Pollen and Seed Morphology as Taxonomic Markers in Verbascum Taxa Based on Herbarium Specimens of MARIUM
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Herbaria
2.2. Palynological Investigations
2.3. Pollen Morphology: Principal Component and Correlation Analyses
2.4. Seed Micromorphology Analysis
3. Results
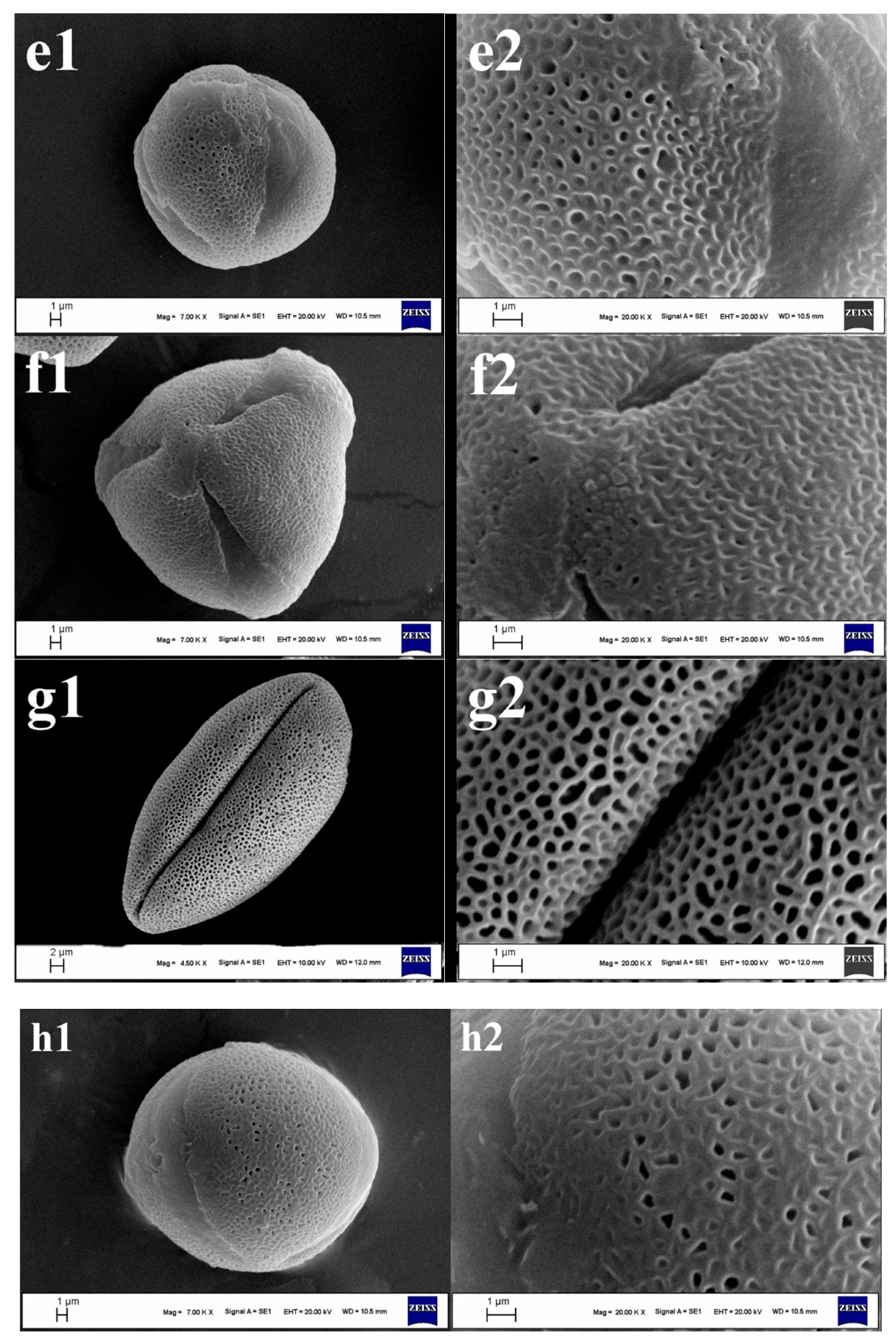
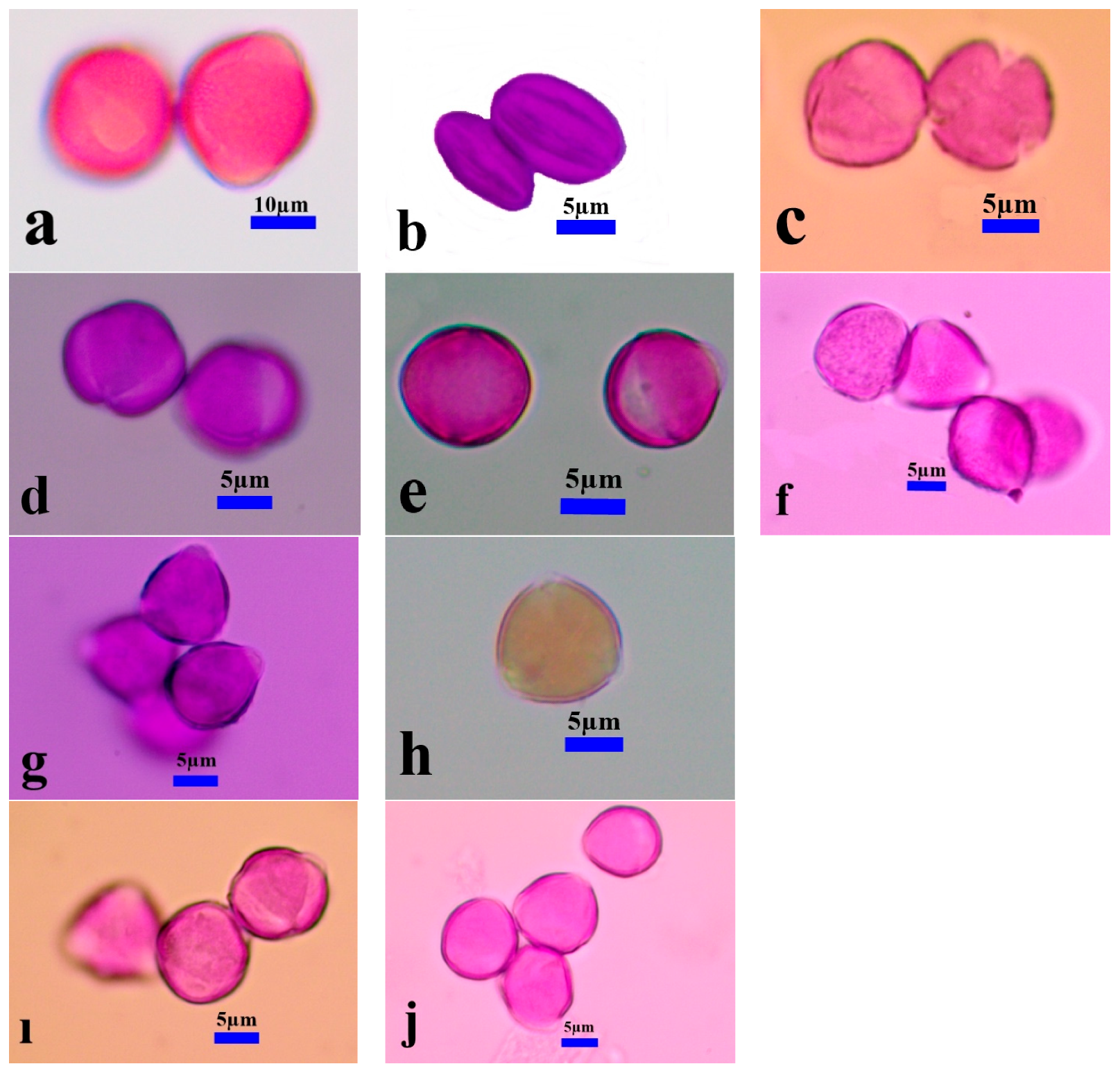
3.1. Pollen Morphology
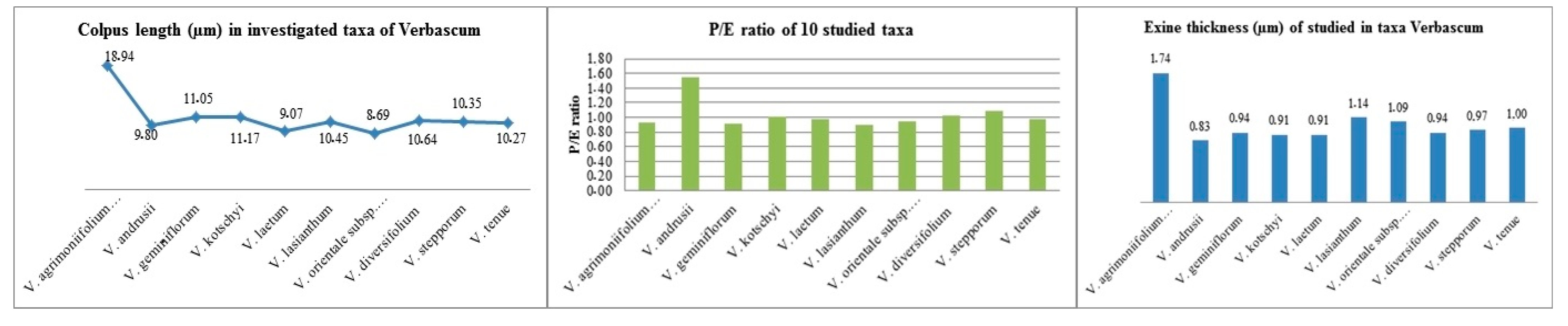
3.1.1. Size, Symmetry, and Shape
3.1.2. Apertures
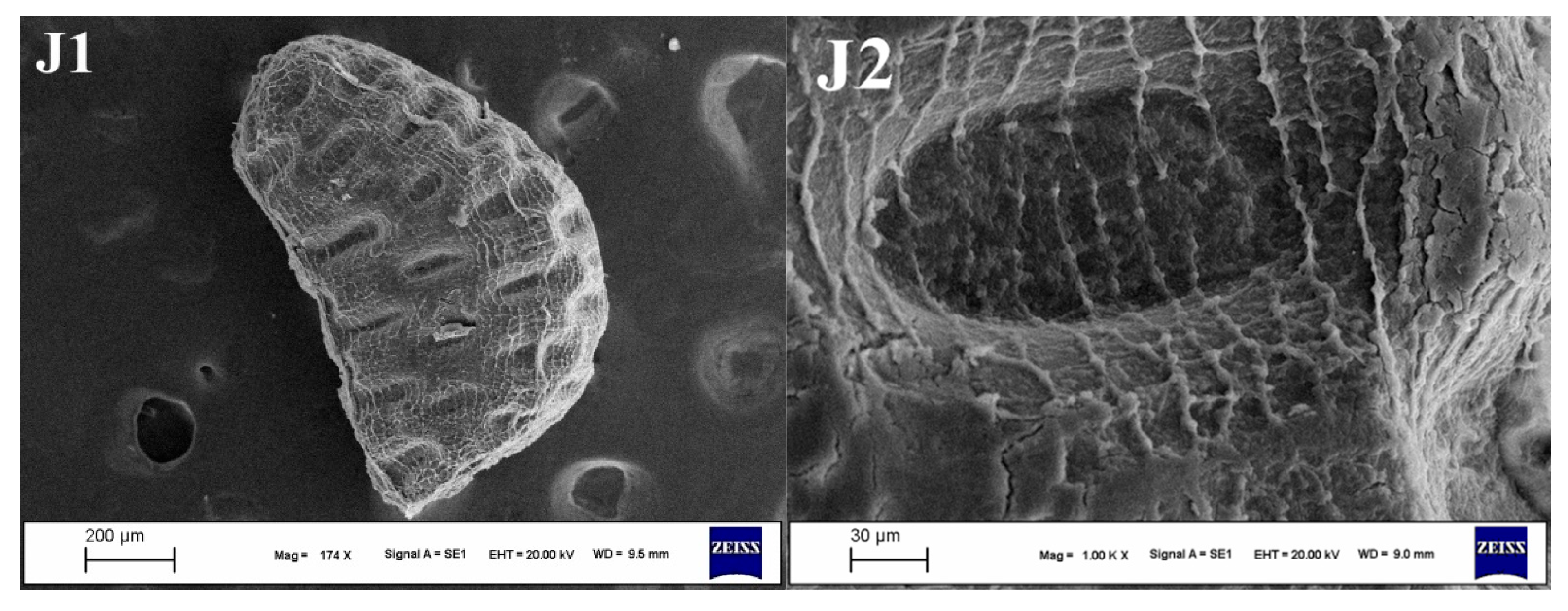
3.1.3. Exine, Intine, and Ornamentation
3.1.4. Pollen Morphology: Principal Component Analysis
3.1.5. Pollen Morphology: Correlation Analysis
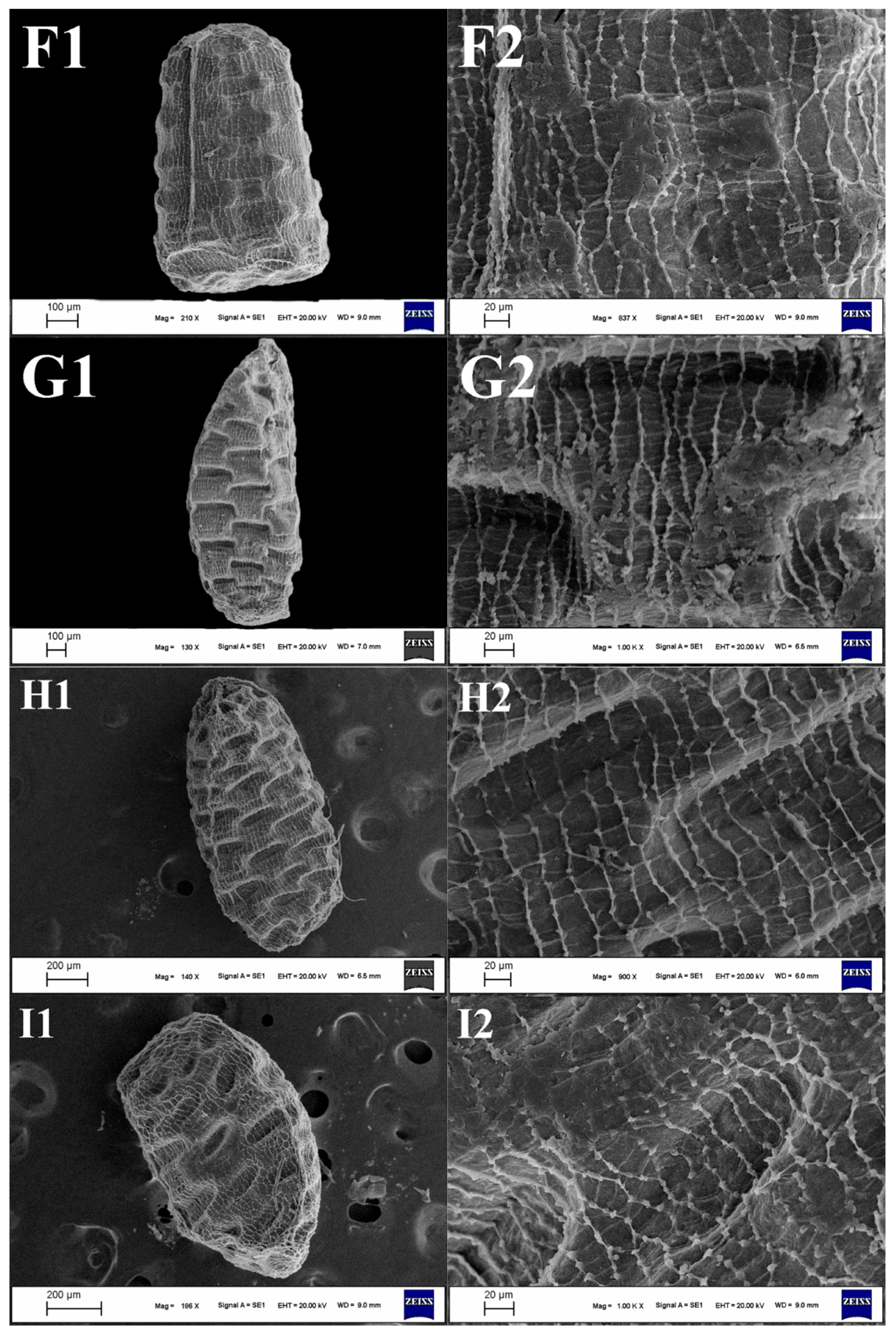
3.2. Seed Morphology
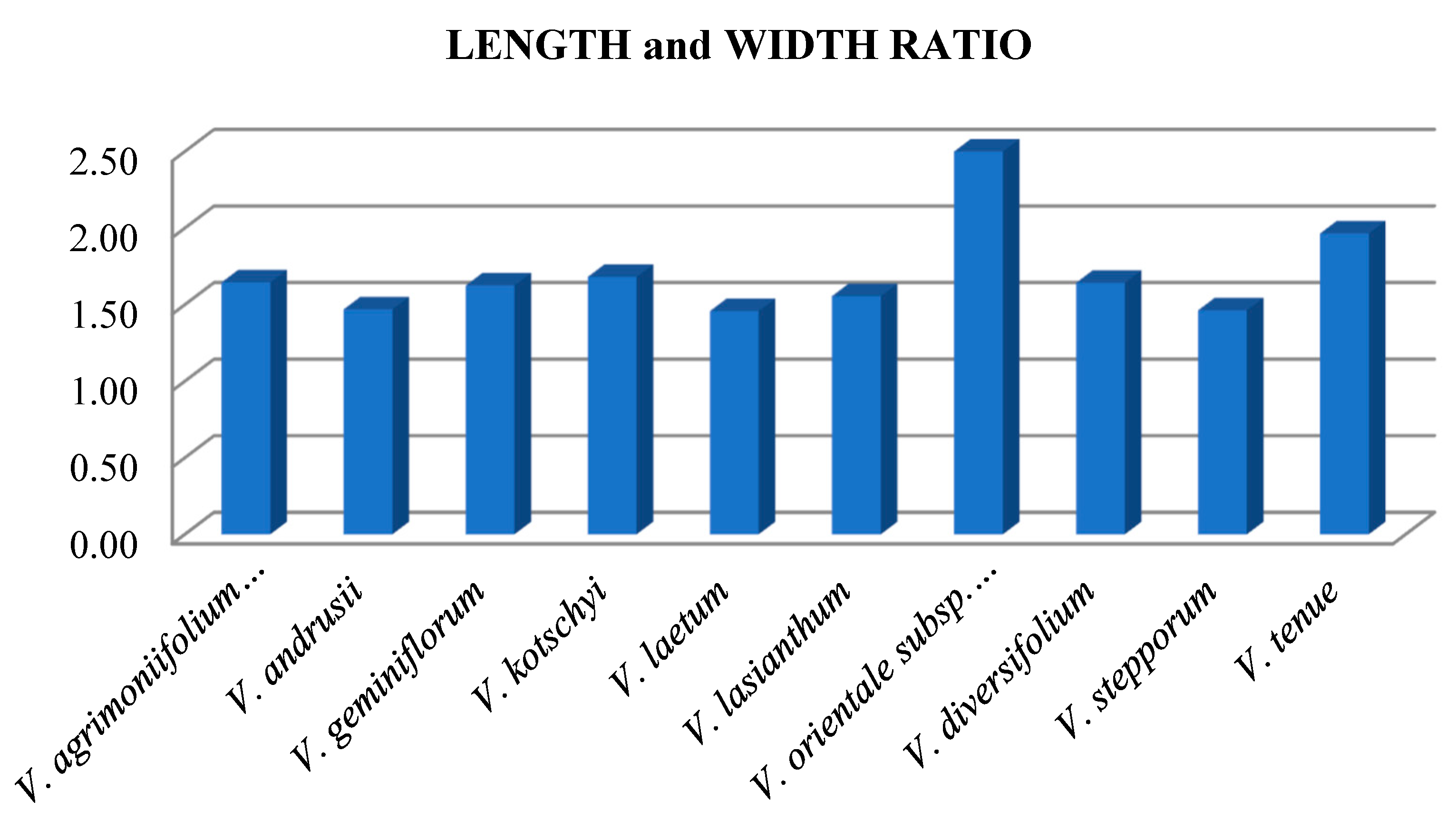
3.2.1. Seed Size
3.2.2. Seed Shape
3.2.3. Apex of the Seeds
3.2.4. Seed Color
3.2.5. Seed Ornamentation
4. Discussion
4.1. Essential Role of Herbarium in Taxonomic Studies
4.2. Pollen Morphology: A Useful Tool with Limitations
4.3. Exine Sculpturing: A Promising Taxonomic Marker
4.4. Taxonomic Key Based on Qualitative and Quantitative Characters of Pollen
4.5. Quantitative Pollen Traits and Species Relationships
4.6. Correlations between Pollen Traits
4.7. Seed Morphology and Taxonomic Significance in Verbascum
4.8. Taxonomic Key Based on Qualitative and Quantitative Morphological Features of Seed
5. Conclusions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meineke, E.K.; Davis, C.C.; Davies, T.J. The unrealized potential of herbaria for global change biology. Ecol. Monogr. 2018, 88, 505–525. [Google Scholar] [CrossRef]
- Meineke, E.K.; Davies, T.J.; Daru, B.H.; Davis, C.C. Biological collections for understanding biodiversity in the Anthropocene. Phil. Trans. R. Soc. B 2019, 374, 20170386. [Google Scholar] [CrossRef] [PubMed]
- Heberling, J.M.; Prather, L.A.; Tonsor, S.J. The changing uses of herbarium data in an era of global change: An overview using automated content analysis. BioScience 2019, 69, 812–822. [Google Scholar] [CrossRef]
- Bieker, V.C.; Martin, M.D. Implications and future prospects for evolutionary analyses of DNA in historical herbarium collections. Bot. Lett. 2018, 165, 409–418. [Google Scholar] [CrossRef]
- Funk, V.A. Collections-based science in the 21st century. J. Syst. Evol. 2018, 56, 175–193. [Google Scholar] [CrossRef]
- Carine, M.A.; Cesar, E.A.; Ellis, L.; Hunnex, J.; Paul, A.M.; Prakash, R.; Rumsey, F.J.; Wajer, J.; Wilbraham, J.; Yesilyurt, J.C. Examining the spectra of herbarium uses and users. Bot. Lett. 2018, 165, 328–336. [Google Scholar] [CrossRef]
- Bebber, D.P.; Carine, M.A.; Wood, J.R.; Wortley, A.H.; Harris, D.J.; Prance, G.T.; Davidse, G.; Paige, J.; Pennington, T.D.; Robson, N.K.; et al. Herbaria are a major frontier for species discovery. Proc. Natl. Acad. Sci. USA 2010, 107, 22169–22171. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, Z.A.; Muñoz-Rodríguez, P.; Harris, D.J.; Wells, T.; Wood, J.R.; Filer, D.; Scotland, R.W. How long does it take to discover a species? Syst. Biodivers. 2020, 18, 784–793. [Google Scholar] [CrossRef]
