Fish of Low Commercial Value in Lakes of Different Trophic Status (Poland)
Abstract
1. Introduction
2. Materials and Methods
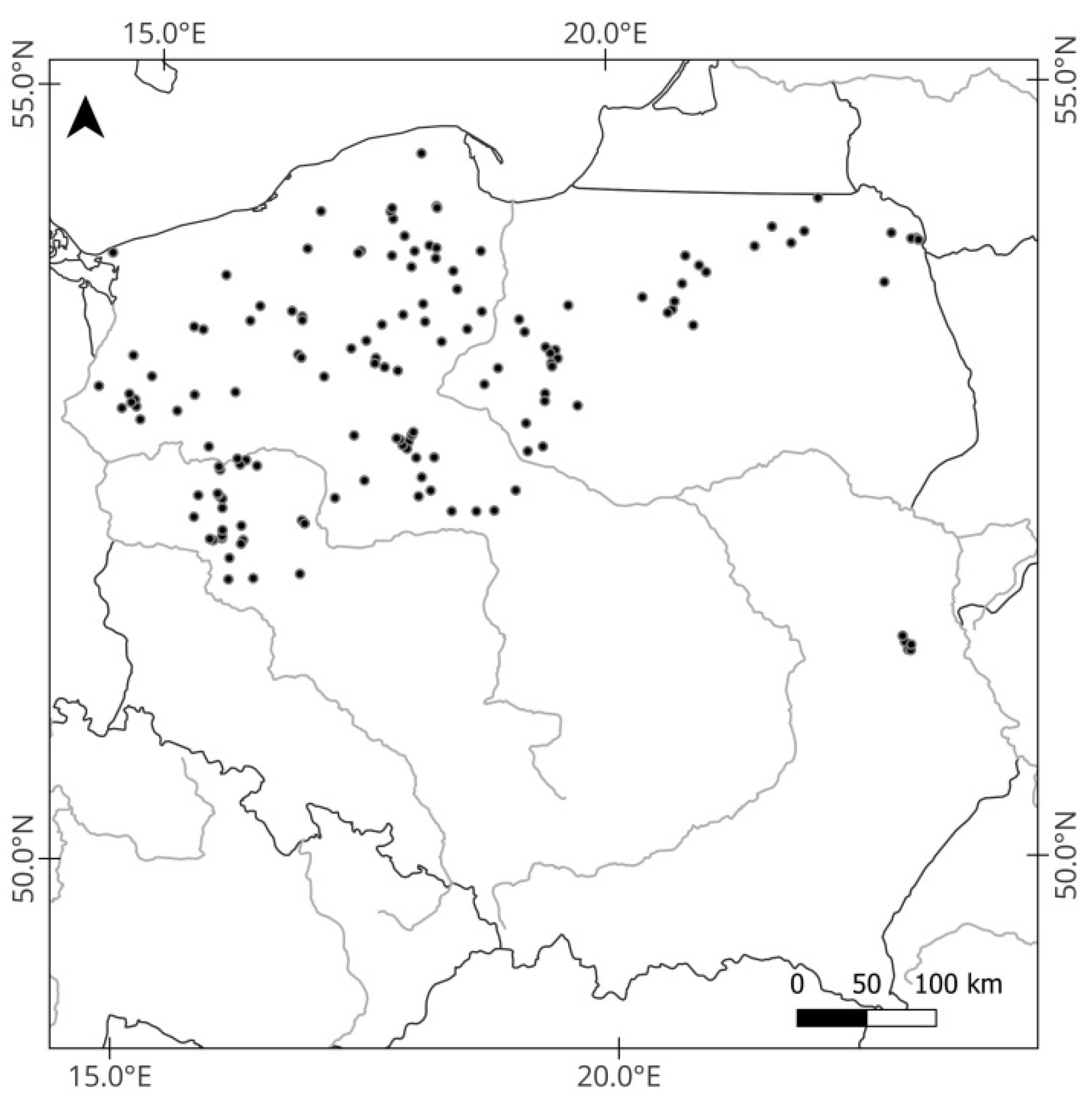
2.1. Study Area
2.2. Fish Sampling
2.3. Data Analyses
3. Results
3.1. Fish Communities in Lakes of Different Trophic Status
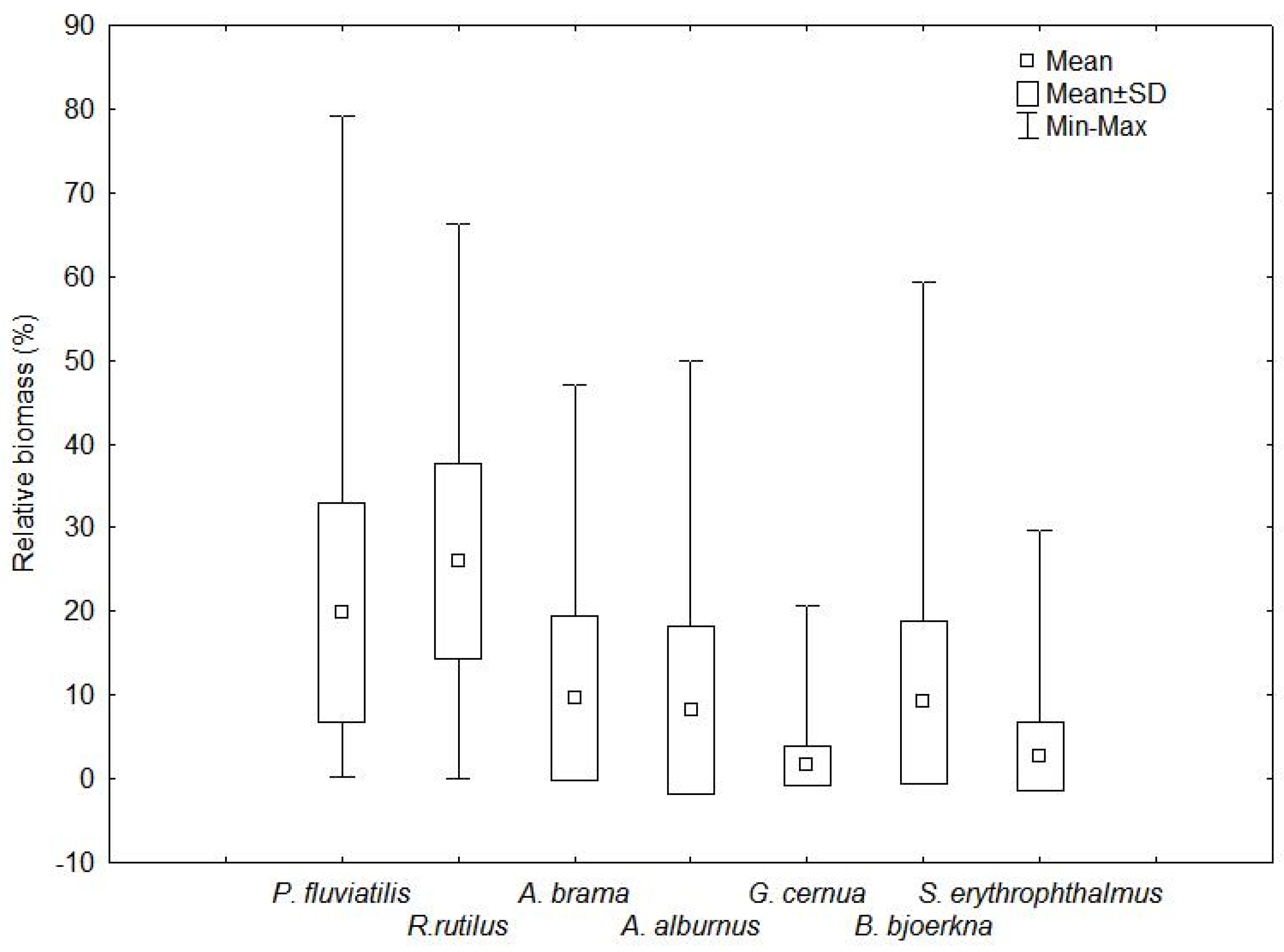
3.2. Low-Value Species Occurrence, Abundance, Contribution, and Dominance
3.3. Total Abundance and Contribution of Low-Value Species
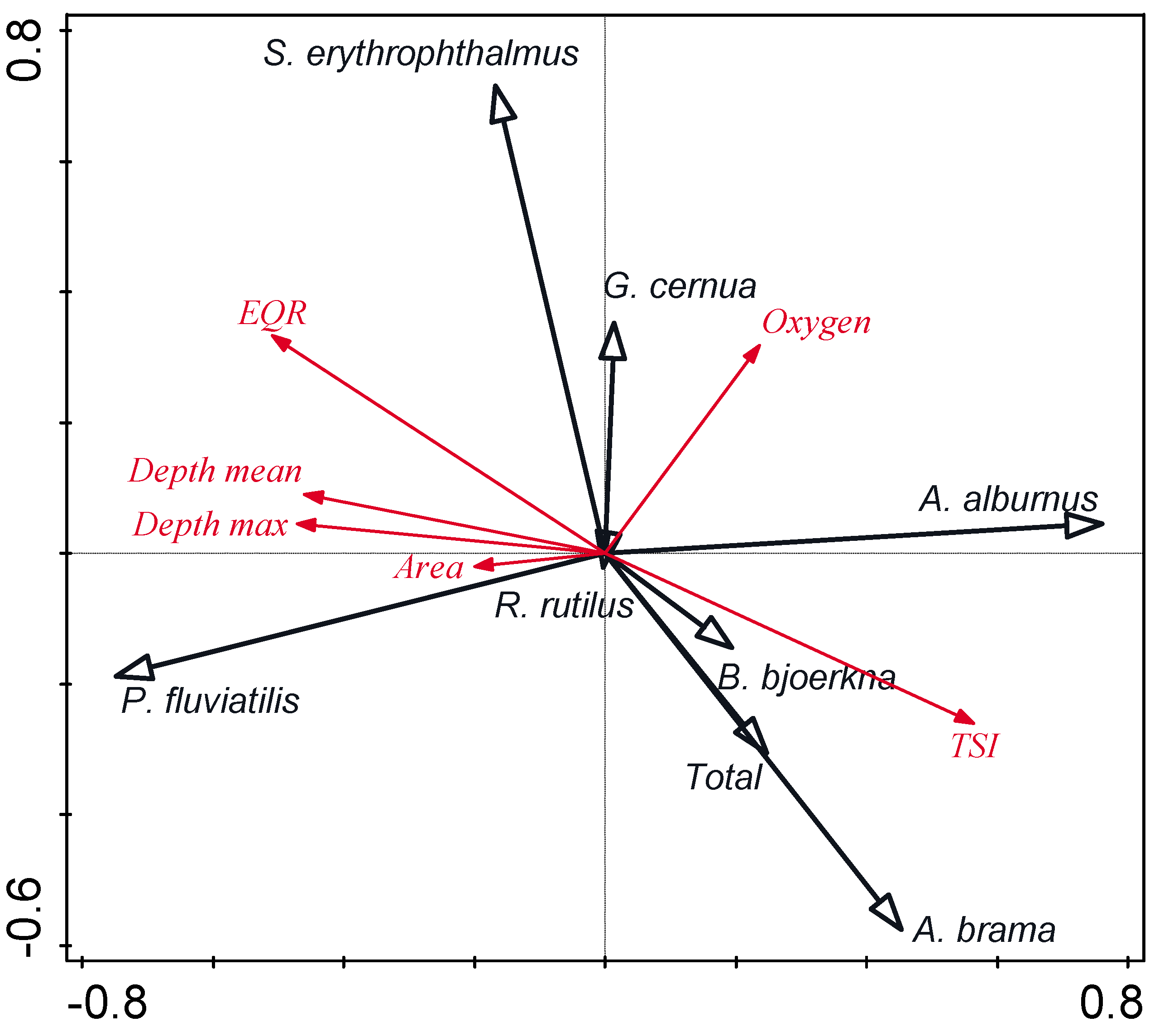
3.4. Relationships between Low-Value Fish and Environmental Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dayton, L.A.; Freeberg, M.H.; Murawski, S.A.; Pope, J.G. A Global Assessment of Fisheries Bycatch and Discards; FAO Fisheries Technical Paper 339; FAO: Rome, Italy, 1996; 233p. [Google Scholar]
- Funge-Smith, S.; Lindebo, E.; Staples, D. Asian Fisheries Today: The Production and Use of Low Value/Trash Fish from Marine Fisheries in the Asia-Pacific Region; Rap Publication 2005/16; Asia Pacific Fishery Commission: Bangkok, Thailand, 2005. [Google Scholar]
- Tacon, A.G.; Metian, M. Fishing for aquaculture: Non-food use of small pelagic forage fish—A global perspective. Rev. Fish. Sci. 2009, 17, 305–317. [Google Scholar] [CrossRef]
- Racioppo, A.; Speranza, B.; Campaniello, D.; Sinigaglia, M.; Corbo, M.R.; Bevilacqua, A. Fish loss/waste and low-value fish challenges: State of Art, Advances, and Perspectives. Foods 2021, 10, 2725. [Google Scholar] [CrossRef]
