Seasonal Variability in Non-Structural Carbohydrate Content of Warm-Adapted Zostera noltei and Zostera marina Populations
Abstract
1. Introduction
2. Materials and Methods
2.1. Habitat Description and Sample Collection
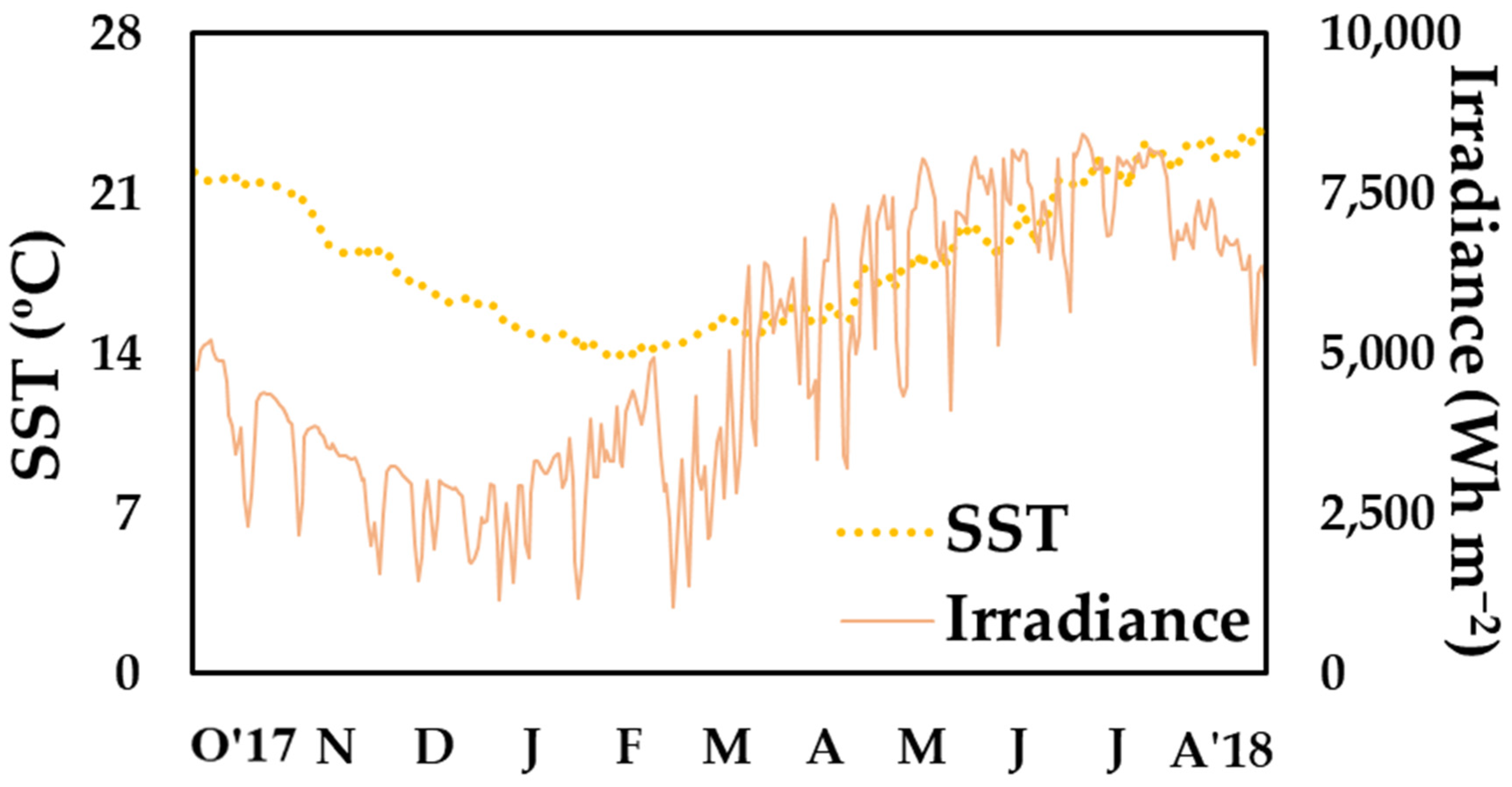
2.2. Environmental Data
2.3. Biochemical Analysis
2.4. Relative Growth Rate
2.5. Statistical Analysis
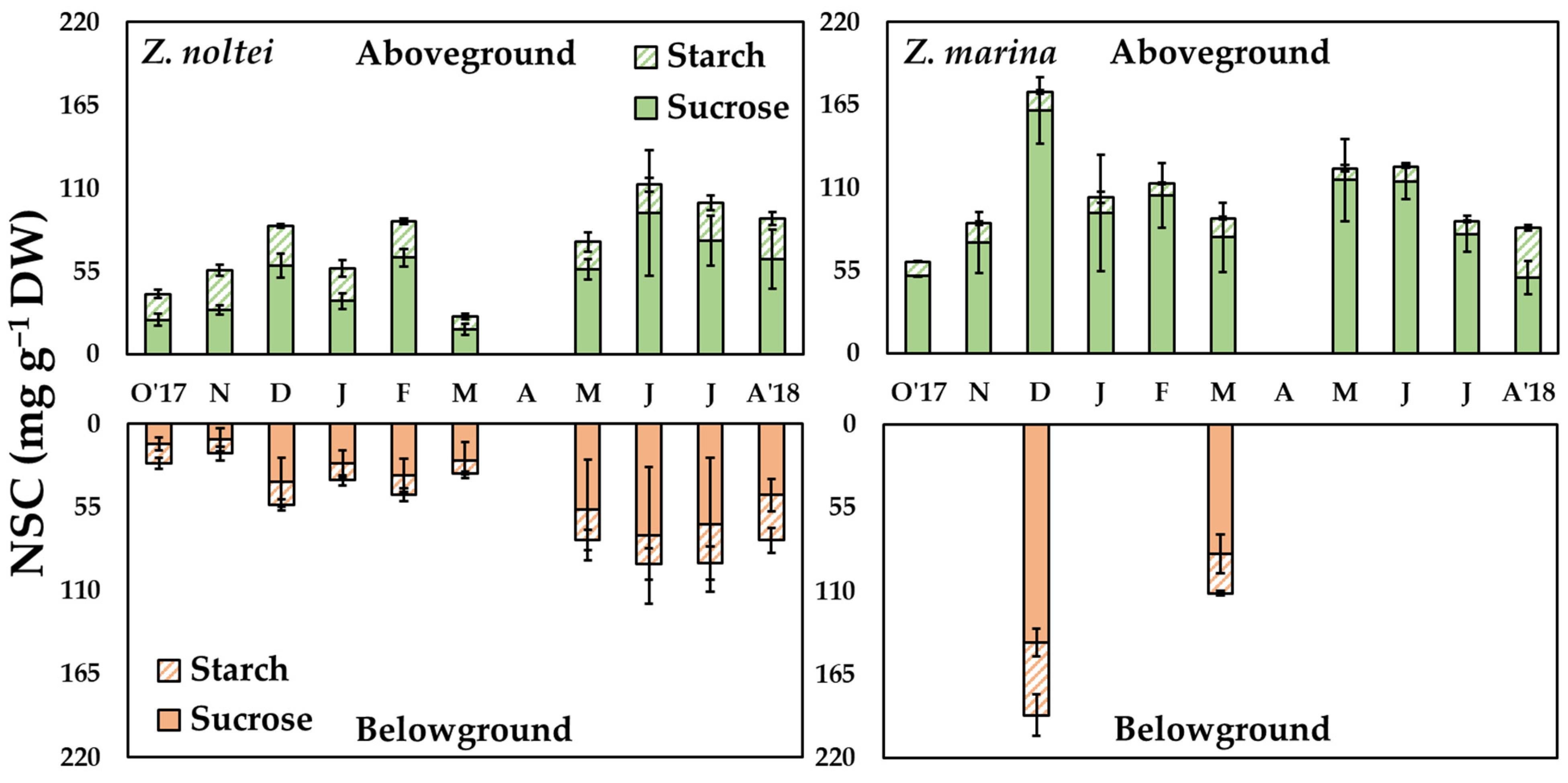
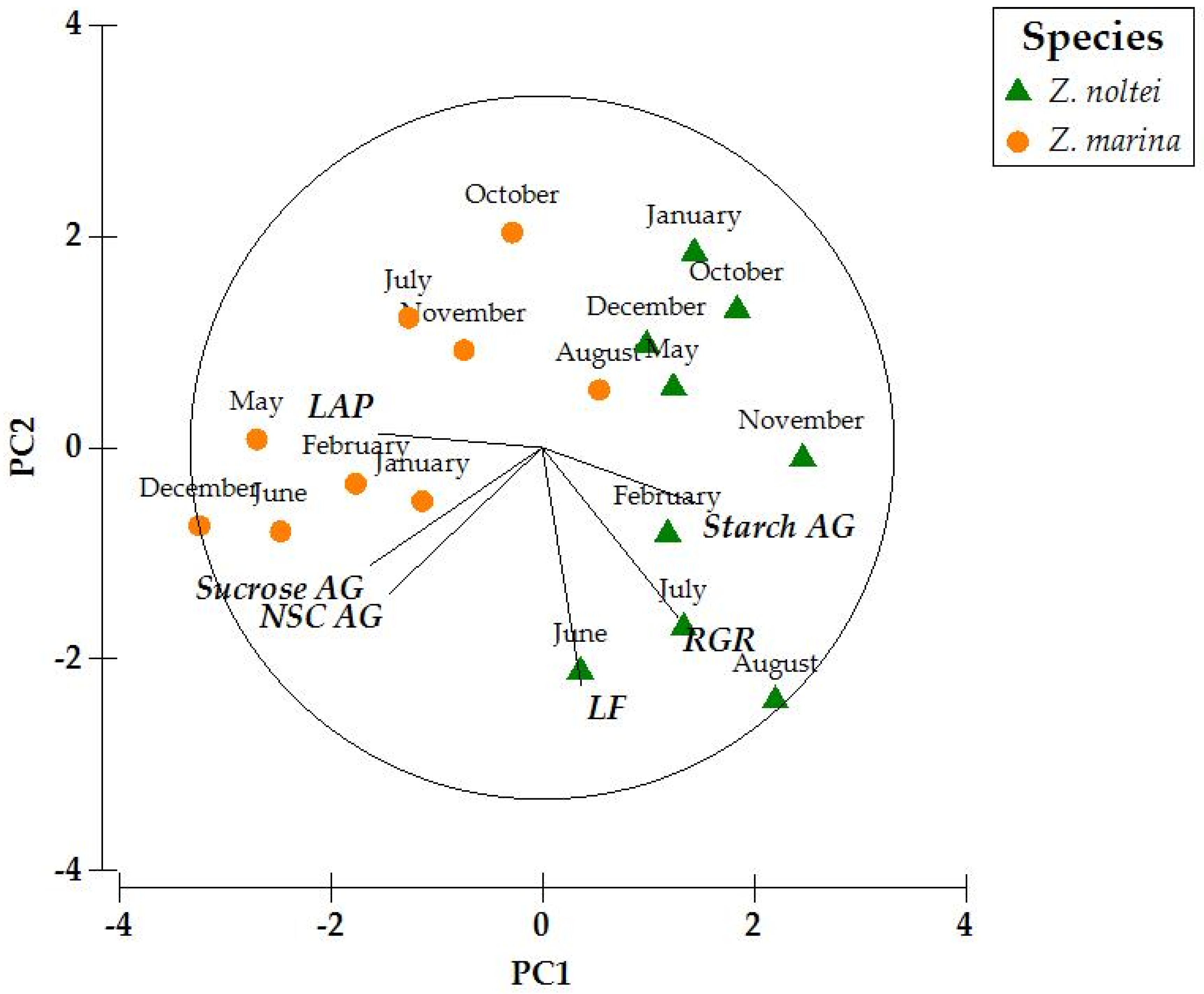
3. Results
4. Discussion
4.1. Seasonal Variability in NSC Content
4.2. Differences in NSC Accumulation among Tissues
4.3. Species-Specific Differences
4.4. Conservation Status of Seagrass Populations in Cadiz Bay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hemminga, M.A.; Duarte, C.M. Seagrass Ecology; Cambridge University Press: Cambridge, UK, 2000. [Google Scholar]
- Short, F.T.; Polidoro, B.; Livingstone, S.R.; Carpenter, K.E.; Bandeira, S.; Bujang, J.S.; Calumpong, H.P.; Carruthers, T.J.B.; Coles, R.G.; Dennison, W.C.; et al. Extinction risk assessment of the world’s seagrass species. Biol. Conserv. 2011, 144, 1961–1971. [Google Scholar] [CrossRef]
- Waycott, M.; Duarte, C.M.; Carruthers, T.J.; Orth, R.J.; Dennison, W.C.; Olyarnik, S.; Calladine, A.; Fourqurean, J.W.; Keck, K.L.; Hughes, A.R.; et al. Accelerating loss of seagrasses across the globe threatens coastal ecosystems. Proc. Natl. Acad. Sci. USA 2009, 106, 12377–12381. [Google Scholar] [CrossRef] [PubMed]
- Orth, R.J.; Carruthers, T.J.; Dennison, W.C.; Duarte, C.M.; Fourqurean, J.W.; Heck, K.L.; Hughes, A.R.; Kendrick, G.A.; Kenworthy, W.J.; Olyarnik, S.; et al. A global crisis for seagrass ecosystems. Bioscience 2006, 56, 987–996. [Google Scholar] [CrossRef]
