Abstract
Benthic diatoms have been studied in different areas of the Mediterranean Sea, but no data have been available for Israeli coastal waters until the present time. In this work, the composition, ecology, and phytogeography of diatoms of the macrophytes epiphyton are presented for the first time. Altogether, 85 diatom taxa were found among the epiphyton of 25 species of green, brown, and red macroalgae from the Israeli coast between March and May of 2021. These diatoms represent three classes, 17 orders, 26 families, and 41 genera. The taxonomic composition, ecology, and phytogeography of species are discussed. The distribution of diatoms are compared to that of other macrophytes and anthropogenic loads across the shoreline. The dominant species are given. Ecological characteristics and abundance in communities of revealed species are represented and statistically analyzed. The index of saprobity S varies between 1.69–2.71. Sites that stressed aquatic communities are indicated. The influence of the anthropogenic loads on the coastal territories is defined as a major factor that stimulated diatom species richness. Sites with anthropogenic stress for aquatic communities are indicated. Based on the composition of bioindicators, it is concluded that the section of the Israeli coast studied is oligo-mesotrophic compared to the eutrophic Gulf of Tartus.
1. Introduction
The degree of knowledge of the phytobenthos of the coastal seas of the Mediterranean basin varies. There is a bibliographic review of 320 works on the phytobenthos of the Mediterranean Sea for the period 1978–1980 [1]. It included issues of taxonomy, cytology, floristry, ecology, biology, biochemistry, protection, and use of Mediterranean phytobenthos off the coast of Italy and France and mentioned that research has begun in the 1980s on important but little-studied groups: diatoms, cyanobacteria, fungi, and lichens.
A checklist of marine macroalgal species described for the Mediterranean coast of Israel is presented in a review work on the study of more 300 species of red (179 taxa), brown (52), and green algae (76) with taxonomical and ecological approaches [2], but diatom microalgae were not included in this work. The first data on benthic diatom species of the Mediterranean basin are given for specific areas: the port of Ischia, which is a volcanic island in the Tyrrhenian Sea, part of the Mediterranean Sea off the western coast of Italy [3]; Gulf of Trieste in Northern Adriatic [4]; the Mediterranean coastal wetlands of Spain [5]; epiphyton of macrophytes of Greece and the island of Rhodes in the Aegean Sea [6,7,8], and Egyptian coastal waters of the Eastern Mediterranean near Port Said [7]; the Suez Canal [9] and the coastal waters of Tartus [10]. For Italian coastal waters, a benthic microalgae checklist was compiled [11] and their seasonal dynamics of abundance and biomass were studied [12]. The study of the primary production of microphytobenthos of the French Mediterranean coast showed that their highest values reached at a depth of 0.5 m in the winter-spring season with a maximum 60 mg O2·m−2·h−1 and mean biomass as high as 65 mg Chl a m−2 in the Westerschelde [13,14].
A list of algal species and cyanobacteria found in the continental waters of Israel in 1898–2022 with progress in 2000–2022 increased from 1261 to 1628 species which belong to 14 taxonomic phyla. Taxonomic analysis shows that diatoms, cyanobacteria, and green algae predominate in continental waters of this region [15]. Seaweed, microalgae, and cyanobacteria are known to be important biological indicators of pollution and saprobity of water [16,17,18,19,20], which can be used to assess the quality of water. The study of indicator species of marine benthic diatoms lags behind freshwater species and is still poorly understood.
Methods of bioindication have been developed for the various components of the biota of aquatic ecosystems. Benthic diatoms are one of the most sensitive components of the aquatic ecosystem [21,22]. Therefore, it is especially important to study the taxonomic composition, floristics, and ecology of algae in the phytobenthos, not only in fresh and brackish but also in marine waters, which are poorly studied in comparison. It is known that the indicator significance of individual species depends substantially on a number of regional factors, which can produce an essential error in the calculation of the saprobity index and in the definition of the water quality class, respectively [23,24].
In addressing this problem, the primary objective is to create marine microalgae databases and checklists in different areas.
Thus, this study, for the first time for Israeli coastal waters of the Mediterranean Sea, will synthesize data on the taxonomic composition, floristics, and ecology of benthic diatoms to identify indicator properties of Israeli aquatic flora that can be used to monitor the quality of coastal waters. For continental waters, ecological preferences of species are known for ten environmental variables [15]. But the identification of indicator algae for the marine environment is at an initial stage. At the same time, seaweed populations found in the eastern Mediterranean are known to have low biodiversity due to their oligotrophic environment [2], as evidenced by the chlorophyll content of the water [25].
The aim of this study was to determine the taxonomic diversity of diatoms in the Mediterranean coastal waters of Israel and the main environmental factors influencing their richness and diversity, and to identify indicator species that, together with macroalgal data, can serve as an auxiliary tool in coastal zone monitoring.
2. Materials and Methods
2.1. Description of the Study Sites
Altogether, 19 National Parks and Nature Reserves serve as marine protected areas on the Mediterranean coast of Israel [26]. From the mid-1960s to the early 2000s, seven small marine nature reserves were officially declared along Israel’s Mediterranean coast. The State of Israel is a signatory to international conventions in which it is committed to preserve 10% of its marine areas by 2020. At present, less than 0.25% of Israel’s marine areas in the Mediterranean Sea are protected as officially declared nature reserves. It is proposed that Israel officially declare and operate marine nature reserves in a total of 20% of its territorial waters in the Mediterranean Sea. In keeping with the data collected, marine nature reserves are planned that will preserve representative areas of the marine habitats typical of Israel [26].
The coastline of Israel on the Mediterranean Sea is an almost smooth line, stretches from south to north for about 200 km, and has only one bay in the Haifa area (Figure 1). Only a few small streams flow into the sea along the entire coast. The sampling period is the wateriest during the year; however, no significant inflow of fresh water was found. The information on the sampling sites’ positions was taken from the published paper [2] and our field observations. The coastal strip is composed of sand dunes and small areas of sandstone. City beaches and recreational areas, visited by tourists all year round, are sandy areas, occasionally crossed by stone embankments created to strengthen the coast (Figure 2). The climate is typical Mediterranean with weak seasonality and water temperatures from 17 °C in winter to 32 °C in summer. To collect samples, we chose the most favorable climatic period [2], when the winter storms are over [27], and water temperature and solar activity increase and stimulate the development of photosynthetics.
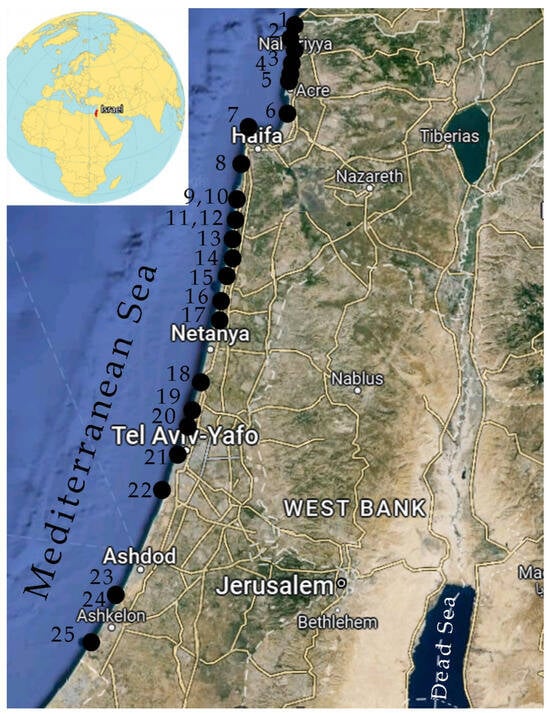
Figure 1.
Map of sampling sites in Israeli coastal waters of the Mediterranean Sea in 2021; black dots are marked with the site numbers as given in Table 1.

Figure 2.
Sampling sites in the Mediterranean coast according to Table 1: (a) 1—Yam Rosh HaNiqra; (b) 2—Yam Kziv; (c) 4—Yam Bustan; (d), 8—Dan Panorama; (e) 9—Yam-Atlit; (f) 10—Yam-Dor Habonim; (g) 16—Yam—Mikhmoret; (h) 21—Bat Yam; (i) 24—Ashkelon north.
The Mediterranean coast of Israel, with 19 sites where R. Enav and A. Israel studied only macrophytes, was supplemented by six more sites for phytobenthos sampling. A total of 25 sites were studied, evenly distributed along the coast (Figure 1 and Figure 2). All sites visited were protected or potentially protected areas (Table 1).

