Changes in Population Densities and Species Richness of Pollinators in the Carpathian Basin during the Last 50 Years (Hymenoptera, Diptera, Lepidoptera)
Abstract
1. Introduction
“The apple trees were coming into bloom but no bees droned among the blossoms, so there was no pollination and there would be no fruit. The roadsides, once so attractive, were now lined with browned and withered vegetation as though swept by fire. These, too, were silent, deserted by all living things. Even the streams were now lifeless”. (Rachel Carson: Silent Spring).
2. Materials and Methods
2.1. Data Selection
2.2. Changes in Methods and Their Statistical Balancing
2.3. Data Processing
2.4. Sampling Sites
3. Results
3.1. Symphyta
3.2. Aculeata
3.3. Bumblebees (Bombus spp.)
3.4. Diptera
3.4.1. Syrphidae (Hoverflies)
3.4.2. Tabanidae (Horseflies)
3.4.3. Bombyliidae (Bee Flies)
3.4.4. Tachinidae (Tachinids)
3.5. Lepidoptera
3.5.1. Nocturnal Macrolepidoptera
3.5.2. Butterflies (Rhopalocera)
4. Discussion
4.1. Symphyta
4.2. Aculeata
4.3. Bumblebees (Bombus spp.)
4.4. Diptera
4.5. Lepidoptera
4.5.1. Nocturnal Macrolepidoptera
4.5.2. Butterflies (Rhopalocera)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Molnár, A.V.; Takács, A. Megporzási válság. A pollináció mint természeti szolgáltatás. Ökológia Természettudományi Közlöny 2016, 147, 303–305. [Google Scholar]
- Rhodes, C.J. Pollinator decline—An ecological calamity in the making? Sci. Prog. 2018, 101, 121–160. [Google Scholar] [CrossRef] [PubMed]
- Halmos, G. A Hosszútávú Vonuló Énekesmadár Fajok Állományváltozásának és Populációdinamikájának Vizsgálata. Ph.D. Thesis, ELTE Department of Systematic Zoology and Ecology, Budapest, Hungary, 2009; 117p. Available online: https://teo.elte.hu/minosites/tezis2009/halmos_g.pdf (accessed on 18 March 2024).
- Potts, S.G.; Imperatriz-Fonseca, V.; Ngo, H.T.; Biesmeijer, J.C.; Breeze, T.D.; Dicks, L.V.; Garibaldi, L.A.; Hill, R.; Settele, J.; Vanbergen, A.J. Summary for Policymakers of the Assessment Report of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services on Pollinators, Pollination and Food Production; Secretariat of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services: Bonn, Germany, 2016; 36p.
- Dicks, L.V.; Breeze, T.D.; Ngo, H.T.; Senapathi, D.; An, J.; Aizen, M.A.; Basu, P.; Buchori, D.; Galetto, L.; Garibaldi, L.A.; et al. A global-scale expert assessment of drivers and risks associated with pollinator decline. Nat. Ecol. Evol. 2021, 5, 1453–1461. [Google Scholar] [CrossRef] [PubMed]
- Kluser, S.; Peduzzi, P. Global Pollinator Decline: A Literature Review. A Scientific Report about the Current Situation, Recent Findings and Potential Solution to Shed Light on the Global Pollinator Crisis; UNEP/DEWA/GRID-Europe: Châtelaine, Switzerland, 2007; 10p. [Google Scholar]
- Marks, R. Native Pollinators. In Fish and Wildlife Habitat Management Leaflet; Wildlife Habitat Council: Bethesda, MD, USA, 2005; Volume 34, pp. 1–10. [Google Scholar]
- Halleux, V. Protecting Pollinators in the EU; European Parliament Briefing; EPRS | European Parliamentary Research Service: Strassbourg, France, 2021; 8p. [Google Scholar]
- Zombori, L. Adatok Nagykovácsi levéldarázsfaunájához I. (Hymenoptera, Symphyta). Folia Entomol. Hung. 1973, 26, 217–224. [Google Scholar]
- Zombori, L. Jegyzetek Nagykovácsi levéldarázs faunájáról (Hymenoptera: Symphyta) II. Folia Entomol. Hung. 1975, 28, 223–229. [Google Scholar]
- Zombori, L. Adatok Nagykovácsi levéldarázs-faunájához (Hymenoptera: Symphyta) III–IV. Folia Entomol. Hung. 1975, 28, 369–381. [Google Scholar]
- Zombori, L. Sawflies from the Agtelek National Park (Hymenoptera: Symphyta). In The Fauna of the Aggtelek National Park, II; Mahunka, S., Ed.; Hungarian Natural History Museum: Budapest, Hungary, 1999; pp. 573–580. [Google Scholar]
- Zombori, L. Sawflies form Fertő-Hanság National Park (Hymenoptera: Symphyta). In The Fauna of the Fertő-Hanság National Park; Mahunka, S., Ed.; Hungarian Natural History Museum: Budapest, Hungary, 2002; pp. 545–552. [Google Scholar]
- Haris, A. Sawflies of the Zselic Hills, SW Hungary Hymenoptera: Symphyta. Nat. Somogyiensis 2009, 15, 127–158. [Google Scholar] [CrossRef]
- Haris, A. Sawflies of the Vértes Mountains Hymenoptera: Symphyta. Nat. Somogyiensis 2010, 17, 209–238. [Google Scholar] [CrossRef]
- Haris, A. Sawflies of the Börzsöny Mountains North Hungary Hymenoptera: Symphyta. Nat. Somogyiensis 2011, 19, 149–176. [Google Scholar] [CrossRef]
- Haris, A. Sawflies of Belső-Somogy (Hymenoptera: Symphyta). Nat. Somogyiensis 2012, 22, 141–162. [Google Scholar] [CrossRef]
- Haris, A. Second contribution to the sawflies of Belső Somogy Hymenoptera: Symphyta. Nat. Somogyiensis 2018, 31, 45–62. [Google Scholar] [CrossRef]
- Haris, A. Sawflies from Külső-Somogy, South-West Hungary (Hymenoptera: Symphyta). Nat. Somogyiensis 2018, 32, 147–164. [Google Scholar] [CrossRef]
- Haris, A. Sawflies of the Keszthely Hills and its surroundings. Nat. Somogyiensis 2019, 33, 107–128. [Google Scholar] [CrossRef]
- Haris, A. Sawflies of Southern part of Somogy county Hymenoptera: Symphyta. Nat. Somogyiensis 2020, 35, 51–70. [Google Scholar] [CrossRef]
- Haris, A. Sawflies of the Cserhát Mountains Hymenoptera: Symphyta. Nat. Somogyiensis 2021, 37, 25–42. [Google Scholar] [CrossRef]
- Haris, A. Second contribution to the knowledge of sawflies of the Zselic Hills (Hymenoptera: Symphyta). A Kaposvári Rippl-Rónai Múzeum Közleményei (Commun. Rippl-Rónai Mus. Kaposvár) 2022, 8, 65–80. [Google Scholar] [CrossRef]
- Haris, A.; Vidlička, L.; Majzlan, O.; Roller, L. Effectiveness of Malaise trap and sweep net sampling in sawfly research (Hymenoptera: Symphyta). Biologia 2024, 79, 1705–1714. [Google Scholar] [CrossRef]
- Roller, L. Sawfly (Hymenoptera, Symphyta) community in the Devínska Kobyla National Nature Reserve. Biologia 1998, 53, 213–221. [Google Scholar]
- Roller, L. Seasonal flight activity of sawflies Hymenoptera, Symphyta in submontane region of the West Carpathians, Central Slovakia. Biologia 2006, 61, 193–205. [Google Scholar] [CrossRef]
- Józan, Z. A Zselic méhszerű (Hymenoptera, Apoidea) faunájának alapvetése. The Apoidea (Hymenoptea) fauna of the Zselic Downs. A Janus Pannon. Múzeum Évkönyve 1989, 34, 81–92. [Google Scholar]
- Józan, Z. A Zselic darázsfaunájának (Hymenoptera, Aculeata) állatföldrajzi és ökofaunisztikai vizsgálata. Zoologeographic and ecofaunistic study of the Aculeata fauna (Hymenoptera, Aculeata) of Zselic. Somogyi Múzeumok Közleményei 1992, 9, 279–292. [Google Scholar]
- Józan, Z. A Béda-Karapancsa Tájvédelmi Körzet fullánkos hártyásszárnyú (Hymenoptera, Aculeata) faunájának alapvetése. Aculeata (Hymenoptera, Aculeata) fauna of the Béda-Karapancsa Landscape Protection Area. Dunántúli Dolg. Természettudományi Sor. 1992, 6, 219–246. [Google Scholar]
- Józan, Z. A Boronka-melléki Tájvédelmi Körzet fullánkos hártyásszárnyú (Hymenoptera, Aculeata) faunájának alapvetése. Aculeata (Hymenoptera, Aculeata) fauna of the Boronka Landscape Protection Area. Dunántúli Dolg. Természettudományi Sor. 1992, 7, 163–210. [Google Scholar]
- Józan, Z. Adatok a tervezett Duna-Dráva Nemzeti Park fullánkos hártyásszárnyú (Hymenoptera, Aculeata) faunájának ismeretéhez. Data to the knowledge of the Aculeata (Hymenoptera, Aculeata) fauna of the planned Danube-Dráva National Park. Dunántúli Dolg. Természettudományi Sor. 1995, 8, 99–115. [Google Scholar]
- Józan, Z. A Mecsek méhszerű faunája (Hymenoptera, Apoidea). The Apoidea (Hymenoptera) fauna of the Mecsek Mountains (Hungary: South Transdanubia). A Janus Pannon. Múzeum Évkönyve 1995, 40, 29–43. [Google Scholar]
- Józan, Z. A Baláta környék fullánkos hártyásszárnyú faunájának (Hym., Aculeata) alapvetése. Aculeata fauna of Lake Baláta (Hym., Aculeata). Somogyi Múzeumok Közleményei 1996, 12, 271–297. [Google Scholar]
- Józan, Z. A Duna-Dráva Nemzeti Park fullánkos hártyásszárnyú (Hymenoptera, Aculeata) faunája.—The Aculeata fauna of the Duna-Dráva National Park, Hungary (Hymenoptera, Aculeata). Dunántúli Dolg. Természettudományi Sor. 1998, 9, 291–327. [Google Scholar]
- Józan, Z. A Villányi-hegység fullánkos hártyásszárnyú (Hymenoptera, Aculeata) faunája. The Aculeata (Hymenoptera) fauna of the Villány Hills, South Hungary. Dunántúli Dolg. Természettudományi Sor. 2000, 10, 267–283. [Google Scholar]
- Józan, Z. A Mecsek kaparódarázs faunájának (Hymenoptera: Sphecoidea) faunisztikai, állatföldrajzi és ökofunisztikai vizsgálata. Faunistical, zoogeographical and ecofaunistical investigation on the Sphecoids fauna of the Mecsek Montains (Hymenoptera, Sphecoidea). Nat. Somogyiensis 2002, 3, 45–56. [Google Scholar] [CrossRef]
- Józan, Z. Az Őrség és környéke fullánkos hártyásszárnyú faunájának alapvetése (Hymenoptera, Aculeata). Aculeata (Hymenoptera, Aculeata) fauna of Őrség and its surroundings. Praenorica Folia Hist.-Nat. 2002, 6, 59–96. [Google Scholar]
- Józan, Z. A Mecsek fullánkos hártyásszárnyú faunája (Hymenoptera, Aculeata). Aculeata fauna of Mecsek Hills (Hymenoptera, Aculeata). Folia Comloensis 2006, 15, 219–238. [Google Scholar]
- Józan, Z. Új kaparódarázs fajok (Hymenoptera, Sphecidae) Magyarország faunájában. New sphecid wasps (Hymenoptera, Sphecidae) in the fauna of Hungary. Somogyi Múzeumok Közleményei 2008, 18, 81–83. [Google Scholar]
- Józan, Z. A Barcsi borókás fullánkos faunája, III. (Hymenoptera: Aculeata). Aculeata fauna of Barcs Juniper Woodland (Hymenoptera: Aculeata). Nat. Somogyiensis 2015, 26, 95–108. [Google Scholar] [CrossRef]
- Tóth, S. Angaben zur Kenntnis der Schwebfliegen-Fauna der Slowakei (Diptera: Syrphidae). Folia Musei Hist.-Nat. Bakony. 1990, 9, 91–108. [Google Scholar]
- Tóth, S. Magyarország zengőlégy faunája (Diptera: Syrphidae). Hoverflies of Hungary (Diptera: Syrphidae). e-Acta Nat. Pannonica 2011, (Suppl. S1), 5–408. [Google Scholar]
- Tóth, S. Magyarország fürkészlégy faunája (Diptera: Tachinidae). Tachinid flies of Hungary (Diptera: Tachinidae). e-Acta Nat. Pannonica 2013, 5 (Suppl. S1), 1–321. [Google Scholar]
- Tóth, S. A hazai bögölyök nyomában (Diptera: Tabanidae). Horse-flies of Hungary (Diptera: Tabanidae). e-Acta Naturalia Pannonica 2024, (Suppl. S4), 1–124. [Google Scholar]
- Ábrahám, L. Biomonitoring of the buttetfly fauna in the Drava region {Lepidopteia: Diurna). Nat. Somogyiensis 2005, 7, 63–74. [Google Scholar] [CrossRef]
- Ábrahám, L. A Boronka-melléki Tájvédelmi Körzet nagylepke faunájának természetvédelmi értékelése I. (Lepidoptera). Nature conservation evaluation of the macrolepidoptera fauna of Boronka Landscape Protected Area (Lepidoptera). Dunántúli Dolg. Természettudományi Sor. 1992, 7, 241–271. [Google Scholar]
- Ábrahám, L. Bakonynána és környéke nagylepke faunája (Lepidoptera) Macrolepidoptera fauna of Bakonynána region. Folia Musei Hist.-Nat. Bakony. 1991, 10, 85–104. [Google Scholar]
- Ábrahám, L. Lápi tarkalepke Euphydryas aurinia (Rottembtirg, 1775). Marsh fritillary Euphydryas aurinia (Rottembtirg, 1775). In Natura 2000 Fajok és Élőhelyek Magyarországon; Haraszthy, L., Ed.; Pro Vértes Közalapítvány: Csákvár, Hungary, 2014; pp. 323–326. [Google Scholar]
- Ábrahám, L.; Herczig, B.; Bürgés, G. Faunisztikai adatok a Keszthelyi-hegység nagylepke faunájának ismeretéhez (Lepidoptera: Macrolepidoptera}. Data to the Macrolepidoptera fauna of Keszthely Hills (Lepidoptera: Macrolepidoptera}. Nat. Somogyiensis 2007, 10, 303–330. [Google Scholar] [CrossRef]
- Ábrahám, L.; Uherkovich, À. A Zselic nagylepkéi (Lepidoptera) 1. Bevezetés és faunisztikai alapvetés. Macrolepidoptera fauna of Zselic (Lepidoptera) Introduction and biomonitoring. A Janus Pannon. Múzeum Évkönyve 1993, 38, 47–59. [Google Scholar]
- Uherkovich, Á. Long-term monitoring of biodiversity by the study of butterflies and larger moths (Lepidoptera) in Sellye region (South Hungary, co. Baranya) in the years 1967–2022. Nat. Somogyiensis 2022, 9, 95–138. [Google Scholar] [CrossRef]
- Pillich, F. Aus der Arthropodenwelt Simontornya’s; Pillich, F. private edition; Simontornya, Hungary, 1914; 172p. [Google Scholar]
- Sáfián, S. Butterflies of Kercaszomor (Őrség), Western Hungary (Lepidoptera: Papilionoidea and Hesperioidea). Nat. Somogyiensis 2011, 19, 251–262. [Google Scholar] [CrossRef]
- Ács, E.; Ronkay, G.; Ronkay, L.; Cs, S.; Varga, Z.; Vojnits, A. The Lepidoptera of the Bátorliget Nature Conservation areas. In The Bátorliget Nature Reserves—After Forty Years; Mahunka, S., Ed.; Hungarian Natural History Museum: Budapest, Hungary, 1991; Volume 2, pp. 505–540. [Google Scholar]
- Čanády, A. Príspevok k poznaniu výskytu denných motýľov (Rhopalocera) v urbánnom prostredí Košíc. (Slovensko). Contribution to the knowledge of the occurrence of butterflies (Rhopalocera) in the urban environment of Košice. (Slovakia). Folia Faun. Slovaca 2014, 19, 235–241. [Google Scholar]
- Dietzel, G. A Bakony nappali lepkéi. Butterflies of Bakony Mountains. In A Bakony Természettudományi Kutatásának Eredményei 21; Bakony Natural History Museum, Ed.; Bakonyi Természettudományi Múzeum: Zirc, Hungary, 1997; pp. 1–212. [Google Scholar]
- Sarvašová, L. Denné motýle (lepidoptera, papilionoidea) lúk kúpeľov sliač a okolia (Slovensko). Butterflies (lepidoptera, papilionoidea) meadow of spas sliač and surroundings (Slovakia). Folia Faun. Slovaca 2016, 21, 63–71. [Google Scholar]
- Gergely, P. A pomázi Majdán-fennsík nappali lepkéinek megfigyelései 2000 és 2020 között (Lepidoptera: Rhopalocera) Observations of butterflies in the Majdan plateau of Pomáz (Hungary) between 2000 and 2020 (Lepidoptera: Rhopalocera). Lepidopterol. Hung. 2021, 17, 99–107. [Google Scholar] [CrossRef]
- Gór, Á. Lepkefaunisztikai kutatások Biatorbágyon és környékén (Lepidoptera). Lepidoptera survey in Biatorbágy (Hungary) and its surrounding areas. eActa Nat. Pannonica 2018, 16, 55–70. [Google Scholar] [CrossRef]
- Schmidt, P. A Csombárdi-rét Természetvédelmi Terület nappali lepkéinek alapállapot felmérése (Lepiodptera). The basic survey of the butterfies in the Csombárd-meadow Nature Conservation Area (Lepidoptera). Nat. Somogyiensis 2017, 30, 179–192. [Google Scholar] [CrossRef]
- Németh, L. Data to the knowledge of the Macrolepidoptera-fauna of the Tapolca basin. Folia Musei Hist.-Nat. Bakony. 1991, 10, 105–136. [Google Scholar]
- Szabóky, C.; Samu, F.; Szeőke, K.; Petrányi, G. Simontornya lepkevilágáról (Lepidoptera). Moths and butterflies of Simontornya. In Simontornya Ízeltlábúi. Arthropods of Simontornya; Hungarian Biodiversity Research Society, Ed.; Magyar Biodiverzitás-kutató Társaság: Budapest, Hungary, 2014; pp. 143–186. [Google Scholar]
- Hudák, T. A nappali lepkefauna vizsgálata Székesfehérváron (Lepidoptera: Rhopalocera). Investigation on the butterfly fauna of Székesfehérvár (Lepidoptera: Rhopalocera). Nat. Somogyiensis 2018, 31, 113–136. [Google Scholar] [CrossRef]
- Varga, J.; Korompai, T.; Horokán, K.; Hirka, A.; Gáspár, C.; Kozma, P.; Csóka, G.; Csuzdi, C. Analysis of the Macrolepidoptera fauna in Répáshuta based on the catches of a light-trap between 2014–2019. Acta Univ. Esterházy Sect. Biol. 2022, 47, 59–75. [Google Scholar]
- Árnyas, E.; Szabó, S.; Tóthmérész, B.; Varga, Z. Lepkefaunisztikai vizsgálatok fénycsapdás gyűjtéssel az Aggteleki Nemzeti Parkban. Moth fauna studies with light trap collection in the Aggtelek National Park. Természetvédelmi Közl. 2004, 11, 34–42. [Google Scholar]
- Kovács, L. Bátorliget nagylepke-faunája. Macrolepidoptera fauna of Bátorliget. In Bátorliget Élővilága. (Die Tier- und Pflanzenwelt des Naturschutzgebietes von Bátorliget und seiner Umgebung); Székessy, V., Ed.; Akadémiai kiadó: Budapest, Hungary, 1953; 486p. [Google Scholar]
- Infusino, M.; Brehm, G.; Di Marco, C.; Scalercio, S. Assessing the efficiency of UV LEDs as light sources for sampling the diversity of macro-moths (Lepidoptera). Eur. J. Entomol. 2017, 114, 25–33. [Google Scholar] [CrossRef]
- Pan, H.; Liang, G.; Lu, Y. Response of Different Insect Groups to Various Wavelengths of Light under Field Conditions. Insects 2021, 12, 427. [Google Scholar] [CrossRef] [PubMed]
- Goulet, H. Herbicides, Beetles, and the Decline of Insectivorous Birds. Canada’s Oldest Field Naturalist Club. 2023. 35p. Available online: https://ofnc.ca/wp-content/uploads/2018/01/Herbicides_beetles_birds.pdf (accessed on 30 March 2024).