- Short, F.; Carruthers, T.; Dennison, W.; Waycott, M. Global seagrass distribution and diversity: A bioregional model. J. Exp. Mar. Biol. Ecol. 2007, 350, 3–20. [Google Scholar] [CrossRef]
- Pazzaglia, J.; Reusch, T.B.; Terlizzi, A.; Marín-Guirao, L.; Procaccini, G. Phenotypic plasticity under rapid global changes: The intrinsic force for future seagrasses survival. Evol. Appl. 2021, 14, 1181–1201. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.S.; Park, S.R.; Kim, Y.K. Effects of irradiance, temperature, and nutrients on growth dynamics of seagrasses: A review. J. Exp. Mar. Biol. Ecol. 2007, 350, 144–175. [Google Scholar] [CrossRef]
- Touchette, B.W.; Burkholder, J.M. Overview of the physiological ecology of carbon metabolism in seagrasses. J. Exp. Mar. Biol. Ecol. 2000, 250, 169–205. [Google Scholar] [CrossRef]
- Pirc, H. Seasonal changes in soluble carbohydrates, starch, and energy content in Mediterranean seagrasses. Mar. Ecol. 1989, 10, 97–105. [Google Scholar] [CrossRef]
- Brun, F.G.; Hernández, I.; Vergara, J.J.; Pérez-Lloréns, J.L. Growth, carbon allocation and proteolytic activity in the seagrass Zostera noltii shaded by Ulva canopies. Funct. Plant Biol. 2003, 30, 551–560. [Google Scholar] [CrossRef]
- Alcoverro, T.; Manzanera, M.; Romero, J. Annual metabolic carbon balance of the seagrass Posidonia oceanica: The importance of carbohydrate reserves. Mar. Ecol. Prog. Ser. 2001, 211, 105–116. [Google Scholar] [CrossRef]
- Beca-Carretero, P.; Marín, C.; Azcárate-García, T.; Cara, C.L.; Brun, F.G.; Stengel, D.B. Ecotype-specific and correlated seasonal responses of biomass production, non-structural carbohydrates, and fatty acids in Zostera marina. Plants 2024, 13, 396. [Google Scholar] [CrossRef] [PubMed]