Table 1.
Sampling sites in the Israel coastline with GPS coordinates, abbreviation, and sampling date. P—Protected; PP—Potentially protected.
2.2. Sampling and Material Processing
In the present study, samples were collected from natural protected or potentially protected areas along the Mediterranean coast of Israel from Rosh HaNiqra to Ashkelon (Table 1), of which 19 points replicate macroalgae collection sites [2].
The study was carried out at 25 stations of the Israel coastal waters of the Mediterranean Sea from 6 March to 6 May 2021 at a depth of 0.3–0.5 m at the coordinates provided in Table 1. The collection of algae and water samples was carried out in parallel with the measurement of environmental variables. The temperature, conductivity, and pH of water were measured at the sampling time using a HANNA HI 9813 Waterproof Portable pH/Temperature meter, HANNA Instruments (Hanna Instruments Ltd., Leighton Buzzard, Bedfordshire UK). Water samples for the nitrate nitrogen analysis were collected at the sampling site in 50 mL plastic tubes and transported to the lab in an icebox. The nitrate nitrogen concentration was measured using a HANNA HI 93,728 and HANNA kits according to a spectrophotometric method in the Laboratory of Algae Biodiversity and Ecology, Institute of Evolution, University of Haifa (Israel). The water salinity was measured in psu with a salinity refractometer, Seawater Salinity Meter (Ade Advanced Optics, Oregon City, OR, USA). The GPS coordinates of sampling sites were determined with a GARNMIN GISMAP 64 (Garmin International Inc, Kansas City, Olathe, KS, USA).
Overall, 50 samples of green, brown, and red macrophytes and 25 water samples were collected in each mentioned site, placed in 50 mL plastic tubes, fixed in 4% neutral formaldehyde, and aliquots of the same samples were not fixed for direct live algae study and moved to the Institute of Evolution, University of Haifa in an ice box. Each sample received its unique number and was stored in the Collection of Institute of Evolution for later studies. Metadata associated with each sample were inserted into our database in Microsoft Access. The first direct microscopic examination was performed on fresh samples in the Laboratory of Algae Biodiversity and Ecology, Institute of Evolution, University of Haifa (Israel). For each collected sample, the diatoms present were documented by microphotograph, while the macroalgae were measured and photographed using an OMAX 3D-500 digital camera (OMAX Manufacturer, Kent, WA, USA) and a Leica DM2500 (Leica Microsystems EMEA, Milton Keynes, UK) light microscope under 400–1000x magnification and photographed by an Omax 9.0 MP USB Digital Camera (OMAX Manufacturer, Kent, WA, USA).
The samples were then dried and sent by mail to the co-authors at the Department of Aquaculture and Marine Pharmacology Kovalevsky Institute of Biology of Southern Seas of the Russian Academy of Sciences for study of species composition of microalgae fouling the different macrophytes and for scanning electron microscope (SEM) definition. Dried algae samples were placed in Petri dishes which were then filled with distilled water. After soaking of the macrophyte fragments, they were preliminarily examined under a light microscope to assess their colonization with diatoms. Then, microalgae were washed off the macrophytes with a scraper, and the suspension was placed in glass containers for further processing of these samples. Further, the resulting washings were poured into containers, thoroughly mixed, and aqueous preparations of microalgae were used for observation in light microscopes (LM: Axioskop 40 C. Zeiss with software AxioVisionRel. 4.6, Jena, Germany and an Olympus BX53F, Tokyo, Japan) at magnifications of 10 × 40, 10 × 100 (with Olympus immersion oil of n = 1.518). To analyze the fine structures of diatom valves and frustules, their preparations were studied under a scanning electron microscope (SEM: Hitachi SU3500, Tokyo, Japan). Samples for analysis were prepared mainly according to the methods given in [28] with our modification [29]. In addition, permanent slides were prepared according to the method indicated in [30], which were examined with the immersion oil indicated above. Species were identified whenever possible, down to the genus or species level, based on established morphological features using literary sources, including handbooks and an atlas [31,32,33,34,35,36,37,38,39,40,41,42,43,44]. The ecological and phytogeographic characteristics of diatoms were determined according to [7,19,44,45].
In addition to qualitative samples of diatom processing, a semi-quantitative analysis was also made. The occurrence of diatoms was assessed visually according to the following criteria of the number of cells in the preparation [18,46] (Table 2). Each sample was counted in three replicates.
Anthropogenic pollution load was determined using the description of Einav, Israel, 2008 [2], confirmed and scored by us (Table 3).
The BioDiversity Pro 2.0 program was used for similarity calculation [47]. The Pearson correlation tree was constructed using [48].

Table 2.
Semi-quantitative scores for assessment of species abundance in communities [46] adopted by Barinova, 2017 [49].
Table 2.
Semi-quantitative scores for assessment of species abundance in communities [46] adopted by Barinova, 2017 [49].
| Abundance | Score | Cell Number of Each Species, % | Cell Numbers of Periphyton per Slide (20 × 20 mm) |
|---|---|---|---|
| Occasional | 1 | >1 | 1–10 cells per slide |
| Rare | 2 | 2–10 | 10–20 cells per slide |
| Common | 3 | 10–40 | 20–30 cells per slide |
| Frequent | 4 | 40–60 | 1 cell over a slide transect |
| Very frequent | 5 | 60–80 | Several cells over a slide transect |
| Abundant | 6 | 100 | One or more cells in each field of view ×40 |

Table 3.
Anthropogenic pollution load according to [2], scored by us.
Table 3.
Anthropogenic pollution load according to [2], scored by us.
| Score, Our | Characteristic of Pollution Anthropogenic Load According Einav, Israel, 2008 [2] |
|---|---|
| 1 | Low |
| 2 | Low-to-middle |
| 3 | Good |
| 4 | Polluted–improved |
| 5 | Polluted |
| 6 | High polluted |
The index of saprobity S was calculated for each algal community according to V. Sládeček [16] as a function of the number of saprobic species and their relative abundances and species-specific saprobity index using Equation (1):
where S is the index of saprobity for the algal community (unitless), s is the species-specific saprobity index, and n is the cell density of each species in the sample.
Categorical groups according to Sládeček indicate the degree of self-purification of waters and correspond to species-specific indices of saprobity, which were used to calculate the number of species representing water quality classes and to deduce the degree of self-purification of waters.
Bioindicator analysis was performed with species-specific ecological preferences of the diatom community found at each sampling station to reveal the influence of external factors such as temperature, salinity, pH, and organic pollution level on the collected community [49], according to the bioindicator methods given in [17].
We also calculated the WESI index [44,49,50] to assess the toxic pollution influence on the aquatic ecosystems using Equation (2):
WESI = Rank Index S/Rank N-NO3
The index values vary from 0 to 5. If the index values are less than one, then the ecosystem is exposed to toxic pollution, which inhibits photosynthesis.
3. Results
3.1. Environmental Variables
In our sampling sites of the Israeli coast, the values of salinity ranged from 39 psu to 41 psu during the study period, whereas salinity in the Mediterranean Sea increased from west to east from 36 to 39.5 psu. The water temperature was the same 18.6 °C during the sampling period at all sites, while pH fluctuated from 7.1 to 8.0 depending on the place of study, and nitrate N-NO3 ranged from 0–3.9 mg/L (Table 4).

Table 4.
Averaged environmental variables measured at the sampling sites on the Israeli coastline in 2021.
Our screening analysis of water quality parameters at 25 sites along the Mediterranean coast from north to south showed (Figure 3) that the amount of nitric nitrogen is highest at the northern sites and decreases towards the southern ones as confirmed by the trend line. Water pH is the lowest in the densely populated coastal areas from Haifa to Bat Yam. Electrical conductivity decreases from north to south, repeating in general the distribution of nitrate nitrogen. Salinity varies within small limits along the entire coastline with minimal values approximately at sites located near river mouths. It can be seen that even though the variable values fluctuated from site to site, the trend lines’ directions are the same.
