First Records of Feather Mites and Haemosporidian Parasites in the Isabelline Wheatear (Oenanthe isabellina) from the Westernmost Part of the Species Breeding Range
Abstract
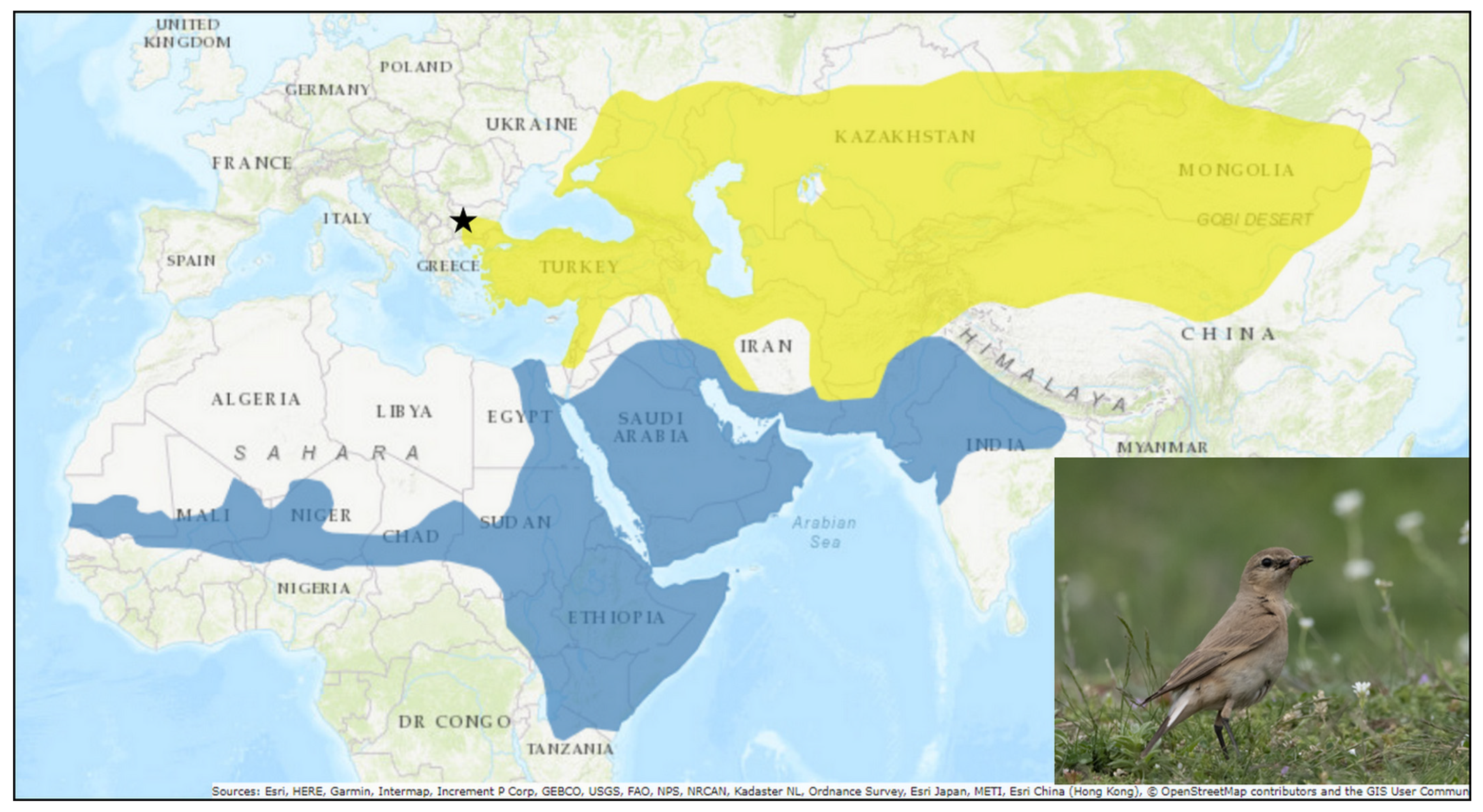
1. Introduction
2. Materials and Methods
2.1. Feather Mites’ Collection and Identification
2.2. Collection of Blood Samples and Preparation of Blood Smears
2.3. Microscopic Examination of the Blood Smears
2.4. Molecular Identification of Haemosporidian Parasites
3. Results
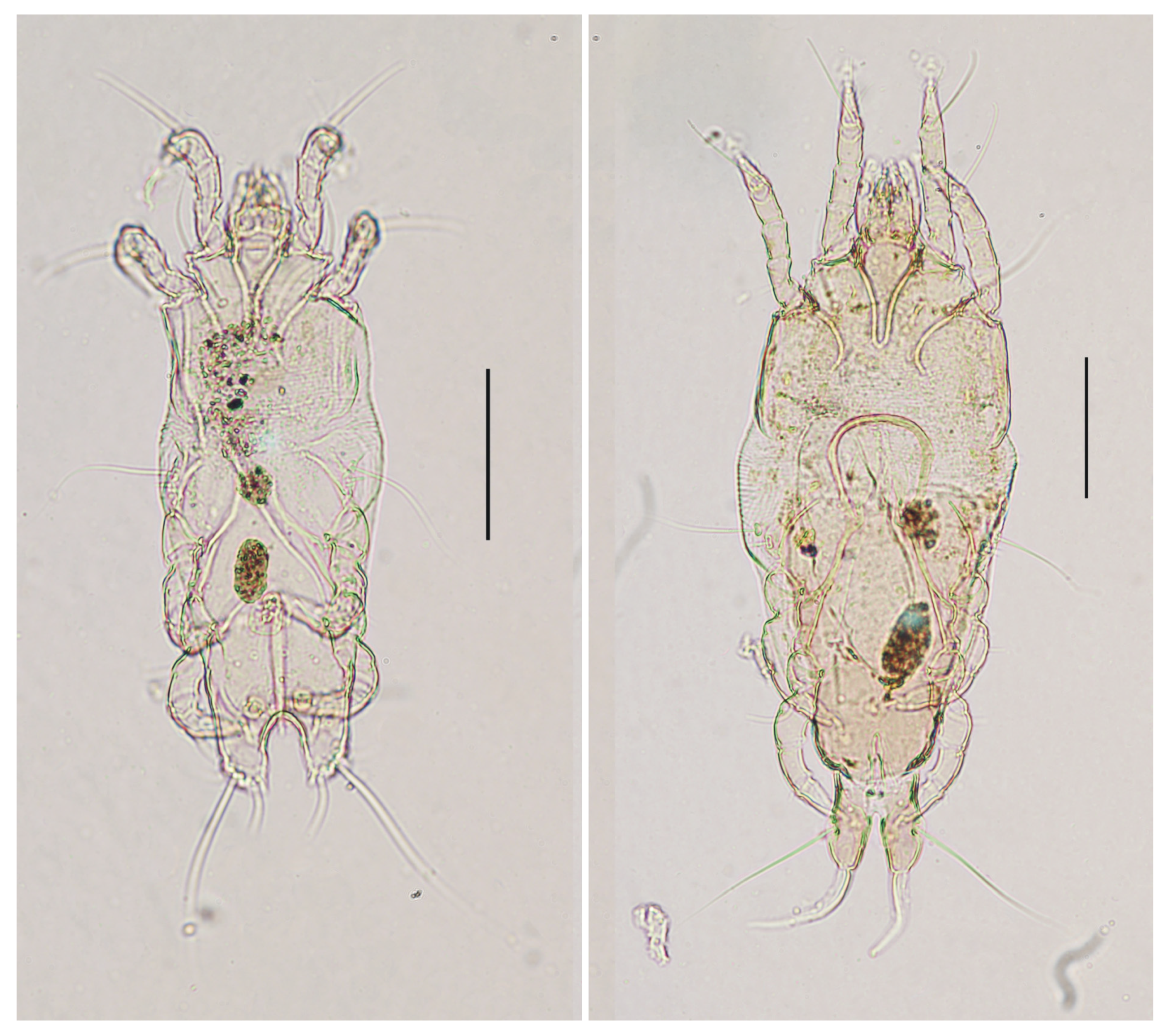
3.1. Feather Mites
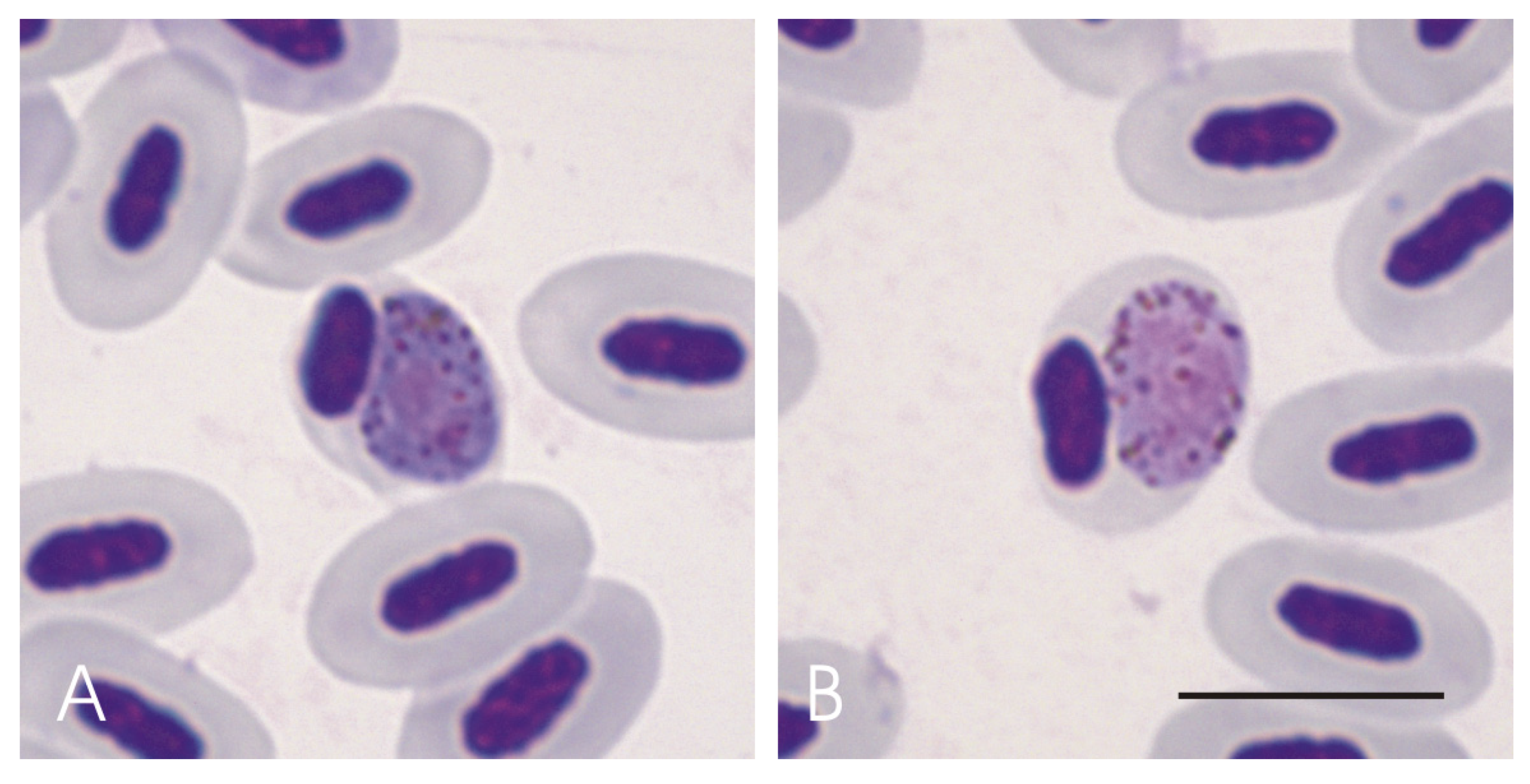
3.2. Haemosporidian Parasites
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lafferty, K.D.; Dobson, A.P.; Kuris, A.M. Parasites Dominate Food Web Links. Proc. Natl. Acad. Sci. USA 2006, 103, 11211–11216. [Google Scholar] [CrossRef] [PubMed]
- Morand, S. (Macro-) Evolutionary Ecology of Parasite Diversity: From Determinants of Parasite Species Richness to Host Diversification. Int. J. Parasitol. Parasites Wildl. 2015, 4, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Larsen, B.B.; Miller, E.C.; Rhodes, M.K.; Wiens, J.J. Inordinate Fondness Multiplied and Redistributed: The Number of Species on Earth and the New Pie of Life. Q. Rev. Biol. 2017, 92, 229–265. [Google Scholar] [CrossRef]
- Valkiūnas, G. Avian Malaria Parasites and Other Haemosporidia; CRC Press: Boca Raton, FL, USA; London, UK; New York, NY, USA; Washington, DC, USA, 2005; ISBN 0415300975. [Google Scholar]
- Bensch, S.; Hellgren, O.; Pérez-Tris, J. MalAvi: A Public Database of Malaria Parasites and Related Haemosporidians in Avian Hosts Based on Mitochondrial Cytochrome b Lineages. Mol. Ecol. Resour. 2009, 9, 1353–1358. [Google Scholar] [CrossRef] [PubMed]
