An Integrative Taxonomic Revision of the Freshwater Atyid Shrimps (Crustacea: Decapoda: Caridea) of Micronesia
Abstract
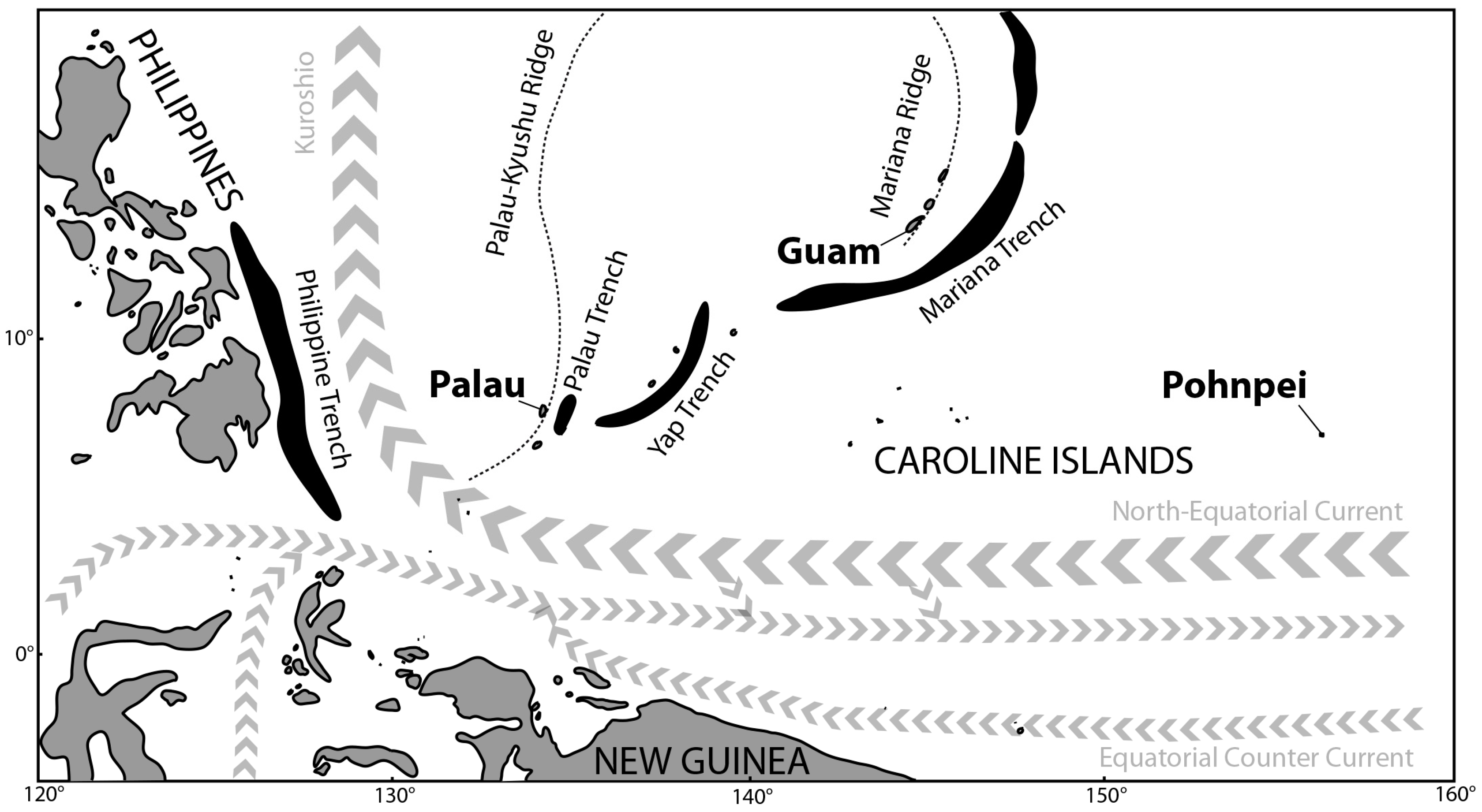
1. Introduction
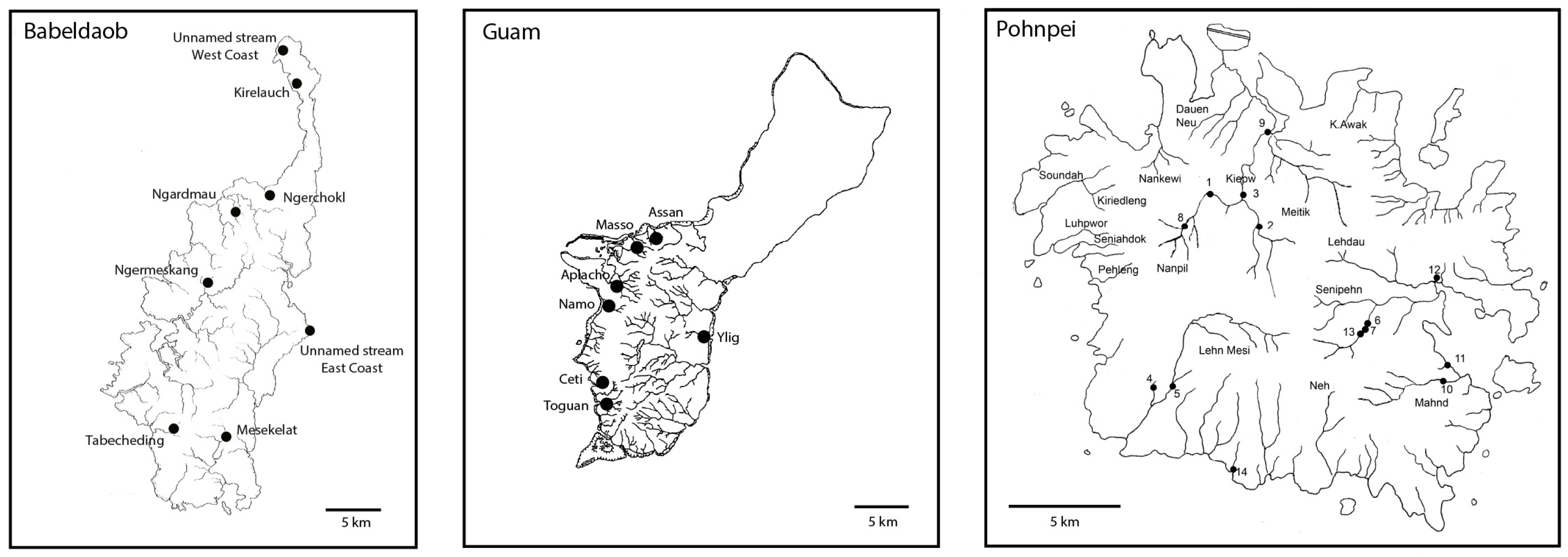
2. Materials and Methods
3. Results
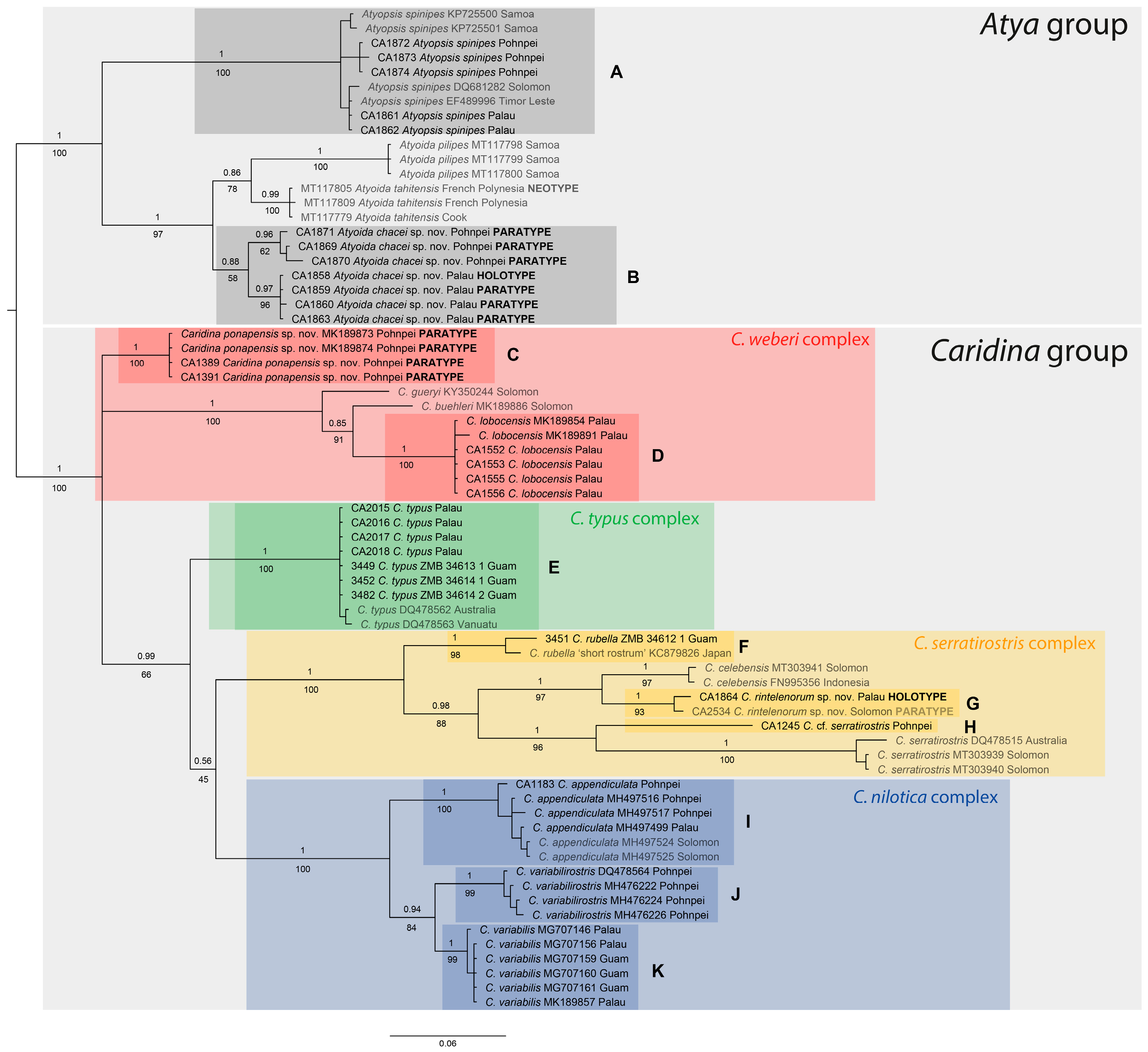
3.1. Molecular Analyses
3.2. Morphological Analyses
3.3. Taxonomic Account
- Family Atyidae De Haan, 1849
- Genus Atyopsis Chace, 1983
- Atyopsis spinipes (Newport, 1847)
- -
- Atya spinipes Newport 1847: 159 [type locality: Philippine Islands];
- -
- Atyopsis spinipes—Chace 1983 [44]: 35, Figures 20–22.
