Current Status and Resources of Alhagi pseudalhagi (Fabaceae) in the Atyrau Region, Western Kazakhstan
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Areas
2.2. Floristic and Population Analyses
2.3. Assessment of Plant Resources
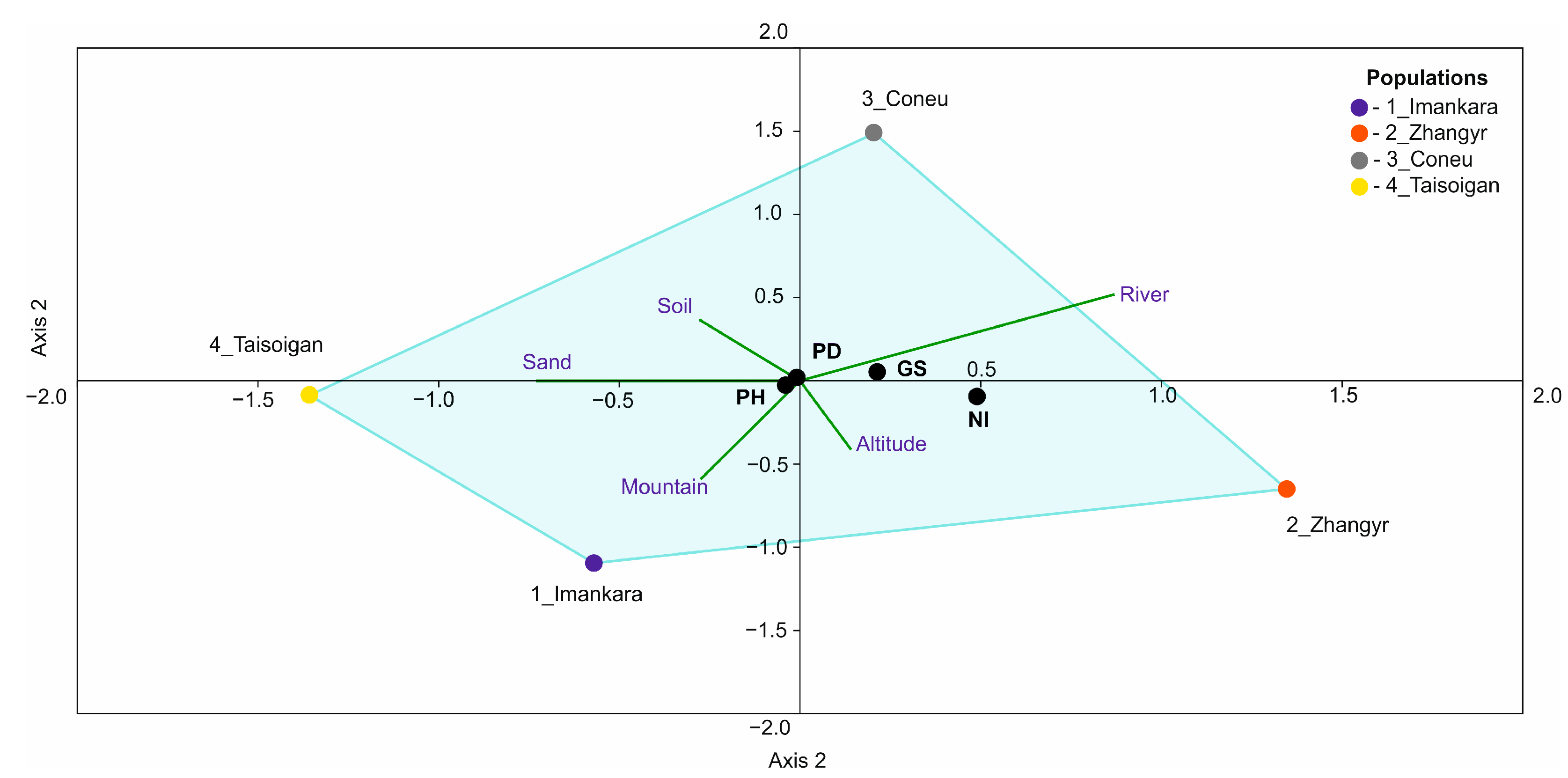
2.4. Statistical Processing
3. Results
3.1. Structure of the Populations of Alhagi pseudalhagi
3.2. Floristic Composition of A. pseudalhagi Populations
3.3. Morphological Differences between Populations and Resource Potential of A. pseudalhagi
3.4. Resource Potential of the Populations A. pseudalhagi
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amirkhosravi, A.; Asri, Y.; Assadi, M.; Mehregan, I. Systematics of Alhagi: Molecular phylogeny and morphology revisited. Rostaniha 2020, 21, 174–184. [Google Scholar] [CrossRef]
- Ye, Z.; Li, T.; Qing, D.; Sun, Y.; Chen, H.; Yu, Q.; Yan, C. Structural elucidation and osteogenic activity of a novel heteropolysaccharide from Alhagi pseudalhagi. Int. J. Biol. Macromol. 2021, 171, 185–197. [Google Scholar] [CrossRef]
- Svetlana, Z.Y.; Nadezhda, G.G.; Mihaela, B.I. Northern Tien Shan medicinal herbs. J. Lucr. Științifice Manag. Agricol. 2019, 20, 399. [Google Scholar]
- Patsaev, A.K.; Makhatov, B.K.; Kucherbaev, K.D.; Bukharbaeva, A.E.; Anes, A.T. The study of medicinal plants of southern Kazakhstan. Bull. Kyrg. State Med. Acad. Named I.K. Akhunbaev 2017, 5, 96–97. [Google Scholar]
- Amini, M.H.; Ahmady, A.; Zhakfar, A.M.; Sediqi, M.N.; Babak, G. Preliminary Phytochemical Profile, in vitro Antioxidant and Sun Protective Activities of Alhagi pseudalhagi and Elaeagnus angustifolia L. J. Pharm. Res. Int. 2019, 12, 1–13. [Google Scholar] [CrossRef]
- Srivastava, B.; Sharma, H.; Dey, Y.N.; Wanjari, M.M.; Jadhav, A.D. Alhagi pseudalhagi: A review of its phytochemistry, pharmacology, folklore claims and Ayurvedic studies. Int. J. Herb. Med. 2014, 2, 47–51. [Google Scholar]
- Myrzagalieva, A.B. Comparative analysis of the species composition and resources of medicinal plants of the Kazakhstan Altai. Bull. Kaznu Biol. Ser. 2013, 2, 3–9. [Google Scholar]
- Xu, X.; Zhang, J.; Chen, L.; Sun, Y.; Qing, D.; Xin, X.; Yan, C. Alhagi pseudalhagiExtract Exerts Protective Effects Against Intestinal Inflammation in Ulcerative Colitis by Affecting TLR4-Dependent NF-κB Signaling Pathways. Front. Pharmacol. 2021, 12, 764602. [Google Scholar] [CrossRef]