- Pigeault, R.; Vézilier, J.; Cornet, S.; Zélé, F.; Nicot, A.; Perret, P.; Gandon, S.; Rivero, A. Avian Malaria: A New Lease of Life for an Old Experimental Model to Study the Evolutionary Ecology of Plasmodium. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140300. [Google Scholar] [CrossRef] [PubMed]
- Doña, J.; Proctor, H.; Serrano, D.; Johnson, K.P.; Oploo, A.O.; Huguet-Tapia, J.C.; Ascunce, M.S.; Jovani, R. Feather Mites Play a Role in Cleaning Host Feathers: New Insights from DNA Metabarcoding and Microscopy. Mol. Ecol. 2019, 28, 203–218. [Google Scholar] [CrossRef] [PubMed]
- Mironov, S.V.; Zabashta, A.V.; Malyshev, L.L. Biodiversity of Feather Mites Parasitizing Passerines of the Lower Don Area and Quantitative Characteristics of Their Invasion. Entomol. Rev. 2023, 103, 573–599. [Google Scholar] [CrossRef]
- BWPi. The Birds of the Western Palearctic Interactive; 2006 Upgra.; DVD Birdguides: Shrewsbury, UK, 2006. [Google Scholar]
- Collar, N. Isabelline Wheatear (Oenanthe isabellina). In Birds of the World; del Hoyo, J., Elliott, A., Sargatal, J., Christie, D., de Juana, E., Eds.; Cornell Lab of Ornithology: Ithaca, NY, USA, 2020. [Google Scholar]
- Shurulinkov, P.S.; Nikolov, I.P.; Daskalova, G.N.; Nikolov, B.P.; Stoyanov, G.P. Further Range Expansion of the Isabelline Wheatear Oenanthe isabellina in Bulgaria. Ciconia 2008, 16, 49–56. [Google Scholar]
- Li, S.; Peng, W. Nest-Site Selection by Isabelline Wheatears Oenanthe isabellina on the Tibet Plateau. Forktail 2014, 30, 132–133. [Google Scholar]
