Associations between Epiphytic Orchids and Their Hosts and Future Perspectives of These in the Context of Global Warming
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Epiphytic Orchid–Host Relationships
2.3. Data Interpolation and Analysis
2.4. Species Threatened by Climate Change
3. Results
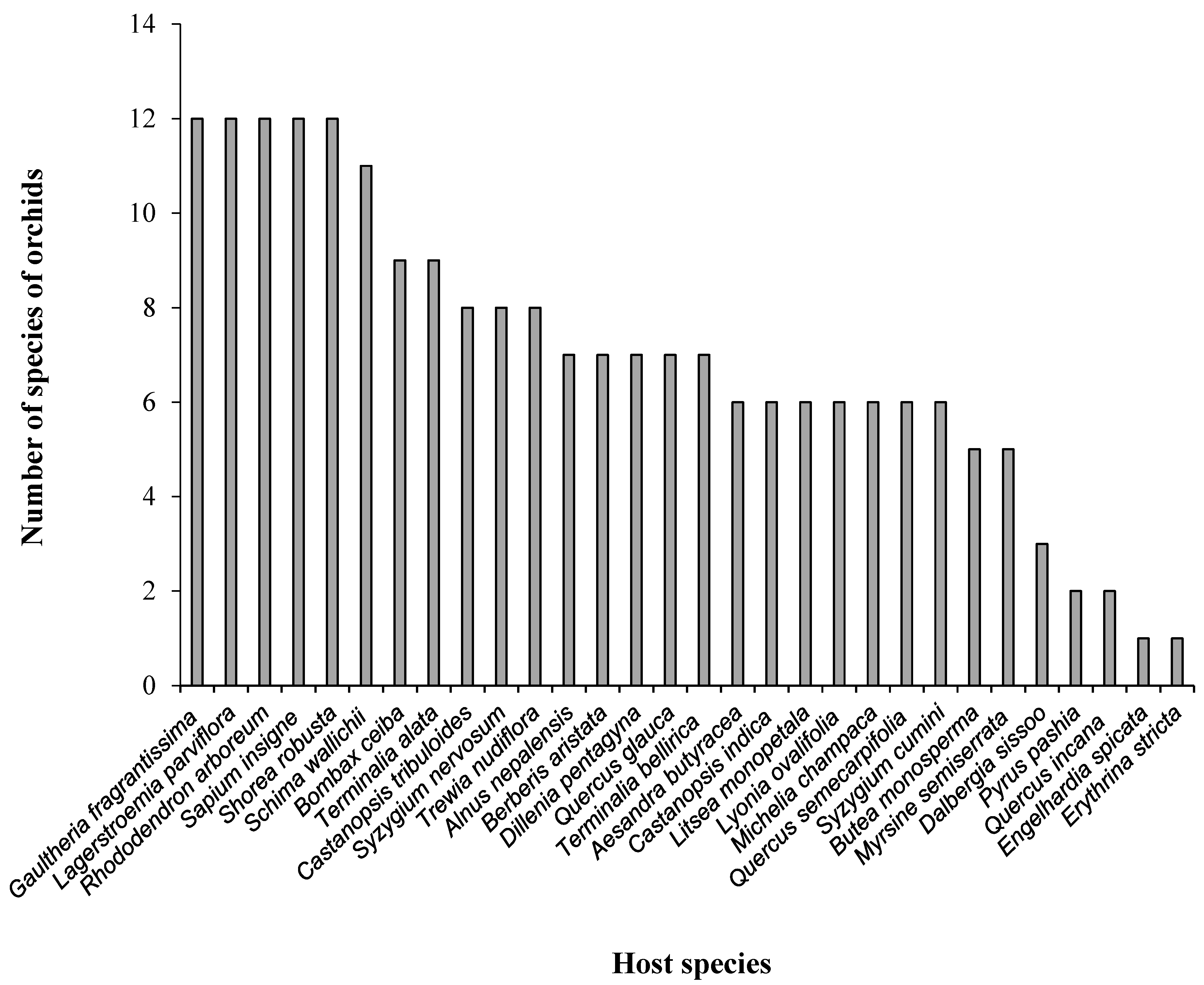
3.1. Associations of Epiphytic Orchids and Their Host Species
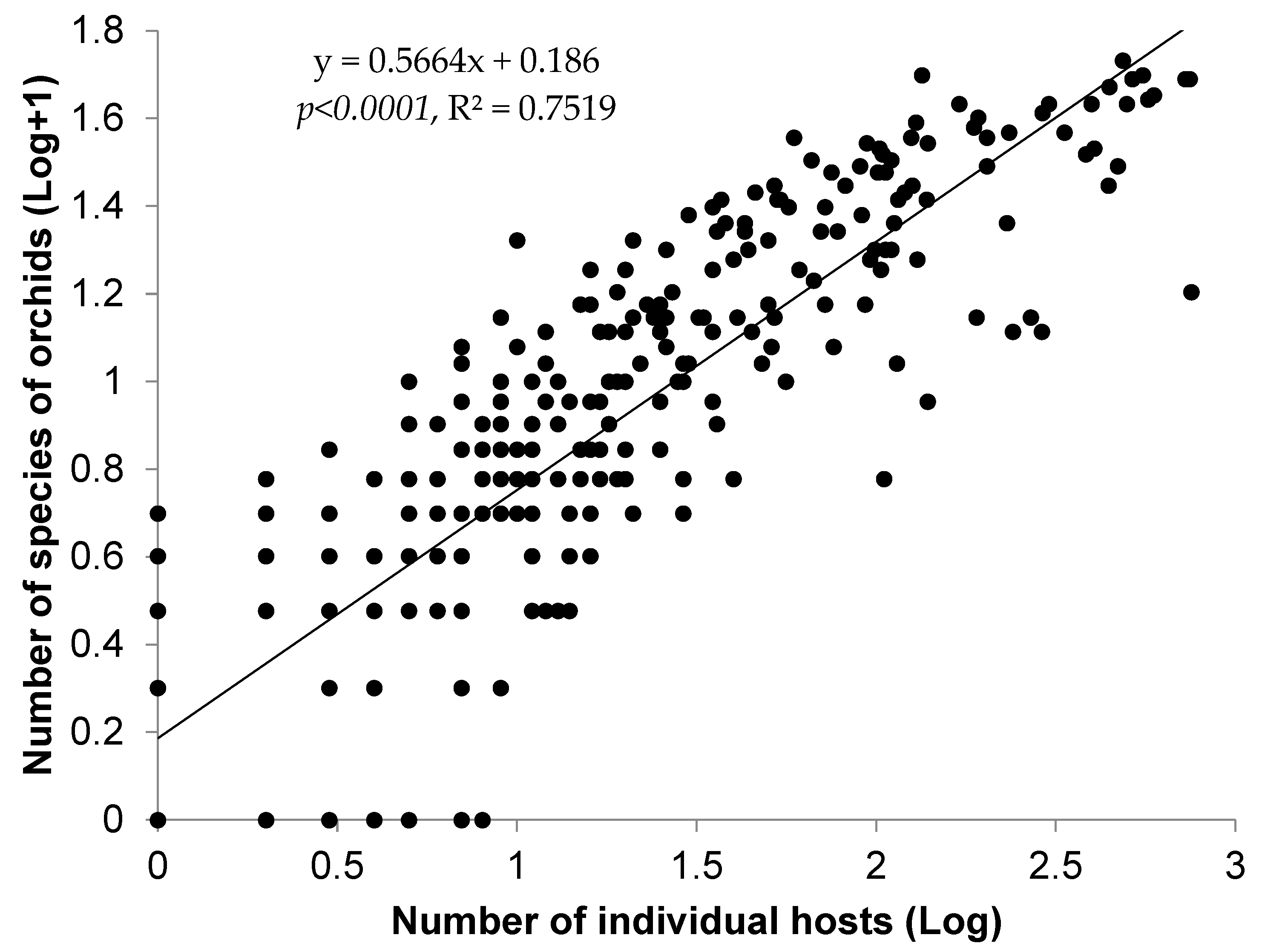
3.2. Orchid–Host Relationship and Distribution Patterns
3.3. The Effect of Temperature Increases on the Number of Plant Species
4. Discussion
4.1. Associations of Epiphytic Orchids and Their Host Species
4.2. Orchid–Host Relationship and Distribution Patterns
4.3. The Predicted Effect of an Increase in Temperature on the Number of Plant Species
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, X.; Li, Y.; Song, X.; Meng, Q.; Zhu, J.; Zhao, Y.; Yu, W. Influence of Host Tree Species on Isolation and Communities of Mycorrhizal and Endophytic Fungi from Roots of a Tropical Epiphytic Orchid, Dendrobium sinense (Orchidaceae). Mycorrhiza 2017, 27, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Alghamdi, S.A. Influence of Mycorrhizal Fungi on Seed Germination and Growth in Terrestrial and Epiphytic Orchids. Saudi J. Biol. Sci. 2017, 26, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, Y.P.; Fischer, A.; Fischer, H.S.; Rokaya, M.B.; Bhattarai, P.; Gruppe, A. Diversity, Composition and Host-Species Relationships of Epiphytic Orchids and Ferns in Two Forests in Nepal. J. Mt. Sci. 2017, 14, 1065–1075. [Google Scholar] [CrossRef]
