Spatial, Temporal, and Interspecific Differences in Composition of Stable Isotopes in Fishes in Maryland Coastal Bays
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sample Collection
2.3. Stable Isotope Analyses
2.4. Data Analyses
3. Results
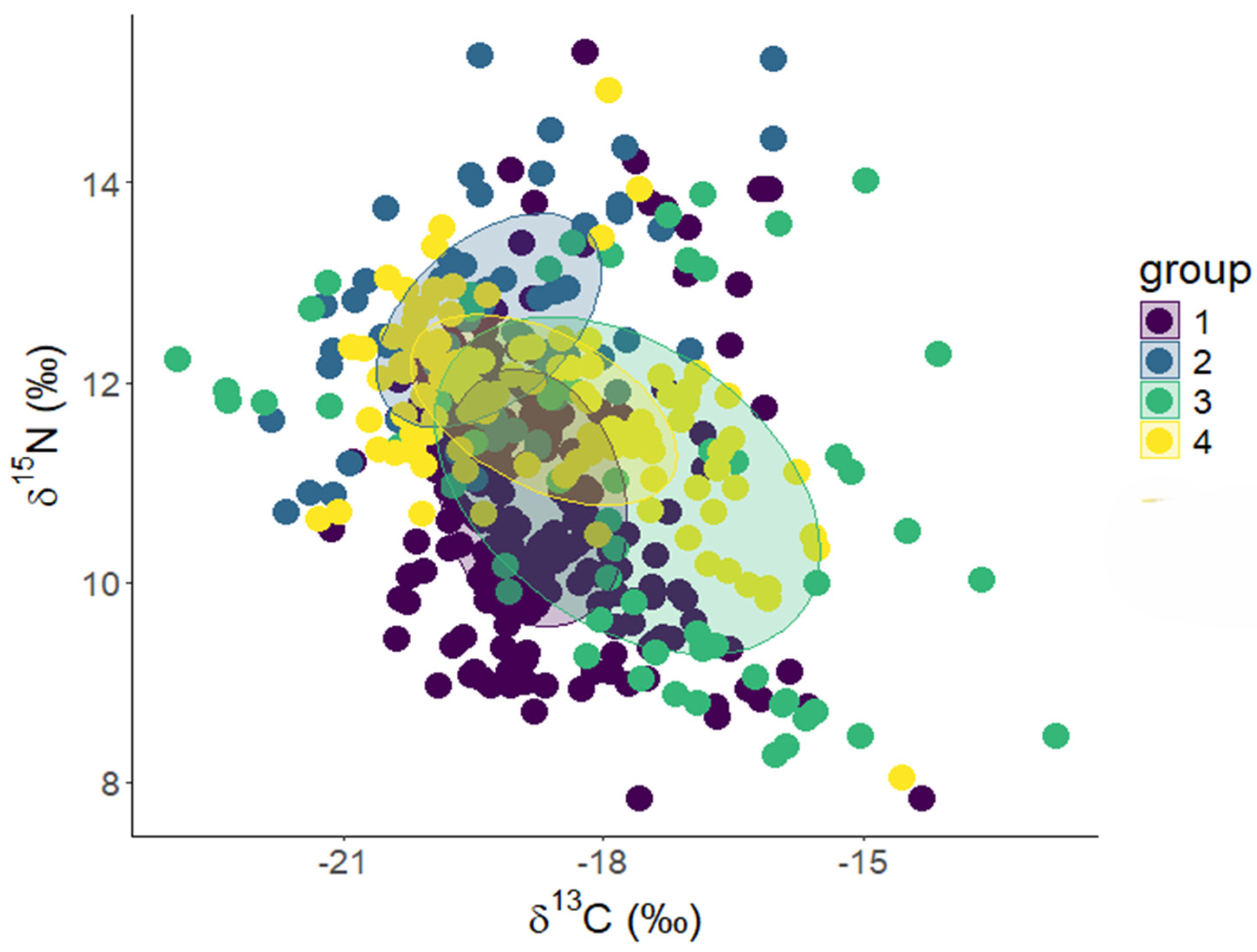
3.1. Interspecific Differences in Isotopic Compositions of the Four Fish Species in MCBs
3.2. Interannual Differences in Isotopic Composition of Each Fish Species in MCBs
3.3. Seasonal Differences in Isotopic Composition of Each Fish Species in MCBs
3.4. Spatial Variation in Stable Isotopes of the Four MCB Fish Species
4. Discussion
4.1. Interspecific Differences in Stable Isotope Composition of the Four MCB Fish Species
4.2. Temporal Variations in Stable Isotope Composition of the Four MCB Fish Species
4.3. Spatial Variations in Stable Isotopes of the Four MCB Fish Species
4.4. Trophic Levels and Niche Overlap of the Four MCB Fish Species
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Layman, C.A.; Araujo, M.S.; Boucek, R.; Hammerschlag-Peyer, C.M.; Harrison, E.; Jud, Z.R.; Matich, P.; Rosenblatt, A.E.; Vaudo, J.J.; Yeager, L.A.; et al. Applying stable isotopes to examine food-web structure: An overview of analytical tools. Biol. Rev. 2012, 87, 545–562. [Google Scholar] [CrossRef] [PubMed]
- Michener, R.H.; Kaufman, L. Stable isotope ratios as tracers in marine food webs: An update. Stable Isot. Ecol. Environ. Sci. 2007, 2, 238–282. [Google Scholar]
- Post, D.M. Using stable isotopes to estimate trophic position: Models, methods, and assumptions. Ecology 2002, 83, 703–718. [Google Scholar] [CrossRef]
- Gannes, L.Z.; Del Rio, C.M.; Koch, P. Natural abundance variations in stable isotopes and their potential uses in animal physiological ecology. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 1998, 119, 725–737. [Google Scholar] [CrossRef] [PubMed]
- Davenport, S.R.; Bax, N.J. A trophic study of a marine ecosystem off southeastern Australia using stable isotopes of carbon and nitrogen. Can. J. Fish. Aquat. Sci. 2002, 59, 514–530. [Google Scholar] [CrossRef]
- Peterson, B.J.; Fry, B. Stable isotopes in ecosystem studies. Annu. Rev. Ecol. Syst. 1987, 18, 293–320. [Google Scholar] [CrossRef]
- Buchheister, A.; Latour, R.J. Trophic ecology of summer flounder in lower Chesapeake Bay inferred from stomach content and stable isotope analyses. Trans. Am. Fish. Soc. 2011, 140, 1240–1254. [Google Scholar] [CrossRef]
- Winemiller, K.O.; Akin, S.; Zeug, S.C. Production sources and food web structure of a temperate tidal estuary: Integration of dietary and stable isotope data. Mar. Ecol. Prog. Ser. 2007, 343, 63–76. [Google Scholar] [CrossRef]
- Black, R.B. Dietary Habits of Selected Species of Fish Resident or Transitory to the MCBs and Their Importance to Understanding Food Webs. Master's Thesis, University of Maryland Eastern Shore, Princess Anne, MD, USA, 2009. [Google Scholar]