- Salim, S.S.; Geetha, R.; Sathiadhas, R. Low value fishes for nuritional security: The case with trawl landings in Kerala. Mysore J. Agric. Sci. 2012, 46, 339–344. [Google Scholar]
- Simeone, M.; Scarpato, D. The low commercial value fish. How can we increase its consumption? Aquac. Econ. Rev. 2014, 15, 43–59. [Google Scholar]
- Kolding, J.; van Zwieten, P.; Marttin, F.; Funge-Smith, S.; Poulain, F. Freshwater Small Pelagic Fish and Their Fisheries in Major African Lakes and Reservoirs in Relation to Food Security and Nutrition; FAO Fisheries and Aquaculture Technical Paper 642; FAO: Rome, Italy, 2019; 124p. [Google Scholar]
- Kapusta, A. Ryby małocenne pod względem środowiskowym. Komun. Ryb. 2022, 4, 2–3. [Google Scholar]
- Kabahenda, M.K.; Amega, R.; Okalany, E.; Husken, S.M.C.; Heck, S. Protein and micronutrient composition of low value fish products commonly marketed in the Lake Victoria region. World J. Agric. Sci. 2011, 7, 521–526. [Google Scholar]
- Tacon, A.G.; Metian, M. Fish matters: Importance of aquatic foods in human nutrition and global food supply. Rev. Fish. Sci. 2013, 21, 22–38. [Google Scholar] [CrossRef]
- Byrd, K.A.; Thilsted, S.H.; Fiorella, K.J. Fish nutrient composition: A review of data from poorly assessed inland and marine species. Public Health Nutr. 2020, 3 (Suppl. S1), S1–S11. [Google Scholar] [CrossRef]
