Phytoseiid Mites: Trees, Ecology and Conservation
Abstract
1. Introduction
1.1. Mites—Diversity and Ecology
1.2. Plants as Mite Habitat
1.3. Phytoseiid Mites
1.4. The Present Study
2. Materials and Methods
2.1. Data Sources for the Study
2.2. Location, Biogeography and Climate of Samsun Province, Türkiye
2.3. Host Tree Species Investigated for Phytoseiids
2.4. Identification of Phytoseiid Species
2.5. Diversity Assessments
2.5.1. Shannon Diversity Index
2.5.2. Jaccard Similarity Index
3. Results
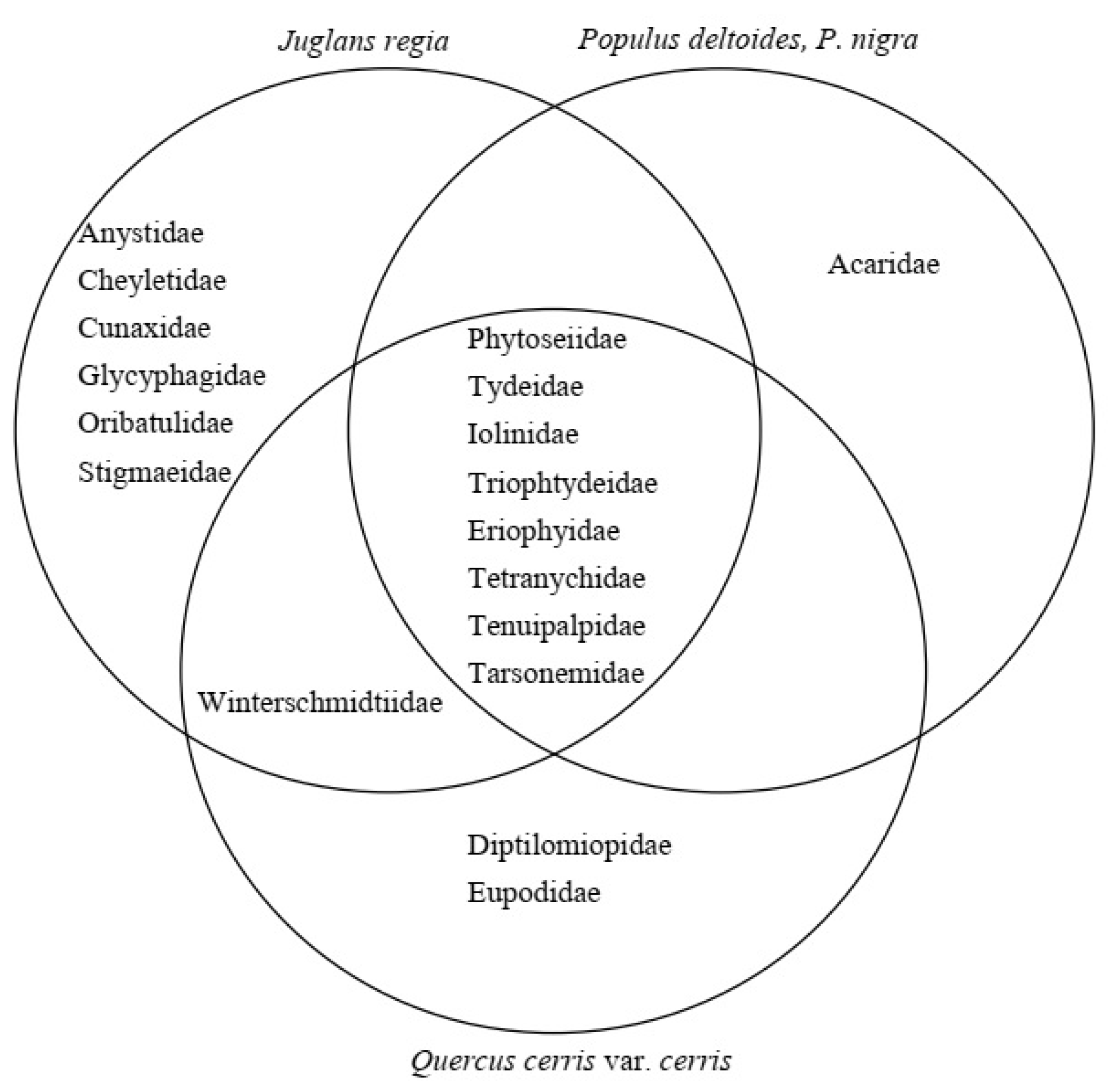
3.1. Mite Families
3.2. Phytoseiid Genera and Species
3.3. Diversity Assessments
3.3.1. Shannon Diversity Index Comparisons
3.3.2. Jaccard Similarity Index Comparisons
4. Discussion
4.1. Mite Habitat and Microhabitat on the Foliage of Trees
4.2. Comparison of Diversities of Mite Families, and Phytoseiid Genera and Species, Collected from Walnut, Poplar and Oak Trees
4.3. Factors Determining the Phytoseiid Species and Their Densities on Walnut, Poplar and Oak Trees
4.4. Lifestyles of the Phytoseiids Reported in This Study
4.5. Phytoseiid Mites on Walnut, Poplar and Oak Trees across Turkish and International Studies
4.5.1. Walnut Trees
4.5.2. Poplar Trees
4.5.3. Oak Trees
4.6. Sampling
4.6.1. Sampling Effort
4.6.2. Sampling Efficiency
5. Concluding Remarks
5.1. An Overview of This Study
5.2. Additional Considerations
5.2.1. Loss of Taxonomic Expertise
5.2.2. Conservation Status of Mite Species, Including Phytoseiids
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wilson, E.O. The biological diversity crisis. BioScience 1985, 35, 700–706. [Google Scholar] [CrossRef]
- Wilson, E.O. The Diversity of Life; Harvard University Press: Cambridge, MA, USA, 2010. [Google Scholar]
- Krantz, G.W. Introduction. In A Manual of Acarology; Krantz, G.W., Walter, D.E., Eds.; Texas Tech University Press: Lubbock, TX, USA, 2009; pp. 1–2. [Google Scholar]
- Walter, D.E.; Proctor, H.C. Mites: Ecology, Evolution and Behaviour: Life at a Microscale, 2nd ed.; Springer: Dordrecht, The Netherlands, 2013. [Google Scholar] [CrossRef]
- Stork, N.E. How many species of insects and other terrestrial arthropods are there on earth? Annu. Rev. Entomol. 2018, 63, 31–45. [Google Scholar] [CrossRef] [PubMed]
- Seeman, O. Mites on insects; the other, other 99%. Entomol. Soc. Qld. News Bull. 2020, 48, 56–65. [Google Scholar]
- Sullivan, G.T.; Ozman-Sullivan, S.K. Alarming evidence of widespread mite extinctions in the shadows of plant, insect and vertebrate extinctions. Austral Ecol. 2021, 46, 163–176. [Google Scholar] [CrossRef]
- Stanton, N.L. Patterns of species diversity in temperate and tropical litter mites. Ecology 1979, 60, 295–304. [Google Scholar] [CrossRef]
- Walter, D.E.; Proctor, H.C. Predatory mites in tropical Australia: Local species richness and complementarity. Biotropica 1998, 30, 72–81. [Google Scholar] [CrossRef]
- Walter, D.E.; Seeman, O.; Rodgers, D.; Kitching, R.L. Mites in the mist: How unique is a rainforest canopy knockdown fauna? Aust. J. Ecol. 1998, 23, 501–508. [Google Scholar] [CrossRef]