- Mayor, E.D. Population dynamics of mysids and their role in the trophic ecology of fishes in Maryland Coastal Bays. Doctoral Dissertation, University of Maryland Eastern Shore, Princess Anne, MD, USA, 2015. [Google Scholar]
- Casey, J.D.; Doctor, S. Status of finfish populations in the Maryland coastal bays. In Maryland’s Coastal Bays: Ecosystem Health Assessment; Document Number DNR-12-1202-0009; Maryland Department of Natural Resources: Annapolis, MD, USA, 2004; pp. 8–34. [Google Scholar]
- Bolinger, A.; Doctor, S.; Luettel, A.; Luisi, M.; Tyler, G. Investigation of Maryland’s Coastal Bays and Atlantic Ocean Finfish Stocks 2007 Report; Document Number F-50-R-30; Maryland Department of Natural Resources: Annapolis, MD, USA, 2007. [Google Scholar]
- Pincin, J.; Wilberg, M.J.; Harris, L.; Willey, A. Trends in abundance indices of fishes in Maryland’s Coastal Bays during 1972–2009. Estuaries Coasts 2014, 37, 791–800. [Google Scholar] [CrossRef]
- Doctor, S.; Tyler, G.; Weedon, C.; Willey, A. Investigation of Maryland’s Coastal Bays and Atlantic Ocean Finfish Stocks Report; Document Number F-50-R-27; Maryland Department of Natural Resources: Annapolis, MD, USA, 2018. [Google Scholar]
- Luo, J. Life History of the Bay Anchovy, Anchoa Mitchilli, in Chesapeake Bay; The College of William and Mary: Williamsburg, VA, USA, 1991. [Google Scholar]
- Chao, L.N.; Musick, J.A. Life-history, feeding-habits, and functional-morphology of juvenile Sciaenid fishes in York River Estuary, Virginia. Fish. Bull. 1977, 75, 657. [Google Scholar]
- Waggy, G.L.; Peterson, M.S.; Comyns, B.H. Feeding habits and mouth morphology of young Silver Perch (Bairdiella chrysoura) from the north-central Gulf of Mexico. Southeast. Nat. 2007, 6, 743–751. [Google Scholar] [CrossRef]
- Hales, L.S.; Van Den Avyle, M.J. Species Profile: Life Histories and Environmental Requirements of Coastal Fishes and Invertebrates (South Atlantic): Spot; U.S. Fish and Wildlife Service Biological Report Biological Report U.S. Army Corps of Engineers TR EL-82-4; Fish and Wildlife Service, US Department of the Interior: Washington, DC, USA, 1989; Volume 82, 24p. [Google Scholar]
- Grimes, B.H. Summer and Winter Flounder; Fish and Wildlife Service, US Department of the Interior: Washington, DC, USA, 1989. [Google Scholar]
- Packer, D.B.; Hoff, T. Life history, habitat parameters, and essential habitat of mid-Atlantic summer flounder. Am. Fish. Soc. Symp. 1999, 22, 76–92. [Google Scholar]
- Rogers, S.G.; Van Den Avyle, M.J. Species Profiles: Life Histories and Environmental Requirements of Coastal Fishes and Invertebrates (South Atlantic): Summer flounder; United States Fish and Wildlife Service FWS/OBS-82/11.15; U.S. Army Corps of Engineers, TR EL-82-4; Fish and Wildlife Service, US Department of the Interior: Washington, DC, USA, 1983; 14p. [Google Scholar]
- NOAA National Centers for Environmental information, Climate at a Glance: Statewide Time Series, Published November 2021. Available online: https://www.ncdc.noaa.gov/cag/ (accessed on 1 December 2021).
