Terrestrial Tardigrada (Water Bears) of the Słowiński National Park (Northern Poland)
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling and Sample Processing
2.2. Species Identification
2.3. Microscopy and Imaging
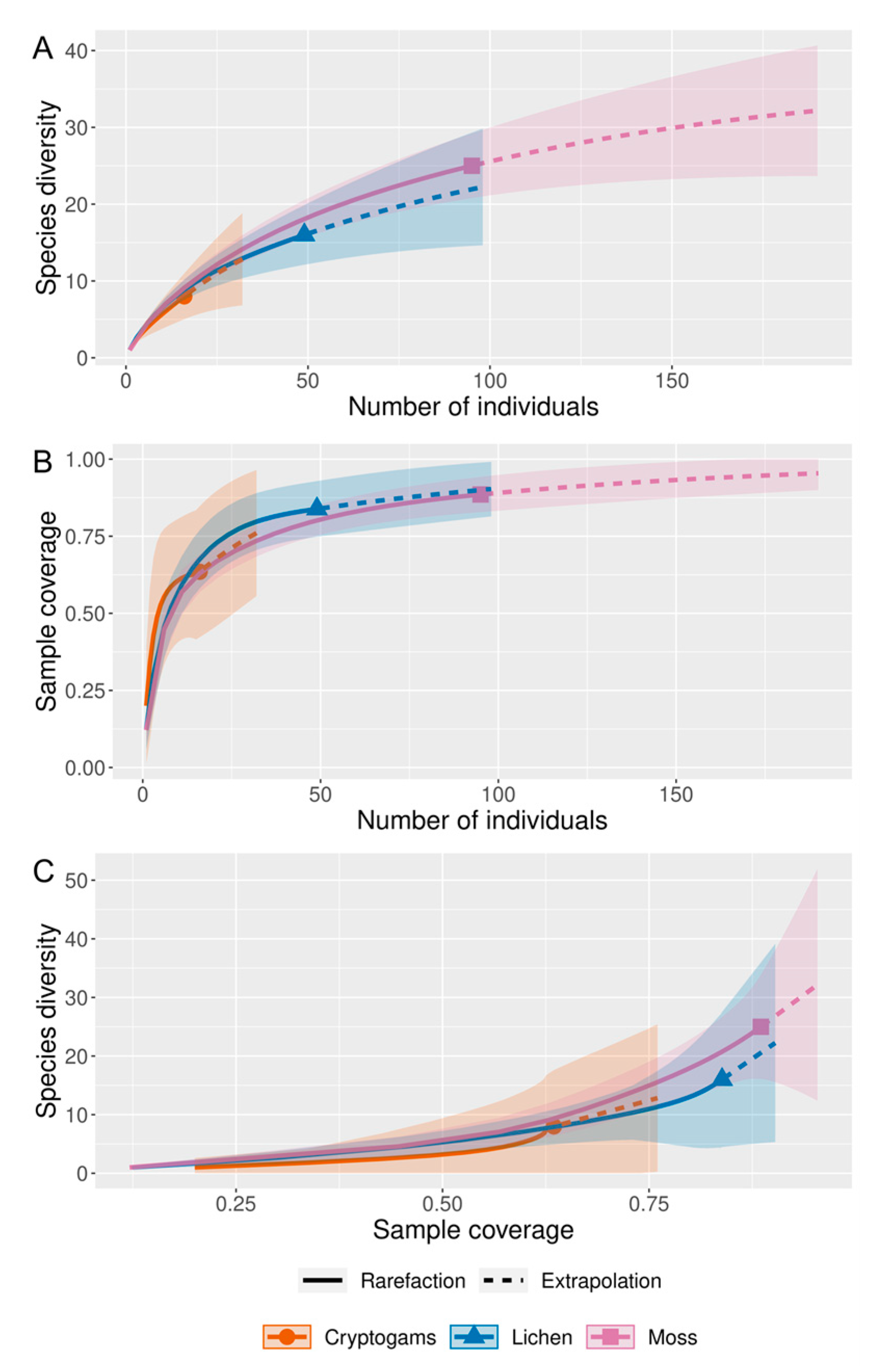
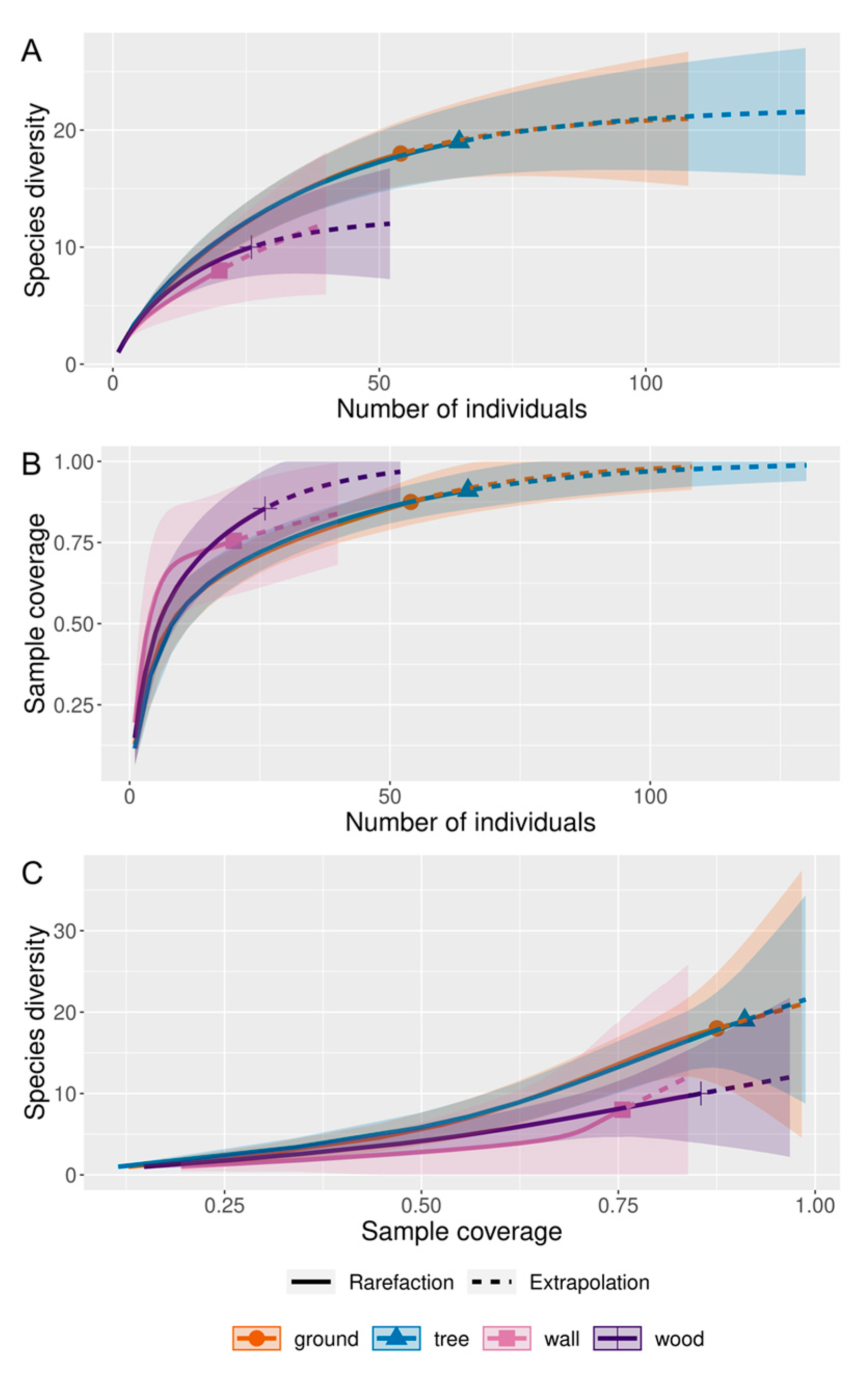
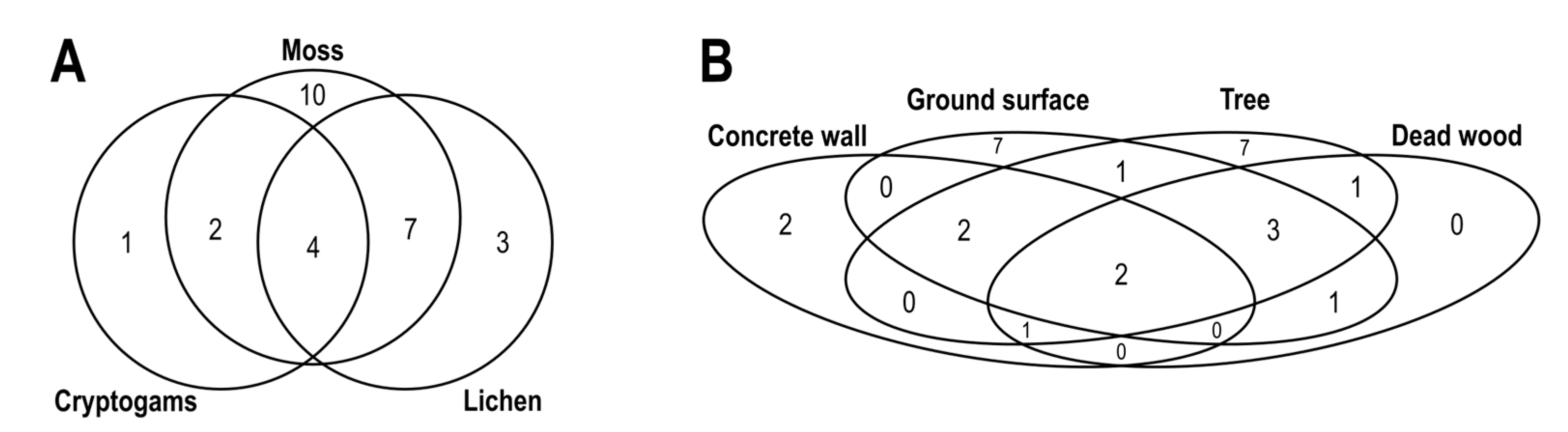
2.4. Statistical Analysis
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Nelson, D.R.; Guidetti, R.; Rebecchi, L. Phylum Tardigrada. In Thorp and Covich’s Freshwater Invertebrates; Thorp, J., Rogers, C., Eds.; Elsevier: London, UK, 2015; pp. 347–380. ISBN 978-0-12-385026-3. [Google Scholar]
- Degma, P.; Guidetti, R. Actual Checklist of Tardigrada Species; Università di Modena e Reggio Emilia: Modena, Italy, 2019; v42. [Google Scholar] [CrossRef]
- Jakubski, A. Opis fauny wrotków (Rotatoria) Powiatu Sokalskiego z uwzględnieniem gromad brzuchorzęsków (Gartroprioga) i niesporczaków (Tardigrada). Wiadom Mus. Dzieduszyckich Lwów 1915, 1, 1–166. [Google Scholar]
- Kaczmarek, Ł.; Kosicki, J.; Roszkowska, M. Tardigrada of Bory Tucholskie National Park, Zaborski Landscape Park, and Their Surroundings (Pomerania Province, Poland). Turk. J. Zool. 2018, 42, 6–17. [Google Scholar] [CrossRef]
- Nowak, B.; Stec, D. The First Record of Macrobiotus vladimiri Bertolani, Biserov, Rebecchi & Cesari, 2011 (Tardigrada: Eutardigrada: Macrobiotidae: hufelandi group) from Poland. Turk. J. Zool. 2017, 41, 558–567. [Google Scholar] [CrossRef]