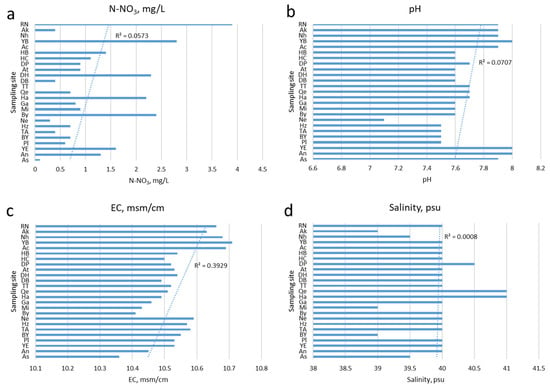
Figure 3.
Distribution of environmental variables values defined in April–May 2021 at the sites of the coastline located from north to south with the linear trend lines: (a) Nitrate-nitrogen; (b) Water pH; (c) Electrical conductivity; (d) Water salinity.
3.2. Species Richness, Ecology, and Distribution of Diatoms
Altogether, 85 species of pennate diatoms were found in the green, brown, and red algae epiphyton in the Israeli coastal waters of the Mediterranean Sea (Table 5, Appendix A, Table A1, Figure A1, Figure A2, Figure A3 and Figure A4), representing three classes, 17 orders, 26 families, and 41 genera. Of these, 94% were benthic forms. Among macroalgae in the Israeli coastal waters, green algae were predominant; others were found in samples fragmentarily. The autecology of revealed species is shown in Table 5. Most diatom species-indicators of the water salinity were affiliated to the group of marine species (53%), and 26% were brackish-marine. The species-indicators of organic pollution with saprobity index s show the domination of the β-mesosaprobic taxa which were also found. In terms of phytogeographical distribution, cosmopolitan species were predominant among the detected diatoms and 21% were arctic-boreal-tropical (Table 5).

Table 5.
Species composition, ecological and phytogeographical characteristics of epiphyton diatoms on the Israeli coastline of the Mediterranean Sea in 2021.
Table 6 indicates that green algae macrophytes were the most common substrate for diatoms, hosting the highest number of species. Diatom species richness fluctuated from one in Bet Yanai (site 17) to 27 in Hadera Tal Afar (site 14) with the dominant species at each site listed in Table 7. Two sites (7 and 8) in Haifa Bat Galim Casino and Dan Panorama were also species-rich, with 25 diatom species each. It should be noted that the number of diatom species in macrophyte epiphytic communities correlated with abundance scores (R = 0.778, p = 0.00001). We compared the dynamics of both these parameters with the intensity of the anthropogenic load and the organic pollution index S calculated by us. The results are visible in Figure 4, where large correlations can be seen between species richness and abundance scores, but not with the saprobity index. However, visually the saprobity index often fluctuates similarly. At the same time, species richness does not have a significant correlation with measured environmental variables (Appendix A, Table A2).

Table 6.
Sampled substrates, scores of anthropogenic loads (according to Table 3), species richness and abundance scores of diatoms at the sampling sites on the Israeli coastline in 2021. Note: “-“, no data.

Table 7.
The dominant species according to the abundance scores of diatoms at the sampling sites on the Israeli coastline in 2021.
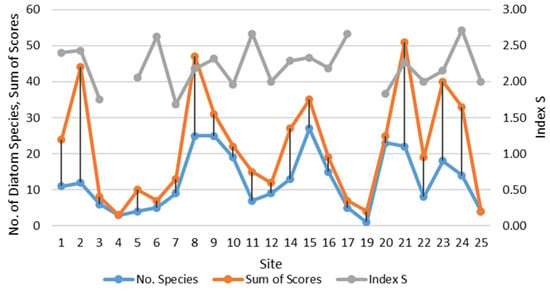
Figure 4.
Dynamics of diatom species number, sum of abundance scores in community and saprobity index S at the sampling sites on the Israeli coastline in 2021.
The contribution of diatom species richness in known algae communities in the sites of the Israeli Mediterranean coast can be seen in Figure 5 where the long-term studied Rhodophyta algae had the largest number of species in the vast majority of the sites in comparison to the diatom species richness the studies of which have just started. In the same histogram, the fluctuation of saprobity index S and anthropogenic load scores can be seen. Visually, both variables are correlated and demonstrate the tendency to increase from North to South. It is remarkable that the saprobity index S was higher near the harbors on the coast up to 3.0 at the sites Haifa, Mikhmoret, and Palmachim where the macrophyte species number also increased.
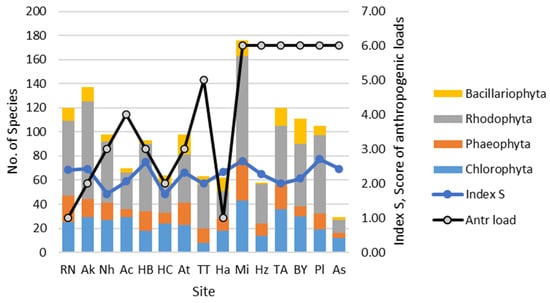
Figure 5.
Dynamics of macrophytes from [2] and diatom species numbers and saprobity index S at the sampling sites on the Israeli coastline in 2021.
The WESI index, which reflects photosynthesis stress (Figure 6), was indicated as “below normal” in only a few sites; these sites were Rosh HaNiqra, Haifa Bat Galim Casino, Yam—Dor Habonim, Hadera, and Yam Evtah, most of which are located near harbors or touristic attractions. All these sites contain low species numbers and abundance, which might suggest that some toxic substances were present and stressed the diatom community. One of these substances may be an oil spill that occurred during the sampling period. The influence of oil as the main impact factor is supported by the fact that suppression of diatom communities was observed even at the Rosh HaNiqra site, where there is no anthropogenic impact, but we noted clots of oil on the coastal rocks.
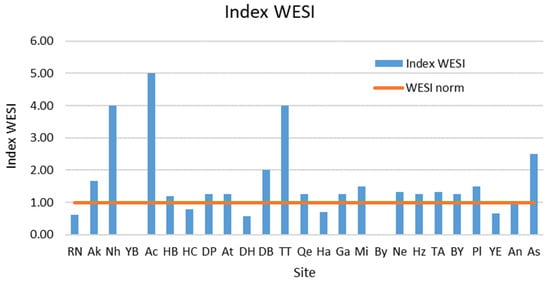
Figure 6.
Dynamics of the WESI index at the sampling sites on the Israeli coastline in 2021.
The tree of similarity of the species composition of diatoms in epiphyton communities is shown in Figure 7 and represents a certain continuum, which indicates the evenness of the conditions in which diatom communities are formed.
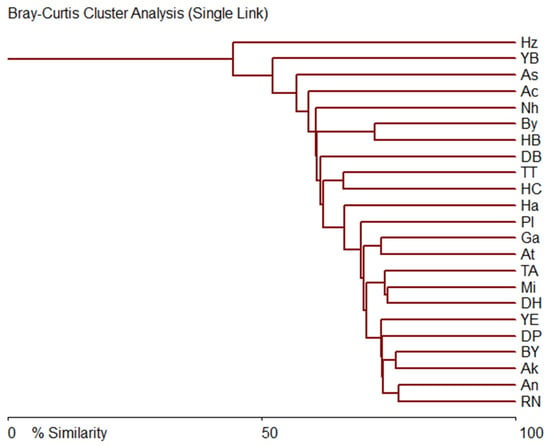
Figure 7.
Bray–Curtis similarity tree of diatom species comparison in communities on the sampling sites in the Israel coastline in 2021.
At the same time, if we include the number of species, abundance scores (Appendix A, Table A1) and chemical indicators in the analysis, then in Figure 8 we can see two main clusters, the defining indicator of which is species richness and abundance, and two sites, Mikhmoret (harbor) and Ashkelon (harbor), remain unlike the rest of the sites.
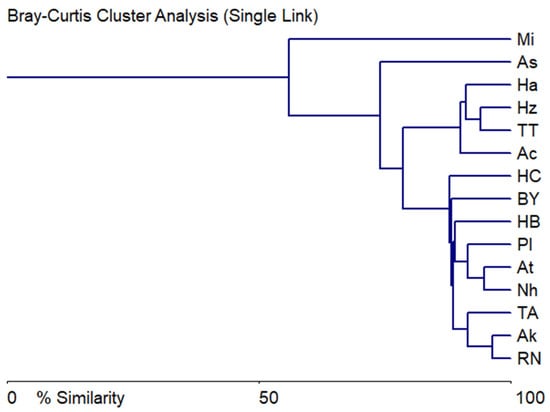
Figure 8.
Bray–Curtis similarity tree of diatom communities’ abundance scores, saprobity index S, and chemical variables comparison at the sampling sites on the Israeli coastline in 2021.