- Walter, D.E. Achilles and the mite: Zeno’s paradox and rainforest mite diversity. In Acarology: Proceedings of the 10th International Congress; Halliday, R.B., Walter, D.E., Proctor, H.C., Norton, R.A., Colloff, M.J., Eds.; CSIRO Publishing: Melbourne, Australia, 2001; pp. 113–120. [Google Scholar]
- Basset, Y.; Cizek, L.; Cuenoud, P.; Didham, R.K.; Guilhaumon, F.; Missa, O.; Novotny, V.; Ødegaard, F.; Roslin, T.; Schmidl, J.; et al. Arthropod diversity in a tropical forest. Science 2012, 338, 1481–1484. [Google Scholar] [CrossRef]
- Basset, Y.; Cizek, L.; Cuénoud, P.; Didham, R.K.; Novotny, V.; Ødegaard, F.; Roslin, T.; Tishechkin, A.K.; Schmidl, J.; Winchester, N.N.; et al. Arthropod distribution in a tropical rainforest: Tackling a four dimensional puzzle. PLoS ONE 2015, 10, e0144110. [Google Scholar] [CrossRef]
- Sullivan, G.T.; Ozman-Sullivan, S.K. Global mite diversity is in crisis: What can we do about it? Zoosymposia 2022, 22, 089–093. [Google Scholar] [CrossRef]
- Ozman-Sullivan, S.K.; Sullivan, G.T. Coextinction is magnifying the current extinction crisis, as illustrated by the eriophyoid mites and their host plants. Acarologia 2023, 63, 169–179. [Google Scholar] [CrossRef]
- Krantz, G.W. Habits and habitats. In A Manual of Acarology; Krantz, G.W., Walter, D.E., Eds.; Texas Tech University Press: Lubbock, TX, USA, 2009; pp. 64–82. [Google Scholar]
- Bruce-Oliver, S.J.; Hoy, M.A.; Yaninek, J.S. Effect of some food sources associated with cassava in Africa on the development, fecundity and longevity of Euseius fustis (Pritchard and Baker) (Acari: Phytoseiidae). Exp. Appl. Acarol. 1996, 20, 73–85. [Google Scholar] [CrossRef]
- Christenhusz, M.J.M.; Byng, J.W. The number of known plant species in the world and its annual increase. Phytotaxa 2016, 261, 201–217. [Google Scholar] [CrossRef]
- Pimm, S.L.; Joppa, L.N. How many plant species are there, where are they, and at what rate are they going extinct? Ann. Mo. Bot. Gard. 2015, 100, 170–176. [Google Scholar] [CrossRef]
- Corlett, R.T. Plant diversity in a changing world: Status, trends, and conservation needs. Plant Divers. 2016, 38, 10–16. [Google Scholar] [CrossRef] [PubMed]
- O’Dowd, D.J.; Willson, M.F. Leaf domatia and mites on Australian plants: Ecological and evolutionary implications. Biol. J. Linn. Soc. 1989, 37, 191–236. [Google Scholar] [CrossRef]
- Walter, D.E. Dancing on the head of a pin: Mites in the rainforest canopy. Rec. West. Aust. Mus. 1995, 52, 49–53. [Google Scholar]
- Schmidt, R.A. Leaf structures affect predatory mites (Acari: Phytoseiidae) and biological control: A review. Exp. Appl. Acarol. 2014, 62, 1–17. [Google Scholar] [CrossRef] [PubMed]
- O’Dowd, D.J.; Brew, C.R.; Christophel, D.C.; Norton, R.A. Mite-plant associations from the Eocene of southern Australia. Science 1991, 252, 99–101. [Google Scholar] [CrossRef]
- Walter, D.E.; O’Dowd, D.J. Leaves with domatia have more mites. Ecology 1992, 73, 1514–1518. [Google Scholar] [CrossRef]
- Walter, D.E.; O’Dowd, D.J. Leaf morphology and predators: Effect of domatia on the distribution of phytoseiid mites (Acari: Phytoseiidae). Environ. Entomol. 1992, 21, 478–484. [Google Scholar] [CrossRef]