- Szulecka, O. Farsze rybne—możliwości wykorzystania ryb małocennych. In Działalność Podmiotów Rybackich i Wędkarskich w 2021 Roku. Uwarunkowania Gospodarcze, Ekonomiczne, Środowiskowe i Klimatyczne; Cejko, A., Wołos, A., Eds.; IRS: Olsztyn, Poland, 2022; pp. 209–219. [Google Scholar]
- Thorseng, H.; Gondolf, U.H. Contribution of Iron from Esomus longimanus to the Diet: Studies on Content and In Vitro Availability. Master’s Thesis, The Royal Veterinary and Agricultural University, Copenhagen, Denmark, 2005. [Google Scholar]
- Roos, N.; Wahab, M.A.; Chamnan, C.; Thilsted, S.H. The role of fish in food-based strategies to combat vitamin A and mineral deficiencies in developing countries. J. Nutr. 2007, 137, 1106–1109. [Google Scholar] [CrossRef]
- Kawarazuka, N.; Béné, C. The potential role of small fish species in improving micronutrient deficiencies in developing countries: Building evidence. Public Health Nutr. 2011, 14, 1927–1938. [Google Scholar] [CrossRef]
- Clarke, S.B.; Nesbitt, W.A.; Efitre, J.; Masette, M.; Chapman, L.J. Elemental composition of small pelagic fishes in three East African lakes: Implications for nutritional security. Fish. Res. 2022, 256, 106479. [Google Scholar] [CrossRef]
- Blabolil, P.; Logez, M.; Ricard, D.; Prchalová, M.; Říha, M.; Sagouis, A.; Peterka, J.; Kubečka, J.; Argillier, C. An assessment of the ecological potential of Central and Western European reservoirs based on fish communities. Fish. Res. 2016, 173, 80–87. [Google Scholar] [CrossRef]
