Microhabitat Structure Affects Ground-Dwelling Beetle Communities More than Temperature along an Urbanization Gradient
Abstract
1. Introduction
2. Materials and Methods
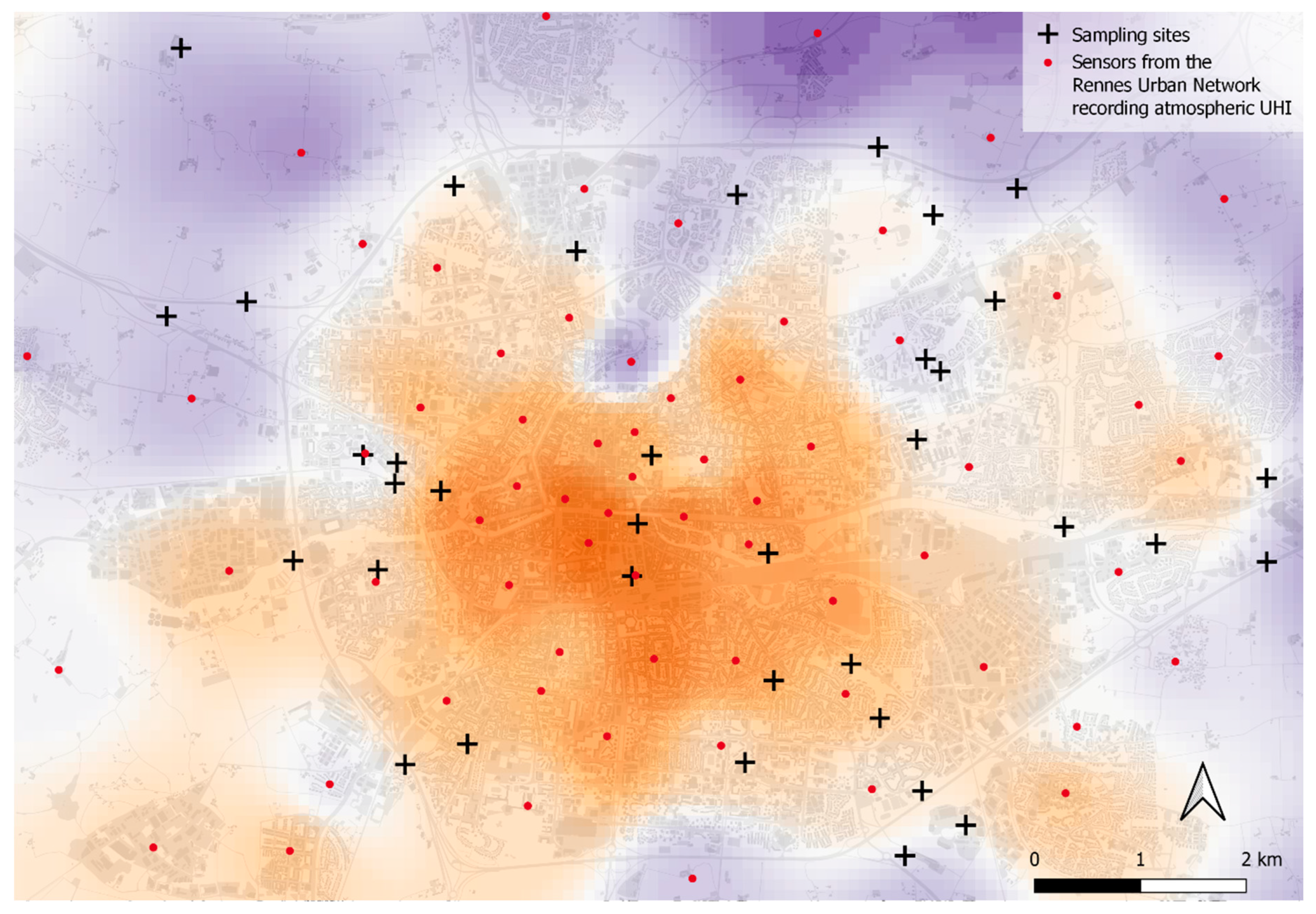
2.1. Sampling Sites
2.2. Arthropod Sampling
2.3. Community Indices
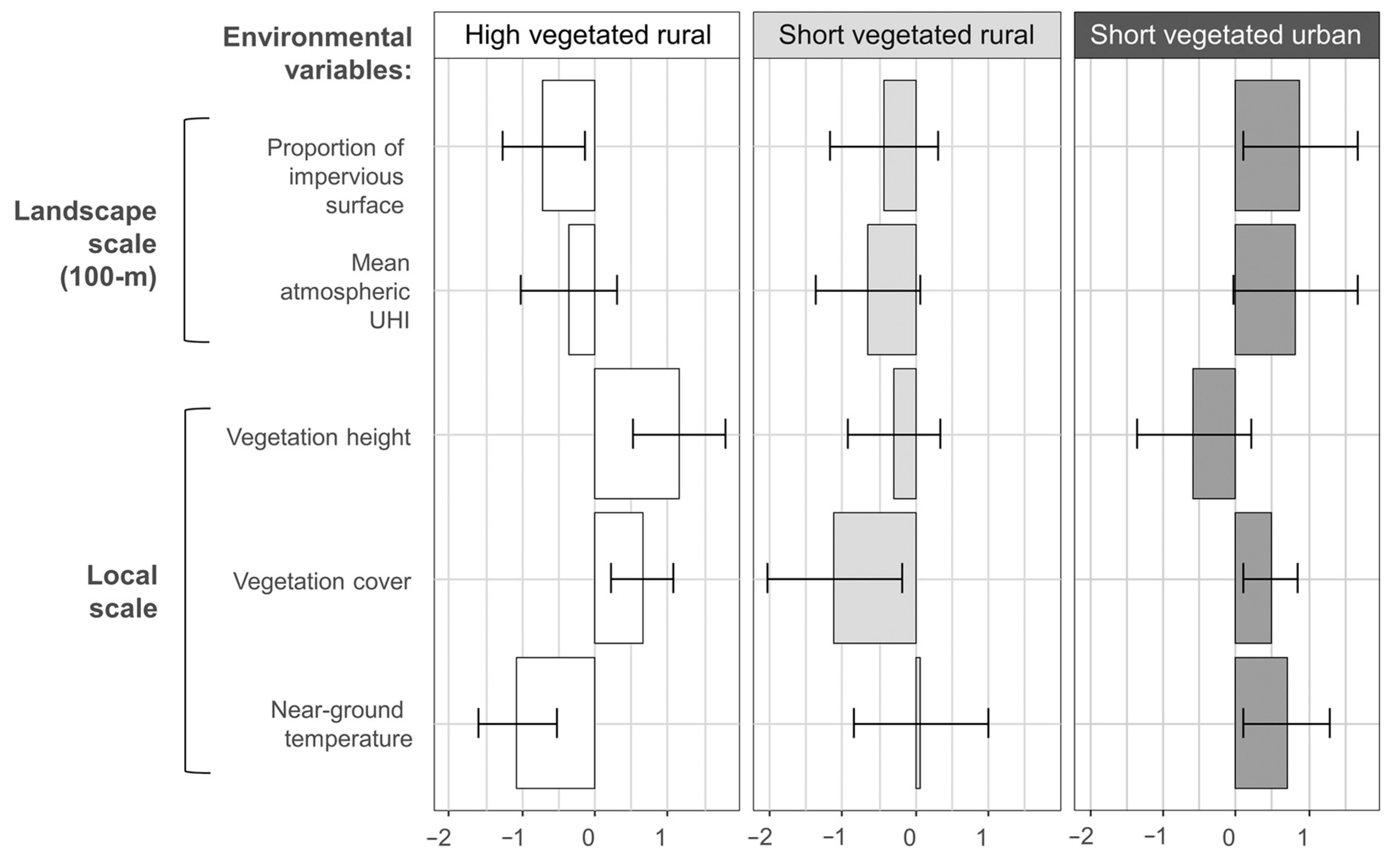
2.4. Environmental Variables
2.5. Data Analysis
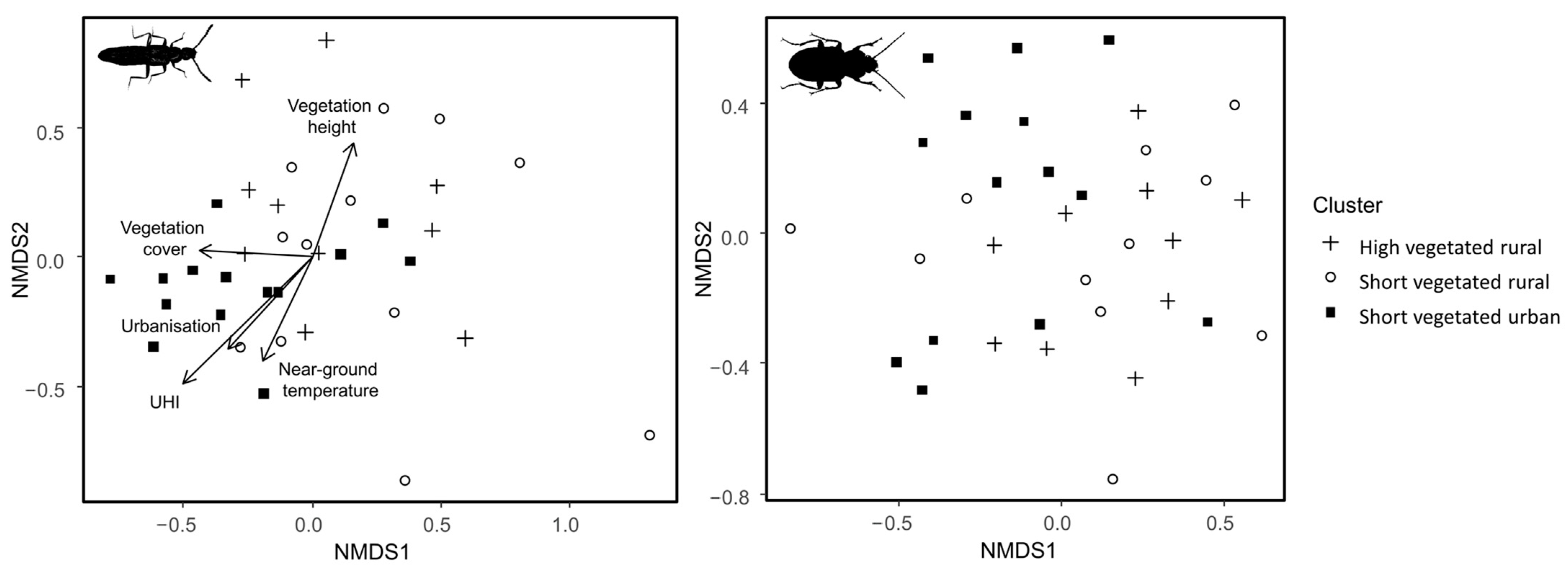
2.5.1. Community Composition Analysis
2.5.2. Community Diversity Analysis
3. Results
3.1. Community Composition
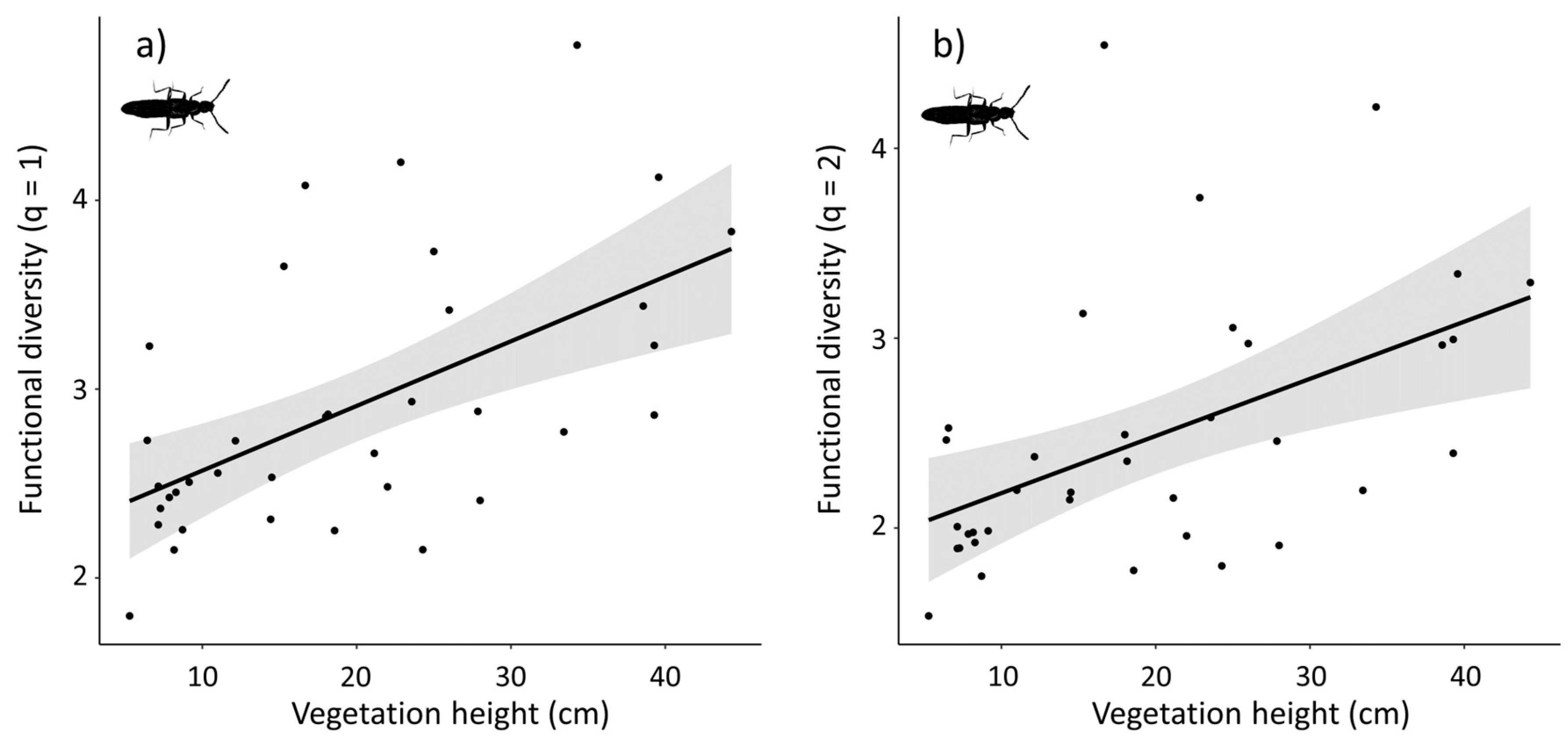
3.2. Density and Diversity Indices
4. Discussion
4.1. Density and Diversity Indices
4.2. Absence of Temperature Effect
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Norton, B.A.; Thomson, L.J.; Williams, N.S.G.; McDonnell, M.J. The Effect of Urban Ground Covers on Arthropods: An Experiment. Urban Ecosyst. 2014, 17, 77–99. [Google Scholar] [CrossRef]
- Philpott, S.; Cotton, J.; Bichier, P.; Friedrich, R.; Moorhead, L.; Uno, S.; Valdez, M. Local and Landscape Drivers of Arthropod Abundance, Richness, and Trophic Composition in Urban Habitats. Urban Ecosyst. 2014, 17, 513–532. [Google Scholar] [CrossRef]
- Otoshi, M.D.; Bichier, P.; Philpott, S.M. Local and Landscape Correlates of Spider Activity Density and Species Richness in Urban Gardens. Environ. Entomol. 2015, 44, 1043–1051. [Google Scholar] [CrossRef]