- Walter, D.E.; O’Dowd, D.J. Beneath biodiversity: Factors influencing the diversity and abundance of canopy mites. Selbyana 1995, 16, 12–20. [Google Scholar]
- Walter, D.E.; O’Dowd, D.J. Life on the forest phylloplane: Hairs, little houses, and myriad mites. In Forest Canopies; Lowman, M.D., Nadkarni, N.M., Eds.; Academic Press: Sydney, Australia, 1995; pp. 325–351. [Google Scholar]
- Walter, D.E. Living on leaves: Mites, tomenta, and leaf domatia. Annu. Rev. Entomol. 1996, 41, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Tixier, M.-S. Predatory mites (Acari: Phytoseiidae) in agro-ecosystems and conservation biological control: A review and explorative approach for forecasting plant-predatory mite interactions and mite dispersal. Front. Ecol. Evol. 2018, 6, 192. [Google Scholar] [CrossRef]
- Chant, D.A.; McMurtry, J.A. A review of the subfamilies Phytoseiinae and Typhlodrominae (Acari: Phytoseiidae). Int. J. Acarol. 1994, 20, 223–310. [Google Scholar] [CrossRef]
- Walter, D.E.; Beard, J.J. A review of the Australian Phytoseiinae (Acari: Mesostigmata: Phytoseiidae). Invertebr. Syst. 1997, 11, 823–860. [Google Scholar] [CrossRef]
- de Moraes, G.J.; McMurtry, J.A.; Denmark, H.A.; Campos, C.B. A revised catalog of the mite family Phytoseiidae. Zootaxa 2004, 434, 1–494. [Google Scholar] [CrossRef]
- de Castro, T.M.M.G.; de Moraes, G.J. Mite diversity on plants of different families found in the Brazilian Atlantic Forest. Neotrop. Entomol. 2007, 36, 774–782. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tixier, M.-S.; Kreiter, S. Arthropods in biodiversity hotspots: The case of the Phytoseiidae (Acari: Mesostigmata). Biodivers. Conserv. 2009, 18, 507–527. [Google Scholar] [CrossRef]
- Tixier, M.-S.; Kreiter, S.; Douin, M.; Moraes, G.J. Rates of description of Phytoseiidae (Acari: Mesostigmata): Space, time and body size variations. Biodivers. Conserv. 2012, 21, 993–1013. [Google Scholar] [CrossRef]
- Kreiter, S.; Payet, R.-M.; Douina, M.; Fontainec, O.; Fillâtred, J.; Le Bellece, F. Phytoseiidae of La Réunion Island (Acari: Mesostigmata): Three new species and two males described, new synonymies, and new records. Acarologia 2020, 60, 111–195. [Google Scholar] [CrossRef]
- Kar, A.; Karmakar, K. Description of eleven new species of phytoseiid mites (Acari: Mesostigmata) from Meghalaya state, north eastern India. Zootaxa 2021, 5068, 301–354. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.M.; Molla, M.I.H.; Karmakar, K.; Demite, P.R. Description of four new species of phytoseiid mites (Acari: Mesostigmata: Phytoseiidae) from Andhra Pradesh, India. Int. J. Acarol. 2022, 48, 407–417. [Google Scholar] [CrossRef]
- Biswas, S.; Karmakar, K. Descriptions of five new species of phytoseiid mites (Acari: Mesostigmata) from Andaman and Nicobar Islands. Int. J. Acarol. 2023, 49, 34–48. [Google Scholar] [CrossRef]
- Chant, D.A. Phytoseiid mites (Acarina: Phytoseiidae). Part, I. Bionomics of seven species in southeastern England. Part II. A taxonomic review of the family Phytoseiidae, with descriptions of 38 new species. Can. Entomol. Suppl. 1959, 12, 5–166. [Google Scholar] [CrossRef]