- Yu, J.; Zhen, W.; Kong, L.; He, H.; Zhang, Y.; Yang, X.; Chen, F.; Zhang, M.; Liu, Z.; Jeppesen, E. Changes in pelagic fish community composition, abundance, and biomass along a productivity gradient in subtropical lakes. Water 2021, 13, 858. [Google Scholar] [CrossRef]
- Guo, C.; Li, S.; Ke, J.; Liao, C.; Hansen, A.G.; Jeppesen, E.; Zhang, T.; Li, W.; Liu, J. The feeding habits of small-bodied fishes mediate the strength of top-down effects on plankton and water quality in shallow subtropical lakes. Wat. Res. 2023, 233, 119705. [Google Scholar] [CrossRef]
- Wołos, A. Kompleksowe przyczyny spadku odłowów gospodarczych z jezior. In Zrównoważone Korzystanie z Zasobów Rybackich na tle ich Stanu w 2014 Roku; Mickiewicz, M., Wołos, A., Eds.; IRS: Olsztyn, Poland, 2015; pp. 125–134. [Google Scholar]
- Szulecka, O. Czy ryby małocenne mogą być cenne?—możliwości wykorzystania ryb małocennych. Wiad. Ryb. 2021, 7–8, 17–18. [Google Scholar]
- Wołos, A. Wstęp. In Zmniejszenie Negatywnego Wpływu Rybactwa Śródlądowego na Środowisko Wodne Poprzez Innowacyjne Zagospodarowanie Małocennych Gatunków; Raport z realizacji I etapu operacji; Wołos, A., Czerwiński, T., Draszkiewicz-Mioduszewska, H., Kapusta, A., Mickiewicz, M., Trella, M., Eds.; Raport z realizacji I etapu operacji; IRS: Olsztyn, Poland, 2021; 125p. [Google Scholar]
- Mieczan, T.; Płaska, W.; Adamczuk, M.; Toporowska, M.; Bartkowska, A. Effects of the invasive fish species Ameiurus nebulosus on microbial communities in peat pools. Water 2022, 14, 815. [Google Scholar] [CrossRef]