- Erdmann, W.; Kosicki, J.Z.; Kayastha, P.; Mioduchowska, M.; Kaczmarek, Ł. An Integrative Description of Mesobiotus mandalori sp. nov. (Eutardigrada, Macrobiotoidea) from Poland. Eur. Zool. J. 2024. after review. [Google Scholar]
- Doyère, L. Mémoire Sur Les Tardigrades. Ann. Des Sci. Nat. 1840, 14, 269–361. [Google Scholar]
- Vecchi, M.; Cesari, M.; Bertolani, R.; Jönsson, K.I.; Rebecchi, L.; Guidetti, R. Integrative Systematic Studies on Tardigrades from Antarctica Identify New Genera and New Species within Macrobiotoidea and Echiniscoidea. Invert. Syst. 2016, 30, 303–322. [Google Scholar] [CrossRef]
- Kaczmarek, Ł.; Parnikoza, I.; Gawlak, M.; Esefeld, J.; Peter, H.-U.; Kozeretska, I.; Roszkowska, M. Tardigrades from Larus dominicanus Lichtenstein, 1823 Nests on the Argentine Islands (Maritime Antarctic). Polar. Biol. 2018, 41, 283–301. [Google Scholar] [CrossRef]
- Kaczmarek, Ł.; Michalczyk, Ł. The Macrobiotus hufelandi Group (Tardigrada) Revisited. Zootaxa 2017, 4363, 101–123. [Google Scholar] [CrossRef]
- Morek, W.; Surmacz, B.; López-López, A.; Michalczyk, Ł. “Everything Is Not Everywhere”: Time-Calibrated Phylogeography of the Genus Milnesium (Tardigrada). Mol. Ecol. 2021, 30, 3590–3609. [Google Scholar] [CrossRef] [PubMed]
- Stec, D. An Integrative Description of Two New Mesobiotus Species (Tardigrada: Eutardigrada: Macrobiotidae) with Updated Genus Phylogeny. Zool. Stud. 2022, 61, 1–30. [Google Scholar] [CrossRef]
- Słowiński National Park. Official Website of Słowiński National Park. Available online: https://www.slowinskipn.pl (accessed on 26 October 2022).