- Chapman, A.D. Uses of Primary Species-Occurrence Data; Global Biodiversity Information Facility: Copenhagen, Denmark, 2005. [Google Scholar]
- Culley, T.M. Why vouchers matter in botanical research. Appl. Plant Sci. 2013, 1, 1300076. [Google Scholar] [CrossRef]
- Heberling, J.M.; Isaac, B.L. Herbarium specimens as exaptations: New uses for old collections. Am. J. Bot. 2017, 104, 963–965. [Google Scholar] [CrossRef]
- Soltis, P.S. Digitization of herbaria enables novel research. Am. J. Bot. 2017, 104, 1281–1284. [Google Scholar] [CrossRef] [PubMed]
- Willis, C.G.; Ellwood, E.R.; Primack, R.B.; Davis, C.C.; Pearson, K.D.; Gallinat, A.S.; Yost, J.M.; Nelson, G.; Mazer, S.J.; Rossington, N.L.; et al. Old plants, new tricks: Phenological research using herbarium specimens. Trends Ecol. Evol. 2017, 32, 531–546. [Google Scholar] [CrossRef]
- Heywood, V.H. Flowering Plants of the World; Oxford University Press: Oxford, UK, 1993. [Google Scholar]
- Judd, W.S.; Campbell, S.C.; Kellogg, E.A.; Stevens, P.F.; Donoghue, J.M. Plant Systematics: A Phylogenetic Approach, 2nd ed.; Sinauer Associates, Inc.: Sunderland, MA, USA, 2002; p. 576. [Google Scholar]
- Huber-Morath, A. Verbascum L. In Flora of Turkey and the East Aegean Islands; Davis, P.H., Ed.; Edinburgh University Press: Edinburgh, Scotland, 1978; Volume 6, pp. 461–603. [Google Scholar]
- Davis, P.H.; Mill, R.R.; Tan, K. Flora of Turkey and the East Aegean Islands; (Suppl. 1); Edinburgh University Press: Edinburgh, Scotland, 1988; Volume 10, pp. 191–193. [Google Scholar]
- Karaveliogullari, F.A.; Yüce, E.; Başer, B. Verbascum duzgunbabadagensis (Scrophulariaceae), a new species from eastern Anatolia, Turkey. Phytotaxa 2014, 181, 47–53. [Google Scholar] [CrossRef]
- Ekim, T. Verbascum L. In Flora of Turkey and the East Aegean Islands; (Suppl. 2); Güner, A., Ed.; Edinburgh University Press: Edinburgh, Scotland, 2000; Volume 11, p. 193. [Google Scholar]
- Karavelioğulları, F.A. A new record Verbascum szovitsianum Boiss. var. szovitsianum (Scrophulariaceae) from Turkey. Biol. Divers. Conserv. 2009, 2, 68–70. [Google Scholar]
- Karavelioğulları, F.A. Verbascum L. In Türkiye Bitkileri Listesi (Damarlı Bitkiler), Nezahat Gökyiğit Botanik Bahçesi ve Flora Araştırmaları Derneği Yayını; Güner, A., Ed.; Edinburgh University Press: Edinburgh, Scotland, 2012; pp. 850–870. [Google Scholar]
- Firat, M. Verbascum zerdust (Scrophulariaceae), a new species from Bitlis province (Turkey) belonging to section Bothrosperma. Nord. J. Bot. 2022, 2022, e03649. [Google Scholar] [CrossRef]
- Öztürk, A.; Güney, K.B.; Bani, B.; Güney, K.; Karaveliogullari, F.A.; Pinar, N.M.; Çeter, T. Pollen morphology of some Verbascum (Scrophulariaceae) taxa in Turkey. Phytotaxa 2018, 333, 209–218. [Google Scholar] [CrossRef]
- Minkin, J.P.; Hardy-Eshbaugh, W. Pollen morphology of the Orobanchaceae and Rhinanthoid Scrophulariaceae. Grana 1989, 28, 1–18. [Google Scholar] [CrossRef]
- Juan, R.; Fernandez, I.; Pastor, J. Systematic consideration of microcharacters of fruits and seeds in the genus Verbascum (Scrophulariaceae). Ann. Bot. 1997, 80, 591–598. [Google Scholar] [CrossRef]