- Mutailifu, P.; Nuerxiati, R.; Lu, C.; Huojiaaihemaiti, H.; Abuduwaili, A.; Yili, A. Extraction, purification, and characterization of polysaccharides from Alhagy pseudalhagi with antioxidant and hypoglycemic activities. Process Biochem. 2022, 121, 339–348. [Google Scholar] [CrossRef]
- Tavassoli, A.P.; Anushiravani, M.; Hoseini, S.M.; Nikakhtar, Z.; Baghdar, H.N.; Ramezani, M.; Ayati, Z.; Amiri, M.S.; Sahebkar, A.; Emami, S.A. Phytochemistry and therapeutic effects of Alhagi spp. and Taganda bin in traditional and modern medicine: A review. J. Herbmed Pharm. 2020, 9, 86–104. [Google Scholar] [CrossRef]
- Zeinullina, A.; Zargar, M.; Dyussibayeva, E.; Orazov, A.; Zhirnova, I.; Yessenbekova, G.; Zotova, L.; Rysbekova, A.; Hu, Y.-G. Agro-Morphological Traits and Molecular Diversity of Proso Millet (Panicum miliaceum L.) Affected by Various Colchicine Treatments. Agronomy 2023, 13, 2973. [Google Scholar] [CrossRef]
- Ishmuratova, M.Y.; Murzalieva, G.T.; Kraschanovskaya, T.R.; Sivolobova, O.A.; Kaldybaeva, A.K.; Temireeva, K.S.; Tukubaeva, G.N.; Isina, J.A.; Amanzhan, A. Wild Medicinal Plants of Karaganda Region; Monograph; Bolashak-Baspa: Karaganda, Kazakhstan, 2016; 205p. (In Russian) [Google Scholar]
- Ishmuratova, M.Y.; Zhunussova, M.A.; Tyrzhanova, S.S.; Silant’eva, M.M. Study of spreading and plant resources of herbs Scabiosa ochroleuca L. and Scabiosa isetensis L. on the territory of Karaganda region. Bull. Karaganda Univ. 2020, 97, 47–53. [Google Scholar] [CrossRef]
- Li, N.; Zhang, G.; Xiong, Y.; Makhabel, B.; Li, X.; Jia, X. New isoflavonolignan with quinone reductase inducing activity from Alhagi pseudalhagi (MB). Fitoterapia 2010, 81, 1058–1061. [Google Scholar] [CrossRef]
- Karshibaev, K.K. Reproduction characteristics of some species Alhagi Gagnev. in the arid zones of Uzbekistan. Arid. Ecosyst. 2014, 4, 127–133. [Google Scholar] [CrossRef]
- Kubentayev, S.A.; Suleimenov, A.N.; Kotukhov, J.A.; Danilova, A.N.; Sumbembayev, A.A. Phytocenotic characteristics and stock of the main medicinal plants of the South-Western Altai (East Kazakhstan). Eurasian J. BioScience 2018, 12, 355–368. [Google Scholar]
- Zhivotovsky, L.A. Typification of plant populations on the basis of their ontogenetic spectra. Contemp. Probl. Ecol. 2023, 16, 265–273. [Google Scholar] [CrossRef]
- Kuanbay, Z.I.; Abiyev, S.A.; Ishmuratova, M.Y.; Admanova, G.B.; Kukenov, Z.Z.; Maksutbekova, G.T. The analysis of the Dongyztau chink flora (Aktobe region). EurAsian J. BioSciences 2020, 14, 249–254. [Google Scholar]