- Thulin, G. Beitrag zur Kenntnis der Tardigradenfauna Schwedens. Ark. Zool. 1911, 7, 1–60. [Google Scholar]
- Dastych, H. The Tardigrada of Poland; Monografie Fauny Polski; Polskie Wyd. Naukowe: Kraków, Poland, 1988; ISBN 978-83-01-08149-2. [Google Scholar]
- Gąsiorek, P.; Stec, D.; Morek, W.; Zawierucha, K.; Kaczmarek, Ł.; Lachowska-Cierlik, D.; Michalczyk, Ł. An Integrative Revision of Mesocrista Pilato, 1987 (Tardigrada: Eutardigrada: Hypsibiidae). J. Nat. Hist. 2016, 50, 2803–2828. [Google Scholar] [CrossRef]
- Stec, D.; Smolak, R.; Kaczmarek, Ł.; Michalczyk, Ł. An Integrative Description of Macrobiotus paulinae sp. nov. (Tardigrada: Eutardigrada: Macrobiotidae: Hufelandi Group) from Kenya. Zootaxa 2015, 4052, 501–536. [Google Scholar] [CrossRef] [PubMed]
- Fontoura, P.; Pilato, G. Diphascon (Diphascon) faialense sp. nov. a New Species of Tardigrada (Eutardigrada, Hypsibiidae) from the Azores and a Key to the Species of the D. pingue Group. Zootaxa 2007, 1589, 47–55. [Google Scholar] [CrossRef]
- Gąsiorek, P.; Stec, D.; Morek, W.; Michalczyk, Ł. An Integrative Redescription of Hypsibius dujardini (Doyère, 1840), the Nominal Taxon for Hypsibioidea (Tardigrada: Eutardigrada). Zootaxa 2018, 4415, 45–75. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, Ł.; Bartylak, T.; Stec, D.; Kulpa, A.; Kepel, M.; Kepel, A.; Roszkowska, M. Revisiting the Genus Mesobiotus Vecchi et al., 2016 (Eutardigrada, Macrobiotidae)—Remarks, Updated Dichotomous Key and an Integrative Description of New Species from Madagascar. Zool. Anz. 2020, 287, 121–146. [Google Scholar] [CrossRef]
- Kaczmarek, Ł.; Kayastha, P.; Roszkowska, M.; Gawlak, M.; Mioduchowska, M. Integrative Redescription of the Minibiotus intermedius (Plate, 1888)—The Type Species of the Genus Minibiotus R.O. Schuster, 1980. Diversity 2022, 14, 356. [Google Scholar] [CrossRef]
- Kaczmarek, Ł.; Michalczyk, Ł. Redescription of Hypsibius microps Thulin, 1928 and H. pallidus Thulin, 1911 (Eutardigrada: Hypsibiidae) Based on the Type Material from the Thulin Collection. Zootaxa 2009, 2275, 60–68. [Google Scholar] [CrossRef]
- Lisi, O.; Binda, M.G.; Pilato, G. Eremobiotus ginevrae sp. nov. and Paramacrobiotus pius sp. nov., Two New Species of Eutardigrada. Zootaxa 2016, 4103, 344. [Google Scholar] [CrossRef] [PubMed]
- Morek, W.; Gąsiorek, P.; Stec, D.; Blagden, B.; Michalczyk, Ł. Experimental Taxonomy Exposes Ontogenetic Variability and Elucidates the Taxonomic Value of Claw Configuration in Milnesium Doyère, 1840 (Tardigrada: Eutardigrada: Apochela). CTOZ 2016, 85, 173–200. [Google Scholar] [CrossRef]
- Ramazzotti, G.; Maucci, W. Il Phylum Tardigrada—III Edizione. Mem. Ist. Ital. Idrobiol. 1983, 41, 1–1012. [Google Scholar]
- Tumanov, D.V. Integrative Redescription of Hypsibius pallidoides Pilato et al., 2011 (Eutardigrada: Hypsibioidea) with the Erection of a New Genus and Discussion on the Phylogeny of Hypsibiidae. EJT 2020, 681, 1–37. [Google Scholar] [CrossRef]
- Bertolani, R.; Guidetti, R.; Marchioro, T.; Altiero, T.; Rebecchi, L.; Cesari, M. Phylogeny of Eutardigrada: New Molecular Data and Their Morphological Support Lead to the Identification of New Evolutionary Lineages. Mol. Phylogenetics Evol. 2014, 76, 110–126. [Google Scholar] [CrossRef] [PubMed]
- Perry, E.; Miller, W.R.; Kaczmarek, Ł. Recommended Abbreviations for the Names of Genera of the Phylum Tardigrada. Zootaxa 2019, 4608, 145–154. [Google Scholar] [CrossRef]
- Perry, E.; Miller, W.R.; Kaczmarek, Ł. Additional Recommended Abbreviations for the Names of Genera of the Phylum Tardigrada. Zootaxa 2021, 4981, 398400. [Google Scholar] [CrossRef] [PubMed]
- Chao, A.; Chiu, C.-H. Species Richness: Estimation and Comparison. In Wiley StatsRef: Statistics Reference Online; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2016; pp. 1–26. ISBN 978-1-118-44511-2. [Google Scholar]
- Chao, A.; Gotelli, N.J.; Hsieh, T.C.; Sander, E.L.; Ma, K.H.; Colwell, R.K.; Ellison, A.M. Rarefaction and Extrapolation with Hill Numbers: A Framework for Sampling and Estimation in Species Diversity Studies. Ecol. Monogr. 2014, 84, 45–67. [Google Scholar] [CrossRef]
- Chao, A.; Ma, K.H.; Hsieh, T.C. iNEXT (iNterpolation and EXTrapolation) Online: Software for Interpolation and Extrapolation of Species Diversity. Program and User’s Guide. 2016. Available online: http://Chao.Stat.Nthu.Edu.Tw/Wordpress/Software_download/Inext-Online/ (accessed on 7 January 2024).