- Dane, F.; Yılmaz, G. Palynological study on some Verbascum L. species. In Proceedings of the Third International Conference “Falz Fein Reading” Training University, Kherson, Ukraine, 25–27 April 2002; p. 151. [Google Scholar]
- Kheyri, S. Identification of breeding system of some species of Verbascum (Scrophulariaceae) in north-west of Iran on the basis of the ratio of pollen to ovule number. Biol. J. Islam. Azad Univ. Garmsar Branch 2009, 4, 67–74. [Google Scholar]
- Asmat, T.; Khan, M.A.; Ahmed, M.; Zafar, M.; Manzoor, F.; Munir, M.; Akhtar, K.; Bashir, S.; Mukhtar, T.; Ambreen, M.; et al. Pollen morphology of selected species of Scrophulariaceae of District Dir Upper. Pak. J. Med. Plant Res. 2011, 5, 6423–6428. [Google Scholar] [CrossRef]
- Al-Hadeethy, M.; Al-Mashhadani, A.; Al-Khesraji, T.; Barusrux, S.; Al-Jewari, H.; Theerakulpisut, P.; Pornpongrungrueng, P. Pollen morphology of Verbascum L. (Scrophulariaceae) in Northern and Central Iraq. Bang. J. Plant Tax. 2014, 21, 159–165. [Google Scholar] [CrossRef]
- Aktas, K. Morphology, Anatomy, Palynology and Seed Micromorphology of Turkish Endemic Verbascum X splendidum Boiss. (Scrophulariaceae). Fres. Environ. Bull. 2019, 28, 10004–10010. [Google Scholar]
- Aktas, K.; Özdemir, C.; Özdemir, B. Morphology, Anatomy, Palynology and Seed Micromorphology of Turkish Endemic Verbascum exuberans Hub.-Mor. (Scrophulariaceae). Planta Daninha 2020, 38, e020191125. [Google Scholar] [CrossRef]
- Baser, B. Pollen and Seed Morphology of Verbascum Species (Group D) (Scrophulariaceae) in Turkey. Fres. Environ. Bull. 2021, 30, 8978–8987. [Google Scholar]
- Petkovié, B.; Delié, G.; Tatié, B. Variation in Verbascum phoeniceum (Scrophulariaceae) in Serbia as affected by geological substratum. Bocconea 1997, 5, 647–654. [Google Scholar]
- Attar, F.; Keshvari, A.; Ghahreman, A.; Zarre, S.; Aghabeigi, F. Micromorphological studies on Verbascum (Scrophulariaceae) in Iran with emphasis on seed surface, capsule ornamentation and trichomes. Flora 2007, 202, 169–175. [Google Scholar] [CrossRef]
- Kheiri, S.; Khayami, M.; Mahmoudzadeh, A. Micromorphological and anatomical studies of certain species of Verbascum (Scrophulariaceae) in West Azerbaijan, Iran. Iran. J. Bot. 2009, 15, 105–113. [Google Scholar]
- Karaveliogullari, F.A. Verbascum ergin-hamzaoglui (Scrophulariaceae), a new species from South Anatolia, Turkey. Turk. J. Bot. 2011, 35, 275–283. [Google Scholar]
- Cabi, E.; Baser, B.; Yavru, A.; Polat, F.; Toprak, U.; Karaveliogulları, F.A. Scanning electron microscope (SEM) and Light microscope (LM) studies on the seed morphology of Verbascum taxa (Scrophulariaceae) and their systematic implications. Aust. J. Crop Sci. 2011, 5, 660–667. [Google Scholar]
- Aytaç, Z.; Duman, H. Verbascum hasbenlii (Scrophulariaceae), a new species from Turkey. Turk. J. Bot. 2012, 36, 322–327. [Google Scholar] [CrossRef]
- Duman, H.; Uzunhisarcikli, M.E.; Tan, K. Verbascum mughlaeum (Scrophulariaceae), a new species from SW Anatolia, Turkey. Phytotaxa 2017, 291, 231–236. [Google Scholar] [CrossRef]