- Liston, A.D. Compendium of European Sawflie; List of Species, Modern Nomenclature, Distribution, Foodplants, Identification Literature; Chalastos Forestry: Gottfrieding, Germany, 1995; pp. 1–190. [Google Scholar]
- Roller, L.; Haris, A. Sawflies of the Carpathian Basin, History and Current Research. Nat. Somogyiensis 2008, 11, 1–261. [Google Scholar] [CrossRef]
- Pearce-Higgins, J.W.; Beale, C.M.; Oliver, T.H.; August, T.A.; Carroll, M.; Massimino, D.; Ockendon, N.; Savage, J.; Wheatley, C.J.; Ausden, M.A.; et al. A national-scale assessment of climate change impacts on species: Assessing the balance of risks and opportunities for multiple taxa. Biol. Conserv. 2017, 213, 124–134. [Google Scholar] [CrossRef]
- Smetana, V.; Šima, P.; Bogusch, P.; Erhart, J.; Holý, K.; Macek, J.; Roller, L.; Straka, J. Hymenoptera of the selected localities in the environs of Levice and Kremnica towns. Acta Musei Tekovensis Levice 2015, 10, 44–68. [Google Scholar]
- Šima, P.; Straka, J. First records of Heriades rubicola Pérez, 1890 (Hymenoptera: Megachilidae) and Nomada moeschleri Alfken, 1913 (Hymenoptera: Apidae) from Slovakia. Entomofauna Carpathica 2016, 28, 14–18. [Google Scholar]
- Mielczarek, L. The first records of Chalcosyrphus pannonicus (Ooldenberg, 1916) (Diptera: Syrphidae) in Poland and Slovakia. Dipteron 2014, 30, 50–54. [Google Scholar]
- Tóth, S. A Dél-Dunántúl bögöly faunájáról (Diptera: Tabanidae). Nat. Somogyiensis 2016, 28, 5–16. [Google Scholar]
- Nartshuk, E.P.; Krivokhatsky, V.; Evenhuis, N. First record of a bee fly (Diptera: Bombyliidae) parasitic on antlions (Myrmeleontidae) in Russia. Russ. Entomol. J. 2019, 28, 189–191. [Google Scholar] [CrossRef]
- Ábrahám, L. Micomitra stupida (Diptera, Bombyliidae): A new parasite of Euroleon nostras (Neuroptera, Myrmeleontidae). Dunántúli Dolg. Természettudományi Sor. 1998, 9, 421–422. [Google Scholar]
- Yeates, D.K. The evolutionary pattern of host use in the Bombyliidae (Diptera): A diverse family of parasitoid flies. Biol. J. Linn. Soc. 1997, 60, 149–185. [Google Scholar] [CrossRef]
- Kaplan, E.; Haris, A. Contribution to the knowledge of the sawflies (Hymenoptera: Symphyta) from Turkey. Nat. Somogyiensis 2021, 37, 25–38. [Google Scholar] [CrossRef]
- Barbir, J.; Martín, L.O.; Lloveras, X.R. Impact of Climate Change on Sawfly (Suborder: Symphyta) Polinators in Andalusia Region, Spain. In Handbook of Climate Change and Biodiversity; Walter Leal Filho, W.L., Barbir, J., Preziosi, R., Eds.; Climate Change Management; Springer Nature Switzerland AG: Cham, Switzerland, 2018; 323p. [Google Scholar] [CrossRef]
- Olszewski, P.; Dyderski, M.K.; Dylewski, Ł.; Bogusch, P.; Schmid-Egger, C.; Ljubomirov, T.; Zimmermann, D.; Le Divelec, R.; Wiśniowski, B.; Twerd, L.; et al. European beewolf (Philanthus triangulum) will expand its geographic range as a result of climate warming. Reg. Environ. Chang. 2022, 22, 129. [Google Scholar] [CrossRef]
- Eickermann, M.; Junk, J.; Rapisarda, C. Climate Change and Insects. Insects 2023, 14, 678. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.H. Sphex ichneumoneus and Sphex pensylvanicus (Hymenoptera: Sphecidae) in Atlantic Canada: Evidence of recent range expansion into the region. Can. Field-Nat. 2020, 134, 52–55. [Google Scholar] [CrossRef]
- Vélez, D.; Silva, D.P.; Vivallo, F. Where could Centris nigrescens (Hymenoptera: Apidae) go under climate change? J. Apic. Res. 2023, 62, 1082–1090. [Google Scholar] [CrossRef]
- Zimmermann, D.; Schoder, S.; Zettel, H.; Hainz-Renetzeder, C.; Kratschmer, S. Changes in the wild bee community (Hymenoptera: Apoidea) over 100 years in relation to land use: A case study in a protected steppe habitat in Eastern Austria. J. Insect Conserv. 2023, 27, 625–641. [Google Scholar] [CrossRef]
- Sánchez-Bayo, F.; Wyckhuys, K.A.G. Further evidence for a global decline of the entomofauna. Austral Entomol. 2021, 60, 9–26. [Google Scholar] [CrossRef]
- Powney, G.D.; Carvell, C.; Edwards, M.; Morris, R.K.A.; Roy, H.E.; Woodcock, B.A.; Isaac, N.J.B. Widespread losses of pollinating insects in Britain. Nat. Commun. 2019, 10, 1018. [Google Scholar] [CrossRef] [PubMed]
- Bogusch, P.; Horák, J. Saproxylic bees and wasps. In Saproxylic Insects: Diversity, Ecology and Conservation; Ulyshen, M., Ed.; Springer Books: Bern, Switzerland, 2018; pp. 217–235. [Google Scholar] [CrossRef]
- Bartomeus, I.; Ascher, J.S.; Gibbs, J.; Danforth, B.N.; Wagner, D.L.; Hedtke, S.M.; Winfree, R. Historical changes in northeastern US bee pollinators related to shared ecological traits. Proc. Nat. Acad. Sci. USA 2013, 110, 4656–4660. [Google Scholar] [CrossRef] [PubMed]
- Kells, A.R.; Goulson, D. Preferred nesting sites of bumblebee queens (Hymenoptera: Apidae) in agroecosystems in the UK. Biol. Conserv. 2003, 109, 165–174. [Google Scholar] [CrossRef]
- Day, M.C. Towards the Conservation of Aculeate Hymenoptera in Europe; Council of Europe Press: Strasbourg, France, 1991; 44p. [Google Scholar]
- Williams, P.H. The distribution and decline of British bumblebees (Bombus Latr.). J. Apic. Res. 1982, 121, 236–245. [Google Scholar] [CrossRef]
- Plowright, C.M.S.; Plowright, R.C.; Williams, P.H. Replacement of Bombus muscorum by Bombus pascuorum in northern Britain? Can. Entomol. 1997, 129, 985–990. [Google Scholar] [CrossRef]
- Jakab, D.A.; Tóth, M.; Szarukán, I.; Szanyi, S.; Józan, Z.; Sárospataki, M.; Nagy, A. Long-term changes in the composition and distribution of the Hungarian bumble bee fauna (Hymenoptera, Apidae, Bombus). J. Hymenopt. Res. 2023, 96, 207–237. [Google Scholar] [CrossRef]
- Sárospataki, M.; Novák, J.; Molnár, V. Hazai poszméhfajok (Bombus spp.) veszélyeztetettsége és védelmük szükségessége. Bumblebees of Hungary (Bombus spp.) and their conservation. Természetvédelmi Közlemények 2004, 11, 299–307. [Google Scholar]
- Šima, P.; Smetana, V.; Čížek, J. Records of Bombus (Megabombus) argillaceus (Hymenoptera: Apidae: Bombini) from Slovakia and notes on the record from the Czech Republic. Klapalekiana 2014, 50, 101–106. [Google Scholar]
- Šima, P.; Smetana, V. Bombus (Cullumanobombus) semenoviellus (Hymenoptera: Apidae: Bombini) new species for the bumble bee fauna of Slovakia. Klapalekiana 2012, 48, 141–147. [Google Scholar]
- Smetana, V. Čmele a spoločenské osy (Hymenoptera: Bombini, Polistinae et Vespinae) na vybraných lokalitách v národnom parku Veľká Fatra. Acta Musei Tekovensis Levice 2008, 7, 23–33. [Google Scholar]
- Smetana, V. Výsledky výskumu čmeľovitých (Hymenoptera: Bombidae) na vybraných lokalitách v Čergove, Bachurni a v bradlovom pásme Spišsko-šarišského medzihoria. Nat. Carp. 2000, 41, 59–66. [Google Scholar]
- Šima, P.; Smetana, V. Čmele (Hymenoptera: Bombidae) na vybraných lokalitách Popradskej kotliny. Nat. Tut. 2019, 23, 169–180. [Google Scholar]
- Tkalců, B. Vier für die Slowakei neu festgestellte Bienenarten (Hymenoptera, Apoidea). Biologia 1973, 28, 679–687. [Google Scholar]
- Móczár, M. Magyarország és a környező területek dongóméheinek (Bombus Latr.) rendszere és ökológiája. Taxonomy and ecology of bumblebees (Bombus Latr.) of Hungary and the surrounding areas. Ann. Hist. Nat. Mus. Hung. 1953, 4, 131–159. [Google Scholar]
- Móczár, M. A dongóméhek (Bombus Latr.) faunakatalógusa (Cat. Hym. IV). Faunistic catalogue of Bumblebees (Cat. Hym. IV). Folia Entomol. Hung. 1953, 6, 197–228. [Google Scholar]
- Tanács, L. The Apoidea (Hymenoptera) of the Tisza–dam. Tiscia 1975, 10, 55–66. [Google Scholar]
- Biella, P.; Ćetković, A.; Gogala, A.; Neumayer, J.; Sárospataki, M.; Šima, P.; Smetana, V. Northwestward range expansion of the bumblebee Bombus haematurus into Central Europe is associated with warmer winters and niche conservatism. Insect Sci. 2021, 28, 861–872. [Google Scholar] [CrossRef]
- Šima, P.; Smetana, V. Quo vadis Bombus haematurus? An outline of the ecology and biology of a species expanding in Slovakia. Acta Musei Tekovensis Levice 2018, 11, 41–65. [Google Scholar]
- Biella, P.; Galimberti, A. The spread of Bombus haematurus in Italy and its first DNA barcode reference sequence. Fragm. Entomol. 2020, 52, 67–70. [Google Scholar] [CrossRef]
- Rasmont, P.; Franzen, M.; Lecocq, T.; Harpke, A.; Roberts, S.; Biesmeijer, K.; Castro, L.; Cederberg, B.; Dvorak, L.; Fitzpatrick, U.; et al. Climatic Risk and Distribution Atlas of European Bumblebees. BioRisk 2015, 10, 1–236. [Google Scholar] [CrossRef]
- Miličić, M.; Vujić, A.; Cardoso, P. Effects of climate change on the distribution of hoverflyspecies (Diptera: Syrphidae) in Southeast Europe. Biodivers. Conserv. 2018, 27, 1173–1187. [Google Scholar] [CrossRef]
- IUCN SSC HSG/CPSG. European Hoverflies: Moving from Assessment to Conservation Planning. A report to the European Commission by the IUCN SSC Conservation Planning Specialist Group (CPSG) and the IUCN SSC Hoverfly Specialist Group (HSG); Conservation Planning Specialist Group: Apple Valley, MN, USA, 2022; 84p. [Google Scholar]
- Barendregt, A.; Zeegers, T.; van Steenis, W.; Jongejans, E. Forest hoverfly community collapse: Abundance and species richness drop over four decades. Insect Conserv. Divers. 2022, 15, 510–521. [Google Scholar] [CrossRef]
- Sommaggio, D.; Zanotelli, L.; Vettorazzo, E.; Burgio, G.; Fontana, P. Different Distribution Patterns of Hoverflies (Diptera: Syrphidae) and Bees (Hymenoptera: Anthophila) along Altitudinal Gradients in Dolomiti Bellunesi National Park (Italy). Insects 2022, 13, 293. [Google Scholar] [CrossRef] [PubMed]
- Herczeg, T.; Száz, D.; Blahó, M.; Barta, A.; Gyurkovszky, M.; Farkas, R.; Horváth, G. The effect of weather variables on the flight activity of horseflies (Diptera: Tabanidae) in the continental climate of Hungary. Parasitol. Res. 2015, 114, 1087–1097. [Google Scholar] [CrossRef] [PubMed]
- Dörge, D.D.; Sarah Cunze, S.; Klimpel, S. Incompletely observed: Niche estimation for six frequent European horsefly species (Diptera, Tabanoidea, Tabanidae). Parasites Vectors 2020, 13, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Régnière, J.; Thireau, J.C.; Saint-Amant, R.; Martel, V. Modeling Climatic Influences on Three Parasitoids of Low-Density Spruce Budworm Populations. Part 3: Actia interrupta (Diptera: Tachinidae). Forests 2021, 12, 1471. [Google Scholar] [CrossRef]
- Boesi, R.; Polidori, C.; Andrietti, F. Searching for the Right Target: Oviposition and Feeding Behavior in Bombylius Bee Flies (Diptera: Bombyliidae). Zool. Stud. 2009, 48, 141–150. [Google Scholar]
- Fox, R.J.P. Citizen Science and Lepidoptera Biodiversity Change in Great Britain. Ph.D. Thesis, University of Exeter, Exeter, UK, 2020; 297p. Available online: https://ore.exeter.ac.uk/repository/bitstream/handle/10871/120925/FoxR.pdf?sequence=1 (accessed on 18 March 2024).