- Wazniak, C.; Wells, D.; Hall, M. The Maryland coastal bays ecosystem. In Maryland’s Coastal Bays: Ecosystem Health Assessment; Maryland Department of Natural Resources: Annapolis, MD, USA, 2004; pp. 1–9. [Google Scholar]
- Boynton, W.R.; Murray, L.; Hagy, J.D.; Stokes, C.; Kemp, W.M. A comparative analysis of eutrophication patterns in a temperate coastal lagoon. Estuaries 1996, 19, 408–421. [Google Scholar] [CrossRef]
- Aighewi, I.T.; Nosakhare, O.K.; Ishaque, A.B. Land use–Land cover changes and sewage loading in the lower eastern shore watersheds and coastal bays of Maryland: Implications for surface water quality. J. Coast. Res. 2013, 29, 1073–1082. [Google Scholar] [CrossRef]
- Oseji, O.F.; Fan, C.; Chigbu, P. Composition and dynamics of phytoplankton in the Coastal Bays of Maryland revealed by microscopic counts and diagnostic pigments. Water 2019, 11, 368. [Google Scholar] [CrossRef]
- Morales-Núñez, A.G.; Chigbu, P. Abundance, distribution, and species composition of amphipods associated with macroalgae from shallow waters of the Maryland Coastal Bays, USA. Mar. Biodivers. 2019, 49, 175–191. [Google Scholar] [CrossRef]
- Wyanski, D.M. Patterns of Habitat Utilization in 0-Age Summer Flounder (Paralichthys dentatus). Master’s Thesis, William & Mary, Williamsburg, VA, USA, 1990. [Google Scholar] [CrossRef]
- Pacheco, A.L. Age and growth of spot in lower Chesapeake Bay, with notes on distribution and abundance of juveniles in the York River system. Chesap. Sci. 1962, 3, 18–28. [Google Scholar] [CrossRef]
- Grammer, G.L.; Brown-Peterson, N.J.; Peterson, M.S.; Comyns, B.H. Life history of silver perch Bairdiella chrysoura (Lacepede, 1803) in north-central Gulf of Mexico estuaries. Gulf Mex. Sci. 2009, 27, 7. [Google Scholar] [CrossRef]
- Bassista, T.P.; Hartman, K.J. Reproductive biology and egg mortality of bay anchovy, Anchoa mitchilli, in the Hudson River estuary. Environ. Biol. Fishes 2005, 73, 49–59. [Google Scholar] [CrossRef]
- Sulzman, E.W. Stable isotope chemistry and measurement: A primer. Stable Isot. Ecol. Environ. Sci. 2007, 2, 1–21. [Google Scholar]
- Edje, B.O.; Chigbu, P. Carbon and nitrogen stable isotopes of copepods in a tidal estuarine system in Maryland, USA. Reg. Stud. Mar. Sci. 2021, 42, 101620. [Google Scholar] [CrossRef]
- Minagawa, M.; Wada, E. Stepwise enrichment of 15N along food chains: Further evidence and the relation between δ15N and animal age. Geochim. Cosmochim. Acta 1984, 48, 1135–1140. [Google Scholar] [CrossRef]