- Aidarkhanova, G.S.; Novak, A.P.; Imasheva, B.S.; Tashev, A. Evaluation of resources of medicinal plants in the forests of the Kazakhstan part of the Altai and their ecological state. Bull. Karaganda Univ. Ser. Biol. Med. Geogr. 2019, 95, 73–79. [Google Scholar]
- Ishmuratova, M.Y.; Imanbayeva, A.A.; Tuyakova, A.T.; Kopbaeva, G.B. Study of common licorice (Glycyrrhiza glabra) reserves in Atyrau and Western-Kazakhstan regions. Biosci. Biotechnol. Res. Asia 2016, 13, 1429. [Google Scholar] [CrossRef]
- Orazov, A.; Tustubayeva, S.; Alemseytova, J.; Mukhitdinov, N.; Myrzagaliyeva, A.; Turuspekov, Y.; Sramko, G. Flora accompanying Prunus ledebouriana (Schltdl.) YY Yao in the Tarbagatai State National Park in Kazakhstan. Int. J. Biol. Chem. 2021, 14, 21–34. [Google Scholar] [CrossRef]
- Kubentayev, S.A.; Zhumagul, M.Z.; Kurmanbayeva, M.S.; Alibekov, D.T.; Kotukhov, Y.A.; Sitpayeva, G.T.; Mukhtubayeva, S.K.; Izbastina, K.S. Current state of population of Rhodiola rosea L. (Crassulaceae) in East Kazakhstan. Bot. Stud. 2021, 62, 19. [Google Scholar] [CrossRef] [PubMed]
- Zargar, M.; Dyussibayeva, E.; Orazov, A.; Zeinullina, A.; Zhirnova, I.; Yessenbekova, G.; Rysbekova, A. Microsatellite-based genetic diversity analysis and population structure of Proso Millet (Panicum miliaceum L.) in Kazakhstan. Agronomy 2023, 13, 2514. [Google Scholar] [CrossRef]
- Galaktionova, E.V. Medicinal plants included in the flora of the North Kazakhstan region. Stud. Nat. Sci. 2012, 5. (In Russian) [Google Scholar]
- Orazov, A.; Myrzagaliyeva, A.; Mukhitdinov, N.; Tustubayeva, S. Callus induction with 6-BAP and IBA as a way to preserve Prunus ledebouriana (Rosaceae), and endemic plant of Altai and Tarbagatai, East Kazakhstan. Biodiversitas J. Biol. Divers. 2022, 23, 3178–3184. [Google Scholar] [CrossRef]
- Chen, X.; Jiang, F.Q.; Wang, Y.J.; Li, Y.M.; Hu, R.J. Characteristics of the Eco-geographical pattern in the arid land of central Asia. Arid Zone Res. 2013, 30, 385–390. [Google Scholar]
- Grudzinskaya, L.M.; Gemedjieva, N.G.; Nelina, N.V.; Karzhaubekova, Z. Annotated List of Medicinal Plants of Kazakhstan: A Reference Edition; MEDA-Alliance Publishing House: Willis, TX, USA, 2010; 200p. [Google Scholar]
- Wei, F.; Yang, X.; Pang, K.; Tang, H. Traditional uses, chemistry, pharmacology, toxicology and quality control of Alhagi sparsifolia Shap.: A review. Front. Pharmacol. 2021, 1214, 761811. [Google Scholar] [CrossRef]
- Kotukhov, Y.A.; Danilova, A.N.; Kubentaev, S.A. List of Medicinal Plants Kazakhstan Altai; MEDA-Alliance Publishing House: Ridder, Kazakhstan, 2015; 70p. (In Russian) [Google Scholar]
- Abduraimov, O.S.; Li, W.; Shomurodov, H.F.; Feng, Y. The main medicinal plants in arid regions of Uzbekistan and their traditional use in folk medicine. Plants 2023, 12, 2950. [Google Scholar] [CrossRef]
- Roberson, E. Nature’s Pharmacy, Our Treasure Chest: Why We Must Conserve Our Natural Heritage. A Native Plant Conservation Campaign Report; Center for Biological Diversity: Tuczon, AZ, USA, 2008; 19p. [Google Scholar]
- Imanbaeva, A.A.; Ishmuratova, M.Y.; Tuyakova, A.T. Screening of Mangystau flora for wild relatives of cultivated plants. Cent. Eur. J. Bot. 2015, 1, 12–20. [Google Scholar] [CrossRef]
- Nishanbaev, S.Z.; Shamyanov, I.J.; Bobakulov, K.M.; Sagidullaev, S. Chemical composition and biological activity of metabolites of the genus Alhagi (review). Chem. Plant Raw Mater. 2019, 4, 5–28. [Google Scholar] [CrossRef]
- Orazov, A.; Yermagambetova, M.; Myrzagaliyeva, A.; Mukhitdinov, N.; Tustubayeva, S.; Turuspekov, Y.; Almerekova, S. Plant height variation and genetic diversity between Prunus ledebouriana (Schlecht.) YY Yao and Prunus tenella Batsch based on using SSR markers in East Kazakhstan. PeerJ 2024, 12, e16735. [Google Scholar] [CrossRef]
- UNCTAD. Convention on Biological Diversity and the Nagoya Protocol: Intellectual Property Implications; UNCTAD: Geneva, Switzerland, 2014; 215p. [Google Scholar]
- Janalieva, K.M.; Budnikova, T.I.; Veselov, E.N. Physical Geography of the Republic of Kazakhstan; Al-Farabi Kazak National University: Almaty, Kazakhstan, 1998; 266p. (In Russian) [Google Scholar]
- Zhang, M. A review on the floristic phytogeography in arid northwestern China and Central Asia. Biodivers. Sci. 2017, 25, 147–155. [Google Scholar] [CrossRef][Green Version]
- Smirnova, O.V.; Zaugolnova, L.B.; Ermakova, I.M. Cenopopulation of Plants; Science Publishing House: Moscow, Russia, 1976; p. 217. [Google Scholar]
- Pavlov, N.B. Flora Kazahstana [Flora of Kazakhstan]; Publishing House of the Kazakh Academy of Sciences: Almaty, Kazakhstan, 1956; Volume 1, 347p. [Google Scholar]
- Goloskokov, V.P. (Ed.) Illiustrirovannyi Opredelitel Rastenii Kazakhstana [Illustrated Determinant of Plants of Kazakhstan]; Nauka: Almaty, Kazakhstan, 1972; Volume 2. (In Russian) [Google Scholar]
- Kamelin, R.V. Key to Plants of Central Asia. A Critical Abstract of Flora; Science Publishing House: Leningrad, Russia, 2015; Volume 11. [Google Scholar]
- International Plant Names Index. Available online: www.ipni.org (accessed on 30 January 2024).