- Dar, A.A.; Jamal, K. The decline of moths globally: A review of possible causes. Munis Entomol. Zool. 2021, 16, 317–326. [Google Scholar]
- Szentkirályi, F.; Leskó, K.; Kádár, F. Climatic effects on long-term fluctuations in species richness and abundance level of forest macrolepidopteran assesmblages in a Hungarian mountainous region. Carpth. J. Earth Environ. Sci. 2007, 2, 73–82. [Google Scholar]
- Conrad, K.F.; Warren, M.S.; Fox, R.; Parsons, M.S.; Woiwod, I.P. Rapid declines of common, widespread British moths provide evidence of an insect biodiversity crisis. Biol. Conserv. 2006, 132, 279–291. [Google Scholar] [CrossRef]
- Mikkola, K. Population trends of Finnish Lepidoptera during 1961–1996. Entomol. Fenn. 1997, 8, 121–143. [Google Scholar] [CrossRef]
- Hill, G.M.; Kawahara, A.Y.; Daniels, J.C.; Bateman, C.C.; Scheffers, B.R. Climate change effects on animal ecology: Butterflies and moths as a case study. Biol. Rev. 2021, 96, 2113–2126. [Google Scholar] [CrossRef] [PubMed]
- Mangels, J.K. Land Use and Climate Change: Anthropogenic Effects on Arthropod Communities and Functional Traits. Ph.D. Thesis, Department of Biology, Technical University of Darmstadt (Germany), Darmstadt, Germany, 2017; p. 169. Available online: https://tuprints.ulb.tu-darmstadt.de/7156/1/Dissertation_Mangels.pdf (accessed on 18 March 2024).
- Van Zandt, P.A.; Johnson, D.D.; Hartley, C.; LeCroy, K.A.; Shew, H.W.; Davis, T.B.; Lehnert, M.S. Which Moths Might be Pollinators? Approaches in the Search for the Flower-Visiting Needles in the Lepidopteran Haystack. Ecol. Entomol. 2019, 45, 13–25. [Google Scholar] [CrossRef]
- Habel, J.C.; Schmitt, T.; Gros, P.; Ulrich, W. Breakpoints in butterfly decline in Central Europe over the last century. Sci. Total. Environ. 2022, 851, 158315. [Google Scholar] [CrossRef]
- Warren, M.S.; Maes, D.; van Swaay, C.A.M.; Goffart, P.; Van Dyck, H.; Bourn, N.A.D.; Wynhoff, I.; Hoare, D.; Ellis, S. The decline of butterflies in Europe: Problems, significance, and possible solutions. Proc. Nat. Acad. Sci. USA 2021, 118, e2002551117. [Google Scholar] [CrossRef]
- Turčáni, M.; Kulfan, J.; Minďáš, J. Penetration of the south European butterfly Libythea celtis (Laicharting 1782) northwards: Indication of global man made environmental changes? Ekológia 2003, 33, 28–41. [Google Scholar]
- Bury, J.; Maslo, D.; Obszarny, M.; Paluch, F. Expansion of Iphiclides podalirius (Linnaeus, 1758) (Lepidoptera: Papilionidae) on Podkarpacie Region (SE Poland) in 2010–2014. Park. Nar. Rezerwaty Przyr. 2015, 34, 3–17. [Google Scholar]
- Gavrilov, M.B.; Radaković, M.G.; Sipos, G.; Mezősi, G.; Gavrilov, G.; Lukić, T.; Basarin, B.; Benyhe, B.; Fiala, K.; Kozák, P.; et al. Aridity in the central and southern Pannonian basin. Atmosphere 2020, 11, 1269. [Google Scholar] [CrossRef]
- Hallmann, C.A.; Sorg, M.; Jongejans, E.; Siepel, H.; Hofland, N.; Schwan, H.; Stenmans, W.; Müller, A.; Sumser, H.; Hörren, T.; et al. More than 75 percent decline over 27 years in total flying insect biomass in protected areas. PLoS ONE 2017, 12, e0185809. [Google Scholar] [CrossRef]


| Five-Years Time Period | Five-Year Average Values | ||||
|---|---|---|---|---|---|
| Mean Temp in °C | Moisture in mm | Number of Days above 30 °C | Number of Days below 0 °C | Number of Days with Heatwave | |
| 1970–1974 | 10.0 | 600.0 | 15 | 93 | 2 |
| 2018–2022 | 10.7 | 571.6 | 39 | 98 | 12 |
| Taxon | 1971–1975 | 1986–1998 | 1990–1992 | 1996–1999 | 2009–2012 | 2019–2022 | Lin x coef. | r2 |
|---|---|---|---|---|---|---|---|---|
| Tenthredo mesomela (Linné, 1758) | 27 | 32 | 11 | 2 | 9 | 0 | −6.09 | 0.75 |
| Tenthredo atra (Linné, 1758) | 57 | 0 | 7 | 0 | 32 | 5 | −4.88 | 0.16 |
| Temthredopsis tarsata (Fabricius, 1804) | 29 | 7 | 2 | 0 | 18 | 1 | −3.11 | 0.25 |
| Macrophya blanda (Fabricius, 1775) | 21 | 20 | 0 | 0 | 17 | 1 | −3.11 | 0.31 |
| Athalia ancilla Serville, 1823 | 20 | 0 | 0 | 0 | 3 | 0 | −2.60 | 0.37 |
| Tenthredo solitaria Scopoli, 1763 | 28 | 3 | 1 | 2 | 22 | 0 | −2.34 | 0.13 |
| Tenthredopsis nassata (Linné, 1767) | 24 | 0 | 6 | 10 | 11 | 1 | −2.20 | 0.23 |
| Pachyprotasis rapae (Linné, 1767) | 25 | 39 | 16 | 10 | 47 | 13 | −1.20 | 0.02 |
| Macrophya ribis (Schrank, 1781) | 6 | 2 | 18 | 10 | 6 | 0 | −0.74 | 0.05 |
| Tenthredo campestris (Linné, 1758) | 0 | 23 | 9 | 0 | 11 | 5 | −0.57 | 0.02 |
| Tenthredo omissa (Förster, 1844) | 3 | 0 | 8 | 1 | 5 | 0 | −0.20 | 0.01 |
| Arge enodis (Linné, 1767) | 2 | 0 | 15 | 8 | 56 | 26 | −0.20 | 0.01 |
| Polynematus annulatus (Gimmerthal, 1834) | 1 | 0 | 0 | 0 | 6 | 0 | 0.37 | 0.08 |
| Ametastegia glabrata (Fallén, 1808) | 0 | 1 | 1 | 8 | 2 | 2 | 0.57 | 0.14 |
| Tenthredopsis litterata (Geoffroy, 1785) | 3 | 0 | 6 | 2 | 8 | 4 | 0.71 | 0.21 |
| Athalia lugens (Klug, 1815) | 1 | 0 | 4 | 21 | 4 | 1 | 0.82 | 0.04 |
| Pristiphora pallidiventris (Fallén, 1808) | 0 | 4 | 0 | 0 | 7 | 4 | 0.82 | 0.27 |
| Allantus cinctus (Linné, 1758) | 4 | 3 | 2 | 7 | 8 | 6 | 0.85 | 0.46 |
| Tenthredo zonula Klug, 1814 | 1 | 21 | 9 | 0 | 34 | 2 | 1.00 | 0.02 |
| Tenthredo “arcuata” | 2 | 4 | 7 | 0 | 22 | 1 | 1.20 | 0.08 |
| Tenthredopsis ornata (Serville, 1823) | 0 | 0 | 0 | 0 | 8 | 4 | 1.25 | 0.49 |
| Macrophya duodecimpunctata (Linné, 1758) | 18 | 8 | 18 | 13 | 46 | 5 | 1.26 | 0.03 |
| Arge berberidis Schrank, 1802 | 1 | 2 | 2 | 0 | 12 | 5 | 1.37 | 0.24 |
| Arge pagana (Panzer, 1797) | 1 | 1 | 3 | 0 | 16 | 3 | 1.49 | 0.21 |
| Tenthredo vespa Retzius, 1783 | 0 | 0 | 17 | 0 | 20 | 2 | 1.51 | 0.09 |
| Arge nigripes (Retzius, 1783) | 1 | 6 | 2 | 0 | 12 | 10 | 1.74 | 0.43 |
| Megalodontes plagiocephalus (Fabricius, 1804) | 1 | 4 | 0 | 1 | 18 | 5 | 1.80 | 0.25 |
| Arge ochropus (Gmelin, 1790) | 2 | 0 | 0 | 0 | 16 | 9 | 2.37 | 0.45 |
| Tenthredo temula Scopoli, 1763 | 16 | 10 | 6 | 0 | 44 | 14 | 2.45 | 0.09 |
| Tenthredo amoena Gravenhorst, 1807 | 0 | 0 | 4 | 0 | 30 | 0 | 2.45 | 0.15 |
| Tenthredo bifasciata rossii (Panzer, 1803) | 9 | 5 | 12 | 0 | 31 | 13 | 2.46 | 0.19 |
| Stethomostus fuliginosus (Schrank, 1781) | 1 | 6 | 4 | 27 | 17 | 7 | 2.46 | 0.22 |
| Tenthredo distinguenda (R. Stein, 1885) | 0 | 0 | 4 | 0 | 21 | 8 | 2.83 | 0.41 |
| Tenthredopsis stigma (Fabricius, 1798) | 3 | 4 | 6 | 0 | 19 | 19 | 3.40 | 0.58 |
| Tenthredo marginella Fabricius, 1793 | 0 | 2 | 9 | 0 | 14 | 19 | 3.49 | 0.67 |
| Monophadnus pallescens (Gmelin, 1790) | 1 | 36 | 6 | 8 | 41 | 25 | 3.90 | 0.19 |
| Tenthredopsis sordida (Klug, 1817) | 19 | 9 | 12 | 2 | 37 | 42 | 5.40 | 0.4 |
| Eutomostethus ephippium (Panzer, 1798) | 31 | 34 | 31 | 18 | 62 | 82 | 9.31 | 0.53 |
| Aglaostigma aucupariae (Klug, 1817) | 5 | 13 | 12 | 10 | 56 | 49 | 9.90 | 0.7 |
| Arge cyanocrocea (Forster, 1771) | 6 | 3 | 6 | 0 | 37 | 58 | 10.17 | 0.64 |
| Arge melanochra (Gmelin, 1790) | 9 | 8 | 12 | 0 | 101 | 40 | 12.06 | 0.35 |
| Aglaostigma fulvipes (Scopoli, 1763) | 6 | 28 | 11 | 68 | 74 | 113 | 20.86 | 0.86 |
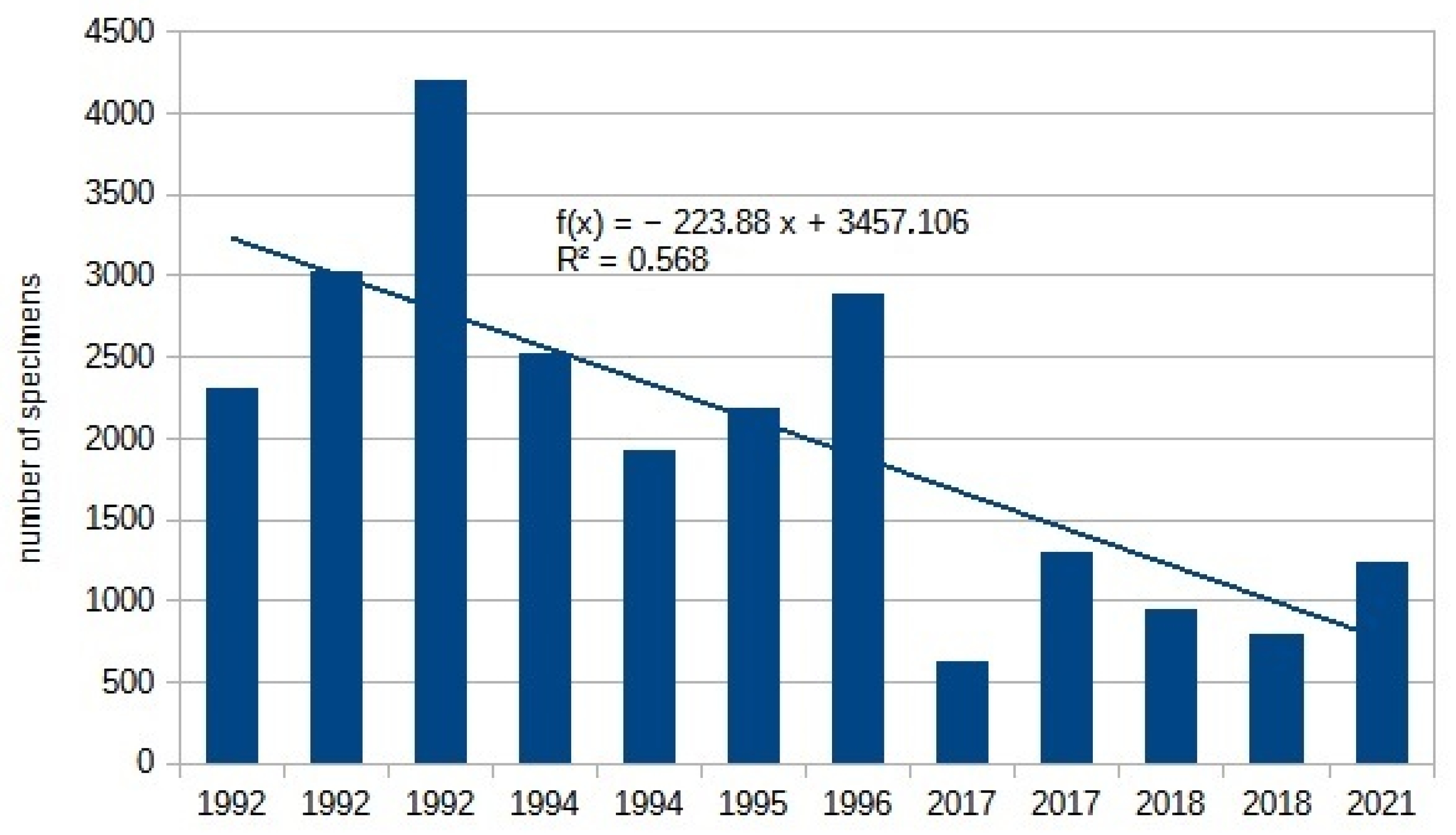
| Taxon | 1992 | 1992 | 1992 | 1994 | 1994 | 1995 | 1996 | 2017 | 2017 | 2018 | 2018 | 2021 | Lin x coef. | r2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pachyprotasis rapae (Linné, 1767) | 112 | 111 | 1154 | 12 | 6 | 10 | 617 | 0 | 0 | 0 | 1 | 0 | −34.16 | 0.12 |
| Empria sexpunctata (Serville, 1823) | 29 | 220 | 0 | 14 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | −8.28 | 0.22 |
| Cladius pectinicornis (Geoffroy, 1785) | 174 | 0 | 12 | 85 | 86 | 36 | 38 | 31 | 40 | 57 | 0 | 2 | −6.87 | 0.25 |
| Tenthredo mesomela (Linné, 1758) | 0 | 60 | 205 | 0 | 0 | 0 | 22 | 0 | 0 | 0 | 0 | 0 | −6.83 | 0.17 |
| Pteronidea myosotidis (Fabricius, 1804) | 79 | 1 | 71 | 112 | 72 | 108 | 180 | 1 | 3 | 1 | 18 | 6 | −6.38 | 0.15 |
| Ametastegia carpini (Hartig, 1837) | 27 | 4 | 95 | 171 | 8 | 0 | 0 | 25 | 0 | 0 | 4 | 3 | −6.06 | 0.17 |
| Claremontia tenuicornis (Klug, 1816) | 0 | 195 | 0 | 0 | 0 | 0 | 80 | 0 | 0 | 0 | 0 | 1 | −5.82 | 0.13 |
| Ametastegia tenera (Fallén, 1808) | 7 | 0 | 10 | 267 | 6 | 271 | 0 | 2 | 1 | 0 | 34 | 1 | −5.04 | 0.03 |
| Birka cinereipes (Klug, 1816) | 1 | 94 | 71 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | −4.81 | 0.28 |
| Tenthredopsis tarsata (Fabricius, 1804) | 115 | 0 | 0 | 4 | 16 | 0 | 2 | 0 | 0 | 0 | 1 | 0 | −4.62 | 0.26 |
| Aglaostigma aucupariae (Klug, 1817) | 106 | 0 | 10 | 23 | 61 | 2 | 7 | 2 | 1 | 24 | 6 | 0 | −4.53 | 0.26 |
| Macrophya alboannulata Costa, 1859 | 135 | 0 | 0 | 9 | 14 | 1 | 1 | 0 | 0 | 19 | 32 | 0 | −4.02 | 0.14 |
| Dolerus aeneus Hartig, 1837 | 1 | 110 | 26 | 1 | 0 | 3 | 52 | 0 | 0 | 0 | 0 | 0 | −3.98 | 0.18 |
| Tenthredopsis ornata (Serville, 1823) | 73 | 0 | 0 | 16 | 66 | 35 | 12 | 4 | 5 | 3 | 5 | 0 | −3.50 | 0.24 |
| Athalia cordata Serville, 1823 | 31 | 0 | 12 | 114 | 121 | 568 | 163 | 38 | 40 | 18 | 21 | 13 | −3.47 | 0.01 |
| Allantus cinctus (Linné, 1758) | 151 | 0 | 2 | 14 | 36 | 5 | 36 | 46 | 46 | 49 | 14 | 1 | −3.41 | 0.09 |
| Tenthredopsis sordida (Klug, 1817) | 60 | 5 | 0 | 31 | 39 | 4 | 0 | 3 | 5 | 0 | 0 | 1 | −3.27 | 0.35 |
| Empria liturata (Gmelin, 1790) | 32 | 60 | 4 | 33 | 3 | 5 | 18 | 0 | 0 | 0 | 4 | 29 | −2.54 | 0.23 |
| Tenthredo campestris (Linné, 1758) | 0 | 2 | 84 | 0 | 0 | 1 | 7 | 0 | 0 | 0 | 0 | 0 | −2.10 | 0.10 |
| Pristiphora pallidiventris (Fallén, 1808) | 3 | 18 | 15 | 85 | 2 | 54 | 27 | 2 | 1 | 3 | 2 | 10 | −2.09 | 0.08 |
| Allantus didymus (Klug, 1818) | 1 | 0 | 0 | 0 | 5 | 3 | 17 | 78 | 30 | 0 | 24 | 0 | −2.06 | 0.10 |
| Pareophora pruni (Linné, 1758) | 63 | 0 | 3 | 10 | 43 | 7 | 2 | 10 | 6 | 18 | 11 | 4 | −1.99 | 0.14 |
| Tenthredopsis nassata (Linné, 1767) | 8 | 1 | 78 | 20 | 11 | 0 | 133 | 1 | 4 | 4 | 4 | 0 | −1.94 | 0.03 |