4. Discussion
The study of diatoms of the macrophyte epiphyton of the Israeli Mediterranean coast not only expands our knowledge of the Mediterranean Sea flora, but also clearly demonstrates the possibility of using microalgae for water quality indication. This is especially true for benthic species leading a substrate-attached lifestyle. As a main result of our research, the influence of the anthropogenic loads of the coastal water areas can be defined as a major factor that stimulated diatom species richness.
Comparison of our data with the literature obtained for other regions of the Mediterranean basin showed some similarity between diatom floras. Despite the different ecological conditions of the studied Mediterranean basin, a general trend in the development of the diatom flora of microphytobenthos has been revealed. During a cruise on the R/V «Akademik Kovalevsky» on the thalli green alga Bryopsis plumosa (Huds.) C. Agardh from Rhodes Island of the Aegean Sea and Port Said of the Eastern Mediterranean were processed [6,7,8]. On the banks of the Aegean Sea, Licmophora (17 species) penetrate to a considerable depth; in particular, we noted species of Licmophora abbreviata, Cocconeis scutellum, and Tabularia tabulata at 97 m [6]. There are some common species of diatoms from Israeli shallow waters and about Rhodes; these are Licmophora abbreviata, Achnanthes brevipes, Pleurosigma elongatum, as well as different species of the genera Synedrosphenia, Amphora, etc. [8]. The salinity of the water, close to that of the Aegean Sea, reached 37 psu, but its transparency varied, due to the high turbidity caused by the presence of suspension from the flow of the Nile River.
In the coastal sediments of the Mediterranean Sea near Port Said and in the northern part of the Gulf of Suez at depths from 0.3 to 16 m, A. Zalat found a rather rich and diverse diatom flora (167 and 119 taxa, respectively) [9]. When comparing the species composition of diatom benthos in deep and shallow water areas of the Egypt coast, 20 common species were noted. The difference in the composition and number of species can be explained by different habitats (season, depth, type of substrate, etc.) and sampling methods. Diatom species Achnanthes brevipes and Licmophora abbreviata, found on the Israeli coastline, were also indicated in the sea near Port Said as dominants [9]. A. Zalat identified 11 diatom assemblages which characterize Mediterranean samples and contain a high abundance of polyhalobous taxa: Petroneis monilifera, Rhaphoneis rhombica, R. amphiceros, Cymatosira lorenziana, Gyrosigma strigile, Biddulphia alternans, Podosira stelligera along with a frequent occurrence of Amphora montana, Eupodiscus radiatus, Licmophora abbreviata, L. ehrenbergii, Mastogloia pumila, Synedrosphenia crystallina. The mesohalobous species Campylodiscus echeneis and Achnanthes brevipes were also common in the Mediterranean sediments. In [6,7], 109 epiphyton diatom taxa were found in the Aegean Sea and 46 in the Egyptian sector of the Mediterranean Sea with 31% similarity in species composition. In addition, in these water areas diatoms were studied on the green algae Caulerpa prolifera (Forsk.) Lamour and Codium bursa (L.) C. Agardh. Altogether, 15 species of diatoms were found, of which six species (five genera) were very rarely deposited on the thalli of C. prolifera and C. bursa. Comparison of the epiphyton diatom species composition of these water areas with our data revealed 42 common species. The coefficient of floristic similarity, or Chekanovsky–Sørensen commonality, of species from the Black and Aegean seas, taking into account our own and the literature data, was 64% [6,7]. In addition, among the species we found, as well as in the Aegean Sea and in the Egyptian sector of the Mediterranean Sea, raphid diatoms predominate in epiphyton communities during colonization of substrates compared to araphid species of pennate diatoms, as indicators of their survival in the sea, and their mobility is necessary to achieve optimal lighting conditions during the sexual process [51]. The list of previous investigation of diatoms in the coastal waters of Tartus [10] contain 17 species that are common with our list of 85 species (Table 5); nevertheless, the total numbers of revealed diatoms were comparable (61 and 85) and therefore the total list of the Eastern Mediterranean diatoms now contains 148 species. Comparison of both species lists’ autecology shows that Tartus species demonstrated a eutrophic water condition whereas the Israeli coastal species were indicators of oligotrophic or slightly mesotrophic environments.
5. Conclusions
Altogether, 85 species of benthic diatoms were found in 25 samples of the epiphyton of green, brown, and red macroalgae from Israeli coastal waters in March–May 2021 at salinity from 39 psu to 41 psu, at a depth of 0.3–0.5 m, and at a temperature of 18.6 °C. The taxa of diatoms belonged to three classes, 17 orders, 26 families, and 41 genera. Of these, 94% are benthic forms. The green algae substrates prevailed from all macrophytes; others were found in samples fragmentarily. Altogether, 53% were marine and 26% brackish-marine species. The saprobity index S shows the domination of β-mesosaprobic taxa. In terms of phytogeography, diatoms with a wide distribution prevailed: cosmopolites—30%, arctic-boreal-tropical species—21%. The high species similarity of communities with significant differences in saprobity indicator S indicates and confirms the need for a comprehensive assessment of water quality, taking into account regional characteristics of the properties of species. Perhaps for the same reason, we observed a lack of correlation between species richness, saprobity index, WESI, anthropogenic loads, and environmental variables. Therefore, to solve these problems, it is necessary to create checklists of species depending on salinity, water depth, temperature, and the type of substrate. Thus, the presented results confirm the need for an integrated approach for a more objective assessment of the state of marine ecosystems. Hence, a checklist of diatoms taking into account species composition, ecology, and phytogeography is important for creating a database. As was mentioned in the paper of R. Einav and A. Israel ([2], page 145), “We hope the current database for Israeli seaweeds will serve as an incentive for future environmental and conservation studies on the biodiversity of the marine environment in general and seaweeds in particular” and we agree. As a result of our investigations, the regional list of the marine diatom species in the Eastern Mediterranean was enriched sufficiently and contains 148 taxa. We hope that the Israeli marine macroalgae database, enriched with data on diatom diversity, will stimulate future ecological and conservation research on marine biodiversity in general and seaweeds in particular.
Author Contributions
Conceptualization, S.B. and L.R.; methodology, S.B. and L.R.; software, S.B.; validation, S.B., L.R., A.B., A.S. and D.B.; formal analysis, S.B., L.R., D.B., A.B. and A.S.; investigation, S.B. and E.C.; resources, S.B.; data curation, S.B., L.R., D.B. and A.S.; writing—original draft preparation, S.B., L.R., D.B., A.B. and A.S.; writing—review and editing, S.B., L.R., D.B., A.B. and A.S.; visualization, S.B.; supervision, S.B.; project administration, S.B.; funding acquisition, S.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received financial support within the governmental research assignments № 124022400152-1 “Comprehensive study of mechanism functioning of marine biotechnological complexes for the purpose of obtaining biologically active substances from hydrobionts”. We are grateful to the Leading Engineer of IBSS RAS of V.N. Lishaev for his help in preparing electronic photographs. This work was partly support by the Israeli Ministry of Aliyah and Integration.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A

Table A1.
Abundance scores of diatom species revealed at the sampling sites on the Israeli coastline in 2021. “-”, no data.
Table A1.