- Erdtman, G. Pollen Morphology and Plant Taxonomy: Angiosperms; Chronica Botanica Co.: Waltham, MA, USA, 1952; pp. 1–553. [Google Scholar]
- Wodehouse, R.P. Pollen Grains, Their Structure, Identification and Significance in Science and Medicine; Hafner Publishing Company: New York, NY, USA, 1935. [Google Scholar]
- Punt, W.; Hoen, P.P.; Blackmore, S.; Nilsson, S.; Le-Thomas, A. Glossary of pollen and spore terminology. Rev. Palaeobot. Palynol. 2007, 143, 1–81. [Google Scholar] [CrossRef]
- Sutton, D.A. A Revision of the Tribe Antirrhineae (Scrophulariaceae); Oxford University Press: Oxford, UK, 1988. [Google Scholar]
- Sukhorukov, A.P.; Kushunina, M. Taxonomic Revision and Distribution of Herbaceous Paramollugo (Molluginaceae) in the Eastern Hemisphere. PhytoKeys 2018, 73, 93–116. [Google Scholar] [CrossRef] [PubMed]
- Herpin, U.; Markert, B.; Weckert, V.; Berlekamp, J.; Friese, K.; Siewers, U.; Lieth, H. Retrospective Analysis of Heavy Metal Concentrations at Selected Locations in the Federal Republic of Germany Using Moss Material from a Herbarium. Sci. Total Environ. 1997, 205, 1–12. [Google Scholar] [CrossRef]
- Körner, C.; Leuzinger, S.; Riedl, S.; Siegwolf, R.T.; Streule, L. Carbon and Nitrogen Stable Isotope Signals for an Entire Alpine Flora, Based on Herbarium Samples. Alp. Bot. 2016, 126, 153–166. [Google Scholar] [CrossRef]
- Nielsen, T.F.; Larsen, J.R.; Michelsen, A.; Bruun, H.H. Are Herbarium Mosses Reliable Indicators of Historical Nitrogen Deposition? Environ. Poll. 2017, 231, 1201–1207. [Google Scholar] [CrossRef] [PubMed]
- Dentant, C.; Lavergne, S.; Malécot, V. Taxonomic Revision of West-Alpine Cushion Plant Species Belonging to Androsace Subsect. Aretia. Bot. Lett. 2018, 165, 337–351. [Google Scholar] [CrossRef]
- Henning, T.; Plitzner, P.; Güntsch, A.; Berendsohn, W.G.; Müller, A.; Kilian, N. Building Compatible and Dynamic Character Matrices–Current and Future Use of Specimen-Based Character Data. Bot. Lett. 2018, 165, 352–360. [Google Scholar] [CrossRef]
- Espinosa, F.; Pinedo Castro, M. On the Use of Herbarium Specimens for Morphological and Anatomical Research. Bot. Lett. 2018, 165, 361–367. [Google Scholar] [CrossRef]
- Chen, Y.; Jabbour, F.; Novikov, A.; Wang, W.; Gerber, S. A Study of Floral Shape Variation in Delphinieae (Ranunculaceae) Using Geometric Morphometrics on Herbarium Specimens. Bot. Lett. 2018, 165, 368–376. [Google Scholar] [CrossRef]
- Çakir, T.; Bagci, E. A taxonomical study on the Verbascum euphraticum Bentham and Verbascum melitenense Boiss (Scrophulariaceae). Sci. Eng. J. Firat. Univ. 2006, 18, 445–458. [Google Scholar]
- Kheiri, S.; Khayami, M.; Osaloo, S.K.; Mahmoodzadeh, A. Pollen morphology of some species of Verbascum L. (Scrophulari-aceae) in Urmia. Pak. J. Bio. Sci. 2006, 9, 434–436. [Google Scholar] [CrossRef]
- Pehlivan, S.; Baser, B.; Karaveliogullari, F.A. Pollen morphology of the genus Verbascum L. (Group A) in Turkey. Biol. Divers. Conserv. 2008, 1, 1–24. [Google Scholar]