| Pteronidea oligospila (Förster, 1854) | 0 | 83 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 28 | −1.52 | 0.05 |
| Aglaostigma fulvipes (Scopoli, 1763) | 51 | 0 | 4 | 5 | 16 | 1 | 86 | 0 | 2 | 18 | 2 | 0 | −1.48 | 0.04 |
| Allantus cingulatus (Scopoli, 1763) | 29 | 10 | 31 | 47 | 5 | 121 | 40 | 14 | 28 | 10 | 1 | −1.47 | 0.02 | |
| Pristiphora armata (Thomson, 1863) | 18 | 0 | 11 | 56 | 11 | 89 | 10 | 2 | 10 | 12 | 16 | 0 | −1.34 | 0.03 |
| Halidamia affinis (Fallén, 1807) | 47 | 0 | 9 | 10 | 26 | 0 | 10 | 3 | 12 | 10 | 17 | 4 | −1.27 | 0.12 |
| Tenthredopsis stigma (Fabricius, 1798) | 18 | 0 | 0 | 4 | 131 | 0 | 0 | 58 | 2 | 0 | 8 | 0 | −1.24 | 0.01 |
| Dolerus gonager (Fabricius, 1781) | 24 | 9 | 25 | 31 | 4 | 2 | 3 | 3 | 0 | 26 | 20 | 0 | −1.10 | 0.11 |
| Tenthredopsis litterata (Geoffroy, 1785) | 4 | 0 | 0 | 1 | 111 | 0 | 16 | 4 | 4 | 3 | 2 | 0 | −1.03 | 0.01 |
| Monophadnus pallescens (Gmelin, 1790) | 4 | 5 | 33 | 5 | 0 | 2 | 7 | 0 | 1 | 0 | 1 | 9 | −0.79 | 0.10 |
| Tenthredella atra (Linné, 1758) | 0 | 14 | 16 | 1 | 1 | 1 | 24 | 2 | 1 | 0 | 0 | 0 | −0.74 | 0.11 |
| Eutomostethus ephippium (Panzer, 1798) | 19 | 0 | 13 | 3 | 0 | 0 | 5 | 0 | 0 | 0 | 0 | 10 | −0.70 | 0.15 |
| Euura mucronata (Hartig, 1837) | 0 | 16 | 9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | −0.64 | 0.21 |
| Ametastegia glabrata (Fallén, 1808) | 11 | 0 | 5 | 8 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | −0.57 | 0.30 |
| Claremontia alternipes (Klug, 1816) | 28 | 12 | 5 | 1 | 0 | 2 | 16 | 1 | 0 | 10 | 3 | 17 | −0.54 | 0.05 |
| Arge berberidis Schrank, 1802 | 9 | 0 | 6 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | −0.50 | 0.38 |
| Tenthredo solitaria Scopoli, 1763 | 4 | 0 | 10 | 1 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | −0.41 | 0.23 |
| Tenthredo “arcuata” | 0 | 0 | 18 | 1 | 0 | 2 | 22 | 0 | 0 | 0 | 0 | 0 | −0.39 | 0.03 |
| Macrophya annulata (Geoffroy, 1785) | 23 | 0 | 7 | 4 | 3 | 5 | 2 | 5 | 27 | 11 | 0 | 1 | −0.34 | 0.02 |
| Athalia lugens (Klug, 1815) | 0 | 0 | 3 | 4 | 1 | 12 | 1 | 1 | 0 | 0 | 0 | 0 | −0.18 | 0.04 |
| Athalia bicolor Serville, 1823 | 8 | 0 | 14 | 14 | 0 | 0 | 21 | 2 | 11 | 1 | 17 | 0 | −0.05 | 0.00 |
| Arge melanochra (Gmelin, 1790) | 2 | 0 | 1 | 3 | 0 | 0 | 0 | 2 | 6 | 0 | 5 | 0 | 0.13 | 0.05 |
| Macrophya duodecimpunctata (Linné, 1758) | 1 | 0 | 0 | 1 | 0 | 0 | 4 | 0 | 6 | 0 | 0 | 4 | 0.22 | 0.14 |
| Tenthredo zonula Klug, 1814 | 0 | 0 | 0 | 0 | 4 | 9 | 9 | 3 | 1 | 9 | 1 | 0 | 0.26 | 0.06 |
| Dolerus puncticollis Thomson, 1871 | 6 | 0 | 1 | 3 | 8 | 2 | 0 | 4 | 0 | 0 | 20 | 0 | 0.27 | 0.03 |
| Ardis sulcata (Cameron, 1882) | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 2 | 0 | 13 | 16 | 0 | 0.81 | 0.28 |
| Stethomostus fuliginosus (Schrank, 1781) | 3 | 0 | 11 | 2 | 2 | 0 | 0 | 0 | 0 | 1 | 0 | 37 | 1.01 | 0.12 |
| Sawflies Total | 2315 | 3033 | 4203 | 2532 | 1929 | 2184 | 2894 | 637 | 1311 | 953 | 795 | 1236 | −223.89 | 0.57 |
| Species under Detection Level (Pannon Biogeographic Region) | Detected in the High Altitudes | Species under Detection Level (Pannon Biogeographic Region) | Detected in the High Altitudes |
|---|---|---|---|
| Abia candens Konow, 1887 | yes | Pteronidea pavida (Serville, 1823) | yes |
| Aglaostigma lichtwardti (Konow, 1892) | yes | Pteronidea ribesii (Scopoli, 1763) | yes |
| Amauronematus histrio (Serville, 1823) | yes | Hypolaepus vicinus (Serville, 1823) | yes |
| Arge fuscipennis (Herrich-Schäffer, 1835) | not | Pamphilius betulae (Linné, 1758) | not |
| Arge gracilicornis (Klug, 1814) | yes | Pikonema pallescens (Hartig, 1837) | yes |
| Cephalcia abietis (Linné, 1758) | yes | Pontania bridgmanii (Cameron, 1883) | yes |
| Cephalcia arvensis Panzer, 1803 | not | Eupontania pedunculi (Hartig, 1837) | yes |
| Cephalcia erythrogaster (Hartig, 1837) | yes | Pristiphora geniculata (Hartig, 1840) | not |
| Claremontia tenuicornis (Klug, 1816) | yes | Pristiphora maesta (Zaddach, 1876) | not |
| Dolerus pratensis (Linné, 1758) | yes | Rhogogaster punctulata (Klug, 1817) | yes |
| Empria longicornis (Thomson, 1871) | yes | Siobla sturmi (Klug, 1817) | yes |
| Empria pumila (Konow, 1896) | yes | Tenthredo bipunctula Klug, 1817 | yes |
| Euura testaceipes (Brischke, 1883) | not | Tenthredo crassa Scopoli, 1763 | yes |
| Gilpina polytoma (Hartig, 1834) | yes | Tenthredo ferruginea Schrank, 1776 | yes |
| Hoplocampa brevis (Klug, 1816) | not | Tenthredo koehleri Klug, 1817 | yes |
| Janus cynosbati (Linné, 1758) | not | Tenthredo olivacea Klug, 1817 | yes |
| Janus luteipes (Lepeletier, 1823) | yes | Tenthredo rubricoxis (Enslin, 1912) | yes |
| Megalodontes cephalotes (Fabricius, 1781) | yes | Tenthredo sulphuripes (Kriechbaumer, 1869) | not |
| Monostegia cingulata (Konow, 1891) | not | Tenthredo trabeata Klug, 1817 | yes |
| Pteronidea leucotrocha (Hartig, 1837) | yes | Tenthredo velox Fabricius, 1798 | yes |
| Pteronidea papillosa (Retzius, 1783) | yes |
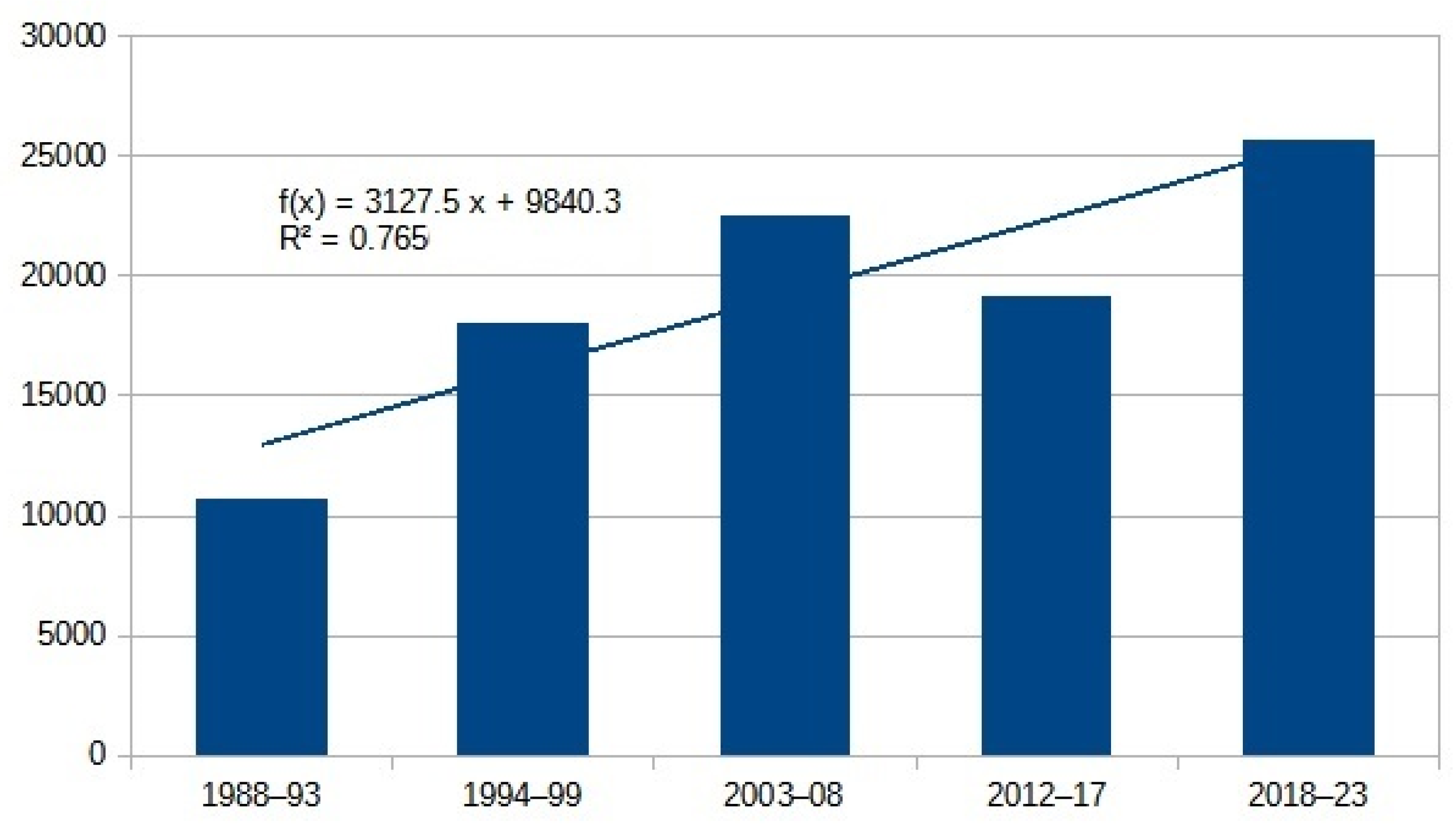
| Genera | 1988–1993 | 1994–1999 | 2003–2008 | 2012–2017 | 2018–2023 | Lin. x coef. | r2 | Changes |
|---|---|---|---|---|---|---|---|---|
| Sceliphron Klug 1801 | 15 | 18 | 28 | 17 | 64 | 9.7 | 0.56 | 4.3 |
| Cerceris Latreille, 1802 | 287 | 367 | 536 | 548 | 1084 | 177.5 | 0.81 | 3.8 |
| Gorytes Latreille, 1805 | 72 | 74 | 180 | 247 | 209 | 44.7 | 0.78 | 2.9 |
| Oxybelus Latreille, 1797 | 245 | 250 | 348 | 447 | 659 | 102.5 | 0.89 | 2.7 |
| Priocnemis Schiødte, 1837 | 33 | 33 | 127 | 68 | 83 | 13.5 | 0.26 | 2.5 |
| Hedychrum Latreille, 1806 | 157 | 162 | 207 | 287 | 372 | 56.5 | 0.91 | 2.4 |
| Crossocerus Lepeletier & Brullé, 1834 | 195 | 1430 | 1064 | 288 | 370 | −79.2 | 0.06 | 1.9 |
| Chrysis Linné, 1761 | 481 | 496 | 758 | 1089 | 903 | 143.7 | 0.75 | 1.9 |
| Scolia Fabricius, 1775 | 68 | 65 | 49 | 107 | 126 | 15.8 | 0.60 | 1.9 |
| Diodontus Curtis, 1834 | 316 | 541 | 883 | 503 | 552 | 43.4 | 0.11 | 1.7 |
| Ectemnius Dahlbom, 1845 | 241 | 245 | 540 | 305 | 255 | 8.8 | 0.01 | 1.1 |
| Crabro Fabricius, 1775 | 32 | 32 | 35 | 17 | 34 | −1.1 | 0.07 | 1.1 |
| Ammophila W. Kirby, 1798 | 87 | 78 | 101 | 106 | 82 | 1.8 | 0.06 | 0.9 |
| Megascolia Betrem, 1928 | 0 | 0 | 0 | 1 | 3 | 0.7 | 0.72 | NA |
| Family | 1988–1993 | 1994–1999 | 2003–2008 | 2012–2017 | 2018–2023 | Lin. x coef. | r2 | Changes |
| Philanthidae | 338 | 419 | 581 | 600 | 1163 | 183.1 | 0.80 | 3.4 |
| Bembicidae | 82 | 124 | 250 | 324 | 278 | 59.2 | 0.82 | 3.4 |
| Psenidae | 89 | 357 | 280 | 196 | 274 | 20.9 | 0.11 | 3.1 |
| Halictidae | 2225 | 3320 | 4524 | 4122 | 6633 | 961.8 | 0.86 | 3.0 |
| Chrysididae | 612 | 954 | 1454 | 1824 | 1707 | 306.0 | 0.89 | 2.8 |
| Sphecidae | 130 | 157 | 220 | 308 | 345 | 58.1 | 0.97 | 2.7 |
| Colletidae | 1132 | 1538 | 1817 | 1834 | 2660 | 335.2 | 0.89 | 2.3 |
| Anthophoridae | 837 | 838 | 1401 | 1301 | 1927 | 264.3 | 0.85 | 2.3 |
| Crabronidae | 2110 | 5235 | 5503 | 3698 | 4405 | 305.3 | 0.13 | 2.1 |
| Pemphredonidae | 190 | 441 | 396 | 232 | 309 | 2.9 | 0.00 | 1.6 |
| Andrenidae | 1447 | 1518 | 2615 | 1573 | 2295 | 175.1 | 0.27 | 1.6 |
| Megachilidae | 1234 | 1421 | 1237 | 1522 | 1860 | 135.3 | 0.69 | 1.5 |
| Aculeata total | 10,681 | 18,010 | 22,516 | 19,167 | 25,740 | 3127.5 | 0.77 | 2.4 |
| Species | 1988–1993 | 1994–1999 | 2003–2008 | 2012–2017 | 2018–2023 | Linear x Coeff. | r2 | Change | Species | 1988–1993 | 1994–1999 | 2003–2008 | 2012–2017 | 2018–2023 | Linear x Coeff. | r2 | Change |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nomada bifasciata Olivier, 1811 | 18 | 15 | 123 | 172 | 305 | 73 | 0.92 | 4.1 | Andrena labialis (Kirby, 1802) | 3 | 5 | 19 | 2 | 2 | −0.5 | 0.01 | 0.6 |
| Lasioglossum politum (Schenck, 1853) | 122 | 365 | 878 | 424 | 436 | 68.7 | 0.16 | 3.6 | Andrena labiata Fabricius, 1781 | 13 | 13 | 12 | 8 | 10 | −1.1 | 0.65 | 0.8 |
| Hylaeus brevicornis Nylander, 1852 | 221 | 265 | 274 | 347 | 481 | 60.2 | 0.87 | 2.2 | Chelostoma campanularum (Kirby, 1802) | 17 | 23 | 16 | 7 | 19 | −1.2 | 0.1 | 1.1 |
| Nomada goodeniana (Kirby, 1802) | 21 | 4 | 50 | 137 | 232 | 55.5 | 0.85 | 11.0 | Megachile centuncularis (Linné, 1758) | 61 | 39 | 29 | 31 | 57 | −1.6 | 0.03 | 0.9 |
| Andrena minutuloides Rcl. Perkins, 1914 | 95 | 110 | 179 | 177 | 333 | 54.3 | 0.83 | 3.5 | Andrena subopaca Nylander, 1848 | 49 | 13 | 24 | 16 | 35 | −2.2 | 0.5 | 0.7 |
| Lasioglossum marginatum (Brullé 1832) | 210 | 360 | 349 | 423 | 370 | 38.3 | 0.58 | 1.8 | Chelostoma florisomne (Linné, 1758) | 26 | 55 | 13 | 25 | 30 | −2.2 | 0.05 | 1.2 |
| Andrena flavipes Panzer, 1799 | 161 | 176 | 188 | 198 | 320 | 34 | 0.71 | 2.0 | Xylocopa valga Gerstäcker, 1872 | 13 | 20 | 6 | 4 | 6 | −3 | 0.51 | 0.5 |
| Megachile pilidens Alfken, 1924 | 28 | 39 | 31 | 86 | 151 | 29.3 | 0.78 | 5.4 | Lasioglossum calceatum (Scopoli, 1763) | 48 | 63 | 57 | 46 | 40 | −3.3 | 0.32 | 0.8 |
| Hylaeus communis Nylander, 1852 | 136 | 151 | 186 | 157 | 263 | 26 | 0.67 | 1.9 | Hylaeus variegatus (Fabricius, 1798) | 55 | 75 | 54 | 29 | 57 | −4.2 | 0.16 | 1.0 |
| Heriades truncorum (Linné, 1758) | 110 | 100 | 63 | 89 | 236 | 24.1 | 0.32 | 2.1 | Andrena limata Smith, 1853 | 35 | 38 | 30 | 18 | 13 | −6.4 | 0.87 | 0.4 |
| Sphecodes ephippius (Linné, 1767) | 39 | 38 | 110 | 46 | 150 | 23 | 0.51 | 3.8 | Colletes cunicularius (Linné, 1761) | 52 | 104 | 139 | 52 | 45 | −6.6 | 0.06 | 0.9 |
| Halictus sexcinctus Fabricius, 1775 | 10 | 37 | 29 | 47 | 117 | 22.4 | 0.75 | 11.7 | Dasypoda hirtipes (Fabricius, 1793) | 51 | 44 | 29 | 39 | 18 | −7.1 | 0.75 | 0.4 |
| Halictus subauratus (Rossi, 1792) | 60 | 53 | 84 | 85 | 153 | 21.8 | 0.76 | 2.6 | Hoplitis adunca (Panzer, 1798) | 52 | 14 | 21 | 29 | 6 | −7.7 | 0.48 | 0.1 |