- Serebryakov, I.G. Ecological Morphology of Plants. Life Forms of the Overgrowths and Conifers; High School: Moscow, Russia, 1982; 380p. (In Russian) [Google Scholar]
- Shay, J.E.; Pennington, L.K.; Mandussi Montiel-Molina, J.A.; Toews, D.J.; Hendrickson, B.T.; Sexton, J.P. Rules of plant species ranges: Application for conservation strategies. Front. Ecol. Evol. 2021, 9, 700962. [Google Scholar] [CrossRef]
- Kew Royal Botanical Garden. Plants of the World Online. Available online: www.powo.science.kew.org (accessed on 30 January 2024).
- Kalacska, M.; Sanchez-Azofeifa, G.A.; Calvo-Alvarado, J.C.; Quesada, M.; Rivard, B.; Janzen, D.H. Species composition, similarity and diversity in three successional stages of a seasonally dry tropical forest. For. Ecol. Manag. 2004, 200, 227–247. [Google Scholar] [CrossRef]
- Komarov, A.S.; Palenova, M.M.; Smirnova, O.V. The concept of discrete description of plant ontogenesis and cellular automata models of plant populations. Ecol. Model. 2003, 170, 427–439. [Google Scholar] [CrossRef]
- Fedorova, S.V. Methodological approaches in population botany and plant ecology. Am. J. BioScience 2020, 8, 73–90. [Google Scholar] [CrossRef]
- Elzinga, C.L.; Salzer, D.W.; Willoughby, J.W. Measuring & Monitoring Plant Populations; Bureau of Land Management, US Department of the Interior: Washington, DC, USA, 2019; 497p. [Google Scholar]
- Kuziev, R.K.; Sektimenko, V.E. Soils of Uzbekistan; Extremum Press Publishing House: Tashkent, Uzbekistan, 2009; p. 351. (In Russian) [Google Scholar]
- Kuziev, R.K.; Yuldashev, G.Y.U.; Akramov, I.A. Bonitization of Soils; The Way of Science Publishing House: Tashkent, Uzbekistan, 2004; p. 127. [Google Scholar]
- Joshi, S.P.; Gupta, V.S.; Aggarwal, R.K.; Ranjekar, P.K.; Brar, D.S. Genetic diversity and phylogenetic relationship revealed by inter simple sequence repeat (ISSR) polymorphism in the genus Oryza. Theor. Appl. Genet. 2000, 100, 1311–1320. [Google Scholar] [CrossRef]
- Rohlf, F. NTSYSpc: Numerical Taxonomy and Multivariate Analysis System, Version 2.02; Exeter Software: Setauket, NY, USA, 1998. [Google Scholar]
- Yaghmaee, F. Evaluation of biological activity of meadow spittlebug Philaenus spumarius (L.) (Cercopide: Homoptera) on Alhagi pseudalhagi (M. Bieb.) Desv. camel thorn plant in Mashhad region, Khorasan Razavi province, Iran. Res. J. Biol. Sci. 2008, 3, 845–849. [Google Scholar]
- Zeng, F.; Zhang, X.; Foetzki, A.; Li, X.; Li, X.; Runge, M. Water relation characteristics of Alhagi sparsifolia and consequences for a sustainable management. Sci. China Ser. D Earth Sci. 2002, 45, 125–131. [Google Scholar] [CrossRef]
- Yan, F.; Wenyong, W.; Zhongwei, H.; Tianyi, Z. Notice of Retraction: An alhagi pseudalhagi roots model based on l-systems in virtual plant research. In Proceedings of the 2010 International Conference on Computer Application and System Modeling (ICCASM 2010), Taiyuan, China, 22–24 October 2010; Volume 14. [Google Scholar]