- Mill, A.C. Stable isotope data as reef food-web descriptors in a dynamic tropical environment. Doctoral Dissertation, Newcastle University, Newcastle upon Tyne, UK, 2007. [Google Scholar]
- Skinner, M.M.; Martin, A.A.; Moore, B.C. Is lipid correction necessary in the stable isotope analysis of fish tissues? Rapid Commun. Mass Spectrom. 2016, 30, 881–889. [Google Scholar] [CrossRef] [PubMed]
- D’Ambra, I.; Graham, W.M.; Carmichael, R.H.; Hernandez, F.J., Jr. Dietary overlap between jellyfish and forage fish in the northern Gulf of Mexico. Mar. Ecol. Prog. Ser. 2018, 587, 31–40. [Google Scholar] [CrossRef]
- Matley, J.K.; Tobin, A.J.; Simpfendorfer, C.A.; Fisk, A.T.; Heupel, M.R. Trophic niche and spatio-temporal changes in the feeding ecology of two sympatric species of coral trout (Plectropomus leopardus and P. laevis). Mar. Ecol. Prog. Ser. 2017, 563, 197–210. [Google Scholar] [CrossRef]
- Cybulski, J.D.; Skinner, C.; Wan, Z.; Wong, C.K.; Toonen, R.J.; Gaither, M.R.; Soong, K.; Wyatt, A.S.; Baker, D.M. Improving stable isotope assessments of inter-and intra-species variation in coral reef fish trophic strategies. Ecol. Evol. 2022, 12, e9221. [Google Scholar] [CrossRef] [PubMed]
- France, R.L. Carbon-13 enrichment in benthic compared to planktonic algae: Food web implications. Mar. Ecol. Prog. Ser. 1995, 124, 307–312. [Google Scholar] [CrossRef]
- Adams, D.H.; Paperno, R. Stable isotopes and mercury in a model estuarine fish: Multibasin comparisons with water quality, community structure, and available prey base. Sci. Total Environ. 2012, 414, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Buchheister, A. Stable isotope dynamics in summer flounder tissues, with application to dietary assessments in Chesapeake Bay. Master’s Thesis, William & Mary, Williamsburg, VA, USA, 2008. [Google Scholar]
- Chouvelon, T.; Caurant, F.; Cherel, Y.; Simon-Bouhet, B.; Spitz, J.; Bustamante, P. Species-and size-related patterns in stable isotopes and mercury concentrations in fish help refine marine ecosystem indicators and provide evidence for distinct management units for hake in the Northeast Atlantic. ICES J. Mar. Sci. 2014, 7, 1073–1087. [Google Scholar] [CrossRef]
- MacKenzie, K.M.; Trueman, C.N.; Palmer, M.R.; Moore, A.; Ibbotson, A.T.; Beaumont, W.R.; Davidson, I.C. Stable isotopes reveal age-dependent trophic level and spatial segregation during adult marine feeding in populations of salmon. ICES J. Mar. Sci. 2012, 69, 1637–1645. [Google Scholar] [CrossRef]