- Lipaev, V.M.; Kozlovskaya, O.L.; Surkov, V.S.; Derevich, S.M.; Busoedova, N.M.; Antipeva, O.A. Wheatears of North-Western Mongolia (a Contribution to Their Distribution, Ecology and Epizootological Importance). In Proceedings of the International and National Aspects of the Epidemiological Surveillance of Plague, Scientific Conference, Irkutsk, Russia, 10–13 October 1978; Safonova, A.D., Ed.; Ministerstvo Zdravookhraneniya SSSR: Irkutsk, Russia, 1977; pp. 78–82. [Google Scholar]
- Martynov, G.A.; Shevchenko, V.L.; Stepanov, V.M.; Martynova, N.V.; Grazhdanov, A.K.; Zharinova, L.K. Experimental Infection of Isabelline Wheatears, Oenanthe isabellina Temm., 1829, with the Plague Agent. Meditsinskaya Parazitol. Parazit. Bolezn. 1983, 52, 37–41. [Google Scholar]
- Shevchenko, V.L. Fleas and Mites of the Isabelline Wheatear (Oenanthe isabellina Temm., 1829) and Its Nests in Northern Cis-Caspia. Meditsinskaya Parazitol. Parazit. Bolezn. 1983, 52, 41–46. [Google Scholar]
- Dubinin, V. Feather Mites (Analgesoidea). Part I. In troduction in Their Study. In Fauna USSR, Paukoobraznye; Akademia Nauk SSSR: Moscow, Russia; Leningrad, Russia, 1951; pp. 1–363. [Google Scholar]
- Chirov, P.A. Mites of the Superfamily Analgoidea Which Live on Birds in Kirgizia. Entomol. Issled. Kirg. Akad. Kirg. SSR 1979, 13, 49–54. [Google Scholar]
- Burdejnaja, S.J.; Kivganov, D.A. Taxonomic Characteristic of Mites of the Family Proctophyllodidae from Birds Migrating via Zmeiny Island. Zapovidna Sprav. Ukr. 2009, 15, 71–75. [Google Scholar]
- BirdLife International Species Factsheet: Isabelline Wheatear Oenanthe isabellina. Available online: https://datazone.birdlife.org/species/factsheet/isabelline-wheatear-oenanthe-isabellina (accessed on 27 March 2024).
- Paperna, I.; Rózsa, L.; Yosef, R. Avian Haemosporidian Blood Parasite Infections at a Migration Hotspot in Eilat, Israel. Eur. J. Ecol. 2016, 2, 47–52. [Google Scholar] [CrossRef][Green Version]
