Abstract
Mites from the Demodecidae and Psorergatidae can optimally use mammalian hosts by inhabiting a number of different microhabitats in their skin. Hence, in individual hosts, several species of parasites from these groups have been described in different microhabitats. There are few data on their co-occurrence either at the host species level or at the host individual level. Most research has addressed the co-occurrence of Demodecidae in carnivorans, ungulates, soricomorphs, and rodents, while the co-occurrence of both families was found in bats. The present study examines the possibility of their co-occurrence in a Eurasian rodent—Apodemus flavicollis. It is a suitable model for such analyses, because representatives of both families have been demonstrated here so far, and our findings extend the list of specific Demodecidae in A. flavicollis with two new species: Demodex tenuis sp. nov. from the lip region and D. mediocris sp. nov. from the chin region. The study also includes the first record of Psorergates muricola in this host, which occurred in the genital–anal region. Therefore, the findings confirm the possibility that different Demodecidae and Psorergatidae species can co-occur in the same host in different body regions. This paper also includes a checklist of Demodecidae and Psorergatidae in rodents around the world.
1. Introduction
Skin mites (Acariformes: Trombidiformes: Prostigmata) parasitizing mammals constitute a species-rich assemblage with co-occurring species often specialized to different microhabitats (location) of the host skin, as well as other tissues and organs [1]. Such a model of parasitism, where several different mite species coexist on the host species, and the host individual levels, has been described for Demodecidae in carnivorous mammals. It has been described both in domestic animals, e.g., dog Canis lupus familiaris Linnaeus, 1758 and cat Felis catus Linnaeus, 1758 models, and in wild animals like the European polecat Mustela putorius Linnaeus, 1758 [2,3,4]. Similar observations have also been made in ungulates, e.g., European bison Bison bonasus (Linnaeus, 1758) [5,6], or soricomorphs [7,8,9]. Similarly, in rodents, such a co-occurrence has been reported in Muridae, including Apodemus spp. [10,11], the brown rat Rattus norvegicus (Berkenhout, 1769) [12,13], or the house mouse Mus musculus [14,15,16]. However, there are fewer data on the co-occurrence of Demodecidae and the closely related Psorergatidae, which has only been described to date in bats [17]. Most importantly, no similar studies have been conducted on rodents, despite the fact that they constitute the largest group of mammals in which the largest number of species from these mite families has been described so far. Previous research indicates that representatives of these groups may occur in the same host species [1,18]. However, this does not confirm their co-occurrence at the individual level, because certain mite species may have different geographical ranges or occur in different host populations. Additionally, there may be mechanisms that favor the colonization of the host by mites from one group/species, while excluding the presence of others in neighboring anatomic microhabitats. Unfortunately, such observations are limited by the lack of full recognition of the acarofauna of individual host species: Demodecidae and Psorergatidae are known only in some mammals, often from single records [1,19].
The present study examines the possibility that several mite species from these families may co-occur in the yellow-necked field mouse Apodemus flavicollis (Rodentia: Muridae). The yellow-necked field mouse is an appropriate model for such analyses, as it has been found to host several representatives of both mite families [1,18,20], and the occurrence of three Demodex species has been noted in hosts from the same region, Poland [11,21,22]. Our findings expand the list of Demodecidae inhabiting this mammal with the description of two new species. In addition, this paper compiles an updated list of Demodecidae and Psorergatidae in rodents around the world, which shows the possibility of co-occurrence at the species level.
2. Material and Methods
Twenty specimens of dead yellow-necked field mice Apodemus flavicollis (Poland, Pomeranian Voivodeship, Gdynia, 54°32’00” N/18°28’30” E, four mice; Kartuzy, 54°19’43” N/18°21’31” E, four mice; Tczew, 54°04’55” N 18°47’05” E, three mice; Ulkowy, 54°10’44” N/18°38’01” E, nine mice), collected from September 2019 to September 2022, were examined for the presence of skin mites.
The mites were isolated using the digestion method developed for the detection of mammalian skin mites [23], with a modification to suit the examined host. Fragments of the skin, 1 cm2 each, were taken from various areas of the body, including the head (around the eyes, nose, area of vibrissae, lips, chin, cheeks, ear pinnae, and vertex), neck, abdomen, back, limbs, tail, and the genital–anal area. Skin samples were preserved in 70% ethanol and subjected to digestion in 10% potassium hydroxide solution. The digested material was decanted, mounted, and examined under phase-contrast microscopy (Nikon Eclipse 50i). The mites were placed in a polyvinyl-lactophenol solution and measured; all measurements are given in micrometers.
The specimen depositories are cited using the following abbreviation: UGDIZP, University of Gdańsk, Department of Invertebrate Zoology and Parasitology, Gdańsk, Poland [24]. The description of the species adopted the nomenclature commonly used for the family Demodecidae [25] and was completed with the nomenclature proposed by Bochkov [26] for the superfamily Cheyletoidea (Acariformes: Prostigmata) and by Izdebska and Rolbiecki [16]. The scientific and common names of the hosts follow Wilson and Reeder [27] and the Integrated Taxonomic Information System [28].
To define the level of host infection, the following main parasitological parameters were measured: prevalence (percentage of hosts infected), mean intensity (mean number of parasites in infected hosts), and intensity range (minimum and maximum number of parasite individuals per host) [29].
The checklist has been compiled based on manuscripts published during the period 1842–2024. It also contains a new record, marked as the present study. The list includes all formally described mite species known to date and other functioning specific names. The study also includes information on the dates of host species, as well as the occurrence and microhabitats of mites. However, no host records related to unidentified Demodex spp. and Psorergates spp. are included.
3. Results
3.1. Systematics
Demodex tenuis sp. nov. Izdebska, Cierocka et Rolbiecki

Table 1.
Body size (micrometers) for adults of Demodex tenuis sp. nov.
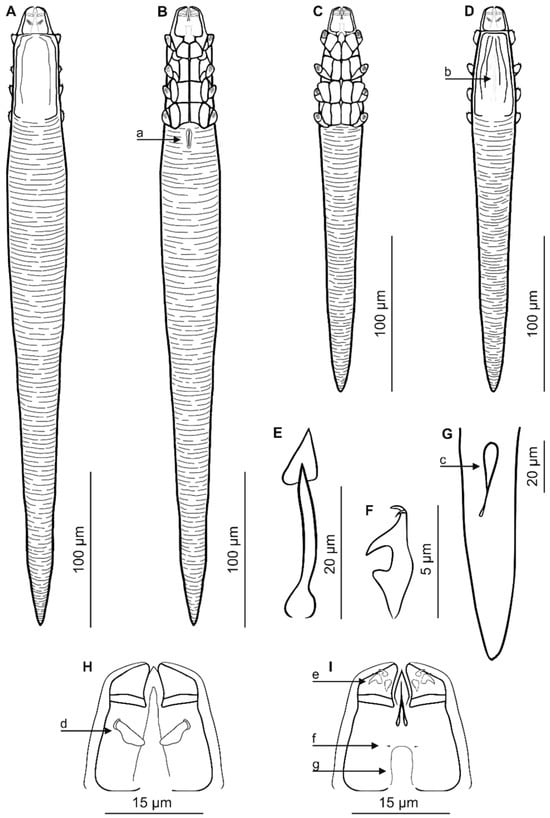
Figure 1.
Demodex tenuis sp. nov.: female, dorsal view (A); female, ventral view (B); male, ventral view (C); male, dorsal view (D); aedeagus (E); claw on the leg (F); posterior part of opisthosoma with visible opisthosomal organ, male (G); gnathosoma, male, dorsal view (H); gnathosoma, male, ventral view (I). Abbreviations: a—vulva, b—aedeagus, c—opisthosomal organ, d—supracoxal spine (seta elc.p), e—spines on palps, f—subgnathosomal seta (seta n), g—pharyngeal bulb.