- Kostecki, C.; Le Loc’h, F.; Roussel, J.M.; Desroy, N.; Huteau, D.; Riera, P.; Le Bris, H.; Le Pape, O. Dynamics of an estuarine nursery ground: The spatio-temporal relationship between the river flow and the food web of the juvenile common sole (Solea solea, L.) as revealed by stable isotopes analysis. J. Sea Res. 2010, 64, 54–60. [Google Scholar] [CrossRef]
- Edje, B.O.; Ishaque, A.B.; Chigbu, P. Spatial and Temporal Patterns of δ13C and δ15N of Suspended Particulate Organic Matter in Maryland Coastal Bays, USA. Water 2020, 12, 2345. [Google Scholar] [CrossRef]
- Brush, J.M.; Fisk, A.T.; Hussey, N.E.; Johnson, T.B. Spatial and seasonal variability in the diet of round goby (Neogobius melanostomus): Stable isotopes indicate that stomach contents overestimate the importance of dreissenids. Can. J. Fish. Aquat. Sci. 2012, 69, 573–586. [Google Scholar] [CrossRef]
- Kang, S.; Kim, J.H.; Kim, D.; Song, H.; Ryu, J.S.; Ock, G.; Shin, K.H. Temporal variation in riverine organic carbon concentrations and fluxes in two contrasting estuary systems: Geum and Seomjin, South Korea. Environ. Int. 2019, 133, 105126. [Google Scholar] [CrossRef] [PubMed]
- Sacco, M.; Blyth, A.J.; Humphreys, W.F.; Cooper, S.J.B.; White, N.E.; Campbell, M.; Mousavi-Derazmahalleh, M.; Hua, Q.; Mazumder, D.; Smith, C.; et al. Rainfall as a trigger of ecological cascade effects in an Australian groundwater ecosystem. Sci. Rep. 2021, 11, 3694. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Won, E.J.; Cho, H.E.; Lee, J.; Shin, K.H. New insight into biomagnification factor of mercury based on food web structure using stable isotopes of amino acids. Water Res. 2023, 245, 120591. [Google Scholar] [CrossRef]
- St. John Glew, K.; Graham, L.J.; McGill, R.A.; Trueman, C.N. Spatial models of carbon, nitrogen and sulphur stable isotope distributions (isoscapes) across a shelf sea: An INLA approach. Methods Ecol. Evol. 2019, 10, 518–531. [Google Scholar] [CrossRef]
- Oseji, O.F.; Chigbu, P.; Oghenekaro, E.; Waguespack, Y.; Chen, N. Spatial and temporal patterns of phytoplankton composition and abundance in the Maryland Coastal Bays: The influence of freshwater discharge and anthropogenic activities. Estuar. Coast. Shelf Sci. 2018, 207, 119–131. [Google Scholar] [CrossRef]
- Estrada, J.A.; Lutcavage, M.; Thorrold, S.R. Diet and trophic position of Atlantic bluefin tuna (Thunnus thynnus) inferred from stable carbon and nitrogen isotope analysis. Mar. Biol. 2005, 147, 37–45. [Google Scholar] [CrossRef]