- Ehara, S. Some phytoseiid mites from Japan, with descriptions of thirteen new species (Acarina: Mesotigmata). Mushi 1972, 46, 137–173. [Google Scholar]
- Chant, D.A.; McMurtry, J.A. Illustrated Keys and Diagnoses for the Genera and Subgenera of the Phytoseiidae of the World (Acari: Mesostigmata); Indira Publishing House: West Bloomfield, MI, USA, 2007. [Google Scholar]
- Ueckermann, E.A.; Zannou, I.D.; de Moraes, G.J.; Oliveira, A.R.; Hanna, R.; Yaninek, J.S. Phytoseiid mites of the tribe Typhlodromini (Acari: Phytoseiidae) from sub-Saharan Africa. Zootaxa 2008, 1901, 1–122. [Google Scholar] [CrossRef]
- Demite, P.R.; McMurtry, J.A.; de Moraes, G.J. Phytoseiidae database: A website for taxonomic and distributional information on phytoseiid mites (Acari). Zootaxa 2014, 3795, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Demite, P.R.; de Moraes, G.J.; McMurtry, J.A.; Denmark, H.A.; Castilho, R.C. Phytoseiidae Database. 2024. Available online: http://www.lea.esalq.usp.br/phytoseiidae/ (accessed on 29 February 2024).
- Döker, I.; Stathakis, T.I.; Kazak, C.; Karut, K.; Papadoulis, G.T. Four new records and two new species of Phytoseiidae (Acari: Mesostigmata) from Turkey, with a key to the Turkish species. Zootaxa 2014, 3827, 331–342. [Google Scholar] [CrossRef][Green Version]
- Nesbitt, H.H.J. A Taxonomic Study of the Phytoseiinae (Family Laelaptidae) Predaceous upon Tetranychidae of Economic Importance. Ph.D. Dissertation, Carleton College, Ottawa, ON, Canada, 1951. [Google Scholar]
- Șekeroğlu, E. Phytoseiid mites (Acarina, Mesostigmata) of southern Anatolia, their biology, and effectiveness as a biological control agent on strawberry plants. Doğa Bilim Dergisi 1984, 8, 320–336. [Google Scholar]
- Kostiainen, T.; Hoy, M.A. Egg-harvesting allows large scale rearing of Amblyseius finlandicus (Acari: Phytoseiidae) in the laboratory. Exp. Appl. Acarol. 1994, 18, 155–165. [Google Scholar] [CrossRef]
- Tixier, M.-S.; Kreiter, S.; Auger, P.; Weber, M. Colonization of Languedoc vineyards by phytoseiid mites (Acari: Phytoseiidae): Influence of wind and crop environment. Exp. Appl. Acarol. 1998, 22, 523–542. [Google Scholar] [CrossRef]
- Cakmak, I.; Janssen, A.; Sabelis, M.W. Intraguild interactions between the predatory mites Neoseiulus californicus and Phytoseiulus persimilis. Exp. Appl. Acarol. 2006, 38, 33–46. [Google Scholar] [CrossRef]
- Beaulieu, F.; Weeks, A.R. Free-living mesostigmatic mites in Australia: Their roles in biological control and bioindication. Aust. J. Exp. Agric. 2007, 47, 460–478. [Google Scholar] [CrossRef]
- Denmark, H.A. Phytoseiid mites (Acari: Phytoseiidae). In Encyclopedia of Entomology; Capinera, J.L., Ed.; Springer: Dordrecht, The Netherlands, 2008; pp. 2876–2881. [Google Scholar] [CrossRef]
- Lorenzon, M.; Pozzebon, A.; Duso, C. Effects of potential food sources on biological and demographic parameters of the predatory mites Kampimodromus aberrans, Typhlodromus pyri and Amblyseius andersoni. Exp. Appl. Acarol. 2012, 58, 259–278. [Google Scholar] [CrossRef] [PubMed]