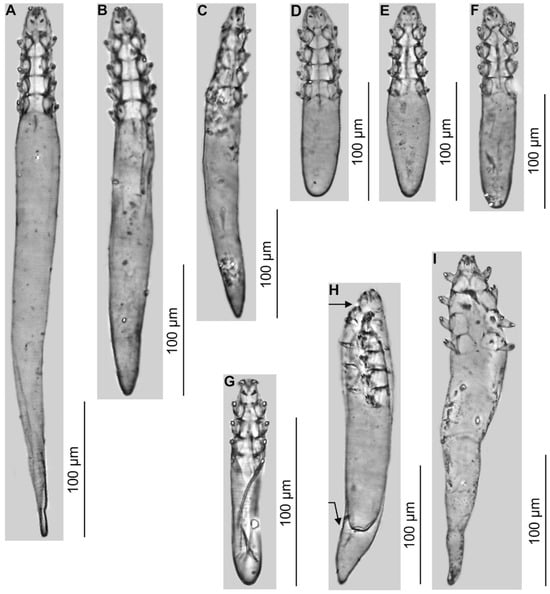
Figure 2.
Demodecidae from Apodemus flavicollis. Demodex tenuis sp. nov.: female, various morphotypes (A,B); male (C); Demodex mediocris sp. nov.: female (D); males, various morphotypes (E,F); Demodex corniculatus: male (G); adult Demodex mediocris sp. nov. with visible remains of nymphal exuviae, arrow (H); Demodex mollis: male (I).
Male (n = 13 and 1 holotype). Body elongated, narrow, slender, with distinctly separated gnathosoma; 253 (218–280) long and 31 (30–32) wide (holotype, 264 × 30). Gnathosoma trapezoidal, with length equal to or slightly less than width at base; on dorsal side in central part of basal segments, pair of wedge-shaped supracoxal spines (setae elc.p) present, ca. 4–5 long (holotype, 4.0), directed obliquely, posteromedially. Palps 3-segmented, terminating in three hooked spines (one small and two larger) on tibio-tarsus. On ventral side, horseshoe-shaped pharyngeal bulb with pair of conical subgnathosomal setae (setae n), situated anterior on both sides. Podosoma trapezoidal, widening posterior end; four pairs of short legs, with coxa integrated into ventral idiosomal wall and five free, overlapping segments (trochanter–tarsus); conical spine on tibiae; two bifurcated claws, ca. 5 long (holotype, 5.0), with sharp, slightly curved downwards spur and triangular bulge on each tarsus. Epimeral plates (coxal fields) distinctly sclerotized, trapezoidal; all epimeral plates connect medially. On the dorsal side of podosoma, podosomal shield present, reaching level of legs IV. Opisthosoma strongly elongated, conical, tapered towards end, constitutes 66 (63–69%) of body length (holotype, 69%). Whole opisthosoma clearly, densely annulated; annuli relatively wide at ca. 1.5–2.0 µm. Opisthosomal organ club-shaped, ca. 20 in length and is located in posterior part of opisthosoma. Aedeagus elongated, 29 (27–30) long (holotype, 29), on dorsal surface, located between epimeral plates II and III. Genital opening located on dorsal surface, at level of anterior margin of epimeral plate II.
Female (n = 17). Distinctly longer than male, 377 (293–434) long, 33 (29–35) wide, with very long opisthosoma. Gnathosoma shape similar to male; usually length less than width at base. Pharyngeal bulb and morphological details of gnathosoma similar to those in male. Also shape of podosoma and legs similar to those in male. Epimeral plates (coxal fields) I–III distinctly sclerotized, pair IV weakly sclerotized; all epimeral plates connect medially. On dorsal side of podosoma, podosomal shield, reaches level of legs IV. Opisthosoma relatively longer than males, narrow, conical, clearly narrows at end to sharp point; constitutes 75% (69–80%) of body length; distinctly annulated, annuli relatively wide at ca. 1.5–2.0 µm. Opisthosomal organ not visible. Vulva 11 (10–13) long, located below posterior arched incision of epimeral plates IV.
Immature stages. Not found; only adults were found in the remains of nymphal exuviae.
Material deposition: Male holotype (reg. no. UGDIZPRAfDDt01m), 13 male paratypes (reg. no. UGDIZPRAfDDt02m–14m) and 17 female paratypes (reg. no. UGDIZPRAfDDt01f –17f); skin of the lips; host Apodemus flavicollis (reg. no. MRMAf11/2019a, MRMAf11/2019b, MRMAf05/2021, MRMAf07/2021a, MRMAf07/2021b, MRMAf08/2021, MRMAf08/2022, MRMAf09/2022a, MRMAf09/2022b); Gdynia, Kartuzy, Ulkowy, Poland; November 2019, May 2021, July 2021, August 2021, August 2022, and September 2022; coll. J.N. Izdebska, K. Cierocka and L. Rolbiecki; the whole-type material (mounted microscope slides) deposited within the framework of the Collection of Extant Invertebrates in Department of Invertebrate Zoology and Parasitology, University of Gdańsk, Poland.
Infection and location in the host: Demodex tenuis sp. nov. was found in 45% of examined yellow-necked field mice, with a mean intensity of 2.4 and an intensity range 1–6; 31 (14 males and 17 females) individuals were noted. The demodecid mites were found in the lips of the examined yellow-necked field mice, and no lesions were observed in the examined hosts.
Etymology: The specific epithet tenuis (slender, slim, narrow) refers to the shape of the body.
Differential diagnosis: Demodex tenuis sp. nov. from the yellow-necked field mouse is morphologically similar to D. gracilentus from the striped field mouse A. agrarius (Pallas, 1771) and D. longior from the common field mouse A. sylvaticus (Linnaeus, 1758) [10]. Demodex tenuis, especially the female, is on average larger than other Demodex species, and has different body proportions (Table 2). Similarly to D. longior, it shows clear dimorphism, with the female being much longer than the male; in contrast, no such differences are found in D. gracilentus, in which individuals of both sexes are of similar length. The differences between these species concern the important structures of the gnathosoma. The supracoxal spines are wedge-shaped, directed obliquely, posteromedially in D. tenuis sp. nov., while they are conical, directed horizontally, medially in D. gracilentus and are club-like, directed obliquely, anterolaterally in D. longior. All mites possess three spines on the terminal segments of the palpi, but they are hook-shaped, one small and two large, in D. tenuis sp. nov., while they are large, overlapping and often visible as a single structure in D. gracilentus, and are small and three-armed in D. longior. The subgnathosomal setae in D. tenuis sp. nov. are located on both sides of the pharyngeal bulb, relatively higher than in D. gracilentus and D. longior. The opisthosomal organ is club-shaped in D. tenuis sp. nov., rhomboidal in D. gracilentus and fusiform in D. longior. The distinctiveness of the species is also confirmed by parasitological data regarding host specificity and location preferences: D. gracilentus is associated with the vibrissae region of the striped field mouse, D. longior in the analogous region of the head in the common field mouse, and D. tenuis sp. nov. exclusively around the lips in the yellow-necked field mouse.

Table 2.
Morphometric comparison between Demodex tenuis sp. nov. and Demodex gracilentus, and Demodex longior.
Demodex mediocris sp. nov. Izdebska, Cierocka et Rolbiecki

Table 3.
Body size (micrometers) for adults of Demodex mediocris sp. nov.
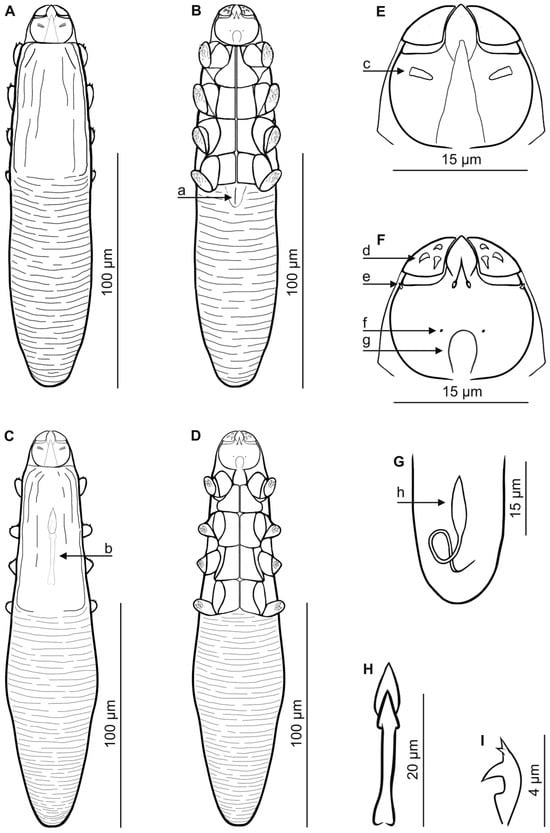
Figure 3.
