Selecting Monitoring Methods for Endangered Trout Populations
Abstract
1. Introduction
- (1)
- Do trout counts differ among methods?
- (2)
- How are sampling methods affected by environmental variables?
2. Methods
2.1. Study System
2.2. Sampling Design
2.3. Environmental Variables
2.4. Statistical Analysis
2.4.1. Do Trout Counts Differ among Methods?
2.4.2. How Are Methods Affected by Environmental Variables?
3. Results
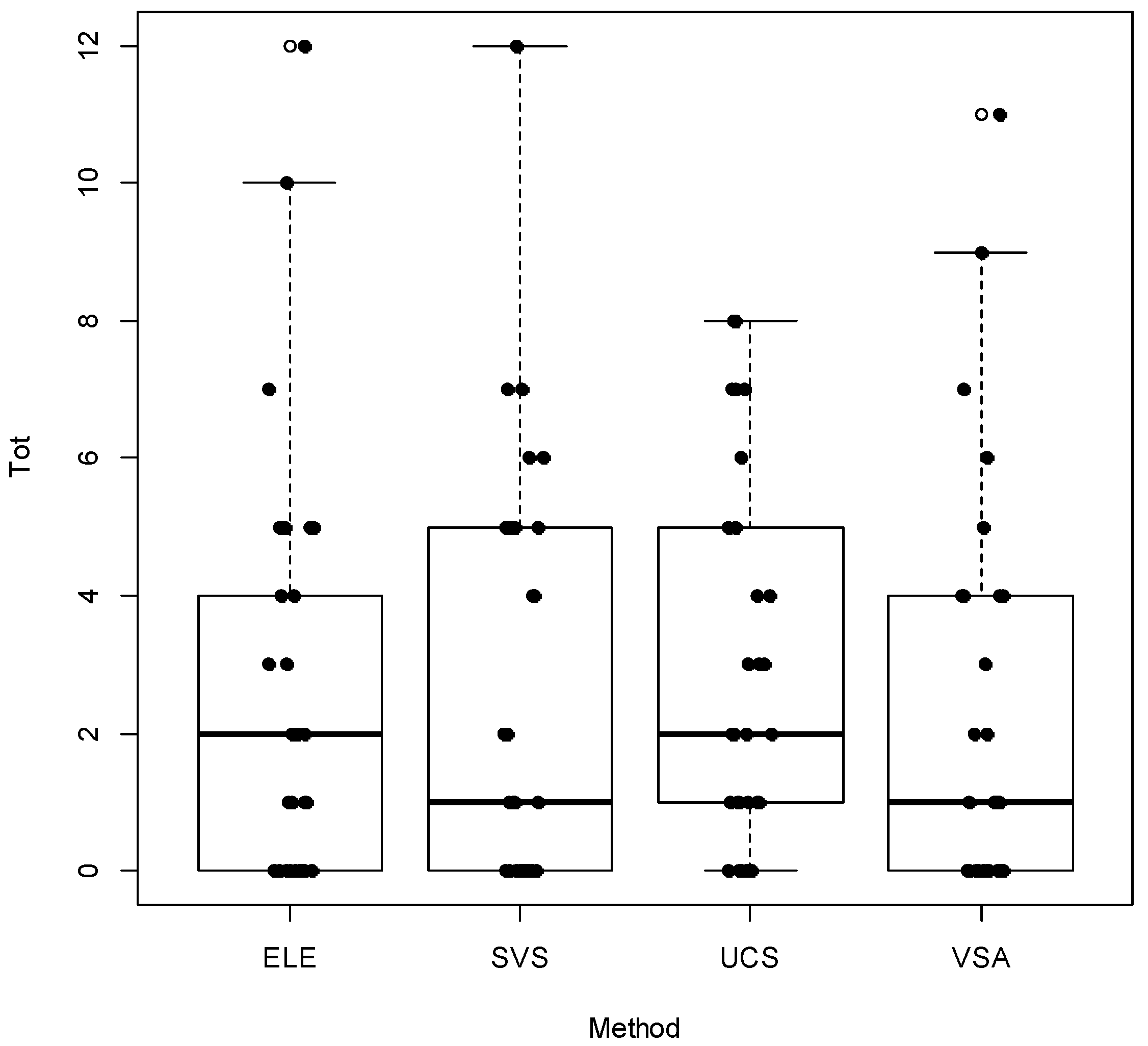
3.1. Do Trout Counts Differ among Methods?
3.2. How Are Methods Affected by Environmental Variables?
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Nichols, J.D.; Williams, B.K. Monitoring for Conservation. Trends Ecol. Evol. 2006, 21, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, E. A General Framework for Analyzing Sustainability of Social-Ecological Systems. Science 2009, 325, 419–422. [Google Scholar] [CrossRef] [PubMed]
- Anadón, J.D.; Giménez, A.; Ballestar, R.; Pérez, I. Evaluation of Local Ecological Knowledge as a Method for Collecting Extensive Data on Animal Abundance. Conserv. Biol. 2009, 23, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Casula, P.; Vignoli, L.; Luiselli, L.; Lecis, R. Local Abundance and Observer’s Identity Affect Visual Detectability of Sardinian Mountain Newts. Herpetol. J. 2017, 27, 258–265. [Google Scholar]
- McKinley, D.C.; Miller-Rushing, A.J.; Ballard, H.L.; Bonney, R.; Brown, H.; Cook-Patton, S.C.; Evans, D.M.; French, R.A.; Parrish, J.K.; Phillips, T.B.; et al. Citizen Science Can Improve Conservation Science, Natural Resource Management, and Environmental Protection. Biol. Conserv. 2017, 208, 15–28. [Google Scholar] [CrossRef]
- Conrad, C.C.; Hilchey, K.G. A Review of Citizen Science and Community-Based Environmental Monitoring: Issues and Opportunities. Env. Monit. Assess. 2010, 176, 273–291. [Google Scholar] [CrossRef] [PubMed]
- Brownscombe, J.W.; Hyder, K.; Potts, W.; Wilson, K.L.; Pope, K.L.; Danylchuk, A.J.; Cooke, S.J.; Clarke, A.; Arlinghaus, R.; Post, J.R. The Future of Recreational Fisheries: Advances in Science, Monitoring, Management, and Practice. Fish. Res. 2019, 211, 247–255. [Google Scholar] [CrossRef]
- Ellender, B.R.; Becker, A.; Weyl, O.L.F.; Swartz, E.R. Underwater Video Analysis as a Non-Destructive Alternative to Electrofishing for Sampling Imperilled Headwater Stream Fishes. Aquat. Conserv. Mar. Freshw. Ecosyst. 2012, 22, 58–65. [Google Scholar] [CrossRef]