- Material examined
- Short diagnosis
- Distribution
- Habitat
- Genus Atyoida Randall, 1840
- Atyoida chacei sp. nov.
- -
- -
- Atya serrata—Miyake, 1938 [10]: 111;
- -
- Atyoida pilipes—Chace, 1983 [44]: 13, Figures 5–8 (part).—Maciolek and Ford, 1987 [13]: 628.—Nelson et al., 1996 [14]: 11.—Buden et al., 2001 [15]: 260.—Leberer and Cai, 2003 [18]: 354.—March et al., 2003 [20]: 126.—Page et al., 2007 [21]: 139, Figure 1 (part); 2008 [46]: 73, Figure 4 (part).—Benstead et al., 2009 [16]: 459.
- Material examined
- Type material:
- Comparative material:
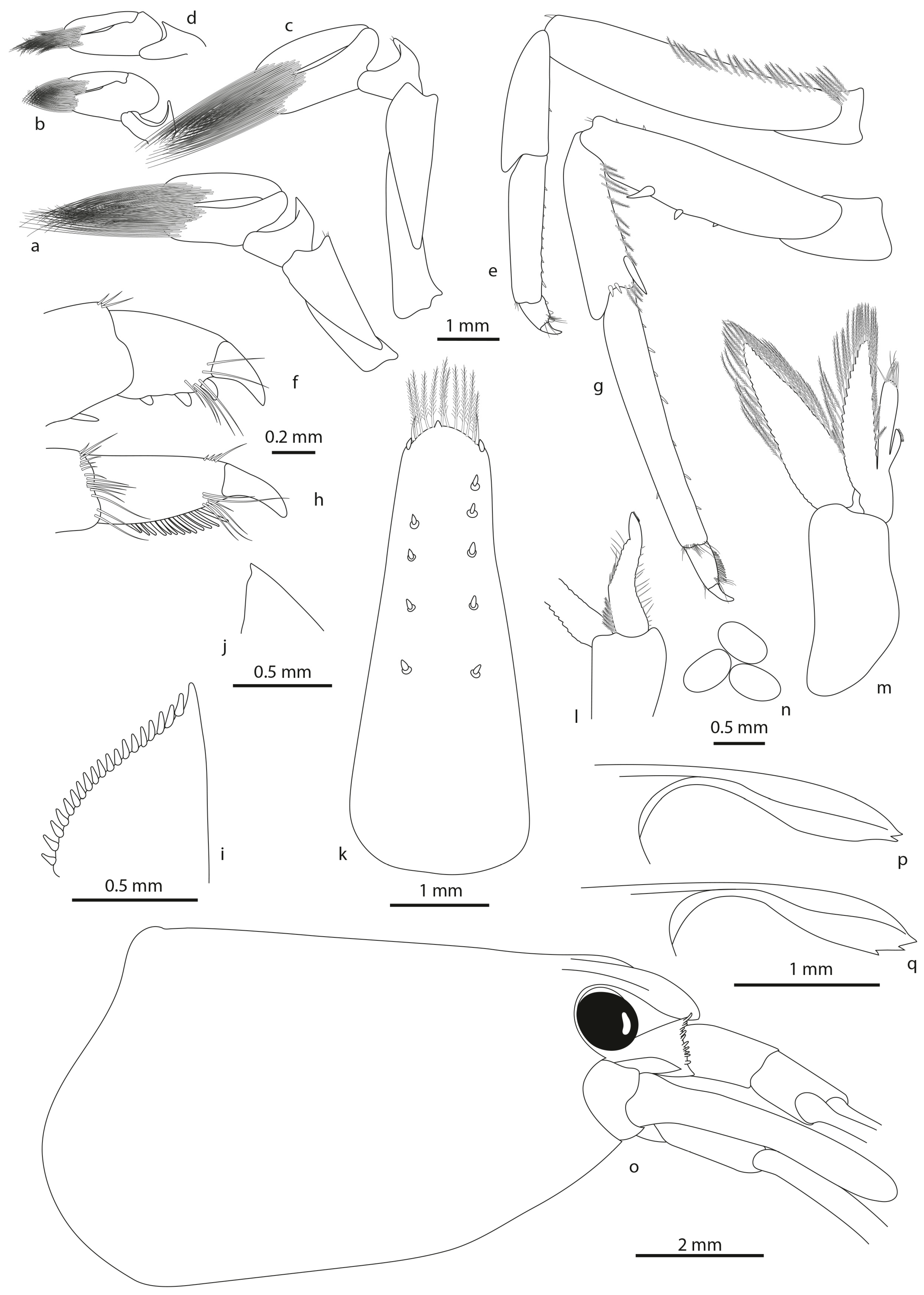
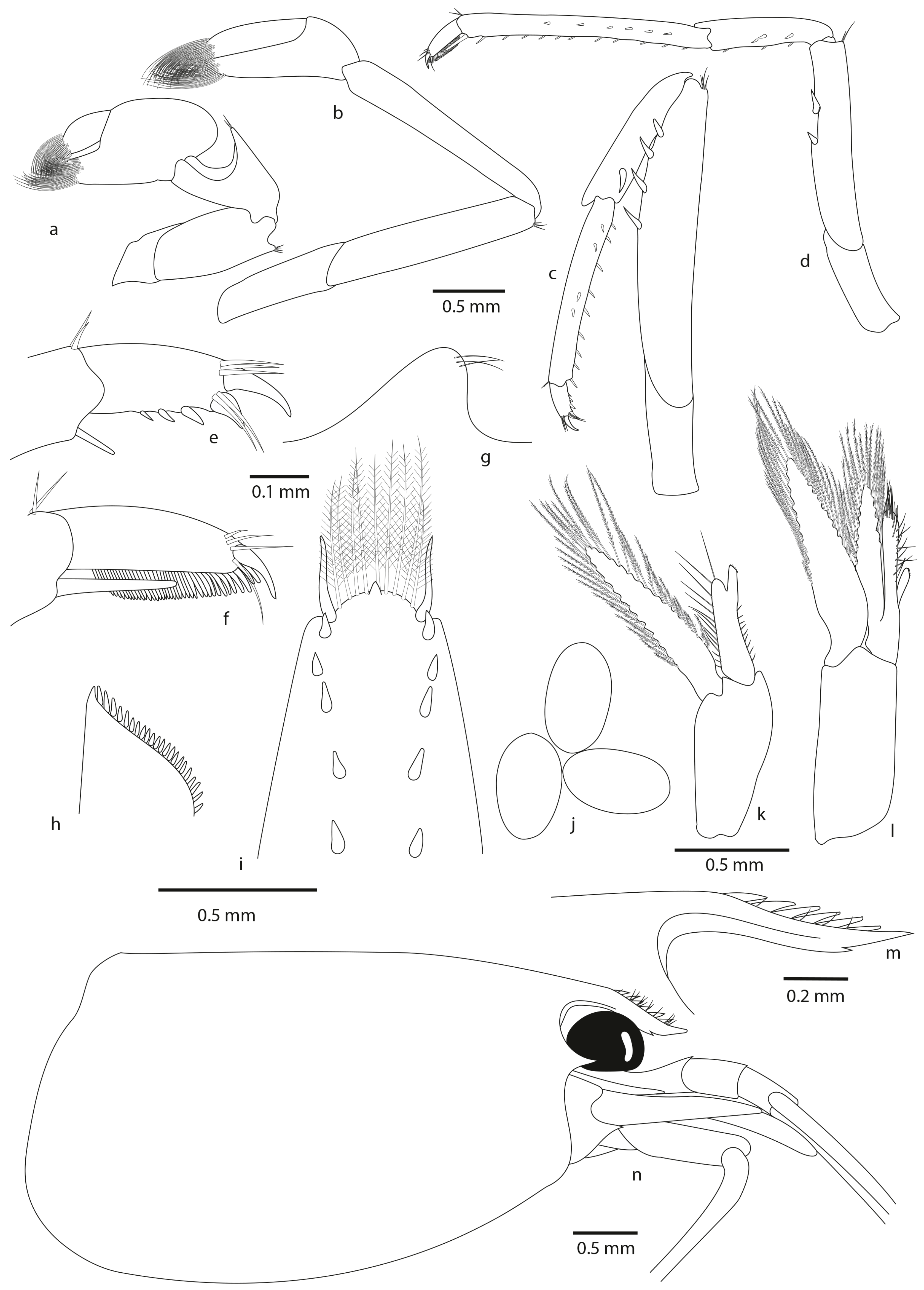
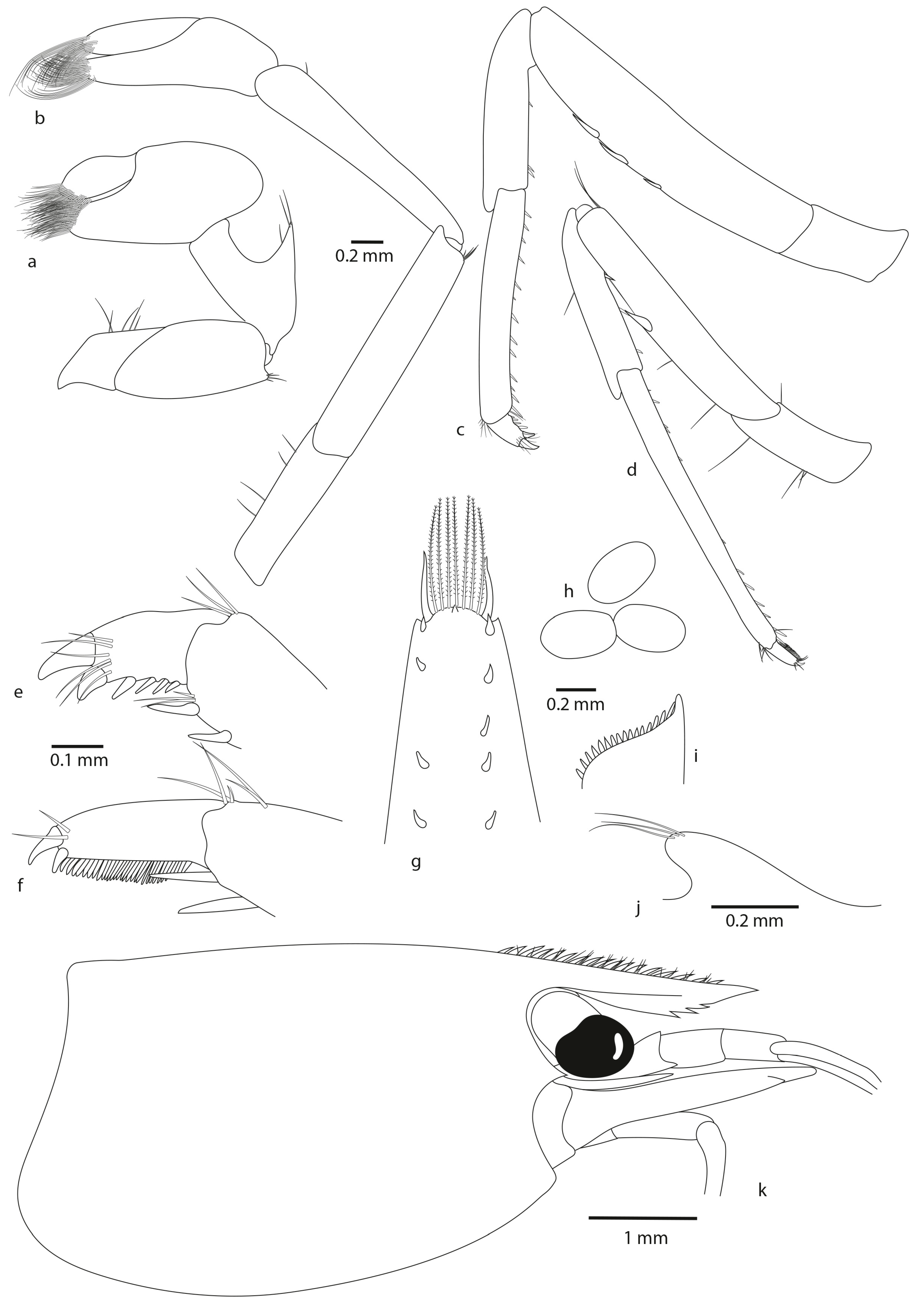
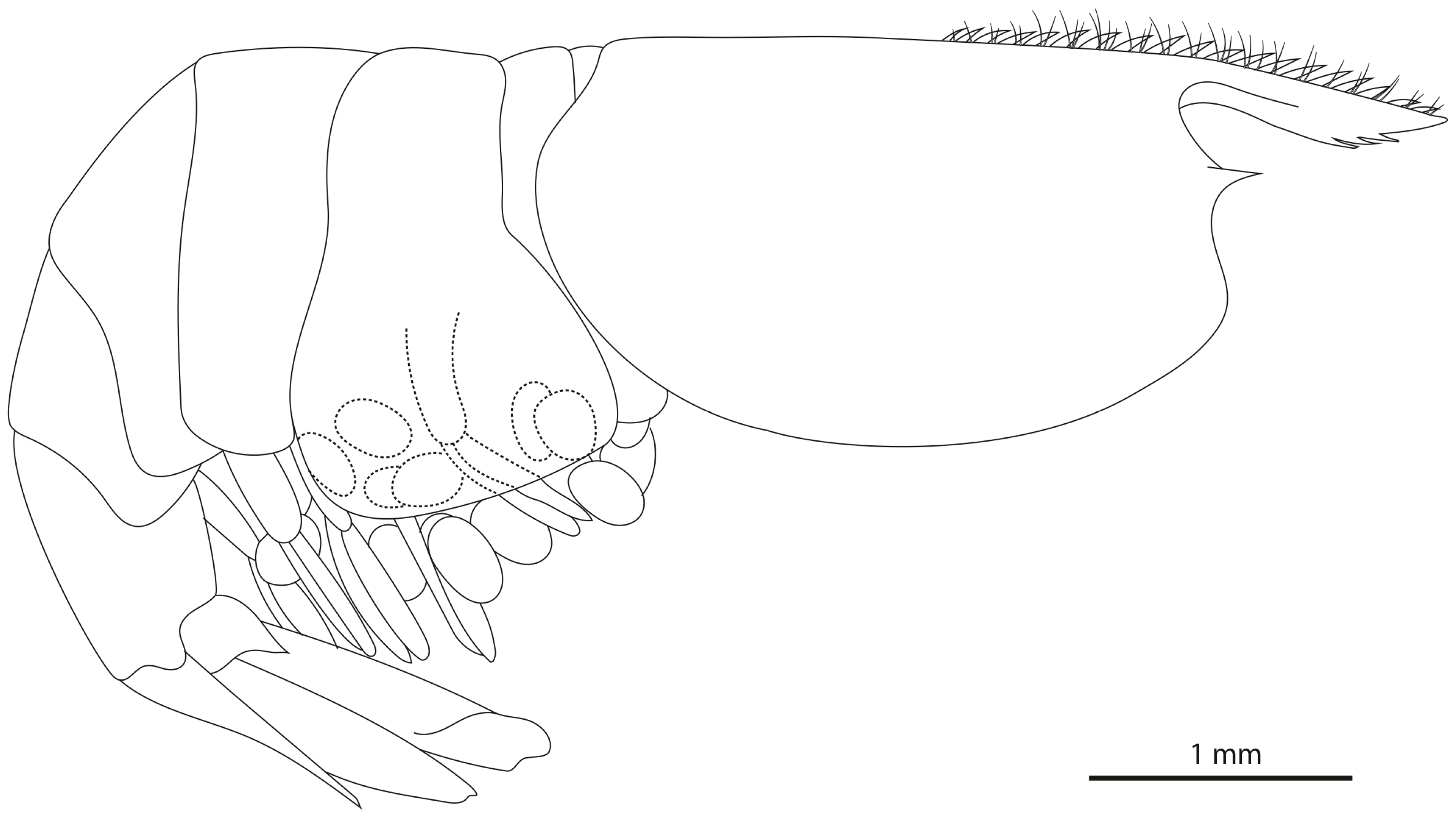
- Description
- Etymology
- Colour pattern
- Distribution
- Habitat
- Remarks
- -
- Frequently, presence of one or more teeth on the ventral margin of the rostrum, vs. always 0 in A. pilipes and A. tahitensis. This character was already highlighted by Chace (1983): “Of 613 specimens with complete rostra from the Palau Islands, 270 have no ventral rostral teeth, 240 have 1, 97 have 2, 5 have 3, and 1 has 4”;
- -
- Two–eight pairs of dorsal spinules, often not in pairs for A. chacei sp. nov. (vs. one–five pairs of dorsal spinules, often not in pairs for A. pilipes, vs. from two to four pairs of dorsal spinules, often in pairs for A. tahitensis);
- -
- Posterior margin rounded with from 8 to 11 plumose intermediate setae distinctly longer than lateral ones (vs. 4 to 9 plumose intermediate setae distinctly longer than lateral ones for A. pilipes, vs. with 6 to 10 plumose intermediate setae distinctly longer than lateral ones for A. tahitensis);
- -
- By the size of undeveloped eggs (0.51–0.52 mm × 0.29–0.31 mm (vs. 0.47–0.60 mm × 0.31–0.39 mm for A. pilipes, vs. 0.55–0.70 mm × 0.29–0.54 mm for A. tahitensis)).