- Ciloglu, A.; Ergen, A.G.; Inci, A.; Dik, B.; Duzlu, O.; Onder, Z.; Yetismis, G.; Bensch, S.; Valkiūnas, G.; Yildirim, A. Prevalence and Genetic Diversity of Avian Haemosporidian Parasites at an Intersection Point of Bird Migration Routes: Sultan Marshes National Park, Turkey. Acta Trop. 2020, 210, 105465. [Google Scholar] [CrossRef]
- Hussein, I.O.; Mirshamsi, O.; Mohammadiankalat, T.; Aliabadian, M. Prevalence of Avian Haemosporidian Parasites: A Comparative Study between Resident and Migratory Birds of Iraq. Iran. J. Anim. Biosyst. 2023, 19, 115–126. [Google Scholar] [CrossRef]
- Černy, V. Deux Especes Nouvelles d’Acariens Plumicoles. Acarologia 1963, 5, 649–652. [Google Scholar]
- Mironov, S.V. A New Genus of the Feather Mite Subfamily Pterodectinae (Analgoidea; Proctophyllodidae). Parazitologiya 1996, 30, 398–403. [Google Scholar]
- Hellgren, O.; Waldenström, J.; Bensch, S. A New PCR Assay for Simultaneous Studies of Leucocytozoon, Plasmodium and Haemoproteus from Avian Blood. J. Parasitol. 2004, 90, 797–802. [Google Scholar] [CrossRef]
- Gaud, J. Acariens Plumicoles (Analgesoidea) Parasites Des Oiseaux Du Maroc. I. Proctophyllodidae. Bull. Société Sci. Nat. Phys. Maroc 1957, 37, 105–136. [Google Scholar]
- Černy, V. Feather Mites (Analgesoidea) from Birds Trapped at the Falsterbo Bird Station, Southern Sweden. Acta Univ. Lund. Sect. II Medica Math. Sci. Rerum Nat. 1965, 8, 1–8. [Google Scholar]
- Kolarova, N.T.; Mitov, P.G. Feather Mites of the Superfamily Analgoidea (Acari: Astigmata) from Passerines (Aves: Passeriformes) in South Dobrudzha, Bulgaria. Acta Zool. Bulg. 2008, 2, 91–102. [Google Scholar]