Demodex mediocris sp. nov.: female, dorsal view (A); female, ventral view (B); male, dorsal view (C); male, ventral view (D); gnathosoma, male, dorsal view (E); gnathosoma, male, ventral view (F); posterior part of opisthosoma with visible opisthosomal organ, male (G); aedeagus (H); claw on the leg (I). Abbreviations: a—vulva, b—aedeagus, c—supracoxal spine (seta elc.p), d—spines on palps, e—setae v”F, f—subgnathosomal seta (seta n), g—pharyngeal bulb, h—opisthosomal organ.
Male (n = 16 and 1 holotype). Body cylindrical, sometimes spindle-shaped, with distinctly separated gnathosoma; 172 (151–187) long and 33 (29–37) wide (holotype, 184 × 30). Gnathosoma oval, length less than width at base; on dorsal side in central part of basal segments, pair of peg-like supracoxal spines (setae elc.p) present, ca. 3–3.5 long (holotype, 3.0), directed obliquely, medially. Palps 3-segmented, terminating in three spines (one small, two larger) on tibio-tarsus; also, small setae v”F present on middle segment (trochanter-femur-tarsus). On ventral surface of gnathosoma, horseshoe-shaped pharyngeal bulb with pair of small, conical subgnathosomal setae (setae n), situated anterior on both sides. Podosoma rectangular; four pairs of short legs, with coxa integrated into ventral idiosomal wall and five free, overlapping segments (trochanter–tarsus); two bifurcated claws, ca. 4.0 long (holotype, 4.0 μm) with sharp spur and triangular bulge on each tarsus; two small knobs at base of each claw. Epimeral plates (coxal fields) I–IV distinctly sclerotized, trapezoidal; all epimeral plates connect medially. On the dorsal side of podosoma, podosomal shield reaches level of legs IV. Opisthosoma fusiform or cylindrical, rounded at end, constitutes 56 (51–61%) of body length (holotype, 58%). Whole opisthosoma clearly, densely annulated; annuli relatively wide at ca. 1.5 µm. Opisthosomal organ present; anterior part of opisthosomal organ, spindle-shaped and posterior part filamentous, spirally twisted; located in posterior part of opisthosoma. Aedeagus elongated, stocky, 25 (24–27) long (holotype, 26), on dorsal side, located between epimeral plates II and III. Genital opening located on dorsal side, at level of anterior edge of epimeral plate II.
Female (n = 12). Similar to male, cylindrical, 166 (155–184) long, 31 (30–34) wide. Gnathosoma oval, length usually less than width at base. Pharyngeal bulb and morphological details of gnathosoma similar to those in male. Also, shape of podosoma and legs similar to those in male. Epimeral plates I–IV distinctly sclerotized, trapezoidal; all epimeral plates connect medially. On the dorsal side of podosoma, podosomal shield reaches level of legs IV. Opisthosoma similar to male, usually cylindrical, rounded at end, constitutes 54 (51–58%) of body length. Whole opisthosoma distinctly annulated; annuli relatively wide at ca. 1.5–2.0 µm. Opisthosomal organ absent. Vulva 7 (6–8) long, located below posterior adges of epimeral plates IV.
Immature stages. Not found; only adults were found in the remains of nymphal exuviae.
Material deposition: Male holotype (reg. no. UGDIZPRAfDDm08m), 16 male paratypes (reg. no. UGDIZPRAfDDm01m–07m, UGDIZPRAfDDm09m–17m) and 12 female paratypes (reg. no. UGDIZPRAfDDt01f–12f); skin of the chin; host Apodemus flavicollis (reg. no. MRMAf09/2019, MRMAf11/2019, MRMAf07/2021a, MRMAf07/2021b, MRMAf08/2021, MRMAf08/2022a, MRMAf08/2022b); Gdynia, Kartuzy, Tczew, Ulkowy, Poland; September 2019, November 2019, July 2021, August 2021, and August 2022; coll. J.N. Izdebska, K. Cierocka and L. Rolbiecki; the whole-type material (mounted microscope slides) is deposited within the framework of the Collection of Extant Invertebrates in Department of Invertebrate Zoology and Parasitology, University of Gdańsk, Poland.
Infection and location in the host: Demodex mediocris sp. nov. was found in 35% of examined yellow-necked field mouse, with a mean intensity of 3.9 and an intensity range 1–11; 29 (17 males and 12 females) individuals were noted. The demodecid mites were found in the chin of the examined yellow-necked field mice. The observed mites did not cause any lesions in examined hosts.
Etymology: The specific epithet mediocris refers to metric features and morphological structures that are average for demodecid mites.
Differential diagnosis: Demodex mediocris sp. nov. from the yellow-necked field mouse resembles D. corniculatus from the same host in terms of size and shape [22]. However, D. corniculatus shows clear sexual dimorphism: males are smaller and cylindrical; also, females have a spindle-shaped opisthosoma. In D. mediocris sp. nov., the sizes of both sexes are similar, and spindle-shaped opisthosoma are sometimes observed in males (Table 4). The gnathosoma of D. mediocris sp. nov. is oval and shorter than the width at the base, while that of D. corniculatus is rectangular and longer than the width at the base. The supracoxal spines in D. mediocris sp. nov. are smaller (3–3.5 µm), peg-like, while in D. corniculatus they are massive, larger (5–6 µm), and wedge-shaped. Both species present three spines on the terminal segments of the palpi, but in D. mediocris sp. nov., the spines are smaller (one small, two slightly larger) and conical; in D. corniculatus they are larger, massive, as if forming a single bifurcated structure. The subgnathosomal setae in D. mediocris sp. nov are located on both sides of the pharyngeal bulb at the level of its anterior edge, while in D. corniculatus they are slightly above its anterior edge. The opisthosomal organ in D. mediocris sp. nov. is very complex, i.e., composed of an upper spindle-shaped structure and a lower spirally twisted one, while in D. corniculatus it is arc-shaped. Furthermore, the aedeagus of the male D. mediocris sp. nov. is located at the level of epimeral plates II–III, while in D. corniculatus it is located at the level of epimeral plates III–IV. These species also demonstrate distinct location preferences: D. mediocris sp. nov. was found only in the less hairy region of the chin, while D. corniculatus occurs in the hairy skin of the entire body.

Table 4.
Morphometric comparison between Demodex mediocris sp. nov. and Demodex corniculatus.
3.2. New Record of Psorergatidae
Psorergates muricola was found in three out of the nine examined yellow-necked mouse (Table 5, Figure 4). A total of 291 mites were found (90 females, 28 males and 173 nymphs). The prevalence was 33%, with a mean intensity of 97.3. All individuals of P. muricola were recorded in the genital–anal area. No skin lesions were observed in infected hosts.

Table 5.
Body size (mean, range and ±SD, in µm) for Psorergates muricola.
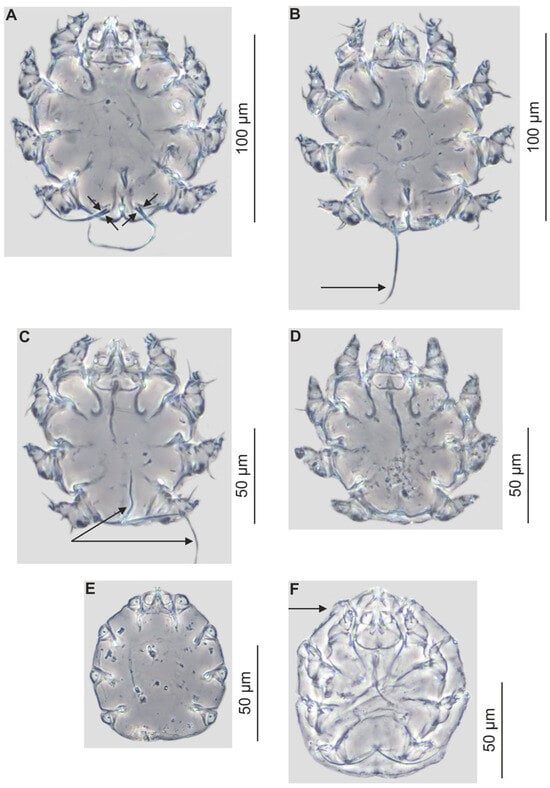
Figure 4.