Abundance scores of diatom species revealed at the sampling sites on the Israeli coastline in 2021. “-”, no data.
| No. | Taxa | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 14 | 15 | 16 | 17 | 19 | 20 | 21 | 22 | 23 | 24 | 25 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Achnanthes brevipes C.A. Agardh 1824 | 4 | 4 | 1 | - | 6 | - | - | - | - | 1 | - | - | 2 | 1 | - | - | - | 1 | 2 | - | - | 1 | - |
| 2 | Achnanthes sp. | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 2 | - | - | - | - | 1 |
| 3 | Amphora bigibba Grunow 1875 | - | - | - | - | - | - | - | - | - | - | - | 1 | - | - | - | - | - | 1 | - | - | - | - | - |
| 4 | Amphora graeffeana Hendey 1973 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 1 | - | - | - | - | - |
| 5 | Amphora jostesorum Witkowski, Lange-Bertalot & Metzeltin 2000 | - | - | - | - | - | - | - | - | 1 | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 6 | Amphora marina W. Smith 1856 | - | - | - | - | - | - | 1 | - | 1 | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 7 | Amphora ovalis (Kützing) Kützing 1844 | - | - | 1 | - | - | - | 1 | - | - | 1 | - | - | - | - | 1 | - | - | - | 3 | - | - | - | - |
| 8 | Amphora proteus Gregory 1857 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 1 | - | - |
| 9 | Berkeleya rutilans (Trentepohl ex Roth) Grunow 1880 | - | - | 1 | - | - | - | 1 | - | - | 1 | - | - | - | 1 | - | - | - | - | - | - | 1 | - | - |
| 10 | Caloneis formosa var. densestriata Proschkina-Lavrenko 1963 | - | - | - | - | - | - | - | - | 1 | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 11 | Caloneis liber (W. Smith) P.T. Cleve 1894 | - | - | - | - | - | - | - | 1 | 1 | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 12 | Carinasigma rectum (Donkin) G. Reid 2012 | - | - | - | - | - | - | - | - | - | - | - | - | 1 | - | - | - | - | - | 1 | - | - | - | - |
| 13 | Climacosphenia elongata Bailey 1854 | - | - | - | - | - | - | - | 1 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 14 | Cocconeis costata W. Gregory 1855 | - | - | - | - | - | - | - | - | - | 1 | - | - | - | 1 | - | - | - | 1 | 1 | - | - | - | - |
| 15 | Cocconeis distans W. Gregory 1855 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 1 | - | - | - | - |
| 16 | Cocconeis pinnata W. Gregory ex Greville 1859 | - | - | - | - | - | 1 | - | - | - | 1 | - | - | - | 1 | - | - | - | - | - | - | - | - | - |
| 17 | Cocconeis placentula Ehrenberg 1838 | - | - | - | - | - | - | - | - | 1 | 1 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 18 | Cocconeis scutellum Ehrenberg 1838 | 1 | 6 | - | - | - | - | 3 | 3 | - | 1 | 1 | - | - | 3 | 2 | - | - | 2 | 6 | 1 | 6 | - | 1 |
| 19 | Cocconeis speciosa W. Gregory 1855 | - | - | - | - | - | - | - | - | - | 1 | - | - | - | - | - | - | - | 1 | 1 | - | 1 | - | - |
| 20 | Cocconeis sp. | - | - | - | - | - | - | - | 5 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 21 | Coscinodiscus granii L.F. Gough 1905 | - | - | - | - | - | - | 1 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 22 | Cyclotella stylorum Brightwell 1860 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 1 | - | - | - | - | - | - | - | - |
| 23 | Delphineis minutissima (Hustedt) Simonsen 1987 | - | - | - | - | - | - | - | - | - | - | - | - | 1 | - | - | - | - | 1 | - | - | - | - | - |
| 24 | Diploneis smithii (Brébisson) P.T. Cleve 1894 var. smithii | - | - | - | - | - | - | - | - | 1 | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 25 | Diploneis smithii var. pumila (Grunow) Hustedt 1937 | - | - | - | - | - | - | - | 2 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 26 | Diploneis splendida P.T. Cleve 1894 | - | - | - | - | - | - | - | 1 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 27 | Encyonema perpusillum (A. Cleve-Euler) D.G. Mann 1990 | 1 | 1 | - | - | - | - | - | 2 | - | - | - | - | 1 | - | 2 | - | - | - | 3 | - | - | 1 | - |
| 28 | Entomoneis paludosa (W. Smith) Reimer 1975 | - | - | - | - | - | - | - | 1 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 29 | Falcula media var. subsalina Proschkina-Lavrenko 1963 | - | - | - | - | - | - | - | - | 1 | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 30 | Fallacia forcipata (Greville) A.J. Stickle et D.G. Mann 1990 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 1 | - | - | - | - |
| 31 | Gomphonemopsis pseudexigua (Simonsen) Medlin 1986 | - | - | - | 1 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 32 | Grammatophora marina (Lyngbye) Kützing 1844 | 2 | 4 | 3 | - | - | - | - | 1 | 1 | 1 | - | 1 | - | 2 | 2 | - | - | - | 4 | 5 | - | 2 | 1 |
| 33 | Halamphora acutiuscula (Kützing) Z. Levkov 2009 | - | - | - | - | - | - | - | - | - | 1 | - | - | - | - | 1 | 1 | - | - | - | - | - | - | - |
| 34 | Halamphora coffeiformis (C.A. Agardh) Levkov 2009 | - | 4 | - | - | - | - | - | 3 | - | 1 | 1 | - | 1 | 2 | 1 | 2 | - | - | 3 | - | 1 | - | - |
| 35 | Halamphora hyalina (Kützing) Rimet et R. Jahn 2018 | - | - | - | - | - | - | 3 | 2 | 1 | - | - | - | - | - | - | - | - | 1 | - | - | - | - | - |
| 36 | Halamphora tenerrima (Aleem et Hustedt) Z. Levkov 2009 | - | - | - | - | - | - | - | - | 1 | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 37 | Hantzschia sp. | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 2 | - | - |
| 38 | Haslea ostrearia (Gaillon) Simonsen 1974 | - | - | - | - | - | - | - | - | 1 | - | - | - | - | 1 | - | - | - | - | - | - | 5 | - | - |
| 39 | Haslea subagnita (Proschkina-Lavrenko) Makarova et Karayeva 1985 | - | - | 1 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 40 | Hyalosira delicatula Kützing 1844 = Striatella delicatula | - | - | - | - | - | - | - | 1 | - | 1 | - | - | - | - | - | - | - | 1 | 4 | 5 | 3 | - | 1 |
| 41 | Licmophora abbreviata C.A. Agardh 1831 | 4 | 3 | - | 1 | 1 | - | - | 5 | 3 | 1 | - | - | - | 1 | - | - | - | 1 | 2 | 3 | 2 | 5 | - |
| 42 | Licmophora flabellata (Greville) C.A. Agardh 1830 | 1 | - | - | - | - | - | - | - | - | - | - | - | - | 1 | - | - | - | - | - | - | - | - | - |
| 43 | Lyrella lyroides (Hendey) D.G. Mann 1990 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 1 | - | - | - | - | - |
| 44 | Nanofrustulum shiloi (J.J. Lee, Reimer et McEnery) Round, Hallsteinsen et Paasche 1999 | - | - | - | - | - | - | - | - | - | - | - | 4 | - | 1 | - | - | 4 | - | - | - | 4 | - | - |
| 45 | Navicula ammophila var. intermedia Grunow 1862 | 3 | 4 | - | - | - | - | - | 2 | 1 | 4 | - | - | 3 | - | 1 | - | - | - | 4 | 2 | 4 | 4 | - |
| 46 | Navicula antonii Lange-Bertalot 2000 | - | - | - | - | - | - | - | - | - | - | - | 1 | - | - | 1 | 1 | - | 1 | - | - | - | - | - |
| 47 | Navicula cancellata Donkin 1872 | - | - | - | - | - | - | - | - | - | - | 1 | - | - | - | - | - | - | - | - | - | - | 1 | - |