| Nomiapis diversipes (Latreille, 1806) | 1 | 7 | 66 | 91 | 66 | 21.4 | 0.72 | 66.0 | Lasioglossum morio (Fabricius, 1793) | 78 | 149 | 131 | 64 | 81 | −7.9 | 0.11 | 1.0 |
| Andrena ovatula (Kirby, 1802) | 78 | 73 | 82 | 121 | 154 | 20.2 | 0.82 | 2.0 | Andrena minutula (Kirby, 1802) | 88 | 84 | 172 | 83 | 48 | −8.1 | 0.07 | 0.5 |
| Colletes daviesanus Smith, 1846 | 14 | 14 | 38 | 85 | 70 | 18.3 | 0.8 | 5.0 | Ceratina cyanea (Kirby, 1802) | 71 | 71 | 49 | 10 | 43 | −11.7 | 0.54 | 0.6 |
| Megachile ericetorum Lepeletier, 1841 | 58 | 35 | 40 | 100 | 117 | 18.3 | 0.62 | 2.0 | Osmia rufa (Linné, 1758) | 54 | 138 | 49 | 32 | 44 | −12.6 | 0.23 | 0.8 |
| Nomada distinguenda Morawitz, 1874 | 3 | 21 | 56 | 73 | 62 | 17 | 0.82 | 20.7 | Anthophora plumipes (Pallas, 1772) | 36 | 95 | 14 | 18 | 8 | −13.3 | 0.35 | 0.2 |
| Hylaeus confusus Nylander, 1852 | 122 | 105 | 73 | 44 | 97 | 11 | 0.33 | 0.8 | Lasioglossum malachurum (Kirby, 1802) | 59 | 163 | 82 | 31 | 36 | −17.8 | 0.28 | 0.6 |
| Andrena nitidiuscula Schenck, 1853 | 34 | 53 | 22 | 14 | 107 | 10.7 | 0.21 | 3.1 | Crossocerus elongatulus (Vander Linden, 1829) | 21 | 319 | 174 | 90 | 48 | −17.5 | 0.05 | 2.2 |
| Lasioglossum leucozonium (Schrank, 1781) | 34 | 55 | 55 | 44 | 90 | 10.1 | 0.57 | 2.6 | Ectemnius continuus (Fabricius, 1804) | 128 | 106 | 135 | 91 | 80 | −11.1 | 0.56 | 0.6 |
| Andrena proxima (Kirby, 1802) | 43 | 29 | 51 | 35 | 89 | 9.9 | 0.43 | 2.1 | Polistes nimpha (Christ, 1791) | 68 | 45 | 11 | 21 | 57 | −4.6 | 0.09 | 0.8 |
| Colletes similis Schenck, 1853 | 15 | 14 | 23 | 31 | 54 | 9.5 | 0.84 | 3.6 | Auplopus carbonarius (Scopoli, 1763) | 50 | 103 | 95 | 63 | 53 | −3.4 | 0.05 | 1.0 |
| Sphecodes albilabris (Fabricius, 1793) | 31 | 28 | 44 | 72 | 56 | 9.4 | 0.66 | 1.8 | Sceliphron destillatorium (Illiger 1807) | 15 | 11 | 18 | 11 | 4 | −2.2 | 0.44 | 0.3 |
| Sphecodes monilicornis (Kirby, 1802) | 83 | 98 | 85 | 89 | 134 | 9.3 | 0.49 | 1.6 | Vespula germanica (Fabricius, 1793) | 44 | 15 | 23 | 30 | 29 | −1.5 | 0.05 | 0.7 |
| Panurgus calcaratus (Scopoli, 1763) | 53 | 55 | 83 | 50 | 99 | 9.2 | 0.41 | 1.9 | Diodontus minutus (Fabricius 1793) | 259 | 366 | 503 | 315 | 229 | −1.1 | 0.03 | 0.9 |
| Coelioxys afra Lepeletier, 1841 | 31 | 51 | 54 | 48 | 76 | 8.7 | 0.73 | 2.5 | Tiphia femorata Fabricius 1775 | 103 | 177 | 134 | 122 | 152 | 4.3 | 0.06 | 1.5 |
| Anthidium manicatum (Linnaeus, 1758) | 20 | 17 | 28 | 34 | 51 | 7.9 | 0.85 | 2.6 | Ancistrocerus gazella (Panzer, 1798) | 4 | 26 | 61 | 21 | 28 | 4.3 | 0.11 | 7.0 |
| Coelioxys conoidea (Illiger, 1806) | 3 | 8 | 18 | 18 | 30 | 6.4 | 0.94 | 10 | Ammophila sabulosa (Linné, 1758) | 36 | 28 | 46 | 56 | 50 | 5.6 | 0.63 | 1.4 |
| Osmia aurulenta (Panzer, 1799) | 44 | 31 | 56 | 57 | 59 | 5.6 | 0.56 | 1.3 | Psenulus pallipes (Panzer, 1798) | 82 | 309 | 187 | 129 | 201 | 5.8 | 0.01 | 2.5 |
| Amegilla salviae (Morawitz, 1876) | 17 | 22 | 35 | 14 | 47 | 5.2 | 0.36 | 2.8 | Cerceris arenaria (Linné, 1758) | 94 | 143 | 143 | 111 | 145 | 7 | 0.27 | 1.5 |
| Anthidium oblongatum (Illiger, 1806) | 57 | 20 | 26 | 57 | 63 | 4.9 | 0.15 | 1.1 | Anoplius viaticus paganus (Dahlbom, 1843) | 7 | 28 | 56 | 37 | 39 | 7.3 | 0.42 | 5.6 |
| Andrena hattorfiana (Fabricius, 1775) | 19 | 7 | 13 | 35 | 29 | 4.8 | 0.44 | 1.5 | Chrysis ignita Linné, 1761 | 39 | 49 | 96 | 57 | 72 | 7.4 | 0.27 | 1.8 |
| Hylaeus annularis (Kirby, 1802) | 31 | 36 | 25 | 39 | 53 | 4.7 | 0.5 | 1.7 | Bembecinus tridens (Fabricius, 1781) | 90 | 106 | 72 | 172 | 94 | 7.4 | 0.09 | 1.0 |
| Andrena bimaculata (Kirby, 1802) | 13 | 54 | 84 | 49 | 37 | 4.3 | 0.07 | 2.8 | Priocnemis perturbator (Harris, 1780) | 1 | 10 | 25 | 32 | 34 | 8.8 | 0.94 | 34.0 |
| Andrena haemorrhoa (Fabricius, 1781) | 78 | 64 | 103 | 18 | 61 | 4.3 | 0.07 | 0.8 | Scolia hirta (Schrank, 1781) | 6 | 21 | 18 | 35 | 53 | 10.8 | 0.9 | 8.8 |
| Andrena symphyti Schmiedeknecht, 1883 | 1 | 15 | 11 | 17 | 20 | 4 | 0.74 | 20 | Pseudomalus pusillus (Fabricius, 1804) | 34 | 58 | 98 | 74 | 88 | 12.4 | 0.6 | 2.6 |
| Andrena nitida (Müller, 1776) | 22 | 17 | 26 | 32 | 33 | 3.8 | 0.71 | 1.5 | Pemphredon lethifer (Shuckard, 1837) | 166 | 275 | 212 | 184 | 274 | 12.5 | 0.15 | 1.7 |
| Tetralonia malvae (Rossi, 1790) | 25 | 32 | 25 | 25 | 47 | 3.7 | 0.38 | 1.9 | Tachysphex tarsinus (Lepeletier, 1845) | 0 | 0 | 51 | 120 | 70 | 26 | 0.66 | NA |
| Lasioglossum villosulum (Kirby, 1802) | 4 | 25 | 32 | 25 | 20 | 3.2 | 0.23 | 5.0 | Gorytes quinquecinctus (Fabricius, 1793) | 63 | 41 | 102 | 136 | 146 | 26.1 | 0.83 | 2.3 |
| Melitta nigricans Alfken, 1905 | 2 | 8 | 24 | 9 | 12 | 2.1 | 0.16 | 6.0 | Hedychrum nobile (Scopoli, 1763) | 66 | 66 | 82 | 120 | 189 | 30 | 0.83 | 2.9 |
| Eucera nigrescens Pérez, 1879 | 35 | 48 | 72 | 35 | 48 | 1.8 | 0.03 | 1.4 | Lestica clypeata (Schreber, 1759) | 100 | 88 | 110 | 135 | 229 | 30.5 | 0.72 | 2.3 |
| Stelis breviuscula (Nylander, 1848) | 1 | 18 | 13 | 14 | 12 | 1.8 | 0.2 | 12.0 | Cerceris sabulosa (Panzer, 1799) | 88 | 110 | 179 | 187 | 322 | 54.5 | 0.89 | 3.7 |
| Stelis punctulatissima (Kirby, 1802) | 3 | 9 | 25 | 10 | 10 | 1.5 | 0.08 | 3.3 | Oxybelus quatuordecimnotatus Jurine, 1807 | 90 | 105 | 69 | 216 | 310 | 55.1 | 0.73 | 3.4 |
| Tetraloniella salicariae (Lepeletier, 1841) | 3 | 10 | 8 | 4 | 1 | 1 | 0.18 | 0.3 | Philanthus triangulum (Fabricius, 1775) | 51 | 52 | 45 | 52 | 79 | 5.6 | 0.44 | 1.5 |
| Taxon | 1988–1993 | 1994–1999 | 2003–2008 | 2012–2017 | 2018–2023 | Linear x coef | r2 |
|---|---|---|---|---|---|---|---|
| Chrysis taczanovskii Radoszkowski, 1876 | 0 | 1 | 0 | 42 | 110 | 26.10 | 0.74 |
| Cerceris rubida (Jurine, 1807) | 1 | 6 | 45 | 116 | 276 | 66.00 | 0.83 |
| Diodontus brevilabris de Beaumont 1967 | 9 | 100 | 177 | 107 | 209 | 40.70 | 0.69 |
| Colletes hederae Schmidt & Westrich, 1993 | 0 | 0 | 0 | 0 | 5 | NA | NA |
| Nomiapis bispinosa (Brullé, 1832) | 0 | 0 | 0 | 0 | 11 | NA | NA |
| Pasites maculatus Jurine, 1807 | 1 | 0 | 13 | 18 | 58 | 13.20 | 0.78 |
| Scolia hirta (Schrank, 1781) | 6 | 21 | 18 | 35 | 53 | 10.80 | 0.9 |
| Megascolia maculata (Drury, 1773) | 0 | 0 | 0 | 1 | 3 | 0.70 | 0.72 |
| Sceliphron curvatum (F.Smith, 1870) | 0 | 8 | 10 | 6 | 13 | 2.40 | 0.62 |
| Sceliphron caementarium (Drury, 1773) | 0 | 0 | 0 | 0 | 47 | NA | NA |
| Isodontia mexicana (Saussure, 1867) | 0 | 0 | 2 | 37 | 89 | 21.50 | 0.77 |
| Taxon | 2000–2004 | 2005–2009 | 2010–2014 | 2015–2019 | 2020–2023 | Linear x coef | r2 |
| Colletes hederae Schmidt & Westrich, 1993 | 0 | 0 | 0 | 77 | 25 | 5.52 | 0.34 |
| Sceliphron caementarium (Drury, 1773) | 0 | 1 | 0 | 4 | 15 | 1.22 | 0.45 |
| Sceliphron curvatum (F.Smith, 1870) | 0 | 4 | 75 | 4 | 8 | 3.30 | 0.14 |
| Isodontia mexicana (Saussure, 1867) | 0 | 1 | 0 | 5 | 2 | 0.40 | 0.42 |
| Megascolia maculata (Drury, 1773) | 0 | 1 | 1 | 5 | 7 | 0.72 | 0.59 |
| Taxon/Data of the Last 2 Decades | 2005–2009 | 2010–2014 | 2015–2019 | 2020–2023 | Linear x coeff | r2 |
|---|---|---|---|---|---|---|
| Bombus (Bombias) confusus Schenck, 1859 | 2 | 0 | 0 | 0 | Recently under detection limit | |
| Bombus (Subterraneobombus) subterraneus (Linné, 1758) | 0 | 0 | 0 | 0 | ||
| Bombus (Thoracobombus) pomorum (Panzer, 1805) | 0 | 0 | 0 | 0 | ||
| Bombus (Bombus) terrestis (Linné, 1758) | 944 | 888 | 479 | 205 | −262.6 | 0.94 |
| Bombus (Melanobombus) lapidarius (Linné, 1758) | 835 | 291 | 481 | 45 | −218.0 | 0.72 |
| Bombus (Thoracobombus) pascuorum (Scopoli, 1763) | 692 | 944 | 401 | 284 | −176.7 | 0.60 |
| Bombus (Megabombus) hortorum (Linné, 1761) | 173 | 357 | 106 | 11 | −73.7 | 0.42 |
| Bombus (Thoracobombus) ruderarius (Müller, 1776) | 174 | 151 | 56 | 13 | −57.8 | 0.95 |
| Bombus (Thoracobombus) humilis Illiger, 1806 | 75 | 7 | 32 | 16 | −15.2 | 0.42 |
| Bombus (Bombus) lucorum (Linné, 1761) | 41 | 104 | 31 | 7 | −17.5 | 0.30 |
| Bombus (Pyrobombus) hypnorum (Linné, 1758) | 31 | 18 | 12 | 7 | −7.8 | 0.95 |
| Bombus (Megabombus) ruderatus (Fabricius, 1775) | 18 | 11 | 0 | 0 | −6.5 | 0.90 |
| Bombus (Thoracobombus) sylvarum (Linné, 1761) | 77 | 98 | 24 | 65 | −1.7 | 0.39 |
| Bombus (Thoracobombus) muscorum (Linné, 1758) | 6 | 1 | 1 | 2 | −1.2 | 0.42 |
| Bombus (Pyrobombus) pratorum (Linné, 1761) | 1 | 28 | 18 | 2 | −0.7 | 0.00 |
| Bombus (Pyrobombus) haematurus Kriechbaumer, 1870 | 80 | 93 | 103 | 116 | 11.8 | 1.00 |
| Bombus (Megabombus) argillaceus (Scopoli, 1763) | 4 | 28 | 8 | 31 | 61.0 | 0.33 |
| Bombus (Psithyrus) vestalis (Geoffroy, 1785) | 60 | 49 | 55 | 13 | −13.5 | 0.67 |
| Bombus (Psithyrus) rupestris (Fabricius, 1793) | 12 | 56 | 6 | 0 | −8.6 | 0.19 |
| Bombus (Psithyrus) bohemicus (Seidl, 1837) | 3 | 29 | 21 | 0 | −1.7 | 0.02 |
| Bombus (Psithyrus) barbutellus (Kirby, 1802) | 4 | 1 | 0 | 0 | −1.3 | 0.79 |
| Bombus (Psithyrus) campestris (Panzer, 1801) | 2 | 4 | 1 | 0 | −0.9 | 0.46 |
| Bombus (Psithyrus) maxillosus Klug, 1817 | 1 | 0 | 0 | 0 | under detection limit | |
| Taxon/Historic Data | 1980–1984 | 1985–1989 | 1990–1994 | 1995–1999 | 2000–2004 | |
| Bombus (Bombias) confusus Schenck, 1859 | 71 | 45 | 1 | 0 | 0 | |
| Bombus (Subterraneobombus) subterraneus (Linné, 1758) | 38 | 3 | 0 | 0 | 0 | |
| Bombus (Thoracobombus) pomorum (Panzer, 1805) | 25 | 0 | 0 | 0 | 0 | |
| Bombus (Megabombus) argillaceus (Scopoli, 1763) | 0 | 0 | 0 | 0 | 0 | |
| Bombus (Megabombus) ruderatus (Fabricius, 1775) | NA | NA | NA | NA | 6 | |
| Bombus (Megabombus) hortorum (Linné, 1761) | 26 | NA | NA | NA | 16 | |
| Bombus (Thoracobombus) muscorum (Linné, 1758) | 27 | 0 | 27 | 0 | 50 | |
| Bombus (Thoracobombus) ruderarius (Müller, 1776) | 33 | NA | NA | NA | 54 | |
| Bombus (Thoracobombus) sylvarum (Linné, 1761) | 129 | NA | NA | NA | 145 | |