- Semerdjieva, I.; Yankova-Tsvetkova, E.; Zheljazkov, V.D.; Koleva-Valkova, L.H.; Nikolova, R. Reproductive Capacity and Scanning Electron Microscopy (SEM) Analyses of the Micromorphological Surfaces of Three Endemic Satureja Species from Bulgaria. Plants 2023, 12, 24–36. [Google Scholar] [CrossRef] [PubMed]
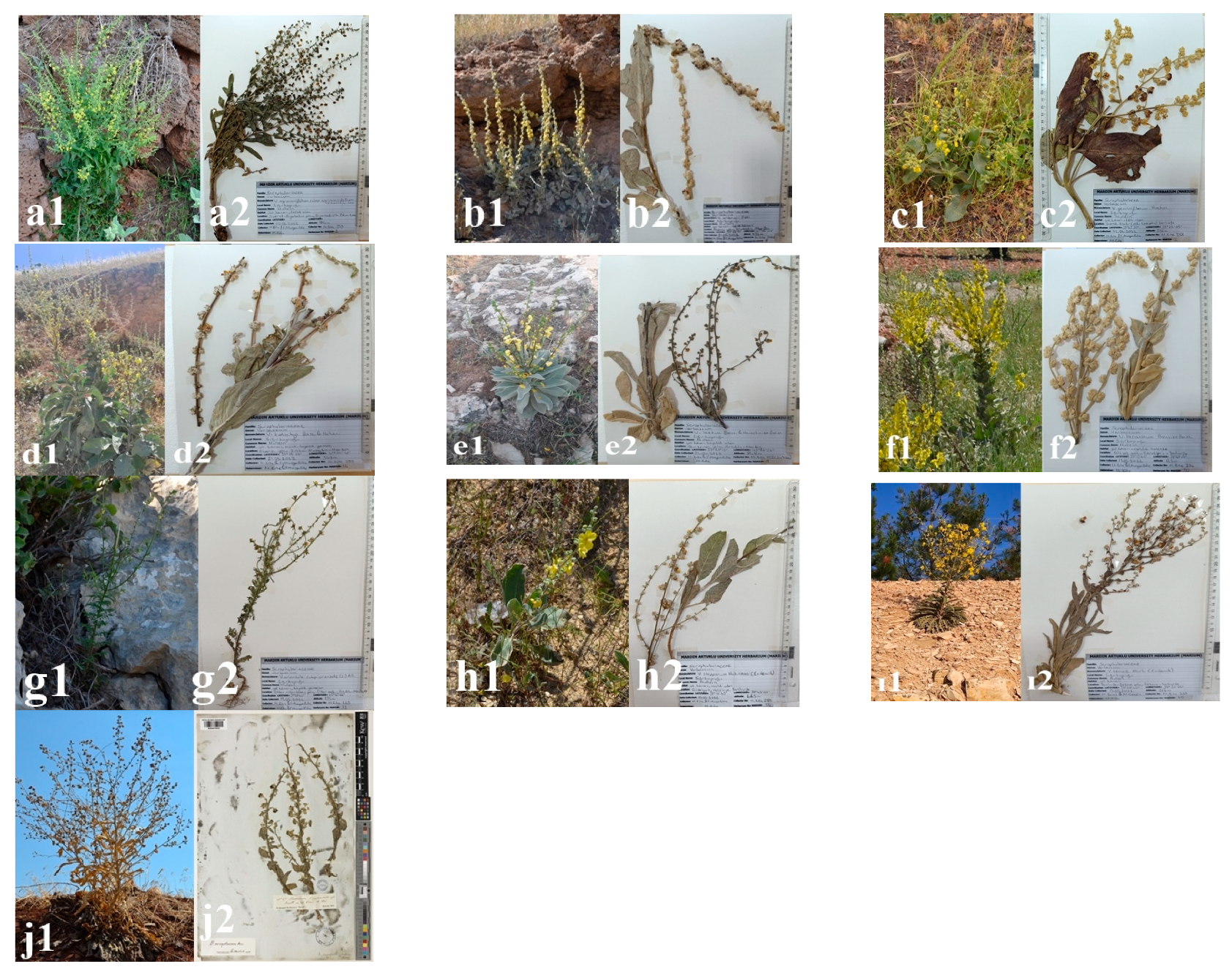






| Species | Group | Collection Areas and Habitat | Date | Altitude (m) | Coordinates | Collector | Herbarium No (MARIUM) |
|---|---|---|---|---|---|---|---|
| V. agrimoniifolium subsp. agrimoniifolium | A | C8 Diyarbakır: Siverek-Diyarbakır road, 35 km from Diyarbakır, roadside, rocky slope | 2 June 2022 | 1014 | Not available | F Mungan Kılıç M.Kılıç | 1 |
| C8 Mardin: Artuklu, roadside | 5 June 2022 | 971 | 37°22′22″ N 40°42′11″ E | F Mungan Kılıç M.Kılıç | 2 | ||
| C7 Şanlıurfa: Karaköprü, roadside | 19 May 2022 | 554 | 37°11′22″ N 38°48′49″ E | F Mungan Kılıç M.Kılıç | 3 | ||
| V. andrusii | K | Mardin: Artuklu, roadside | 9 May 2022 | 695 | 37°17′46″ N 40°42′47″ E | F Mungan Kılıç M.Kılıç | 4 |
| Mardin: Nusaybin, roadside | 21 May 2022 | 536 | 37°08′11″ N 41°04′44″ E | F Mungan Kılıç M.Kılıç | 5 | ||
| Mardin: Ömerli, roadside | 24 May 2022 | 774 | 37°23′58″ N 38°52′13″ E | F Mungan Kılıç M.Kılıç | 6 | ||
| Mardin: Midyat, roadside | 24 May 2022 | 1001 | 37°28′15″ N 41°07′18″ E | F Mungan Kılıç M.Kılıç | 7 | ||
| Mardin: Dargeçit, roadside | 24 May 2022 | 933 | 37°33′08″ N 41°40′16″ E | F Mungan Kılıç M.Kılıç | 6 | ||
| Mardin: Kızıltepe, roadside | 28 May 2022 | 655 | 37°16′31″ N 40°32′41″ E | F Mungan Kılıç M.Kılıç | 7 | ||
| Mardin: Yeşilli, roadside | 29 May 2022 | 733 | 37°18′31″ N 40°49’54″ E | F Mungan Kılıç M.Kılıç | 8 | ||
| V. geminiflorum | I | Mardin: Artuklu, roadside | 16 May 2022 | 591 | 37°16’22″ N 40°40’49″ E | F Mungan Kılıç M.Kılıç | 9 |
| Şanlıurfa: Siverek, roadside | 2 June 2022 | 867 | 37°43’30″ N 39°25’05″ E | F Mungan Kılıç M.Kılıç | 10 | ||
| V. kotschyi | K | Mardin: Artuklu, roadside | 10 May 2022 | 828 | 37°19’38″ N 40°47’48″ E | F Mungan Kılıç M.Kılıç | 11 |
| Mardin: Nusaybin, roadside | 21 May 2022 | 546 | 37°08’06″ N 41°04’41″ E | F Mungan Kılıç M.Kılıç | 12 | ||
| Mardin: Yeşilli, roadside | 24 May 2022 | 1158 | 37°22’17″ N 40°51’38″ E | F Mungan Kılıç M.Kılıç | 13 | ||
| Mardin: Savur, roadside | 31 May 2022 | 1113 | 37°31’10″ N 40°55’56″ E | F Mungan Kılıç M.Kılıç | 14 | ||
| Şanlıurfa: Karaköprü, roadside | 2 August 2022 | 621 m | 37°12′34″ N 38°47′28″ E | F Mungan Kılıç M.Kılıç | 15 | ||
| V. laetum | C | Mardin: Artuklu, roadside | 24 April 2022 | 848 | 37°20′12″ N 40°46′26″ E | F Mungan Kılıç M.Kılıç | 16 |
| Mardin: Mazıdağı, roadside | 15 May 2022 | 939 | 37°30′14″ N 40°31′19″ E | F Mungan Kılıç M.Kılıç | 17 | ||
| Mardin: Midyat, roadside | 24 May 2022 | 928 | 37°26′15″ N 41°18′07″ E | F Mungan Kılıç M.Kılıç | 18 | ||
| Mardin: Savur, roadside | 31 May 2022 | 933 | 37°31′31″ N 40°53′37″ E | F Mungan Kılıç M.Kılıç | 19 | ||
| Şanlıurfa: Viranşehir-Urfa road, 25 km after Viranşehir, roadside, stony slopes | 19 May 2022 | - | Not available | F Mungan Kılıç M.Kılıç | 20 | ||
| V. lasianthum | L | Diyarbakır: Çınar, roadside | 2 June 2022 | 725 | 37°40′47″ N 40°27′28″ E | F Mungan Kılıç M.Kılıç | 21 |