- Brun, F.G.; Vergara, J.J.; Pérez-Lloréns, J.L.; Ramírez, C.; Morris, E.P.; Peralta, G.; Hernández, I. Diversidad de angiospermas marinas en la bahía de Cádiz: Redescubriendo a Zostera marina. Chron. Naturae 2015, 5, 45–56. [Google Scholar]
- Ruiz, J.M.; Guillén, J.E.; Ramos-Segura, A.; Otero, M.M. Atlas de Praderas Marinas de España; Instituto Español de Oceanografía: Madrid, Spain, 2015. [Google Scholar]
- Beca-Carretero, P.; Guihéneuf, F.; Krause-Jensen, D.; Stengel, D.B. Seagrass fatty acid profiles as a sensitive indicator of climate settings across seasons and latitudes. Mar. Environ. Res. 2020, 161, 105075. [Google Scholar] [CrossRef] [PubMed]
- Azcárate-García, T.; Beca-Carretero, P.; Cara, C.L.; Villamayor, B.; Cosnett, E.; Bermejo, R.; Hernández, I.; Brun, F.G.; Stengel, D.B. Seasonal plant development and meadow structure of Irish and southern Spanish seagrass populations. Aquat. Bot. 2022, 183, 103569. [Google Scholar] [CrossRef]
- Brun, F.G.; Hernández, I.; Vergara, J.J.; Peralta, G.; Pérez-Lloréns, J.L. Assessing the toxicity of ammonium pulses to the survival and growth of Zostera noltii. Mar. Ecol. Prog. Ser. 2002, 225, 177–187. [Google Scholar] [CrossRef]
- Peralta, G.; Pérez-Lloréns, J.L.; Hernández, I.; Vergara, J.J. Effects of light availability on growth, architecture and nutrient content of the seagrass Zostera noltii Hornem. J. Exp. Mar. Biol. Ecol. 2002, 269, 9–26. [Google Scholar] [CrossRef]
- Olivé, I.; Brun, F.G.; Vergara, J.J.; Pérez-Lloréns, J.L. Effects of light and biomass partitioning on growth, photosynthesis and carbohydrate content of the seagrass Zostera noltii Hornem. J. Exp. Mar. Biol. Ecol. 2007, 345, 90–100. [Google Scholar] [CrossRef]