Psorergates muricola: female with four terminal setae visible, arrows (A); female with one terminal seta, arrow (B); male with two terminal setae visible, arrows (C); male without terminal setae (D); nymph (E); adult with visible remains of nymphal exuviae, arrow (F).
3.3. The Co-Occurrence of Demodecidae and Psorergatidae
In the 20 examined yellow-necked mice, 5 species of skin mites were found: Demodex corniculatus (prevalence 100%, mean intensity 18.5, intensity range 2–48), D. tenuis sp. nov. (45%, 2.4, 1–6), D. mollis (40%, 6.0, 2–16), D. mediocris sp. nov. (35%, 3.9, 1–11), and Psorergates muricola (15%, 97.0, 5–280). In 1 mouse, mites from 5 species co-occurred (a total of 351 specimens), in 3 mice—4 mite species co-occurred, in another 3 mice—3 mite species, in 7 mice—2 mite species, while in 5 mice, only 1 mite species was noted (Figure 5 and Figure 6).
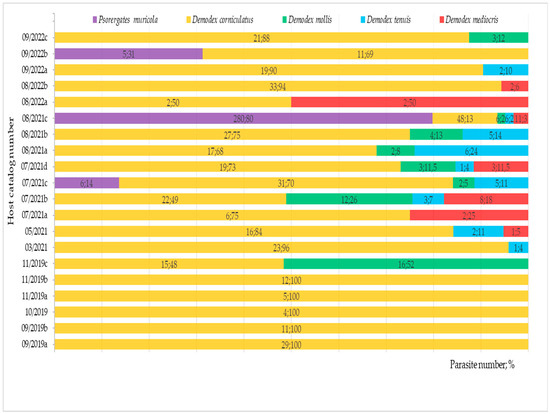
Figure 5.
The co-occurrence (number, %) of Demodecidae and Psorergatidae in the examined Apodemus flavicollis.
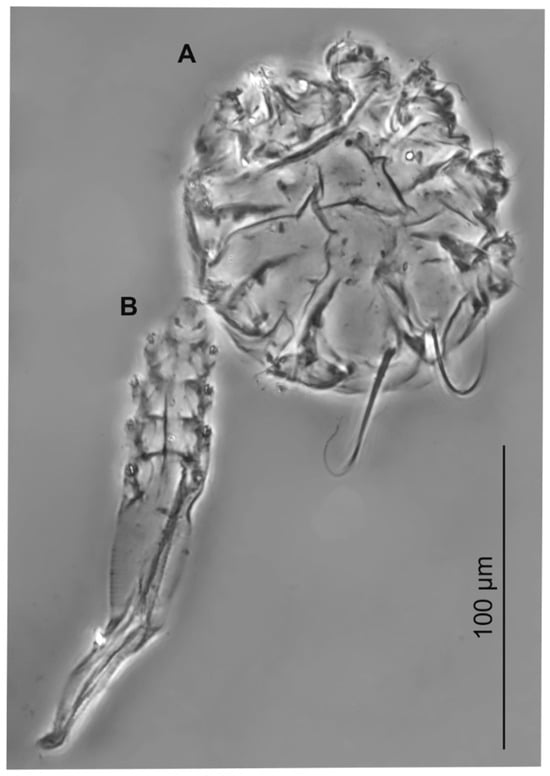
Figure 6.
The co-occurrence of Psorergates muricola (A) and Demodex corniculatus (B) in the same skin fragment.
The dominant parasite was D. corniculatus, found in all mice, in various locations of the hairy skin of the body (a total of 371 specimens). However, the highest density was found for P. muricola—291 individuals were found only in the genital–anal areas in three mice. The level of infection with this mite was varied—in 1 mouse 280 specimens were found, and in the other 2 mice only 6 and 5 mites were found. No skin lesions were observed in the mice.
3.4. Biodiversity of Demodecidae and Psorergatidae in Rodentia
Of the 54 rodent species (from 8 families) studied so far, 46 Demodecidae and 34 Psorergatidae species were documented. The highest species richness (10) was found in the house mouse (Table 6).

Table 6.
A checklist of Demodecidae and Psorergatidae reported in rodents.
4. Discussion
The biodiversity of mammalian skin mites remains poorly understood, with most species described from rodents [1,18,19].
In the case of Demodecidae, the co-occurrence of several host-specific (monoxenic) species in different host microhabitats is commonly observed, with the largest number of synhospital species being identified in the Muridae: seven in M. musculus, five each in A. sylvaticus and R. norvegicus, and four in A. agrarii [1]. Until now, three species have been identified in A. flavicollis, including D. rosus from the tongue, D. corniculatus from the hairy skin of the body, and D. mollis from the eyelid region [11,21,22,65]. A large number of species from various microhabitats in other murids indicated the likely occurrence of further, unknown mite species in A. flavicollis. However, their discovery was complicated by the low levels of infection intensity, asymptomatic infection, the microscopic size and hidden lifestyle of these mites, as well as their spatially limited microhabitat within the host and low prevalences of infection. The two new species described in the present study, D. tenuis sp. nov. and D. mediocris sp. nov., have very narrow microhabitats, similarly to the previously identified D. rosus or D. mollis. Hence, they probably show much lower infection parameters than D. corniculatus [11,22]. In turn, their narrow spectrum of microhabitats and low intensity of infection limits the possibility of co-occurrence; as such, the co-occurrence of various species with D. corniculatus was most often observed, the most numerous and most widely distributed in the skin of this host.
In turn, the parasites of the family Psorergatidae often are oligoxenic: among the 72 known species, the largest number (34 species) has been shown in rodents, with most being found in Cricetidae and Muridae, and 5 species, the largest number, observed in the common vole Microtus arvalis [117].
In the present study, P. muricola obtained from the yellow-necked field mouse was described based on individuals obtained from the abscess behind the ear in the dark-colored brush-furred rat Lophuromys aquilus and in the ear pinna of a Southern African vlei rat Otomys irroratus from the Democratic Republic of the Congo [30]. It was subsequently found in other representatives of Muridae, both from Africa and Europe: Peters’s hybomys Hybomys univittatus, house mouse, laboratory mouse, common field mouse, and the rusty-bellied brush-furred rat Lophuromys sikapusi. The parasite was detected mainly in the ears or their vicinity, but also in the inter alia regions of the eyes, vibrissae, nose, and in the skin all over the body [20,31].
The occurrence of P. muricola was often correlated with the appearance of skin lesions in the form of skin nodules [31], which is quite a characteristic symptom of occurrence in the case of the family Psorergatidae [33,57,59,83,87,88,101,102,103,104,105,115]. Most species of Psorergatidae have been found and described in hosts in which skin lesions have been observed. Conversely, cases of asymptomatic Psorergatidae infections have been reported only rarely [17,20,112]. This may be related to their small size and difficulties in preparation, or the lack of data on their preferences for location in the host body, frequency of occurrence, and level of infection. Identification of these mites may also be problematic. Certain features merit particular consideration in the diagnosis, differentiating P. muricola from other representatives of the genus. For example, for females of P. muricola and P. oettlei, it is only a difference in dimensions (e.g., the length and width of the body). In turn, for males of these species, the criterion is the lack of terminal setae in P. oettlei. Intact terminal setae, located at the end of the adult idiosoma, are characterized by considerable length [19] and may be subject to damage. During the current studies on Psorergatidae, damaged setae, or their complete absence, were repeatedly observed, which is also confirmed by earlier observations [17,19,112].
The current literature data confirm that Psorergatidae are characterized by different ranges of host specificity: they can be monoxenic or oligoxenic (Table 6) [19,26]. However, most species are known from a few records, without parasitological analyses based on larger numbers of hosts. Therefore, the degree of specificity and the host range require verification. The last time the Psorergatidae were subjected to meticulous analyses was over 30 years ago [19], and as such, the taxonomy and biology of the group are very poorly known. For example, it is also questionable whether Psorergates simplex, P. apodemi and P. musculinus possess a wide range of host specificity, although the species have been recorded in several members of the Cricetidae and Muridae families. Importantly, the wide host range of Psorergates muricola, including Eurasian, cosmopolitan and African species, is also relatively uncertain. It is possible that this taxon may represent two species with different geographical ranges, but to confirm this requires further criteria for differentiation. It is possible that different species exhibit different parasitism strategies, with different levels of association with the host, showing conservatism or plasticity in establishing host–parasite relationships with rodent species.