| Diyarbakır: Yenişehir, 28 km from Ergani, roadside, cultivated fields | 2 June 2022 | 766 | Not available | F Mungan Kılıç M.Kılıç | 22 | ||
| Diyarbakır: Ergani, roadside | 2 June 2022 | 809 | 38°11′53″ N 39°49′57″ E | F Mungan Kılıç M.Kılıç | 23 | ||
| Mardin: Yeşilli, roadside | 29 May 2022 | 676 | 37°16′53″ N 40°50′46″ E | F Mungan Kılıç M.Kılıç | 24 | ||
| Mardin: Savur, roadside | 31 May 2022 | 903 | 37°29′05″ N 40°49′40″ E | F Mungan Kılıç M.Kılıç | 25 | ||
| Mardin: Artuklu, roadside | 3 August 2022 | 1132 | 37°22′55″ N 40°39′07″ E | F Mungan Kılıç M.Kılıç | 26 | ||
| Mardin: Ömerli, 6 km after Ömerli, the roadside | 4 August 2022 | - | Not available | F Mungan Kılıç M.Kılıç | 27 | ||
| Şanlıurfa: Karaköprü, roadside | 19 May 2022 | 665 | 37°13′46″ N 38°49′06″ E | F Mungan Kılıç M.Kılıç | 28 | ||
| Şanlıurfa: Hilvan, Near Hilvan location, roadside | 2 August 2022 | 592 | Not available | F Mungan Kılıç M.Kılıç | 29 | ||
| V. orientale subsp. orientale | A | Mardin: Artuklu, roadside | 13 May 2022 | 835 | 37°20′44″ N 40°43′44″ E | F Mungan Kılıç M.Kılıç | 30 |
| V. diversifolium (E) | G | Türkiye, C7 Şanlıurfa: Siverek, Hilvan-Siverek road, 29 km from Siverek, the roadside cultivated area | 2 August 2022 | 604 | 37°36′32″ N 39°05′19″ E | F Mungan Kılıç M.Kılıç | 31 |
| V. stepporum (E) | K | Şanlıurfa: Viranşehir-Urfa road, roadside right–left stony area, around the highway (400-26/042) sign | 19 May 2022 | 660 | Not available | F Mungan Kılıç M.Kılıç | 32 |
| Şanlıurfa: Haliliye, North of Güzelyurt village, roadside | 19 May 2022 | 665 | 37°12′49″ K 38°49′05″ D | F Mungan Kılıç M.Kılıç | 33 | ||
| Şanlıurfa: Karaköprü, Borsa İstanbul Secondary School south, roadside | 19 May 2022 | 606 | 37°12′26″ K 38°47′30″ D | F Mungan Kılıç M.Kılıç | 34 | ||
| Şanlıurfa: Karaköprü, Gölpınar Nature Park south, roadside | 19 May 2022 | 704 | 37°14′05″ K 38°49′30″ D | F Mungan Kılıç M.Kılıç | 35 | ||
| Şanlıurfa: Bozova, Yassıca recreation area | 19 May 2022 | 553 | 37°27′25″ K 38°23′26″ D | M Balos C Çeçen | 36 | ||
| Şanlıurfa: Hilvan, 24 km from Hilvan, the roadside cultivated area | 2 August 2022 | 774 | 37°23′58″ K 38°52′13″ D | F Mungan Kılıç M.Kılıç | 37 | ||
| V. tenue (E) | F | Şanlıurfa: Şanlıurfa-Adıyaman highway, Mardin, Habur junction, roadside, calcareous slope | 19 May 2022 | 717 | 37°11′55″ K 38°48′01″ D | F Mungan Kılıç M.Kılıç | 38 |
| Şanlıurfa: Bozova, Şanlıurfa-Bozova road, before reaching Bozova, opposite Opet, by the roadside | 19 May 2022 | 616 | 37°20′40″ K 38°32′02″ D | M Balos C Çeçen | 39 |
| Species | P min (Mean) Max | E min (Mean) Max | P/E Ratio | Shape | Clg min (Mean) Max | Clt Min (Mean) Max | Plg Min (Mean) Max | Plt Min (Mean) Max | Ex Min (Mean) Max | In Min (Mean) Max | Apt | Or |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| V. agrimoniifolium subsp. agrimoniifolium | 21.71 (23.15) 25.93 | 22.11 (24.85) 27.16 | 0.93 | Oblate spheroidal | 17.15 (18.94) 22.58 | 4.72 (6.65) 8.92 | 6.33 (7.75) 9.95 | 5.12 (6.89) 9.02 | 1.32 (1.74) 2.30 | 0.73 (0.93) 1.40 | 90% Tricolporate 10% Tricolpate | Reticulate |