| Bombus (Thoracobombus) humilis Illiger, 1806 | NA | NA | NA | NA | 7 | |
| Bombus (Thoracobombus) pascuorum (Scopoli, 1763) | 71 | NA | NA | NA | 42 | |
| Bombus (Pyrobombus) haematurus Kriechbaumer, 1870 | 0 | 0 | 0 | 0 | 1 | |
| Bombus (Pyrobombus) hypnorum (Linné, 1758) | 7 | NA | NA | NA | 1 | |
| Bombus (Pyrobombus) pratorum (Linné, 1761) | 3 | NA | NA | NA | NA | |
| Bombus (Bombus) terrestis (Linné, 1758) | 349 | NA | 157 | 96 | 134 | |
| Bombus (Bombus) lucorum (Linné, 1761) | 2 | NA | NA | NA | 4 | |
| Bombus (Melanobombus) lapidarius (Linné, 1758) | 103 | NA | NA | NA | 188 | |
| Bombus (Psithyrus) rupestris (Fabricius, 1793) | NA | NA | 0 | 0 | NA | |
| Bombus (Psithyrus) campestris (Panzer, 1801) | NA | NA | 0 | 0 | NA | |
| Bombus (Psithyrus) vestalis (Geoffroy, 1785) | 1 | NA | 2 | 1 | 1 | |
| Bombus (Psithyrus) bohemicus (Seidl, 1837) | NA | NA | NA | NA | NA | |
| Bombus (Psithyrus) barbutellus (Kirby, 1802) | NA | NA | 2 | 2 | 1 | |
| Bombus (Psithyrus) maxillosus Klug, 1817 | NA | NA | NA | NA | 1 | |
| Syrphidae (Hoverflies) | 1980–1984 | 1985–1989 | 1990–1994 | 1995–1999 | 2000–2004 | 2005–2010 | Linear x coef | r2 | ln x coef. | r2 | Change |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sphaerophoria scripta (Linné, 1758) | 3509 | 2049 | 557 | 581 | 39 | 109 | −657.31 | 0.81 | −2008.2 | 0.95 | 0.03 |
| Cheilosia variabilis (Panzer, 1798) | 392 | 5 | 21 | 1 | 20 | 13 | −53.43 | 0.41 | −187.76 | 0.64 | 0.03 |
| Syrphus torvus Osten Sacken, 1875 | 359 | 43 | 14 | 14 | 10 | 15 | −51.97 | 0.49 | −178.25 | 0.72 | 0.04 |
| Syrphus vitripennis Meigen, 1822 | 846 | 176 | 210 | 121 | 24 | 48 | −129.57 | 0.63 | −417.9 | 0.82 | 0.06 |
| Episyrphus balteatus (De Geer, 1776) | 1587 | 909 | 652 | 2050 | 85 | 94 | −243.97 | 0.33 | 0.06 | ||
| Melanostoma mellinum (Linné, 1758) | 1168 | 1257 | 420 | 321 | 73 | 75 | −260.45 | 0.85 | 0.06 | ||
| Pipizella viduata (Linné, 1758) | 364 | 426 | 97 | 82 | 48 | 33 | −80.11 | 0.75 | 0.09 | ||
| Eristalis arbustorum (Linné, 1758) | 1259 | 767 | 411 | 156 | 86 | 116 | −228.94 | 0.85 | −691.94 | 0.96 | 0.09 |
| Myathropa florea (Linné, 1758) | 93 | 132 | 45 | 20 | 17 | 9 | −22.57 | 0.73 | 0.10 | ||
| Eristalis pertinax (Scopoli, 1763) | 160 | 41 | 15 | 25 | 5 | 19 | −22.94 | 0.54 | −76.95 | 0.77 | 0.12 |
| Syritta pipiens (Linné, 1758) | 501 | 588 | 415 | 109 | 36 | 78 | −116.48 | 0.82 | 0.16 | ||
| Scaeva pyrastri (Linné, 1758) | 210 | 85 | 106 | 17 | 16 | 34 | −33.6 | 0.71 | 0.16 | ||
| Platycheirus albimanus (Fabricius, 1781) | 162 | 46 | 33 | 121 | 33 | 27 | −17.89 | 0.34 | 0.17 | ||
| Volucella zonaria (Poda, 1761) | 6 | 0 | 7 | 1 | 36 | 1 | 2.2 | 0.09 | 0.17 | ||
| Eristalis tenax (Linné, 1758) | 1115 | 509 | 270 | 89 | 122 | 196 | −169 | 0.67 | 0.18 | ||
| Syrphus ribesii (Linné, 1758) | 344 | 114 | 32 | 156 | 23 | 63 | −44.4 | 0.48 | 0.18 | ||
| Volucella pellucens (Linné, 1758) | 64 | 8 | 15 | 8 | 9 | 12 | −7.54 | 0.4 | 0.19 | ||
| Eupeodes luniger (Meigen, 1822) | 36 | 16 | 21 | 58 | 7 | 8 | −3.71 | 0.13 | 0.22 | ||
| Cheilosia impressa (Loew, 1840) | 74 | 89 | 81 | 11 | 20 | 28 | −14.49 | 0.62 | 0.38 | ||
| Cheilosia soror (Zetterstedt, 1843) | 26 | 59 | 55 | 7 | 19 | 23 | −5.23 | 0.22 | 0.88 |
| Family | 1980–1984 | 1985–1989 | 1990–1994 | 1995–1999 | 2000–2004 | 2005–2009 | 2010–2014 | 2014–2019 | Lin. x coeff | r2 | Change |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Syrphidae | 11,602 | 9226 | 4415 | 5365 | 2587 | 2708 | NA | NA | −1812.49 | 0.86 | 0.2 |
| Bombilidae | 530 | 533 | 569 | 615 | 452 | 701 | 928 | 980 | 63.79 | 0.65 | 1.8 |
| Tabanidae | 978 | 1046 | 977 | 1387 | NA | 1614 | 571 | NA | 136.93 | 0.84 | 0.6 |
| Tachinidae | 2473 | 1826 | 4477 | 725 | 233 | 516 | NA | NA | −523.31 | 0.37 | 0.2 |
| Bombyliidae | Syrphidae |
|---|---|
| Bombylosoma unicolor (Loew, 1955) | Brachyopa panzeri Goffe, 1945 |
| Bombylius fuliginosus Wiedemann in Meigen, 1820 | Brachyopa vittata Shummel, 1834 |
| Bombylius quadrifarius Loew, 1855 | Brachypalpus chrysites Egger, 1859 |
| Heteralonia dispar (Loew, 1869) | Callicera macquarti Rondani, 1944 |
| Spogostylum aethiops (Fabricius, 1781) | Callicera rufa Schummel, 1842 |
| Callicera spinolae Rondani, 1844 | |
| Tabanidae | Chalcosyrphus curvipes (Loew, 1854) |
| Pangonius pyritosus (Loew, 1859) | Cheilosia bracusi Vujic & Claussen, 1994) |
| Hybomitra arpadi (Szilády, 1923) | Cheilosia brunnipennis (Becker, 1894) |
| Hybomitra aterrima (Meigen, 1820) | Cheilosia hypena (Becker, 1894) |
| Hybomitra expollicata (Pandellé, 1883) | Cheilosia insignis (Loew, 1857) |
| Hybomitra montana (Meigen, 1820) | Cheilosia melanopa (Zetterstedt, 1843) |
| Hybomitra nigricornis (Zetterstedt, 1842) | Cheilosia melanura (Becker, 1894) |
| Hybomitra tarandina (Linné, 1758) | Cheilosia pictipennis Egger, 1860 |
| Cheilosia sahlbergi (Becker, 1894) | |
| Tachinidae | Cheilosia subpictipennis (Claussen, 1898) |
| Amelibaea tultschensis (Brauer & Bergenstamm, 1891) | Chrysogaster basalis (Loew, 1857) |
| Anthomyiopsis nigrisquamata (Zetterstedt, 1838) | Cliorhina pachymera (Egger, 1858) |
| Anthomyiopsis plagiodera Mesnil, 1972 | Epistrophe obscuripes (Strobl, 1910) |
| Aphria xyphias Pandellé, 1896 | Eristalis vitripennis (Strobl, 1893) |
| Besseria dimidiata (Zetterstedt, 1844) | Eumerus hungaricus (Szilády, 1940) |
| Besseria melanura (Meigen, 1824) | Eumerus longicornis (Loew, 1855) |
| Bithia acanthophora (Rondani, 1861) | Eumerus ruficornis (Meigen, 1822) |
| Blepharomyia pagana (Meigen, 1824) | Eumerus sabulosum (Fallén, 1817) |
| Cadurciella tritaeniata (Rondani, 1859) | Eumerus tauricus (Stackelberg, 1952) |
| Campylochaeta latigena Mesnil, 1974 | Eupeodes lucasi (Marcos-García & Láska, 1983) |
| Catharosia albisquama (Villeneuve, 1932) | Hammerschmidtia ferruginea (Fallén, 1817) |
| Ceranthia tristella Herting, 1966 | Helophilus affinis Wahlberg, 1844 |
| Chetoptilia puella (Rondani, 1862) | Lejota ruficornis (Wahlberg, 1843) |
| Conogaster pruinosa (Meigen, 1824) | Melanogaster curvistylus (Vujić-Stuke, 1998) |
| Phytomyptera abnormis (Stein, 1924) | Melanostoma dubium (Zetterstedt, 1837) |
| Eloceria delecta (Meigen, 1824) | Milesia crabroniformis (Fabricius, 1775) |
| Estheria acuta (Portschinsky, 1881) | Orthonevra tristis (Loew, 1871) |
| Gonia bimaculata Wiedemann, 1819 | Paragus medeae Stanescu, 1991 |
| Heraultia albipennis Villeneuve, 1920 | Paragus punctulatus (Zetterstedt, 1838) |
| Ligeriella aristata (Villeneuve, 1911) | Pipiza fenestrata (Meigen, 1822) |
| Minthodes pictipennis Brauer & Bergenstamm, 1889 | Pipizella pennina (Goeldlin de Tiefenau, 1974) |
| Psalidoxena transsylvanica (Villeneuve, 1929) | Platycheirus complicatus (Becker, 1889) |
| Siphona confusa Mesnil, 1961 | Platycheirus immarginatus (Zetterstedt, 1849) |
| Siphona ingerae Andersen, 1982 | Platycheirus jaerensis Nielsen, 1971 |
| Therobia leonidei Mesnil, 1964 | Platycheirus nielseni Vockeroth, 1990 |
| Vibrissina debilitata (Pandellé, 1896) | Platycheirus perpallidus (Verrall, 1901) |
| Winthemia bohemani (Zetterstedt, 1844) | Rhingia austriaca (Meigen, 1830) |
| Scaeva albomaculata (Macquart, 1842) | |
| Sphaerophoria shircan Violovits, 1957 | |
| Sphiximorpha binominata (Verrall, 1901) | |
| Syrphus nitidifrons Becker, 1921 | |
| Syrphus sexmaculatus (Zetterstedt, 1838) | |
| Trichopsomyia joratensis Goeldlin de Tiefenau, 1997 | |
| Xylota coeruleiventris (Zetterstedt, 1838) |
| Tabanidae (Horseflies) | 1980–1984 | 1985–1989 | 1990–1994 | 1995–1999 | 2005–2009 | 2010–2014 | Lin. x coeff. | r2 | Change |
|---|---|---|---|---|---|---|---|---|---|
| Tabanus bromius Linné, 1758 | 212 | 128 | 187 | 134 | 116 | 62 | −23.97 | 0.71 | 0.3 |
| Haematopota pluvialis (Linné, 1758) | 189 | 228 | 205 | 760 | 161 | 70 | −6.88 | 0.03 | 0.4 |
| Atylotus rusticus (Linné, 1758) | 116 | 13 | 13 | 40 | 72 | 61 | −2.03 | 0.01 | 0.5 |
| Chrysops viduatus (Fabricius, 1794) | 74 | 20 | 29 | 59 | 90 | 48 | 3.24 | 0.05 | 0.6 |
| Heptatoma pellucens (Fabricius, 1776) | 16 | 4 | 4 | 11 | 42 | 21 | 4.17 | 0.3 | 1.3 |
| Tabanus autumnalis Linné, 1761 | 29 | 18 | 14 | 28 | 67 | 41 | 6.31 | 0.38 | 1.4 |
| Silvius alpinus (Scopoli, 1763) | 5 | 0 | 1 | 1 | 31 | 9 | 3.22 | 0.26 | 1.8 |
| Therioplectes gigas (Herbst, 1787) | 5 | 1 | 3 | 5 | 34 | 14 | 4.17 | 0.3 | 2.8 |
| Chrysops caecutiens (Linné, 1758) | 14 | 16 | 13 | 33 | 83 | 41 | 10.17 | 0.05 | 2.9 |
| Haematopota italica. Meigen, 1804. | 19 | 114 | 227 | 42 | 85 | 58 | −2.2 | 0 | 3.1 |
| Tabanus bovinus Linné, 1758 | 8 | 13 | 20 | 14 | 81 | 40 | 10.23 | 0.38 | 5.0 |
| Bombyliidae (Bee Flies) | 1980–1984 | 1985–1989 | 1990–1994 | 1995–1999 | 2000–2004 | 2005–2009 | 2010–2014 | 2016–2020 | Lin. x coeff | r2 | Change |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Apolysis szappanosi Papp, 2005 | 0 | 1 | 0 | 0 | 1 | 0 | 5 | 0 | 0.25 | 0.13 | NA |
| Exoprosopa jacchus (Fabricius, 1805) | 1 | 2 | 7 | 1 | 8 | 27 | 23 | 18 | 3.46 | 0.67 | 18.0 |
| Lomatia sabaea (Fabricius, 1781) | 3 | 0 | 15 | 3 | 18 | 13 | 23 | 38 | 4.39 | 0.73 | 12.7 |
| Bombylius medius Linné, 1758 | 7 | 7 | 9 | 14 | 10 | 34 | 34 | 70 | 7.7 | 0.73 | 10.0 |
| Bombylius discolor Mikan, 1796 | 13 | 14 | 8 | 41 | 41 | 23 | 31 | 91 | 8.04 | 0.55 | 7.0 |
| Conophorus virescens (Fabricius, 1787) | 9 | 32 | 19 | 45 | 38 | 57 | 72 | 60 | 7.9 | 0.83 | 6.7 |
| Bombylius fimbriatus Meigen, 1820 | 8 | 31 | 18 | 40 | 41 | 13 | 26 | 52 | 3.2 | 0.27 | 6.5 |
| Bombylius cinerascens Mikan, 1796 | 13 | 33 | 19 | 24 | 28 | 51 | 27 | 79 | 6.33 | 0.53 | 6.1 |