| 48 | Navicula dumontiae Baardseth et Taasen 1973 | - | - | - | - | - | - | - | - | - | - | - | - | - | 1 | - | - | - | - | - | - | 1 | - | - |
| 49 | Navicula pennata A. Schmidt 1876 | - | - | - | - | - | - | - | 1 | 1 | - | - | 1 | - | 1 | - | - | - | 1 | - | 1 | - | - | - |
| 50 | Navicula perminuta Grunow 1880 | - | - | - | 1 | - | 1 | - | - | - | 1 | 1 | 1 | 1 | 1 | 1 | - | - | 1 | - | - | - | - | - |
| 51 | Navicula ramosissima (Agardh) P.T. Cleve 1895 | 2 | - | - | - | - | 1 | - | - | 1 | 1 | - | 1 | - | 2 | 2 | 1 | - | 1 | - | - | - | 3 | - |
| 52 | Navicula sp. | - | - | - | - | - | - | - | - | 1 | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 53 | Nitzschia distans W. Gregory 1857 | - | - | - | - | - | - | - | - | 1 | - | - | - | - | - | - | - | - | - | 1 | - | - | - | - |
| 54 | Nitzschia frustulum (Kützing) Grunow in P.T. Cleve et Grunow 1880 | - | 3 | - | - | - | - | - | 1 | - | 1 | - | - | - | - | - | - | - | 1 | - | - | 1 | - | - |
| 55 | Nitzschia hybrida Grunow 1880 | - | - | - | - | - | - | - | - | - | - | - | - | 1 | - | - | - | - | - | - | - | - | 1 | - |
| 56 | Nitzschia inconspicua Grunow 1862 | - | - | - | - | - | - | - | - | - | - | - | - | 1 | 1 | - | - | - | - | - | - | - | - | - |
| 57 | Nitzschia lanceolata W. Smith 1853 | - | 4 | - | - | 2 | - | - | 2 | - | - | - | - | 3 | - | - | - | - | - | - | - | - | - | - |
| 58 | Nitzschia ovalis H.J. Arnott 1880 | - | - | - | - | - | - | - | - | - | - | - | - | - | 1 | - | - | - | 1 | - | - | - | - | - |
| 59 | Nitzschia scalpelliformis (Grunow) Grunow 1880 | - | - | - | - | - | - | - | - | - | - | - | - | 6 | - | - | - | - | - | 2 | - | - | - | - |
| 60 | Nitzschia sigma (Kützing) W. Smith 1853 | - | - | - | - | - | 1 | 1 | - | 2 | - | - | - | 5 | 1 | - | - | - | 1 | 1 | - | 1 | 1 | - |
| 61 | Nitzschia spathulata Brébisson 1853 | - | - | - | - | - | - | - | 1 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 62 | Parlibellus delognei (Van Heurck) E.J. Cox 1988 | - | - | - | - | - | - | - | 1 | - | - | - | - | - | - | 1 | - | - | 1 | - | - | - | 1 | - |
| 63 | Platessa saxonica (Krasske ex Hustedt) C.E. Wetzel, Lange-Bertalot et Ector 2017 | - | - | - | - | - | - | - | - | - | - | - | - | - | 1 | - | - | - | - | - | - | - | - | - |
| 64 | Pleurosigma aestuarii (Brébisson ex Kützing) W. Smith 1853 | - | - | - | - | - | - | - | - | 1 | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 65 | Pleurosigma angulatum (Queckett) W. Smith 1853 | - | - | - | - | - | - | - | - | 1 | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 66 | Pleurosigma elongatum W. Smith 1852 | - | - | - | - | - | - | - | 1 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 67 | Pleurosigma inflatum Shadbolt 1853 | - | - | - | - | - | - | - | - | 1 | - | - | - | - | - | - | - | - | - | 1 | - | - | - | - |
| 68 | Pleurosigma salinarum (Grunow) Grunow 1880 | - | - | - | - | - | - | 1 | 1 | - | - | - | - | - | 1 | - | - | - | - | - | - | - | - | - |
| 69 | Podosira hormoides (Montagne) Kutzing 1844 | - | - | - | - | - | - | - | - | - | - | - | - | - | 1 | - | - | - | - | - | - | - | - | - |
| 70 | Psammodictyon panduriforme var. minor (Grunow) L.I. Ryabushko 2006 | - | - | - | - | - | - | - | - | - | - | - | - | - | 1 | 1 | - | - | 1 | - | - | - | 1 | - |
| 71 | Rhoicosphenia marina (W. Smith) M. Schmidt 1889 | - | 1 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 1 | - | - |
| 72 | Seminavis ventricosa (Gregory) M. Garcia-Baptista 1993 | - | - | - | - | - | - | 1 | - | - | - | - | 1 | 1 | - | - | - | - | - | - | - | - | - | - |
| 73 | Staurosirella martyi (Héribaud) E.A. Morales & K.M. Manoylov 2006 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 1 | - | - | - |
| 74 | Striatella unipunctata (Lyngbye) C.A. Agardh 1832 | - | - | - | - | - | - | - | - | 1 | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 75 | Striatella sp. | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 1 | - | - | - | - | - |
| 76 | Synedrosphenia crystallina (C. Agardh) Lobban & Ashworth 2022 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 1 | - | 1 | - | - |
| 77 | Tabularia affinis (Kützing) Snoeijs 1992 | - | - | - | - | - | - | - | - | - | - | - | - | - | 1 | - | - | - | - | - | - | 1 | - | - |
| 78 | Tabularia fasciculata (C.A. Agardh) Williams et Round 1986 | 2 | 4 | - | - | 1 | 3 | - | 3 | 3 | 1 | 6 | 1 | - | 2 | 1 | - | - | - | 5 | 1 | 4 | 5 | - |
| 79 | Tabularia parva (Kützing) D. Williams et Round 1990 | 3 | 6 | - | - | - | - | - | 4 | 2 | - | 4 | - | - | 3 | 1 | 2 | - | - | 3 | - | - | 6 | - |
| 80 | Tabularia tabulata (C.A. Agardh) Snoijes 1992 | - | - | - | - | - | - | - | - | - | - | 1 | - | - | 1 | - | - | - | - | - | - | - | - | - |
| 81 | Thalassiosira eccentrica (Ehrenberg) P.T. Cleve 1904 | - | - | - | - | - | - | - | 1 | - | - | - | - | - | - | - | - | - | - | - | - | - | 1 | - |
| 82 | Trachyneis aspera (Ehrenberg) P.T. Cleve 1894 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 1 | - | - | - | - |
| 83 | Tryblionella coarctata (Grunow) D.G. Mann 1990 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 1 | - | - | - | - | - |
| 84 | Tryblionella punctata W. Smith 1853 | 1 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 85 | Undatella lineolata (Ehrenberg) L.I. Ryabushko 2006 | - | - | 1 | - | - | - | - | 1 | 1 | 1 | - | - | - | 1 | - | - | - | - | - | - | - | - | - |

Table A2.
The similarity/distance table of the Pearson’s correlation coefficient for species richness and environmental variables at the sampling sites on the Israeli Mediterranean coast, 2021. Significance values are marked, * p < 0.05.
Table A2.
The similarity/distance table of the Pearson’s correlation coefficient for species richness and environmental variables at the sampling sites on the Israeli Mediterranean coast, 2021. Significance values are marked, * p < 0.05.
| Variable | No. of Species | N-NO3, mg L−1 | pH | EC msm cm−1 | Salinity, psu |
|---|---|---|---|---|---|
| No of Species | 0 | 0.09 * | −0.28 * | 0.15 * | −0.13 * |
| N-NO3, mg L−1 | 0.775 | 0.000 | 0.12 * | −0.14 * | 0.23 * |
| pH | 0.359 | 0.702 | 0.000 | 0.43 * | 0.07 * |
| EC sm cm−1 | 0.622 | 0.640 | 0.144 | 0.000 | −0.29 * |
| Salinity, psu | 0.672 | 0.450 | 0.815 | 0.339 | 0 |
Appendix B
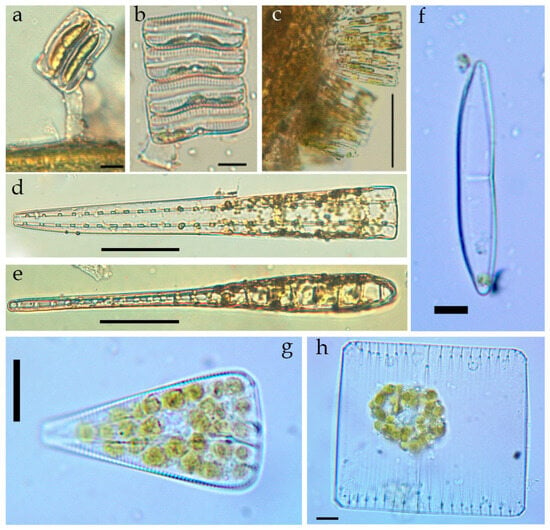
Figure A1.
LM. Live microphotographs of some diatom species in the macrophytes epiphyton of the Israeli sector of the Mediterranean Sea: Achnanthes brevipes (a,b) on the macrophyte thallus (a); colonies of Tabularia parva on the macrophyte thallus (c); Climacosphenia elongata, girdle view (d), valve view (e); Amphora jostesorum (f); Licmophora abbreviata (g); Striatella unipunctata (h). Scale bar: (a–c,f) = 10 µm; (d,e) = 50 µm; (g,h) = 20 µm.