- Hoeber, V.; Klinghardt, M.; Zotz, G. Drought Resistance Does Not Explain Epiphytic Abundance of Accidental Epiphytes. Plant Ecol. Divers. 2020, 13, 175–187. [Google Scholar] [CrossRef]
- Moreno-Chacón, M.; Saldaña, A. α, β and γ-Diversity of Vascular Epiphytes along the Climatic Gradient of Continental Chile. N. Z. J. Bot. 2019, 57, 18–31. [Google Scholar] [CrossRef]
- Taylor, A.; Saldaña, A.; Zotz, G.; Kirby, C.; Díaz, I.; Burns, K. Composition Patterns and Network Structure of Epiphyte–Host Interactions in Chilean and New Zealand Temperate Forests. N. Z. J. Bot. 2016, 54, 204–222. [Google Scholar] [CrossRef]
- Callaway, R.M.; Reinhart, K.O.; Moore, G.W.; Moore, D.J.; Pennings, S.C. Epiphyte Host Preferences and Host Traits: Mechanisms for Species-Specific Interactions. Oecologia 2002, 132, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, R.L.; Castro, J.V. Circular Distribution of an Epiphytic Herb on Trees in a Subtropical Rain Forest. Trop. Ecol. 2009, 50, 211. [Google Scholar]
- Zhang, Z.; Yan, Y.; Tian, Y.; Li, J.; He, J.-S.; Tang, Z. Distribution and Conservation of Orchid Species Richness in China. Biol. Conserv. 2015, 181, 64–72. [Google Scholar] [CrossRef]
- Wagner, K.; Mendieta-Leiva, G.; Zotz, G. Host Specificity in Vascular Epiphytes: A Review of Methodology, Empirical Evidence and Potential Mechanisms. AoB Plants 2015, 7, plu092. [Google Scholar] [CrossRef]