- Pappas, M.L.; Xanthis, C.; Samaras, K.; Koveos, D.S.; Broufas, G.D. Potential of the predatory mite Phytoseius finitimus (Acari: Phytoseiidae) to feed and reproduce on greenhouse pests. Exp. Appl. Acarol. 2013, 61, 387–401. [Google Scholar] [CrossRef] [PubMed]
- Grafton-Cardwell, E.; Bentley, W.; Bianchi, M.; Cave, F.; Elkins, R.; Godfrey, L.; Gu, P.; Haviland, D.R.; Headrick, D.; Hoddle, M.; et al. Surveys of 12 California crops for phytoseiid predatory mites show changes compared to earlier studies. Calif. Agr. 2020, 74, 129–137. [Google Scholar] [CrossRef]
- Cruz-Miralles, J.; Cabedo-Lopez, M.; Guzzo, M.; Perez-Hedo, M.; Flors, V.; Jaques, J.A. Plant defense responses triggered by phytoseiid predatory mites (Mesostigmata: Phytoseiidae) are species-specific, depend on plant genotype and may not be related to direct plant feeding. BioControl 2021, 66, 381–394. [Google Scholar] [CrossRef]
- Mills, N.J.; Grafton-Cardwell, E.E.; Tollerup, K.E. An exploratory analysis of the structure of tetranychid and phytoseiid assemblages in walnut orchards in California. Exp. Appl. Acarol. 2024, 92, 739–758. [Google Scholar] [CrossRef]
- Jung, C.; Croft, B.A. Aerial dispersal of phytoseiid mites (Acari: Phytoseiidae): Estimating falling speed and dispersal distance of adult females. Oikos 2003, 94, 182–190. [Google Scholar] [CrossRef]
- Novljan, M.; Bohinc, T.; Kreiter, S.; Doker, I.; Trdan, S. The indigenous species of predatory mites (Acari: Phytoseiidae) as biological control agents of plant pests in Slovenia. Acarologia 2023, 63, 1048–1061. [Google Scholar] [CrossRef]
- Şekeroğlu, E.; Kazak, C. First record of Phytoseiulus persimilis (Acari: Phytoseiidae) in Turkey. Entomophaga 1993, 38, 343–345. [Google Scholar] [CrossRef]
- Çobanoğlu, S. New phytoseiid mites (Acarina: Mesostigmata) for Turkish fauna. Turk. J. Agric. For. 1997, 21, 361–370. [Google Scholar] [CrossRef]
- Ozman, S.K.; Cobanoğlu, S. Current status of hazelnut mites in Turkey. Acta Hortic. 2001, 556, 479–487. [Google Scholar] [CrossRef]
- Cobanoglu, S.; Ozman, S.K. Beneficial mite species of hazelnut orchard ecosystems from the Black Sea Region of Turkey. In Proceedings of the 2nd Meeting of WG 4, Bio-Control of Arthropod Pests in the Stored Products, Prague, Czech Republic, 30–31 May 2002; pp. 91–99. [Google Scholar]
- Faraji, F.; Çobanoğlu, S.; Çakmak, I. A checklist and a key for the Phytoseiidae species of Turkey with two new species records (Acari: Mesostigmata). Int. J. Acarol. 2011, 37 (Suppl. 1), 221–243. [Google Scholar] [CrossRef]
- Yeşilayer, A.; Çobanoğlu, S. The distribution of predatory mite species (Acari: Phytoseiidae) on ornamental plants and parks of Istanbul, Turkey. Turk. Entomol. Bult. 2011, 1, 135–143. [Google Scholar]
- Döker, I.; Kazak, C.; Karut, K. The genus Amblyseius Berlese (Acari: Phytoseiidae) in Turkey with discussion on the identity of Amblyseius meridionalis. Syst. Appl. Acarol. 2020, 25, 1395–1420. [Google Scholar]
- Döker, I.; Bas, H.; Ozman-Sullivan, S.K. A supplementary description of Typhlodromina conspicua (Garman) (Acari: Phytoseiidae) from Türkiye, with comments on its taxonomic status. Int. J. Acarol. 2024, 50, 81–86. [Google Scholar] [CrossRef]