- Castañeda, R.A.; Van Nynatten, A.; Crookes, S.; Ellender, B.R.; Heath, D.D.; MacIsaac, H.J.; Mandrak, N.E.; Weyl, O.L.F. Detecting Native Freshwater Fishes Using Novel Non-Invasive Methods. Front. Environ. Sci. 2020, 8, 29. [Google Scholar] [CrossRef]
- Palmas, F.; Casula, P.; Curreli, F.; Podda, C.; Cabiddu, S.; Sabatini, A. Exploring Less Invasive Visual Surveys to Assess the Spatial Distribution of Endangered Mediterranean Trout Population in a Small Intermittent Stream. Biology 2023, 12, 1000. [Google Scholar] [CrossRef]
- Nielsen, J.L. Scientific Sampling Effects: Electrofishing California’s Endangered Fish Populations. Fisheries 1998, 23, 6–12. [Google Scholar] [CrossRef]
- Weyl, O.L.F.; Pattrick, P.; Ellender, B.R.; Miya, T.; Woodford, D.J.; Bennett, R.H.; Wasserman, R.J.; Mäkinen, T. Ethical Considerations for Field Research on Fishes. Koedoe Afr. Prot. Area Conserv. Sci. 2016, 58, 1–15. [Google Scholar] [CrossRef]
- Sutherland, J.W. Ecological Census Techniques: A Handbook; Cambridge University Press: Cambridge, UK, 1996; ISBN 978-0-521-47815-1. [Google Scholar]
- Meyer, K.A.; High, B. Accuracy of Removal Electrofishing Estimates of Trout Abundance in Rocky Mountain Streams. N. Am. J. Fish. Manag. 2011, 31, 923–933. [Google Scholar] [CrossRef]
- Snyder, D.E. Invited Overview: Conclusions from a Review of Electrofishing and Its Harmful Effects on Fish. Rev. Fish. Biol. Fish. 2003, 13, 445–453. [Google Scholar] [CrossRef]
- Bozek, M.A.; Rahel, F.J. Comparison of Streamside Visual Counts to Electrofishing Estimates of Colorado River Cutthroat Trout Fry and Adults. N. Am. J. Fish. Manag. 1991, 11, 38–42. [Google Scholar] [CrossRef]
- Brownscombe, J.W.; Danylchuk, A.J.; Chapman, J.M.; Gutowsky, L.F.G.; Cooke, S.J. Best Practices for Catch-and-Release Recreational Fisheries—Angling Tools and Tactics. Fish. Res. 2017, 186, 693–705. [Google Scholar] [CrossRef]
- Hickey, M.A.; Closs, G.P. Evaluating the Potential of Night Spotlighting as a Method for Assessing Species Composition and Brown Trout Abundance: A Comparison with Electrofishing in Small Streams. J. Fish. Biol. 2006, 69, 1513–1523. [Google Scholar] [CrossRef]
- Thurow, R.F.; Dolloff, C.A.; Marsden, J.E. Chapter 17. Visual Observation of Fishes and Aquatic Habitat. In Fisheries Techniques; American Fisheries Society: Bethesda, MD, USA, 2012; pp. 781–817. [Google Scholar]
- Splendiani, A.; Palmas, F.; Sabatini, A.; Caputo Barucchi, V. The Name of the Trout: Considerations on the Taxonomic Status of the Salmo trutta L., 1758 Complex (Osteichthyes: Salmonidae) in Italy. Eur. Zool. J. 2019, 86, 432–442. [Google Scholar] [CrossRef]