| Villa hottentotta (Linné, 1758) | 20 | 6 | 72 | 33 | 36 | 70 | 73 | 99 | 10.54 | 0.65 | 5.0 |
| Bombylius canescens Mikan, 1796 | 8 | 6 | 15 | 27 | 13 | 32 | 19 | 33 | 3.29 | 0.59 | 4.1 |
| Bombylius fulvescens Meigen & Wiedemann, 1820 | 7 | 20 | 15 | 21 | 9 | 23 | 27 | 27 | 2.23 | 0.51 | 3.9 |
| Bombylius major Linné, 1758 | 54 | 156 | 105 | 160 | 63 | 26 | 28 | 199 | 0.49 | 0 | 3.7 |
| Anthrax anthrax (Schrank, 1781) | 5 | 1 | 9 | 0 | 1 | 9 | 10 | 18 | 1.63 | 0.43 | 3.6 |
| Anthrax leucogaster Meigen & Wiedemann, 1820 | 5 | 2 | 12 | 20 | 6 | 21 | 15 | 17 | 1.93 | 0.43 | 3.4 |
| Bombylius pictus Panzer, 1794 | 2 | 5 | 3 | 4 | 3 | 10 | 21 | 4 | 1.36 | 0.27 | 2.0 |
| Hemipenthes morio (Linné, 1758) | 67 | 42 | 90 | 254 | 31 | 78 | 89 | 124 | 4.46 | 0.02 | 1.9 |
| Bombylella atra (Scopoli, 1763) | 23 | 39 | 22 | 53 | 3 | 45 | 36 | 7 | −1.28 | 0.03 | 0.3 |
| Tachinidae (Tachinids) | 1980–1984 | 1985–1989 | 1990–1994 | 1995–1999 | 2000–2004 | 2005–2009 | 2010–2014 | Lin. x coeff. | r2 | Change |
|---|---|---|---|---|---|---|---|---|---|---|
| Blondelia nigripes (Fallén, 1810) | 52 | 29 | 69 | 12 | NA | 12 | NA | −9.7 | 0.37 | 0.2 |
| Chetogena filipalpis Rondani, 1859 | 4 | 0 | 18 | 5 | NA | 0 | NA | −0.3 | 0 | 0.0 |
| Compsilura concinnata (Meigen, 1824) | 29 | 44 | 216 | 13 | NA | 3 | NA | −8.3 | 0.22 | 0.1 |
| Exorista larvarum (Linné, 1758) | 29 | 33 | 80 | 5 | NA | 7 | NA | −7.2 | 0.14 | 0.2 |
| Gymnosoma clavatum (Rohdendorf, 1947) | 15 | 5 | 30 | 7 | NA | 4 | NA | −2 | 0.06 | 0.3 |
| Gymnosoma dolycoridis Dupuis 1961 | 31 | 10 | 71 | 19 | NA | 0 | NA | −5.3 | 0.09 | 0.0 |
| Gymnosoma rotundatum (Linné, 1758) | 28 | 29 | 125 | 10 | NA | 7 | NA | −6.1 | 0.04 | 0.3 |
| Linnaemya frater (Rondani, 1859) | 11 | 75 | 11 | 1 | NA | 7 | NA | −8.2 | 0.18 | 0.6 |
| Phasia pusilla Meigen, 1824 | 53 | 45 | 39 | 0 | NA | 1 | NA | −14.9 | 0.87 | 0.0 |
| Tachina fera (Linné, 1761) | 55 | 19 | 232 | 42 | NA | 26 | NA | −3.5 | 0 | 0.5 |
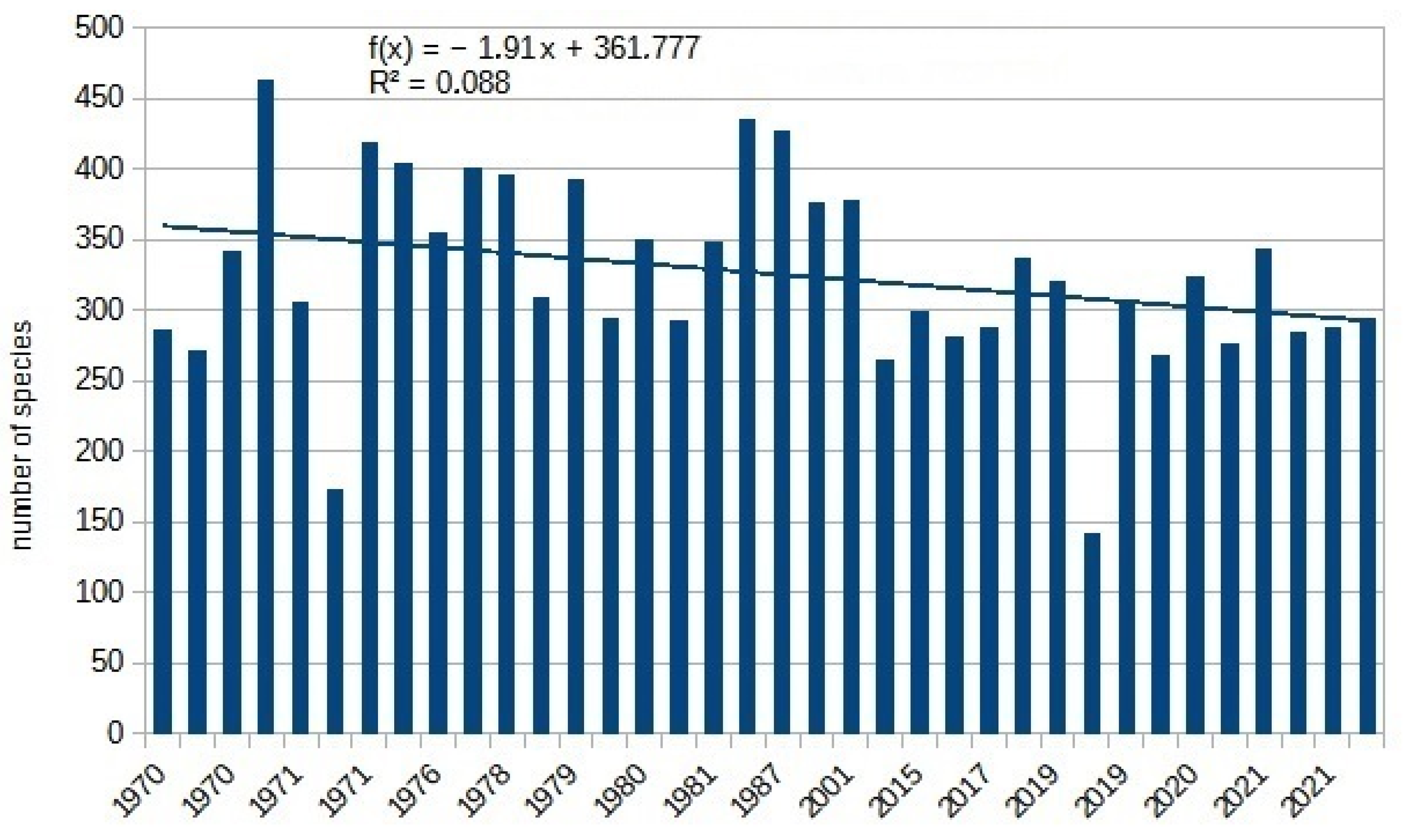
| Taxon | 1970 | 1970 | 1970 | 1970 | 1973 | 1976 | 1977 | 1978 | 1979 | 1979 | 1980 | 1980 | 1981 | 1981 | 1986 | 1987 | 2019 | 2019 | 2020 | 2020 | 2021 | 2021 | 2021 | 2021 | 2022 | Lin. x coeff (1970) | r2 | Lin. x ceff (1981) | r2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phragmatobia fuliginosa (Linnaeus, 1758) | 0 | 13 | 0 | 207 | 1028 | 261 | 221 | 193 | 145 | 110 | 121 | 407 | 116 | 472 | 315 | 428 | 0 | 20 | 45 | 7 | 10 | 22 | 11 | 34 | 23 | −9.28 | 0.09 | −28.44 | 0.42 |
| Xestia c-nigrum (Linnaeus, 1758) | 1049 | 309 | 132 | 532 | 186 | 674 | 189 | 230 | 7 | 7 | 14 | 189 | 248 | 273 | 224 | 147 | 2 | 55 | 49 | 21 | 14 | 61 | 62 | 70 | 5 | −20.52 | 0.38 | −17.64 | 0.52 |
| Mythimna pallens Linné, 1758 | 16 | 35 | 18 | 41 | 139 | 229 | 62 | 96 | 11 | 0 | 8 | 34 | 27 | 216 | 113 | 90 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | −3.13 | 0.12 | −10.77 | 0.40 |
| Eilema lurideola (Zincken, 1817) | 64 | 0 | 112 | 24 | 0 | 2 | 6 | 0 | 16 | 53 | 59 | 218 | 471 | 7 | 11 | 3 | 2 | 581 | 3 | 181 | 0 | 228 | 2 | 121 | 2 | 4.07 | 0.04 | −9.14 | 0.03 |
| Diachrysia chrysitis (Linné, 1758) | 59 | 35 | 18 | 39 | 111 | 143 | 103 | 45 | 9 | 118 | 16 | 113 | 19 | 61 | 89 | 59 | 0 | 9 | 0 | 13 | 1 | 8 | 0 | 11 | 0 | −3.18 | 0.27 | −6.42 | 0.52 |
| Spilosoma lubricipeda (Linné, 1758) | 0 | 85 | 0 | 156 | 303 | 369 | 151 | 27 | 68 | 47 | 85 | 88 | 26 | 86 | 57 | 130 | 33 | 9 | 18 | 22 | 27 | 7 | 0 | 2 | 8 | −5.88 | 0.21 | −6.25 | 0.41 |
| Melanchra pisi (Linné, 1758) | 0 | 0 | 0 | 3 | 8 | 65 | 46 | 18 | 12 | 3 | 7 | 19 | 2 | 171 | 44 | 24 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | −0.52 | 0.01 | −6.12 | 0.25 |
| Arctia caja (Linné, 1758) | 5 | 2 | 20 | 11 | 121 | 72 | 13 | 22 | 30 | 32 | 12 | 51 | 37 | 32 | 32 | 21 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | −1.66 | 0.19 | −3.14 | 0.67 |
| Lacanobia oleracea (Linné, 1758) | 13 | 25 | 0 | 37 | 38 | 226 | 22 | 13 | 3 | 7 | 5 | 27 | 25 | 73 | 34 | 28 | 0 | 14 | 3 | 14 | 0 | 40 | 30 | 1 | 2 | −1.34 | 0.14 | −2.01 | 0.17 |
| Melanchra persicariae Linné, 1761 | 0 | 0 | 1 | 25 | 27 | 132 | 9 | 16 | 0 | 2 | 1 | 26 | 35 | 15 | 21 | 19 | 0 | 9 | 34 | 29 | 15 | 0 | 0 | 1 | 2 | −0.64 | 0.03 | −2.00 | 0.35 |
| Smerinthus ocellata Linné, 1758 | 14 | 0 | 8 | 12 | 16 | 52 | 23 | 5 | 0 | 0 | 1 | 29 | 11 | 36 | 15 | 12 | 0 | 0 | 1 | 2 | 0 | 6 | 0 | 1 | 0 | −0.65 | 0.13 | −1.70 | 0.41 |
| Macdunnoughia confusa (Stephens, 1850) | 3 | 3 | 0 | 46 | 27 | 50 | 73 | 39 | 3 | 4 | 1 | 15 | 27 | 27 | 22 | 17 | 0 | 9 | 8 | 4 | 1 | 10 | 12 | 5 | 1 | −0.90 | 0.12 | −1.68 | 0.47 |
| Mythimna turca Linné, 1761 | 2 | 210 | 3 | 118 | 461 | 833 | 75 | 44 | 1 | 4 | 3 | 65 | 85 | 36 | 34 | 14 | 8 | 42 | 48 | 55 | 26 | 10 | 85 | 4 | 17 | −8.61 | 0.12 | −1.53 | 0.05 |
| Abrostola triplasia (Linné, 1758) | 1 | 21 | 0 | 6 | 14 | 25 | 5 | 4 | 0 | 26 | 16 | 7 | 2 | 12 | 30 | 14 | 0 | 43 | 9 | 42 | 3 | 22 | 0 | 1 | 3 | 0.15 | 0.01 | −0.77 | 0.04 |
| Abrostola tripartita (Hufnagel, 1766) | 1 | 16 | 2 | 32 | 8 | 11 | 3 | 7 | 9 | 20 | 22 | 9 | 16 | 11 | 3 | 5 | 0 | 0 | 0 | 1 | 6 | 3 | 3 | 5 | 2 | −0.47 | 0.19 | −0.59 | 0.24 |
| Drymonia obliterata (Esper, 1785) | 0 | 0 | 0 | 2 | 0 | 0 | 3 | 6 | 0 | 1 | 1 | 13 | 1 | 7 | 4 | 13 | 0 | 114 | 5 | 301 | 19 | 1 | 0 | 1 | 26 | 2.37 | 0.08 | 1.19 | 0 |
| Protodeltote pygarga (Hufnagel, 1766) | 3 | 899 | 10 | 200 | 431 | 127 | 41 | 25 | 6 | 28 | 20 | 61 | 115 | 53 | 61 | 64 | 209 | 225 | 444 | 375 | 95 | 95 | 331 | 10 | 99 | −2.12 | 0.01 | 5.07 | 0.02 |
| Eilema depressa (Esper, 1786) | 2 | 0 | 24 | 0 | 0 | 1 | 0 | 0 | 2 | 0 | 1 | 1 | 0 | 1 | 99 | 35 | 0 | 79 | 20 | 38 | 0 | 31 | 1 | 315 | 1 | 3.78 | 0.18 | 8.1 | 0.13 |
| Colocasia coryli (Linné, 1758) | 3 | 7 | 20 | 253 | 155 | 42 | 35 | 45 | 9 | 9 | 16 | 74 | 18 | 25 | 16 | 29 | 50 | 217 | 17 | 1034 | 52 | 142 | 0 | 105 | 69 | 5.96 | 0.05 | 10.12 | 0.02 |
| Athetis furvula (Hübner, 1808) | 0 | 1654 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 371 | 1 | 0 | −10.8 | 0.06 | 10.31 | 0.15 |
| Zanclognatha lunalis (Scopoli, 1763) | 5 | 986 | 2 | 51 | 8 | 0 | 2 | 0 | 6 | 4 | 1 | 1 | 2 | 0 | 1 | 1 | 0 | 108 | 268 | 171 | 30 | 17 | 433 | 21 | 92 | −1.45 | 0 | 15.05 | 0.19 |
| Earias vernana (Fabricius, 1787) | 0 | 87 | 0 | 7 | 2 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 2 | 0 | 0 | 18 | 0 | 21 | 0 | 546 | 0 | 0 | 4.04 | 0.07 | 15.1 | 0.15 |
| Paracolax tristalis (Fabricius, 1794) | 48 | 2049 | 29 | 28 | 33 | 13 | 8 | 11 | 14 | 3 | 5 | 5 | 7 | 4 | 3 | 3 | 0 | 365 | 210 | 777 | 210 | 167 | 1594 | 342 | 277 | 9.1 | 0.02 | 67.41 | 0.35 |
| Eilema sororcula (Hufnagel, 1767) | 0 | 13 | 1 | 2923 | 5151 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 69 | 0 | 1352 | 0 | 414 | 1243 | 1860 | 24 | 184 | −16.3 | 0.01 | 86.13 | 0.27 |
| Family | 1970 | 1970 | 1970 | 1970 | 1971 | 1971 | 1973 | 1973 | 1975 | 1976 | 1976 | 1977 | 1978 | 1979 | 1979 | 1980 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Geometridae | 2999 | 11,723 | 1409 | 10,538 | 5853 | 909 | 4513 | 7299 | 901 | 1749 | 1378 | 1013 | 1217 | 2853 | 2954 | 1669 |
| Noctuidae | 2192 | 5431 | 1169 | 7987 | 2009 | 1305 | 5897 | 7599 | 3301 | 6560 | 5872 | 3481 | 3077 | 1032 | 3560 | 1059 |
| Lasiocampidae | 37 | 15 | 115 | 269 | 65 | 21 | 211 | 393 | 126 | 206 | 81 | 94 | 45 | 42 | 43 | 54 |
| Saturniidae | 2 | 0 | 0 | 4 | 0 | 0 | 4 | 12 | 56 | 75 | 16 | 29 | 25 | 1 | 2 | 0 |
| Sphingidae | 16 | 5 | 38 | 182 | 2 | 0 | 218 | 161 | 237 | 570 | 205 | 142 | 81 | 15 | 202 | 12 |
| Drepanidae | 14 | 7 | 15 | 660 | 42 | 2 | 141 | 674 | 108 | 153 | 119 | 28 | 47 | 34 | 43 | 22 |
| Notodontidae | 51 | 321 | 64 | 1828 | 106 | 282 | 485 | 2160 | 319 | 598 | 278 | 99 | 191 | 50 | 233 | 22 |
| Erebidae | 579 | 7020 | 785 | 6150 | 1604 | 909 | 4845 | 12,153 | 989 | 2864 | 3259 | 884 | 735 | 1501 | 1668 | 1498 |
| Nolidae | 6 | 158 | 153 | 1031 | 12 | 55 | 185 | 529 | 30 | 151 | 142 | 20 | 43 | 91 | 153 | 109 |
| Thyatridae | 13 | 545 | 18 | 543 | 29 | 103 | 92 | 502 | 18 | 109 | 151 | 57 | 40 | 66 | 26 | 62 |
| Total | 5896 | 25,228 | 3770 | 28,830 | 9824 | 4828 | 16,606 | 31,002 | 6115 | 13,104 | 11,501 | 5847 | 5533 | 5693 | 8901 | 4511 |
| Family | 1980 | 1981 | 1981 | 1986 | 1987 | 2000 | 2001 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | 2019 | 2019 | 2020 |
| Geometridae | 1088 | 2552 | 1398 | 2573 | 1831 | 3924 | 2217 | 570 | 1073 | 838 | 693 | 1105 | 1021 | 525 | 3578 | 984 |
| Noctuidae | 2488 | 1065 | 1990 | 3605 | 4094 | NA | NA | 4619 | 2053 | 2587 | 2359 | 2862 | 5236 | 459 | 1972 | 1874 |
| Lasiocampidae | 35 | 42 | 47 | 221 | 55 | 364 | 471 | 18 | 6 | 34 | 60 | 104 | 160 | 3 | 31 | 96 |
| Saturniidae | 1 | 8 | 2 | 24 | 11 | 1 | 0 | 2 | 0 | 2 | 6 | 3 | 22 | 0 | 29 | 5 |
| Sphingidae | 87 | 10 | 95 | 215 | 85 | 288 | 353 | 78 | 73 | 169 | 193 | 164 | 213 | 2 | 18 | 103 |
| Drepanidae | 18 | 31 | 39 | 74 | 29 | 158 | 115 | NA | NA | NA | NA | NA | NA | 36 | 195 | 47 |
| Notodontidae | 85 | 35 | 92 | 376 | 96 | 102 | 77 | 197 | 153 | 182 | 134 | 184 | 166 | 36 | 284 | 144 |
| Erebidae | 1283 | 1709 | 1409 | 2415 | 1939 | NA | NA | 1313 | 3240 | 2527 | 1899 | 3552 | 2694 | 683 | 3355 | 1363 |
| Nolidae | 71 | 135 | 73 | 143 | 110 | NA | NA | 7 | 58 | 52 | 24 | 8 | 14 | 58 | 94 | 18 |
| Thyatridae | 25 | 235 | 31 | 76 | 39 | 53 | 38 | NA | NA | NA | NA | NA | NA | 46 | 205 | 36 |
| Total | 5191 | 5839 | 5203 | 9786 | 8308 | 12,732 | 11,422 | 6914 | 6876 | 6450 | 5488 | 8121 | 9638 | 1842 | 9761 | 4675 |
| Family | 2020 | 2020 | 2020 | 2020 | 2021 | 2021 | 2021 | 2021 | 2022 | lin x coef 1970 | r2 | lin x coef 2014 | r2 | AVG | MD | |