- Cakir, S. Determination of Mite Species in Walnut Orchards in Samsun Province, Turkey. Master’s Thesis, Ondokuz Mayis University, Samsun, Türkiye, 2020. [Google Scholar]
- Cakir, S.; Tixier, M.-S.; Ozman-Sullivan, S.K. Phytoseiid species (Acari: Phytoseiidae) on walnut trees in Samsun Province, Turkey. Acarol. Stud. 2020, 2, 24–33. [Google Scholar]
- Bas, H.; Döker, İ.; Ozman-Sullivan, S.K. New records and complementary descriptions of three Phytoseiidae (Acari: Mesostigmata) species from Turkey. Int. J. Acarol. 2022, 48, 393–400. [Google Scholar] [CrossRef]
- Bas, H.; Ozman-Sullivan, S.K.; Ueckermann, E.A.; Chetverikov, P.E.; Doker, I. Population dynamics of mite species on poplar trees in the Black Sea region of Turkey. Zoosymposia 2022, 22, 168. [Google Scholar] [CrossRef]
- Saglam, D.; Doker, I.; Ozman-Sullivan, S.K. Survey of phytoseiid mite species in an oak forest in Samsun Province, Turkey. In Proceedings of the 9th Symposium of the EurAAc, Bari, Italy, 12–15 July 2022; pp. 57–58. [Google Scholar]
- Saglam, D.; Doker, I.; Ozman-Sullivan, S.K. Re-description of the female of Kampimodromus langei Wainstein & Arutunjan (Acari: Phytoseiidae) based on normal and abnormal specimens, with the first description of its male. Acarologia 2022, 62, 446–453. [Google Scholar] [CrossRef]
- Bas, H. The Determination of Mite Species and Their Population Densities on Poplar Trees in Samsun Province, Türkiye. Master’s Thesis, Ondokuz Mayis University, Samsun, Türkiye, 2023. [Google Scholar]
- Saglam, D. The Determination of Mite Species and Their Population Densities on Oak Trees on the Campus of Ondokuz Mayis University in Samsun, Türkiye. Master’s Thesis, Ondokuz Mayis University, Samsun, Türkiye, 2023. [Google Scholar]
- Saglam, D.; Ueckermann, E.A.; Döker, I.; Magowski, W.L.; Ozman-Sullivan, S.K. Non-phytophagous mite species in remnant oak forests in Samsun Province, Türkiye. Integr. Control. Plant-Feed. Mites IOBC-WPRS Bull. 2024, 169, 87–88. [Google Scholar]
- Ozman-Sullivan, S.K.; Doker, I.; Chetverikov, P.E.; Sullivan, G.T.; Kaplan, E. Co-occurring complexes of phytoseiid and gall-forming eriophyoid mites on broad-leaved trees in Türkiye. Integr. Control. Plant-Feed. Mites IOBC-WPRS Bull. 2024, 169, 74–75. [Google Scholar]
- Ozyazici, M.; Dengiz, O.; Saglam, M.; Demirağ Turan, I. Choosing suitable site for some forage legumes using multi-criteria assessment and geostatistical approach. PONTE 2016, 72, 139–154. [Google Scholar] [CrossRef]
- Anonymous. Samsun Climate and Temperature. 2023. Available online: https://www.samsun.climatemps.com/ (accessed on 8 December 2023).
- Anonymous. Vezirkopru, Samsun, Turkey Climate. 2023. Available online: https://weatherandclimate.com/turkey/samsun/vezirkopru (accessed on 8 December 2023).
- Rain, R. Shannon Diversity Index Calculator. Available online: https://www.omnicalculator.com/ecology/shannon-index (accessed on 6 June 2024).
- Statistics How To. Available online: https://www.statisticshowto.com/jaccard-index/ (accessed on 14 June 2024).