- Kalinowska, K.; Ulikowski, D.; Traczuk, P.; Rechulicz, J. Changes in native fish communities in response to the presence of alien brown bullhead (Ameiurus nebulosus) in four lakes (Poland). Biol. Invasions 2023, 25, 2891–2900. [Google Scholar] [CrossRef]
- Colby, P.J.; Spangler, G.R.; Hurley, D.A.; McCombie, A.M. Effects of eutrophication on salmonid communities in oligotrophic lakes. Can. J. Fish. Aquat. Sci. 1972, 29, 975–983. [Google Scholar] [CrossRef]
- Jeppesen, E.; Jensen, J.P.; Søndergaard, M.; Lauridsen, T.; Landkildehus, F. Trophic structure, species richness and biodiversity in Danish lakes: Changes along a phosphorus gradient. Freshw. Biol. 2000, 45, 201–218. [Google Scholar] [CrossRef]
- Mehner, T.; Diekmann, M.; Brämick, U.; Lemcke, R. Composition of fish communities in German lakes as related to lake morphology, trophic state, shore structure and human use intensity. Freshw. Biol. 2005, 50, 70–85. [Google Scholar] [CrossRef]
- Jeppesen, E.; Søndergaard, M.; Kanstrup, E.; Petersen, B.; Eriksen, R.B.; Hammershøj, M.; Mortensen, E.; Jensen, J.P.; Have, A. Does the impact of nutrients on the biological structure and function of brackish and freshwater lakes differ? Hydrobiologia 1994, 276, 15–30. [Google Scholar] [CrossRef]
- Carlson, R.F. A trophic state index for lakes. Limnol. Oceanogr. 1977, 22, 361–369. [Google Scholar] [CrossRef]
- EN 14757: 2015; CEN. Water quality—Sampling of Fish with Multimesh Gillnets; European Standard. European Committee for Standardization: Brussels, Belgium, 2015.
- Kottelat, M.; Freyhof, J. Handbook of European Freshwater Fishes; Kottelat, Cornol, Swittzerland and Freyhof: Berlin, Germany, 2007; 646p. [Google Scholar]
- Poikane, S.; Ritterbusch, D.; Argillier, C.; Białokoz, W.; Blabolil, P.; Breine, J.; Jaarsma, N.G.; Krause, T.; Kubečka, J.; Lauridsen, T.L.; et al. Response of fish communities to multiple pressures: Development of a total anthropogenic pressure intensity index. Sci. Total Environ. 2017, 586, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Regulation. Regulation of the Minister of the Environment of 25 June 2021 on status classification of surface water bodies and environmental quality standards for CRediT authorship contribution statement priority substances. Official Journal of the Laws of 2021, Item 1475. 264p. Available online: https://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20210001475 (accessed on 4 June 2024). (In Polish)
- ter Braak, C.J.F.; Šmilauer, P. Canoco Reference Manual and User’s Guide: Software for Ordination (Version 5.0); Microcomputer Power: Ithaca, NY, USA, 2012. [Google Scholar]
- Heng, G.K.; Kim, T.L.L. Maximizing the utilization of fish catch for human consumption. Fish People 2008, 6, 27–30. [Google Scholar]
- Argillier, C.; Caussé, S.; Gevrey, M.; Pédron, S.; De Bortoli, J.; Brucet, S.; Emmrich, M.; Jeppesen, E.; Lauridsen, T.; Mehner, T.; et al. Development of a fish-based index to assess the eutrophication status of European lakes. Hydrobiologia 2013, 704, 193–211. [Google Scholar] [CrossRef]
- Spirkovski, Z.; Ilik-Boeva, D.; Talevski, T.; Trajcevski, B.; Palluqi, A.; Flloko, A.; Miraku, T.; Kapedani, E.; Pietrock, M.; Ritterbusch, D. Fish and Fisheries, Lake Ohrid. In Implementing the EU Water Framework Directive in South-Eastern Europe; Deutsche Gesellschaft für Internationale Zusammenarbeit (GIZ) GmbH: Bonn/Eschborn, Germany, 2017; 99p. [Google Scholar]
- Kalinowska, K.; Ulikowski, D.; Traczuk, P.; Kozłowski, M.; Kapusta, A. Fish species richness in Polish lakes. Diversity 2023, 15, 164. [Google Scholar] [CrossRef]
- Vainikka, A.; Turunen, A.; Salgado-Ismodes, A.; Lotsari, E.; Olin, M.; Ruuhijärvi, J.; Huuskonen, H.; Arzel, C.; Nummi, P.; Kahilainen, K.K. Biomass and sustainable yields of Eurasian perch (Perca fluviatilis) in small boreal lakes with respect to lake properties and water quality. Fish. Res. 2024, 271, 106922. [Google Scholar] [CrossRef]
- Hutorowicz, A.; Ulikowski, D.; Tunowski, J. Comparison of size distribution of fish obtained from gill netting and the distributions of echoes from hydroacoustics in Lake Dejguny. Water 2023, 15, 1117. [Google Scholar] [CrossRef]
- Mickiewicz, M. Problem zagospodarowania ryb małocennych w jeziorowych gospodarstwach rybackich. Magazyn Przem. Ryb. 2000, 1, 47–49. [Google Scholar]
- Krzywiński, T.; Domiszewski, Z.; Tokarczyk, G.; Bienkiewicz, G. Ocena przydatności mięsa ryb małocennych do produkcji żywności przekąskowej. Żywn. Nauka Technol. Jakość 2014, 5, 111–123. [Google Scholar]
- Czerwiński, T. Ryby małocenne pod względem konsumenckim. Analiza gospodarki rybami małocennymi w wybranych zbiornikach zaporowych w latach 2002–2018. In Zmniejszenie Negatywnego Wpływu Rybactwa Śródlądowego na Środowisko Wodne Poprzez Innowacyjne Zagospodarowanie Małocennych Gatunków; Wołos, A., Czerwiński, T., Draszkiewicz-Mioduszewska, H., Kapusta, A., Mickiewicz, M., Trella, M., Eds.; Raport z realizacji I etapu operacji; IRS: Olsztyn, Poland, 2021; 125p. [Google Scholar]
- Jeppesen, E.; Meerhoff, M.; Holmgren, K.; Gonzalez-Bergonzoni, I.; Mello, F.T.; Declerck, S.A.J.; Meester, L.D.; Søndergaard, M.; Lausidsen, T.L.; Bjerring, R.; et al. Impacts of climate warming on lake fish community structure and potential effects on ecosystem function. Hydrobiologia 2010, 646, 73–90. [Google Scholar] [CrossRef]
- Lawton, J.H. Species richness and population dynamics of animal assemblages: Patterns on body size: Abundance space. Philos. Trans. R. Soc. Lond. Ser. B 1991, 330, 283–291. [Google Scholar]
- Olin, M.; Rask, M.; Ruuhljärvi, J.; Kurkilahti, M.; Ala-Opas, P.; Ylönen, O. Fish community structure in mesotrophic and eutrophic lakes of southern Finland: The relative abundances of percids and cyprinids along a trophic gradient. J. Fish Biol. 2002, 60, 593–612. [Google Scholar] [CrossRef]
- Mehner, T.; Holmgren, K.; Lauridsen, T.L.; Jeppesen, E.; Diekmann, M. Lake depth and geographical position modify lake fish assemblages of the European ‘Central Plains’ ecoregion. Freshw. Biol. 2007, 52, 2285–2297. [Google Scholar] [CrossRef]
- Gaye-Siessegger, J. The great Cormorant (Phalacrocorax carbo) at lower lake Constance/Germany: Dietary composition and impact on commercial fisheries. Knowl. Manag. Aquat. Ecosyst. 2014, 414, 4. [Google Scholar] [CrossRef][Green Version]
- Traczuk, P.; Ulikowski, D.; Kalinowska, K. Stomach contents of the great cormorant Phalacrocorax carbo inhabiting north-eastern Poland. Fish. Aquat. Life 2021, 29, 202–210. [Google Scholar]
- European Commission. Directive of the European Parliament and of the Council 2000/60/EC Establishing a Framework for Community Action in the Field of Water Policy—Official Journal 2000 L 327/1; European Commission: Brussels, Belgium, 2000; 72p. [Google Scholar]
- Carpenter, S.R.; Kitchell, J.F.; Hodgson, J.R. Cascading trophic interactions and lake productivity. BioScience 1985, 35, 634–639. [Google Scholar] [CrossRef]
- Gulati, R.D.; Lammens, E.H.H.R.; Meijer, M.-L.; van Donk, E. Biomanipulation tool for water management: Proceedings of an International Conference held in Amsterdam, The Netherlands, 8–11 August, 1989 (Vol. 61); Springer Science & Business Media, B.V.: Berlin, Germany, 1989. 628p.
- Søndergaard, M.; Liboriussen, L.; Pedersen, A.R.; Jeppesen, E. Lake restoration by fish removal: Short- and long-term effects in 36 Danish lakes. Ecosystems 2008, 11, 1291–1305. [Google Scholar] [CrossRef]
- Mehner, T.; Benndorf, J.; Kasprzak, P.; Koschel, R. Biomanipulation of lake ecosystems: Successful applications and expanding complexity in the underlying science. Freshw. Biol. 2002, 47, 2453–2465. [Google Scholar] [CrossRef]
- Jeppesen, E.; Søndergaard, M.; Lauridsen, T.L.; Davidson, T.A.; Liu, Z.; Mazzeo, N.; Trochine, C.; Özkan, K.; Jensen, H.S.; Trolle, D.; et al. Biomanipulation as a restoration tool to combat eutrophication: Recent advances and future challenges. Adv. Ecol. Res. 2012, 47, 411–488. [Google Scholar]
- Setubal, R.B.; Riccardi, N. Long term effects of fish biomanipulation and macrophyte management on zooplankton functional diversity and production in a temperate shallow lake. Limnology 2020, 21, 305–317. [Google Scholar] [CrossRef]
- McQueen, D.J.; Post, J.R.; Mills, E.L. Trophic relationships in freshwater pelagic ecosystems. Can. J. Fish. Aquat. Sci. 1986, 43, 1571–1581. [Google Scholar] [CrossRef]
- Aldin-Lundgren, E. Role of Low Value Fish for Consumption and Possible Interactions/Conflicts with the Aquaculture in Cambodia. Master’s Thesis, Department of Systems Ecology, Stockholm University, Stockholm, Sweden, 2008; 50p. [Google Scholar]
- Bruno, S.F.; Ekorong, F.J.A.A.; Karkal, S.S.; Cathrine, M.S.B.; Kudre, T.G. Green and innovative techniques for recovery of valuable compounds from seafood by products and discards: A Review. Trends Food Sci. Technol. 2019, 85, 1022. [Google Scholar] [CrossRef]
- Kim, D.H. Low-value fish used as feed is a source of disease in farmed fish. Fish. Aquat. Sci. 2015, 18, 203–209. [Google Scholar] [CrossRef][Green Version]
- Illera-Vives, M.; Seoane Labandeira, S.; Brito, L.M.; López-Fabal, A.; López-Mosquera, M.E. Evaluation of compost from seaweed and fish waste as a fertilizer for horticultural use. Sci. Hortic. 2015, 186, 101–107. [Google Scholar] [CrossRef]

| Trophy | Number of Lakes | Area (ha) | Depth Max (m) |
|---|---|---|---|
| Oligotrophy | 3 | 83.5–156.1 | 6.5–40.5 |
| Mesotrophy | 39 | 52.0–765.3 | 2.6–45.0 |
| Eutrophy | 84 | 50.0–790.7 | 0.4–45.0 |
| Hypertrophy | 19 | 51.8–461.3 | 1.4–23.1 |
| Total | 145 | 50.0–790.7 | 0.4–45.0 |
| Trophy | Number of Species | NPUE (inds./100 m2) | WPUE (kg/100 m2) |
|---|---|---|---|
| Oligotrophy | 7–15 | 53–312 | 1.2–7.2 |
| Mesotrophy | 9–16 | 28–502 | 0.7–9.1 |
| Eutrophy | 6–17 | 23–1926 | 1.0–24.1 |
| Hypertrophy | 1–18 | 16–3237 | 0.5–28.9 |
| Total | 1–18 | 16–3237 | 0.5–28.9 |