- Kyrö, K.; Brenneisen, S.; Kotze, D.J.; Szallies, A.; Gerner, M.; Lehvävirta, S. Local Habitat Characteristics Have a Stronger Effect than the Surrounding Urban Landscape on Beetle Communities on Green Roofs. Urban For. Urban Green. 2018, 29, 122–130. [Google Scholar] [CrossRef]
- Oke, T.R.; Mills, G.; Christen, A.; Voogt, J.A. Urban Climates; Cambridge University Press: Cambridge, UK, 2017; ISBN 978-0-521-84950-0. [Google Scholar]
- Foissard, X.; Dubreuil, V.; Quenol, H. Defining Scales of the Land Use Effect to Map the Urban Heat Island in a Mid-Size European City: Rennes (France). Urban Clim. 2019, 29, 100490. [Google Scholar] [CrossRef]
- Piano, E.; Bona, F.; Isaia, M. Urbanization Drivers Differentially Affect Ground Arthropod Assemblages in the City of Turin (NW-Italy). Urban Ecosyst. 2020, 23, 617–629. [Google Scholar] [CrossRef]
- Cabon, V.; Quénol, H.; Dubreuil, V.; Ridel, A.; Bergerot, B. Urban Heat Island and Reduced Habitat Complexity Explain Spider Community Composition by Excluding Large and Heat-Sensitive Species. Land 2024, 13, 83. [Google Scholar] [CrossRef]
- Bohac, J. Staphylinid Beetles as Bioindicators. Agric. Ecosyst. Environ. 1999, 74, 357–372. [Google Scholar] [CrossRef]
- Rainio, J.; Niemelä, J. Ground Beetles (Coleoptera: Carabidae) as Bioindicators. Biodivers. Conserv. 2003, 12, 487–506. [Google Scholar] [CrossRef]
- Michaels, K.F. Using Staphylinid and Tenebrionid Beetles as Indicators of Sustainable Landscape Management in Australia: A Review. Aust. J. Exp. Agric. 2007, 47, 435–449. [Google Scholar] [CrossRef]
- Niemelä, J.; Kotze, D.J.; Venn, S.; Penev, L.; Stoyanov, I.; Spence, J.; Hartley, D.; de Oca, E.M. Carabid Beetle Assemblages (Coleoptera, Carabidae) across Urban-Rural Gradients: An International Comparison. Landsc. Ecol. 2002, 17, 387–401. [Google Scholar] [CrossRef]
- Deichsel, R. Species Change in an Urban Setting—Ground and Rove Beetles (Coleoptera: Carabidae and Staphylinidae) in Berlin. Urban Ecosyst. 2006, 9, 161–178. [Google Scholar] [CrossRef]
- Vergnes, A.; Le Viol, I.; Clergeau, P. Green Corridors in Urban Landscapes Affect the Arthropod Communities of Domestic Gardens. Biol. Conserv. 2012, 145, 171–178. [Google Scholar] [CrossRef]
- Magura, T.; Nagy, D.; Tóthmérész, B. Rove Beetles Respond Heterogeneously to Urbanization. J. Insect Conserv. 2013, 17, 715–724. [Google Scholar] [CrossRef]
- Piano, E.; De Wolf, K.; Bona, F.; Bonte, D.; Bowler, D.E.; Isaia, M.; Lens, L.; Merckx, T.; Mertens, D.; Van Kerckvoorde, M.; et al. Urbanization Drives Community Shifts towards Thermophilic and Dispersive Species at Local and Landscape Scales. Glob. Chang. Biol. 2017, 23, 2554–2564. [Google Scholar] [CrossRef] [PubMed]
- Lövei, G.L.; Magura, T. Body Size and the Urban Heat Island Effect Modulate the Temperature–Size Relationship in Ground Beetles. J. Biogeogr. 2022, 49, 1618–1628. [Google Scholar] [CrossRef]
- Irmler, U.; Lipkow, E. Effect of Environmental Conditions on Distribution Patterns of Rove Beetles. In Biology of Rove Beetles (Staphylinidae): Life History, Evolution, Ecology and Distribution; Betz, O., Irmler, U., Klimaszewski, J., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 117–144. ISBN 978-3-319-70257-5. [Google Scholar]
- Magura, T.; Lövei, G.L. Consequences of Urban Living: Urbanization and Ground Beetles. Curr. Landsc. Ecol. Rep. 2021, 6, 9–21. [Google Scholar] [CrossRef]
- Vergnes, A.; Pellissier, V.; Lemperiere, G.; Rollard, C.; Clergeau, P. Urban Densification Causes the Decline of Ground-Dwelling Arthropods. Biodivers. Conserv. 2014, 23, 1859–1877. [Google Scholar] [CrossRef]
- Croci, S.; Butet, A.; Georges, A.; Aguejdad, R.; Clergeau, P. Small Urban Woodlands as Biodiversity Conservation Hot-Spot: A Multi-Taxon Approach. Landsc. Ecol. 2008, 23, 1171–1186. [Google Scholar] [CrossRef]
- Faeth, S.H.; Bang, C.; Saari, S. Urban Biodiversity: Patterns and Mechanisms. Ann. N. Y. Acad. Sci. 2011, 1223, 69–81. [Google Scholar] [CrossRef]
- Beninde, J.; Veith, M.; Hochkirch, A. Biodiversity in Cities Needs Space: A Meta-Analysis of Factors Determining Intra-Urban Biodiversity Variation. Ecol. Lett. 2015, 18, 581–592. [Google Scholar] [CrossRef] [PubMed]