- Carneiro, E.; Mielke, O.H.H.; Casagrande, M.M.; Fiedler, K. Skipper Richness (Hesperiidae) along Elevational Gradients in Brazilian Atlantic Forest. Neotrop. Entomol. 2014, 43, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Plate, L. Beiträge Zur Naturgeschichte Der Tardigraden. Zool. Jahrb. 1888, 3, 487–550. [Google Scholar] [CrossRef]
- Biserov, V.I. New species of Tardigrada in the USSR fauna. Zool. Zhurnal 1990, 69, 17–25. [Google Scholar]
- Vincenzi, J.; Cesari, M.; Kaczmarek, Ł.; Roszkowska, M.; Mioduchowska, M.; Rebecchi, L.; Kiosya, Y.; Guidetti, R. The Xerophilic Genera Xerobiotus and Pseudohexapodibius (Macrobiotidae; Tardigrada): Biodiversity, Biogeography and Phylogeny. Zool. J. Linn. Soc. 2024, 200, 111–141. [Google Scholar] [CrossRef]
- Richters, F. Faune Des Mousses: Tardigrades. In Duc d’Orleans Campagne Arctique de 1907; Bulens: Brussels, Belgium, 1911. [Google Scholar]
- Kaczmarek, Ł.; Michalczyk, Ł.; Mcinnes, S.J. Annotated Zoogeography of Non-Marine Tardigrada. Part III: North America and Greenland. Zootaxa 2016, 4203, 1. [Google Scholar] [CrossRef] [PubMed]
- McInnes, S.J. Zoogeographic Distribution of Terrestrial/Freshwater Tardigrades from Current Literature. J. Nat. Hist. 1994, 28, 257–352. [Google Scholar] [CrossRef]
- Mcinnes, S.J.; Michalczyk, Ł.; Kaczmarek, Ł. Annotated Zoogeography of Non-Marine Tardigrada. Part IV: Africa. Zootaxa 2017, 4284, 1–74. [Google Scholar] [CrossRef]
- Murray, J. The Tardigrada of the Forth Valley. Ann. Scott. Nat. Hist. 1905, 55, 160–164. [Google Scholar]
- Kaczmarek, Ł.; Michalczyk, Ł.; McInnes, S.J. Annotated Zoogeography of Non-Marine Tardigrada. Part I: Central America. Zootaxa 2014, 3763, 1–62. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, Ł.; Michalczyk, Ł.; Mcinnes, S.J. Annotated Zoogeography of Non-Marine Tardigrada. Part II: South America. Zootaxa 2015, 3923, 1–107. [Google Scholar] [CrossRef] [PubMed]
- Michalczyk, Ł.; Kaczmarek, Ł.; Mcinnes, S.J. Annotated Zoogeography of Non-Marine Tardigrada. Part V: Australasia. Zootaxa 2022, 5107, 1–119. [Google Scholar] [CrossRef] [PubMed]
- Richters, F. Beiträge Zur Kenntnis der Fauna der Umgebung von Frankfurt a M. In Bericht der Senckenbergische Naturforschende Gesselschaft in Frankfurt am Main; Senckenberg Gesellschaft für Naturforschung: Frankfurt am Main, Germany, 1902; pp. 23–26. [Google Scholar]
- Marcus, E. Tardigrada. Tierreich 1936, 66, 1–340. [Google Scholar]
- Pilato, G.; Binda, M.G. A Comparison of Diphascon (D.) alpinum Murray, 1906, D. (D.) chilenense Plate, 1889 and D. (D.) pingue (Marcus, 1936) (Tardigrada), and Description of a New Species. Zool. Anz. 1998, 236, 181–185. [Google Scholar]
- Pilato, G.; Binda, M.G. Three New Species of Diphascon of the pingue group (Eutardigrada, Hypsibiidae) from Antarctica. Polar Biol. 1999, 21, 335–342. [Google Scholar] [CrossRef]
- Pilato, G.; Binda, M.G.; Bertolani, R.; Lisi, O. Four New Species of the Diphascon nobilei group (Eutardigrada, Hypsibiidae). J. Nat. Hist. 2005, 39, 1029–1041. [Google Scholar] [CrossRef]
- Thulin, G. Über Die Phylogenie Und Das System Der Tardigraden. Hereditas 1928, 11, 207–266. [Google Scholar] [CrossRef]
- Urbanowicz, C. Sur La Variabilité de Macrobiotus oberhaeuseri. Bull. Biol. Fr. Belg. 1925, 59, 124–142. [Google Scholar]
- Cuénot, L. Description d’un Tardigrade Nouveau de La Faune Francaise. Arch. d’Anatomie Microsc. 1929, 25, 121–125. [Google Scholar]
- Zawierucha, K.; Dziamięcki, J.; Jakubowska, N.; Michalczyk, Ł.; Kaczmarek, Ł. New Tardigrade Records for the Baltic States with a Description of Minibiotus formosus sp. n. (Eutardigrada, Macrobiotidae). ZooKeys 2014, 408, 81–105. [Google Scholar] [CrossRef]
- Schultze, C.A.S. Macrobiotus Hufelandii Animal e Crustaceorum Classe Novum, Reviviscendi Post Diuturnam Asphixiam et Aridiatem Potens; Carolum Curths: Berlin, Germany, 1834. [Google Scholar]
- Bertolani, R.; Biserov, V.; Rebecchi, L.; Cesari, M. Taxonomy and Biogeography of Tardigrades Using an Integrated Approach: New Results on Species of the Macrobiotus hufelandi Group. Invert. Zool 2011, 8, 23–36. [Google Scholar] [CrossRef]
- Richters, F. Nordische Tardigraden. Zool. Anz. 1903, 27, 168–172. [Google Scholar]
- Kaczmarek, Ł.; Jakubowska, N.; Michalczyk, Ł. Current Knowledge on Turkish Tardigrades with a Description of Milnesium beasleyi sp. nov. (Eutardigrada: Apochela: Milnesiidae, the granulatum group). Zootaxa 2012, 3589, 49. [Google Scholar] [CrossRef]
- Michalczyk, Ł.; Wełnicz, W.; Frohme, M.; Kaczmarek, Ł. Redescriptions of Three Milnesium Doyère, 1840 Taxa (Tardigrada: Eutardigrada: Milnesiidae), Including the Nominal Species for the Genus. Zootaxa 2012, 3154, 1. [Google Scholar] [CrossRef]
- Tumanov, D.V. Five New Species of the Genus Milnesium (Tardigrada, Eutardigrada, Milnesiidae). Zootaxa 2006, 1122, 1. [Google Scholar] [CrossRef]
