Diversity of Freshwater Mollusks from Lake Pampulha, Municipality of Belo Horizonte, Minas Gerais, Brazil
Abstract
1. Introduction
2. Materials and Methods
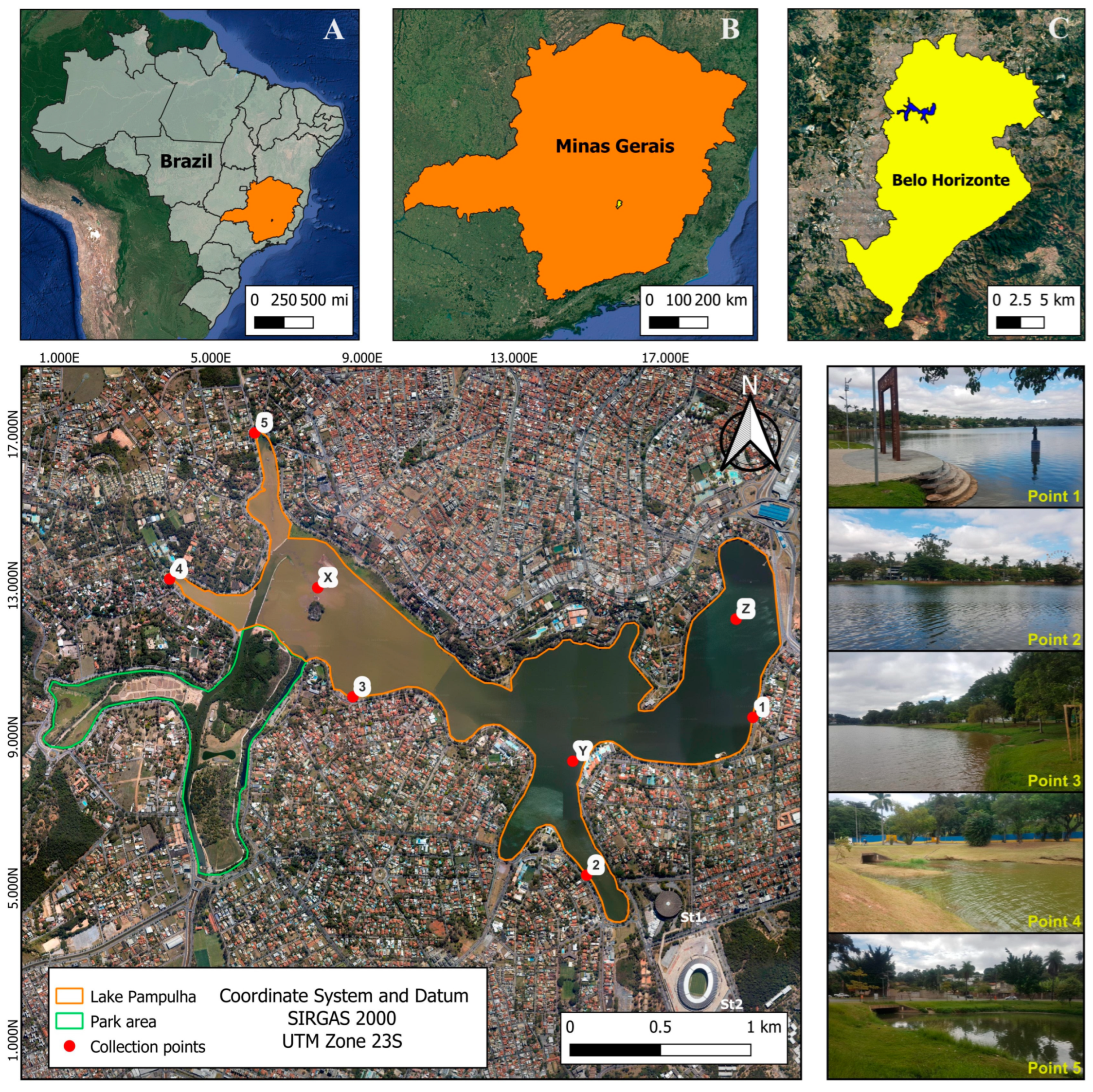
2.1. Study Area
2.2. Methodological Framework for Mollusk Analysis: From Sampling Techniques to Statistical Insights
2.2.1. Sampling Methodology and Georeferencing Techniques
2.2.2. Uniform Collection Practices and Environmental Considerations
2.2.3. Collection Instruments and Statistical Evaluation of Mollusk Shells
2.3. Molluska Taxonomy
2.4. Community Ecology Analysis
2.5. Parasitological: Evaluation of Trematodes
2.6. Water Quality Assessment
3. Results
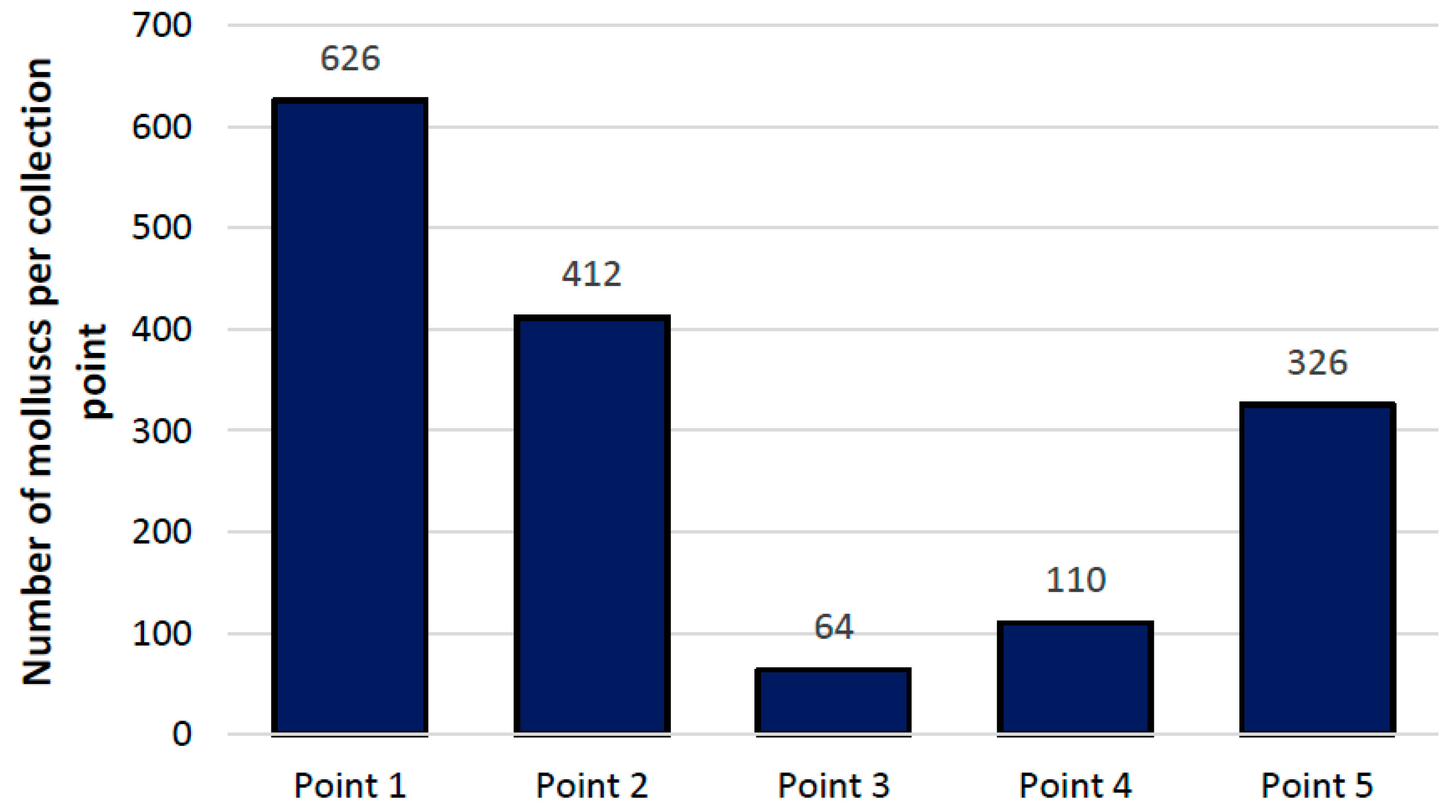
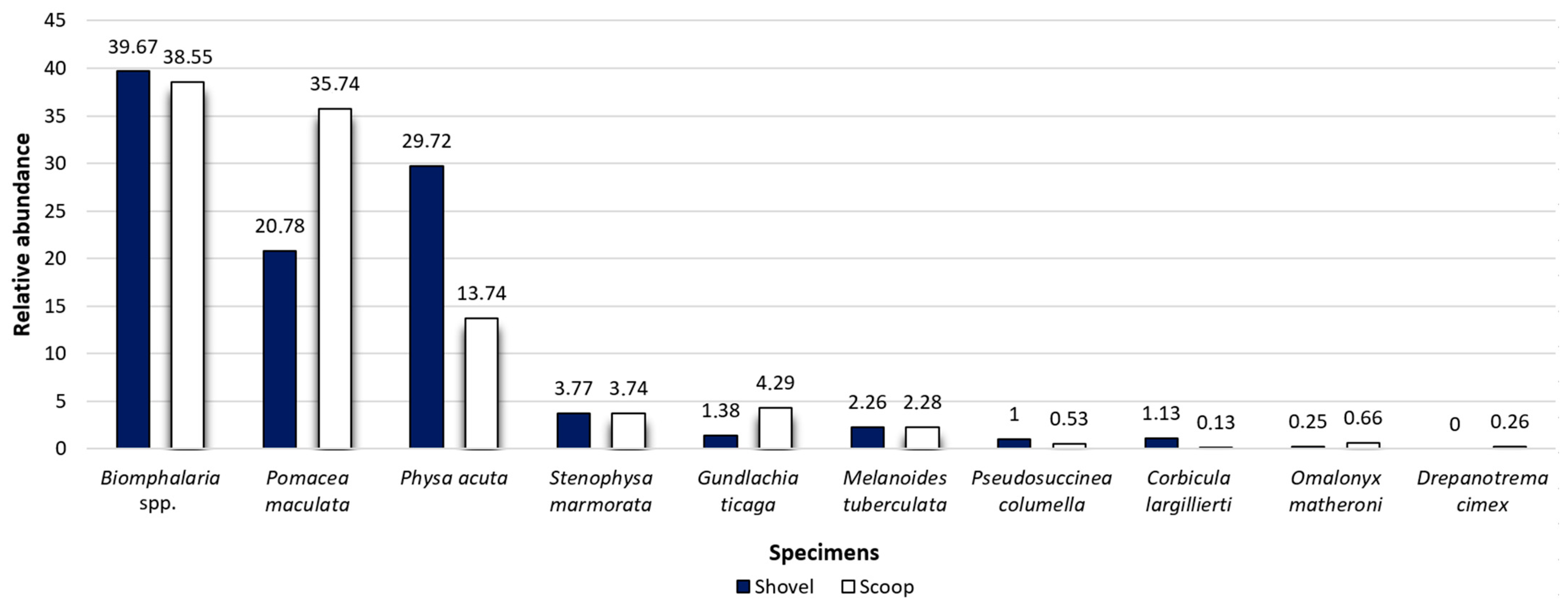
3.1. Malacology
3.2. Community Ecology Analysis
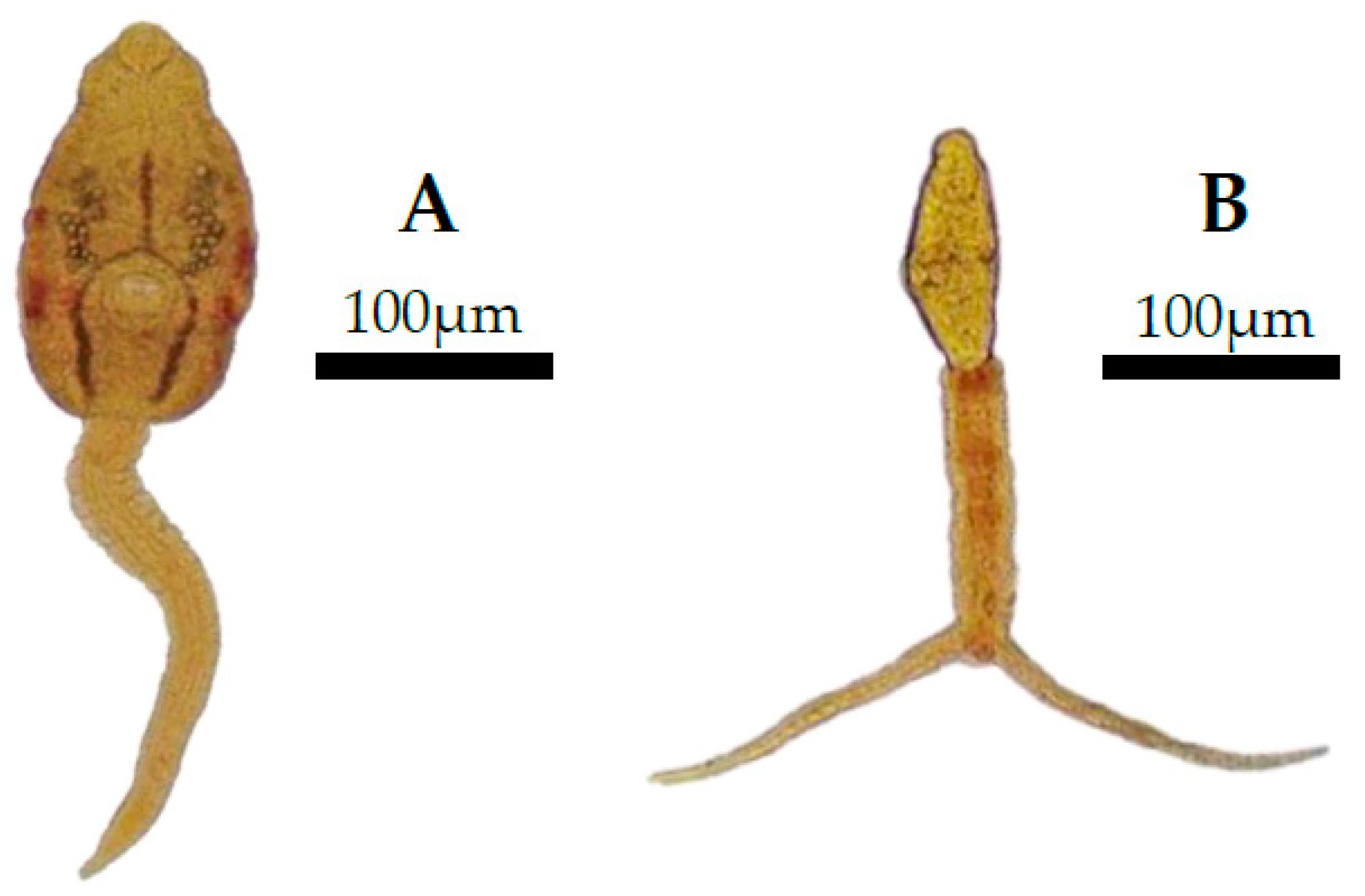
3.3. Evaluation of Parasitic Trematodes
3.4. Descriptive Characterization of Collection Environments and Additional Water Parameters
4. Discussion
4.1. Malacology
4.1.1. Invasive Mollusks
4.1.2. Taxonomic Complexities and Unexplored Diversity
4.2. Sampling Challenges
4.3. Parasitology: Trematode Infections
4.4. Eutrophication and Water Quality