- Arutunjan, E.S.; Mironov, S.V. New and Little Known Species of Analgoid-Mites from the USSR. Acad. Sci. Armen. SSR Inst. Zool. Zool. Pap. 1983, 19, 319–336. [Google Scholar]
- Mironov, S.V. Feather Mites from Passerines of the North-West of Russia. Parazitologiya 1996, 30, 521–539. [Google Scholar]
- Doña, J.; Serrano, D.; Mironov, S.; Montesinos-Navarro, A.; Jovani, R. Unexpected Bird–Feather Mite Associations Revealed by DNA Metabarcoding Uncovers a Dynamic Ecoevolutionary Scenario. Mol. Ecol. 2019, 28, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Haiba, M.H. Plasmodia of Common Egyptian Birds. J. Comp. Pathol. Ther. 1948, 58, 81–93, IN7–IN8. [Google Scholar] [CrossRef]
- Valkiūnas, G.; Iezhova, T.A.; Mironov, S.V. High Prevalence and Diversity of Blood Parasites of Passerine Birds in Southern Turkmenistan. Parazitologiya 2001, 35, 135–141. [Google Scholar]
- Nourani, L.; Aliabadian, M.; Dinparast Djadid, N.; Mirshamsi, O. Occurrence of Haemoproteus spp. (Haemosporida: Haemoproteidae) in New Host Records of Passerine Birds from the East of Iran. Iran. J. Parasitol. 2018, 13, 267–274. [Google Scholar] [PubMed]
- Drovetski, S.V.; Aghayan, S.A.; Mata, V.A.; Lopes, R.J.; Mode, N.A.; Harvey, J.A.; Voelker, G. Does the Niche Breadth or Trade-off Hypothesis Explain the Abundance-Occupancy Relationship in Avian Haemosporidia? Mol. Ecol. 2014, 23, 3322–3329. [Google Scholar] [CrossRef]
- Nourani, L.; Aliabadian, M.; Mirshamsi, O.; Djadid, N.D. Molecular Detection and Genetic Diversity of Avian Haemosporidian Parasites in Iran. PLoS ONE 2018, 13, e0206638. [Google Scholar] [CrossRef]