- Proske, A.; Lokatis, S.; Rolff, J. Impact of Mowing Frequency on Arthropod Abundance and Diversity in Urban Habitats: A Meta-Analysis. Urban For. Urban Green. 2022, 76, 127714. [Google Scholar] [CrossRef]
- Díaz, S.; Lavorel, S.; Chapin, F.S.; Tecco, P.A.; Gurvich, D.E.; Grigulis, K. Functional Diversity—At the Crossroads between Ecosystem Functioning and Environmental Filters. In Terrestrial Ecosystems in a Changing World; Canadell, J.G., Pataki, D.E., Pitelka, L.F., Eds.; Global Change—The IGBP Series; Springer: Berlin/Heidelberg, Germany, 2007; pp. 81–91. ISBN 978-3-540-32730-1. [Google Scholar]
- Dubreuil, V.; Foissard, X.; Nabucet, J.; Thomas, A.; Quénol, H. Fréquence et intensité des îlots de chaleur à rennes: Bilan de 16 années d’observations (2004–2019). Climatologie 2020, 17, 6. [Google Scholar] [CrossRef]
- Chollet, S.; Brabant, C.; Tessier, S.; Jung, V. From Urban Lawns to Urban Meadows: Reduction of Mowing Frequency Increases Plant Taxonomic, Functional and Phylogenetic Diversity. Landsc. Urban Plan. 2018, 180, 121–124. [Google Scholar] [CrossRef]
- Ward, D.F.; New, T.R.; Yen, A.L. Effects of Pitfall Trap Spacing on the Abundance, Richness and Composition of Invertebrate Catches. J. Insect Conserv. 2001, 5, 47–53. [Google Scholar] [CrossRef]
- Lott, D.A. The Staphylinidae (Rove Beetles) of Britain and Ireland. Part 5: Scaphidiinae, Piestinae, Oxytelinae; Royal Entomological Society: London, UK, 2009; Volume 12, ISBN 978-0-901546-90-6. [Google Scholar]
- Lott, D.A.; Anderson, R. The Staphylinidae (Rove Beetles) of Britain and Ireland. Parts 7 & 8: Oxyporinae, Steninae, Euaesthetinae, Pseudopsinae, Paederinae, Staphylininae; Royal Entomological Society: London, UK, 2011; Volume 12, ISBN 978-0-901546-92-0. [Google Scholar]
- Jeannel, R. Faune de France—Colèoptères Carabiques; Office Central de Faunistique; Office Central de Faunistique; Paul LECHEVALIER—PARIS 1949: Paris, France, 1949. [Google Scholar]
- Clough, Y.; Kruess, A.; Tscharntke, T. Organic versus Conventional Arable Farming Systems: Functional Grouping Helps Understand Staphylinid Response. Agric. Ecosyst. Environ. 2007, 118, 285–290. [Google Scholar] [CrossRef]
- Hill, M.O. Diversity and Evenness: A Unifying Notation and Its Consequences. Ecology 1973, 54, 427–432. [Google Scholar] [CrossRef]
- Gower, J.C. A General Coefficient of Similarity and Some of Its Properties. Biometrics 1971, 27, 857–871. [Google Scholar] [CrossRef]
- Maechler, M.; Rousseeuw, P.; Struyf, A.; Hubert, M.; Hornik, K.; Roudier, P.; Gonzalez, J.; Kozlowski, K.; Schubert, E.; Murphy, K. Cluster: “Finding Groups in Data”: Cluster Analysis Extended Rousseeuw et al., R Package Version 2.1.6; 2023. Available online: https://cran.r-project.org/web/packages/cluster/cluster.pdf (accessed on 12 January 2024).