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Machado, F.M.; Miranda, M.S.; Salvador, R.B.; Pimenta, A.D.; Côrtes, M.O.; Gomes, J.A.; Miyahira, I.C.; Agudo-Padrón, I.; Oliveira, C.D.C.; Caetano, C.H.S.; et al. How many species of Mollusca are there in Brazil? A collective taxonomic effort to reveal this still unknown diversity. Zoologia 2023, 40, e23026. [Google Scholar] [CrossRef]
- Strong, E.E.; Gargominy, O.; Ponder, W.F.; Bouchet, P. Global diversity of gastropods (Gastropoda; Mollusca) in freshwater. Hydrobiologia 2008, 595, 149–166. [Google Scholar] [CrossRef]
- Amaral, A.C.Z.; Ribeiro, C.V.; Mansur, M.C.D.; Paiva, A.W.E.; Mattews-Cascon, H.; Leite, F.P.P.; de Melo, G.A.S.; Coelho, P.A.; Buckup, L.; Ventura, C.R.R. A situação de ameaça dos invertebrados aquáticos do Brasil. Livro Vermelho Fauna Bras. Ameaçada Extinção 2008, 1, 156–165. [Google Scholar]
- Santos, S.B.; Thiengo, S.C.; Fernandez, M.A.; Miyahira, I.C.; Gonçalves, I.C.B.; Ximenes, R.D.F.; Mansur, M.C.D.; Pereira, D. Espécies de Moluscos Límnicos Invasores No Brasil. In Moluscos Límnicos Invasores No Brasil. Biologia, Prevenção, Controle; Mansur, M.C.D., Santos, C.P., Pereira, D., Paz, I.C.P., Zurita, M.L.L., Rodriguez, M.T.R., Nehrke, M.V., Bergonci, P.E.A., Eds.; Redes Editora: Porto Alegre, Brazil, 2012; pp. 25–49. ISBN 978-85-61638-46-7. [Google Scholar]