- Bianco, P.G.; Caputo Barucchi, V.; Lorenzoni, M.; Nonnis Marzano, F.; Stefani, F.; Sabatini, A.; Tancioni, L. Salmo Cettii. In Lista Rossa Vertebrati Italiani; Rondinini, C., Battistoni, A., Peronace, V., Teofili, C., Eds.; (IUCN) International Union for Conservation of Nature: Gland, Switzerland, 2013. [Google Scholar]
- Clavero, M.; Hermoso, V.; Levin, N.; Kark, S. Geographical Linkages between Threats and Imperilment in Freshwater Fish in the Mediterranean Basin. Divers. Distrib. 2010, 16, 744–754. [Google Scholar] [CrossRef]
- Berrebi, P.; Caputo Barucchi, V.; Splendiani, A.; Muracciole, S.; Sabatini, A.; Palmas, F.; Tougard, C.; Arculeo, M.; Marić, S. Brown Trout (Salmo trutta L.) High Genetic Diversity around the Tyrrhenian Sea as Revealed by Nuclear and Mitochondrial Markers. Hydrobiologia 2019, 826, 209–231. [Google Scholar] [CrossRef]
- Splendiani, A.; Righi, T.; Fioravanti, T.; Sabatini, A.; Palmas, F.; Tougard, C.; Berrebi, P.; Talarico, L.; Caputo Barucchi, V. Population Genetics, Demography and Conservation of Mediterranean Brown Trout from Sardinia. Aquat. Conserv. Mar. Freshw. Ecosyst. 2024, 34, e4099. [Google Scholar] [CrossRef]
- Araguas, R.M.; Sanz, N.; Fernández, R.; Utter, F.M.; Pla, C.; García-Marín, J.-L. Role of Genetic Refuges in the Restoration of Native Gene Pools of Brown Trout. Conserv. Biol. 2009, 23, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Larios-López, J.E.; de Figueroa, J.M.T.; Alonso-González, C.; Sanz, B.N. Distribution of Brown Trout (Salmo trutta Linnaeus, 1758) (Teleostei: Salmonidae) in Its Southwesternmost European Limit: Possible Causes. Ital. J. Zool. 2015, 82, 404–415. [Google Scholar] [CrossRef][Green Version]
- Skoulikidis, N.T.; Sabater, S.; Datry, T.; Morais, M.M.; Buffagni, A.; Dörflinger, G.; Zogaris, S.; del Mar Sánchez-Montoya, M.; Bonada, N.; Kalogianni, E.; et al. Non-Perennial Mediterranean Rivers in Europe: Status, Pressures, and Challenges for Research and Management. Sci. Total Environ. 2017, 577, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Palmas, F.; Righi, T.; Musu, A.; Frongia, C.; Podda, C.; Serra, M.; Splendiani, A.; Caputo Barucchi, V.; Sabatini, A. Pug-Headedness Anomaly in a Wild and Isolated Population of Native Mediterranean Trout Salmo trutta L., 1758 Complex (Osteichthyes: Salmonidae). Diversity 2020, 12, 353. [Google Scholar] [CrossRef]
- Sabatini, A.; Podda, C.; Frau, G.; Cani, M.V.; Musu, A.; Serra, M.; Palmas, F. Restoration of Native Mediterranean Brown Trout Salmo cettii Rafinesque, 1810 (Actinopterygii: Salmonidae) Populations Using an Electric Barrier as a Mitigation Tool. Eur. Zool. J. 2018, 85, 137–149. [Google Scholar] [CrossRef]