- Brun, F.G.; Pérez-Lloréns, J.L.; Hernández, I.; Vergara, J.J. Patch distribution and within-patch dynamics of the seagrass Zostera noltii Hornem. in los Toruños Salt-Marsh, Cádiz Bay, Natural Park, Spain. Bot. Mar. 2003, 46, 513–524. [Google Scholar] [CrossRef]
- Cebrián, J.; Duarte, C.M.; Agawin, N.S.R.; Merino, M. Leaf growth response to simulated herbivory: A comparison among seagrass species. J. Exp. Mar. Biol. Ecol. 1998, 220, 67–81. [Google Scholar] [CrossRef]
- Short, F.T.; Duarte, C.M. Methods for the measurement of seagrass growth and production. In Global Seagrass Research Methods; Elsevier: Amsterdam, The Netherlands, 2001; pp. 155–198. [Google Scholar]
- Burke, M.K.; Dennison, W.C.; Moore, K.A. Non-structural carbohydrate reserves of eelgrass Zostera marina. Mar. Ecol. Prog. Ser. 1996, 137, 195–201. [Google Scholar] [CrossRef]
- Buia, M.C.; Zupo, V.; Mazzella, L. Primary production and growth dynamics. Mar. Ecol. 1992, 13, 2–16. [Google Scholar] [CrossRef]
- Soissons, L.M.; Haanstra, E.P.; van Katwijk, M.M.; Asmus, R.; Auby, I.; Barillé, L.; Brun, F.G.; Cardoso, P.G.; Desroy, N.; Fournier, J.; et al. Latitudinal patterns in European seagrass carbon reserves: Influence of seasonal fluctuations versus short-term stress and disturbance events. Front. Plant Sci. 2018, 9, 88. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, K.; Kawasaki, Y.; Sato, S. Effect of seasonal changes on the carbohydrate levels of eelgrass Zostera marina at Odawa Bay. Fish Sci. 2001, 67, 755–757. [Google Scholar] [CrossRef]
- Brun, F.G.; Vergara, J.J.; Navarro, G.; Hernández, I.; Pérez-Lloréns, J.L. Effect of shading by Ulva rigida canopies on growth and carbon balance of the seagrass Zostera noltii. Mar. Ecol. Prog. Ser. 2003, 265, 85–96. [Google Scholar] [CrossRef][Green Version]
