The Effects of Land Use Changes on the Distribution of the Chinese Endemic Species of Brown-Eared Pheasant
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Data Source
2.2.1. Brown Pheasant Occurrence Point
2.2.2. Sources of Environmental Variables
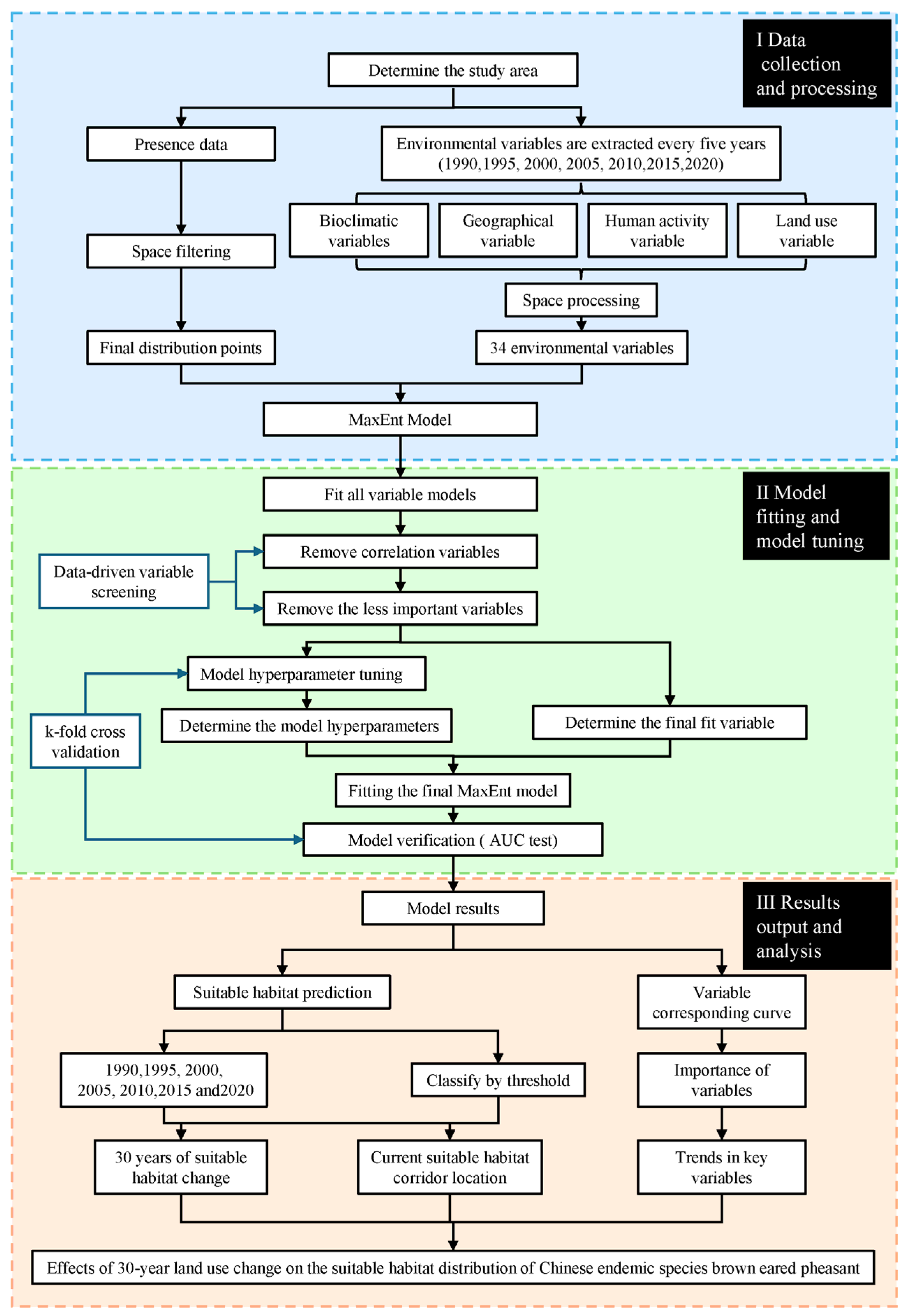
2.3. Methodologies
2.3.1. Data Processing
2.3.2. Modeling Analysis and Model Tuning
2.3.3. Analysis of the Influence of Suitable Habitat Distribution
2.3.4. Corridor Analysis
3. Results
3.1. Modeling Results and Model Evaluation
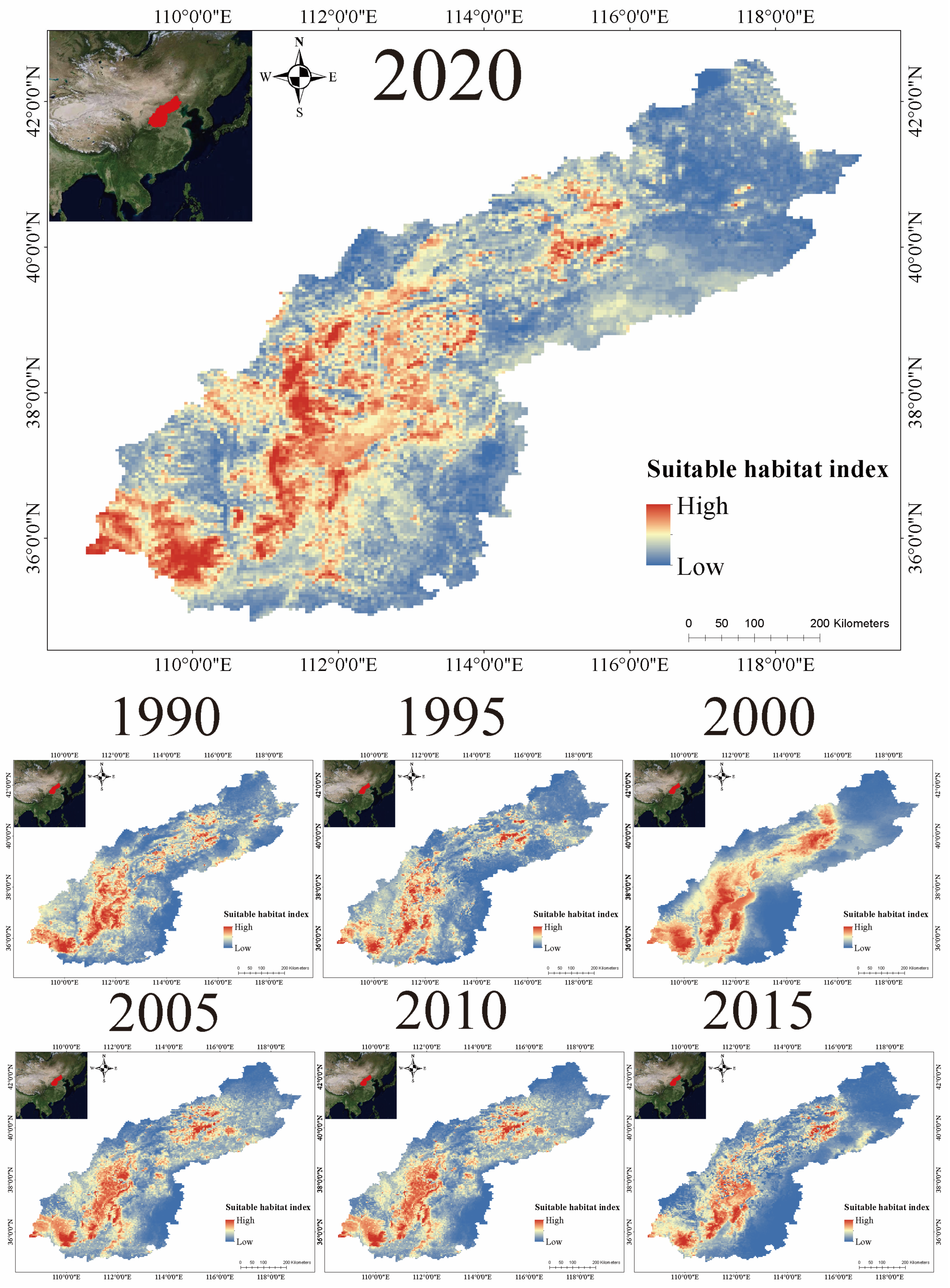
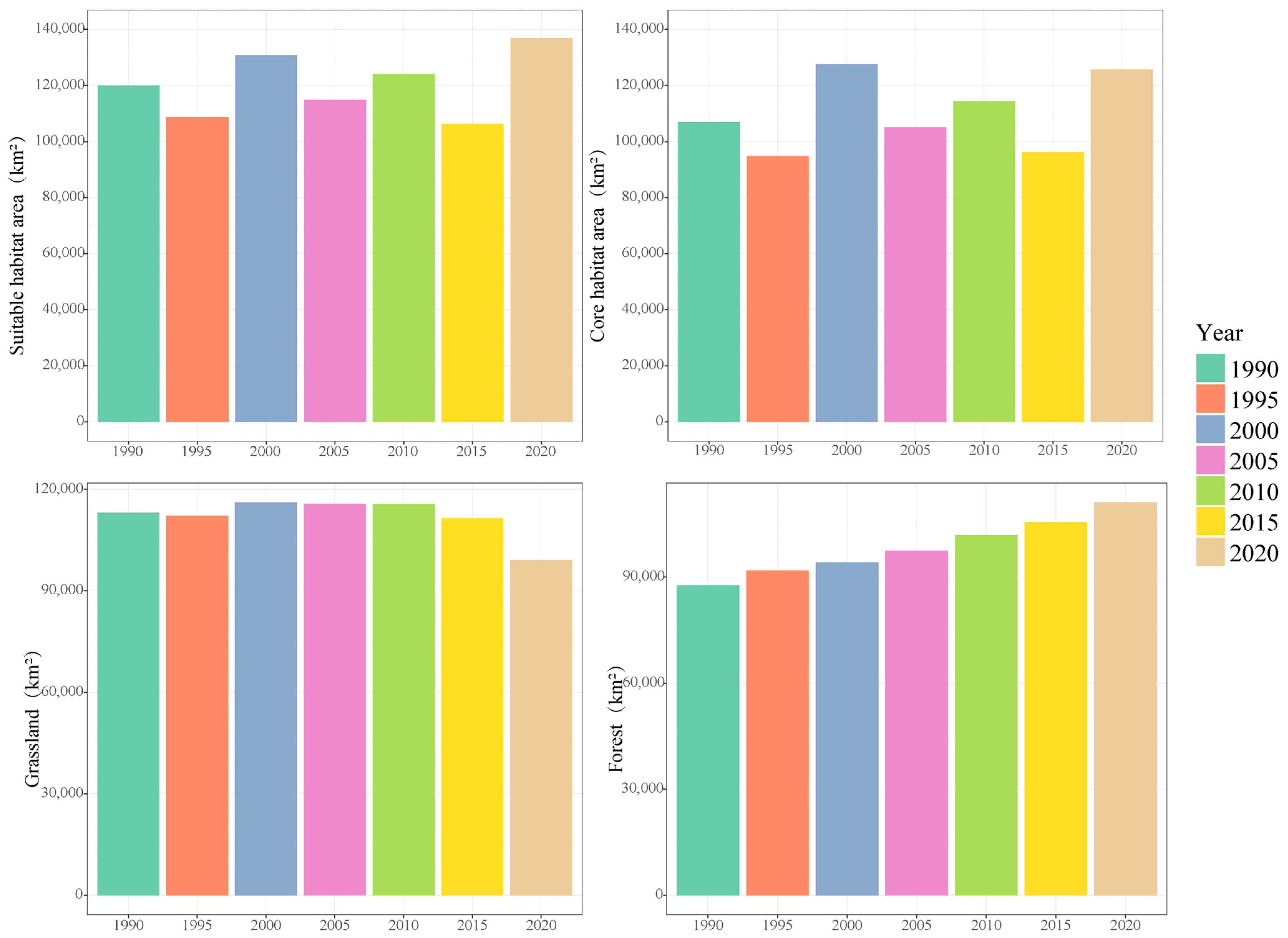
3.2. Distribution of Suitable Habitats
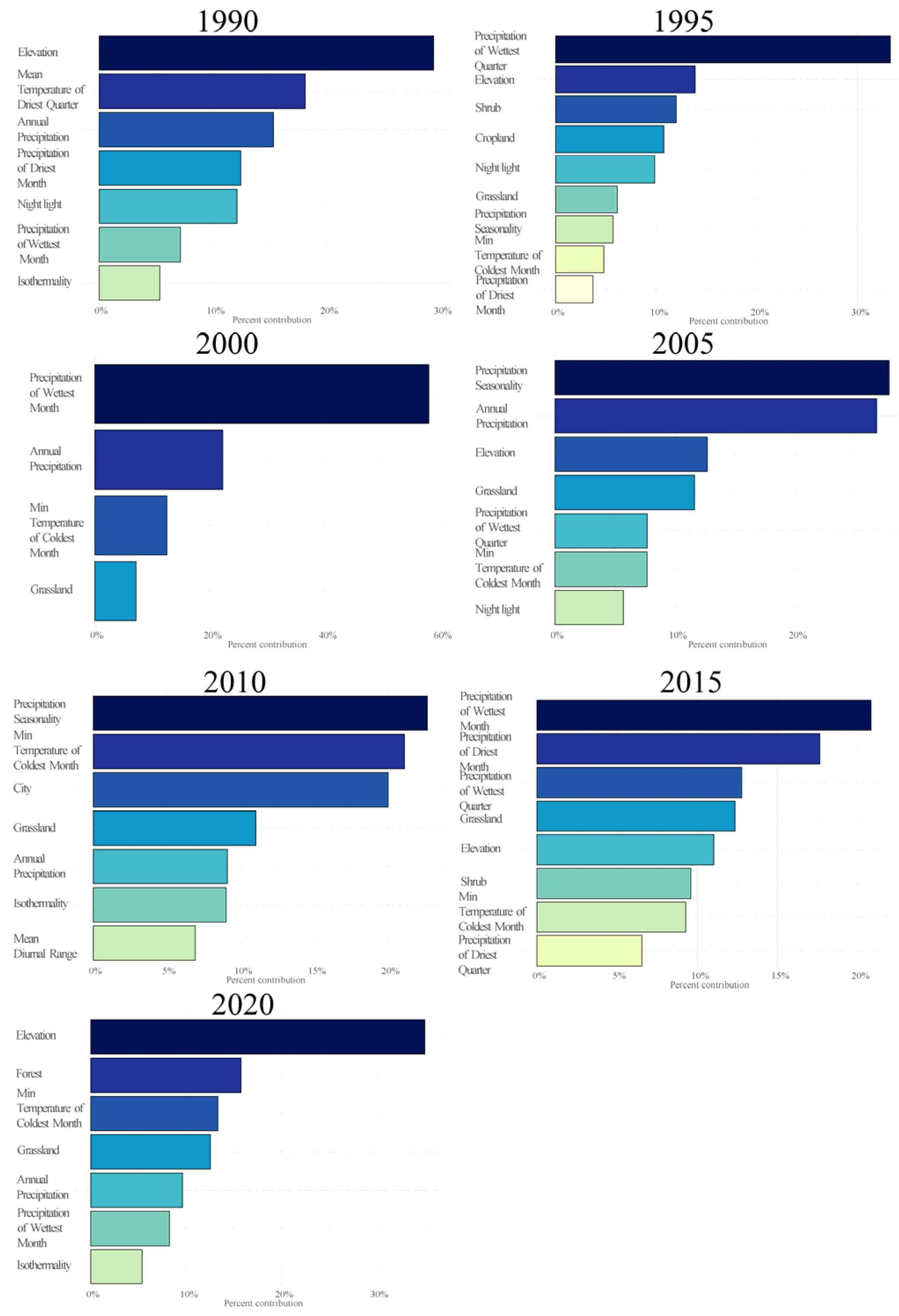
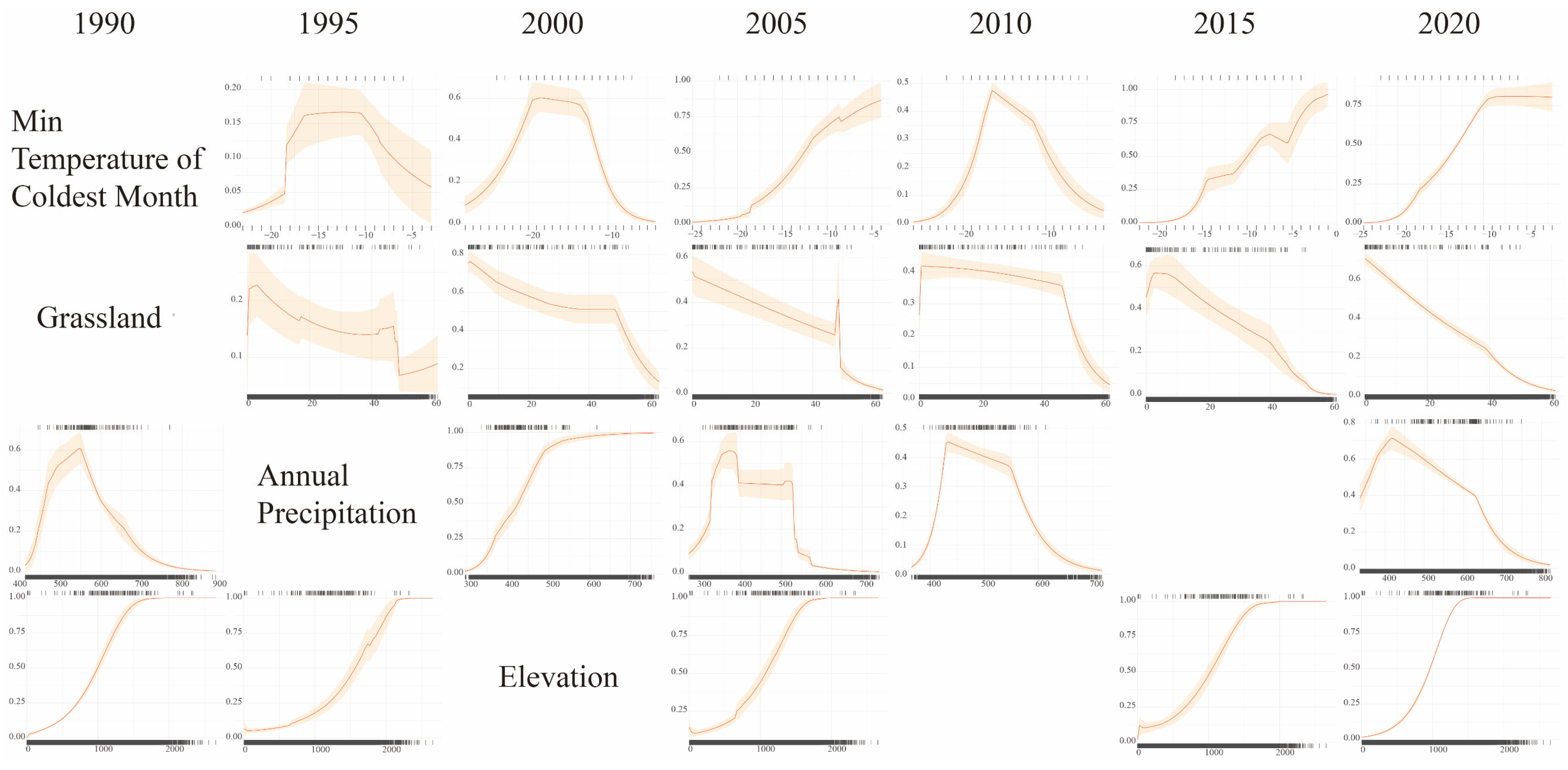
3.3. The Influence of Environmental Variables
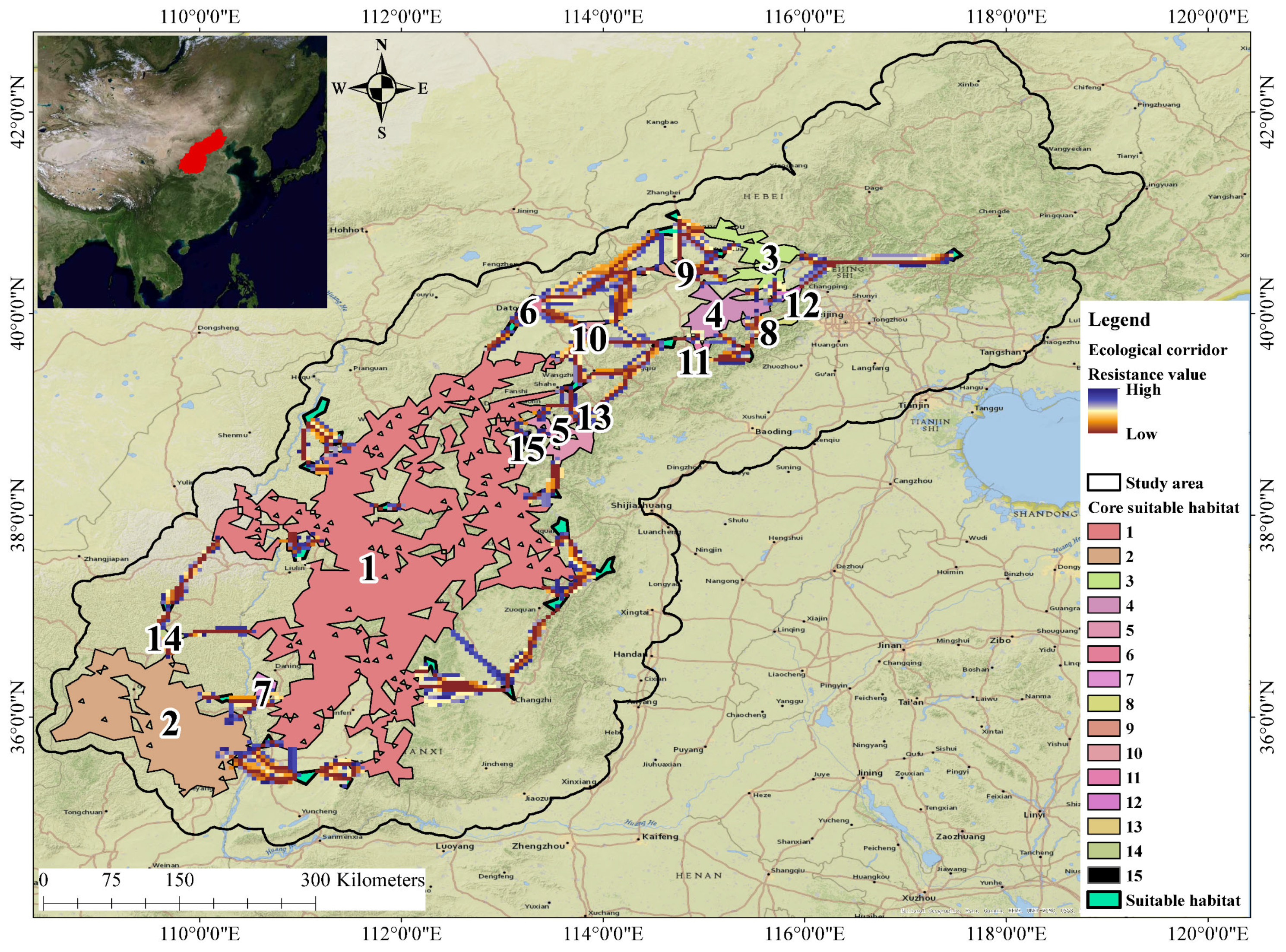
3.4. Results of the Current Analysis of the Construction of the Corridor between Suitable Habitats
4. Discussion
4.1. Distribution Dynamics of Suitable Habitat for Brown-Eared Pheasant
4.2. Effects of Critical Variables on Habitat Suitability of Brown-Eared Pheasant
4.3. The Current Status of Suitable Habitats for Brown-Eared Pheasants and Corridor Planning
4.4. Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Malik, A.A.; Puissant, J.; Buckeridge, K.M.; Goodall, T.; Jehmlich, N.; Chowdhury, S.; Gweon, H.S.; Peyton, J.M.; Mason, K.E.; Van Agtmaal, M.; et al. Land Use Driven Change in Soil pH Affects Microbial Carbon Cycling Processes. Nat. Commun. 2018, 9, 3591. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yu, L.; Du, Z.; Xu, Y.; Zhao, J.; Zhao, H.; Zhang, G.; Peng, D.; Gong, P. Distribution of Ecological Restoration Projects Associated with Land Use and Land Cover Change in China and Their Ecological Impacts. Sci. Total Environ. 2022, 825, 153938. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Chen, L.; Yu, X. Impact of China’s Grain for Green Project on the Landscape of Vulnerable Arid and Semi-arid Agricultural Regions: A Case Study in Northern Shaanxi Province. J. Appl. Ecol. 2009, 46, 536–543. [Google Scholar] [CrossRef]
- Choi, C.; Shi, X.; Shi, J.; Gan, X.; Wen, C.; Zhang, J.; Jackson, M.V.; Fuller, R.A.; Gibson, L. China’s Ecological Conservation Redline Policy Is a New Opportunity to Meet Post-2020 Protected Area Targets. Conserv. Lett. 2022, 15, e12853. [Google Scholar] [CrossRef]