- Freitas, J.R.; Bedê, L.C.; Marco, P., Jr.; Rocha, L.A.; Santos, M.B.L. Population dynamics of aquatic snails in Pampulha Reservoir. Mem. Inst. Oswaldo Cruz 1987, 82 (Suppl. SIV), 299–305. [Google Scholar] [CrossRef] [PubMed]
- Böhm, M.; Dewhurst-Richman, N.I.; Seddon, M.; Ledger, S.E.; Albrecht, C.; Allen, D.; Bogan, A.E.; Cordeiro, J.; Cummings, K.S.; Cuttelod, A.; et al. The conservation status of the world’s freshwater molluscs. Hydrobiologia 2021, 848, 3231–3254. [Google Scholar] [CrossRef]
- Seddon, M.; Appleton, C.; Van Damme, D.; Graf, D. Freshwater Molluscs of Africa: Diversity, Distribution, and Conservation. The Diversity of Life in African Freshwaters: Underwater, under Threat. An Analysis of the Status and Distribution of Freshwater Species throughout Mainland Africa; IUCN: Gland, Switzerland, 2011; pp. 92–125. [Google Scholar]
- Min, F.; Wang, J.; Liu, X.; Yuan, Y.; Guo, Y.; Zhu, K.; Chai, Z.; Zhang, Y.; Li, S. Environmental factors affecting freshwater snail intermediate hosts in Shenzhen and adjacent region, South China. Trop. Med. Infect. Dis. 2022, 7, 426. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.V.; Versiani, W. Schistossomose mansoni em Belo Horizonte. Braz. Med. 1938, 52, 471–472. [Google Scholar]
- Martins, A.V.; Falcão, A.L. Distribuição Geográfica dos Planorbídeos e Seus Índices de Infestação Pelas Cercárias de Schistosoma Mansoni No Município de Belo Horizonte; Congresso Brasileiro de Higiene: Curitiba, Brazil, 1953; Volume 10. [Google Scholar]
- Milward-de-Andrade, R. O problema da esquistossomose mansoni no lago artificial da Pampulha, Belo Horizonte, Minas Gerais (Brasil). Rev. Bras. Mal. Doenças Trop. 1959, 11, 653–674. [Google Scholar]
- Milward-de-Andrade, R. Nota ecológica sôbre o lago da Pampulha (Belo Horizonte, MG). Com especial referência aos planorbídeos (Pulmonata, Planorbidae). Rev. Bras. Mal. Doenças Trop. 1969, 21, 59–116. [Google Scholar]
- Milward-de-Andrade, R. Primeiro encontro de Biomphalaria tenagophila (d’Orbigny, 1835) no lago da Pampulha, Belo Horizonte, MG. Ciênc. Cult. 1972, 24, 375. [Google Scholar]
- Carvalho, O.S.; Guimarães, C.T.; Massara, C.L.; Bonésio, J.E.R. Situação atual da esquistossomose mansoni no lago da Pampulha, Belo Horizonte, MG, Brasil. Rev. Saude Publica 1985, 19, 270–277. [Google Scholar] [CrossRef][Green Version]