| V. andrusii | 11.08 (12.88) 14.36 | 6.68 (8.34) 11.78 | 1.54 | Subprolate | 8.09 (9.80) 11.47 | 2.00 (3.12) 4.44 | 3.15 (3.72) 4.55 | 2.19 (3.05) 4.89 | 0.56 (0.83) 1.03 | 0.30 (0.48) 0.70 | 53% Tricolporate 47% Tricolpate | Reticulate |
| V. geminiflorum | 12.25 (13.31) 14.66 | 13.53 (14.52) 15.21 | 0.91 | Oblate spheroidal | 9.30 (11.05) 12.66 | 2.51 (3.19) 3.75 | 2.53 (3.47) 4.22 | 3.09 (3.88) 4.62 | 0.64 (0.94) 1.11 | 0.35 (0.53) 0.75 | 68% Tricolporate 32% Tricolpate | Reticulate |
| V. kotschyi | 12.22 (13.81) 17.09 | 10.39 (13.73) 15.83 | 1.01 | Prolate spheroidal | 9.19 (11.17) 14.84 | 1.91 (3.03) 4.36 | 3.59 (4.42) 5.82 | 2.64 (3.57) 4.35 | 0.69 (0.91) 1.15 | 0.35 (0.52) 0.64 | 63% Tricolporate 37% Tricolpate | Microreticulate |
| V. laetum | 9.75 (11.08) 12.91 | 9.67 (11.33) 13.58 | 0.97 | Oblate spheroidal | 7.80 (9.07) 10.89 | 1.87 (2.86) 3.99 | 2.44 (3.86) 5.09 | 2.25 (3.31) 4.88 | 0.71 (0.91) 1.13 | 0.32 (0.48) 0.60 | 73% Tricolporate 27% Tricolpate | Reticulate |
| V. lasianthum | 10.27 (13.16) 15.41 | 10.85 (14.66) 17.45 | 0.90 | Oblate spheroidal | 7.74 (10.45) 12.78 | 2.68 (3.84) 4.97 | 3.44 (4.81) 5.88 | 2.78 (4.06) 5.13 | 0.71 (1.14) 1.54 | 0.45 (0.64) 0.88 | 80% Tricolporate 20% Tricolpate | Microreticulate |
| V. orientale subsp. orientale | 9.34 (11.03) 13.42 | 8.53 (11.68) 14.89 | 0.94 | Oblate spheroidal | 6.92 (8.69) 11.17 | 2.20 (2.93) 3.89 | 2.83 (3.94) 4.73 | 2.76 (3.33) 4.07 | 0.75 (1.09) 1.40 | 0.32 (0.52) 0.66 | 70% Tricolporate 30% Tricolpate | Reticulate |
| V. diversifolium | 11.79 (13.27) 15.87 | 11.83 (12.99) 15.17 | 1.02 | Prolate spheroidal | 8.74 (10.64) 13.44 | 2.02 (2.70) 3.80 | 1.88 (2.77) 3.71 | 2.14 (3.10) 3.88 | 0.71 (0.94) 1.24 | 0.32 (0.46) 0.64 | 86% Tricolporate 14% Tricolpate | Reticulate |
| V. stepporum | 11.60 (13.55) 17.23 | 10.71 (12.60) 14.40 | 1.08 | Prolate spheroidal | 8.02 (10.35) 14.77 | 2.06 (3.02) 4.03 | 3.23 (4.43) 6.53 | 2.98 (3.88) 4.86 | 0.64 (0.97) 1.25 | 0.27 (0.48) 0.70 | 83% Tricolporate 17% Tricolpate | Reticulate |
| V. tenue | 11.23 (13.09) 15.57 | 12.20 (13.42) 15.27 | 0.97 | Oblate spheroidal | 9.12 (10.27) 12.15 | 2.01 (2.99) 4.20 | 3.39 (4.46) 6.35 | 2.26 (3.67) 4.59 | 0.56 (1.00) 1.50 | 0.32 (0.57) 0.78 | 88% Tricolporate 12% Tricolpate | Reticulate |
| P | E | P/E | Clg | Clt | Plg | Plt | Ex | |
|---|---|---|---|---|---|---|---|---|
| E | 0.9174 ** | |||||||
| P/E | −0.1405 | −0.5184 | ||||||
| Clg | 0.9948 ** | 0.94 | −0.2051 | |||||
| Clt | 0.9455 | 0.911 ** | −0.2037 | 0.9458 ** | ||||
| Plg | 0.8756 | 0.857 | −0.2384 | 0.8638 ** | 0.9348 | |||
| Plt | 0.9482 ** | 0.9586 ** | −0.3376 | 0.9528 ** | 0.9732 ** | 0.9419 ** | ||
| Ex | 0.8822 ** | 0.9268 ** | −0.3909 | 0.8857 ** | 0.9528 ** | 0.9115 ** | 0.9508 ** | |
| In | 0.9039 ** | 0.9289 ** | −0.3323 | 0.9092 ** | 0.9755 ** | 0.9418 ** | 0.9618 ** | 0.9639 ** |
| Species | Group | Length Min (Mean) Max | Width Min (Mean) Max | Color | Shape | Seed Surface |
|---|---|---|---|---|---|---|
| V. agrimoniifolium subsp. agrimoniifolium | A | 0.41 (0.51) 0.62 | 0.25 (0.31) 0.37 | Black | Prismatic and oblong with shallow alveolate, truncated and acute beaks. | Irregular rectangular cells, with densely and distinct vesicles. |