- Walter, D.E. Leaf surface structure and the distribution of Phytoseius mites (Acarina: Phytoseiidae) in south-east Australian forests. Aust. J. Zool. 1992, 40, 593–603. [Google Scholar] [CrossRef]
- Lundström, A.N. Planzenbiologische Studien. II. Die Anpassungen der Planzen an Thiere. Nova Acta Regiae Soc. Sci. Ups. 1887, 13, 1–88. [Google Scholar]
- Agwaral, A.A. Do leaf domatia mediate a plant–mite mutualism? An experimental test of the effects on predators and herbivores. Ecol. Entomol. 1997, 22, 371–376. [Google Scholar] [CrossRef]
- Agwaral, A.A.; Karban, R. Domatia mediate plant–arthropod mutualism. Nature 1997, 387, 562–563. [Google Scholar] [CrossRef]
- Schausberger, P.; Croft, B.A. Cannibalism and intraguild predation among phytoseiid mites: Are aggressiveness and prey preference related to diet specialization? Exp. Appl. Acarol. 2000, 24, 709–725. [Google Scholar] [CrossRef]
- McMurtry, J.A.; Croft, B.A. Lifestyles of phytoseiid mites and their roles in biological control. Annu. Rev. Entomol. 1997, 42, 291–321. [Google Scholar] [CrossRef]
- Croft, B.A.; Blackwood, J.S.; McMurtry, J.A. Classifying life-style types of phytoseiid mites: Diagnostic traits. Exp. Appl. Acarol. 2004, 33, 247–260. [Google Scholar] [CrossRef]
- McMurtry, J.A.; de Moraes, G.J.; Sourassou, N.F. Revision of the lifestyles of phytoseiid mites (Acari: Phytoseiidae) and implications for biological control strategies. Syst. Appl. Acarol. 2013, 18, 297–320. [Google Scholar] [CrossRef]
- Dicke, M.; Sabelis, M.W.; de Jong, M. Analysis of prey preference in phytoseiid mites by using an olfactometer, predation models and electrophoresis. Exp. Appl. Acarol. 1988, 5, 225–241. [Google Scholar] [CrossRef]
- Schausberger, P. Comparative investigations on the effect of different foods on development and reproduction of Amblyseius aberrans Oud. and A. finlandicus Oud. (Acarina, Phytoseiidae). J. Appl. Entomol. 1992, 113, 476–486. [Google Scholar] [CrossRef]
- Duso, C.; Ahmad, S.; Trello, P.; Pozzebon, A.; Klaric, V.; Baldessari, M.; Malagnini, V.; Angeli, G. The impact of insecticides applied in apple orchards on the predatory mite Kampimodromus aberrans (Acari: Phytoseiidae). Exp. Appl. Acarol. 2014, 62, 391–414. [Google Scholar] [CrossRef]
- Duso, C.; de Lillo, E. Grape. In Eriophyoid Mites: Their Biology, Natural Enemies and Control; Lindquist, E.E., Sabelis, M.W., Bruin, J., Eds.; Elsevier: Amsterdam, The Netherlands, 1996; pp. 571–582. [Google Scholar]
- Ozman-Sullivan, S.K. Life history of Kampimodromus aberrans as a predator of Phytoptus avellanae (Acari: Phytoseiidae, Phytoptidae). Exp. Appl. Acarol. 2006, 38, 15–23. [Google Scholar] [CrossRef]
- Jeppson, L.R.; Keifer, H.H.; Baker, E.W. Mites Injurious to Economic Plants; University of California Press: Berkeley, CA, USA, 1975. [Google Scholar]
- Kozlowski, J.; Kozlowska, M. Notes on Aculus schlechtendali as food for predatory mites. In Modern Acarology; Dusbabek, F., Bukva, V., Eds.; Academia: Prague, Czechoslovakia; SPB Academic Publishing BV: The Hague, The Netherlands, 1991; Volume 2, pp. 675–678. [Google Scholar]
- Roda, A.; Nyrop, J.; English-Loeb, G. Leaf pubescence mediates the abundance of non-prey food and the density of the predatory mite Typhlodromus pyri. Exp. Appl. Acarol. 2003, 29, 193–211. [Google Scholar] [CrossRef]
- Kasap, İ.; Atlıhan, R.; Özgökçe, M.S.; Kaydan, M.B.; Polat, E.; Yarımbatman, A. Harmful mite species and their predators in the walnut orchards around Van Lake. Yuz. Yil Univ. J. Agric. Sci. 2008, 18, 99–102. (In Turkish) [Google Scholar]
- Kasap, İ.; Atlıhan, R.; Özgökçe, M.S.; Kaydan, M.B.; Polat, E.; Yarımbatman, A. Population density of the important harmful mites and their predatories in the walnut orchards of around Van Lake. Turk. J. Entomol. 2009, 33, 305–314. (In Turkish) [Google Scholar]
- Denizhan, E.; Çobanoğlu, S. Eriophyid mites of walnut trees (Juglans regia L.) and their predators in Ankara. Yuz. Yil Univ. J. Agric. Sci. 2009, 19, 33–37. (In Turkish) [Google Scholar]