- Castañeda, R.A.; Weyl, O.L.F.; Mandrak, N.E. Using Occupancy Models to Assess the Effectiveness of Underwater Cameras to Detect Rare Stream Fishes. Aquat. Conserv. Mar. Freshw. Ecosyst. 2020, 30, 565–576. [Google Scholar] [CrossRef]
- Ebner, B.C.; Starrs, D.; Morgan, D.L.; Fulton, C.J.; Donaldson, J.A.; Doody, J.S.; Cousins, S.; Kennard, M.; Butler, G.; Tonkin, Z.; et al. Emergence of Field-Based Underwater Video for Understanding the Ecology of Freshwater Fishes and Crustaceans in Australia. J. R. Soc. West. Aust. 2014, 97, 287–296. [Google Scholar]
- Elliott, J.M. Pools as Refugia for Brown Trout during Two Summer Droughts: Trout Responses to Thermal and Oxygen Stress. J. Fish. Biol. 2000, 56, 938–948. [Google Scholar] [CrossRef]
- Neter, J.; Kutner, M.H.; Nachtsheim, C.J.; Wasserman, W. Applied Linear Statistical Models, 4th ed.; McGraw-Hill: New York, NY, USA, 1996. [Google Scholar]
- Hilborn, R.; Mangel, M. The Ecological Detective: Confronting Models with Data; Princeton University Press: Princeton, NJ, USA, 1997; ISBN 0-691-03497-4. [Google Scholar]
- Delignette-Muller, M.L.; Dutang, C. Fitdistrplus: An R Package for Fitting Distributions. J. Stat. Softw. 2015, 64, 1–34. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2018. [Google Scholar]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S-PLUS; Springer Science & Business Media: New York, NY, USA, 2013; ISBN 978-1-4757-3121-7. [Google Scholar]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach; Springer: Berlin, Germany, 2002. [Google Scholar]
- Zeileis, A.; Hothorn, T. Diagnostic Checking in Regression Relationships. R News 2002, 2, 7–10. [Google Scholar]
- Hitt, N.P.; Rogers, K.M.; Snyder, C.D.; Dolloff, C.A. Comparison of Underwater Video with Electrofishing and Dive Counts for Stream Fish Abundance Estimation. Trans. Am. Fish. Soc. 2021, 150, 24–37. [Google Scholar] [CrossRef]
- Richter, I.A.; Giacomini, H.C.; De Kerckhove, D.T.; Jackson, D.A.; Jones, N.E. Correcting for Size Bias in Electrofishing Removal Samples. Ecol. Model. 2022, 467, 109929. [Google Scholar] [CrossRef]
- Calcagno, V.; de Mazancourt, C. Glmulti: An R Package for Easy Automated Model Selection with (Generalized) Linear Models. J. Stat. Softw. 2010, 34, 1–29. [Google Scholar] [CrossRef]
- Mac Nally, R. Regression and Model-Building in Conservation Biology, Biogeography and Ecology: The Distinction between—And Reconciliation of—“predictive” and “Explanatory” Models. Biodivers. Conserv. 2000, 9, 655–671. [Google Scholar] [CrossRef]