- De Rosa, S.; Zavodnik, N.; De Stefano, S.; Fiaccavento, R.; Travizi, A. Seasonal Changes of Biomass and Soluble Carbohydrates in the Seagrass Zostera noltii Hornem; Walter de Gruyter: Berlin, Germany, 1990. [Google Scholar]
- Vermaat, J.E.; Verhagen, F.C. Seasonal variation in the intertidal seagrass Zostera noltii Hornem.: Coupling demographic and physiological patterns. Aquat Bot. 1996, 52, 259–281. [Google Scholar] [CrossRef]
- Vichkovitten, T.; Holmer, M.; Frederiksen, M.S. Spatial and temporal changes in non-structural carbohydrate reserves in eelgrass (Zostera marina L.) in Danish coastal waters. Bot. Mar. 2007, 50, 75–87. [Google Scholar] [CrossRef]
- Alcoverro, T.; Zimmerman, R.C.; Kohrs, D.G.; Alberte, R.S. Resource allocation and sucrose mobilization in light-limited eelgrass Zostera marina. Mar. Ecol. Prog. Ser. 1999, 187, 121–131. [Google Scholar] [CrossRef]
- Longstaff, B.J.; Loneragan, N.R.; O’donohue, M.J.; Dennison, W.C. Effects of light deprivation on the survival and recovery of the seagrass Halophila ovalis (R.Br.) Hook. J. Exp. Mar. Biol. Ecol. 1999, 234, 1–27. [Google Scholar] [CrossRef]
- Cabaço, S.; Santos, R. Effects of burial and erosion on the seagrass Zostera noltii. J. Exp. Mar. Biol. Ecol. 2007, 340, 204–212. [Google Scholar] [CrossRef]
- Cabaço, S.; Machás, R.; Vieira, V.; Santos, R. Impacts of urban wastewater discharge on seagrass meadows (Zostera noltii). Estuar. Coast. Shelf Sci. 2008, 78, 1–13. [Google Scholar] [CrossRef]
- Garmendia, J.M.; Valle, M.; Borja, Á.; Chust, G.; Rodríguez, J.G.; Franco, J. Estimated footprint of shellfishing activities in Zostera noltei meadows in a northern Spain estuary: Lessons for management. Estuar. Coast. Shelf Sci. 2021, 254, 107320. [Google Scholar] [CrossRef]