- Morek, W.; Stec, D.; Gąsiorek, P.; Surmacz, B.; Michalczyk, Ł. Milnesium tardigradum Doyère, 1840: The First Integrative Study of Interpopulation Variability in a Tardigrade Species. J. Zool. Syst. Evol. Res. 2019, 57, 1–23. [Google Scholar] [CrossRef]
- Sugiura, K.; Minato, H.; Matsumoto, M.; Suzuki, A.C. Milnesium (Tardigrada: Apochela) in Japan: The First Confirmed Record of Milnesium tardigradum s.s. and Description of Milnesium pacificum sp. nov. Zool. Sci. 2020, 37, 1. [Google Scholar] [CrossRef]
- Ciobanu, D.A.; Roszkowska, M.; Kaczmarek, Ł. Two New Tardigrade Species from Romania (Eutardigrada: Milnesiidae, Macrobiotidae), with Some Remarks on Secondary Sex Characters in Milnesium dornensis sp. nov. Zootaxa 2015, 3941, 542–564. [Google Scholar] [CrossRef] [PubMed]
- Gąsiorek, P.; Stec, D.; Morek, W.; Marnissi, J.; Michalczyk, Ł. The Tardigrade Fauna of Tunisia, with an Integrative Description of Bryodelphax maculatus sp. nov. (Heterotardigrada: Echiniscidae). Afr. Zool. 2017, 52, 77–89. [Google Scholar] [CrossRef]
- Claxton, S.K. A Revision of the Genus Minibiotus (Tardigrada: Macrobiotidae) with Descriptions of Eleven New Species from Australia. Rec. Aust. Mus. 1998, 50, 125–160. [Google Scholar] [CrossRef]
- Pilato, G.; Kiosya, Y.; Lisi, O.; Inshina, V.; Biserov, V. Annotated List of Tardigrada Records from Ukraine with the Description of Three New Species. Zootaxa 2011, 3123, 1. [Google Scholar] [CrossRef]
- Schill, R.O.; Förster, F.; Dandekar, T.; Wolf, M. Using Compensatory Base Change Analysis of Internal Transcribed Spacer 2 Secondary Structures to Identify Three New Species in Paramacrobiotus (Tardigrada). Org. Divers. Evol. 2010, 10, 287–296. [Google Scholar] [CrossRef]
- Kaczmarek, Ł.; Mioduchowska, M.; Kačarević, U.; Kubska, K.; Parnikoza, I.; Gołdyn, B.; Roszkowska, M. New Records of Antarctic Tardigrada with Comments on Interpopulation Variability of the Paramacrobiotus fairbanksi Schill, Förster, Dandekar and Wolf, 2010. Diversity 2020, 12, 108. [Google Scholar] [CrossRef]
- Kayastha, P.; Mioduchowska, M.; Warguła, J.; Kaczmarek, Ł. A Review on the Genus Paramacrobiotus (Tardigrada) with a New Diagnostic Key. Diversity 2023, 15, 977. [Google Scholar] [CrossRef]
- Kayastha, P.; Szydło, W.; Mioduchowska, M.; Kaczmarek, Ł. Morphological and Genetic Variability in Cosmopolitan Tardigrade Species—Paramacrobiotus fairbanksi Schill, Förster, Dandekar & Wolf, 2010. Sci. Rep. 2023, 13, 17672. [Google Scholar] [CrossRef] [PubMed]
- Iharos, G. Neue Tardigraden-Arten Aus Ungarn (Neuere Beiträge Zur Kenntnis Der Tardigraden-Fauna Ungarns, IV). Acta Zool. Acad. Sci. Hung. 1966, 12, 111–122. [Google Scholar]
- Guil, N.; Rodrigo, E.; Machordom, A. Soil Tardigrade Biodiversity with the Description of a New Eutardigrade Genus and Its Phylogenetic Position. Syst. Biodivers. 2015, 13, 234–256. [Google Scholar] [CrossRef]
- Dastych, H. Niesporczaki (Tardigrada) Tatrzańskiego Parku Narodowego; Monografie Fauny Polski; PWN: Krakow, Poland, 1980. [Google Scholar]
- Kaczmarek, Ł.; Michalczyk, Ł. Niesporczaki (Tardigrada) Wielkopolskiego Parku Narodowego (środkowo-zachodnia Polska). Rocz. nauk. PTOP "Salamdra" 2003, 7, 55–64. [Google Scholar]
- Michalczyk, Ł.; Kaczmarek, Ł. Water Bears (Tardigrada) of the Bieszczady Mountains (Poland, West Carpathians). Zootaxa 2003, 242, 1–24. [Google Scholar] [CrossRef]
- Dastych, H. Niesporczaki (Tardigrada) Wolińskiego Parku Narodowego. Bad. Fizjogr. Pol. Zach. Ser. B 1972, 25, 37–45. [Google Scholar]
- Dastych, H. Niesporczaki (Tardigrada) Ojcowskiego Parku Narodowego. Bad. Fizjogr. Pol. Zach. 1979, 32, 97–104. [Google Scholar]
- Hęciak, S. Niesporczaki (Tardigrada) Gór Świętokrzyskich. Bad. Fizjogr. Pol. Zach. 1976, 29, 111–128. [Google Scholar]
- Kaczmarek, Ł.; Gawlak, M.; Bartels, P.J.; Nelson, D.R.; Roszkowska, M. Revision of the Genus Paramacrobiotus Guidetti et al., 2009 with the Description of a New Species, Re-Descriptions and a Key. Ann. Zool. 2017, 67, 627–656. [Google Scholar] [CrossRef]
- Kaczmarek, Ł.; Rutkowski, T.; Zacharyasiewicz, M.; Surmacki, A.; Osiejuk, T.S.; Kayastha, P. New Species of Macrobiotidae (Eutardigrada) from Cameroon (Africa), Characteristics of Macrobiotus Morpho-Groups and a Key to the nelsonae group. Ann. Zool. 2023, 73, 1–15. [Google Scholar] [CrossRef]
- Guidetti, R.; Bertolani, R.; Nelson, D. Ecological and Faunistic Studies on Tardigrades in Leaf Litter of Beech Forests. Zool. Anz. 1999, 238, 215–223. [Google Scholar]
- Nelson, D.R.; Bartels, P.J. Species Richness of Soil and Leaf Litter Tardigrades in the Great Smoky Mountains National Park (North Carolina/Tennessee, USA). J. Limnol. 2013, 72, 144–151. [Google Scholar] [CrossRef]
- Kaczmarek, Ł.; GoŁdyn, B.; Wełnicz, W.; Michalczyk, Ł. Ecological Factors Determining Tardigrada Distribution in Costa Rica. J. Zool. Syst. Evol. Res. 2011, 49, 78–83. [Google Scholar] [CrossRef]
- Nelson, D.R.; Bartels, P.J.; Fegley, S.R. Environmental Correlates of Tardigrade Community Structure in Mosses and Lichens in the Great Smoky Mountains National Park (Tennessee and North Carolina, USA). Zool. J. Linn. Soc. 2020, 188, 913–924. [Google Scholar] [CrossRef]
- Guidetti, R.; Ingemar Jönsson, K.; Kaczmarek, Ł.; Meier, T.; Speed, J.D.M.; Prestø, T.; Stur, E.; Topstad, L.; Cesari, M.; Roszkowska, M.; et al. Tardigrade Diversity and Community Composition across Norwegian Boreal Forests. Zool. J. Linn. Soc. 2024, 200, 156–171. [Google Scholar] [CrossRef]
- Guil, N.; Sánchez-Moreno, S.; Machordom, A. Local Biodiversity Patterns in Micrometazoans: Are Tardigrades Everywhere? Syst. Biodivers. 2009, 7, 259–268. [Google Scholar] [CrossRef]
- Ito, M.T. Ecological Distribution, Abundance and Habitat Preference of Terrestrial Tardigrades in Various Forests on the Northern Slope of Mt. Fuji, Central Japan. Zool. Anz. 1999, 238, 225–234. [Google Scholar]
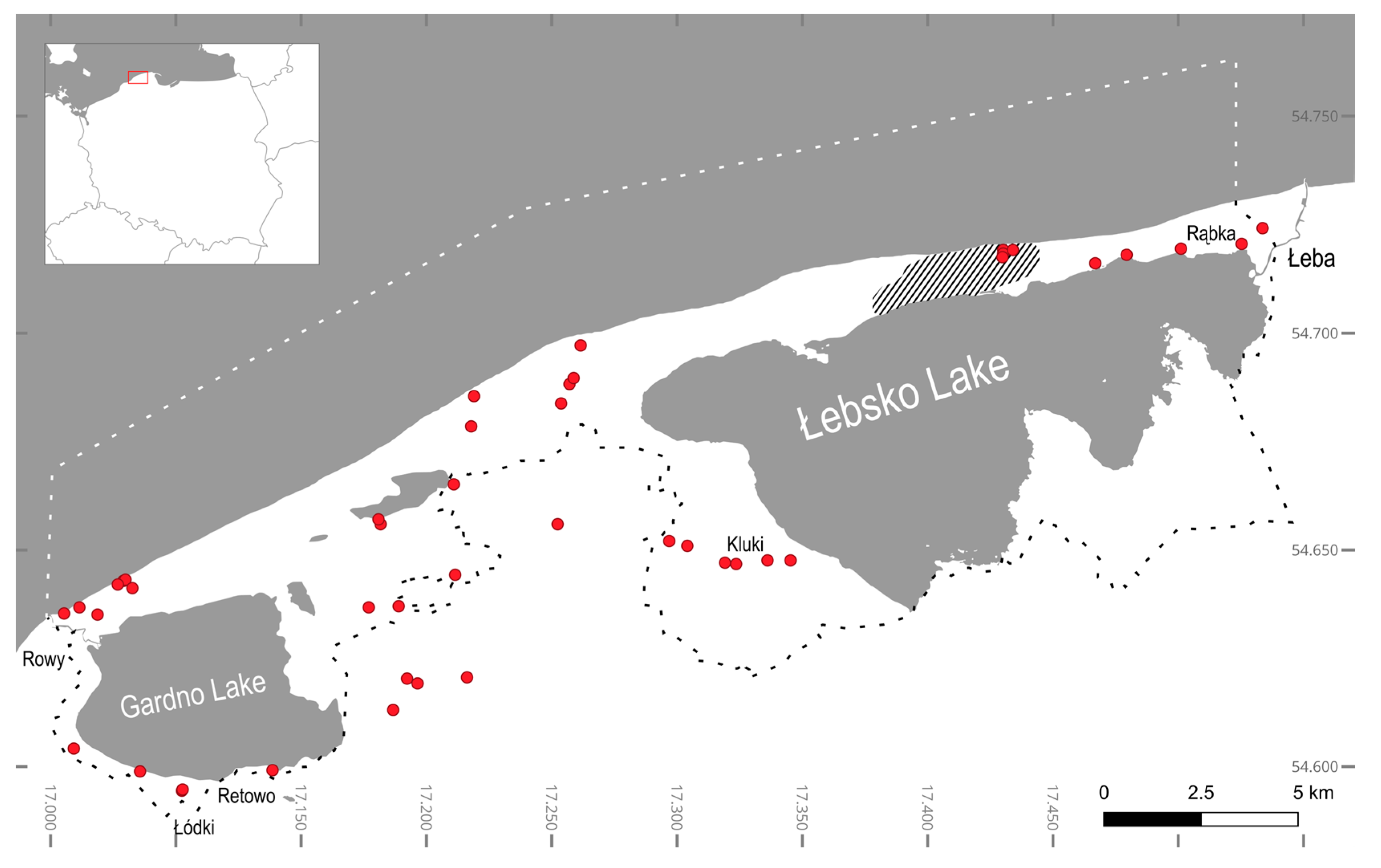
| Sample | Location | Habitat | Substrate | Description |
|---|---|---|---|---|
| 1 | 54.629167, 17.103056 | lichen | tree | Outskirts of Łódki village, willow tree |
| 2A | 54.633611, 17.086389 | moss | tree | Between Retowo and Rowy villages, near bridge, birch tree |
| 2B | 54.633611, 17.086389 | moss | ground surface | Between Retowo and Rowy villages, near bridge |
| 3 | 54.690556, 17.182222 | moss | concrete wall | Forest path to Dołgie Duże lake |
| 4 | 54.691667, 17.181389 | moss | tree | Forest path to Dołgie Duże lake, beech tree |
| 23 | 54.647778, 17.187222 | lichen | tree | Road between Smołdzin and Gardna Wielka |
| 24A | 54.678889, 17.211944 | moss | ground surface | Pine forest |
| 25A | 54.655278, 17.216667 | moss | tree | Road along Łupawa river, near graveyard |
| 25B | 54.655278, 17.216667 | moss | ground surface | Road along Łupawa river, near graveyard |
| 25C | 54.655278, 17.216667 | moss | concrete wall | Road along Łupawa river, near graveyard |
| 25D | 54.655278, 17.216667 | moss | tree | Road along Łupawa river, near graveyard, pine tree |
| 26 | 54.671667, 17.189444 | moss | tree | Mixed forest, oak tree |
| 27A | 54.629444, 17.103333 | moss | tree | Outskirts of Łódki village, willow tree |
| 28B | 54.655000, 17.192778 | moss | tree | Pine forest |
| 29B | 54.653889, 17.196944 | moss | ground surface | Pine forest |
| 29C | 54.653889, 17.196944 | moss | ground surface | Pine forest |
| 30 | 54.638889, 17.060000 | moss | concrete wall | Water canal lock |
| 33B | 54.633889, 17.139167 | lichen | tree | Camping field near Retowo village, near lake |
| 34 | 54.677500, 17.079722 | lichen | dead wood | White dune, near beach |
| 35 | 54.677778, 17.080556 | moss | ground surface | White dune, near beach |
| 37 | 54.671389, 17.177500 | moss | ground surface | Spruce forest |
| 39 | 54.676667, 17.077500 | lichen | ground surface | White dune, near beach |
| 41B | 54.670000, 17.056111 | lichen | tree | Rowy, white dune, birch tree |
| 42B | 54.671389, 17.062222 | lichen | tree | White dune, aspen tree |
| 42C | 54.671389, 17.062222 | moss | ground surface | White dune, near beach |
| 43 | 54.669722, 17.069444 | cryptogams | ground surface | Gray dune |
| 44A | 54.675833, 17.083333 | moss | tree | Oak forest, birch tree |
| 45 | 54.682222, 17.345556 | moss | tree | Kluki, path to watchtower, oak tree in a meadow |
| 50A | 54.722778, 17.257500 | cryptogams | ground surface | Czołpińska dune |
| 51A | 54.713056, 17.218333 | moss | concrete wall | Old military proving ground |
| 51B | 54.713056, 17.218333 | moss | concrete wall | Old military proving ground |
| 52A | 54.718333, 17.254167 | moss | tree | Pine forest, pine tree |
| 53A | 54.720000, 17.219444 | lichen | tree | Pine branches |
| 53B | 54.720000, 17.219444 | lichen | dead wood | Dead pine |
| 53C | 54.720000, 17.219444 | cryptogams | tree | Pine tree |
| 54B | 54.724167, 17.259167 | lichen | ground surface | Czołpińska dune |
| 55B | 54.681389, 17.323889 | lichen | concrete wall | Kluki, 18th century graveyard, gravestone |
| 55C | 54.681389, 17.323889 | moss | concrete wall | Kluki, 18th century graveyard, gravestone |
| 57C | 54.731667, 17.261944 | moss | ground surface | Seaside path near dunes, mountain pine scrubs |
| 59 | 54.699722, 17.211389 | moss | tree | Near Dołgie Duże lake, oak tree |
| 61A | 54.685556, 17.304444 | moss | dead wood | Outskirts of Kluki village, submerged dead birch |
| 61B | 54.685556, 17.304444 | lichen | dead wood | Outskirts of Kluki village, dead birch |
| 62A | 54.690556, 17.252778 | moss | tree | Smołdziński forest, oak tree |
| 64A | 54.690556, 17.252778 | moss | tree | Czołpińska dune |
| 66B | 54.686667, 17.297222 | moss | tree | Kluki, oak-beech forest, beech tree |
| 67A | 54.682222, 17.336389 | moss | dead wood | Kluki, museum building roof |
| 72A | 54.681667, 17.319444 | moss | dead wood | Outskirts of Kluki village, oak forest |
| 72B | 54.681667, 17.319444 | moss | tree | Outskirts of Kluki village, birch tree |
| 73 | 54.755000, 17.525278 | moss | concrete wall | Outskirts of Rąbka village, shrine |
| 76A | 54.753611, 17.430278 | lichen | ground surface | Łącka dune, beach |
| 77 | 54.752778, 17.430278 | moss | ground surface | Łącka dune, between two pine trees, dune crevice, sea facing |
| 78 | 54.752500, 17.479444 | moss | tree | Alder carr, alder tree |
| 79 | 54.753889, 17.501111 | moss | tree | Alder carr, alder tree |
| 85 | 54.751944, 17.430000 | lichen | tree | Łącka dune, pine forest, dune crevice, sea facing |
| 86 | 54.758611, 17.533611 | moss | concrete wall | Outskirts of Rąbka village, curb |
| 87 | 54.750556, 17.466944 | moss | dead wood | Path to Łącka dune, missile launcher site, wooden roof |
| 88 | 54.753611, 17.434167 | moss | ground surface | Mountain pine scrub |
| Taxon | Sample (Number of Specimens + Number of Eggs) | Remarks |
|---|---|---|
| Adropion belgicae (Richters, 1911) [37] | 53C (1) | Holarctic species [38,39,40]. In Poland known from southern regions, mainly from Tatra Mountains [15]. |
| Adropion scoticum scoticum (Murray, 1905) [41] | 25B (5); 53C (1); 57C (1) | Probably a cosmopolitan complex of very similar species [38,39,40,42,43,44]. Very common in Poland with wide distribution, especially in coniferous forests [15]. |
| Dianea sattleri (Richters, 1902) [45] | 39 (1) | Probably a cosmopolitan complex of very similar species [38,39,40,42,43,44]. Widely distributed in Poland [15]. |
| Diphascon pingue pingue (Marcus, 1936) [46] | 27A (1); 78 (5) | The pingue group is a complex of extremely similar species [18]. Nominal Diphascon pingue is probably restricted to the Holarctic [47,48]. Common in Poland. Strongly connected with forest habitats [15]. |
| Diphascon sp. | 39 (1) | Probably a new species from the Diphascon nobilei group [49]. For more details, see Discussion. |
| Eremobiotus ginevrae Lisi, Binda and Pilato, 2016 [23] | 35 (4) | Species new to Poland. Up to now reported only from type locality in Italy [23]. |
| Guidettion prorsirostre (Thulin, 1928) [50] | 25B (1) | Species with Holarctic distribution with only few localities in other geographic regions, suggesting a species complex [38,39,40,43,44]. In Poland, common and widely distributed [15]. |
| Hypsibius dujardini (Doyère, 1840) [7] | 25D (1); 53A (9) | Hypsibius dujardini is a species complex [22] with a global distribution [38,39,40,42,43,44]. Widely reported in Poland [15]. However, the distribution of nominal species of the complex, redescribed in 2018, is at present unknown and up to now the only confirmed locality is Paris, France [19]. Taking this into account, this report should be considered as new for Poland. |
| Hypsibius convergens (Urbanowicz, 1925) [51] | 26 (1) | Hypsibius convergens is a species complex [22] with a global distribution [38,39,40,42,43,44] and nominal species-type locality in Lithuania. The nominal species still needs a modern redescription. |
| Hypsibius pallidus | 24A (2); 29C (1) | This is a species complex. Hypsibius pallidus is a common European species, but it has also been reported from non-European localities [38,39,40,42,43]. Very common in Poland and widely distributed [15]. |
| Hypsibius scabropygus Cuénot, 1929 [52] | 2B (1); 61B (1) | Species with wide Holarctic distribution [38,39,40]. The original description is uncertain and the species needs a modern redescription [53]. Species new for the Polish fauna. |
| Macrobiotus cf. hufelandi | 1 (1); 2A (3); 2B (12); 3 (1); 23 (17); 25C (3); 25D (1); 29B (6); 29C (1); 30 (2); 37 (2); 33B (9); 43 (4); 50A (2); 52A (2); 53A (6); 53B (3); 53C (16); 55C (10); 57C (5); 66B (18); 67A (75); 72A (1); 72B (1); 73 (8); 77 (19); 79 (10); 86 (66); 87 (30) | Accurate identification of these specimens was impossible due to lack of eggs. |
| Macrobiotus hufelandi C.A.S. Schultze, 1834 [54] | 4 (5+1); 25B (75+1); 51A (3+1); 59 (32+1); 62A (3+1) | This species belongs to a cosmopolitan and species-rich complex [10]. In the past, Mac. hufelandi sensu stricto was reported almost everywhere [39], but its range is probably much more restricted. However, at present, its true distribution is unknown. In Poland, it was reported from many localities [15], but most of the reports are old and it is obvious that some of them belong to other members of the hufelandi group. |
| Macrobiotus vladimiri Bertolani, Biserov, Rebecchi and Cesari, 2011 [55] | 55B (0+1) | To date, species known only from Italy (type locality) and Poland [4,5,55]. |
| Mesobiotus sp. | 24A (1); 25A (1); 27A (1); 29C (1); 59 (2) | Accurate identification of these specimens was impossible due to lack of eggs. |
| Mesobiotus sp. | 61A (11+1) | Probably a new species, but more material is necessary for a formal description of it. See Discussion for more details. |
| Mesocrista revelata | 1 (1); 37 (4); 25B (4); 53B (1); 53C (10); 72A (3) | It is known from few localities in Poland. However, it is highly probable that most if not all reports of Mec. spitzbergensis [56] in Poland should be considered as Mec. revelata [4,16]. If this is the case, this species is probably widely distributed in Poland. |
| Milnesium beasleyi Kaczmarek, Jakubowska and Michalczyk, 2012 [57] | 30 (1); 34 (8); 41B (5); 42B (4) | This species was reported from Turkey (type locality) and Poland [4,57]. |
| Milnesium tardigradum tardigradum Doyère, 1840 [7] | 51B (13) | In the past, species considered as cosmopolitan and reported around the world. However, following redescriptions [58,59] and genetic analyses, confirmed localities are in Africa (Republic of South Africa), Asia (Japan) and Europe (Denmark, France, Germany, Great Britan, Hungary, Poland, Russia and Switzerland) [11,60,61]. |
| Milnesium dornensis Ciobanu, Roszkowska and Kaczmarek, 2015 [62] | 1 (1); 25B (2); 27A (1); 33B (1); 42B (2); 42C (2); 43 (1); 51B (2); 53A (4); 61A (1); 72A (1); 79 (1); 85 (2); 86 (2) | The species is known only from Poland and Romania (type locality) [4,62] and recently probably found in Tunisia (reported as Mil. Cf. dornensis) [63]. |
| Minibiotus intermedius (Plate, 1888) [34] | 61A (1); 53B (1); 78 (1) | Minibiotus intermedius used to be considered cosmopolitan for many years [39]. However, modern taxonomy has shown that it is a species complex [64]. It was recently redescribed based on material from type locality in Germany [21]. In Poland, it was reported from many localities [15]. Its distribution needs to be confirmed based on a recent redescription of this species. In this situation, the only confirmed locality should be considered Germany. Taking it into account, this record should be considered as the first confirmed locality of this species from Poland. |
| Notahypsibius pallidoides (Pilato, Kiosya, Lisi, Inshina and Biserov, 2011) [65] | 33B (1); 34 (2); 39 (2); 50A (1); 54B (4); 64A (2); 67A (2); 85 (1); 87 (1); 88 (2) | Up to now, species known from Austria, Croatia, Russia and Ukraine [26,65], however some reports of Hys. pallidus may in fact belong to this species. Species new for Poland. |
| Paramacrobiotus fairbanksi Schill, Förster, Dandekar and Wolf, 2010 [66] | 28B (5+3); 45 (14+1); 53C (26) | A true cosmopolitan species, confirmed based on morphological and genetic studies [67,68,69]. |
| Paramacrobiotus sp. | 44A (1) | Accurate identification of this specimen was impossible due to lack of eggs and overall low number of specimens. |
| Pseudohexapodibius degenerans | 35 (2); 39 (2+1); 76A (33+15); 88 (6+1) | Species up to recently only known from type locality in Ukraine [35]. Recorded by [36] using data collected in this study. |
| Ramazzottius sp. | 23 (21); 25B (1); 33B (4); 34 (5); 41B (4); 42B (15); 51B (6); 88 (3) | Accurate identification of the species was impossible due to lack of eggs. |
| Xerobiotus pseudohufelandi (Iharos, 1966) [70] | 35 (2); 39 (32+16) | Known from few localities in Europe, Russia and Tunisia [39,71]. In Poland, known only from few localities [15,36] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartylak, T.; Kayastha, P.; Polishchuk, A.; Roszkowska, M.; Bartylak, M.M.; Rutkowski, T.; Zacharyasiewicz, M.; Kaczmarek, Ł. Terrestrial Tardigrada (Water Bears) of the Słowiński National Park (Northern Poland). Diversity 2024, 16, 239. https://doi.org/10.3390/d16040239
Bartylak T, Kayastha P, Polishchuk A, Roszkowska M, Bartylak MM, Rutkowski T, Zacharyasiewicz M, Kaczmarek Ł. Terrestrial Tardigrada (Water Bears) of the Słowiński National Park (Northern Poland). Diversity. 2024; 16(4):239. https://doi.org/10.3390/d16040239
Chicago/Turabian StyleBartylak, Tomasz, Pushpalata Kayastha, Anastasiia Polishchuk, Milena Roszkowska, Magdalena Maria Bartylak, Tomasz Rutkowski, Michał Zacharyasiewicz, and Łukasz Kaczmarek. 2024. "Terrestrial Tardigrada (Water Bears) of the Słowiński National Park (Northern Poland)" Diversity 16, no. 4: 239. https://doi.org/10.3390/d16040239
APA StyleBartylak, T., Kayastha, P., Polishchuk, A., Roszkowska, M., Bartylak, M. M., Rutkowski, T., Zacharyasiewicz, M., & Kaczmarek, Ł. (2024). Terrestrial Tardigrada (Water Bears) of the Słowiński National Park (Northern Poland). Diversity, 16(4), 239. https://doi.org/10.3390/d16040239