- Iqbal, U.; Ali, A.; Daad, A.; Aslam, M.U.; Rehman, F.U.; Farooq, U.; Gul, M.F. Unraveling the defensive strategies of camel thorn Alhagi maurorum medik. For thriving in arid and semi-arid environments. J. Arid. Environ. 2023, 219, 105076. [Google Scholar] [CrossRef]
- El-Hak, A.; Hassan, H.Z. Genetic variation within and among the wild populations of Alhagi graecorum using ISSR markers. Taeckholmia 2019, 39, 67–85. [Google Scholar] [CrossRef]
- Brock, J.H. Ecology and management of Alhagi maurorum in a pine-oak forest in north-central Arizona, USA. In Plant Invasions: Human Perception, Ecological Impacts and Management; Backhuys Publishers: Leiden, The Netherlands, 2008; pp. 93–100. [Google Scholar]






| Name | Geographical Location | Coordinates | Height Above Sea Level | Administrative Location |
|---|---|---|---|---|
| Population 1 | The vicinity of Imankara Mountain | 47°19′49″ N 54°22′12″ E | 370 m above sea level | Atyrau region, Zhylyoysky district |
| Population 2 | Zhangyr River valley | 46°40′05″ N 49°23′50″ E | 281 m below sea level | Atyrau region, Kurmangazinsky district |
| Population 3 | Coneu River valley | 46°40′00″ N 49°23′50″ E | 284 m below sea level | Atyrau region, Kurmangazinsky district |
| Population 4 | Taisoigan sands | 48°49′23″ N 53°44′36″ E | 225 m above sea level | Atyrau region, Kzylkoginsky district |
| Name | Generative Plant Height, cm | Number of Generative Individuals per 1 m2, Pcs. | Number of Generative Shoots per Individual, Pcs. |
|---|---|---|---|
| Populations 1 | 24.4 ± 1.2 | 0.8 ± 0.02 | 3.2 ± 0.5 |
| Populations 2 | 28.3 ± 1.3 | 2.6 ± 0.03 | 5.6 ± 0.8 |
| Populations 3 | 30.5 ± 1.5 | 2.2 ± 0.01 | 6.1 ± 0.03 |
| Populations 4 | 26.2 ± 1.4 | 0.5 ± 0.02 | 2.9 ± 0.4 |
| Populations | Area, ha | Yield, kg/ha | Operational Reserve, tons | The Volume of Possible Harvested Raw Materials, tons |
|---|---|---|---|---|
| Population 1 | 96.0 | 2847 ± 180 | 273.30 | 136.65 |
| Population 2 | 52.0 | 976 ± 42 | 50.76 | 25.38 |
| Population 3 | 80.0 | 850 ± 94 | 68.04 | 34.02 |
| Population 4 | 12.3 | 2148 ± 122 | 26.42 | 13.21 |
| Total | 240.3 | - | 418.52 | 209.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sagyndykova, M.; Imanbayeva, A.; Gassanova, G.; Ishmuratova, M. Current Status and Resources of Alhagi pseudalhagi (Fabaceae) in the Atyrau Region, Western Kazakhstan. Diversity 2024, 16, 219. https://doi.org/10.3390/d16040219
Sagyndykova M, Imanbayeva A, Gassanova G, Ishmuratova M. Current Status and Resources of Alhagi pseudalhagi (Fabaceae) in the Atyrau Region, Western Kazakhstan. Diversity. 2024; 16(4):219. https://doi.org/10.3390/d16040219
Chicago/Turabian StyleSagyndykova, Meruert, Akzhunis Imanbayeva, Gulnara Gassanova, and Margarita Ishmuratova. 2024. "Current Status and Resources of Alhagi pseudalhagi (Fabaceae) in the Atyrau Region, Western Kazakhstan" Diversity 16, no. 4: 219. https://doi.org/10.3390/d16040219
APA StyleSagyndykova, M., Imanbayeva, A., Gassanova, G., & Ishmuratova, M. (2024). Current Status and Resources of Alhagi pseudalhagi (Fabaceae) in the Atyrau Region, Western Kazakhstan. Diversity, 16(4), 219. https://doi.org/10.3390/d16040219