| V. andrusii | K | 0.66 (0.94) 1.26 | 0.48 (0.64) 0.81 | Brown | Prismatic ovate and oblong with ±shallow alveolate, deep and broad ridges, truncated beaks. | Irregular polygonal cells, with densely and distinct vesicles. |
| V. geminiflorum | I | 0.75 (1.04) 1.63 | 0.37 (0.64) 0.86 | Brown | Prismatic ovate and oblong with ±shallow alveolate, obtuse beaks, striped from apex to hilum. | Irregular polygonal cells, with densely and distinct vesicles. |
| V. kotschyi | K | 0.80 (1.16) 1.60 | 0.51 (0.69) 0.88 | Light brown | Prismatic ovate and oblong with shallow alveolate, ±deep and broad ridges, truncated beaks, approximately striped from apex to hilum. | Irregular polygonal cells, with densely and distinct vesicles. |
| V. laetum | C | 0.53 (0.86) 1.10 | 0.39 (0.59) 0.83 | Dark brown | Prismatic, prismatic ovate and oblong with ±shallow alveolate, truncated beaks, striped between apex and hilum. | Irregular and exserted rectangular cells with vesicles only on the angles of the walls cells. |
| V. lasianthum | L | 0.49 (0.70) 1.01 | 0.29 (0.45) 0.59 | Brown | Prismatic and oblong with ±shallow alveolate, deep ridges, truncated beaks, striped from apex to hilum. | Irregular polygonal cells, with densely and distinct vesicles. |
| V. orientale subsp. orientale | A | 1.01 (1.40) 1.70 | 0.32 (0.56) 0.80 | Brown | Prismatic, elliptic to oblong and like a boot with ±shallow alveolate, deep ridges, truncated beaks. | Irregular small rectangular cells, with densely and distinct vesicles. |
| V. diversifolium (E) | G | 0.58 (0.92) 1.20 | 0.33 (0.56) 0.72 | Light brown-brown | Prismatic oblong with ±shallow alveolate, often with truncated beaks, and some with broad beaks | Irregular polygonal cells, with densely and distinct vesicles |
| V. stepporum (E) | K | 0.63 (0.79) 1.00 | 0.30 (0.54) 0.70 | Brown | Prismatic oblong with ±shallow alveolate, truncated beak | Irregular polygonal cells, with densely and distinct vesicles |
| V. tenue (E) | F | 0.75 (1.12) 1.34 | 0.38 (0.57) 0.84 | Dark brown | Prismatic oblong and reniform with shallow alveolate, truncated beak | Irregular polygonal cells, with densely and indistinct vesicles |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mungan Kılıç, F. Pollen and Seed Morphology as Taxonomic Markers in Verbascum Taxa Based on Herbarium Specimens of MARIUM. Diversity 2024, 16, 443. https://doi.org/10.3390/d16080443
Mungan Kılıç F. Pollen and Seed Morphology as Taxonomic Markers in Verbascum Taxa Based on Herbarium Specimens of MARIUM. Diversity. 2024; 16(8):443. https://doi.org/10.3390/d16080443
Chicago/Turabian StyleMungan Kılıç, Fatma. 2024. "Pollen and Seed Morphology as Taxonomic Markers in Verbascum Taxa Based on Herbarium Specimens of MARIUM" Diversity 16, no. 8: 443. https://doi.org/10.3390/d16080443
APA StyleMungan Kılıç, F. (2024). Pollen and Seed Morphology as Taxonomic Markers in Verbascum Taxa Based on Herbarium Specimens of MARIUM. Diversity, 16(8), 443. https://doi.org/10.3390/d16080443