- Timsina, B.; Rokaya, M.B.; Münzbergová, Z.; Kindlmann, P.; Shrestha, B.; Bhattarai, B.; Raskoti, B.B. Diversity, Distribution and Host-Species Associations of Epiphytic Orchids in Nepal. Biodivers. Conserv. 2016, 25, 2803–2819. [Google Scholar] [CrossRef]
- Körner, C. Why Are There Global Gradients in Species Richness? Mountains Might Hold the Answer. Trends Ecol. Evol. 2000, 15, 513–514. [Google Scholar] [CrossRef]
- McCain, C.M.; Grytnes, J.-A. Elevational Gradients in Species Richness. In Encyclopedia of Life Sciences; John Wiley & Sons, Ltd.: Chichester, UK, 2010; ISBN 978-0-470-01617-6. [Google Scholar]
- Acharya, K.P.; Vetaas, O.R.; Birks, H.J.B. Orchid Species Richness along Himalayan Elevational Gradients. J. Biogeogr. 2011, 38, 1821–1833. [Google Scholar] [CrossRef]
- Rokaya, M.B.; Münzbergová, Z.; Shrestha, M.R.; Timsina, B. Distribution Patterns of Medicinal Plants along an Elevational Gradient in Central Himalaya, Nepal. J. Mt. Sci. 2012, 9, 201–213. [Google Scholar] [CrossRef]
- Li, M.; Feng, J. Biogeographical Interpretation of Elevational Patterns of Genus Diversity of Seed Plants in Nepal. PLoS ONE 2015, 10, e0140992. [Google Scholar] [CrossRef]
- Rokaya, M.B.; Raskoti, B.B.; Timsina, B.; Münzbergová, Z. An Annotated Checklist of the Orchids of Nepal. Nord. J. Bot. 2013, 31, 511–550. [Google Scholar] [CrossRef]
- Adhikari, Y.P.; Fischer, A.; Fischer, H.S. Epiphytic Orchids and Their Ecological Niche under Anthropogenic Influence in Central Himalayas, Nepal. J. Mt. Sci. 2016, 13, 774–784. [Google Scholar] [CrossRef]
- Adhikari, Y.P.; Fischer, A.; Fischer, H.S. Micro-Site Conditions of Epiphytic Orchids in a Human Impact Gradient in Kathmandu Valley, Nepal. J. Mt. Sci. 2012, 9, 331–342. [Google Scholar] [CrossRef]