- Dormann, C.F.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carré, G.; Marquéz, J.R.G.; Gruber, B.; Lafourcade, B.; Leitão, P.J.; et al. Collinearity: A Review of Methods to Deal with It and a Simulation Study Evaluating Their Performance. Ecography 2013, 36, 27–46. [Google Scholar] [CrossRef]
- Ayllón, D.; Almodóvar, A.; Nicola, G.G.; Elvira, B. Ontogenetic and Spatial Variations in Brown Trout Habitat Selection. Ecol. Freshw. Fish. 2010, 19, 420–432. [Google Scholar] [CrossRef]
- Wilson, K.L.; Allen, M.S.; Ahrens, R.N.M.; Netherland, M.D. Use of Underwater Video to Assess Freshwater Fish Populations in Dense Submersed Aquatic Vegetation. Mar. Freshw. Res. 2014, 66, 10–22. [Google Scholar] [CrossRef]
- Castañeda, R.A.; Mandrak, N.E.; Barrow, S.; Weyl, O.L.F. Occupancy Dynamics of Rare Cyprinids after Invasive Fish Eradication. Aquat. Conserv. Mar. Freshw. Ecosyst. 2020, 30, 1424–1436. [Google Scholar] [CrossRef]
- Kellner, K.F.; Swihart, R.K. Accounting for Imperfect Detection in Ecology: A Quantitative Review. PLoS ONE 2014, 9, e111436. [Google Scholar] [CrossRef]
- Lindenmayer, D.B.; Likens, G.E. Adaptive Monitoring: A New Paradigm for Long-Term Research and Monitoring. Trends Ecol. Evol. 2009, 24, 482–486. [Google Scholar] [CrossRef] [PubMed]




| Variable Name and Code | Unit | Min | Max | Mean | SD |
|---|---|---|---|---|---|
| Pool maximum depth (MDepth) | meters | 0.5 | 2.5 | 1.09 | 0.49 |
| Pool length (Length) | meters | 2 | 31 | 13.36 | 7.72 |
| Pool maximum width (MWidth) | meters | 2 | 10 | 4.77 | 2.23 |
| Pool area (Area) | m2 | 8 | 140 | 63.87 | 44.85 |
| Turbidity (Turb) | NTU | 0.2 | 1.85 | 0.65 | 0.45 |
| Pool shading (Shade) | % cover | 20 | 100 | 79 | 28.93 |
| Refuges (Refuge) | % cover | 5 | 60 | 21.83 | 14.17 |
| Water temperature (WTemp) | °Celsius | 16.20 | 25.53 | 21.59 | 3.01 |
| Trout count (N) | N°individuals | 0 | 10 | 2.53 | 2.34 |
| Data | Rank | Model Structure | AICc | ΔAICc | wi |
|---|---|---|---|---|---|
| Tot (J + SA + AD) | 1 | Tot ~ 1 + Stream + N | 453.5055 | 0 | 0.8763 |
| 2 | Tot ~ 1 + Stream + N + Method | 457.4694 | 3.964 | 0.1208 | |
| 3 | Tot ~ 1 + N | 465.6514 | 12.146 | 0.002 | |
| 4 | Tot ~ 1 + Stream | 468.6184 | 15.1129 | 0.0005 | |
| 5 | Tot ~ 1 + N + Method | 469.0052 | 15.4997 | 0.0004 | |
| 6 | Tot ~ 1 + Stream + Method | 473.2471 | 19.7416 | 0 | |
| 7 | Tot ~ 1 | 525.9969 | 72.4914 | 0 | |
| 8 | Tot ~ 1 + Method | 531.3003 | 77.7949 | 0 | |
| A + SA | 1 | ASA ~ 1 + Stream + N | 351.7343 | 0 | 0.7429 |