| Species | Number of Lakes | Frequency (%) | NPUE (inds./100 m2) | CVN (%) | WPUE (g/100 m2) | CVW (%) |
|---|---|---|---|---|---|---|
| Perca fluviatilis | 145 | 100.0 | 100.1 ± 111.4 | 111 | 974.5 ± 962.0 | 99 |
| (0.4–852.8) | (8.3–8054.1) | |||||
| Rutilus rutilus | 144 | 99.3 | 90.2 ± 109.0 | 121 | 1477.3 ± 1459.7 | 99 |
| (3.9–653.3) | (59.8–10,965.1) | |||||
| Abramis brama | 137 | 94.5 | 26.1 ± 44.4 | 170 | 672.7 ± 1087.4 | 162 |
| (0.1–244.6) | (0.2–9819.7) | |||||
| Alburnus alburnus | 135 | 93.1 | 88.1 ± 212.2 | 241 | 766.4 ± 1678.4 | 219 |
| (0.2–1341.7) | (1.5–12,022.4) | |||||
| Gymnocephalus cernua | 135 | 93.1 | 11.4 ± 18.2 | 160 | 88.1 ± 121.4 | 138 |
| (0.2–118.2) | (1.2–775.9) | |||||
| Blicca bjoerkna | 132 | 91.0 | 39.5 ± 74.2 | 188 | 675.6 ± 1244.5 | 184 |
| (0.1–512.1) | (7.6–10,764.4) | |||||
| Scardinius erythrophthalmus | 122 | 84.1 | 4.2 ± 6.2 | 148 | 142.9 ± 195.0 | 137 |
| (0.1–30.6) | (0.6–1043.3) |
| Species | Area | Depth Max | Depth Mean | Oxygen | TSI | EQR |
|---|---|---|---|---|---|---|
| Perca fluviatilis | ns | ns | ns | ns | ns | 0.19 * |
| Rutilus rutilus | ns | −0.32 **** | −0.34 **** | 0.32 **** | 0.27 *** | ns |
| Abramis brama | ns | −0.27 ** | −0.33 *** | ns | 0.31 *** | −0.18 * |
| Alburnus alburnus | ns | −0.31 *** | −0.32 **** | 0.37 **** | 0.40 **** | −0.20 * |
| Gymnocephalus cernua | ns | −0.24 ** | −0.25 ** | 0.32 **** | 0.44 **** | −0.35 **** |
| Blicca bjoerkna | ns | −0.28 *** | −0.29 *** | 0.32 **** | 0.39 **** | −0.18 * |
| Scardinius erythrophthalmus | ns | −0.19 * | −0.17 * | 0.26 ** | ns | 0.42 **** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalinowska, K.; Ulikowski, D.; Kozłowski, M.; Traczuk, P.; Szkudlarek, M.; Stawecki, K.; Kapusta, A. Fish of Low Commercial Value in Lakes of Different Trophic Status (Poland). Diversity 2024, 16, 437. https://doi.org/10.3390/d16080437
Kalinowska K, Ulikowski D, Kozłowski M, Traczuk P, Szkudlarek M, Stawecki K, Kapusta A. Fish of Low Commercial Value in Lakes of Different Trophic Status (Poland). Diversity. 2024; 16(8):437. https://doi.org/10.3390/d16080437
Chicago/Turabian StyleKalinowska, Krystyna, Dariusz Ulikowski, Michał Kozłowski, Piotr Traczuk, Maciej Szkudlarek, Konrad Stawecki, and Andrzej Kapusta. 2024. "Fish of Low Commercial Value in Lakes of Different Trophic Status (Poland)" Diversity 16, no. 8: 437. https://doi.org/10.3390/d16080437
APA StyleKalinowska, K., Ulikowski, D., Kozłowski, M., Traczuk, P., Szkudlarek, M., Stawecki, K., & Kapusta, A. (2024). Fish of Low Commercial Value in Lakes of Different Trophic Status (Poland). Diversity, 16(8), 437. https://doi.org/10.3390/d16080437