- O’Brien, M.H.P. Marine influence on juvenile fish trophic ecology and community dynamics in Maryland’s northern coastal lagoons. Master’s Thesis, University of Maryland, College Park, MD, USA, 2013. [Google Scholar]
- Mancinelli, G.; Glamuzina, B.; Petrić, M.; Carrozzo, L.; Glamuzina, L.; Zotti, M.; Raho, D.; Vizzini, S. The trophic position of the Atlantic blue crab Callinectes sapidus Rathbun 1896 in the food web of Parila Lagoon (South Eastern Adriatic, Croatia): A first assessment using stable isotopes. Mediterr. Mar. Sci. 2016, 17, 634–643. [Google Scholar] [CrossRef]
- Ou, C.; Montaña, C.G.; Winemiller, K.O. Body size–trophic position relationships among fishes of the lower Mekong basin. R. Soc. Open Sci. 2017, 4, 160645. [Google Scholar] [CrossRef] [PubMed]
- Cummings, D.O.; Buhl, J.; Lee, R.W.; Simpson, S.J.; Holmes, S.P. Estimating niche width using stable isotopes in the face of habitat variability: A modelling case study in the marine environment. PLoS ONE 2012, 7, e40539. [Google Scholar] [CrossRef]
- Gaspar, C.; Giménez, J.; Andonegi, E.; Astarloa, A.; Chouvelon, T.; Franco, J.; Goñi, N.; Corrales, X.; Spitz, J.; Bustamante, P.; et al. Trophic ecology of northern gannets Morus bassanus highlights the extent of isotopic niche overlap with other apex predators within the Bay of Biscay. Mar. Biol. 2022, 169, 105. [Google Scholar] [CrossRef]
- Flaherty, E.A.; Ben-David, M. Overlap and partitioning of the ecological and isotopic niches. Oikos 2010, 119, 1409–1416. [Google Scholar] [CrossRef]
- Shaw, A. Dietary niche overlap of an estuarine predatory fish community in South Carolina assessed by stable isotope analysis. Master’s Thesis, College of Charleston, Charleston, SC, USA, 2013. [Google Scholar]
- Pelage, L.; Lucena-Fredou, F.; Eduardo, L.N.; Le Loc’h, F.; Bertrand, A.; Lira, A.S.; Fredou, T. Competing with each other: Fish isotopic niche in two resource availability contexts. Front. Mar. Sci. 2022, 9, 975091. [Google Scholar] [CrossRef]
- Gonzalez, J.G.; Ménard, F.; Le Loc’h, F.; De Andrade, H.A.; Viana, A.P.; Ferreira, V.; Frédou, F.L.; Lira, A.S.; Munaron, J.M.; Frédou, T. Trophic resource partitioning of two snook fish species (Centropomidae) in tropical estuaries in Brazil as evidenced by stable isotope analysis. Estuar. Coast. Shelf Sci. 2019, 226, 106287. [Google Scholar] [CrossRef]