- Adhikari, Y.P.; Fischer, H.S.; Fischer, A. Host Tree Utilization by Epiphytic Orchids in Different Land-Use Intensities in Kathmandu Valley, Nepal. Plant Ecol. 2012, 213, 1393–1412. [Google Scholar] [CrossRef]
- Adhikari, Y.P.; Fischer, A. Distribution Pattern of the Epiphytic Orchid Rhynchostylis Retusa under Strong Human Influence in Kathmandu Valley, Nepal. Bot. Orient. J. Plant Sci. 2011, 8, 90–99. [Google Scholar] [CrossRef]
- Raskoti, B.B. The Orchids of Nepal, 1st ed.; Bhakta Bahadur Raskoti and Rita Ale: Kathmandu, Nepal, 2009. [Google Scholar]
- Rajbhandari, K.R. Beautiful Orchids of Nepal; Keshab R. Rajbhandari: Kathmandu, Nepal, 2001; ISBN 978-99933-51-83-2. [Google Scholar]
- Press, J.R.; Shrestha, K.K.; Sutton, D.A. Annotated Checklist of the Flowering Plants of Nepal; Natural History Museum: London, UK, 2000. [Google Scholar]
- POWO Plants of the World Online. Facilitated by the Royal Botanic Gardens, Kew. Available online: http://www.plantsoftheworldonline.org/ (accessed on 3 April 2024).
- Sokal, R.R.; Rohlf, F.J. Biometry: The Principles and Practice of Statistics in Biological Research; W.H. Freeman and Company: New York, NY, USA, 1995; Volume 3. [Google Scholar]
- Chytrý, M.; Tichý, L.; Holt, J.; Botta-Dukát, Z. Determination of Diagnostic Species with Statistical Fidelity Measures. J. Veg. Sci. 2002, 13, 79–90. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2021; Available online: https://www.r-project.org/ (accessed on 18 March 2018).