- Chao, A.; Henderson, P.A.; Chiu, C.-H.; Moyes, F.; Hu, K.-H.; Dornelas, M.; Magurran, A.E. Measuring Temporal Change in Alpha Diversity: A Framework Integrating Taxonomic, Phylogenetic and Functional Diversity and the iNEXT.3D Standardization. Methods Ecol. Evol. 2021, 12, 1926–1940. [Google Scholar] [CrossRef]
- Delgado de la Flor, Y.A.; Burkman, C.E.; Eldredge, T.K.; Gardiner, M.M. Patch and Landscape-Scale Variables Influence the Taxonomic and Functional Composition of Beetles in Urban Greenspaces. Ecosphere 2017, 8, e02007. [Google Scholar] [CrossRef]
- Chatelain, M.; Rüdisser, J.; Traugott, M. Urban-Driven Decrease in Arthropod Richness and Diversity Associated with Group-Specific Changes in Arthropod Abundance. Front. Ecol. Evol. 2023, 11, 980387. [Google Scholar] [CrossRef]
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R Package for Multivariate Analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Maechler, M.; Rousseeuw, P.; Struyf, A.; Hubert, M.; Hornik, K.; Studer, M.; Roudier, P.; Gonzalez, J. Package ‘Cluster’, R Package Version 2.1.6; 2023. Available online: https://cran.r-project.org/web/packages/cluster/index.html (accessed on 12 January 2024).
- Oksanen, J.; Simpson, G.L.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Solymos, P.; Stevens, M.H.H.; Szoecs, E.; et al. Vegan: Community Ecology Package. R Package Version 2.6-6.1. 2022. Available online: https://cran.r-project.org/web/packages/vegan/index.html (accessed on 12 January 2020).
- Peres-Neto, P.R.; Legendre, P.; Dray, S.; Borcard, D. Variation Partitioning of Species Data Matrices: Estimation and Comparison of Fractions. Ecology 2006, 87, 2614–2625. [Google Scholar] [CrossRef] [PubMed]
- Legendre, P.; Oksanen, J.; ter Braak, C.J.F. Testing the Significance of Canonical Axes in Redundancy Analysis. Methods Ecol. Evol. 2011, 2, 269–277. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2022. [Google Scholar]
- Alaruikka, D.; Kotze, D.J.; Matveinen, K.; Niemelä, J. Carabid Beetle and Spider Assemblages along a Forested Urban–Rural Gradient in Southern Finland. J. Insect Conserv. 2002, 6, 195–206. [Google Scholar] [CrossRef]
- Magura, T.; Horváth, R.; Tóthmérész, B. Effects of Urbanization on Ground-Dwelling Spiders in Forest Patches, in Hungary. Landsc. Ecol. 2010, 25, 621–629. [Google Scholar] [CrossRef]
- Buchholz, S.; Hannig, K.; Moller, M.; Schirmel, J. Reducing Management Intensity and Isolation as Promising Tools to Enhance Ground-Dwelling Arthropod Diversity in Urban Grasslands. Urban Ecosyst. 2018, 21, 1139–1149. [Google Scholar] [CrossRef]
- Varet, M.; Burel, F.; Lafage, D.; Pétillon, J. Age-Dependent Colonization of Urban Habitats: A Diachronic Approach Using Carabid Beetles and Spiders. Anim. Biol. 2013, 63, 257–269. [Google Scholar] [CrossRef]
- Harvey, J.A.; van der Putten, W.H.; Turin, H.; Wagenaar, R.; Bezemer, T.M. Effects of Changes in Plant Species Richness and Community Traits on Carabid Assemblages and Feeding Guilds. Agric. Ecosyst. Environ. 2008, 127, 100–106. [Google Scholar] [CrossRef]