- Genus Caridina H. Milne Edwards, 1837
- Caridina weberi species group
- Caridina ponapensis sp. nov.
- -
- -
- Non Caridina weberi parvirostris—Maciolek & Ford, 1987 [13]: 628;
- -
- Material examined
- Type material
- Comparative material
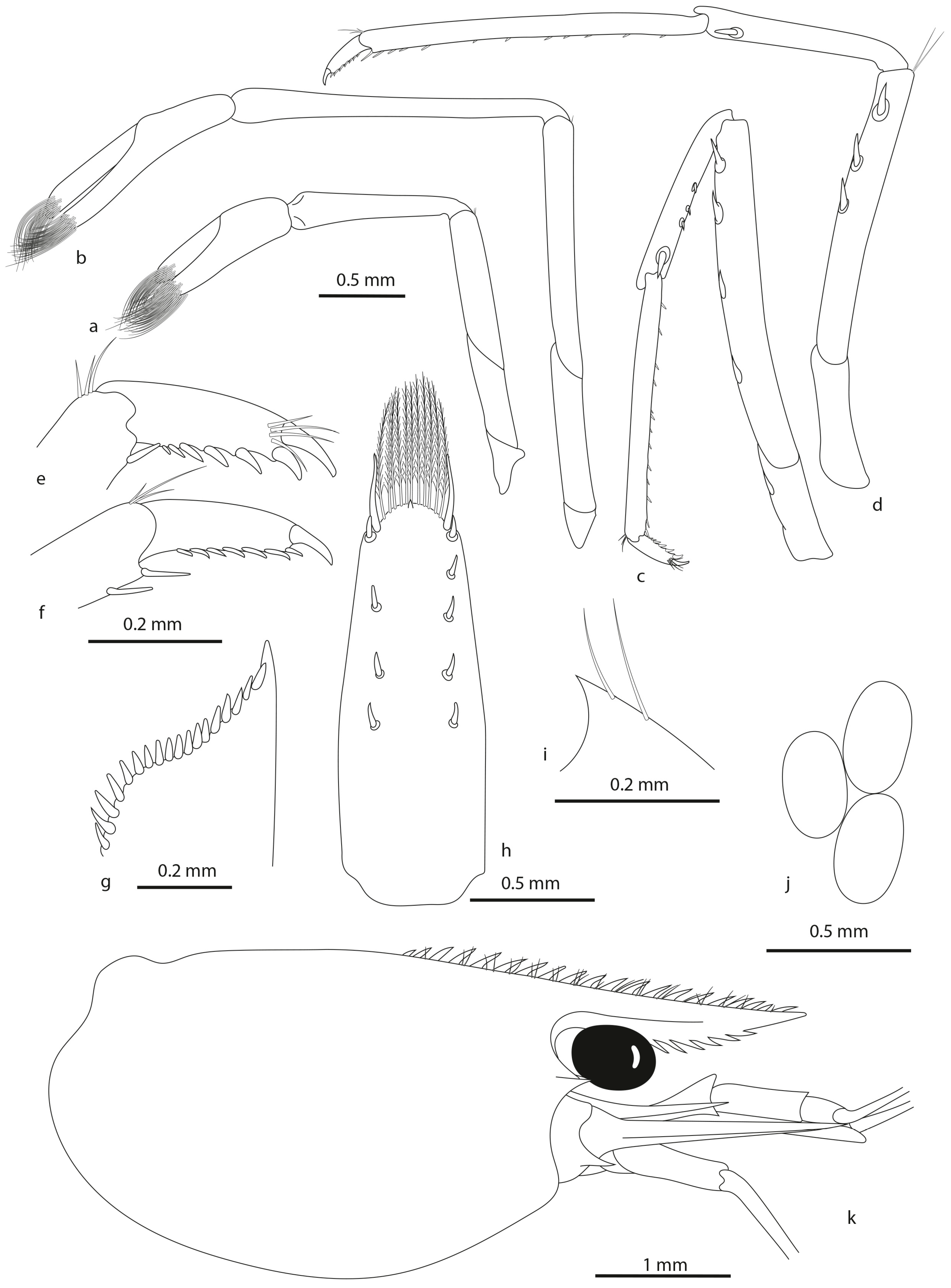
- Description
- Habitat
- Etymology
- Colour pattern (Figure 9C)
- Distribution
- Remarks
- Caridina lobocensis Cai, Choy & Ng, 2009
- -
- Caridina lobocensis Cai, Choy, and Ng, 2009: 68, Figures 2–4 [type locality: Tributary of Loboc River, Loboc (approx. 9°38.206′ N, 124°1.807′ E), Bohol Island, Philippines];
- -
- ? Caridina lobocensis—de Mazancourt et al., 2019 [47]: 166, Figures 2–5.
- Material examined
- Description
- Habitat
- Colour pattern
- Distribution
- Remarks
- Caridina typus species group
- Caridina typus H. Milne Edwards, 1837
- -
- Caridina typus H. Milne Edwards, 1837: 363 [type locality unknown].
- Material examined
- Distribution
- Remarks
- Caridina serratirostris species group
- Caridina cf. serratirostris
- Material examined
- Distribution
- Remarks
- Caridina rintelenorum sp. nov.
- Material examined
- Type material
- Comparative material
- Description
- Habitat
- Etymology
- Colour pattern
- Distribution
- Remarks
- Caridina rubella Fujino & Shokita, 1975
- -
- Caridina rubella Fujino and Shokita, 1975: 102, Figure 6 [type locality: Izaga Cave, Miyako Island, Ryukyu, Japan].—Shokita and Nishijima, 1976 [52]: 71.—Shokita, 1979 [53]: 203; 2003 [54]: 250, Figures 18F and 19I; 2006 [55]: 61.—Maciolek, 1983 [25]: 607.—Cai and Anker, 2004 [56]: 243, Figures 6 and 7.—Fujita and Shokita, 2005 [57]: 203.—Cai and Shokita, 2006 [51]: 2145, Figures 6F–J, 7 and 8.—Fujita, 2007 [58]: 92; 2018: 16.—Cai and Ng, 2009 [59]: 1094.—Weese et al., 2012 [60]: 109.—De Grave et al., 2022 [61]: 711, Figures 1 and 2.
- -
- Caridina sp. 1—Marquet et al., 2003 [62]: 74.
- Material examined
- Distribution
- Remarks
- Caridina nilotica species group
- Caridina appendiculata Jalihal & Shenoy, 1998
- -
- -
- Material examined
- Distribution
- Habitat
- Remarks
- Caridina variabilis de Mazancourt, Marquet, Rogers & Keith, 2018
- Material examined
- Distribution
- Remarks
- Caridina variabilirostris de Mazancourt, Marquet & Keith, 2018
- Material examined
- Distribution
- Remarks
- Caridina gracilirostris species group
- Caridina gracilirostris De Man, 1892
- -
- Caridina gracilirostris De Man, 1892: 399, pl. 25, Figure 31a–d [type locality: River near Maros (approx. 5°0.594′ S, 119°35.381′ E), Sulawesi (Celebes), Indonesia];
- -
- Material examined
- Distribution
- Habitat
- Remarks
4. Discussion
- Key for studied species:
- 1.1
- Pereiopods P1 and P2 carpus identically shaped……………………………….………2
- 1.2
- Pereiopods P1 and P2 carpus differently shaped; P2 carpus is more elongated than that of P1……………………………………………..………………………………………3
- 2.1
- Cephalothorax anterior border bearing a strong spine; straight rostrum rounded at its extremity with 2 to 6 small ventral teeth…………………………Atyopsis spinipes
- 2.2
- Cephalothorax anterior border acute bearing no spine; curved rostrum with 0 to 4 small ventral teeth………………………………………………Atyoida chacei sp. nov.
- 3.1
- Eyes with cornea reduced to a small dark spot………………………Caridina rubella
- 3.2
- Eyes with cornea normally developed…………………………………………………..4
- 4.1
- Teeth absent on dorsal margin of rostrum………………………………Caridina typus
- 4.2
- More or less abundant teeth present on dorsal margin of rostrum……..………………5
- 5.1
- Long stylocerite reaching or exceeding the first article of the antennal peduncle……6
- 5.2
- Short stylocerite, failing to reach the first article of the antennal peduncle……………8
- 6.1
- Dorsal border of rostrum with 1–3 teeth located on the cephalothorax, posterior to the eye; P1 carpus 1.3–1.6 as long as wide…………………………………C. lobocensis
- 6.2
- Dorsal border of rostrum with 7–10 teeth located on the cephalothorax, posterior to the eye; P1 carpus 3.4–4.9 as long as wide…………………………………………..……7
- 7.1
- Rostrum reaching to the base of the third segment of the antennular peduncle with 19–21 dorsal teeth ………………………………………………C. rintelenorum sp. nov.
- 7.2
- Rostrum reaching beyond the end of the antennular peduncle with 21–26 dorsal teeth……………………………………………………………………..C. cf. serratirostris
- 8.1
- Short rostrum, curved downwards, 0.2–0.3 of cl, armed with 4–6 teeth on dorsal margin, 0 of them situated on carapace behind orbital margin……C. ponapensis sp. nov.
- 8.2
- Relatively long rostrum, 0.6–2.1 of cl, armed with 5–26 teeth on dorsal margin, 0–4 of them situated on carapace behind orbital margin………………………………………9
- 9.1
- Uropodal diaeresis with 6–10 spiniform setae and a very long and upcurved rostrum with 5–9 dorsal teeth, widely spaced.……………………………………C. gracilirostris
- 9.2
- Number of spiniform setae on uropodal diaeresis 8–15 and a long or short rostrum with 8–26 dorsal teeth, closely set ……………………………………..…………………10
- 10.1
- Pre-anal carina with a prominent finger-like backward striking tooth……………………………………………………………………….C. appendiculata
- 10.2
- Pre-anal carina unarmed…………………………………………….……………………11
- 11.1
- P5 dactylus with a single terminal spine and a short distal propodus seta; posterior margin of the telson with intermediate setae longer or equal than lateral ones………………………………………………………………………C. variabilirostris
- 11.2
- P5 dactylus with two strong distal spines and a very long distal propodus seta; posterior margin of the telson with intermediate setae shorter or equal than lateral ones…………………………………………………………………….………C. variabilis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kobayashi, K. Origin of the Palau and Yap trench arc-system. Geophys. J. Int. 2004, 157, 1303–1315. [Google Scholar] [CrossRef]
- Ur, R.H.; Hideo, N.; Kei, K. Geological Origin of the Volcanic islands of the Caroline Group in the Federated States of Micronesia, Western Pacific. South Pac. Stud. 2013, 33, 101–118. [Google Scholar]
- Tracey, J.I.; Schlanger, S.O.; Stark, J.T.; Doan, D.B.; May, H.G. General Geology of Guam. Geol. Surv. Prof. Pap. 1964, 403, 1–104. [Google Scholar]
- McDowall, R.M. On amphidromy, a distinct form of diadromy in aquatic organisms. Fish Fish. 2007, 8, 1–13. [Google Scholar] [CrossRef]
- de Mazancourt, V.; Klotz, W.; Marquet, G.; Mos, B.; Rogers, D.C.; Keith, P. New Insights on Biodiversity and Conservation of Amphidromous Shrimps of the Indo-Pacific islands (Decapoda: Atyidae: Caridina). In Recent Advances in Freshwater Crustacean Biodiversity and Conservation; Kawai, T., Rogers, D.C., Eds.; CRC Press: Bocca Raton, FL, USA, 2021; pp. 381–404. [Google Scholar] [CrossRef]
- Schenkel, E. Beitrag zur Kenntnis der Dekapodenfauna von Celebes. Verhandlungen Der Naturforschenden Ges. Basel 1902, 13, 485–585. Available online: https://biodiversitylibrary.org/page/32330331 (accessed on 7 February 2024).
- Bouvier, E.L. Recherches sur la morphologie, les variations, la distribution géographique des Crevettes de la famille des Atyidés. In Encyclopédie Entomologique; Roret: Paris, France, 1925. [Google Scholar]
- de Mazancourt, V.; Marquet, G.; Keith, P. Caridina variabilirostris (Crustacea: Decapoda: Atyidae), a new species of freshwater shrimp from Pohnpei (Micronesia). Eur. J. Taxon. 2018, 453, 1–16. [Google Scholar] [CrossRef]
- de Mazancourt, V.; Marquet, G.; Christopher Rogers, D.; Keith, P. Description of a new species of Caridina (Crustacea: Decapoda: Atyidae) from two Micronesian islands (Guam and Babeldaob). Zootaxa 2018, 4377, 39–50. [Google Scholar] [CrossRef]
- Miyake, S. Notes on decapod crustaceans collected by Prof. Teiso Esaki from Micronesia. Annot. Zool. Jpn. 1938, 17, 107–112. [Google Scholar]
- Best, B.R.; Davidson, C.E. Inventory and Atlas of the Inland Aquatic Ecosystems of the Marianas Archipelago; Technical Report; University of Guam Marine Laboratory: Mangilao, Guam, 1981; Volume 75, 226p. [Google Scholar]
- Roux, J. Über einige Süßwasserdekapoden (Atyidae) des Berliner Zoologischen Museums. Zool. Anz. 1925, 62, 145–154. [Google Scholar]
- Maciolek, J.A.; Ford, J.I. Macrofauna and environment of the Nanpil-Kiepw River, Ponape, Eastern Caroline Islands. Bull. Mar. Sci. 1987, 41, 623–632. [Google Scholar]
- Nelson, S.G.; Camacho, F.A.; Parham, J.E.; Tibbatts, R.B.; Leberer, T.; Smith, B.D. Surveys of the Macrofauna of the Nanpil Kiepw and Lehn Mesi Rivers of Pohnpei; Technical Report 103; University of Guam Marine Laboratory: Mangilao, Guam, 1996; 22p. [Google Scholar]
- Buden, D.W.; Lynch, D.B.; Short, J.W.; Leberer, T. Decapod crustaceans of the headwater streams of Pohnpei, Eastern Caroline Islands, Federated States of Micronesia. Pac. Sci. 2001, 55, 257–265. [Google Scholar] [CrossRef]
- Benstead, J.P.; March, J.G.; Pringle, C.M.; Ewel, K.C.; Short, J.W. Biodiversity and ecosystem function in species-poor communities: Community structure and leaf litter breakdown in a Pacific island stream. J. Nat. Hist. Mus. Inst. Chiba 2009, 28, 454–465. [Google Scholar] [CrossRef]
- Bright, G.R. The Inland Waters of Palau, Caroline Islands; Report: Office Chief Conserv.; Trust Territory of the Pacific Islands: Koror, Palau, 1979; 61p. [Google Scholar]
- Leberer, T.; Cai, Y. Shrimps of the family Atyidae from Guam, Mariana Islands. Micronesica 2003, 35–36, 353–358. [Google Scholar]
- Leberer, T.; Nelson, S.G. Factors Affecting the Distribution of Atyid Shrimps in Two Tropical Insular Rivers. Pac. Sci. 2001, 55, 389–398. [Google Scholar] [CrossRef]
- March, J.G.; Benstead, J.P.; Pringle, C.M.; Luckymis, M. Benthic Community Structure and Invertebrate Drift in a Pacific Island Stream, Kosrae, Micronesia. Biotropica 2003, 35, 125–130. [Google Scholar] [CrossRef]
- Page, T.J.; von Rintelen, K.; Hughes, J.M. An island in the stream: Australia’s place in the cosmopolitan world of Indo-West Pacifi c freshwater shrimp (Decapoda: Atyidae: Caridina). Mol. Phylogenet. Evol. 2007, 43, 645–659. [Google Scholar] [CrossRef] [PubMed]
- Edmondson, C.H. Atyidae of southern Polynesia; Occasional Papers; Bernice P. Bishop Museum: Honolulu, HI, USA, 1935; Volume 11, pp. 1–19. [Google Scholar]
- Bright, G.R. The freshwater decapod crustaceans of Yap, Caroline Islands. In The Inland Aquatic Habitats of Yap; Nelson, S.G., Ed.; University of Guam Marine Laboratory: Mangilao, GU, USA, 1989; pp. 33–36. [Google Scholar]
- Ellis-Neill, L. Distributional and Production Dynamics of Benthic Invertebrates in a Tropical Stream on Guam; University of Guam: Mangilao, GU, USA, 1987. [Google Scholar]
- Maciolek, J.A. Distribution and biology of Indo-Pacific insular hypogeal shrimps. Bull. Mar. Sci. 1983, 33, 606–618. [Google Scholar]
- Keith, P.; Gerbeaux, P.; Marquet, G.; Taillebois, L.; Castelin, M. Freshwater Survey of Babeldaob (Palau); Muséum National d’Histoire Naturelle Report; MNHN: Paris, France, 2011; 32p. [Google Scholar]
- Keith, P.; Gerbeaux, P.; Marquet, G.; Taillebois, L.; Castelin, M. Freshwater Survey of Pohnpei; Muséum national d’Histoire naturelle report; MNHN: Paris, France, 2012; 27p. [Google Scholar]
- Lamarque, P.; Thérezien, Y.; Charlon, N. Étude des conditions de la pêche à l’électricité dans les eaux tropicales. Bois Et Forêts Trop. 1975, 161, 61–63. [Google Scholar]
- Fehlmann, H.A. Ecological Distribution of Freshwater Fishes in a Stream Drainage in the Palau Islands. Ph.D. Thesis, Stanford University, Stanford, CA, USA, 1960; 172p. [Google Scholar]
- Palumbi, S.R. Nucleic Acids II: The Polymerase Chain Reaction. In Molecular Systematics 205–247; Hillis, D.M., Mable, B.K., Eds.; Sinauer Associates: Sunderland, MA, USA, 1996. [Google Scholar]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. JModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef] [PubMed]
- Guindon, S.; Gascuel, O. A Simple, Fast, and Accurate Algorithm to Estimate Large Phylogenies by Maximum Likelihood. Syst. Biol. 2003, 52, 696–704. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML-VI-HPC: Maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 2006, 22, 2688–2690. [Google Scholar] [CrossRef]
- Ronquist, F.; Huelsenbeck, J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef]
- Miller, M.; Pfeiffer, W.; Schwartz, T. Creating the CIPRES Science Gateway for Inference of Large Phylogenetic Trees. In Proceedings of the Gateway Computing Environments Workshop (GCE), New Orleans, LA, USA, 14 November 2010. [Google Scholar]
- Felsenstein, J. Confidence Limits on Phylogenies: An Approach Using the Bootstrap. Evolution 1985, 39, 783. [Google Scholar] [CrossRef]
- de Mazancourt, V.; Marquet, G.; Keith, P. The “Pinocchio-shrimp effect”: First evidence of variation in rostrum length with the environment in Caridina H. Milne-Edwards, 1837 (Decapoda: Caridea: Atyidae). J. Crustac. Biol. 2017, 37, 249–257. [Google Scholar] [CrossRef]
- Coleman, C.O. “Digital inking”: How to make perfect line drawings on computers. Org. Divers. Evol. 2003, 3 (Suppl. S14), 1–14. Available online: http://senckenberg.de/odes/03-14.htm (accessed on 7 February 2024). [CrossRef]
- Coleman, C.O. Substituting time-consuming pencil drawings in arthropod taxonomy using stacks of digital photographs. Zootaxa 2006, 1360, 61–68. [Google Scholar] [CrossRef]
- de Mazancourt, V.; Boseto, D.; Marquet, G.; Keith, P. Solomon’s Gold Mine: Description or redescription of 24 species of Caridina (Crustacea: Decapoda: Atyidae) freshwater shrimps from the Solomon Islands, including 11 new species. Eur. J. Taxon. 2020, 696, 1–86. [Google Scholar] [CrossRef]
- Chace, F.A. The Atya-like shrimps of the Indo-Pacific Region (Decapoda: Atyidae). Smithson. Contrib. Zool. 1983, 384, 1–54. [Google Scholar] [CrossRef]
- Lorang, C.; de Mazancourt, V.; Marquet, G.; Keith, P. Taxonomic study of the freshwater shrimps genus Atyoida Randall, 1840 (Crustacea: Decapoda: Atyidae) in Polynesia with a revalidation of A. tahitensis Stimpson, 1860. Zootaxa 2020, 4751, 55–74. [Google Scholar] [CrossRef]
- Page, T.J.; Cook, B.D.; von Rintelen, T.; von Rintelen, K.; Hughes, J.M. Evolutionary relationships of atyid shrimps imply both ancient Caribbean radiations and common marine dispersals. J. N. Am. Benthol. Soc. 2008, 27, 68–83. [Google Scholar] [CrossRef]
- de Mazancourt, V.; Klotz, W.; Marquet, G.; Mos, B.; Rogers, D.C.; Keith, P. The complex study of complexes: The first well-supported phylogeny of two species complexes within genus Caridina (Decapoda: Caridea: Atyidae) sheds light on evolution, biogeography, and habitat. Mol. Phylogenetics Evol. 2019, 131, 164–180. [Google Scholar] [CrossRef] [PubMed]
- Christodoulou, M.; Antoniou, A.; Magoulas, A.; Koukouras, A. Revision of the freshwater genus Atyaephyra (Crustacea, Decapoda, Atyidae) based on morphological and molecular data. ZooKeys 2012, 229, 53–110. [Google Scholar] [CrossRef] [PubMed]
- Choy, S.; Page, T.J.; Mos, B. Taxonomic revision of the Australian species of Australatya Chace 1983 (Crustacea, Decapoda, Atyidae), and the description of a new species. Zootaxa 2019, 4711, 366–378. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Choy, S.; Ng, P.K.L. Epigean and hypogean freshwater shrimps of Bohol Island, central Philippines (Crustacea: Decapoda: Caridea). Raffles Bull. Zool. 2009, 57, 65–89. [Google Scholar]
- Cai, Y.; Shokita, S. Report on a collection of freshwater shrimps (Crustacea: Decapoda: Caridea) from the Philippines, with descriptions of four new species. Raffles Bull. Zool. 2006, 54, 245–270. [Google Scholar]
- Shokita, S.; Nishijima, S. Aquatic Animal of the “Shiokawa Salty Spring” with a Discussion on the Flaw Mechanism of Salty Water, Investigation of Natural Resource; part 6. A report on the investigation of “Shiokawa Salty Spring” II; Okinawa Prefectural Board of Education: Okinawa, Japan, 1979; Volume II, pp. 68–91. [Google Scholar]
- Shokita, S. The Distribution and Speciation of the Inland Water Shrimps and Prawns from the Ryukyu Islands, II; Bulletin of the College of Science, University of the Ryukyus: Okinawa, Japan, 1979; Volume 28, pp. 193–278. [Google Scholar]
- Shokita, S.; Atyidae. The Flora and Fauna of Inland Waters in the Ryukyu Islands; Nishida, M., Shikatani, N., Shokita, S., Eds.; Tokai University Press: Tokyo, Japan, 2003; Volume 572, pp. 249–254. [Google Scholar]
- Shokita, S. Caridina rubella. In Threatened Wildlife of Japan. Red Data Book, Volume 7, Invertebrate (except Insecta and Mollusca), 2nd ed.; Ministry of the Environment, Ed.; Japan Wildlife Research Center: Tokyo, Japan, 2006; Volume 86, p. 61. [Google Scholar]
- Cai, Y.; Anker, A. On a collection of freshwater shrimps (Crustacea Decapoda Caridea) from the Philippines, with descriptions of five new species. Trop. Zool. 2004, 17, 233–266. [Google Scholar] [CrossRef]
- Fujita, Y.; Shokita, S. Caridina rubella. In Threatened Wildlife in Okinawa, Animals: Red Data, 2nd ed.; Nature Conservation Division, Department of Cultural and Environmental Affairs, Okinawa Prefectural Government: Okinawa, Japan, 2005; p. 203. [Google Scholar]
- Fujita, Y. Decapod crustaceans inhabiting springs on Miyako Islands, the Ryukyus, Japan. Bull. Miyakojima City Mus. 2007, 11, 89–110. [Google Scholar]
- Cai, Y.; Ng, P.K.L. The freshwater shrimps of the genera Caridina and Parisia from karst caves of Sulawesi Selatan, Indonesia, with descriptions of three new species (Crustacea: Decapoda: Caridea: Atyidae). J. Nat. Hist. 2009, 43, 1093–1114. [Google Scholar] [CrossRef]
- Weese, D.A.; Fujita, Y.; Hidaka, M.; Santos, S.R. The long and short it: Variation and Population Structure of the Anchialine Atyid Shrimp Caridina rubella on Miyaho-Jima, Japan. J. Crustac. Biol. 2012, 32, 109–117. [Google Scholar] [CrossRef][Green Version]
- De Grave, S.; Iliffe, T.M.; Christodoulou, M. Further records of the anchialine shrimp Caridina rubella Fujino & Shokita, 1975 (Decapoda, Atyidae). Crustaceana 2022, 95, 709–716. [Google Scholar] [CrossRef]
- Marquet, G.; Keith, P.; Vigneux, E. Atlas des poissons et des crustacés d’eau douce de Nouvelle-Calédonie. Patrim. Nat. 2003, 58, 282. [Google Scholar]
- Fujino, T.; Shokita, S. Report on Some New Atyid Shrimps (Crustacea, Decapoda. Caridea) from the Ryukyu Islands. Bull. Sci. Eng. Div. Univ. Ryukyus. Math. Nat. Sci. 1975, 18, 93–113. [Google Scholar]
- Cai, Y.; Ng, P.K.L. A revision of the Caridina gracilirostris de Man, 1892, species group, with descriptions of two new taxa (Decapoda; Caridea; Atyidae). J. Nat. Hist. 2007, 41, 1585–1602. [Google Scholar] [CrossRef]
- Klotz, W.; Karge, A.; von Rintelen, K. A redescription of two atyid shrimps (Decapoda: Caridina) from Central Sulawesi, Indonesia. Zootaxa 2007, 1466, 1–10. [Google Scholar] [CrossRef]
- de Mazancourt, V.; Klotz, W.; Marquet, G.; Keith, P. Integrative taxonomy helps separate four species of freshwater shrimps commonly overlooked as Caridina longirostris (Crustacea: Decapoda: Atyidae) on Indo-West Pacific islands. Invertebr. Syst. 2018, 32, 1422–1447. [Google Scholar] [CrossRef]
- Short, J.W.; Page, T.J.; Humphrey, C.L. Caridina biyiga sp. nov., a new freshwater shrimp (Crustacea: Decapoda: Atyidae) from Leichhardt Springs, Kakadu National Park, Australia, based on morphological and molecular data, with a preliminary illustrated key to Northern Territory. Zootaxa 2019, 4695, 1–25. [Google Scholar] [CrossRef]
- Hernawati, R.; Nurhaman, U.; Busson, F.; Suryobroto, B.; Hanner, R.; Keith, P.; Wowor, D.; Hubert, N. Exploring community assembly among Javanese and Balinese freshwater shrimps (Atyidae, Palaemonidae) through DNA barcodes. Hydrobiologia 2020, 847, 647–663. [Google Scholar] [CrossRef]
- De Man, J.G. Decapoden des Indischen Archipels. In Zoologische Ergebnisse einer Reise in Niederländisch Ost-Indien; Weber, M., Ed.; Brill: Leiden, The Netherlands, 1892; Volume 2, pp. 265–527. [Google Scholar]
- Holthuis, L.B. A collection of Decapod Crustacea from Sumba, Lesser Sunda Islands, Indonesia. Zool. Verh. 1978, 162, 1–55. [Google Scholar]
- Cook, B.D.; Page, T.J.; Hughes, J.M. Molecular and conservation biogeography of freshwater caridean shrimps in northwestern Australia. In Phylogeography and Population Genetics in Crustacea; Held, C., Koenemann, S., Schubart, C.D., Eds.; Taylor and Francis Group: Boca Raton, FL, USA, 2011; pp. 273–287. [Google Scholar]
- von Rintelen, K.; Karge, A.; Klotz, W. News from a small island—First record of a freshwater shrimp (Decapoda, Atyidae, Caridina) from Peleng, Banggai Islands, Indonesia. J. Nat. Hist. 2008, 42, 2243–2256. [Google Scholar] [CrossRef]
- Craig, D.A.; Currie, D.C.; Joy, D.A. Geographical history of the central western Pacific black fly subgenus Inseliellum (Diptera: Simuliidae: Simulium) based on a reconstructed phylogeny of the species, hot archpelagoes and hydrological considerations. J. Biogeogr. 2001, 28, 1101–1127. [Google Scholar] [CrossRef]
- Mennesson, M.I. Taxonomie, Phylogénie et Dispersion du Genre Eleotris (Teleostei: Gobioidei: Eleotridae) dans l’Indo-Pacifique. Ph.D. Thesis, Muséum national d’Histoire Naturelle, Paris, France, 2016; 351p. [Google Scholar]
- Buden Donald, W.; Lynch, D.B.; Watson, R.E. The Gobiid Fishes (Teleostei: Gobioidei: Sicydiinae) of the Headwater Streams of Pohnpei, Eastern Caroline Islands, Federated States of Micronesia. Micronesica 2001, 34, 1–10. [Google Scholar]
- Kulbicki, M.; Mou-tham, G.; Vigliola, L.; Wantiez, L.; Manaldo, E.; Labrosse, P.; Letourneur, Y. Major Coral Reef Fish Species of the South Pacific with Basic Information on Their Biology and Ecology; Crisp-IRD Report; SPC: Nouméa, New Caledonia, 2011; 107p. [Google Scholar]
- Keith, P.; Lord, C.; Maeda, K. Indo-Pacific Sicydiine Gobies: Biodiversity, Life Traits and Conservation; Société Française d’Icthyologie: Paris, France, 2015; 256p. [Google Scholar]
- Parenti, L.R.; Maciolek, J.A. New Sicydiine Gobies from Ponape and Palau, Micronesia, with comments on Systematics of the Subfamily. Bull. Mar. Sci. 1993, 53, 945–972. [Google Scholar]
- Nelson, S.G.; Parham, J.E.; Brenttibbatts, R.; Camacho, F.A.; Leberer, T.; Smith, B.D. Distributions and Microhabitats of the Amphidromous Gobies in Streams of Micronesia. Micronesica 1997, 30, 83–91. [Google Scholar]
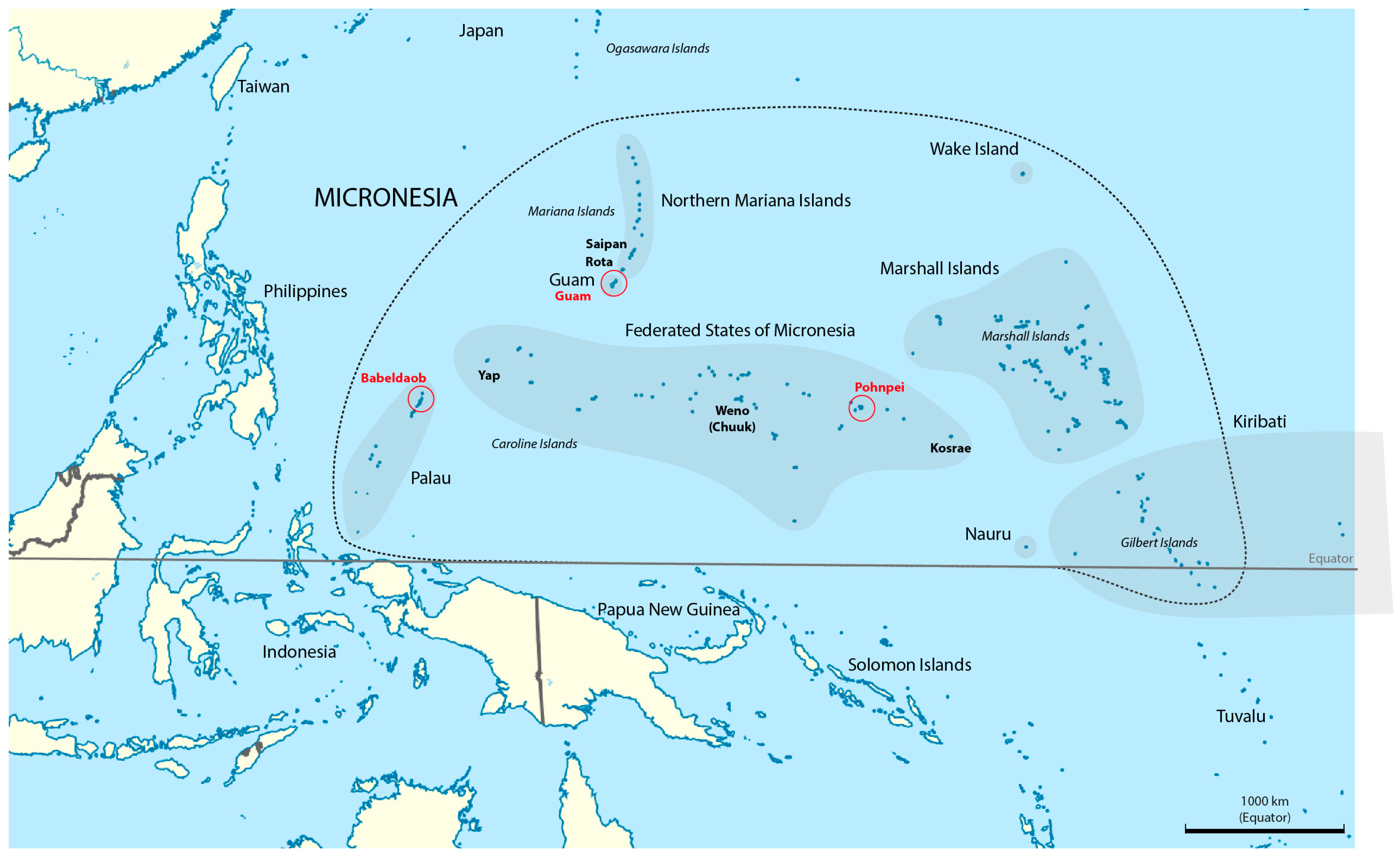


| Country | Palau | USA | Federated States of Micronesia | Remarks | |||
|---|---|---|---|---|---|---|---|
| Island Species | Babeldaob | Guam | Marianas | Yap | Pohnpei | Kosrae | |
| Atyoida pilipes | [17] | [11,18,19] | [7,10] | [13,14,15] | [16,20,21] | 1 | |
| Atyopsis spinipes | [17] | [11,18,19] | [22] | [13] | [16] | ||
| C. gracilirostris | [17] | [23] | |||||
| C. serratirostris | [17] | [23] | [20] | ||||
| C. typus | [17] | [11,18,19,24] | [7,10] | [23] | [13,15] | [16] | 2 |
| C. variabilirostris | [8,13,14,15] | [16] | 3 | ||||
| C. variabilis | [9,17] | [9,11,18,19,24] | [7] | [23] | 4 | ||
| Antecaridina lauensis | [18] | ||||||
| Halocaridinides sp. | [18,25] | 5 | |||||
| Island | Rivers | Localities | Dates | Coordinates | Altitude (m) |
|---|---|---|---|---|---|
| Babeldaob | Tabecheding | Station 1 | 27 February 2011 | 07°27.169′ N 134°31.748′ E | 28 |
| Ngerchokl | Station 2 | 27 February 2011 | 07°36.527′ N 134°36.958′ E | 36 | |
| Kirelauch | Station 3 | 28 February 2011 | 07°41.417′ N 134°37.754′ E | 29 | |
| Unnamed stream (west coast) | Station 4 | 28 February 2011 | 07°43.056′ N 134°36.924′ E | 10 | |
| Unnamed stream (east coast) | Station 5 | 28 February 2011 | 07°29.909′ N 134°38.099′ E | 7 | |
| Ngermeskang | Station 6 | 1 March 2011 | 07°32.484′ N 134°34.670′ E | 75 | |
| Ngardmau (waterfall) | Station 7 | 2 March 2011 | 07°35.482′ N 134°35.573′ E | 41 | |
| Mesekelat | Station 8 | 3 March 2011 | 07°26.305′ N 134°34.328′ E | 20 | |
| Pohnpei | Nanpil | Station 1 | 9 March 2012 | 06°55.242′ N 158°12.265′ E | 127 |
| Station 2 | 9 March 2012 | 06°54.498′ N 158°13.270′ E | 93 | ||
| Station 3 | 9 March 2012 | 06°55.111′ N 158°12.878′ E | 52 | ||
| Station 8 | 13 March 2012 | 06°54.252′ N 158°11.491′ E | 180 | ||
| Station 9 | 13 March 2012 | 06°56.609′ N 158°13.550′ E | 5 | ||
| Lehn mesi | Station 4 | 10 March 2012 | 06°51.055′ N 158°10.890′ E | 197 | |
| Station 5 | 10 March 2012 | 06°51.107′ N 158°11.896′ E | 139 | ||
| Senipehn | Station 6 | 12 March 2012 | 06°51.895′ N 158°15.810′ E | 81 | |
| Station 7 | 12 March 2012 | 06°51.906′ N 158°16.010′ E | 95 | ||
| Station 13 | 15 March 2012 | 06°51.795′ N 158°15.622′ E | 119 | ||
| Mahnd | Station 10 | 14 March 2012 | 06°50.221′ N 158°17.212′ E | 107 | |
| “River 1” | Station 11 | 14 March 2012 | 06°51.120′ N 158°17.854′ E | 5 | |
| “Petroglyphe River” | Station 12 | 14 March 2012 | 06°53.221′ N 158°17.44′ E | 5 | |
| “River 2” | Station 14 | 10 March 2012 | 06°48.579′ N 158°12.639′ E | 5 | |
| Guam | Assan | 30 January–3 February 2011 | 13°28.168′ N 144°42.762′ E | 11-16 | |
| Masso | 30 January–3 February 2011 | 13°27.200′ N 144°41.871′ E | 34 | ||
| Aplacho | 30 January–3 February 2011 | 13°24.345′ N 144°41.187′ E | 58-75 | ||
| Namo | 30 January–3 February 2011 | 13°23.904′ N 144°39.959′ E | 4 | ||
| Ceti | 30 January–3 February 2011 | 13°19.514′ N 144°40.183′ E | 197 | ||
| Toguan | 30 January–3 February 2011 | 13°17.117′ N 144°39.955′ E | 15-41 | ||
| Ylig | 30 January–3 February 2011 | 13°23.589′ N 144°45.636′ E | 2 |
| Atyoida chacei sp. nov. (n = 11) | Atyoida pilipes (n = 39) | Atyoida tahitensis (n = 22) | ||
|---|---|---|---|---|
| P1 | Dactylus length/width | 3.9–5.9 | 3.5–6.1 | 3.9–6.0 |
| Chela length/width | 1.9–4.2 | 1.7–4.7 | 1.8–3.9 | |
| Carpus length/width | 1.0–1.3 | 0.9–1.4 | 0.8–1.2 | |
| P2 | Dactylus length/width | 3.7–6.3 | 3.6–5.3 | 3.9–5.7 |
| Chela length/width | 2.2–3.9 | 2.1–4.3 | 1.9–4.0 | |
| Carpus length/width | 1.0–1.8 | 1.0–1.6 | 1.0–1.5 | |
| P3 | Dactylus spines number | 2–6 | 3–5 | 3–6 |
| Propodus length/dactylus length | 2.5–3.7 | 2.2–3.7 | 1.8–3.1 | |
| Dactylus length/width | 1.7–3.2 | 1.7–3.7 | 2.2–3.3 | |
| Propodus length/width | 3.0–6.6 | 3.9–7.5 | 3.9–5.4 | |
| P5 | Dactylus spines number | 8–18 | 4–14 | 5–13 |
| Propodus length/dactylus length | 3.3–5.6 | 2.9–4.8 | 2.7–5.8 | |
| Dactylus length/width | 2.1–3.5 | 2.1–5.1 | 1.7–4.4 | |
| Propodus length/width | 5.9–13.4 | 6.0–10.4 | 6.0–10.4 | |
| Rostrum | Dorsal teeth number | 0 | 0 | 0 |
| Ventral teeth number | 0–2 | 0 | 0 | |
| Length/carapace length | 0.2–0.4 | 0.2–0.3 | 0.2–0.3 | |
| Uropod | Diaeresis spines number | 15–20 | 15–22 | 14–18 |
| Telson | Distal setae number | 8–11 | 4–9 | 6–10 |
| Length/width | 2.4–2.8 | 2.3–3.1 | 2.5–3.2 | |
| Dorsal spines number (left + right) | 2–5 + 4–8 | 1–5 + 1–4 | 2–4 + 2–3 | |
| Eggs | Size (mm) | 0.51–0.52 × 0.29–0.31 | 0.47–0.60 × 0.31–0.39 | 0.55–0.70 × 0.29–0.54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Mazancourt, V.; Marquet, G.; Keith, P. An Integrative Taxonomic Revision of the Freshwater Atyid Shrimps (Crustacea: Decapoda: Caridea) of Micronesia. Diversity 2024, 16, 200. https://doi.org/10.3390/d16040200
de Mazancourt V, Marquet G, Keith P. An Integrative Taxonomic Revision of the Freshwater Atyid Shrimps (Crustacea: Decapoda: Caridea) of Micronesia. Diversity. 2024; 16(4):200. https://doi.org/10.3390/d16040200
Chicago/Turabian Stylede Mazancourt, Valentin, Gérard Marquet, and Philippe Keith. 2024. "An Integrative Taxonomic Revision of the Freshwater Atyid Shrimps (Crustacea: Decapoda: Caridea) of Micronesia" Diversity 16, no. 4: 200. https://doi.org/10.3390/d16040200
APA Stylede Mazancourt, V., Marquet, G., & Keith, P. (2024). An Integrative Taxonomic Revision of the Freshwater Atyid Shrimps (Crustacea: Decapoda: Caridea) of Micronesia. Diversity, 16(4), 200. https://doi.org/10.3390/d16040200