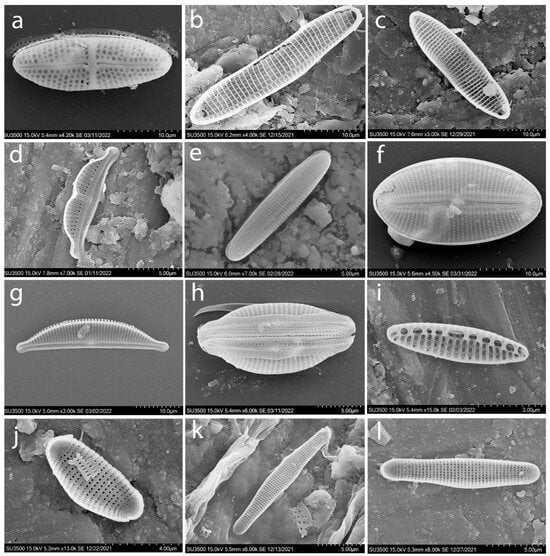
Figure A2.
SEM. Typical species of diatoms found in the macrophytes epiphyton of the Israeli sector of the Mediterranean Sea: Achnanthes brevipes (a–c), Amphora bigibba (d); Berkeleya rutilans (e); Diploneis smithii (f); Halamphora coffeiformis (g,h); Nitzschia inconspicua (i); Hyalosira delicatula (j–l).
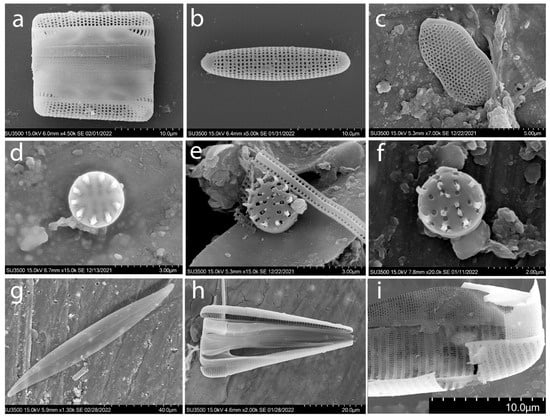
Figure A3.
SEM. Typical species of diatoms found in the macrophytes epiphyton of the Israeli sector of the Mediterranean Sea: Grammatophora marina (a,b); Psammodictyon panduriforme var. minor (c); Nanofrustulum shiloi (d–f); Pleurosigma elongatum (g); Licmophora abbreviata (h); Licmophora abbreviata valve fragment (i).
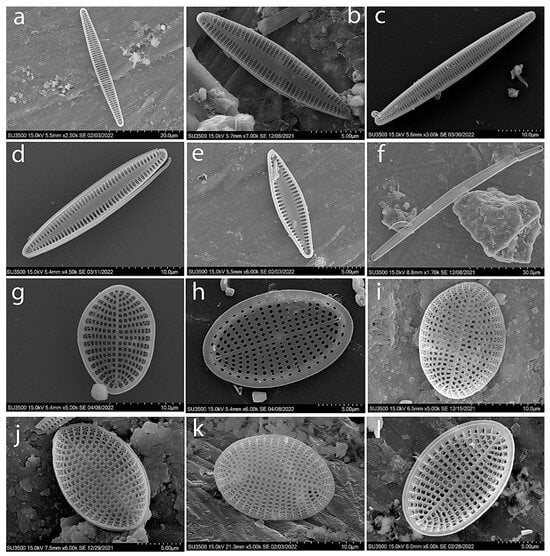
Figure A4.
SEM. Typical species of diatoms found in the macrophytes epiphyton of the Israeli sector of the Mediterranean Sea: Tabularia affinis (a); Tabularia fasciculata (b,c); Tabularia parva (d,e); Tabularia tabulata (f); Cocconeis scutellum (g–l).
References
- Boundouresque, C.F. Rapport bibliographique sui le phytobenthos Mediterraneen (annees 1978 a 1980). Rapp. Proc. Verb. Reun. Commis. Int. Explor. Sci. Mediterr. Monaco 1981, 27, 69–120. (In French) [Google Scholar]
- Einav, R.; Israel, A. Checklist of seaweeds from the Israeli Mediterranean: Taxonomical and ecological approaches. Isr. J. Plant Sci. 2008, 56, 127–184. [Google Scholar] [CrossRef]
- Mazzella, L.; Cinelli, F.; Fresi, E.; Ponticelli, M.P. Ricerche sui popolamenti bentonici di substrato duro del porto di Ischia. Intralitorale fotofilo: II Microflora a diatomee. Giorn. Bot. Ital. 1978, 112, 13–27. [Google Scholar] [CrossRef]
- Munda, I.M. Seasonal fouling by diatoms on artificial substrata at different depths near Piran (Gulf of Trieste, Northern Adriatic). Acta Adriat. 2005, 46, 137–157. [Google Scholar]
- Pujadas, R.T. Ecological Analysis of Periphytic Diatoms in Mediterranean Coastal Wetlands (Empordà Wetlands, NE Spain); Universitat de Girona: Girona, Italy, 2005; pp. 1–171. [Google Scholar]
- Ryabushko, L.I. Comparative analysis of composition and spatial distribution of the bottom microalgae of the Aegean Sea and Black Sea. In Proceedings of the Oceanography of the Eastern Mediterranean and Black Sea. Similarities and Differences of Two Interconnected Basins, Athens, Greece, 23–26 February 1999; pp. 33–34. [Google Scholar]
- Ryabushko, L.I. Microphytobenthos of the Black Sea; Gaevskaya, A.V., Ed.; EKOSI-Gidrofizica: Sevastopol, Russia, 2013; 416p. (In Russian) [Google Scholar]
- Ryabushko, L.I.; Bondarenko, A.V.; Shiroyan, A.G. Diatoms of Bryopsis plumosa (Hudson) C. Agardh (Chlorophyta, Bryopsidales) epiphyton from the Black and Aegean seas. Intern. J. Algae 2019, 21, 321–334. [Google Scholar] [CrossRef]
- Zalat, A.A. Distribution and origin of diatoms in the bottom sediments of the Suez canal lakes and adjacent areas, Egypt. Diat. Res. 2002, 17, 243–266. [Google Scholar] [CrossRef]
- Gerasimyuk, V.P. Microphytobenthos of the gulf of Tartous (Mediterranean Syria). Algologia 2017, 27, 313–322. (In Russian) [Google Scholar] [CrossRef]
- Cibic, T.; Blasutto, O.; Falconi, C.; Uman, F. Microphytobenthic biomass, species composition and nutrient availability in sublittoral sediments of the Gulf of Trieste (northern Adriatic Sea). Estuar. Coast. Shelf Sci. 2007, 75, 50–62. [Google Scholar] [CrossRef]
- Cibic, T.; Facca, C. Microphytobenthos. In: Checklist della flora e della fauna dei mari italiani. Parte II. (Ed. Relini G.). Biol. Mar. Mediterr. 2010, 17 (Suppl. 1), 754–800. [Google Scholar]
- Barranguet, C.; Plante-Cuny, M.R.; Alivon, E. Microphytobenthos production in the Gulf of Fos, French Mediterranean coast. Hydrobiologia 1996, 333, 181–193. [Google Scholar] [CrossRef]
- Barranguet, C.; Herman, P.M.J.; Sinke, J.J. Microphytobenthos biomass and community composition studied by pigment biomarkers: Importance and fate in the carbon cycle of a tidal flat. J. Sea Res. 1997, 38, 59–70. [Google Scholar] [CrossRef]
- Barinova, S.; Smith, T. Flora of Algae and Cyanobacteria of Continental Waters of Israel in the XXI Century: Taxonomy, Autecology and Water Quality Indicators. Diversity 2022, 14, 328. [Google Scholar] [CrossRef]
- Sládeček, V. Diatoms as indicators of organic pollution. Acta Hydroch. Hydrobiol. 1986, 14, 555–566. [Google Scholar] [CrossRef]
- Barinova, S. Essential and practical bioindication methods and systems for the water quality assessment. Int. J. Environ. Sci. Nat. Resour. 2017, 2, 555588. [Google Scholar] [CrossRef]
- Barinova, S. How to Align and Unify the Cell Counting of Organisms for Bioindication. Int. J. Environ. Sci. Nat. Resour. 2017, 2, 555585. [Google Scholar] [CrossRef]
- Barinova, S.; Bondarenko, A.; Ryabushko, L.; Kapranov, S. Microphytobenthos as an indicator of water quality and organic pollution in the western coastal zone of the Sea of Azov. Oceanol. Hydrobiol. Stud. 2019, 48, 125–139. [Google Scholar] [CrossRef]
- Miroshnichenko, E.S.; Barinova, S.S.; Ryabushko, L.I. The first records of Cyanobacteria diversity in the benthos of the Israeli coast of the Mediterranean Sea. Bot. Pacifica J. Plant Sci. Conserv. 2022, 11, 159–167. [Google Scholar] [CrossRef]
- Watanabe, T.; Asai, K.; Houki, A.; Tanaka, S.; Hizuka, T. Saprophilous and Eurysaprobic Diatom Taxa to Organic Water Pollution and Diatom Assemblage Index (DAIpo). Diatom Jpn. J. Diatomol. 1986, 2, 23–73. [Google Scholar]
- Watanabe, T.; Asai, K.; Houki, A.; Sumita, M. Numerical simulation of organic pollution based on the attached diatom assemblage in Lake Biwa (1). Diatom Jpn. J. Diatomol. 1990, 5, 9–20. [Google Scholar]
- Ermolaeva, N.I.; Dvurechenskaya, S.Y. Regional indices of the indicator significance of zooplanktonic organisms in water bodies of southern Western Siberia. Russ. J. Ecol. 2013, 44, 527–531. [Google Scholar] [CrossRef]
- Ermolaeva, N.I.; Dvurechenskaya, S.Y. Developing the Regional Indicator Indexes of Zooplankton for Water Quality Class Determination of Water Bodies in Siberia. In Novel Methods for Monitoring and Managing Land and Water Resources in Siberia; Mueller, L., Sheudshen, A., Eulenstein, F., Eds.; Springer Water: Cham, Switzerland, 2016; pp. 157–183. [Google Scholar] [CrossRef]
- Herut, B.; Almogi-Labin, A.; Jannink, N.; Gertman, I. The seasonal dynamics of nutrient and chlorophyll a concentrations on the SE Mediterranean shelf-slope. Oceanol. Acta 2000, 23, 771–782. [Google Scholar] [CrossRef]
- Israel Nature and Parks Authority. Available online: https://en.parks.org.il/article/%D7%A9%D7%9E%D7%95%D7%A8%D7%95%D7%AA-%D7%98%D7%91%D7%A2-%D7%99%D7%9E%D7%99%D7%95%D7%AA/ (accessed on 16 November 2019).