- Grace, M.K.; Akçakaya, H.R.; Bennett, E.L.; Brooks, T.M.; Heath, A.; Hedges, S.; Hilton-Taylor, C.; Hoffmann, M.; Hochkirch, A.; Jenkins, R.; et al. Testing a Global Standard for Quantifying Species Recovery and Assessing Conservation Impact. Conserv. Biol. 2021, 35, 1833–1849. [Google Scholar] [CrossRef] [PubMed]
- Visconti, P.; Butchart, S.H.M.; Brooks, T.M.; Langhammer, P.F.; Marnewick, D.; Vergara, S.; Yanosky, A.; Watson, J.E.M. Protected Area Targets Post-2020. Science 2019, 364, 239–241. [Google Scholar] [CrossRef]
- Song, K.; Mi, C.-R.; Zhao, Y.-Z.; Yang, N.; Sun, Y.-H.; Xu, J.-L. Zonation of Nature Reserve According to the Habitat Requirement of Conservation Target: A Case Study on the Endangered Brown Eared-Pheasant at Baihuashan Nature Reserve. Glob. Ecol. Conserv. 2021, 32, e01941. [Google Scholar] [CrossRef]
- Li, H.; Liu, X.; Han, Z.; Wu, S.; Cao, C. Foraging and Day-Roosting Sites Selection by the Endangered Brown-Eared Pheasant Crossoptilon Mantchuricum during Autumn in the Huanglong Mountains, Shaanxi Province, China. Pak. J. Zool. 2021, 53, 401–800. [Google Scholar] [CrossRef]
- Huang, T.; Yang, T.; Wang, K.; Huang, W. Assessing the Current and Future Potential Distribution of Solanum rostratum Dunal in China Using Multisource Remote Sensing Data and Principal Component Analysis. Remote Sens. 2024, 16, 271. [Google Scholar] [CrossRef]
- Zhang, J.; Li, H.; Tian, Y.; Qiu, H.; Zhou, X.; Ma, H.; Yuan, L. Assessing Rice Sheath Blight Disease Habitat Suitability at a Regional Scale through Multisource Data Analysis. Remote Sens. 2023, 15, 5530. [Google Scholar] [CrossRef]
- Riddell, E.A.; Iknayan, K.J.; Hargrove, L.; Tremor, S.; Patton, J.L.; Ramirez, R.; Wolf, B.O.; Beissinger, S.R. Exposure to Climate Change Drives Stability or Collapse of Desert Mammal and Bird Communities. Science 2021, 371, 633–636. [Google Scholar] [CrossRef]
- Ditmer, M.A.; Iannarilli, F.; Tri, A.N.; Garshelis, D.L.; Carter, N.H. Artificial Night Light Helps Account for Observer Bias in Citizen Science Monitoring of an Expanding Large Mammal Population. J. Anim. Ecol. 2021, 90, 330–342. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Wang, J.; Zhang, L.; Chen, S.; Zhao, A.; Ning, X.; Fan, G.; Wu, N.; Zhang, L.; Wang, Z. Prediction of the Potentially Suitable Areas of Litsea Cubeba in China Based on Future Climate Change Using the Optimized MaxEnt Model. Ecol. Indic. 2023, 148, 110093. [Google Scholar] [CrossRef]
- Ab Lah, N.Z.; Yusop, Z.; Hashim, M.; Mohd Salim, J.; Numata, S. Predicting the Habitat Suitability of Melaleuca Cajuputi Based on the MaxEnt Species Distribution Model. Forests 2021, 12, 1449. [Google Scholar] [CrossRef]
- Li, J.; Fan, G.; He, Y. Predicting the Current and Future Distribution of Three Coptis Herbs in China under Climate Change Conditions, Using the MaxEnt Model and Chemical Analysis. Sci. Total Environ. 2020, 698, 134141. [Google Scholar] [CrossRef]
- Kang, S.; Eltahir, E.A.B. North China Plain Threatened by Deadly Heatwaves Due to Climate Change and Irrigation. Nat. Commun. 2018, 9, 2894. [Google Scholar] [CrossRef]
- Liu, J.; Rühland, K.M.; Chen, J.; Xu, Y.; Chen, S.; Chen, Q.; Huang, W.; Xu, Q.; Chen, F.; Smol, J.P. Aerosol-Weakened Summer Monsoons Decrease Lake Fertilization on the Chinese Loess Plateau. Nat. Clim. Change 2017, 7, 190–194. [Google Scholar] [CrossRef]
- Ruan, H.; Wu, X.; Wang, S.; Yang, J.; Zhu, H.; Guo, Q.; Wang, L.; Wang, D. The Responses of Different Insect Guilds to Grassland Degradation in Northeastern China. Ecol. Indic. 2021, 133, 108369. [Google Scholar] [CrossRef]
- Kadmon, R.; Farber, O.; Danin, A. Effect of Roadside Bias on the Accuracy of Predictive Maps Produced by Bioclimatic Models. Ecol. Appl. 2004, 14, 401–413. [Google Scholar] [CrossRef]
- Johnston, A.; Hochachka, W.M.; Strimas-Mackey, M.E.; Ruiz Gutierrez, V.; Robinson, O.J.; Miller, E.T.; Auer, T.; Kelling, S.T.; Fink, D. Analytical Guidelines to Increase the Value of Community Science Data: An Example Using eBird Data to Estimate Species Distributions. Divers. Distrib. 2021, 27, 1265–1277. [Google Scholar] [CrossRef]
- Amiri, M.; Tarkesh, M.; Jafari, R.; Jetschke, G. Bioclimatic Variables from Precipitation and Temperature Records vs. Remote Sensing-Based Bioclimatic Variables: Which Side Can Perform Better in Species Distribution Modeling? Ecol. Inform. 2020, 57, 101060. [Google Scholar] [CrossRef]
- Yang, J.; Huang, X. The 30 m Annual Land Cover Dataset and Its Dynamics in China from 1990 to 2019. Earth Syst. Sci. Data 2021, 13, 3907–3925. [Google Scholar] [CrossRef]
- Mills, S.C.; Socolar, J.B.; Edwards, F.A.; Parra, E.; Martínez-Revelo, D.E.; Quintero, J.M.O.; Haugaasen, T.; Freckleton, R.P.; Barlow, J.; Edwards, D.P. High Sensitivity of Tropical Forest Birds to Deforestation at Lower Altitudes. Ecology 2023, 104, e3867. [Google Scholar] [CrossRef] [PubMed]
- Cao, A.; Shi, X. The Effects of Climate Change on Habitat Connectivity: A Case Study of the Brown-Eared Pheasant in China. Land 2022, 11, 806. [Google Scholar] [CrossRef]
- Ghoddousi, A.; Bleyhl, B.; Sichau, C.; Ashayeri, D.; Moghadas, P.; Sepahvand, P.; Hamidi, A.K.; Soofi, M.; Kuemmerle, T. Mapping Connectivity and Conflict Risk to Identify Safe Corridors for the Persian Leopard. Landsc. Ecol. 2020, 35, 1809–1825. [Google Scholar] [CrossRef]
- Merow, C.; Smith, M.J.; Silander, J.A. A Practical Guide to MaxEnt for Modeling Species’ Distributions: What It Does, and Why Inputs and Settings Matter. Ecography 2013, 36, 1058–1069. [Google Scholar] [CrossRef]
- Hijmans, R.J.; Phillips, S.; Leathwick, J.; Elith, J. Dismo: Species Distribution Modeling; R Core Team: Vienna, Austria, 2023. [Google Scholar]
- Vignali, S.; Barras, A.G.; Arlettaz, R.; Braunisch, V. SDMtune: An R Package to Tune and Evaluate Species Distribution Models. Ecol. Evol. 2020, 10, 11488–11506. [Google Scholar] [CrossRef]
- Zhu, M.; Zhao, Y.; Li, W.; Han, X.; Wang, Z.; Yang, X.; Dang, C.; Liu, Y.; Xu, S. Impact of Carbon Neutralization Policy on the Suitable Habitat Distribution of the North China Leopard. Sci. Rep. 2024, 14, 18821. [Google Scholar] [CrossRef]
- Xu, L.; Fan, Y.; Zheng, J.; Guan, J.; Lin, J.; Wu, J.; Liu, L.; Wu, R.; Liu, Y. Impacts of Climate Change and Human Activity on the Potential Distribution of Aconitum Leucostomum in China. Sci. Total Environ. 2024, 912, 168829. [Google Scholar] [CrossRef]
- Vale, C.G.; Tarroso, P.; Brito, J.C. Predicting Species Distribution at Range Margins: Testing the Effects of Study Area Extent, Resolution and Threshold Selection in the Sahara-Sahel Transition Zone. Divers. Distrib. 2014, 20, 20–33. [Google Scholar] [CrossRef]
- Hofman, M.P.G.; Hayward, M.W.; Kelly, M.J.; Balkenhol, N. Enhancing Conservation Network Design with Graph-Theory and a Measure of Protected Area Effectiveness: Refining Wildlife Corridors in Belize, Central America. Landsc. Urban Plan. 2018, 178, 51–59. [Google Scholar] [CrossRef]
- He, F.; Tong, Z.; Hannaway, D.B.; Li, X. Erratic Precipitation and Clipping Frequency Reshape the Community Structure and Species Stability of Leymus Chinensis Steppe. Ecol. Indic. 2021, 133, 108432. [Google Scholar] [CrossRef]
- Zou, B.; Luo, G.; Zhu, B.; Ran, J.; Fang, C. The spatial distribution relationship between three pheasant species and mutual predator, the red fox (Vulpes vulpes), on the Western Sichuan Plateau. Biodivers. Sci. 2021, 29, 918–926. [Google Scholar] [CrossRef]
- Hu, W.; Wang, Y.; Zhang, D.; Yu, W.; Chen, G.; Xie, T.; Liu, Z.; Ma, Z.; Du, J.; Chao, B.; et al. Mapping the Potential of Mangrove Forest Restoration Based on Species Distribution Models: A Case Study in China. Sci. Total Environ. 2020, 748, 142321. [Google Scholar] [CrossRef] [PubMed]
- Kittle, A.M.; Watson, A.C.; Cushman, S.A.; Macdonald, D.W. Forest Cover and Level of Protection Influence the Island-Wide Distribution of an Apex Carnivore and Umbrella Species, the Sri Lankan Leopard (Panthera Pardus Kotiya). Biodivers. Conserv. 2018, 27, 235–263. [Google Scholar] [CrossRef]
- Yi, Z.; Zhou, W.; Razzaq, A.; Yang, Y. Land Resource Management and Sustainable Development: Evidence from China’s Regional Data. Resour. Pol. 2023, 84, 103732. [Google Scholar] [CrossRef]
- Zhu, M.; Zhao, Y.; Wang, M.; Zaman, M.; Jiang, G. Response of Wildlife Communities to Human Activities in the Distribution Area of the North China Leopard. Glob. Ecol. Conserv. 2024, 51, e02872. [Google Scholar] [CrossRef]
- Wang, J.; Lin, Y.; Glendinning, A.; Xu, Y. Land-Use Changes and Land Policies Evolution in China’s Urbanization Processes. Land. Use Policy 2018, 75, 375–387. [Google Scholar] [CrossRef]
- Amburgey, S.M.; Miller, D.A.W.; Rochester, C.J.; Delaney, K.S.; Riley, S.P.D.; Brehme, C.S.; Hathaway, S.A.; Fisher, R.N. The Influence of Species Life History and Distribution Characteristics on Species Responses to Habitat Fragmentation in an Urban Landscape. J. Anim. Ecol. 2021, 90, 685–697. [Google Scholar] [CrossRef]
- Zheng, K.; Wei, J.-Z.; Pei, J.-Y.; Cheng, H.; Zhang, X.-L.; Huang, F.-Q.; Li, F.-M.; Ye, J.-S. Impacts of Climate Change and Human Activities on Grassland Vegetation Variation in the Chinese Loess Plateau. Sci. Total Environ. 2019, 660, 236–244. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Song, Z.; Ding, C. Determining the Distribution Loss of Brown Eared-Pheasant (Crossoptilon Mantchuricum) Using Historical Data and Potential Distribution Estimates. PeerJ 2016, 4, e2556. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, Y.; Guo, D.; Lu, W.; Xu, H. How Does Ecological Protection Redline Policy Affect Regional Land Use and Ecosystem Services? Environ. Impact Assess. Rev. 2023, 100, 107062. [Google Scholar] [CrossRef]
- Beissinger, S.R.; MacLean, S.A.; Iknayan, K.J.; Valpine, P.D. Concordant and Opposing Effects of Climate and Land-Use Change on Avian Assemblages in California’s Most Transformed Landscapes. Sci. Adv. 2023, 9, eabn0250. [Google Scholar] [CrossRef] [PubMed]
- Fourcade, Y.; WallisDeVries, M.F.; Kuussaari, M.; Swaay, C.A.M.; Heliölä, J.; Öckinger, E. Habitat Amount and Distribution Modify Community Dynamics under Climate Change. Ecol. Lett. 2021, 24, 950–957. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Ma, X.; Chen, B.; Shang, Y.; Song, M. Challenges toward Carbon Neutrality in China: Strategies and Countermeasures. Resour. Conserv. Recycl. 2022, 176, 105959. [Google Scholar] [CrossRef]
- Brown, G.S.; DeWitt, P.D.; Dawson, N.; Landriault, L. Threshold Responses in Wildlife Communities and Evidence for Biodiversity Indicators of Sustainable Resource Management. Ecol. Indic. 2021, 133, 108371. [Google Scholar] [CrossRef]
- Ferreira, I.J.M.; Campanharo, W.A.; Fonseca, M.G.; Escada, M.I.S.; Nascimento, M.T.; Villela, D.M.; Brancalion, P.; Magnago, L.F.S.; Anderson, L.O.; Nagy, L.; et al. Potential Aboveground Biomass Increase in Brazilian Atlantic Forest Fragments with Climate Change. Glob. Change Biol. 2023, 29, 3098–3113. [Google Scholar] [CrossRef]
- Fletcher, R.J.; Robertson, E.P.; Poli, C.; Dudek, S.; Gonzalez, A.; Jeffery, B. Conflicting Nest Survival Thresholds across a Wetland Network Alter Management Benchmarks for an Endangered Bird. Biol. Conserv. 2021, 253, 108893. [Google Scholar] [CrossRef]
- Sinha, A.; Menzies, R.K.; Chatterjee, N.; Rao, M.; Naniwadekar, R. Drivers of Taxonomic, Phylogenetic, and Functional Beta Diversity of Himalayan Riverine Birds. Front. Ecol. Evol. 2022, 10, 788184. [Google Scholar] [CrossRef]
- Puri, M.; Srivathsa, A.; Karanth, K.K.; Patel, I.; Kumar, N.S. Links in a Sink: Interplay between Habitat Structure, Ecological Constraints and Interactions with Humans Can Influence Connectivity Conservation for Tigers in Forest Corridors. Sci. Total Environ. 2022, 809, 151106. [Google Scholar] [CrossRef]
- Sun, X.; Shen, J.; Xiao, Y.; Li, S.; Cao, M. Habitat Suitability and Potential Biological Corridors for Waterbirds in Yancheng Coastal Wetland of China. Ecol. Indic. 2023, 148, 110090. [Google Scholar] [CrossRef]
- Tian, Z.; Huo, D.; Yi, K.; Que, J.; Lu, Z.; Hou, J. Evaluation of Suitable Habitats for Birds Based on MaxEnt and Google Earth Engine—A Case Study of Baer’s Pochard (Aythya baeri) in Baiyangdian, China. Remote Sens. 2023, 16, 64. [Google Scholar] [CrossRef]
- Johnston, A.; Matechou, E.; Dennis, E.B. Outstanding Challenges and Future Directions for Biodiversity Monitoring Using Citizen Science Data. Methods Ecol. Evol. 2023, 14, 103–116. [Google Scholar] [CrossRef]



| Variable Class | Variable Description | Unit | Sources |
|---|---|---|---|
| Land use variable | Cropland | Percent | The 30 m annual land cover dataset and its dynamics in China from 1985 to 2022. https://doi.org/10.5281/zenodo.4417809 [22]. |
| Forest | Percent | ||
| Shrub | Percent | ||
| Grassland | Percent | ||
| Water | Percent | ||
| City | Percent | ||
| Bioclimatic variables | Annual Mean Temperature | °C | WorldClim data website (https://www.worldclim.org, accessed on 22 August 2024) |
| Mean Diurnal Range | °C | ||
| Isothermality | Percent | ||
| Temperature Seasonality | °C | ||
| Max Temperature of Warmest Month | °C | ||
| Min Temperature of Coldest Month | °C | ||
| Temperature Annual Range | °C | ||
| Mean Temperature of Wettest Quarter | °C | ||
| Mean Temperature of Driest Quarter | °C | ||
| Mean Temperature of Warmest Quarter | °C | ||
| Mean Temperature of Coldest Quarter | °C | ||
| Annual Precipitation | Mm | ||
| Precipitation of Wettest Month | Mm | ||