- Serrano, O.; Arias-Ortiz, A.; Duarte, C.M.; Kendrick, G.A.; Lavery, P.S. Impact of marine heatwaves on seagrass ecosystems. In Ecosystem Collapse and Climate Change; Springer International Publishing: Cham, Switzerland, 2021; Volume 241, pp. 345–364. [Google Scholar]
- Roca, G.; Romero, J.; Columbu, S.; Farina, S.; Pagès, J.F.; Gera, A.; Inglis, G.; Alcoverro, T. Detecting the impacts of harbour construction on a seagrass habitat and its subsequent recovery. Ecol. Indic. 2014, 45, 9–17. [Google Scholar] [CrossRef]
- Pérez-Lloréns, J.L.; Brun, F.G. “Sea rice”: From traditional culinary customs to sustainable crop for high-end gastronomy? Int. J. Gastron. Food Sci. 2023, 34, 100814. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azcárate-García, T.; Beca-Carretero, P.; Hernández, I.; Brun, F.G. Seasonal Variability in Non-Structural Carbohydrate Content of Warm-Adapted Zostera noltei and Zostera marina Populations. Diversity 2024, 16, 391. https://doi.org/10.3390/d16070391
Azcárate-García T, Beca-Carretero P, Hernández I, Brun FG. Seasonal Variability in Non-Structural Carbohydrate Content of Warm-Adapted Zostera noltei and Zostera marina Populations. Diversity. 2024; 16(7):391. https://doi.org/10.3390/d16070391
Chicago/Turabian StyleAzcárate-García, Tomás, Pedro Beca-Carretero, Ignacio Hernández, and Fernando G. Brun. 2024. "Seasonal Variability in Non-Structural Carbohydrate Content of Warm-Adapted Zostera noltei and Zostera marina Populations" Diversity 16, no. 7: 391. https://doi.org/10.3390/d16070391
APA StyleAzcárate-García, T., Beca-Carretero, P., Hernández, I., & Brun, F. G. (2024). Seasonal Variability in Non-Structural Carbohydrate Content of Warm-Adapted Zostera noltei and Zostera marina Populations. Diversity, 16(7), 391. https://doi.org/10.3390/d16070391