- Spanier, E.; Zviely, D. Key Environmental Impacts along the Mediterranean Coast of Israel in the Last 100 Years. J. Mar. Sci. Eng. 2023, 11, 2. [Google Scholar] [CrossRef]
- Simonsen, R. Untersuchungen zur Systematik und Ökologie der Bodendiatomeen der Westlichcn Ostsee. Int. Rev. Ges. Hydrobiol. Beihefte 1962, 1, 9–144. [Google Scholar]
- Blaginina, A.; Ryabushko, L. Finding of a Rare Species of Diatom Nanofrustulum shiloi (Lee, Reimer et Mcenery) Round, Hallsteinsen et Paasche, 1999 in the Periphyton of the coastal waters of the Black Sea. Intern. J. Algae 2021, 23, 247–256. [Google Scholar] [CrossRef]
- Gleser, S.I.; Jousé, A.P.; Makarova, I.V.; Proschkina-Lavrenko, A.I.; Sheshukova-Poretzkaya, V.S. (Eds.) Diatoms of the USSR (Fossil and Modern); Nauka: Leningrad, Russia, 1974; Volume 1, p. 403. (In Russian) [Google Scholar]
- Smith, W. Synopsis of the British Diatomaceæ: With Remarks on Their Structure, Functions and Distribution; and Instructions for Collecting and Preserving Specimens; Smith and Beck: London, UK, 1853; Volume 1, 89p. [Google Scholar]
- Smith, W. A Synopsis of the British Diatomaceæ: With Remarks on Their Structure, Functions and Distribution; and Instructions for Collecting and Preserving Specimens; Smith and Beck: London, UK, 1856; Volume 2, 107p. [Google Scholar]
- Proschkina-Lavrenko, A.I. (Ed.) Diatom Analysis. The Determinant of Fossil and Contemporary Diatoms. Book 3; Gosizdat of Geological Publishers: Leningrad, Russia, 1950; 398p. (In Russian) [Google Scholar]
- Proshckina-Lavrenko, A.I. Diatoms of the Black Sea Plankton; AS USSR Nauka: Moscow, Russia, 1955; 222p. (In Russian) [Google Scholar]
- Proshckina-Lavrenko, A.I. Diatoms of the Black Sea Benthos; Nauka: Moscow, Russia, 1963; 243p. (In Russian) [Google Scholar]
- Guslyakov, N.E.; Zakordonets, O.A.; Gerasimyuk, V.P. Atlas of Benthic Diatoms North-Western Part of the Black Sea and Adjacent Waters; Nauk Dumka: Kiev, Ukraine, 1992; 112p. (In Russian) [Google Scholar]
- Kuylenstierna, M. Benthic Algal Vegetation in the Nordre Älv Estuary (Swedish West Coast). Ph.D. Thesis, University of Göteborg, Göteborg, Sweden, 1989; 244p. [Google Scholar]
- Kuylenstierna, M. Benthic Algal Vegetation in the Nordre Älv Estuary (Swedish West Coast). Ph.D. Thesis, University of Göteborg, Göteborg, Sweden, 1990; 76p. [Google Scholar]
- Witkowski, A.; Lange-Bertalot, H.; Metzeltin, D. Diatom Flora of Marine Coasts I. Iconongraphia Diatomologica, 7; A.R.G. Gantner Verlag K.G.: Ruggell, Liechtenstein, 2000; 950p. [Google Scholar]
- Toyoda, K.; Cox, E.J.; Sims, P.A.; Williams, D.M. The typification of Achnanthes Bory based on Echinella stipitata Lyngbye, with an account of the morphology and fine structure of Lyngbye’s species. Diatom Res. 2005, 20, 375–386. [Google Scholar] [CrossRef]
- Al-Yamani, F.Y.; Saburova, M.A. Illustrated Guide on the Benthic Diatoms of Kuwait’s Marine Environment; Kuwait Institute for Scientific Research: Safat, Kuwait, 2011; 352p. [Google Scholar]
- Alvarez-Blanco, I.; Blanco, S. Benthic diatoms from Mediterranean coasts. In Bibliotheca Diatomologica; Gebruder Borntraeger Verlagsbuchhandlung: Stuttgart, Germany, 2014; Volume 60, 409p. [Google Scholar]
- Ryabushko, L.I.; Begun, A.A. Diatoms of Microphytobenthos of the Sea of Japan; PK KIA: Sevastopol, Ukraine, 2016; Volume 2, 324p. (In Russian) [Google Scholar]
- Barinova, S.S.; Belous, O.P.; Tsarenko, P.M. Algal Indication of Water Bodies in Ukraine: Methods and Perspectives; Haifa University Publishing House: Kiev, Ukraine, 2019; 367p. (In Russian) [Google Scholar]
- Guiry, M.D.; Guiry, G.M. AlgaeBase. World-wide Electronic Publication, National University of Ireland, Galway. Available online: http://www.algaebase.org (accessed on 2 March 2024).
- Korde, N.V. Methods of biological study of bottom sediments of lakes (field work and biological analysis). In Life of Fresh Waters of the USSR; Publishing House of the Academy of Sciences of the USSR: Moscow, Russia, 1956; Volume 4, pp. 383–413. (In Russian) [Google Scholar]
- McAleece, N.; Gage, J.D.G.; Lambshead, P.J.D.; Paterson, G.L.J. BioDiversity Professional Statistics Analysis Software. Jointly Developed by the Scottish Association for Marine Science and the Natural History Museum London. 1997. Available online: https://www.sams.ac.uk/science/outputs/ (accessed on 2 March 2024).
- Wessa, P. Person Correlation (v1.0.13) in Free Statistics Software (v1.2.1). Office for Research Development and Education. 2017. Available online: https://www.wessa.net/rwasp_correlation.wasp/ (accessed on 2 March 2024).
- Barinova, S. On the classification of water quality from an ecological point of view. Int. J. Environ. Sci. Nat. Resour. 2017, 2, 1–8. [Google Scholar] [CrossRef]
- Barinova, S.S.; Medvedeva, L.A.; Anissimova, O.V. Diversity of Algal Indicators in Environmental Assessment; Pilies Studio: Tel Aviv, Israel, 2006; 495p. (In Russian) [Google Scholar]
- Baumann, W. Verhalten der Diatomeen. Microkosmos 1981, 70, 104–108. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).