| 2 | ASA ~ 1 + N | 354.3142 | 2.5799 | 0.2045 | |
| 3 | ASA ~ 1 + Stream + N + Method | 357.7945 | 6.0602 | 0.0359 | |
| 4 | ASA ~ 1 + N + Method | 359.4606 | 7.7263 | 0.0156 | |
| 5 | ASA ~ 1 + Stream | 364.9632 | 13.2289 | 0.001 | |
| 6 | ASA ~ 1 | 370.038 | 18.3038 | 0.0001 | |
| 7 | ASA ~ 1 + Stream + Method | 371.0232 | 19.2889 | 0 | |
| 8 | ASA ~ 1 + Method | 375.3934 | 23.6591 | 0 | |
| J | 1 | J ~ 1 + Stream + N + Method | 296.397 | 0 | 0.3394 |
| 2 | J ~ 1 + Stream + N | 296.9249 | 0.5279 | 0.2606 | |
| 3 | J ~ 1 + Stream + Method | 297.4489 | 1.0519 | 0.2006 | |
| 4 | J ~ 1 + Stream | 297.4607 | 1.0637 | 0.1994 | |
| 5 | J ~ 1 + N + Method | 347.4382 | 51.0412 | 0 | |
| 6 | J ~ 1 + N | 348.3466 | 51.9497 | 0 | |
| 7 | J ~ 1 | 408.5618 | 112.1648 | 0 | |
| 8 | J ~ 1 + Method | 410.9305 | 114.5335 | 0 |
| Data | Rank | Model Structure | K | AICc | ΔAICc | wi |
|---|---|---|---|---|---|---|
| Tot (J + SA + A) | 1 | Tot ~ N + Stream + Method + Method:Depth | 12 | 452.8375 | 0 | 0.5243 |
| 2 | Tot ~ N + Stream + Method + Method:Area | 12 | 454.8241 | 1.9866 | 0.1942 | |
| 3 | Tot ~ N + Stream + Method + Method:Length | 12 | 455.6747 | 2.8372 | 0.1269 | |
| 4 | Tot ~ N + Stream (Null A) | 5 | 455.6778 | 2.8403 | 0.1267 | |
| 5 | Tot ~ N + Stream + Method (Null B) | 8 | 459.7729 | 6.9354 | 0.0164 | |
| 6 | Tot ~ N + Stream + Method + Method:Refug | 12 | 461.8712 | 9.0336 | 0.0057 | |
| 7 | Tot ~ N + Stream + Method + Method:Turb | 12 | 463.7103 | 10.8728 | 0.0023 | |
| 8 | Tot ~ N + Stream + Method + Method:Shade | 12 | 464.5323 | 11.6947 | 0.0015 | |
| 9 | Tot ~ N + Stream + Method + Method:Temp | 12 | 465.1169 | 12.2794 | 0.0011 | |
| 10 | Tot ~ N + Stream + Method + Method:Width | 12 | 465.6282 | 12.7907 | 0.0009 | |
| A + SA | 1 | ASA ~ N + Stream + Method + Method:Depth | 12 | 348.2256 | 0 | 0.6509 |
| 2 | ASA ~ N + Stream + Method + Method:Area | 12 | 349.9593 | 1.7336 | 0.2736 | |
| 3 | ASA ~ N + Stream (Null A) | 5 | 353.9072 | 5.6815 | 0.038 | |
| 4 | ASA ~ N + Stream + Method + Method:Length | 12 | 354.1168 | 5.8912 | 0.0342 | |
| 5 | ASA ~ N + Stream + Method (Null B) | 8 | 360.0974 | 11.8717 | 0.0017 | |
| 6 | ASA ~ N + Stream + Method + Method:Shade | 12 | 362.4088 | 14.1831 | 0.0005 | |
| 7 | ASA ~ N + Stream + Method + Method:Width | 12 | 362.6081 | 14.3825 | 0.0005 | |
| 8 | ASA ~ N + Stream + Method + Method:Turb | 12 | 364.2744 | 16.0488 | 0.0002 | |
| 9 | ASA ~ N + Stream + Method + Method:Refug | 12 | 364.3348 | 16.1091 | 0.0002 | |
| 10 | ASA ~ N + Stream + Method + Method:Temp | 12 | 365.3122 | 17.0866 | 0.0001 | |