- Freitas, J.R.; Santos, M.B.L.; Rocha, L.A. Situação Atual da Transmissão da Esquistossomose na Pampulha—Ecologia dos Moluscos da Represa; Seminário Sobre a Bacia Hidrográfi ca da Pampulha: Belo Horizonte, Brazil, 1992; pp. 40–69. [Google Scholar]
- Pinto-Coelho, R.M. Monitoramento do Reservatório da Pampulha Convênio 3998-Secretaria Municipal do Meio Ambiente—PBH Fundep—UFMG Relatório Final Período: Abril 2000-Abril 2001. Belo Horizonte, Brazil. 2001. 85p. Available online: http://www.rmpcecologia.com/art_pdf/rf_fundep3998.pdf (accessed on 1 December 2023).
- Ribeiro, J.P.M. Análise da Recarga e da Conexão Hidráulica do Sistema Aquífero Granular-Fissural No Campus Pampulha da UFMG, Belo Horizonte, MG; Dissertação (Mestrado em Geologia)—Instituto de Geociências, Universidade Federal de Minas Gerais: Belo Horizonte, Brazil, 2013; 112p. [Google Scholar]
- Lopes, F.A.; Silveira, J.S.; Leite, A.C.; Piazi, J.; de Azevedo Lopes, N.I. Recreação de contato secundário em lagos urbanos: O caso da Lagoa da Pampulha. Rev. Geogr. 2022, 15, 42–60. [Google Scholar] [CrossRef]
- Silva, T.F.D.G.; Vinçon-Leite, B.; Giani, A.; