The Interaction between Climate Change and Biodiversity Can Be Assessed from a Material Cycle Perspective
Abstract
1. Introduction
1.1. The Interconnection between Climate Change and Biodiversity
1.2. The Complexity of Biodiversity Variability Due to Climate Change
- A comprehensive and multidimensional approach is crucial to examine the complexity of biodiversity. Biodiversity is defined at the genetic, species, and ecosystem levels according to the Convention on Biological Diversity (CBD). While each aspect of biodiversity requires unique assessment indicators, the need to treat biodiversity as a multidimensional structure has been consistently emphasized [59,60,61].
- Relying solely on species diversity, the most widely used indicator, has limitations in directly analyzing environmental stress impacts [62,63,64]. Species diversity does not consistently respond to environmental stress and may increase or decrease when exposed to it. Species-focused assessments may equate communities with few species and high uniformity to those with many species and low uniformity. Additionally, species-rich areas may not be aligned with the habitats of rare or endangered species [65,66,67,68]. Species richness (the number of species in an area) is commonly used when considering multiple species. However, this approach still struggles to resolve issues, such as discrepancies between specific taxonomic groups and functional types [69]. Moreover, species diversity often uses both species richness and evenness. However, the potential inverse relationship between these metrics has led to criticism of composite indices that include both, deeming them potentially inaccurate [70,71]. For instance, communities A and B may have identical Shannon indices for species diversity despite differing richness and evenness. Because of these characteristics, although species diversity can be used to gauge taxonomic richness, ecosystem status, or trends, it may be inadequate for comprehensive biodiversity analysis. This limitation also applies to genetic diversity measurements.
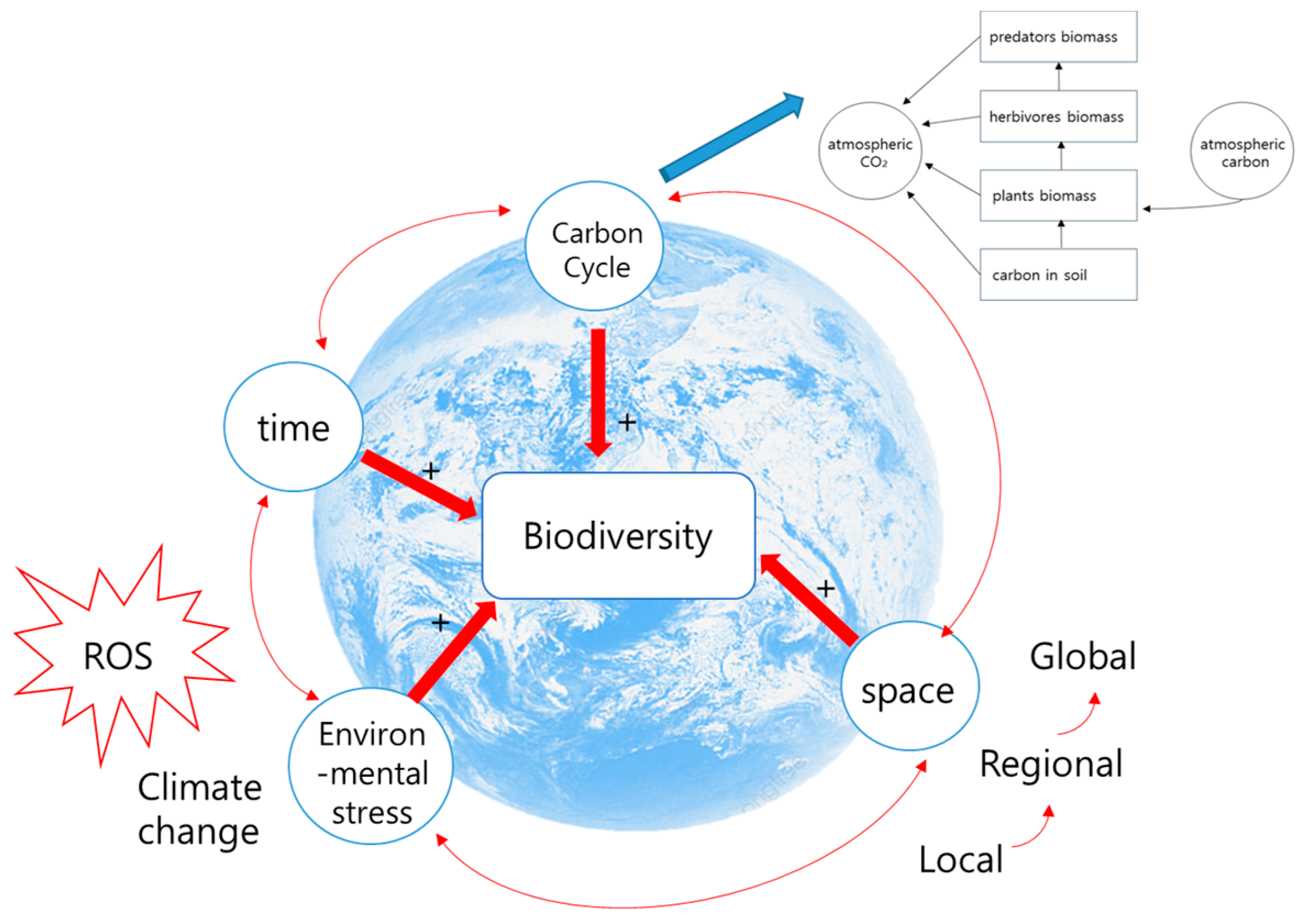
- In open ecosystems, it is crucial to understand changes in biodiversity across time and space [72,73,74,75,76,77]. Continuous carbon emissions from anthropogenic activities have led to increased levels of climate change and greater biodiversity loss [78,79]. Consequently, this has led to a further increase in carbon emissions. This phenomenon represents a self-reinforcing feedback loop that intensifies climate change [12,20]. To understand the flow of materials entering and leaving such an open system, it is necessary not only to predict the exact proportions of each species’ abundance at specific locations and points but also to assess how the relative ratios of rare to common species change [72,80]. This approach may be more conducive to detecting biodiversity changes induced by environmental stress rather than typical methods that measure absolute species quantities.
- Understanding biodiversity from an ecosystem function perspective has become increasingly important as global ecosystems change rapidly due to climate change [81,82]. Although a unified definition of ecosystem function has not yet been established, it may be broadly categorized into two aspects based on its characteristics—patterns and processes. From a pattern perspective, functional traits are considered differences between populations or species independent of time. This approach is commonly used in big data analyses. In contrast, the process aspect considers time as a factor. This includes the phenomena through which communities modify carbon cycling through interactions with the environment. From this perspective, ecosystem function is defined as the transfer or storage of energy or matter from the cellular to the global level [83]. This definition was adopted in the present study. Reconstructing the functional changes in ecosystems over time can aid in predicting the implications of future changes.
1.3. A New Approach to Biodiversity
2. Material Cycling and Environmental Stress
2.1. Carbon Flow and Climate Change as Environmental Stress
2.2. Changes in Organism Migration and Range Shifts Due to Environmental Stress
2.3. Need to Understand Environmental Stress and Diversity Considering Carbon Flow
3. Environmental Stress and Biodiversity
3.1. Macroscopic and Microscopic Environmental Stress
3.2. Current Status and Implications of Arithmetic Indicator-Centered Biodiversity Assessment That Fails to Consider Environmental Stress
3.3. The Interaction between Biodiversity and Environmental Stress within Changes in Organism Distribution and Migration
4. Material Cycling–Environmental Stress–Biodiversity
4.1. Understanding Biodiversity Considering Complex Systems
4.2. Biodiversity Considering Comprehensive Environmental Changes Based on Material Cycling
5. Conclusions and Future Research Directions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
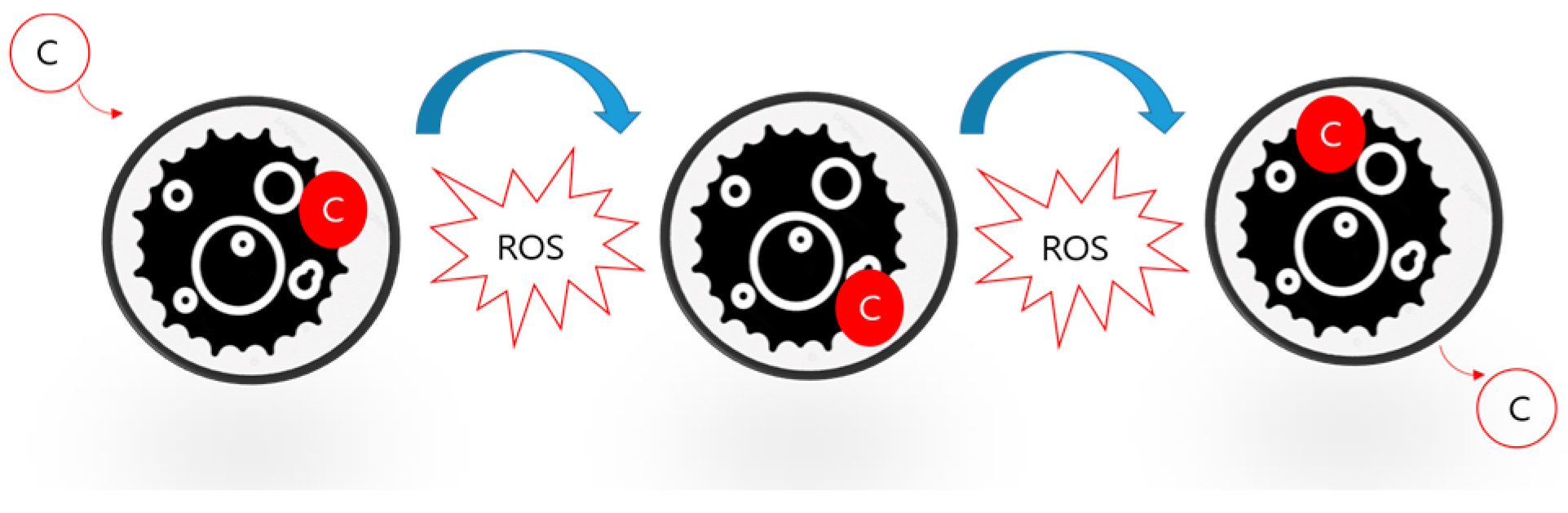
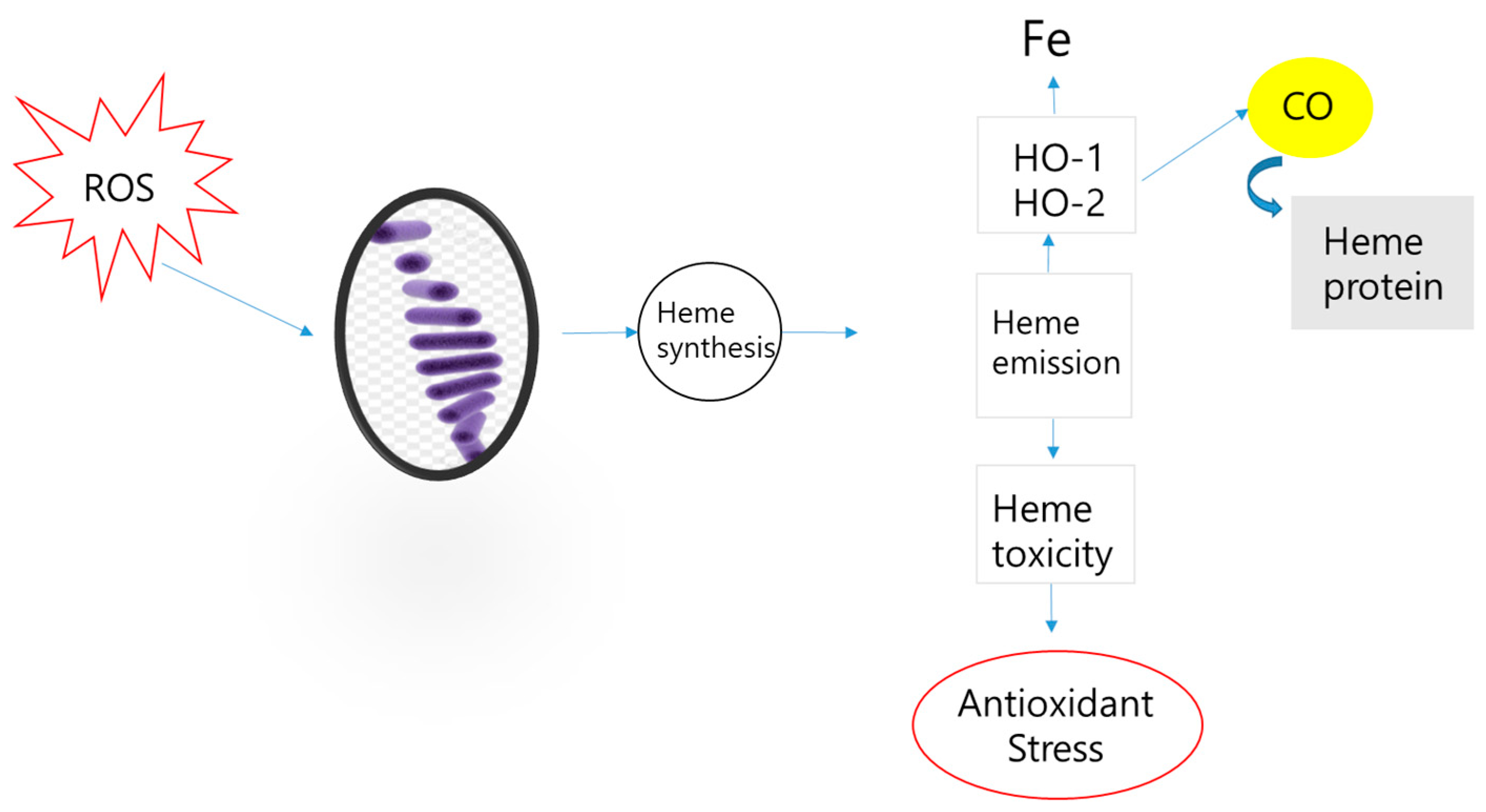
Appendix A. Carbon Cycle Structure and the Role of ROS

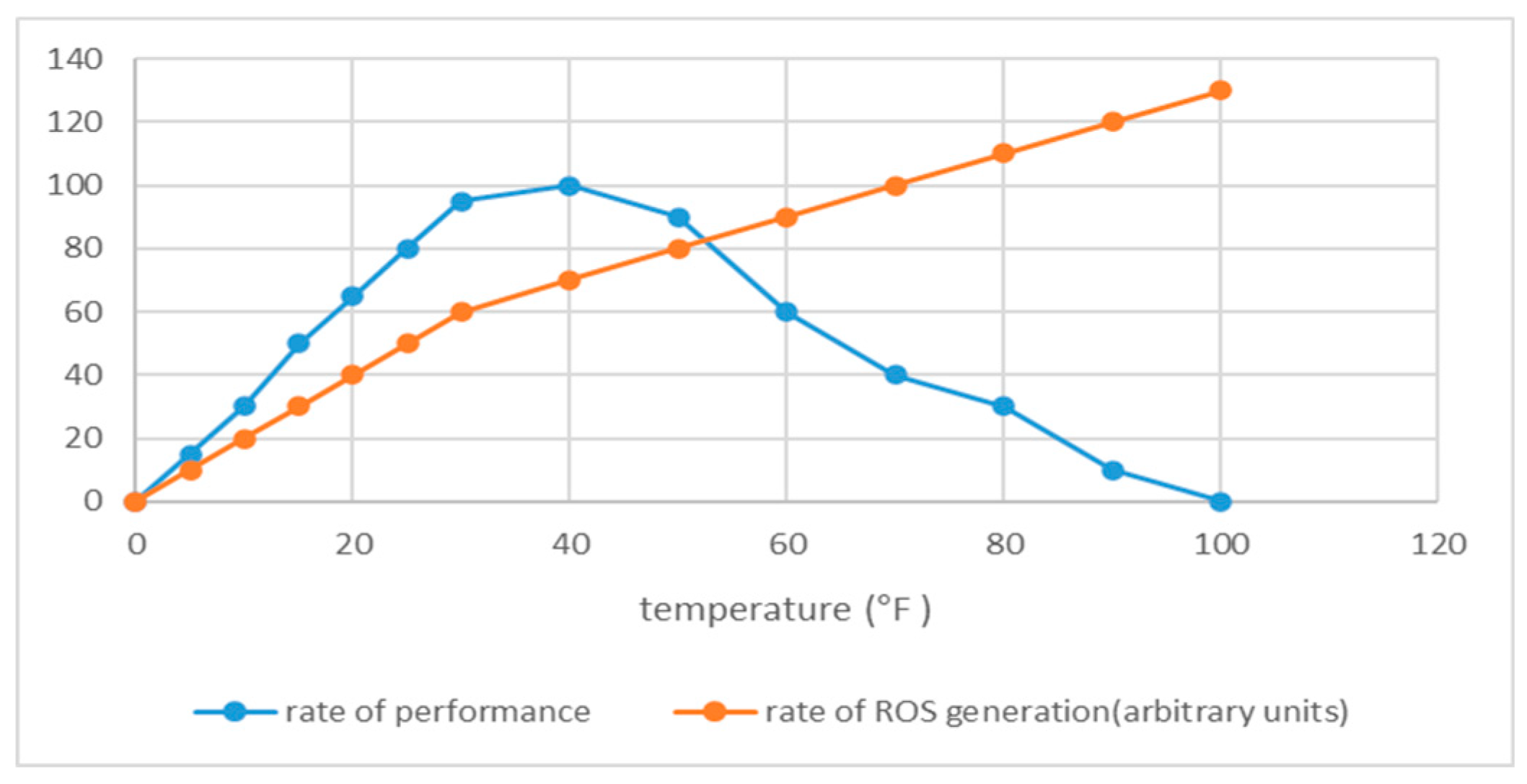
Appendix B. Heat Stress and Dietary Shifts in Animals
References
- Pecl, G.T.; Araújo, M.B.; Bell, J.D.; Blanchard, J.; Bonebrake, T.C.; Chen, I.-C.; Clark, T.D.; Colwell, R.K.; Danielsen, F.; Evengård, B. Biodiversity redistribution under climate change: Impacts on ecosystems and human well-being. Science 2017, 355, eaai9214. [Google Scholar] [CrossRef] [PubMed]
- Zandalinas, S.I.; Fritschi, F.B.; Mittler, R. Global Warming, Climate Change, and Environmental Pollution: Recipe for a Multifactorial Stress Combination Disaster. Trends Plant Sci. 2021, 26, 588–599. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Calvin, K.; Dasgupta, D.; Krinner, G.; Mukherji, A.; Thorne, P.; Trisos, C.; Romero, J.; Aldunce, P.; Barret, K. IPCC, 2023: Climate Change 2023: Synthesis Report, Summary for Policymakers. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Core Writing Team, Lee, H., Romero, J., Eds.; IPCC: Geneva, Switzerland, 2023. [Google Scholar]
- Romanuk, T.N.; Vogt, R.J.; Young, A.; Tuck, C.; Carscallen, M.W. Maintenance of positive diversity-stability relations along a gradient of environmental stress. PLoS ONE 2010, 5, e10378. [Google Scholar] [CrossRef] [PubMed]
- Schulte, P.M. What is environmental stress? Insights from fish living in a variable environment. J. Exp. Biol. 2014, 217, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Koolhaas, J.M.; Bartolomucci, A.; Buwalda, B.; de Boer, S.F.; Flügge, G.; Korte, S.M.; Meerlo, P.; Murison, R.; Olivier, B.; Palanza, P. Stress revisited: A critical evaluation of the stress concept. Neurosci. Biobehav. Rev. 2011, 35, 1291–1301. [Google Scholar] [CrossRef]
- Tortosa, M.; Velasco, P.; Rodríguez, V.M.; Cartea, M.E. Changes in Brassica oleracea leaves infected with Xanthomonas campestris pv. campestris by proteomics analysis. Front. Plant Sci. 2022, 12, 781984. [Google Scholar] [CrossRef] [PubMed]
- Geyer, J.; Kiefer, I.; Kreft, S.; Chavez, V.; Salafsky, N.; Jeltsch, F.; Ibisch, P.L. Classification of climate-change-induced stresses on biological diversity. Conserv. Biol. 2011, 25, 708–715. [Google Scholar] [CrossRef]
- Jing, X.; Sanders, N.J.; Shi, Y.; Chu, H.; Classen, A.T.; Zhao, K.; Chen, L.; Shi, Y.; Jiang, Y.; He, J.-S. The links between ecosystem multifunctionality and above-and belowground biodiversity are mediated by climate. Nat. Commun. 2015, 6, 8159. [Google Scholar] [CrossRef]
- Singh, A.; Mehta, S.; Yadav, S.; Nagar, G.; Ghosh, R.; Roy, A.; Chakraborty, A.; Singh, I.K. How to cope with the challenges of environmental stresses in the era of global climate change: An update on ROS stave off in plants. Int. J. Mol. Sci. 2022, 23, 1995. [Google Scholar] [CrossRef]
- Chaudhry, S.; Sidhu, G.P.S. Climate change regulated abiotic stress mechanisms in plants: A comprehensive review. Plant Cell Rep. 2022, 41, 1–31. [Google Scholar] [CrossRef]
- Soto-Navarro, C.; Ravilious, C.; Arnell, A.; De Lamo, X.; Harfoot, M.; Hill, S.; Wearn, O.; Santoro, M.; Bouvet, A.; Mermoz, S. Mapping co-benefits for carbon storage and biodiversity to inform conservation policy and action. Philos. Trans. R. Soc. B 2020, 375, 20190128. [Google Scholar] [CrossRef] [PubMed]
- Mehmood, I.; Bari, A.; Irshad, S.; Khalid, F.; Liaqat, S.; Anjum, H.; Fahad, S. Carbon cycle in response to global warming. Environ. Clim. Plant Veg. Growth 2020, 1–15. [Google Scholar] [CrossRef]
- Rosenzweig, C.; Karoly, D.; Vicarelli, M.; Neofotis, P.; Wu, Q.; Casassa, G.; Menzel, A.; Root, T.L.; Estrella, N.; Seguin, B. Attributing physical and biological impacts to anthropogenic climate change. Nature 2008, 453, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Schlesinger, W.H. Biogeochemistry: An Analysis of Global Change; Academic Press: San Diego, CA, USA, 1997. [Google Scholar]
- Kabir, M.; Habiba, U.E.; Khan, W.; Shah, A.; Rahim, S.; Patricio, R.; Ali, L.; Shafiq, M. Climate change due to increasing concentration of carbon dioxide and its impacts on environment in 21st century; a mini review. J. King Saud Univ.-Sci. 2023, 35, 102693. [Google Scholar] [CrossRef]
- Pugnaire, F.I.; Morillo, J.A.; Peñuelas, J.; Reich, P.B.; Bardgett, R.D.; Gaxiola, A.; Wardle, D.A.; Van Der Putten, W.H. Climate change effects on plant-soil feedbacks and consequences for biodiversity and functioning of terrestrial ecosystems. Sci. Adv. 2019, 5, eaaz1834. [Google Scholar] [CrossRef] [PubMed]
- Stuart Chapin III, F.; McFarland, J.; David McGuire, A.; Euskirchen, E.S.; Ruess, R.W.; Kielland, K. The changing global carbon cycle: Linking plant–soil carbon dynamics to global consequences. J. Ecol. 2009, 97, 840–850. [Google Scholar] [CrossRef]
- Manlick, P.J.; Perryman, N.L.; Koltz, A.M.; Cook, J.A.; Newsome, S.D. Climate warming restructures food webs and carbon flow in high-latitude ecosystems. Nat. Clim. Change 2024, 14, 184–189. [Google Scholar] [CrossRef]
- Weiskopf, S.R.; Isbell, F.; Arce-Plata, M.I.; Di Marco, M.; Harfoot, M.; Johnson, J.; Lerman, S.B.; Miller, B.W.; Morelli, T.L.; Mori, A.S. Biodiversity loss reduces global terrestrial carbon storage. Nat. Commun. 2024, 15, 4354. [Google Scholar] [CrossRef]
- Mori, A.S.; Dee, L.E.; Gonzalez, A.; Ohashi, H.; Cowles, J.; Wright, A.J.; Loreau, M.; Hautier, Y.; Newbold, T.; Reich, P.B. Biodiversity–productivity relationships are key to nature-based climate solutions. Nat. Clim. Change 2021, 11, 543–550. [Google Scholar] [CrossRef]
- Pavoine, S.; Bonsall, M.B. Measuring biodiversity to explain community assembly: A unified approach. Biol. Rev. 2011, 86, 792–812. [Google Scholar] [CrossRef]
- Hamann, E.; Blevins, C.; Franks, S.J.; Jameel, M.I.; Anderson, J.T. Climate change alters plant–herbivore interactions. New Phytol. 2021, 229, 1894–1910. [Google Scholar] [CrossRef]
- Urban, M.C. Accelerating extinction risk from climate change. Science 2015, 348, 571–573. [Google Scholar] [CrossRef] [PubMed]
- Cahill, A.E.; Aiello-Lammens, M.E.; Fisher-Reid, M.C.; Hua, X.; Karanewsky, C.J.; Yeong Ryu, H.; Sbeglia, G.C.; Spagnolo, F.; Waldron, J.B.; Warsi, O. How does climate change cause extinction? Proc. R. Soc. B Biol. Sci. 2013, 280, 20121890. [Google Scholar] [CrossRef]
- Åkesson, A.; Curtsdotter, A.; Eklöf, A.; Ebenman, B.; Norberg, J.; Barabás, G. The importance of species interactions in eco-evolutionary community dynamics under climate change. Nat. Commun. 2021, 12, 4759. [Google Scholar] [CrossRef] [PubMed]
- DeLucia, E.H.; Nabity, P.D.; Zavala, J.A.; Berenbaum, M.R. Climate change: Resetting plant-insect interactions. Plant Physiol. 2012, 160, 1677–1685. [Google Scholar] [CrossRef]
- Forrest, J.R. Complex responses of insect phenology to climate change. Curr. Opin. Insect Sci. 2016, 17, 49–54. [Google Scholar] [CrossRef]
- Reich, P.B.; Tilman, D.; Isbell, F.; Mueller, K.; Hobbie, S.E.; Flynn, D.F.; Eisenhauer, N. Impacts of biodiversity loss escalate through time as redundancy fades. Science 2012, 336, 589–592. [Google Scholar] [CrossRef] [PubMed]
- Meyer, S.T.; Ebeling, A.; Eisenhauer, N.; Hertzog, L.; Hillebrand, H.; Milcu, A.; Pompe, S.; Abbas, M.; Bessler, H.; Buchmann, N. Effects of biodiversity strengthen over time as ecosystem functioning declines at low and increases at high biodiversity. Ecosphere 2016, 7, e01619. [Google Scholar] [CrossRef]
- Olsson, M.; Schwartz, T.; Wapstra, E.; Uller, T.; Ujvari, B.; Madsen, T.; Shine, R. Climate change, multiple paternity and offspring survival in lizards. Evolution 2011, 65, 3323–3326. [Google Scholar] [CrossRef]
- Koleček, J.; Reif, J.; Šálek, M.; Hanzelka, J.; Sottas, C.; Kubelka, V. Global population trends in shorebirds: Migratory behaviour makes species at risk. Sci. Nat. 2021, 108, 9. [Google Scholar] [CrossRef]
- Buse, J.; Griebeler, E.M.; Niehuis, M. Rising temperatures explain past immigration of the thermophilic oak-inhabiting beetle Coraebus florentinus (Coleoptera: Buprestidae) in south-west Germany. Biodivers. Conserv. 2013, 22, 1115–1131. [Google Scholar] [CrossRef]
- Alabia, I.D.; García Molinos, J.; Saitoh, S.I.; Hirawake, T.; Hirata, T.; Mueter, F.J. Distribution shifts of marine taxa in the Pacific Arctic under contemporary climate changes. Divers. Distrib. 2018, 24, 1583–1597. [Google Scholar] [CrossRef]
- Losapio, G.; Schöb, C. Resistance of plant–plant networks to biodiversity loss and secondary extinctions following simulated environmental changes. Funct. Ecol. 2017, 31, 1145–1152. [Google Scholar] [CrossRef]
- DeGregorio, B.A.; Westervelt, J.D.; Weatherhead, P.J.; Sperry, J.H. Indirect effect of climate change: Shifts in ratsnake behavior alter intensity and timing of avian nest predation. Ecol. Model. 2015, 312, 239–246. [Google Scholar] [CrossRef]
- Cannizzo, Z.J.; Griffen, B.D. Changes in spatial behaviour patterns by mangrove tree crabs following climate-induced range shift into novel habitat. Anim. Behav. 2016, 121, 79–86. [Google Scholar] [CrossRef]
- Yu, X.-T.; Yang, F.-L.; Da, W.; Li, Y.-C.; Xi, H.-M.; Cotton, A.M.; Zhang, H.-H.; Duan, K.; Xu, Z.-B.; Gong, Z.-X. Species Richness of Papilionidae Butterflies (Lepidoptera: Papilionoidea) in the Hengduan Mountains and Its Future Shifts under Climate Change. Insects 2023, 14, 259. [Google Scholar] [CrossRef] [PubMed]
- Karell, P.; Ahola, K.; Karstinen, T.; Valkama, J.; Brommer, J.E. Climate change drives microevolution in a wild bird. Nat. Commun. 2011, 2, 208. [Google Scholar] [CrossRef]
- Zimova, M.; Mills, L.S.; Nowak, J.J. High fitness costs of climate change-induced camouflage mismatch. Ecol. Lett. 2016, 19, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Campbell, D.R.; Powers, J.M. Natural selection on floral morphology can be influenced by climate. Proc. R. Soc. B Biol. Sci. 2015, 282, 20150178. [Google Scholar] [CrossRef] [PubMed]
- Taylor, B.W.; Flecker, A.S.; Hall, R.O., Jr. Loss of a harvested fish species disrupts carbon flow in a diverse tropical river. Science 2006, 313, 833–836. [Google Scholar] [CrossRef]
- Eilers, E.J.; Kremen, C.; Smith Greenleaf, S.; Garber, A.K.; Klein, A.-M. Contribution of pollinator-mediated crops to nutrients in the human food supply. PLoS ONE 2011, 6, e21363. [Google Scholar] [CrossRef]
- Millard, J.; Outhwaite, C.L.; Ceaușu, S.; Carvalheiro, L.G.; da Silva e Silva, F.D.; Dicks, L.V.; Ollerton, J.; Newbold, T. Key tropical crops at risk from pollinator loss due to climate change and land use. Sci. Adv. 2023, 9, eadh0756. [Google Scholar] [CrossRef]
- Jentsch, A.; Kreyling, J.; Elmer, M.; Gellesch, E.; Glaser, B.; Grant, K.; Hein, R.; Lara, M.; Mirzae, H.; Nadler, S.E. Climate extremes initiate ecosystem-regulating functions while maintaining productivity. J. Ecol. 2011, 99, 689–702. [Google Scholar] [CrossRef]
- Fordham, D.A.; Resit Akçakaya, H.; Araújo, M.B.; Elith, J.; Keith, D.A.; Pearson, R.; Auld, T.D.; Mellin, C.; Morgan, J.W.; Regan, T.J. Plant extinction risk under climate change: Are forecast range shifts alone a good indicator of species vulnerability to global warming? Glob. Change Biol. 2012, 18, 1357–1371. [Google Scholar] [CrossRef]
- Van Strien, A.; Soldaat, L.; Gregory, R. Desirable mathematical properties of indicators for biodiversity change. Ecol. Indic. 2012, 14, 202–208. [Google Scholar] [CrossRef]
- Fleishman, E.; Noss, R.F.; Noon, B.R. Utility and limitations of species richness metrics for conservation planning. Ecol. Indic. 2006, 6, 543–553. [Google Scholar] [CrossRef]
- Lamb, E.G.; Bayne, E.; Holloway, G.; Schieck, J.; Boutin, S.; Herbers, J.; Haughland, D.L. Indices for monitoring biodiversity change: Are some more effective than others? Ecol. Indic. 2009, 9, 432–444. [Google Scholar] [CrossRef]
- Konopiński, M.K. Shannon diversity index: A call to replace the original Shannon’s formula with unbiased estimator in the population genetics studies. PeerJ 2020, 8, e9391. [Google Scholar] [CrossRef] [PubMed]
- Saura, S.; Estreguil, C.; Mouton, C.; Rodríguez-Freire, M. Network analysis to assess landscape connectivity trends: Application to European forests (1990–2000). Ecol. Indic. 2011, 11, 407–416. [Google Scholar] [CrossRef]
- Müller, F.; Burkhard, B. The indicator side of ecosystem services. Ecosyst. Serv. 2012, 1, 26–30. [Google Scholar] [CrossRef]
- Reza, M.I.H.; Abdullah, S.A. Regional Index of Ecological Integrity: A need for sustainable management of natural resources. Ecol. Indic. 2011, 11, 220–229. [Google Scholar] [CrossRef]
- LaRue, E.A.; Hardiman, B.S.; Elliott, J.M.; Fei, S. Structural diversity as a predictor of ecosystem function. Environ. Res. Lett. 2019, 14, 114011. [Google Scholar] [CrossRef]
- Lee, A.C.; Mishler, B. Phylogenetic diversity and endemism: Metrics for identifying critical regions of conifer conservation in Australia. Berkeley Sci. J. 2014, 18. [Google Scholar] [CrossRef]
- Isaac, N.J.; Turvey, S.T.; Collen, B.; Waterman, C.; Baillie, J.E. Mammals on the EDGE: Conservation priorities based on threat and phylogeny. PLoS ONE 2007, 2, e296. [Google Scholar] [CrossRef]
- Rosauer, D.; Laffan, S.W.; Crisp, M.D.; Donnellan, S.C.; Cook, L.G. Phylogenetic endemism: A new approach for identifying geographical concentrations of evolutionary history. Mol. Ecol. 2009, 18, 4061–4072. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, G.S.; Parker, I.M. Phylogenetic distance metrics for studies of focal species in communities: Quantiles and cumulative curves. Diversity 2022, 14, 521. [Google Scholar] [CrossRef]
- Duelli, P.; Obrist, M.K. Biodiversity indicators: The choice of values and measures. Agric. Ecosyst. Environ. 2003, 98, 87–98. [Google Scholar] [CrossRef]
- Soto-Navarro, C.; Harfoot, M.; Hill, S.; Campbell, J.; Mora, F.; Campos, C.; Pretorius, C.; Pascual, U.; Kapos, V.; Allison, H. Towards a multidimensional biodiversity index for national application. Nat. Sustain. 2021, 4, 933–942. [Google Scholar] [CrossRef]
- Feld, C.K.; Martins da Silva, P.; Paulo Sousa, J.; De Bello, F.; Bugter, R.; Grandin, U.; Hering, D.; Lavorel, S.; Mountford, O.; Pardo, I. Indicators of biodiversity and ecosystem services: A synthesis across ecosystems and spatial scales. Oikos 2009, 118, 1862–1871. [Google Scholar] [CrossRef]
- Cao, Y.; Hawkins, C.P. Weighting effective number of species measures by abundance weakens detection of diversity responses. J. Appl. Ecol. 2019, 56, 1200–1209. [Google Scholar] [CrossRef]
- Feld, C.K.; Sousa, J.P.; Da Silva, P.M.; Dawson, T.P. Indicators for biodiversity and ecosystem services: Towards an improved framework for ecosystems assessment. Biodivers. Conserv. 2010, 19, 2895–2919. [Google Scholar] [CrossRef]
- Blowes, S.A.; Belmaker, J.; Chase, J.M. Global reef fish richness gradients emerge from divergent and scale-dependent component changes. Proc. R. Soc. B Biol. Sci. 2017, 284, 20170947. [Google Scholar] [CrossRef]
- Prendergast, J.; Wood, S.; Lawton, J.; Eversham, B. Correcting for variation in recording effort in analyses of diversity hotspots. Biodivers. Lett. 1993, 1, 39–53. [Google Scholar] [CrossRef]
- Balmford, A.; Crane, P.; Dobson, A.; Green, R.E.; Mace, G.M. The 2010 challenge: Data availability, information needs and extraterrestrial insights. Philos. Trans. R. Soc. B Biol. Sci. 2005, 360, 221–228. [Google Scholar] [CrossRef]
- Soininen, J.; Passy, S.; Hillebrand, H. The relationship between species richness and evenness: A meta-analysis of studies across aquatic ecosystems. Oecologia 2012, 169, 803–809. [Google Scholar] [CrossRef]
- Hillebrand, H.; Blasius, B.; Borer, E.T.; Chase, J.M.; Downing, J.A.; Eriksson, B.K.; Filstrup, C.T.; Harpole, W.S.; Hodapp, D.; Larsen, S. Biodiversity change is uncoupled from species richness trends: Consequences for conservation and monitoring. J. Appl. Ecol. 2018, 55, 169–184. [Google Scholar] [CrossRef]
- Tucker, C.M.; Cadotte, M.W.; Carvalho, S.B.; Davies, T.J.; Ferrier, S.; Fritz, S.A.; Grenyer, R.; Helmus, M.R.; Jin, L.S.; Mooers, A.O. A guide to phylogenetic metrics for conservation, community ecology and macroecology. Biol. Rev. 2017, 92, 698–715. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, Z.G.; Nielsen, S.E.; Acorn, J.H. Negative relationships between species richness and evenness render common diversity indices inadequate for assessing long-term trends in butterfly diversity. Biodivers. Conserv. 2017, 26, 617–629. [Google Scholar] [CrossRef]
- Gauthier, J.; Derome, N. Evenness-richness scatter plots: A visual and insightful representation of shannon entropy measurements for ecological community analysis. Msphere 2021, 6, 01019–01020. [Google Scholar] [CrossRef]
- Magurran, A.E. Biological diversity. Curr. Biol. 2005, 15, R116–R118. [Google Scholar] [CrossRef] [PubMed]
- McGlinn, D.J.; Xiao, X.; May, F.; Gotelli, N.J.; Engel, T.; Blowes, S.A.; Knight, T.M.; Purschke, O.; Chase, J.M.; McGill, B.J. Measurement of Biodiversity (MoB): A method to separate the scale-dependent effects of species abundance distribution, density, and aggregation on diversity change. Methods Ecol. Evol. 2019, 10, 258–269. [Google Scholar] [CrossRef]
- Tucker, C.M.; Cadotte, M.W. Unifying measures of biodiversity: Understanding when richness and phylogenetic diversity should be congruent. Divers. Distrib. 2013, 19, 845–854. [Google Scholar] [CrossRef]
- Zillio, T.; Condit, R. The impact of neutrality, niche differentiation and species input on diversity and abundance distributions. Oikos 2007, 116, 931–940. [Google Scholar] [CrossRef]
- Magurran, A.E.; Henderson, P.A. Explaining the excess of rare species in natural species abundance distributions. Nature 2003, 422, 714–716. [Google Scholar] [CrossRef] [PubMed]
- McGill, B.J. A test of the unified neutral theory of biodiversity. Nature 2003, 422, 881–885. [Google Scholar] [CrossRef]
- Habibullah, M.S.; Din, B.H.; Tan, S.-H.; Zahid, H. Impact of climate change on biodiversity loss: Global evidence. Environ. Sci. Pollut. Res. 2022, 29, 1073–1086. [Google Scholar] [CrossRef]
- Mantyka-Pringle, C.S.; Visconti, P.; Di Marco, M.; Martin, T.G.; Rondinini, C.; Rhodes, J.R. Climate change modifies risk of global biodiversity loss due to land-cover change. Biol. Conserv. 2015, 187, 103–111. [Google Scholar] [CrossRef]
- McGill, B.J.; Etienne, R.S.; Gray, J.S.; Alonso, D.; Anderson, M.J.; Benecha, H.K.; Dornelas, M.; Enquist, B.J.; Green, J.L.; He, F. Species abundance distributions: Moving beyond single prediction theories to integration within an ecological framework. Ecol. Lett. 2007, 10, 995–1015. [Google Scholar] [CrossRef] [PubMed]
- Bellwood, D.R.; Streit, R.P.; Brandl, S.J.; Tebbett, S.B. The meaning of the term ‘function’ in ecology: A coral reef perspective. Funct. Ecol. 2019, 33, 948–961. [Google Scholar] [CrossRef]
- Stewart, P.S.; Voskamp, A.; Santini, L.; Biber, M.F.; Devenish, A.J.; Hof, C.; Willis, S.G.; Tobias, J.A. Global impacts of climate change on avian functional diversity. Ecol. Lett. 2022, 25, 673–685. [Google Scholar] [CrossRef]
- Bellwood, D.R.; Pratchett, M.S.; Morrison, T.H.; Gurney, G.G.; Hughes, T.P.; Álvarez-Romero, J.G.; Day, J.C.; Grantham, R.; Grech, A.; Hoey, A.S. Coral reef conservation in the Anthropocene: Confronting spatial mismatches and prioritizing functions. Biol. Conserv. 2019, 236, 604–615. [Google Scholar] [CrossRef]
- Streck, C. Synergies between the Kunming-Montreal Global Biodiversity Framework and the Paris Agreement: The role of policy milestones, monitoring frameworks and safeguards. Clim. Policy 2023, 23, 800–811. [Google Scholar] [CrossRef]
- Strassburg, B.B.; Kelly, A.; Balmford, A.; Davies, R.G.; Gibbs, H.K.; Lovett, A.; Miles, L.; Orme, C.D.L.; Price, J.; Turner, R.K. Global congruence of carbon storage and biodiversity in terrestrial ecosystems. Conserv. Lett. 2010, 3, 98–105. [Google Scholar] [CrossRef]
- Wardle, D.A.; Jonsson, M.; Bansal, S.; Bardgett, R.D.; Gundale, M.J.; Metcalfe, D.B. Linking vegetation change, carbon sequestration and biodiversity: Insights from island ecosystems in a long-term natural experiment. J. Ecol. 2012, 100, 16–30. [Google Scholar] [CrossRef]
- Gan, J.; McCarl, B.A. Measuring transnational leakage of forest conservation. Ecol. Econ. 2007, 64, 423–432. [Google Scholar] [CrossRef]
- Labrière, N.; Locatelli, B.; Vieilledent, G.; Kharisma, S.; Basuki, I.; Gond, V.; Laumonier, Y. Spatial congruence between carbon and biodiversity across forest landscapes of northern Borneo. Glob. Ecol. Conserv. 2016, 6, 105–120. [Google Scholar] [CrossRef]
- Steudel, B.; Hector, A.; Friedl, T.; Löfke, C.; Lorenz, M.; Wesche, M.; Kessler, M. Biodiversity effects on ecosystem functioning change along environmental stress gradients. Ecol. Lett. 2012, 15, 1397–1405. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, U.N.; Ayres, E.; Wall, D.H.; Bardgett, R.D. Soil biodiversity and carbon cycling: A review and synthesis of studies examining diversity–function relationships. Eur. J. Soil Sci. 2011, 62, 105–116. [Google Scholar] [CrossRef]
- Mokany, K.; Ware, C.; Harwood, T.D.; Schmidt, R.K.; Ferrier, S. Habitat-based biodiversity assessment for ecosystem accounting in the Murray-Darling Basin. Conserv. Biol. 2022, 36, e13915. [Google Scholar] [CrossRef]
- Maes, J.; Teller, A.; Erhard, M.; Condé, S.; Vallecillo, S.; Barredo, J.I.; Paracchini, M.L.; Abdul Malak, D.; Trombetti, M.; Vigiak, O.; et al. Mapping and Assessment of Ecosystems and Their Services: An EU Wide Ecosystem Assessment in Support of the EU Biodiversity Strategy; Publications Office of the European Union: Ispra, Italy, 2020. [Google Scholar] [CrossRef]
- Keenan, T.; Williams, C. The terrestrial carbon sink. Annu. Rev. Environ. Resour. 2018, 43, 219–243. [Google Scholar] [CrossRef]
- Gonzalez-Meler, M.A.; Taneva, L.; Trueman, R.J. Plant respiration and elevated atmospheric CO2 concentration: Cellular responses and global significance. Ann. Bot. 2004, 94, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Huntley, M.; Lopez, M.; Karl, D. Top predators in the Southern Ocean: A major leak in the biological carbon pump. Science 1991, 253, 64–66. [Google Scholar] [CrossRef]
- Dangal, S.R.; Tian, H.; Zhang, B.; Pan, S.; Lu, C.; Yang, J. Methane emission from global livestock sector during 1890–2014: Magnitude, trends and spatiotemporal patterns. Glob. Change Biol. 2017, 23, 4147–4161. [Google Scholar] [CrossRef] [PubMed]
- Hessen, D.O.; Ågren, G.I.; Anderson, T.R.; Elser, J.J.; De Ruiter, P.C. Carbon sequestration in ecosystems: The role of stoichiometry. Ecology 2004, 85, 1179–1192. [Google Scholar] [CrossRef]
- Meunier, C.L.; Liess, A.; Andersson, A.; Brugel, S.; Paczkowska, J.; Rahman, H.; Skoglund, B.; Rowe, O.F. Allochthonous carbon is a major driver of the microbial food web–A mesocosm study simulating elevated terrestrial matter runoff. Mar. Environ. Res. 2017, 129, 236–244. [Google Scholar] [CrossRef]
- Schmitz, O.J.; Leroux, S.J. Food webs and ecosystems: Linking species interactions to the carbon cycle. Annu. Rev. Ecol. Evol. Syst. 2020, 51, 271–295. [Google Scholar] [CrossRef]
- Steinberg, D.K.; Landry, M.R. Zooplankton and the ocean carbon cycle. Annu. Rev. Mar. Sci. 2017, 9, 413–444. [Google Scholar] [CrossRef] [PubMed]
- Middelburg, J.J. Marine Carbon Biogeochemistry: A Primer for Earth System Scientists; Springer Nature: London, UK, 2019; p. 118. [Google Scholar] [CrossRef]
- Schmitz, O.J.; Wilmers, C.C.; Leroux, S.J.; Doughty, C.E.; Atwood, T.B.; Galetti, M.; Davies, A.B.; Goetz, S.J. Animals and the zoogeochemistry of the carbon cycle. Science 2018, 362, eaar3213. [Google Scholar] [CrossRef]
- Thompson, R.M.; Brose, U.; Dunne, J.A.; Hall, R.O.; Hladyz, S.; Kitching, R.L.; Martinez, N.D.; Rantala, H.; Romanuk, T.N.; Stouffer, D.B. Food webs: Reconciling the structure and function of biodiversity. Trends Ecol. Evol. 2012, 27, 689–697. [Google Scholar] [CrossRef]
- Monroe, J.G.; Markman, D.W.; Beck, W.S.; Felton, A.J.; Vahsen, M.L.; Pressler, Y. Ecoevolutionary dynamics of carbon cycling in the anthropocene. Trends Ecol. Evol. 2018, 33, 213–225. [Google Scholar] [CrossRef]
- De Deyn, G.B.; Cornelissen, J.H.; Bardgett, R.D. Plant functional traits and soil carbon sequestration in contrasting biomes. Ecol. Lett. 2008, 11, 516–531. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, O.J.; Sylvén, M.; Atwood, T.B.; Bakker, E.S.; Berzaghi, F.; Brodie, J.F.; Cromsigt, J.P.; Davies, A.B.; Leroux, S.J.; Schepers, F.J. Trophic rewilding can expand natural climate solutions. Nat. Clim. Change 2023, 13, 324–333. [Google Scholar] [CrossRef]
- Malhi, Y.; Lander, T.; le Roux, E.; Stevens, N.; Macias-Fauria, M.; Wedding, L.; Girardin, C.; Kristensen, J.Å.; Sandom, C.J.; Evans, T.D. The role of large wild animals in climate change mitigation and adaptation. Curr. Biol. 2022, 32, R181–R196. [Google Scholar] [CrossRef] [PubMed]
- Berzaghi, F.; Chami, R.; Cosimano, T.; Fullenkamp, C. Financing conservation by valuing carbon services produced by wild animals. Proc. Natl. Acad. Sci. USA 2022, 119, e2120426119. [Google Scholar] [CrossRef]
- Mariani, G.; Cheung, W.W.; Lyet, A.; Sala, E.; Mayorga, J.; Velez, L.; Gaines, S.D.; Dejean, T.; Troussellier, M.; Mouillot, D. Let more big fish sink: Fisheries prevent blue carbon sequestration—Half in unprofitable areas. Sci. Adv. 2020, 6, eabb4848. [Google Scholar] [CrossRef]
- Martin, A.H.; Pearson, H.C.; Saba, G.K.; Olsen, E.M. Integral functions of marine vertebrates in the ocean carbon cycle and climate change mitigation. One Earth 2021, 4, 680–693. [Google Scholar] [CrossRef]
- Rodil, I.F.; Lohrer, A.M.; Attard, K.M.; Thrush, S.F.; Norkko, A. Positive contribution of macrofaunal biodiversity to secondary production and seagrass carbon metabolism. Ecology 2022, 103, e3648. [Google Scholar] [CrossRef]
- Cassia, R.; Nocioni, M.; Correa-Aragunde, N.; Lamattina, L. Climate change and the impact of greenhouse gasses: CO2 and NO, friends and foes of plant oxidative stress. Front. Plant Sci. 2018, 9, 273. [Google Scholar] [CrossRef]
- Kohli, S.K.; Khanna, K.; Bhardwaj, R.; Abd_Allah, E.F.; Ahmad, P.; Corpas, F.J. Assessment of subcellular ROS and NO metabolism in higher plants: Multifunctional signaling molecules. Antioxidants 2019, 8, 641. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Bai, L. Elevated CO2 and reactive oxygen species in stomatal closure. Plants 2021, 10, 410. [Google Scholar] [CrossRef]
- Qiu, Q.-S.; Huber, J.L.; Booker, F.L.; Jain, V.; Leakey, A.D.; Fiscus, E.L.; Yau, P.M.; Ort, D.R.; Huber, S.C. Increased protein carbonylation in leaves of Arabidopsis and soybean in response to elevated [CO2]. Photosynth. Res. 2008, 97, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.; Schlauch, K.; Tam, R.; Cortes, D.; Torres, M.A.; Shulaev, V.; Dangl, J.L.; Mittler, R. The plant NADPH oxidase RBOHD mediates rapid systemic signaling in response to diverse stimuli. Sci. Signal. 2009, 2, ra45. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R.; Vanderauwera, S.; Suzuki, N.; Miller, G.; Tognetti, V.B.; Vandepoele, K.; Gollery, M.; Shulaev, V.; Van Breusegem, F. ROS signaling: The new wave? Trends Plant Sci. 2011, 16, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Taub, D.R.; Wang, X. Why are nitrogen concentrations in plant tissues lower under elevated CO2? A critical examination of the hypotheses. J. Integr. Plant Biol. 2008, 50, 1365–1374. [Google Scholar] [CrossRef] [PubMed]
- Beligni, M.V.; Lamattina, L. Nitric oxide counteracts cytotoxic processes mediated by reactive oxygen species in plant tissues. Planta 1999, 208, 337–344. [Google Scholar] [CrossRef]
- Correa-Aragunde, N.; Foresi, N.; Lamattina, L. Nitric oxide is a ubiquitous signal for maintaining redox balance in plant cells: Regulation of ascorbate peroxidase as a case study. J. Exp. Bot. 2015, 66, 2913–2921. [Google Scholar] [CrossRef] [PubMed]
- Paital, B.; Panda, S.K.; Hati, A.K.; Mohanty, B.; Mohapatra, M.K.; Kanungo, S.; Chainy, G.B.N. Longevity of animals under reactive oxygen species stress and disease susceptibility due to global warming. World J. Biol. Chem. 2016, 7, 110. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R. ROS are good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Belcher, J.D.; Beckman, J.D.; Balla, G.; Balla, J.; Vercellotti, G. Heme degradation and vascular injury. Antioxid. Redox Signal. 2010, 12, 233–248. [Google Scholar] [CrossRef]
- Hermes-Lima, M.; Zenteno-Savın, T. Animal response to drastic changes in oxygen availability and physiological oxidative stress. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2002, 133, 537–556. [Google Scholar] [CrossRef]
- Metcalfe, N.B.; Monaghan, P. Does reproduction cause oxidative stress? An open question. Trends Ecol. Evol. 2013, 28, 347–350. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P.; Holmgren, A.; Larsson, N.-G.; Halliwell, B.; Chang, C.J.; Kalyanaraman, B.; Rhee, S.G.; Thornalley, P.J.; Partridge, L.; Gems, D. Unraveling the biological roles of reactive oxygen species. Cell Metab. 2011, 13, 361–366. [Google Scholar] [CrossRef]
- Schulte, P.M. The effects of temperature on aerobic metabolism: Towards a mechanistic understanding of the responses of ectotherms to a changing environment. J. Exp. Biol. 2015, 218, 1856–1866. [Google Scholar] [CrossRef] [PubMed]
- Vitikainen, E.I.; Cant, M.A.; Sanderson, J.L.; Mitchell, C.; Nichols, H.J.; Marshall, H.H.; Thompson, F.J.; Gilchrist, J.S.; Hodge, S.J.; Johnstone, R.A. Evidence of oxidative shielding of offspring in a wild mammal. Front. Ecol. Evol. 2016, 4, 58. [Google Scholar] [CrossRef]
- Smith, S.M.; Nager, R.G.; Costantini, D. Meta-analysis indicates that oxidative stress is both a constraint on and a cost of growth. Ecol. Evol. 2016, 6, 2833–2842. [Google Scholar] [CrossRef]
- Ritchie, D.J.; Friesen, C.R. Invited review: Thermal effects on oxidative stress in vertebrate ectotherms. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2022, 263, 111082. [Google Scholar] [CrossRef]
- Speakman, J.R.; Blount, J.D.; Bronikowski, A.M.; Buffenstein, R.; Isaksson, C.; Kirkwood, T.B.; Monaghan, P.; Ozanne, S.E.; Beaulieu, M.; Briga, M. Oxidative stress and life histories: Unresolved issues and current needs. Ecol. Evol. 2015, 5, 5745–5757. [Google Scholar] [CrossRef] [PubMed]
- Vedder, O.; Bouwhuis, S.; Sheldon, B.C. Quantitative assessment of the importance of phenotypic plasticity in adaptation to climate change in wild bird populations. PLoS Biol. 2013, 11, e1001605. [Google Scholar] [CrossRef]
- Martin, R.A.; da Silva, C.R.; Moore, M.P.; Diamond, S.E. When will a changing climate outpace adaptive evolution? Wiley Interdiscip. Rev. Clim. Change 2023, 14, e852. [Google Scholar] [CrossRef]
- Menzel, A.; Sparks, T.H.; Estrella, N.; Koch, E.; Aasa, A.; Ahas, R.; Alm-Kübler, K.; Bissolli, P.; Braslavská, O.g.; Briede, A. European phenological response to climate change matches the warming pattern. Glob. Change Biol. 2006, 12, 1969–1976. [Google Scholar] [CrossRef]
- Waldvogel, A.-M.; Feldmeyer, B.; Rolshausen, G.; Exposito-Alonso, M.; Rellstab, C.; Kofler, R.; Mock, T.; Schmid, K.; Schmitt, I.; Bataillon, T. Evolutionary genomics can improve prediction of species’ responses to climate change. Evol. Lett. 2020, 4, 4–18. [Google Scholar] [CrossRef]
- Sarode, R.M.; Das, A.; Verma, A.K.; Singh, P.; Saini, M.; Bhardwaj, Y.; Sharma, A.K. Partial replacement of dietary buffalo meat on the bone with chicken carcass improves serum antioxidant profile of zoo-housed Indian leopards (Panthera pardus fusca). Zoo Biol. 2019, 38, 292–304. [Google Scholar] [CrossRef]
- Zeng, Z.; Zdzieblik, D.; Centner, C.; Brauchle, C.; Gollhofer, A.; König, D. Changing dietary habits increases the intake of antioxidant vitamins and reduces the concentration of reactive oxygen species in blood: A pilot study. Int. J. Food Prop. 2020, 23, 1337–1346. [Google Scholar] [CrossRef]
- Durge, S.M.; Das, A.; Saha, S.K.; Pande, A.; Thakuria, D.; Saxena, A.; Bhardvaj, Y.; Verma, A.K. Dietary lutein supplementation improves immunity and antioxidant status of captive Indian leopards (Panthera fusca). Zoo Biol. 2022, 41, 328–339. [Google Scholar] [CrossRef]
- Casagrande, S.; DeMoranville, K.J.; Trost, L.; Pierce, B.; Bryła, A.; Dzialo, M.; Sadowska, E.T.; Bauchinger, U.; McWilliams, S.R. Dietary antioxidants attenuate the endocrine stress response during long-duration flight of a migratory bird. Proc. R. Soc. B 2020, 287, 20200744. [Google Scholar] [CrossRef]
- Beaulieu, M.; Haas, A.; Schaefer, H.M. Self-supplementation and effects of dietary antioxidants during acute thermal stress. J. Exp. Biol. 2014, 217, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Bešlo, D.; Golubić, N.; Rastija, V.; Agić, D.; Karnaš, M.; Šubarić, D.; Lučić, B. Antioxidant activity, metabolism, and bioavailability of polyphenols in the diet of animals. Antioxidants 2023, 12, 1141. [Google Scholar] [CrossRef]
- Monaghan, P.; Metcalfe, N.B.; Torres, R. Oxidative stress as a mediator of life history trade-offs: Mechanisms, measurements and interpretation. Ecol. Lett. 2009, 12, 75–92. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, H.M.; McGraw, K.; Catoni, C. Birds use fruit colour as honest signal of dietary antioxidant rewards. Funct. Ecol. 2008, 22, 303–310. [Google Scholar] [CrossRef]
- Zhang, F.P.; Cai, X.H.; Wang, H.; Ren, Z.X.; Larson-Rabin, Z.; Li, D.Z. Dark purple nectar as a foraging signal in a bird-pollinated Himalayan plant. New Phytol. 2012, 193, 188–195. [Google Scholar] [CrossRef]
- Alan, R.R.; McWilliams, S.R.; McGraw, K.J. The importance of antioxidants for avian fruit selection during autumn migration. Wilson J. Ornithol. 2013, 125, 513–525. [Google Scholar] [CrossRef]
- Bolser, J.A.; Alan, R.R.; Smith, A.D.; Li, L.; Seeram, N.P.; McWilliams, S.R. Birds select fruits with more anthocyanins and phenolic compounds during autumn migration. Wilson J. Ornithol. 2013, 125, 97–108. [Google Scholar] [CrossRef]
- Skrip, M.M.; Bauchinger, U.; Goymann, W.; Fusani, L.; Cardinale, M.; Alan, R.R.; McWilliams, S.R. Migrating songbirds on stopover prepare for, and recover from, oxidative challenges posed by long-distance flight. Ecol. Evol. 2015, 5, 3198–3209. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; Møller, A.P. Long-lived birds suffer less from oxidative stress. Avian Res. 2018, 9, 41. [Google Scholar] [CrossRef]
- Beaulieu, M.; Schaefer, H.M. Rethinking the role of dietary antioxidants through the lens of self-medication. Anim. Behav. 2013, 86, 17–24. [Google Scholar] [CrossRef]
- Catoni, C.; Peters, A.; Schaefer, H.M. Life history trade-offs are influenced by the diversity, availability and interactions of dietary antioxidants. Anim. Behav. 2008, 76, 1107–1119. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed]
- Selman, C.; McLaren, J.S.; Meyer, C.; Duncan, J.S.; Redman, P.; Collins, A.R.; Duthie, G.G.; Speakman, J.R. Life-long vitamin C supplementation in combination with cold exposure does not affect oxidative damage or lifespan in mice, but decreases expression of antioxidant protection genes. Mech. Ageing Dev. 2006, 127, 897–904. [Google Scholar] [CrossRef]
- Skrip, M.M.; McWilliams, S.R. Oxidative balance in birds: An atoms-to-organisms-to-ecology primer for ornithologists. J. Field Ornithol. 2016, 87, 1–20. [Google Scholar] [CrossRef]
- Skrip, M.M. Oxidative State of Songbirds during Migration and Best Practices to Communicate the Science. Ph.D. Dissertation, University of Rhode Island, Kingston, RI, USA, 2016. [Google Scholar]
- Moskaug, J.Ø.; Carlsen, H.; Myhrstad, M.C.; Blomhoff, R. Polyphenols and glutathione synthesis regulation. Am. J. Clin. Nutr. 2005, 81, 277S–283S. [Google Scholar] [CrossRef]
- Yeh, C.-T.; Ching, L.-C.; Yen, G.-C. Inducing gene expression of cardiac antioxidant enzymes by dietary phenolic acids in rats. J. Nutr. Biochem. 2009, 20, 163–171. [Google Scholar] [CrossRef]
- Spanier, G.; Xu, H.; Xia, N.; Tobias, S.; Deng, S.; Wojnowski, L.; Forstermann, U.; Li, H. Resveratrol reduces endothelial oxidative stress by modulating the gene expression of superoxide dismutase 1 (SOD1), glutathione peroxidase 1 (GPx1) and NADPH oxidase subunit (Nox4). J. Physiol. Pharmacol. 2009, 60, 111–116. [Google Scholar] [PubMed]
- Slifka, K.A.; Bowen, P.E.; Stacewicz-Sapuntzakis, M.; Crissey, S.D. A survey of serum and dietary carotenoids in captive wild animals. J. Nutr. 1999, 129, 380–390. [Google Scholar] [CrossRef]
- Yvon-Durocher, G.; Allen, A.P. Linking community size structure and ecosystem functioning using metabolic theory. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 2998–3007. [Google Scholar] [CrossRef] [PubMed]
- Crissey, S.D.; Jacobsen, K.L.; Slifka, K.A.; Ange, K.D.; Bowen, P.E.; Stacewicz-Sapuntzakis, M.; Langman, C.B.; Sadler, W.; Kahn, S.; Ward, A. Serum concentrations of lipids, vitamin D metabolites, retinol, retinyl esters, tocopherols and selected carotenoids in twelve captive wild felid species at four zoos. J. Nutr. 2003, 133, 160–166. [Google Scholar] [CrossRef]
- Møller, A.P.; Erritzøe, J.; Karadas, F. Levels of antioxidants in rural and urban birds and their consequences. Oecologia 2010, 163, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.A.; McGraw, K.J.; Robinson, W.D. Serum antioxidant levels in wild birds vary in relation to diet, season, life history strategy, and species. Oecologia 2009, 161, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Galván, I.; Erritzøe, J.; Karadaş, F.; Møller, A.P. High levels of liver antioxidants are associated with life-history strategies characteristic of slow growth and high survival rates in birds. J. Comp. Physiol. B 2012, 182, 947–959. [Google Scholar] [CrossRef] [PubMed]
- Walther, G.-R. Community and ecosystem responses to recent climate change. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 2019–2024. [Google Scholar] [CrossRef] [PubMed]
- Blois, J.L.; Zarnetske, P.L.; Fitzpatrick, M.C.; Finnegan, S. Climate change and the past, present, and future of biotic interactions. Science 2013, 341, 499–504. [Google Scholar] [CrossRef]
- Carbeck, K.M.; DeMoranville, K.J.; D’Amelio, P.B.; Goymann, W.; Trost, L.; Pierce, B.; Bryła, A.; Dzialo, M.; Bauchinger, U.; McWilliams, S.R. Environmental cues and dietary antioxidants affect breeding behavior and testosterone of male European starlings (Sturnus vulgaris). Horm. Behav. 2018, 103, 36–44. [Google Scholar] [CrossRef]
- Dowling, D.K.; Simmons, L.W. Reactive oxygen species as universal constraints in life-history evolution. Proc. R. Soc. B Biol. Sci. 2009, 276, 1737–1745. [Google Scholar] [CrossRef] [PubMed]
- Metcalfe, N.B.; Alonso-Alvarez, C. Oxidative stress as a life-history constraint: The role of reactive oxygen species in shaping phenotypes from conception to death. Funct. Ecol. 2010, 24, 984–996. [Google Scholar] [CrossRef]
- Garratt, M.; Brooks, R.C. Oxidative stress and condition-dependent sexual signals: More than just seeing red. Proc. R. Soc. B Biol. Sci. 2012, 279, 3121–3130. [Google Scholar] [CrossRef] [PubMed]
- Ito, H.; Kurokawa, H.; Matsui, H. Mitochondrial reactive oxygen species and heme, non-heme iron metabolism. Arch. Biochem. Biophys. 2021, 700, 108695. [Google Scholar] [CrossRef]
- Piantadosi, C.A. Carbon monoxide, reactive oxygen signaling, and oxidative stress. Free Radic. Biol. Med. 2008, 45, 562–569. [Google Scholar] [CrossRef]
- Zgliczynski, B.J.; Sandin, S.A. Size-structural shifts reveal intensity of exploitation in coral reef fisheries. Ecol. Indic. 2017, 73, 411–421. [Google Scholar] [CrossRef]
- McCauley, D.J.; Gellner, G.; Martinez, N.D.; Williams, R.J.; Sandin, S.A.; Micheli, F.; Mumby, P.J.; McCann, K.S. On the prevalence and dynamics of inverted trophic pyramids and otherwise top-heavy communities. Ecol. Lett. 2018, 21, 439–454. [Google Scholar] [CrossRef]
- Beck, J.; Ballesteros-Mejia, L.; Buchmann, C.M.; Dengler, J.; Fritz, S.A.; Gruber, B.; Hof, C.; Jansen, F.; Knapp, S.; Kreft, H. What’s on the horizon for macroecology? Ecography 2012, 35, 673–683. [Google Scholar] [CrossRef]
- Bromham, L.; Hua, X.; Cardillo, M. Macroevolutionary and macroecological approaches to understanding the evolution of stress tolerance in plants. Plant Cell Environ. 2020, 43, 2832–2846. [Google Scholar] [CrossRef]
- Buckley, Y.M.; Puy, J. The macroecology of plant populations from local to global scales. New Phytol. 2022, 233, 1038–1050. [Google Scholar] [CrossRef] [PubMed]
- Nevo, E. Evolution under environmental stress at macro-and microscales. Genome Biol. Evol. 2011, 3, 1039–1052. [Google Scholar] [CrossRef]
- Travis, J. Climate change and habitat destruction: A deadly anthropogenic cocktail. Proc. R. Soc. London. Ser. B Biol. Sci. 2003, 270, 467–473. [Google Scholar] [CrossRef]
- Mair, L.; Hill, J.K.; Fox, R.; Botham, M.; Brereton, T.; Thomas, C.D. Abundance changes and habitat availability drive species’ responses to climate change. Nat. Clim. Change 2014, 4, 127–131. [Google Scholar] [CrossRef]
- Boag, T.H.; Gearty, W.; Stockey, R.G. Metabolic tradeoffs control biodiversity gradients through geological time. Curr. Biol. 2021, 31, 2906–2913.e2903. [Google Scholar] [CrossRef]
- Chen, I.-C.; Hill, J.K.; Ohlemüller, R.; Roy, D.B.; Thomas, C.D. Rapid range shifts of species associated with high levels of climate warming. Science 2011, 333, 1024–1026. [Google Scholar] [CrossRef]
- Ramalho, Q.; Vale, M.M.; Manes, S.; Diniz, P.; Malecha, A.; Prevedello, J.A. Evidence of stronger range shift response to ongoing climate change by ectotherms and high-latitude species. Biol. Conserv. 2023, 279, 109911. [Google Scholar] [CrossRef]
- Easterling, D.R.; Meehl, G.A.; Parmesan, C.; Changnon, S.A.; Karl, T.R.; Mearns, L.O. Climate extremes: Observations, modeling, and impacts. Science 2000, 289, 2068–2074. [Google Scholar] [CrossRef]
- Weiskopf, S.R.; Rubenstein, M.A.; Crozier, L.G.; Gaichas, S.; Griffis, R.; Halofsky, J.E.; Hyde, K.J.; Morelli, T.L.; Morisette, J.T.; Muñoz, R.C. Climate change effects on biodiversity, ecosystems, ecosystem services, and natural resource management in the United States. Sci. Total Environ. 2020, 733, 137782. [Google Scholar] [CrossRef] [PubMed]
- Segan, D.B.; Murray, K.A.; Watson, J.E. A global assessment of current and future biodiversity vulnerability to habitat loss–climate change interactions. Glob. Ecol. Conserv. 2016, 5, 12–21. [Google Scholar] [CrossRef]
- Laidre, K.L.; Stirling, I.; Lowry, L.F.; Wiig, Ø.; Heide-Jørgensen, M.P.; Ferguson, S.H. Quantifying the sensitivity of Arctic marine mammals to climate-induced habitat change. Ecol. Appl. 2008, 18, S97–S125. [Google Scholar] [CrossRef]
- Hazen, E.L.; Jorgensen, S.; Rykaczewski, R.R.; Bograd, S.J.; Foley, D.G.; Jonsen, I.D.; Shaffer, S.A.; Dunne, J.P.; Costa, D.P.; Crowder, L.B. Predicted habitat shifts of Pacific top predators in a changing climate. Nat. Clim. Change 2013, 3, 234–238. [Google Scholar] [CrossRef]
- Bozinovic, F.; Pörtner, H.O. Physiological ecology meets climate change. Ecol. Evol. 2015, 5, 1025–1030. [Google Scholar] [CrossRef]
- Huang, M.; Ding, L.; Wang, J.; Ding, C.; Tao, J. The impacts of climate change on fish growth: A summary of conducted studies and current knowledge. Ecol. Indic. 2021, 121, 106976. [Google Scholar] [CrossRef]
- Lee, W.-S.; Monaghan, P.; Metcalfe, N.B. Experimental demonstration of the growth rate–lifespan trade-off. Proc. R. Soc. B Biol. Sci. 2013, 280, 20122370. [Google Scholar] [CrossRef] [PubMed]
- Ohlberger, J. Climate warming and ectotherm body size–from individual physiology to community ecology. Funct. Ecol. 2013, 27, 991–1001. [Google Scholar] [CrossRef]
- Scrosati, R.A.; Knox, A.S.; Valdivia, N.; Molis, M. Species richness and diversity across rocky intertidal elevation gradients in Helgoland: Testing predictions from an environmental stress model. Helgol. Mar. Res. 2011, 65, 91–102. [Google Scholar] [CrossRef]
- Scrosati, R.A.; van Genne, B.; Heaven, C.S.; Watt, C.A. Species richness and diversity in different functional groups across environmental stress gradients: A model for marine rocky shores. Ecography 2011, 34, 151–161. [Google Scholar] [CrossRef]
- Pires, A.P.; Srivastava, D.S.; Marino, N.A.; MacDonald, A.A.M.; Figueiredo-Barros, M.P.; Farjalla, V.F. Interactive effects of climate change and biodiversity loss on ecosystem functioning. Ecology 2018, 99, 1203–1213. [Google Scholar] [CrossRef]
- Pires, A.P.; Srivastava, D.S.; Farjalla, V.F. Is biodiversity able to buffer ecosystems from climate change? What we know and what we don’t. Bioscience 2018, 68, 273–280. [Google Scholar] [CrossRef]
- Montoya, D.; Yallop, M.L.; Memmott, J. Functional group diversity increases with modularity in complex food webs. Nat. Commun. 2015, 6, 7379. [Google Scholar] [CrossRef]
- Blowes, S.A.; Daskalova, G.N.; Dornelas, M.; Engel, T.; Gotelli, N.J.; Magurran, A.E.; Martins, I.S.; McGill, B.; McGlinn, D.J.; Sagouis, A. Local biodiversity change reflects interactions among changing abundance, evenness, and richness. Ecology 2022, 103, e3820. [Google Scholar] [CrossRef]
- Hawkins, B.A.; Field, R.; Cornell, H.V.; Currie, D.J.; Guégan, J.-F.; Kaufman, D.M.; Kerr, J.T.; Mittelbach, G.G.; Oberdorff, T.; O’Brien, E.M. Energy, water, and broad-scale geographic patterns of species richness. Ecology 2003, 84, 3105–3117. [Google Scholar] [CrossRef]
- Tews, J.; Brose, U.; Grimm, V.; Tielbörger, K.; Wichmann, M.C.; Schwager, M.; Jeltsch, F. Animal species diversity driven by habitat heterogeneity/diversity: The importance of keystone structures. J. Biogeogr. 2004, 31, 79–92. [Google Scholar] [CrossRef]
- Guégan, J.-F.; Lek, S.; Oberdorff, T. Energy availability and habitat heterogeneity predict global riverine fish diversity. Nature 1998, 391, 382–384. [Google Scholar] [CrossRef]
- Avolio, M.L.; Komatsu, K.J.; Collins, S.L.; Grman, E.; Koerner, S.E.; Tredennick, A.T.; Wilcox, K.R.; Baer, S.; Boughton, E.H.; Britton, A.J. Determinants of community compositional change are equally affected by global change. Ecol. Lett. 2021, 24, 1892–1904. [Google Scholar] [CrossRef] [PubMed]
- O’Gorman, E.J.; Yearsley, J.M.; Crowe, T.P.; Emmerson, M.C.; Jacob, U.; Petchey, O.L. Loss of functionally unique species may gradually undermine ecosystems. Proc. R. Soc. B Biol. Sci. 2011, 278, 1886–1893. [Google Scholar] [CrossRef] [PubMed]
- Storch, D.; Gaston, K.J. Untangling ecological complexity on different scales of space and time. Basic Appl. Ecol. 2004, 5, 389–400. [Google Scholar] [CrossRef]
- Parmesan, C. Ecological and evolutionary responses to recent climate change. Annu. Rev. Ecol. Evol. Syst. 2006, 37, 637–669. [Google Scholar] [CrossRef]
- King, N.G.; Wilmes, S.B.; Smyth, D.; Tinker, J.; Robins, P.E.; Thorpe, J.; Jones, L.; Malham, S.K. Climate change accelerates range expansion of the invasive non-native species, the Pacific oyster, Crassostrea gigas. ICES J. Mar. Sci. 2021, 78, 70–81. [Google Scholar] [CrossRef]
- Bellard, C.; Thuiller, W.; Leroy, B.; Genovesi, P.; Bakkenes, M.; Courchamp, F. Will climate change promote future invasions? Glob. Change Biol. 2013, 19, 3740–3748. [Google Scholar] [CrossRef] [PubMed]
- Gibson-Reinemer, D.K.; Rahel, F.J. Inconsistent range shifts within species highlight idiosyncratic responses to climate warming. PLoS ONE 2015, 10, e0132103. [Google Scholar] [CrossRef]
- Anand, M.; Gonzalez, A.; Guichard, F.; Kolasa, J.; Parrott, L. Ecological systems as complex systems: Challenges for an emerging science. Diversity 2010, 2, 395–410. [Google Scholar] [CrossRef]
- Estrada, E. What is a complex system, after all? Found. Sci. 2023, 1–28. [Google Scholar] [CrossRef]
- Staniczenko, P.P.; Sivasubramaniam, P.; Suttle, K.B.; Pearson, R.G. Linking macroecology and community ecology: Refining predictions of species distributions using biotic interaction networks. Ecol. Lett. 2017, 20, 693–707. [Google Scholar] [CrossRef] [PubMed]
- Ladyman, J.; Lambert, J.; Wiesner, K. What is a complex system? Eur. J. Philos. Sci. 2013, 3, 33–67. [Google Scholar] [CrossRef]
- Kolasa, J. Complexity, system integration, and susceptibility to change: Biodiversity connection. Ecol. Complex. 2005, 2, 431–442. [Google Scholar] [CrossRef]
- Phillips, M.A.; Ritala, P. A complex adaptive systems agenda for ecosystem research methodology. Technol. Forecast. Soc. Change 2019, 148, 119739. [Google Scholar] [CrossRef]
- Roy, F.; Barbier, M.; Biroli, G.; Bunin, G. Complex interactions can create persistent fluctuations in high-diversity ecosystems. PLoS Comput. Biol. 2020, 16, e1007827. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.A.; Frank, K.T.; Leggett, W.C. Dynamic macroecology on ecological time-scales. Glob. Ecol. Biogeogr. 2010, 19, 1–15. [Google Scholar] [CrossRef]
- Kamarainen, A.M.; Grotzer, T.A. Constructing causal understanding in complex systems: Epistemic strategies used by ecosystem scientists. BioScience 2019, 69, 533–543. [Google Scholar] [CrossRef]
- Allen, T.F.; Hoekstra, T.W. Toward a Unified Ecology; Columbia University Press: New York, NY, USA, 2015. [Google Scholar]
- Wilensky, U.; Resnick, M. Thinking in levels: A dynamic systems approach to making sense of the world. J. Sci. Educ. Technol. 1999, 8, 3–19. [Google Scholar] [CrossRef]
- Lachowicz, M. Microscopic, mesoscopic and macroscopic descriptions of complex systems. Probabilistic Eng. Mech. 2011, 26, 54–60. [Google Scholar] [CrossRef]
- Bonan, G.B.; Doney, S.C. Climate, ecosystems, and planetary futures: The challenge to predict life in Earth system models. Science 2018, 359, eaam8328. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Cadotte, M.W.; Wang, P.; Rao, S.; Shi, X.; Ren, S.; Wang, X.; Li, S.p. Multiple dimensions of phylogenetic diversity are needed to explain the complex aboveground–belowground diversity relationships. Oikos 2023, e10474. [Google Scholar] [CrossRef]
- Levin, S.A. Self-organization and the emergence of complexity in ecological systems. Bioscience 2005, 55, 1075–1079. [Google Scholar] [CrossRef]
- Loreau, M. Linking biodiversity and ecosystems: Towards a unifying ecological theory. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Arneth, A.; Harrison, S.P.; Zaehle, S.; Tsigaridis, K.; Menon, S.; Bartlein, P.; Feichter, J.; Korhola, A.; Kulmala, M.; O’donnell, D. Terrestrial biogeochemical feedbacks in the climate system. Nat. Geosci. 2010, 3, 525–532. [Google Scholar] [CrossRef]
- Galvez, M.E.; Fischer, W.W.; Jaccard, S.L.; Eglinton, T.I. Materials and pathways of the organic carbon cycle through time. Nat. Geosci. 2020, 13, 535–546. [Google Scholar] [CrossRef]
- Ivlev, A.A. Global redox cycle of biospheric carbon: Interaction of photosynthesis and earth crust processes. BioSystems 2015, 137, 1–11. [Google Scholar] [CrossRef]
- Allen, J.; Polimene, L. Linking physiology to ecology: Towards a new generation of plankton models. J. Plankton Res. 2011, 33, 989–997. [Google Scholar] [CrossRef][Green Version]
- Carmody, M.; Waszczak, C.; Idänheimo, N.; Saarinen, T.; Kangasjärvi, J. ROS signalling in a destabilised world: A molecular understanding of climate change. J. Plant Physiol. 2016, 203, 69–83. [Google Scholar] [CrossRef] [PubMed]
- Gilroy, S.; Białasek, M.; Suzuki, N.; Górecka, M.; Devireddy, A.R.; Karpiński, S.; Mittler, R. ROS, calcium, and electric signals: Key mediators of rapid systemic signaling in plants. Plant Physiol. 2016, 171, 1606–1615. [Google Scholar] [CrossRef]
- Gilroy, S.; Suzuki, N.; Miller, G.; Choi, W.-G.; Toyota, M.; Devireddy, A.R.; Mittler, R. A tidal wave of signals: Calcium and ROS at the forefront of rapid systemic signaling. Trends Plant Sci. 2014, 19, 623–630. [Google Scholar] [CrossRef]
- Steinhorst, L.; Kudla, J. Calcium and reactive oxygen species rule the waves of signaling. Plant Physiol. 2013, 163, 471–485. [Google Scholar] [CrossRef]
- Hamanaka, R.B.; Chandel, N.S. Mitochondrial reactive oxygen species regulate cellular signaling and dictate biological outcomes. Trends Biochem. Sci. 2010, 35, 505–513. [Google Scholar] [CrossRef]
- Blanco, G.; Hornero-Méndez, D.; Lambertucci, S.A.; Bautista, L.M.; Wiemeyer, G.; Sanchez-Zapata, J.A.; Garrido-Fernandez, J.; Hiraldo, F.; Donázar, J.A. Need and seek for dietary micronutrients: Endogenous regulation, external signalling and food sources of carotenoids in New World vultures. PLoS ONE 2013, 8, e65562. [Google Scholar] [CrossRef]
- Hardison, E.A.; Eliason, E.J. Diet effects on ectotherm thermal performance. Biol. Rev. 2024, 99, 1537–1555. [Google Scholar] [CrossRef]
- Gilbert, A.L.; Miles, D.B. Food, temperature and endurance: Effects of food deprivation on the thermal sensitivity of physiological performance. Funct. Ecol. 2016, 30, 1790–1799. [Google Scholar] [CrossRef]
- Malzahn, A.M.; Doerfler, D.; Boersma, M. Junk food gets healthier when it’s warm. Limnol. Oceanogr. 2016, 61, 1677–1685. [Google Scholar] [CrossRef]
| Elements | Species | Research Content | References |
|---|---|---|---|
| Adaptability to environmental changes | Adaptive C3 and C4 grasses and N-fixing and non-fixing dicotyledonous 16 herb species | Biodiversity loss impacts amplify over time. | [29] |
| Sixty perennial grassland plant species | Current species loss impairs ecosystem functions decades later. | [30] | |
| Sand lizard (Lacerta agilis) | Heterogeneous responses: short-term biodiversity loss and long-term population increase. | [31] | |
| Organism migratory behavior and dispersal | Shorebirds, 194 species (Charadriiformes) | Population decline due to climate-induced migration. | [32] |
| Coraebus florentinus (Coleoptera: Buprestidae) | Habitat range expansion impacts existing species negatively. | [33] | |
| 21 species of marine fish and invertebrates | Velocity of climate change and species migration velocity are not necessarily synchronous. | [34] | |
| Ecological network | Arenaria tetraquetra subsp. amabilis (Bory), Plantago holosteum Scop, Festuca indigesta Boiss | Biodiversity loss alters ecological structures, leading to cascading losses. | [35] |
| Ratsnake, cardinals | Ratsnake habitat shifts increase predation, reducing cardinal populations. | [36] | |
| Aratus pisonii (Decapoda: Sesarmidae) | Habitat alterations affect life history, including increased foraging energy expenditure. | [37] | |
| Natural selection | Papilionidae (Lepidoptera: Papilionoidea), Papilioninae | Despite identical shifts in habitat range, species exhibit differential adaptability. | [38] |
| Strix aluco | Climate warming alters the genetic choice of feather coloration. | [39] | |
| Lepus americanus | Snowpack depletion leads to a mismatch between background and fur color, affecting survival rates. | [40] | |
| Hummingbirds and hawkmoths (Hyles lineata) | Climate conditions influence the intensity of floral trait selection by hummingbirds and hawkmoths. | [41] | |
| Ecosystem functions | Prochilodontidae: Prochilodus mariae | Even in species-rich communities, if nutrient levels are low, species loss impacts ecological functions. | [42] |
| Approximately 150 major global crops with high pollination dependency | Persistent decline in pollinators aggravates global food supply challenges. | [43] | |
| 3080 pollinator species from 2673 locations | Pollinator loss also affects crop production. | [44] | |
| Arrhenatherum elatius, Holcus lanatus, Geranium pratense, Lotus corniculatus, Plantago lanceolate | Ecosystem functions, such as water regulation, primary production, and nutrient cycling, exhibit differential responses. | [45] |
| Type | Characteristics | Methods | Reference |
|---|---|---|---|
| Species Diversity | Measurement of the number of species inhabiting a specific area. | Species richness: the total number of species present within a specific area. | [47,48,49,50] |
| Shannon index: considers both species richness and evenness simultaneously. | |||
| Simpson’s index: measurement of species diversity within a community. | |||
| Sørensen’s similarity coefficient: measurement of species composition similarity between areas. | |||
| Ecosystem Diversity | Diversity of habitats, biotic communities, and ecosystems | Ecosystem integrity index: assesses the capacity to support and maintain a balanced, integrated ecosystem in a specific area. | [51,52,53,54] |
| Ecosystem services indicators: focuses on ecosystem management and policy relationships. | |||
| Landscape connectivity index: measures the degree to which movement between resources is facilitated or impeded. | |||
| Ecosystem extent: measures multiple variables that can serve as proxies for ecosystem functions. | |||
| Phylogenetic Diversity | Sum of branch lengths in phylogenetic trees. | Evolutionary distinctiveness (ED): generation of a list of globally endangered species. | [55,56,57,58] |
| Phylogenetic endemism (PE): combines measures of phylogenetic diversity and endemism. Addresses limitations of weighted endemism. | |||
| Mean pairwise distance (MPD): measures phylogenetic divergence. Reflects deeper branching in the tree of life. | |||
| Mean nearest taxon distance (MNTD): estimates the average phylogenetic distance between each community member and its closest relative. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, A.-Y.; Lee, W.-S.; Son, Y. The Interaction between Climate Change and Biodiversity Can Be Assessed from a Material Cycle Perspective. Diversity 2024, 16, 506. https://doi.org/10.3390/d16080506
Kim A-Y, Lee W-S, Son Y. The Interaction between Climate Change and Biodiversity Can Be Assessed from a Material Cycle Perspective. Diversity. 2024; 16(8):506. https://doi.org/10.3390/d16080506
Chicago/Turabian StyleKim, Ah-Young, Who-Seung Lee, and Yowhan Son. 2024. "The Interaction between Climate Change and Biodiversity Can Be Assessed from a Material Cycle Perspective" Diversity 16, no. 8: 506. https://doi.org/10.3390/d16080506
APA StyleKim, A.-Y., Lee, W.-S., & Son, Y. (2024). The Interaction between Climate Change and Biodiversity Can Be Assessed from a Material Cycle Perspective. Diversity, 16(8), 506. https://doi.org/10.3390/d16080506