| Geometridae | 2050 | 1047 | 1951 | 5336 | 1996 | 1386 | 3838 | 1056 | 2053 | −79.7 | 0.15 | 113.9 | 0.22 | 1908 | 2301 | |
| Noctuidae | 4842 | 1818 | 1127 | 3142 | 715 | 3219 | 7099 | 1664 | 1103 | −45.5 | 0.08 | −26.4 | 0 | 2633 | 3391 | |
| Lasiocampidae | 110 | 56 | 49 | 84 | 34 | 49 | 100 | 23 | 52 | −1.8 | 0.04 | 0.76 | 0.01 | 67 | 73 | |
| Saturniidae | 27 | 13 | 28 | 60 | 18 | 5 | 3 | 10 | 5 | NA | NA | NA | NA | 16 | 3 | |
| Sphingidae | 130 | 111 | 70 | 18 | 81 | 88 | 147 | 113 | 35 | −0.54 | 0 | −3.13 | 0.07 | 99 | 111.5 | |
| Drepanidae | 69 | 52 | 106 | 152 | 208 | 93 | 43 | 53 | 36 | −1.77 | 0.02 | −2.35 | 0.02 | 91 | 42.5 | |
| Notodontidae | 292 | 208 | 106 | 659 | 208 | 159 | 341 | 226 | 223 | −8.64 | 0.06 | 8.06 | 0.11 | 225 | 255.5 | |
| Erebidae | 6623 | 1271 | 2891 | 3486 | 1402 | 2928 | 5369 | 1423 | 1252 | −17.9 | 0.01 | 16.73 | 0 | 2679 | 1553 | |
| Nolidae | 335 | 77 | 195 | 142 | 129 | 35 | 708 | 36 | 56 | −1.91 | 0.01 | 12.47 | 0.15 | 129 | 125.5 | |
| Thyatridae | 133 | 85 | 113 | 262 | 59 | 33 | 165 | 58 | 104 | −2.33 | 0.04 | −0.74 | 0.01 | 108 | 64 | |
| Total | 14,755 | 4757 | 6613 | 13,341 | 4750 | 8123 | 17,911 | 5676 | 5020 | −157 | 0.08 | 146.8 | 0.04 | 9424 | 6876 | |
| Family | 1970 | 1970 | 1970 | 1970 | 1971 | 1971 | 1971 | 1973 | 1976 | 1977 | 1978 | 1979 | 1979 | 1980 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Geometridae | 114 | 107 | 147 | 176 | 120 | 68 | 143 | 139 | 105 | 120 | 124 | 126 | 134 | 109 |
| Noctuidae | 106 | 102 | 120 | 163 | 118 | 61 | 156 | 151 | 133 | 164 | 160 | 98 | 150 | 103 |
| Lasiocampidae | 6 | 3 | 7 | 13 | 5 | 2 | 11 | 13 | 9 | 10 | 10 | 9 | 9 | 8 |
| Saturniidae | 1 | 0 | 0 | 1 | 0 | 0 | 2 | 3 | 3 | 2 | 2 | 1 | 2 | 0 |
| Sphingidae | 2 | 1 | 5 | 10 | 2 | 0 | 11 | 10 | 9 | 11 | 9 | 6 | 10 | 5 |
| Drepanidae | 3 | 2 | 4 | 5 | 3 | 1 | 5 | 5 | 13 | 12 | 12 | 9 | 10 | 8 |
| Notodontidae | 11 | 11 | 10 | 25 | 9 | 14 | 24 | 20 | 24 | 25 | 25 | 9 | 21 | 9 |
| Erebidae | 37 | 33 | 39 | 57 | 42 | 24 | 54 | 54 | 49 | 47 | 44 | 47 | 52 | 49 |
| Nolidae | 3 | 8 | 5 | 8 | 4 | 2 | 7 | 7 | 5 | 5 | 4 | 5 | 6 | 5 |
| Thyatiridae | 4 | 4 | 5 | 6 | 4 | 2 | 6 | 6 | 7 | 6 | 7 | 4 | 6 | 3 |
| Moths total | 287 | 271 | 342 | 464 | 307 | 174 | 419 | 405 | 356 | 401 | 396 | 309 | 393 | 294 |
| Family | 1980 | 1981 | 1981 | 1986 | 1987 | 2000 | 2001 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | 2019 |
| Geometridae | 122 | 122 | 125 | 148 | 144 | 117 | 121 | 115 | 143 | 127 | 127 | 149 | 134 | 61 |
| Noctuidae | 136 | 96 | 132 | 163 | 165 | NA | NA | 73 | 75 | 72 | 79 | 91 | 89 | 36 |
| Lasiocampidae | 7 | 6 | 9 | 11 | 9 | 12 | 9 | 8 | 3 | 9 | 9 | 10 | 9 | 1 |
| Saturniidae | 1 | 1 | 2 | 4 | 1 | 1 | 0 | 1 | 0 | 1 | 2 | 2 | 2 | 0 |
| Sphingidae | 7 | 5 | 9 | 10 | 8 | 10 | 8 | 9 | 8 | 9 | 9 | 9 | 10 | 5 |
| Drepanidae | 10 | 8 | 11 | 12 | 15 | 5 | 5 | 7 | 11 | 7 | 9 | 10 | 10 | 1 |
| Notodontidae | 19 | 10 | 14 | 23 | 23 | 15 | 17 | 17 | 17 | 17 | 17 | 17 | 19 | 10 |
| Erebidae | 44 | 42 | 44 | 55 | 54 | NA | NA | 29 | 34 | 33 | 33 | 43 | 43 | 27 |
| Nolidae | 7 | 5 | 7 | 9 | 8 | NA | NA | 3 | 3 | 2 | 2 | 3 | 1 | 2 |
| Thyatiridae | 5 | 3 | 5 | 6 | 8 | NA | NA | NA | NA | NA | NA | NA | NA | 5 |
| Moths total | 350 | 293 | 349 | 435 | 428 | 377 | 378 | 266 | 300 | 281 | 288 | 337 | 321 | 142 |
| Family | 2019 | 2020 | 2020 | 2021 | 2021 | 2021 | 2021 | 2022 | Lin x coef 1970 | r2 | Lin x coef 2014 | r2 | AVG | MD |
| Geometridae | 103 | 101 | 113 | 110 | 91 | 74 | 77 | 107 | −0.89 | 0.15 | −3.50 | 0.38 | 124 | 122 |
| Noctuidae | 108 | 86 | 113 | 89 | 153 | 128 | 125 | 95 | −0.98 | 0.1 | 4.01 | 0.40 | 128 | 127 |
| Lasiocampidae | 7 | 7 | 9 | 7 | 8 | 4 | 3 | 6 | −0.05 | 0.04 | −0.16 | 0.07 | 8 | 9 |
| Saturniidae | 1 | 1 | 1 | 1 | 2 | 1 | 4 | 2 | NA | NA | NA | NA | 1 | 1 |
| Sphingidae | 2 | 6 | 6 | 4 | 9 | 9 | 8 | 3 | 0.04 | 0.01 | −0.23 | 0.16 | 7 | 8 |
| Drepanidae | 5 | 3 | 5 | 3 | 5 | 2 | 4 | 3 | −0.04 | 0.01 | −0.50 | 0.50 | 7 | 5 |
| Notodontidae | 15 | 11 | 14 | 14 | 16 | 17 | 22 | 14 | −0.02 | 0.00 | −0.04 | 0.00 | 17 | 17 |
| Erebidae | 48 | 42 | 48 | 39 | 50 | 39 | 39 | 52 | −0.06 | 0.01 | 1.08 | 0.40 | 45 | 47 |
| Nolidae | 10 | 10 | 9 | 11 | 5 | 7 | 2 | 6 | 0.01 | 0.00 | 0.35 | 0.21 | 5 | 5 |
| Thyatiridae | 4 | 5 | 5 | 4 | 5 | 4 | 5 | 4 | NA | NA | NA | NA | 5 | 6 |
| Moths total | 304 | 268 | 324 | 277 | 344 | 285 | 289 | 295 | −1.91 | 0.09 | 1.19 | 0.01 | 326 | 315 |
| Species | 1970 | 1970 | 1970 | 1970 | 1973 | 1976 | 1977 | 1978 | 1979 | 1979 | 1980 | 1980 | 1981 | 1981 | 1986 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tarachidia candefacta (Hübner, 1831) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hyphantria cunea (Drury, 1773) | 4 | 1265 | 0 | 14 | 4 | 199 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Antheraea yamamai (Guérin-Méneville, 1861) | 0 | 0 | 0 | 0 | 1 | 9 | 20 | 18 | 1 | 1 | 0 | 0 | 0 | 1 | 15 |
| Helicoverpa armigera (Hübner, 1808) | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Species | 1987 | 2019 | 2019 | 2020 | 2020 | 2020 | 2020 | 2020 | 2021 | 2021 | 2021 | 2021 | 2022 | Lin x coeff | r2 |
| Tarachidia candefacta (Hübner, 1831) | 0 | 0 | 0 | 0 | 0 | 0 | 17 | 0 | 0 | 0 | 51 | 2 | 0 | 1.31 | 0.08 |
| Hyphantria cunea (Drury, 1773) | 2 | 1 | 0 | 0 | 2 | 0 | 8 | 0 | 0 | 0 | 43 | 0 | 0 | −9.41 | 0.1 |
| Antheraea yamamai (Guérin-Méneville, 1861) | 11 | 0 | 29 | 28 | 60 | 0 | 0 | 3 | 18 | 0 | 0 | 4 | 3 | 0.28 | 0.03 |
| Helicoverpa armigera (Hübner, 1808) | 0 | 0 | 63 | 36 | 18 | 13 | 360 | 5 | 7 | 38 | 1380 | 13 | 6 | 10.9 | 0.11 |
| Species | 1980 | 1984 | 1990 | 1991 | 1994 | 1997 | 1997 | 2007 | 2011 | 2011 | 2013 | 2013 | 2015 | 2016 | 2018 | 2018 | 2020 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nymphalidae | |||||||||||||||||
| Vanessa atalanta | 4 | 2 | 4 | 4 | 3 | 4 | 4 | 4 | 3 | 4 | 4 | 4 | 2 | 2 | 4 | 4 | 4 |
| Inachis io | 5 | 4 | 4 | 4 | 3 | 4 | 4 | 4 | 5 | 4 | 4 | 4 | 3 | 4 | 5 | 4 | |
| Vanessa cardui | 4 | 3 | 4 | 3 | 4 | 4 | 3 | 4 | 4 | 4 | 3 | 3 | 3 | 3 | 4 | 5 | 3 |
| Polygonia c-album | 5 | 2 | 4 | 3 | 3 | 3 | 4 | 4 | 2 | 4 | 3 | 4 | 0 | 2 | 4 | 4 | 6 |
| Arachina levana | 5 | 5 | 4 | 5 | 5 | 4 | 5 | 4 | 3 | 4 | 0 | 4 | 4 | 3 | 5 | 5 | 2 |
| Argynnis paphia | 4 | 5 | 4 | 3 | 3 | 4 | 3 | 4 | 2 | 4 | 3 | 3 | 0 | 4 | 4 | 4 | 4 |
| Issoria lathonia | 4 | 4 | 4 | 4 | 3 | 3 | 2 | 4 | 3 | 3 | 2 | 3 | 0 | 2 | 3 | 5 | 4 |
| Melitaea athalia | 5 | 5 | 4 | 4 | 5 | 5 | 5 | 4 | 3 | 4 | 2 | 4 | 4 | 2 | 0 | 2 | 4 |
| Nymphalis urticae | 4 | 5 | 4 | 3 | 3 | 4 | 1 | 2 | 0 | 3 | 0 | 2 | 0 | 0 | 2 | 1 | 1 |
| Euphydryas aurinia | 0 | 0 | 2 | 0 | 0 | 3 | 0 | 3 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Libythea celtis | 1 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 4 | 4 | 4 |
| Pararge aegeria | 5 | 5 | 4 | 3 | 3 | 4 | 4 | 3 | 3 | 4 | 4 | 3 | 3 | 0 | 4 | 3 | 4 |
| Maniola jurtina | 5 | 5 | 5 | 5 | 4 | 5 | 5 | 4 | 5 | 4 | 3 | 3 | 5 | 5 | 4 | 4 | 4 |
| Lasiommata megera | 4 | 2 | 4 | 3 | 3 | 4 | 3 | 4 | 3 | 3 | 3 | 3 | 0 | 0 | 4 | 4 | 4 |
| Neptis hylas | 3 | 0 | 3 | 0 | 3 | 1 | 3 | 0 | 0 | 4 | 0 | 3 | 0 | 0 | 4 | 0 | 4 |
| Pieridae | |||||||||||||||||
| Colias hyale | 5 | 5 | 5 | 2 | 3 | 4 | 2 | 3 | 3 | 2 | 0 | 0 | 0 | 3 | 4 | 3 | 4 |
| Colias croceus | 4 | 1 | 4 | 3 | 3 | 4 | 3 | 3 | 2 | 3 | 0 | 2 | 3 | 0 | 4 | 4 | 4 |
| Anthocharis cardamines | 5 | 4 | 4 | 3 | 4 | 3 | 4 | 4 | 3 | 4 | 0 | 3 | 3 | 0 | 4 | 4 | 4 |
| Leptidea sinapis | 5 | 4 | 4 | 4 | 5 | 3 | 5 | 4 | 4 | 4 | 0 | 3 | 3 | 0 | 4 | 4 | 4 |
| Lycaenidae | |||||||||||||||||
| Polyommatus icarus | 5 | 5 | 5 | 4 | 4 | 4 | 5 | 4 | 5 | 4 | 3 | 5 | 4 | 4 | 4 | 4 | 4 |
| Lycaena dispar rutilus | 4 | 3 | 4 | 3 | 3 | 2 | 3 | 3 | 3 | 4 | 0 | 2 | 0 | 2 | 3 | 3 | 4 |
| Lycaena phlaeas | 4 | 4 | 4 | 3 | 3 | 3 | 3 | 2 | 4 | 0 | 3 | 3 | 3 | 0 | 3 | 3 | 4 |
| Papilionidae | |||||||||||||||||
| Iphicledes podalirius | 2 | 2 | 3 | 2 | 3 | 2 | 4 | 4 | 3 | 4 | 3 | 3 | 2 | 2 | 4 | 4 | 2 |
| Family | Bátorliget 1950 | Bátorliget 1990 | Dráva Valley, 1966–1974 | Dráva Valley 2019–2022 | Simontornya 1914 | Simontornya 2014 |
|---|---|---|---|---|---|---|
| Nymphalidae | 26 | 19 | 35 | 18 | 40 | 20 |
| Lycaenidae | 14 | 8 | 19 | 4 | 27 | 9 |
| Papilionidae | 2 | 2 | 4 | 3 | 3 | 4 |
| Pieridae | 11 | 5 | 10 | 5 | 10 | 12 |
| Hesperiidae | 8 | 6 | 10 | 2 | 11 | 6 |
| Riodinidae | 1 | 1 | 1 | 1 | 1 | 0 |
| Total | 62 | 41 | 79 | 33 | 92 | 51 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haris, A.; Józan, Z.; Roller, L.; Šima, P.; Tóth, S. Changes in Population Densities and Species Richness of Pollinators in the Carpathian Basin during the Last 50 Years (Hymenoptera, Diptera, Lepidoptera). Diversity 2024, 16, 328. https://doi.org/10.3390/d16060328
Haris A, Józan Z, Roller L, Šima P, Tóth S. Changes in Population Densities and Species Richness of Pollinators in the Carpathian Basin during the Last 50 Years (Hymenoptera, Diptera, Lepidoptera). Diversity. 2024; 16(6):328. https://doi.org/10.3390/d16060328
Chicago/Turabian StyleHaris, Attila, Zsolt Józan, Ladislav Roller, Peter Šima, and Sándor Tóth. 2024. "Changes in Population Densities and Species Richness of Pollinators in the Carpathian Basin during the Last 50 Years (Hymenoptera, Diptera, Lepidoptera)" Diversity 16, no. 6: 328. https://doi.org/10.3390/d16060328
APA StyleHaris, A., Józan, Z., Roller, L., Šima, P., & Tóth, S. (2024). Changes in Population Densities and Species Richness of Pollinators in the Carpathian Basin during the Last 50 Years (Hymenoptera, Diptera, Lepidoptera). Diversity, 16(6), 328. https://doi.org/10.3390/d16060328