- Tylianakis, J.M.; Didham, R.K.; Bascompte, J.; Wardle, D.A. Global Change and Species Interactions in Terrestrial Ecosystems. Ecol. Lett. 2008, 11, 1351–1363. [Google Scholar] [CrossRef]
- Diamond, S.E.; Frame, A.M.; Martin, R.A.; Buckley, L.B. Species’ Traits Predict Phenological Responses to Climate Change in Butterflies. Ecology 2011, 92, 1005–1012. [Google Scholar] [CrossRef] [PubMed]
- Sunday, J.M.; Bates, A.E.; Kearney, M.R.; Colwell, R.K.; Dulvy, N.K.; Longino, J.T.; Huey, R.B. Thermal-Safety Margins and the Necessity of Thermoregulatory Behavior across Latitude and Elevation. Proc. Natl. Acad. Sci. USA 2014, 111, 5610–5615. [Google Scholar] [CrossRef] [PubMed]
- Youngsteadt, E.; Ernst, A.F.; Dunn, R.R.; Frank, S.D. Responses of Arthropod Populations to Warming Depend on Latitude: Evidence from Urban Heat Islands. Glob. Chang. Biol. 2017, 23, 1436–1447. [Google Scholar] [CrossRef] [PubMed]
- Merckx, T.; Souffreau, C.; Kaiser, A.; Baardsen, L.F.; Backeljau, T.; Bonte, D.; Brans, K.I.; Cours, M.; Dahirel, M.; Debortoli, N.; et al. Body-Size Shifts in Aquatic and Terrestrial Urban Communities. Nature 2018, 558, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Cabon, V.; Quénol, H.; Deletre, B.; Copin, L.; Dubreuil, V.; Bergerot, B. Body Size Responses to Urban Temperature Variations Are Driven by Life History Traits in Spiders. Funct. Ecol. 2024, 38, 1578–1589. [Google Scholar] [CrossRef]
- Larochelle, A. The Food of Carabid Beetles: (Coleoptera: Carabidae, Including Cicindelinae); Association des Entomologistes Amateurs du Québec: Varennes, QC, Canada, 1990. [Google Scholar]
- Schirmel, J.; Blindow, I.; Buchholz, S. Life-history trait and functional diversity patterns of ground beetles and spiders along a coastal heathland successional gradient. Basic Appl. Ecol. 2012, 13, 606–614. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabon, V.; Laurent, Y.; Georges, R.; Quénol, H.; Dubreuil, V.; Bergerot, B. Microhabitat Structure Affects Ground-Dwelling Beetle Communities More than Temperature along an Urbanization Gradient. Diversity 2024, 16, 504. https://doi.org/10.3390/d16080504
Cabon V, Laurent Y, Georges R, Quénol H, Dubreuil V, Bergerot B. Microhabitat Structure Affects Ground-Dwelling Beetle Communities More than Temperature along an Urbanization Gradient. Diversity. 2024; 16(8):504. https://doi.org/10.3390/d16080504
Chicago/Turabian StyleCabon, Valentin, Yann Laurent, Romain Georges, Hervé Quénol, Vincent Dubreuil, and Benjamin Bergerot. 2024. "Microhabitat Structure Affects Ground-Dwelling Beetle Communities More than Temperature along an Urbanization Gradient" Diversity 16, no. 8: 504. https://doi.org/10.3390/d16080504
APA StyleCabon, V., Laurent, Y., Georges, R., Quénol, H., Dubreuil, V., & Bergerot, B. (2024). Microhabitat Structure Affects Ground-Dwelling Beetle Communities More than Temperature along an Urbanization Gradient. Diversity, 16(8), 504. https://doi.org/10.3390/d16080504