- Ghaemitalab, V.; Mirshamsi, O.; Valkiūnas, G.; Aliabadian, M. Prevalence and Genetic Diversity of Avian Haemosporidian Parasites in Southern Iran. Pathogens 2021, 10, 645. [Google Scholar] [CrossRef] [PubMed]
- Valkiūnas, G.; Zehtindjiev, P.; Dimitrov, D.; Križanauskienė, A.; Iezhova, T.A.; Bensch, S. Polymerase Chain Reaction-Based Identification of Plasmodium (Huffia) elongatum, with Remarks on Species Identity of Haemosporidian Lineages Deposited in GenBank. Parasitol. Res. 2008, 102, 1185–1193. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolarova, N.; Bobeva, A.; Ilieva, M.; Sjöholm, C.; Dimitrov, D. First Records of Feather Mites and Haemosporidian Parasites in the Isabelline Wheatear (Oenanthe isabellina) from the Westernmost Part of the Species Breeding Range. Diversity 2024, 16, 436. https://doi.org/10.3390/d16080436
Kolarova N, Bobeva A, Ilieva M, Sjöholm C, Dimitrov D. First Records of Feather Mites and Haemosporidian Parasites in the Isabelline Wheatear (Oenanthe isabellina) from the Westernmost Part of the Species Breeding Range. Diversity. 2024; 16(8):436. https://doi.org/10.3390/d16080436
Chicago/Turabian StyleKolarova, Nevena, Aneliya Bobeva, Mihaela Ilieva, Christoffer Sjöholm, and Dimitar Dimitrov. 2024. "First Records of Feather Mites and Haemosporidian Parasites in the Isabelline Wheatear (Oenanthe isabellina) from the Westernmost Part of the Species Breeding Range" Diversity 16, no. 8: 436. https://doi.org/10.3390/d16080436
APA StyleKolarova, N., Bobeva, A., Ilieva, M., Sjöholm, C., & Dimitrov, D. (2024). First Records of Feather Mites and Haemosporidian Parasites in the Isabelline Wheatear (Oenanthe isabellina) from the Westernmost Part of the Species Breeding Range. Diversity, 16(8), 436. https://doi.org/10.3390/d16080436