- Tichý, L.; Chytrý, M. Statistical Determination of Diagnostic Species for Site Groups of Unequal Size. J. Veg. Sci. 2006, 17, 809–818. [Google Scholar] [CrossRef]
- Chao, A.; Gotelli, N.J.; Hsieh, T.C.; Sander, E.L.; Ma, K.H.; Colwell, R.K.; Ellison, A.M. Rarefaction and Extrapolation with Hill Numbers: A Framework for Sampling and Estimation in Species Diversity Studies. Ecol. Monogr. 2014, 84, 45–67. [Google Scholar] [CrossRef]
- Bhattarai, K.R.; Vetaas, O.R. Variation in Plant Species Richness of Different Life Forms along a Subtropical Elevation Gradient in the Himalayas, East Nepal. Glob. Ecol. Biogeogr. 2003, 12, 327–340. [Google Scholar] [CrossRef]
- Bhattarai, K.R.; Vetaas, O.R.; Grytnes, J.A. Fern Species Richness along a Central Himalayan Elevational Gradient, Nepal. J. Biogeogr. 2004, 31, 389–400. [Google Scholar] [CrossRef]
- Patterson, B.D.; Pacheco, V.; Solari, S. Distribution of Bats along an Elevational Gradient in the Andes of South-Eastern Peru. J. Zool. 1996, 240, 637–658. [Google Scholar] [CrossRef]
- Rahbek, C. The Relationship Among Area, Elevation, And Regional Species Richness in Neotropical Birds. Am. Nat. 1997, 149, 875–902. [Google Scholar] [CrossRef]
- Subedi, S.C.; Bhattarai, K.R.; Chaudhary, R.P. Distribution Pattern of Vascular Plant Species of Mountains in Nepal and Their Fate against Global Warming. J. Mt. Sci. 2015, 12, 1345–1354. [Google Scholar] [CrossRef]
- Pachauri, R.K.; Allen, M.R.; Barros, V.R.; Broome, J.; Cramer, W.; Christ, R.; Church, J.A.; Clarke, L.; Dahe, Q.; Dasgupta, P.; et al. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2014. [Google Scholar]
- Subedi, A.; Subedi, N.; Chaudhary, R.P. Panchase Forest: An Extraordinary Place for Wild Orchids in Nepal. Pleione 2007, 1, 23–31. [Google Scholar]
- Ghimire, M. Epiphytic Orchids of Nepal. Banko Janakari 2008, 18, 53–63. [Google Scholar] [CrossRef][Green Version]
- Koirala, P.; Pyakurel, D.; Gurung, K. Orchids in Rolpa District of Western Nepal: Documentation, Stock, Trade and Conservation. Banko 2010, 20, 3–13. [Google Scholar] [CrossRef]
- Jiang, Y.; Purvis, A. How Land Use Affects Biodiversity: An Analysis of the Differences in the Effects Recorded on Different Continents. Eur. J. Environ. Sci. 2023, 13, 15–22. [Google Scholar] [CrossRef]
- Preston, F.W. The Canonical Distribution of Commonness and Rarity: Part I. Ecology 1962, 43, 185. [Google Scholar] [CrossRef]
- Grytnes, J.A.; Vetaas, O.R. Species Richness and Altitude: A Comparison between Null Models and Interpolated Plant Species Richness along the Himalayan Altitudinal Gradient, Nepal. Am. Nat. 2002, 159, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Vetaas, O.R.; Grytnes, J.-A. Distribution of Vascular Plant Species Richness and Endemic Richness along the Himalayan Elevation Gradient in Nepal. Glob. Ecol. Biogeogr. 2002, 11, 291–301. [Google Scholar] [CrossRef]
- Grau, O.; Grytnes, J.-A.; Birks, H.J.B. A Comparison of Altitudinal Species Richness Patterns of Bryophytes with Other Plant Groups in Nepal, Central Himalaya. J. Biogeogr. 2007, 34, 1907–1915. [Google Scholar] [CrossRef]
- Baniya, C.B.; SolhøY, T.; Gauslaa, Y.; Palmer, M.W. The Elevation Gradient of Lichen Species Richness in Nepal. Lichenologist 2010, 42, 83. [Google Scholar] [CrossRef]
- Shrestha, U.B.; Bawa, K.S. Impact of Climate Change on Potential Distribution of Chinese Caterpillar Fungus (Ophiocordyceps Sinensis) in Nepal Himalaya. PLoS ONE 2014, 9, e106405. [Google Scholar] [CrossRef] [PubMed]
- Randin, C.F.; Engler, R.; Normand, S.; Zappa, M.; Zimmermann, N.E.; Pearman, P.B.; Vittoz, P.; Thuiller, W.; Guisan, A. Climate Change and Plant Distribution: Local Models Predict High-Elevation Persistence. Glob. Change Biol. 2009, 15, 1557–1569. [Google Scholar] [CrossRef]
- Davis, M.B.; Shaw, R.G. Range Shifts and Adaptive Responses to Quaternary Climate Change. Science 2001, 292, 673–679. [Google Scholar] [CrossRef]
- Telwala, Y.; Brook, B.W.; Manish, K.; Pandit, M.K. Climate-Induced Elevational Range Shifts and Increase in Plant Species Richness in a Himalayan Biodiversity Epicentre. PLoS ONE 2013, 8, e57103. [Google Scholar] [CrossRef] [PubMed]
- Quaresma, A.C.; Jardim, M.A.G. Floristic Composition and Spatial Distribution of Vascular Epiphytes in the Restingas of Maracanã, Brazil. Acta Bot. Bras. 2014, 28, 68–75. [Google Scholar] [CrossRef]
- Steffelová, M.; Traxmandlová, I.; Štípková, Z.; Kindlmann, P. Pollination Strategies of Deceptive Orchids—A Review. Eur. J. Environ. Sci. 2023, 13, 110–116. [Google Scholar] [CrossRef]
- Švecová, M.; Štípková, Z.; Traxmandlová, I.; Kindlmann, P. Difficulties in Determining Distribution of Population Sizes within Different Orchid Metapopulations. Eur. J. Environ. Sci. 2023, 13, 96–109. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Timsina, B.; Münzbergová, Z.; Kindlmann, P.; Bhattarai, B.P.; Shrestha, B.; Raskoti, B.B.; Rokaya, M.B. Associations between Epiphytic Orchids and Their Hosts and Future Perspectives of These in the Context of Global Warming. Diversity 2024, 16, 252. https://doi.org/10.3390/d16040252
Timsina B, Münzbergová Z, Kindlmann P, Bhattarai BP, Shrestha B, Raskoti BB, Rokaya MB. Associations between Epiphytic Orchids and Their Hosts and Future Perspectives of These in the Context of Global Warming. Diversity. 2024; 16(4):252. https://doi.org/10.3390/d16040252
Chicago/Turabian StyleTimsina, Binu, Zuzana Münzbergová, Pavel Kindlmann, Bishnu Prasad Bhattarai, Bikram Shrestha, Bhakta B. Raskoti, and Maan B. Rokaya. 2024. "Associations between Epiphytic Orchids and Their Hosts and Future Perspectives of These in the Context of Global Warming" Diversity 16, no. 4: 252. https://doi.org/10.3390/d16040252
APA StyleTimsina, B., Münzbergová, Z., Kindlmann, P., Bhattarai, B. P., Shrestha, B., Raskoti, B. B., & Rokaya, M. B. (2024). Associations between Epiphytic Orchids and Their Hosts and Future Perspectives of These in the Context of Global Warming. Diversity, 16(4), 252. https://doi.org/10.3390/d16040252