- Gençer Gökçe, P. Determination of Mite Species of Ornamental Plants in Green Areas of Tekirdağ. Master’s Thesis, Namık Kemal University, Graduate School of Natural and Applied Sciences, Tekirdağ, Turkey, 2015. [Google Scholar]
- Rahmani, H.; Kamali, K.; Faraji, F. Predatory mite fauna of Phytoseiidae of northwest Iran (Acari: Mesostigmata). Turk. J. Zool. 2010, 34, 497–508. [Google Scholar] [CrossRef]
- Hajizadeh, J.; Mortazavi, S. The genus Euseius Wainstein (Acari: Phytoseiidae) in Iran, with a revised key to Iranian phytoseiid mites. Int. J. Acarol. 2015, 41, 53–66. [Google Scholar] [CrossRef]
- Nicholson, C.C.; Williams, N.M. Cropland heterogeneity drives frequency and intensity of pesticide use. Environ. Res. Lett. 2021, 16, 074008. [Google Scholar] [CrossRef]
- Kabicek, J. Scarceness of phytoseiid species co-occurrence (Acari: Phytoseiidae) on leaflets of Juglans regia. Plant Protect. Sci. 2010, 46, 79–82. [Google Scholar] [CrossRef]
- Sudo, M.; Osakabe, M. Do plant mites commonly prefer the underside of leaves? Exp. Appl. Acarol. 2011, 55, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Kabicek, J. Broad leaf trees as reservoirs for phytoseiid mites (Acari: Phytoseiidae). Plant Protect. Sci. 2003, 39, 65–69. [Google Scholar] [CrossRef]
- Mladenović, K.D.; Stojnić, B.S.; Milanović, S.D.; Milenković, I.L.; Radulović, Z.B. Predatory mites and spider mites (Acari: Phytoseiidae and Tetranychidae) on oak trees in Serbia. Acta Zool. Bulg. 2021, 73, 179–185. [Google Scholar]
- Kabicek, J. Phytoseiid mites on Quercus cerris in an urban park—Short communication. Plant Protect. Sci. 2017, 53, 181–186. [Google Scholar] [CrossRef]

| Phytoseiid Species | Plant Species | ||
|---|---|---|---|
| Walnut (Juglans regia) | Poplar (Populus deltoides, P. nigra) | Oak (Quercus cerris var. cerris) | |
| Amblydromalus limonicus | _ | X | _ |
| Amblyseius andersoni | X | X | _ |
| Amblyseius bryophilus | _ | X | _ |
| Euseius finlandicus | X | X | |
| Euseius amissibilis | X | X | X |
| Euseius stipulatus | X | X | _ |
| Kampimodromus aberrans | X | _ | _ |
| Kampimodromus langei | _ | _ | X |
| Neoseiulella tiliarum | X | _ | _ |
| Neoseiulus fauveli | _ | X | _ |
| Paraseiulus triporus | X | _ | |
| Phytoseius finitimus | X | _ | X |
| Transeius wainsteini | _ | X | _ |
| Typhlodromina conspicua | _ | X | _ |
| Typhlodromips sessor | X | _ | |
| Typhlodromus (Anthoseius) intercalaris | _ | _ | X |
| Typhlodromus (Anthoseius) rapidus | X | _ | _ |
| Typhlodromus (Anthoseius) sp. | X | _ | _ |
| Typhloseiulus peculiaris | _ | _ | X |
| Mite Taxa | Shannon Index | Walnut (Juglans regia) | Poplar (Populus deltoides and P. nigra) | Oak (Quercus cerris var. cerris) |
|---|---|---|---|---|
| Families | H | 1.60 | 1.63 | 1.81 |
| E | 0.59 | 0.74 | 0.76 | |
| Genera (phytoseiid) | H | 1.15 | 1.53 | 1.02 |
| E | 0.64 | 0.74 | 0.63 | |
| Species (phytoseiid) | H | 1.54 | 1.87 | 1.02 |
| E | 0.70 | 0.81 | 0.57 |
| Mite Taxa | J. regia/Populus spp. | J. regia/Q. cerris var. cerris | Populus spp./Q. cerris var. cerris |
|---|---|---|---|
| Families | 0.50 | 0.53 | 0.67 |
| Genera (phytoseiid) | 0.17 | 0.57 | 0.08 |
| Species (phytoseiid) | 0.19 | 0.25 | 0.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ozman-Sullivan, S.K.; Sullivan, G.T.; Cakir, S.; Bas, H.; Saglam, D.; Doker, I.; Tixier, M.-S. Phytoseiid Mites: Trees, Ecology and Conservation. Diversity 2024, 16, 542. https://doi.org/10.3390/d16090542
Ozman-Sullivan SK, Sullivan GT, Cakir S, Bas H, Saglam D, Doker I, Tixier M-S. Phytoseiid Mites: Trees, Ecology and Conservation. Diversity. 2024; 16(9):542. https://doi.org/10.3390/d16090542
Chicago/Turabian StyleOzman-Sullivan, Sebahat K., Gregory T. Sullivan, Seyma Cakir, Huseyin Bas, Damla Saglam, Ismail Doker, and Marie-Stephane Tixier. 2024. "Phytoseiid Mites: Trees, Ecology and Conservation" Diversity 16, no. 9: 542. https://doi.org/10.3390/d16090542
APA StyleOzman-Sullivan, S. K., Sullivan, G. T., Cakir, S., Bas, H., Saglam, D., Doker, I., & Tixier, M.-S. (2024). Phytoseiid Mites: Trees, Ecology and Conservation. Diversity, 16(9), 542. https://doi.org/10.3390/d16090542