Regardless of the taxonomic doubts related to the species identification of Psorergatidae, our data confirm the co-occurrence of these mites with representatives of Demodecidae. Although the parasites sometimes occupy neighboring microhabitats, they are always characterized by a low infection intensity/density, and hence do not have a significant impact on the host, i.e., no disease symptoms were observed.
Author Contributions
Conceptualization, J.N.I., K.C. and L.R.; sampling, K.C., J.N.I. and L.R.; morphological analysis of Demodecidae: J.N.I., K.C. and L.R.; morphological analysis of Psorergatidae: K.C. and J.N.I.; parasitological analysis: K.C., J.N.I. and L.R.; original draft, J.N.I., K.C. and L.R.; review and editing, K.C., J.N.I. and L.R.; supervision, J.N.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of our article will be made available by the corresponding authors on request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Izdebska, J.N.; Rolbiecki, L. The biodiversity of Demodecid Mites (Acariformes: Prostigmata), specific parasites of mammals with a global checklist and a new finding for Demodex sciurinus. Diversity 2020, 12, 261. [Google Scholar] [CrossRef]
- Izdebska, J.N.; Rolbiecki, L. The status of Demodex cornei: Description of the species and developmental stages, and data on demodecid mites in the domestic dog Canis lupus familiaris. Med. Vet. Entomol. 2018, 32, 346–357. [Google Scholar] [CrossRef] [PubMed]
- Izdebska, J.N.; Rolbiecki, L.; Fryderyk, S. Demodex murilegi and Demodex obliquus, two new specific skin mites from domestic cat Felis catus, with notes on parasitism. Med. Vet. Entomol. 2023, 37, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Izdebska, J.N.; Rolbiecki, L.; Rehbein, S. Two new species of parasitic demodecid mites in the European polecat Mustela putorius and their co-infestation with Miridex putorii (Acariformes: Demodecidae). Eur. Zool. J. 2024, 91, 568–589. [Google Scholar] [CrossRef]
- Izdebska, J.N.; Rolbiecki, L.; Bielecki, W. Demodex bialoviensis sp. nov. (Acariformes, Demodecidae) a new, specific parasite of the European bison Bison bonasus (Artiodactyla, Bovidae). Int. J. Parasitol. Parasites Wildl. 2022, 17, 138–143. [Google Scholar] [CrossRef]
- Izdebska, J.N.; Rolbiecki, L.; Bielecki, W. The first data on parasitic arthropods of the European bison in the summer season with a world checklist. Diversity 2022, 14, 75. [Google Scholar] [CrossRef]
- Cierocka, K.; Izdebska, J.N.; Rolbiecki, L. Demodex foveolator (Acariformes: Demodecidae) from Crocidura suaveolens (Soricomorpha: Soricidae)—The second observation worldwide, and a checklist of the demodecid mites of soricomorphs. Ann. Parasitol. 2019, 65, 329–332. [Google Scholar]
- Cierocka, K.; Izdebska, J.N.; Rolbiecki, L. Demodex crocidurae, a new demodecid mite (Acariformes: Prostigmata) parasitizing the lesser white-toothed shrew and a redescription of Demodex talpae from European mole with data on parasitism in Soricomorpha. Animals 2021, 11, 2712. [Google Scholar] [CrossRef]
- Izdebska, J.N.; Cierocka, K.; Rolbiecki, L. Demodex labialis sp. nov. (Acariformes: Demodecidae) from the European mole Talpa europaea in the background of the Soricomorpha demodecid mites. Ann. Zool. 2024; in press. [Google Scholar]
- Izdebska, J.N.; Rolbiecki, L. A new species of Demodex (Acari, Demodecidae) with data on topical specificity and topography of demodectic mites in the striped field mouse Apodemus agrarius (Rodentia, Muridae). J. Med. Entomol. 2013, 50, 1202–1207. [Google Scholar] [CrossRef]
- Izdebska, J.N.; Rolbiecki, L.; Fryderyk, S.; Mierzyński, Ł. Adult and immature stages of the new species of the genus Demodex (Acariformes: Demodecidae) with data on parasitism, topography, and topical specificity of demodecid mites in the yellow-necked mouse, Apodemus flavicollis (Rodentia: Muridae). J. Parasitol. 2017, 103, 320–329. [Google Scholar] [CrossRef]
- Bukva, V. Demodex species (Acari: Demodecidae) parasitizing the brown rat, Rattus norvegicus (Rodentia): Redescription of Demodex ratti and description of D. norvegicus sp. n. and D. ratticola sp. n. Folia Parasitol. 1995, 42, 149–160. [Google Scholar]
- Izdebska, J.N.; Rolbiecki, L. New species of Demodex (Acari: Demodecidae) with data on parasitism and occurrence of other demodecids of Rattus norvegicus (Rodentia, Muridae). Ann. Entomol. Soc. Am. 2014, 107, 740–747. [Google Scholar] [CrossRef]
- Izdebska, J.N.; Rolbiecki, L. A new species of the genus Demodex Owen, 1843 (Acari: Demodecidae) from the ear canals of the house mouse Mus musculus L. (Rodentia: Muridae). Syst. Parasitol. 2015, 91, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Izdebska, J.N.; Rolbiecki, L. Two new species of Demodex (Acari: Demodecidae) with a redescription of Demodex musculi and data on parasitism in Mus musculus (Rodentia: Muridae). J. Med. Entomol. 2015, 52, 604–613. [Google Scholar] [CrossRef]
- Izdebska, J.N.; Rolbiecki, L. A new genus and species of demodecid mites from the tongue of a house mouse Mus musculus: Description of adult and immature stages with data on parasitism. Med. Vet. Entomol. 2016, 30, 135–143. [Google Scholar] [CrossRef]
- Cierocka, K.; Izdebska, J.N.; Rolbiecki, L.; Ciechanowski, M. The occurrence of skin mites from the Demodecidae and Psorergatidae (Acariformes: Prostigmata) families in bats, with a description of a new species and new records. Animals 2022, 12, 875. [Google Scholar] [CrossRef]
- Bochkov, A.V. A review of mites of the parvorder Eleutherengona (Acariformes: Prostigmata)—Permanent parasites of mammals. Acarina 2009, 1, 1–149. [Google Scholar]
- Giesen, K.M.T. A review of the parasitic mite family Psorergatidae (Cheyletoidea: Prostigmata: Acari) with hypotheses on the phylogenetic relationships of species and species groups. Zool. Verh. 1990, 259, 1–69. [Google Scholar]
- Izdebska, J.N.; Fryderyk, S. New for the fauna of Poland species of Psorergates spp. with the data of occurrence of mites from Psorergatidae family (Acari, Prostigmata) in native mammals. Ann. Parasitol. 2012, 58, 19–22. [Google Scholar]
- Izdebska, J.N.; Fryderyk, S. Recent data on Demodex rosus Bukva, Vitovec et Vlcek, 1985 (Acari, Demodecidae) from oral cavity of yellow-necked field mouse, Apodemus flavicollis (Rodentia, Muridae). Wiad. Parazytol. 2011, 57, 257–260. [Google Scholar]
- Izdebska, J.N. A new Demodecidae species (Acari) from the yellow-necked mouse Apodemus flavicollis (Rodentia, Muridae)—Description with data on parasitism. J. Parasitol. 2012, 98, 1101–1104. [Google Scholar] [CrossRef] [PubMed]
- Izdebska, J.N. Demodex spp. (Acari: Demodecidae) in brown rat (Rodentia: Muridae) in Poland. Wiad. Parazytol. 2004, 50, 333–335. [Google Scholar] [PubMed]
- Zhang, Z.Q. Repositories for mite and tick specimens: Acronyms and their nomenclature. Syst. Appl. Acarol. 2018, 23, 2432–2446. [Google Scholar] [CrossRef]
- Nutting, W.B. Hair follicle mites (Demodex spp.) of medical and veterinary concern. Cornell. Vet. 1976, 66, 214–231. [Google Scholar]
- Bochkov, A.V. New observations on phylogeny of cheyletoid mites (Acari: Prostigmata: Cheyletoidea). Proc. Zool. Inst. RAS 2008, 312, 54–73. [Google Scholar] [CrossRef]
- Wilson, D.E.; Reeder, D.M. (Eds.) Mammals Species of the World: A Taxonomic and Geographic Reference, 3rd ed.; The Johns Hopkins University Press: Baltimore, MD, USA, 2005; Available online: http://www.departments.bucknell.edu/biology/resources/msw3/ (accessed on 8 July 2024).