- Wang, Y.; Huan, Z.; YuWei, C.; Lu, Z.; Guangchun, L. Trophic niche width and overlap of three benthic living fish species in Poyang Lake: A stable isotope approach. Wetlands 2019, 39, 17–23. [Google Scholar] [CrossRef]
- Parra, G.J.; Wojtkowiak, Z.; Peters, K.J.; Cagnazzi, D. Isotopic niche overlap between sympatric Australian snubfin and humpback dolphins. Ecol. Evol. 2022, 12, e8937. [Google Scholar] [CrossRef]

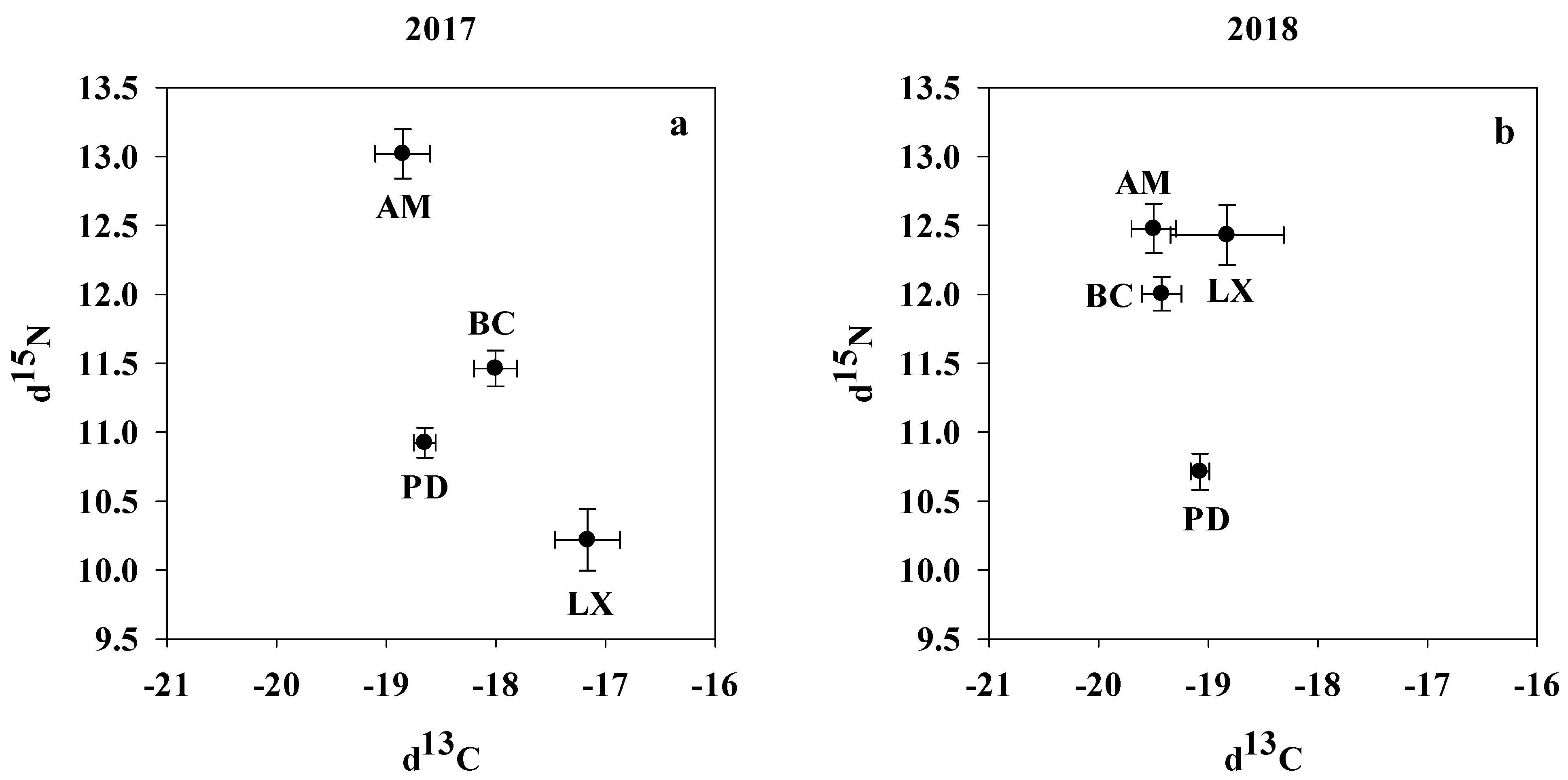
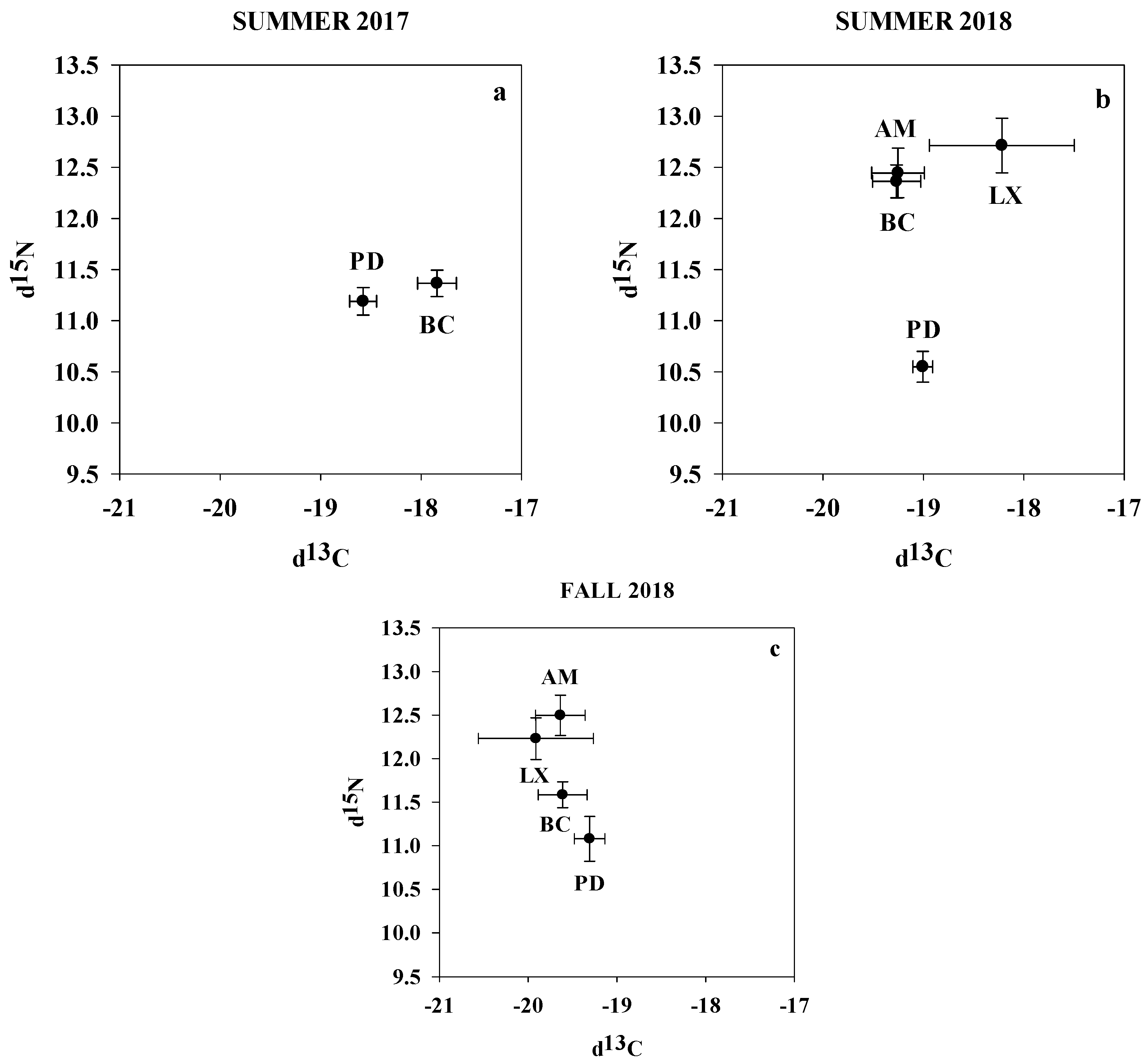
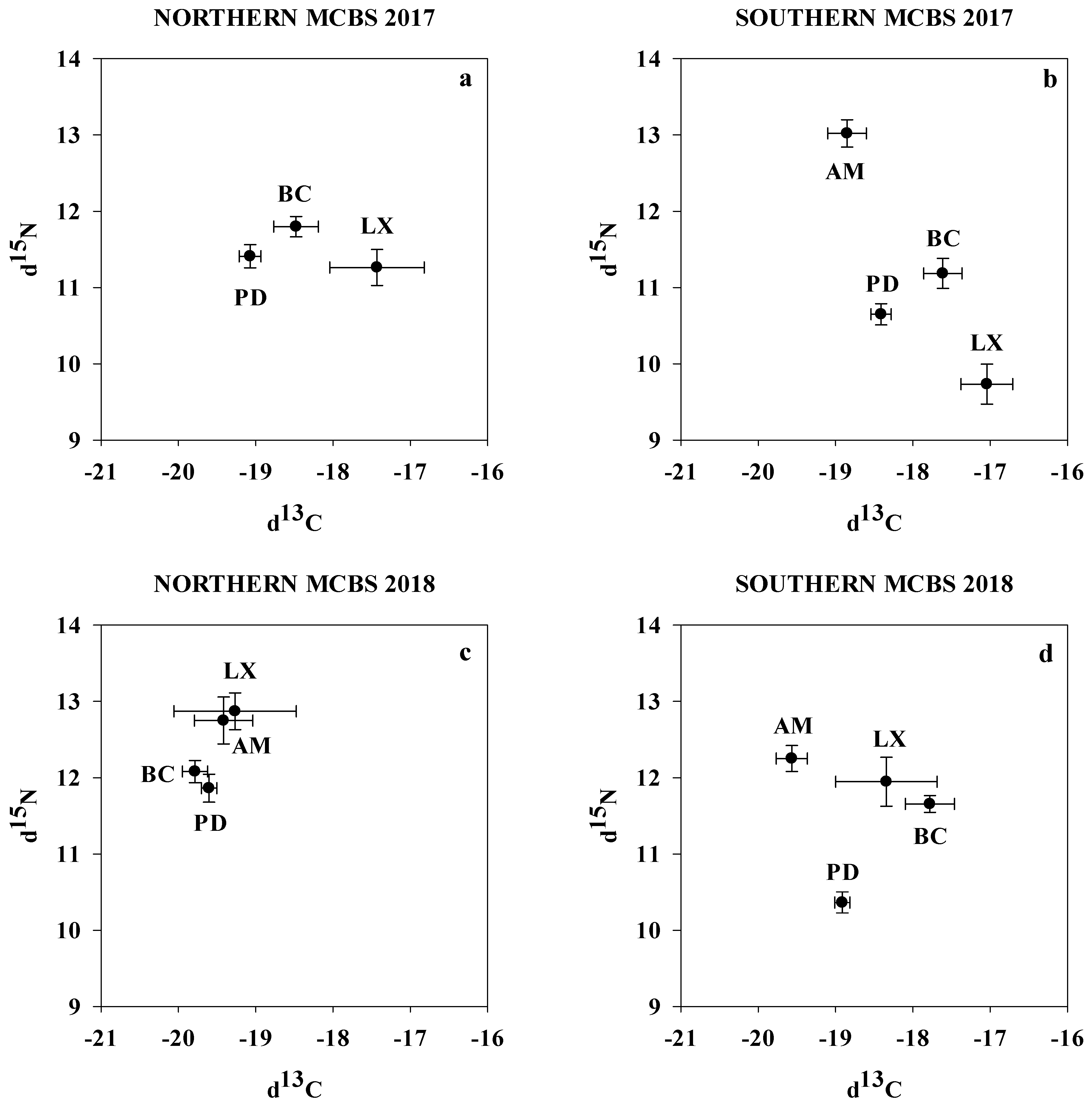
| 2017 (April–October) | 2018 (June–October) | |
| Mean ± SE (n); Range (cm) | Mean ± SE (n); Range (cm) | |
| P. dentatus | 11.9 ± 0.5 (141); 5.5–39.5 | 11.7 ± 0.4 (90); 7.2–33.0 |
| A. mitchilli | 7.3 ± 0.3 (16); 6.1–12.1 | 6.2 ± 0.2 (44); 4.0–9.9 |
| L. xanthurus | 11.4 ± 0.4 (41); 7.1–16.5 | 13.7 ± 0.5 (21); 8.5–18.3 |
| B. chrysoura | 8.4 ± 0.2 (53); 5.5–11.2 | 8.7 ± 0.2 (50); 4.5–15.0 |
| 2017 (North MCBs) | 2017 (South MCBs) | |
| Mean ± SE (n); Range (cm) | Mean ± SE (n); Range (cm) | |
| P. dentatus | 12.0 ± 0.8 (51); 7.7–39.5 | 11.9 ± 0.6 (90); 5.5–35.0 |
| A. mitchilli | - | 7.3 ± 0.3 (16); 6.1–12.1 |
| L. xanthurus | 13.6 ± 0.4 (13); 12.0–16.5 | 10.4 ± 0.4 (28); 7.1–16.3 |
| B. chrysoura | 8.5 ± 0.3 (24); 5.9–11.2 | 8.3 ± 0.2 (29); 5.5–10.5 |
| 2018 (North MCBs) | 2018 (South MCBs) | |
| Mean ± SE (n); Range (cm) | Mean ± SE (n); Range (cm) | |
| P. dentatus | 12.0 ± 1.2 (21); 7.6–33.0 | 11.5 ± 0.4 (69); 7.2–32.4 |
| A. mitchilli | 6.5 ± 0.2 (20); 4.0–8.3 | 5.9 ± 0.3 (24); 4.0–9.9 |
| L. xanthurus | 13.4 ± 0.7 (11); 11.5–18.3 | 13.9 ± 0.7 (10); 8.5–15.7 |
| B. chrysoura | 8.6 ± 0.3 (41); 4.5–15.0 | 9.3 ± 0.8 (9); 6.0–13.9 |
| 2017 | 2017 | 2018 | 2018 | |
|---|---|---|---|---|
| δ13C | δ15N | δ13C | δ15N | |
| P. dentatus | −18.7 ± 0.1 a | 10.9 ± 0.1 c | −19.1 ± 0.1 a | 10.7 ± 0.1 b |
| A. mitchilli | −18.9 ± 0.3 a | 13.0 ± 0.2 a | −19.5 ± 0.2 c | 12.5 ± 0.2 a |
| L. xanthurus | −17.2 ± 0.3 b | 10.2 ± 0.2 d | −18.8 ± 0.5 c | 12.4 ± 0.2 a |
| B. chrysoura | −18.0 ± 0.2 a | 11.5 ± 0.1 b | −19.4 ± 0.2 b | 12.0 ± 0.1 a |
| p-value | <0.001 | <0.001 | 0.013 | <0.001 |
| 2017 | 2018 | 2017 | 2018 | |||
|---|---|---|---|---|---|---|
| δ13C | δ13C | p-Value | δ15N | δ15N | p-Value | |
| P. dentatus | −18.7 ± 0.1 a | −19.1 ± 0.1 b | <0.05 | 10.9 ± 0.1 a | 10.7 ± 0.1 a | >0.05 |
| A. mitchilli | −18.9 ± 0.3 a | −19.5 ± 0.2 b | <0.05 | 13.0 ± 0.2 a | 12.5 ± 0.2 b | <0.05 |