| Precipitation of Driest Month | Mm | ||
| Precipitation Seasonality | Mm | ||
| Precipitation of Wettest Quarter | Mm | ||
| Precipitation of Driest Quarter | Mm | ||
| Precipitation of Warmest Quarter | Mm | ||
| Precipitation of Coldest Quarter | Mm | ||
| Geographical variable | Elevation | Meter | SRTM 90 m digital elevation https://search.earthdata.nasa.gov, accessed on 22 August 2024 |
| Elevation-sd | Meter | ||
| Elevation-range | Meter | ||
| Slope | Degree | ||
| Slope-sd | Degree | ||
| Aspect | Degree | ||
| Aspect-sd | Degree | ||
| Human activity variable | Human footprint | Percent | 20 years of human footprint data (https://wcshumanfootprint.org, accessed on 22 August 2024) |
| Night light | Percent | A prolonged artificial of China (1984–2020). https://doi.org/10.1038/s41597-024-03223-1 [12], accessed on 22 August 2024 |
| Year | Feature Combination | Regularization Multiplier | Number of Iterations | Train AUC | Test AUC |
|---|---|---|---|---|---|
| 2020 | lh | 1.6 | 400 | 0.80 | 0.77 |
| 2015 | lqph | 0.3 | 1600 | 0.87 | 0.79 |
| 2010 | lqph | 1.0 | 300 | 0.84 | 0.79 |
| 2005 | lqpht | 1.0 | 1300 | 0.86 | 0.80 |
| 2000 | h | 1.1 | 200 | 0.83 | 0.80 |
| 1995 | lqpht | 1.0 | 800 | 0.87 | 0.79 |
| 1990 | lqph | 0.4 | 1500 | 0.84 | 0.77 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Dang, C.; Liu, Y.; Xu, S.; Zhu, M. The Effects of Land Use Changes on the Distribution of the Chinese Endemic Species of Brown-Eared Pheasant. Diversity 2024, 16, 514. https://doi.org/10.3390/d16090514
Zhao Y, Dang C, Liu Y, Xu S, Zhu M. The Effects of Land Use Changes on the Distribution of the Chinese Endemic Species of Brown-Eared Pheasant. Diversity. 2024; 16(9):514. https://doi.org/10.3390/d16090514
Chicago/Turabian StyleZhao, Yue, Cuiying Dang, Yaoguo Liu, Shicai Xu, and Mengyan Zhu. 2024. "The Effects of Land Use Changes on the Distribution of the Chinese Endemic Species of Brown-Eared Pheasant" Diversity 16, no. 9: 514. https://doi.org/10.3390/d16090514
APA StyleZhao, Y., Dang, C., Liu, Y., Xu, S., & Zhu, M. (2024). The Effects of Land Use Changes on the Distribution of the Chinese Endemic Species of Brown-Eared Pheasant. Diversity, 16(9), 514. https://doi.org/10.3390/d16090514





