sculptures at the Beishiku Temple. Int. Biodeterior. Biodegrad. 2021, 164, 105290. [Google Scholar] [CrossRef]
- Carlton, J.D.; Langwig, M.V.; Gong, X.; Aguilar-Pine, E.J.; Vázquez-Rosas-Landa, M.; Seitz, K.W.; Baker, B.J.; De Anda, V. Expansion of Armatimonadota through Marine Sediment Sequencing Describes Two Classes with Unique Ecological Roles. ISME Commun. 2023, 3, 64. [Google Scholar] [CrossRef] [PubMed]
- Aghnatios, R.; Drancourt, M. Gemmata Species: Planctomycetes of Medical Interest. Future Microbiol. 2016, 11, 659–667. [Google Scholar] [CrossRef]
- Foesel, B.U.; Mayer, S.; Luckner, M.; Wanner, G.; Rohde, M.; Overmann, J. Occallatibacter riparius gen. nov., sp. nov. and Occallatibacter savannae sp. nov., Acidobacteria Isolated from Namibian Soils, and Emended Description of the Family Acidobacteriaceae. Int. J. Syst. Evol. Microbiol. 2016, 66, 219–229. [Google Scholar] [CrossRef]
- Truu, M.; Nõlvak, H.; Ostonen, I.; Oopkaup, K.; Maddison, M.; Ligi, T.; Espenberg, M.; Uri, V.; Mander, Ü.; Truu, J. Soil Bacterial and Archaeal Communities and Their Potential to Perform N-Cycling Processes in Soils of Boreal Forests Growing on Well-Drained Peat. Front. Microbiol. 2020, 11, 591358. [Google Scholar] [CrossRef]
- Lindström, E.S.; Langenheder, S. Local and Regional Factors Influencing Bacterial Community Assembly. Environ. Microbiol. Rep. 2012, 4, 1–9. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).