| J | 1 | J ~ N + Stream + Method + Method:Shade | 12 | 293.5737 | 0 | 0.6262 |
| 2 | J ~ N + Stream + Method + Method:Turb | 12 | 296.2197 | 2.646 | 0.1668 | |
| 3 | J ~ N + Stream + Method + Method:Width | 12 | 297.4444 | 3.8707 | 0.0904 | |
| 4 | J ~ N + Stream + Method (Null B) | 8 | 298.6943 | 5.1206 | 0.0484 | |
| 5 | J ~ N + Stream (Null A) | 5 | 299.1034 | 5.5297 | 0.0394 | |
| 6 | J ~ N + Stream + Method + Method:Area | 12 | 300.5745 | 7.0009 | 0.0189 | |
| 7 | J ~ N + Stream + Method + Method:Depth | 12 | 302.982 | 9.4084 | 0.0057 | |
| 8 | J ~ N + Stream + Method + Method:Temp | 12 | 304.8883 | 11.3146 | 0.0022 | |
| 9 | J ~ N + Stream + Method + Method:Length | 12 | 305.6803 | 12.1066 | 0.0015 | |
| 10 | J ~ N + Stream + Method + Method:Refug | 12 | 307.6453 | 14.0716 | 0.0006 |
| Data | Rank | Model Structure | K | AICc | ΔAICc | wi |
|---|---|---|---|---|---|---|
| Tot (J + SA + A) | 1 | Tot ~ N + Stream + Method + Method:Depth + Method:Area | 16 | 452.7768 | 0 | 0.275 |
| 2 | Tot ~ N + Stream + Method + Method:Depth | 12 | 452.8375 | 0.0608 | 0.2667 | |
| 3 | Tot ~ N + Stream + Method + Method:Depth + Method:Length | 16 | 453.2807 | 0.5039 | 0.2137 | |
| 4 | Tot ~ N + Stream + Method + Method:Area | 12 | 454.8241 | 2.0473 | 0.0988 | |
| 5 | Tot ~ N + Stream + Method + Method:Length | 12 | 455.6747 | 2.8979 | 0.0646 | |
| 6 | Tot ~ N + Stream | 5 | 455.6778 | 2.9011 | 0.0645 | |
| 7 | Tot ~ N + Stream + Method | 8 | 459.7729 | 6.9962 | 0.0083 | |
| 8 | Tot ~ N + Stream + Method + Method:Depth + Method:Area + Method:Length | 20 | 460.273 | 7.4962 | 0.0065 | |
| 9 | Tot ~ N + Stream + Method + Method:Area + Method:Length | 16 | 462.6188 | 9.842 | 0.002 | |
| A + SA | 1 | ASA ~ N + Stream + Method + Method:Depth | 12 | 348.2256 | 0 | 0.5054 |
| 2 | ASA ~ N + Stream + Method + Method:Depth + Method:Area | 16 | 349.6221 | 1.3965 | 0.2514 | |
| 3 | ASA ~ N + Stream + Method + Method:Area | 12 | 349.9593 | 1.7336 | 0.2124 | |
| 4 | ASA ~ N + Stream | 5 | 353.9072 | 5.6815 | 0.0295 | |
| 5 | ASA ~ N + Stream + Method | 8 | 360.0974 | 11.8717 | 0.0013 | |
| J | 1 | J ~ N + Stream + Method + Method:Shade | 12 | 293.5737 | 0 | 0.4416 |
| 2 | J ~ N + Stream + Method + Method:Shade + Method:Turb | 16 | 294.6245 | 1.0509 | 0.2611 | |
| 3 | J ~ N + Stream + Method + Method:Turb | 12 | 296.2197 | 2.646 | 0.1176 | |
| 4 | J ~ N + Stream + Method + Method:Width | 12 | 297.4444 | 3.8707 | 0.0638 | |
| 5 | J ~ N + Stream + Method | 8 | 298.6943 | 5.1206 | 0.0341 | |
| 6 | J ~ N + Stream + Method + Method:Turb + Method:Width | 16 | 299.0925 | 5.5188 | 0.028 | |
| 7 | J ~ N + Stream | 5 | 299.1034 | 5.5297 | 0.0278 | |
| 8 | J ~ N + Stream + Method + Method:Shade + Method:Width | 16 | 300.0327 | 6.4591 | 0.0175 | |
| 9 | J ~ N + Stream + Method + Method:Shade + Method:Turb + Method:Width | 20 | 301.481 | 7.9073 | 0.0085 |
| Parameter | Best Model Parameter Estimates (Standard Error)Significance | ||
|---|---|---|---|
| Best Tot | Best ASA | Best J | |
| Intercept | 0.088 (0.510) | 0.210 (0.693) | −4.213 (1.111) *** |
| N | 0.227 (0.046) *** | 0.309 (0.064) *** | 0.088 (0.066) |
| Streamfurit | 1.116 (0.304) *** | −0.030 (0.413) | 3.486 (0.537) *** |
| Streampiras | 0.697 (0.282) * | 0.592 (0.356) + | 1.944 (0.556) *** |
| MethodVSA | −1.956 (0.659) ** | −3.793 (0.974) *** | −0.169 (1.401) |
| MethodSVS | −1.808 (0.654) ** | −3.329 (0.958) *** | 0.548 (1.308) |
| MethodUCS | 0.215 (0.608) | −1.099 (0.863) | 0.912 (1.313) |
| MethodELE × Depth | −1.232 (0.411) ** | −1.588 (0.592) ** | |
| MethodVSA × Depth | 0.538 (0.357) | 1.144 (0.490) * | |
| MethodSVS × Depth | 0.302 (0.352) | 0.653 (0.494) | |
| MethodUCS × Depth | −0.486 (0.369) | −0.319 (0.515) | |
| MethodELE × Area | 0.008 (0.004) * | 0.007 (0.005) | |
| MethodVSA × Area | 0.006 (0.004) | 0.013 (0.006) * | |
| MethodSVS × Area | 0.010 (0.004) * | 0.015 (0.006) * | |
| MethodUCS × Area | −0.003 (0.004) | 0.002 (0.006) | |
| MethodELE × Shade | 0.015 (0.010) | ||
| MethodVSA × Shade | 0.018 (0.010) + | ||
| MethodSVS × Shade | 0.007 (0.009) | ||
| MethodUCS × Shade | 0.026 (0.010) ** | ||
| MethodELE × Turb | 0.047 (0.514) | ||
| MethodVSA × Turb | −0.007 (0.514) | ||
| MethodSVS × Turb | 0.346 (0.472) | ||
| MethodUCS × Turb | −1.475 (0.564) ** | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casula, P.; Palmas, F.; Curreli, F.; Sabatini, A. Selecting Monitoring Methods for Endangered Trout Populations. Diversity 2024, 16, 442. https://doi.org/10.3390/d16080442
Casula P, Palmas F, Curreli F, Sabatini A. Selecting Monitoring Methods for Endangered Trout Populations. Diversity. 2024; 16(8):442. https://doi.org/10.3390/d16080442
Chicago/Turabian StyleCasula, Paolo, Francesco Palmas, Francesco Curreli, and Andrea Sabatini. 2024. "Selecting Monitoring Methods for Endangered Trout Populations" Diversity 16, no. 8: 442. https://doi.org/10.3390/d16080442
APA StyleCasula, P., Palmas, F., Curreli, F., & Sabatini, A. (2024). Selecting Monitoring Methods for Endangered Trout Populations. Diversity, 16(8), 442. https://doi.org/10.3390/d16080442