- Integrated Taxonomic Information System (ITIS). Available online: http://www.itis.gov (accessed on 8 August 2024).
- Bush, A.O.; Lafferty, K.D.; Lotz, J.M.; Shostak, A.W. Parasitology meets ecology on its own terms: Margolis et al. revisited. J. Parasitol. 1997, 83, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Fain, A. Notes sur le genre Psorergates Tyrrell. Description de Psorergates ovis Womersley et d’une espèce nouvelle. Acarologia 1961, 3, 60–71. [Google Scholar]
- Fain, A.; Lukoschus, F.; Hallmann, P. Le genre Psorergates chez les muridés. Description de trois espèces nouvelles (Psorergatidae: Trombidiformes). Acarologia 1966, 8, 251–274. [Google Scholar]
- Lukoschus, F.; Fain, A.; Beaujean, M.M.J. Beschreibung neuer Psorergates-Arten (Psorergatidae: Trombidiformes). Tijdschr. Entomol. 1967, 110, 133–181. [Google Scholar]
- Fain, A. Sur un cas de gale chez un rat-taupe (Cryptomys hottentotus) produite par un acarien du genre Psorergates (Psorergatidae: Trombidiformes). Acarologia 1965, 7, 295–300. [Google Scholar]
- Kok, N.J.J.; Lukoschus, F.S.; Clulow, F.V. Psorobia castoris spec. nov. (Acarina: Psorergatidae), a new itch mite from the beaver, Castor canadensis. Can. J. Zool. 1970, 48, 1419–1423. [Google Scholar] [CrossRef]
- Izdebska, J.N.; Fryderyk, S.; Rolbiecki, L. Demodex castoris sp. nov. (Acari: Demodecidae) parasitizing Castor fiber (Rodentia), and other parasitic arthropods associated with Castor spp. Dis. Aquat. Org. 2016, 118, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bacigalupo, J.; Roveda, R.J. Demodex caviae n. sp. Rev. Med. Vet. 1954, 36, 149–153. [Google Scholar]
- Ballweber, L.R.; Harkness, J.E. Parasites of guinea pigs. In Flynn’s Parasites of Laboratory Animals; Baker, D.G., Ed.; Blackwell Publishing: Ames, IA, USA, 2007; pp. 421–449. [Google Scholar]
- Schönfelder, J.; Henneveld, K.; Schönfelder, A.; Hein, J.; Müller, R. Concurrent infestation of Demodex caviae and Chirodiscoides caviae in a guinea pig. A case report. Tierarztl. Prax. Kleintiere. 2010, 38, 28–30. [Google Scholar] [CrossRef][Green Version]
- Hirst, S. On some new or little-known species of Acari, mostly parasitic in habit. Proc. Zool. Soc. Lond. 1921, 1, 357–378. [Google Scholar] [CrossRef]
- Desch, C.E.; Hurley, R.J. Demodex sinocricetuli: New species of hair follicle mite (Acari: Demodecidae) from the Chinese form of the striped hamster, Cricetulus barabensis (Rodentia: Muridae). J. Med. Entomol. 1997, 34, 317–320. [Google Scholar] [CrossRef] [PubMed]
- Hurley, R.J.; Desch, C.E. Demodex cricetuli: New species of hair follicle mite (Acari: Demodecidae) from the Armenian hamster, Cricetulus migratorius (Rodentia: Cricetidae). J. Med. Entomol. 1994, 31, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Nutting, W.B. Demodex aurati sp. nov. and D. criceti, ectoparasites of the golden hamster (Mesocricetus auratus). Parasitology 1961, 51, 515–522. [Google Scholar] [CrossRef]
- Nutting, W.B.; Rauch, H. Distribution of Demodex aurati in the host (Mesocricetus auratus) skin complex. J. Parasitol. 1963, 49, 323–329. [Google Scholar] [CrossRef]
- Owen, D.; Young, C. The occurrence of Demodex aurati and Demodex criceti in the Syrian hamster (Mesocricetus auratus) in the United Kingdom. Vet. Rec. 1973, 17, 282–284. [Google Scholar] [CrossRef]
- Retnasabapathy, A.; Lourdusamy, D. Demodex aurati and Demodex criceti in the golden hamster (Mesocricetus auratus). Southeast. Asian J. Trop. Med. Public Health. 1974, 5, 460. [Google Scholar]
- Cardoso, M.J.L.; Franco, S.R.V.S. Demodicosis in golden hamster (Mesocricetus auratus)—Case in Brasil. Ars Vet. 2003, 19, 126–128. [Google Scholar]
- Karaer, Z.; Kurtdede, A.; Ural, K.; Sari, B.; Cingi, C.C.; Karakurum, M.C.; Haydardedeoglu, A.E. Demodicosis in a golden (Syrian) hamster (Mesocricetus auratus). Ankara Univ. Vet. Fak. Derg. 2009, 56, 227–229. [Google Scholar]
- Brosseau, G. Oral fluralaner as a treatment for Demodex aurati and Demodex criceti in a golden (Syrian) hamster (Mesocricetus auratus). Can. Vet. J. 2020, 61, 135–137. [Google Scholar] [PubMed]
- Nutting, W.B.; Rauch, H. Demodex criceti n. sp. (Acarina: Demodicidae) with notes on its biology. J. Parasitol. 1958, 44, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Fehr, M.; Koestlinger, S. Ectoparasites in small exotic mammals. Vet. Clin. Exot. Anim. 2013, 16, 611–657. [Google Scholar] [CrossRef] [PubMed]
- Hirst, S. Studies on Acari. No. 1. the Genus Demodex, Owen; British Museum (Natural History): London, UK, 1919; pp. 1–44. [Google Scholar]
- Bregetova, N.G.; Bulanova-Zahvatkina, E.M.; Volgin, V.I.; Dubinin, V.B.; Zahvatkin, A.A.; Zemskaâ, A.A.; Lange, A.B.; Pavlovskij, E.N.; Serdûkova, G.V.; Šluger, E.G. Mites of Rodents of the USSR Fauna; Izdatel’stvo Akademii Nauk SSSR: Moskva/Leningrad, Russia, 1955; pp. 1–460. [Google Scholar]
- Michael, A.D. On some unrecorded parasitic Acari found in Great Britain. Zool. J. Linn. Soc. 1889, 20, 400–406. [Google Scholar] [CrossRef][Green Version]
- Dubinin, V.B. Novaya klassifikatsiya kleshchey nadsemeystv Cheyletoidea W. Dub. i Demodicoidea W. Dub. (Acariformes, Trombidiformes). Parazitol. Sb. Zool. Inst. Akad. Nauk SSSR 1957, 17, 71–136. [Google Scholar]
- Izdebska, J.N.; Rolbiecki, L. Demodex microti n. sp. (Acari: Demodecidae) in Microtus arvalis (Pallas) (Rodentia, Cricetidae) with a checklist of the demodecid mites of cricetids. Syst. Parasitol. 2013, 86, 187–196. [Google Scholar] [CrossRef]
- Haitlinger, R. Psorergates dissimilis Fain, Lukos., Hallm. i Psorergates apodemi Fain, Lukos., Hallm. (Psorergatidae; Acarina) dwa nowe gatunki roztoczy dla fauny Polski. Prz. Zool. 1978, 22, 143–145. [Google Scholar]
- Sosnina, E.F. On mites of the genus Psorergates (Trombidiformes: Psorergatidae)—Parasites of Muridae and Cricetidae in the USSR. Parazitologiya 1970, 4, 537–541. [Google Scholar]
- Sosnina, E.F.; Skljar, V.E. On mites of the genus Psorergates (Trombidiformes: Psorergatidae), parasites of rodents from the Donezk district of the Ukraine. Parazitologiya 1971, 5, 291–292. [Google Scholar]
- Neumann, M.G. Sur un acarien (Psorergates simplex Tyrrell) de la souris. Bull. Soc. Hist. Nat. 1893, 27, 13–22. [Google Scholar]
- Lukoschus, F.S.; De Cock, A.W.A.M.; Driesseni, F.M. Four new species of the genus Psorergates Tyrell from European hosts (Acarina, Psorergatidae). Tijdschr. Entomol. 1971, 114, 185–200. [Google Scholar]
- Kok, N.J.J.; Lukoschus, F.S.; Clulow, F.V. Three new itch mites from Canadian small mammals (Acarina Psorergatidae). Can. J. Zool. 1971, 49, 1239–1248. [Google Scholar] [CrossRef]
- Giesen, K.M.T.; Lukoschus, F.S.; Whitaker, J.O.; Gettinger, D. Four new species of itch mites (Acari: Psorergatidae: Prostigmata) from small mammals in North America. J. Med. Entomol. 1983, 20, 164–173. [Google Scholar] [CrossRef]
- Haitlinger, R. Psorergates polonicus sp. n. (Acari, Prostigmata, Psorergatidae) from Pitymys subterraneus (De Sel. Longh.). Pol. Pismo Entomol. 1986, 56, 425–426. [Google Scholar]
- Nutting, W.B.; Emejuaiwe, S.O.; Tisdel, M.O. Demodex gapperi sp. n. (Acari: Demodicidae) from the red-backed vole, Clethrionomys gapperi. J. Parsitol. 1971, 57, 660–665. [Google Scholar] [CrossRef]
- Bukva, V.; Vítovec, J.; Vlcek, M. Demodex rosus sp. n. and D. buccalis sp. n. (Acari: Demodicidae) parasitizing the upper digestive tract of rodents. Folia Parasitol. 1985, 32, 151–162. [Google Scholar]
- Izdebska, J.N.; Kozina, P.; Gólcz, A. The occurrence of Demodex spp. (Acari, Demodecidae) in bank vole Myodes glareolus (Rodentia, Cricetidae) with data on its topographical preferences. Ann. Parasitol. 2013, 59, 129–133. [Google Scholar]
- Grigorjeva, L.A. Peculiarities of the skin lesions in small mammals parasited by Psorergates apodemi and P. dissimilis (Cheyletoidea: Psorergatidae). Parazitologiya 2007, 41, 235–239. [Google Scholar]
- Hughes, S.E.; Nutting, W.B. Demodex leucogasteri n. sp. from Onychomys leucogaster—With notes on its biology and host pathogenesis. Acarologia 1981, 22, 181–186. [Google Scholar]
- Lombert, H.A.P.M.; Lukoschus, F.S.; Whitaker, J.O. Demodex peromysci, n. sp. (Acari: Prostigmata: Demodicidae), from the meibomian glands of Peromyscus leucopus (Rodentia: Cricetidae). J. Med. Entomol. 1983, 20, 377–382. [Google Scholar] [CrossRef]
- Desch, C.E.; Davis, S.L.; Klompen, H. Two new species of Demodex Owen, 1843, the hair follicle mites (Demodecidae), from the dzungarian hamster, Phodopus sungorus (Pallas, 1773) (Rodentia: Muridae). Int. J. Acarol. 2006, 32, 75–80. [Google Scholar] [CrossRef]
- Hirst, S. Remarks on certain species of the genus Demodex, Owen (the Demodex of man, the horse, dog, rat, and mouse). Ann. Mag. Nat. Hist. Ser. 8 1917, 20, 232–235. [Google Scholar] [CrossRef]
- Till, W.M. Two new parasitic mites (Acarina) from the South African porcupine. Parasitology 1957, 47, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Bukva, V. Demodex agrarii sp. n. (Acari: Demodecidae) from cerumen and the sebaceous glands in the ears of the striped field mouse, Apodemus agrarius (Rodentia). Folia Parasitol. 1994, 41, 305–311. [Google Scholar]
- Izdebska, J.N.; Cydzik, K. Occurrence of Demodex spp. (Acari, Demodecidae) in the striped field mouse Apodemus agrarius (Rodentia, Muridae) in Poland. Wiad. Parazytol. 2010, 56, 59–61. [Google Scholar]
- Mertens, L.A.J.M.; Lukoschus, F.S.; Nutting, W.B. Demodex huttereri spec. nov. (Acarina: Prostigmata: Demodicidae) from the meibomian glands of Apodemus agrarius (Rodentia: Muridae). Bonn. Zool. Beitr. 1983, 34, 489–498. [Google Scholar]
- Izdebska, J.N.; Rolbiecki, L.; Fryderyk, S. New data on distribution of Demodex huttereri Mertens, Lukoschus et Nutting, 1983 and topical specificity and topography of demodectic mites in striped field mouse Apodemus agrarius. Wiad. Parazytol. 2011, 57, 261–264. [Google Scholar]
- Beron, P. Catalogue des Acariens parasites et commensaux des Mammifères en Bulgarie. Bull. Inst. Zool. Mus. Sofia. 1973, 37, 167–199. [Google Scholar]
- Hirst, S. XI.—On four new species of the genus Demodex, Owen. Ann. Mag. Nat. Hist. Ser. 9 1918, 2, 145–146. [Google Scholar] [CrossRef]
- Izdebska, J.N.; Rolbiecki, L.; Fryderyk, S. Demodex auricularis sp. nov. (Acari: Demodecidae) from the ear canal of the European wood mouse Apodemus sylvaticus (Rodentia: Muridae). Int. J. Acarol. 2014, 40, 214–219. [Google Scholar] [CrossRef]
- Lukoschus, F.S.; Jongman, R.G.H. Demodex lacrimalis spec. nov. (Demodicidae: Trombidiformes) from the Meibomian glands of the European wood mouse Apodemus sylvaticus. Acarologia 1974, 16, 274–281. [Google Scholar]
- Izdebska, J.N.; Fryderyk, S. New data on the occurrence of Demodex lacrimalis (Acari, Demodecidae) of the wood mouse Apodemus sylvaticus (Rodentia, Muridae). Ann. UMCS Biol. 2012, 67, 7–11. [Google Scholar] [CrossRef][Green Version]
- Bukva, V.; Nutting, W.B.; Desch, C.E. Description of Ophthalmodex apodemi sp.n. (Acari: Demodecidae) from the ocular area of Apodemus sylvaticus (Rodentia: Muridae) with notes on pathogenicity. Int. J. Acarol. 1992, 18, 269–276. [Google Scholar] [CrossRef]
- Rioux, J.A.; Golvan, Y.J. Gale a Psorergates musculinus (Michale 1889) (Acari; Myobiidae) chez les mulots de la région de Montpellier. C. R. Hebd. Seances Acad. Sci. Paris 1961, 232, 86–88. [Google Scholar]
- Izdebska, J.N.; Rolbiecki, L.; Morand, S.; Ribas, A. A new species and new host record of Demodecidae (Acariformes: Prostigmata) associated with the bandicoot rat (Rodentia: Muridae) from Lao PDR with data on parasitism and a checklist of the demodecid mites of rodents. Syst. Appl. Acarol. 2017, 22, 1910–1923. [Google Scholar] [CrossRef]
- Desch, C.E.; Lukoschus, F.S.; Nadchatram, M. A new demodicid (Acari: Demodicidae) from the meibomian glands of Southeast Asian rats (Rodentia: Muridae). Trop. Biomed. 1984, 1, 55–62. [Google Scholar]
- Fain, A.; Lukoschus, F.S.; Rack, G. Notes on parasitic mites from some small mammals in Liberia. Mitt. Hamb. Zool. Mus. Inst. 1974, 71, 165–174. [Google Scholar]
- Lavoipierre, M. New records of Acari from Southern Africa and the Belgian Congo. J. Entomol. Soc. South. Afr. 1946, 9, 78–81. [Google Scholar] [PubMed]
- Till, W.M. Psorergates oettlei n. sp., a new mange-causing mite from the multimammate rat (Acarina, Psorergatidae). Acarologia 1960, 2, 75–79. [Google Scholar]
- Bukva, V. Demodex flagellurus sp. n. (Acari: Demodicidae) from the preputial and clitoral glands of the house mouse, Mus musculus L. Folia Parasitol. 1985, 32, 73–81. [Google Scholar]
- Izdebska, J.N. Nowe gatunki Demodex spp. (Acari, Demodecidae) u Mus musculus w Polsce. Wiad. Parazytol. 2000, 46, 277–280. [Google Scholar] [PubMed]
- Izdebska, J.N.; Rolbiecki, L. Correlation between the occurrence of mites (Demodex spp.) and nematodes in the house mice (Mus musculus Linnaeus, 1758) in the Gdańsk urban agglomeration. Biol. Lett. 2006, 43, 175–178. [Google Scholar]
- Oudemans, A.C. List of Dutch Acari. 7th part: Acarididae Latr. 1806, and Phytoptidae Pagenst. 1861, with synonymical remarks end description of new species etc. Tijdschr. Entomol. 1897, 40, 250–269. [Google Scholar]
- Ventura, J.; Feliu, C.; Foronda, P.; Francino, O.; Sastre, N. First record of the presence of skin mites (Demodex musculi) in wild house mice from the Canary Islands (Spain). Int. J. Acarol. 2020, 46, 1–4. [Google Scholar] [CrossRef]
- Smith, P.C.; Zeiss, C.J.; Beck, A.P.; Scholz, J.A. Demodex musculi infestation in genetically immunomodulated mice. Comp. Med. 2016, 66, 278–285. [Google Scholar]
- Nashat, M.A.; Luchins, K.R.; Riedel, E.R.; Izdebska, J.N.; Lepherd, M.L.; Lipman, N.S. Characterization of Demodex musculi infestation, associated co-morbidities, and its topographical distribution in a mouse strain with defective adaptive immunity. Comp. Med. 2017, 67, 315–329. [Google Scholar]
- Nashat, M.A.; Ricart Arbona, R.J.; Lepherd, M.L.; Santagostino, S.F.; Livingston, R.S.; Riedel, E.R.; Lipman, N.S. Ivermectin-compounded feed compared with topical moxidectin–imidacloprid for eradication of Demodex musculi in laboratory mice. J. Am. Assoc. Lab. Anim. Sci. 2018, 57, 483–497. [Google Scholar] [CrossRef]
- Izdebska, J.N.; Rolbiecki, L.; Fryderyk, S. A new species of Demodex (Acari: Demodecidae) from the skin of the vibrissal area of the house mouse Mus musculus (Rodentia: Muridae), with data on parasitism. Syst. Appl. Acarol. 2016, 21, 1031–1039. [Google Scholar] [CrossRef]
- Beron, P. Sur quelques Acariens (Myobiidae, Psorergatidae, Spinturnicidae, Sarcoptidae et Listrophoroidea) de Bulgarie et de l’île de Crète. Bull. Inst. Zool. 1970, 32, 143–149. [Google Scholar]
- Haitlinger, R. Trichoecius apodeme Fain, Munting, Lukoschus, 1969 and some mite species (Myocoptidae, Myobiidae, Psorergatidae, Haemogamasidae) new for the fauna of Poland. Wiad. Parazytol. 1987, 33, 81–83. [Google Scholar] [PubMed]
- Tyrrell, J.B. On the occurrence in Canada of two species of parasitic mites. Proc. Can. Inst. 1883, 1, 332–342. [Google Scholar]
- Piana, G.P. Cisti cutanee contenenti acari nei topi. Annuario p. anno scolastico 1885–1886. R. Scuola Sup. Med. Vet. 1886, 1, 122–127. [Google Scholar]
- Dubinin, V.B. Parazitofauna mysevidnykh gryzunov i ee izmeneiya v del’te Volgi. Parazitol. Sb. Zool. Inst. Akad. Nauk SSSR 1953, 15, 252–301. [Google Scholar]
- Flynn, R.J.; Jaroslow, B.N. Nidification of a mite (Psorergates simplex Tyrrell, 1883: Myobiidae) in the skin of mice. J. Parasitol. 1956, 42, 49–52. [Google Scholar] [CrossRef]
- Spicka, E.J. First report of Psorergates simplex Tyrrell, 1883 (Acari: Psorergatidae) from wild house mouse, Mus musculus, in the United States. Proc. Ind. Acad. Sci. 1976, 85, 418–422. [Google Scholar]
- Beresford-Jones, W.P. Occurrence of the mite Psorergates simplex in mice. Aust. Vet. J. 1965, 41, 289–290. [Google Scholar] [CrossRef]
- Kamali, K.; Ostovan, H.; Atamehr, A. A catalog of mites and ticks (Acari) of Iran; Islamic Azad University Scientific Publication Center: Tehran, Iran, 2001; pp. 1–196. [Google Scholar]
- Desch, C.E. Redescription of Demodex nanus (Acari: Demodicidae) from Rattus norvegicus and R. rattus (Rodentia). J. Med. Entomol. 1987, 24, 19–23. [Google Scholar] [CrossRef]
- Izdebska, J.N.; Rolbiecki, L. Występowanie Demodex spp. w korelacji z poziomem zarażenia helmintami szczura wędrownego Rattus norvegicus (Berk.) z aglomeracji miejskiej Trójmiasta. In Fauna Miast Europy Środkowej 21. Wieku; Indykiewiecz, P., Barczak, T., Eds.; LOGO: Bydgoszcz, Poland, 2004; pp. 581–584. [Google Scholar]
- Izdebska, J.N.; Rolbiecki, L. Demodectic mites of the brown rat Rattus norvegicus (Berkenhout, 1769) (Rodentia, Muridae) with a new finding of Demodex ratticola Bukva, 1995 (Acari, Demodecidae). Ann. Parasitol. 2012, 58, 71–74. [Google Scholar] [PubMed]
- Izdebska, J.N.; Rolbiecki, L. Topical structure and topography of Demodex spp. (Acari Demodecidae), in brown rat Rattus norvegicus (Rodentia, Muridae). In Arthropods. The Medical and Economic Importance; Buczek, A., Błaszak, C., Eds.; Akapit: Lublin, Poland, 2012; pp. 133–141. [Google Scholar]
- Fain, A.; Goff, M.L. Psorergates rattus (Acari: Psorergatidae), a new species of parasitic mite from Rattus norvegicus in Hawaii. Int. J. Acarol. 1986, 12, 107–110. [Google Scholar] [CrossRef]
- Cierocka, K.; Izdebska, J.N. Psorergatidae mites infestation in the brown rat Rattus norvegicus (Rodentia, Muridae): The first record of Psorergates rattus (Acariformes, Prostigmata) in Europe. Turk. J. Zool. 2019, 43, 314–317. [Google Scholar] [CrossRef]
- Giesen, K.M.T.; Lukoschus, F.S.; Nadchatram, M. Three new itch mites of the family Psorergatidae (Acari, Prostigmata) from Malaysian small mammals. Malay. Nat. J. 1982, 35, 315–328. [Google Scholar]
- Ah, H.S.; Peckham, J.C.; Atyeo, W.T. Psorergates glaucomys sp. n. (Acari: Psorergatidae), a cystogenous mite from the southern flying squirrel (Glaucomys v. volans), with histopathologic notes on a mite-induced dermal cyst. J. Parasitol. 1973, 59, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Giesen, K.M.T.; Lukoschus, F.S. A new itch mite (Acarina: Prostigmata: Psorergatidae) from the South African bush squirrel Paraxerus cepapi. Bull. Inst. R. Sci. Nat. Belg. Entomol. 1982, 53, 1–9. [Google Scholar]
- Hirst, S. On some new or little-known species of Acari. Proc. Zool. Soc. Lond. 1923, 2, 971–1000. [Google Scholar] [CrossRef]
- Beron, P. Acarorum Catalogus VIII. Trombidiformes, Prostigmata, Superfamilia Cheyletoidea (Cheyletidae, Psorergatidae, Demodecidae, Harpyrhynchidae, Syringophilidae), Superfamilia Cloacaroidea (Cloacaridae, Epimyodicidae); Pensoft & National Museum of Natural History: Sofia, Bulgaria, 2021; p. 465. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).