| L. xanthurus | −17.2 ± 0.3 a | −18.8 ± 0.5 b | <0.05 | 10.2 ± 0.2 a | 12.4 ± 0.2 b | <0.05 |
| B. chrysoura | −18.0 ± 0.2 a | −19.5 ± 0.2 b | <0.05 | 11.5 ± 0.1 a | 12.0 ± 0.1 b | <0.05 |
| 2017 | 2018 | |||||
|---|---|---|---|---|---|---|
| δ13C | δ13C | |||||
| North | South | p-Value | North | South | p-Value | |
| P. dentatus | −19.1 ± 0.1 a | −18.4 ± 0.1 b | <0.05 | −19.6 ± 0.1 a | −18.9 ± 0.1 b | <0.001 |
| A. mitchilli | - | −18.9 ± 0.3 | - | −19.4 ± 0.4 a | −19.6 ± 0.2 a | >0.05 |
| L. xanthurus | −17.4 ± 0.6 a | −17.0 ± 0.3 a | >0.05 | −19.3 ± 0.8 a | −18.3 ± 0.7 a | >0.05 |
| B. chrysoura | −18.5 ± 0.3 a | −17.6 ± 0.2 b | <0.05 | −19.8 ± 0.2 a | −17.8 ± 0.3 b | <0.001 |
| δ15N | δ15N | |||||
| North | South | p-value | North | South | p-value | |
| P. dentatus | 11.4 ± 0.2 a | 10.6 ± 0.1 b | <0.05 | 11.9 ± 0.2 a | 10.4 ± 0.1 b | <0.05 |
| A. mitchilli | - | 13.0 ± 0.2 | 12.7 ± 0.3 a | 12.3 ± 0.2 a | >0.05 | |
| L. xanthurus | 11.3 ± 0.2 a | 9.7 ± 0.3 b | <0.05 | 12.9 ± 0.2 a | 11.9 ± 0.3 a | >0.05 |
| B. chrysoura | 11.8 ± 0.1 a | 11.2 ± 0.2 b | <0.05 | 12.1 ± 0.1 a | 11.7 ± 0.1 a | >0.05 |
| Species | SEA (δ2) | SEAc (δ2) |
|---|---|---|
| P. dentatus | 4.2 | 4.3 |
| A. mitchilli | 3.8 | 3.8 |
| L. xanthurus | 10.6 | 10.8 |
| B. chrysoura | 4 | 4 |
| P. dentatus | A. mitchilli | L. xanthurus | B. chrysoura | |
|---|---|---|---|---|
| P. dentatus | 20.1 | 57.2 | 49.8 | |
| A. mitchilli | 22.2 | 76.4 | 48.5 | |
| L. xanthurus | 22.7 | 27.4 | 33.6 | |
| B. chrysoura | 52.3 | 46.1 | 89.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Richardson, C.; Chigbu, P.; Ishaque, A. Spatial, Temporal, and Interspecific Differences in Composition of Stable Isotopes in Fishes in Maryland Coastal Bays. Diversity 2024, 16, 331. https://doi.org/10.3390/d16060331
Richardson C, Chigbu P, Ishaque A. Spatial, Temporal, and Interspecific Differences in Composition of Stable Isotopes in Fishes in Maryland Coastal Bays. Diversity. 2024; 16(6):331. https://doi.org/10.3390/d16060331
Chicago/Turabian StyleRichardson, Chelsea, Paulinus Chigbu, and Ali Ishaque. 2024. "Spatial, Temporal, and Interspecific Differences in Composition of Stable Isotopes in Fishes in Maryland Coastal Bays" Diversity 16, no. 6: 331. https://doi.org/10.3390/d16060331
APA StyleRichardson, C., Chigbu, P., & Ishaque, A. (2024). Spatial, Temporal, and Interspecific Differences in Composition of Stable Isotopes in Fishes in Maryland Coastal Bays. Diversity, 16(6), 331. https://doi.org/10.3390/d16060331





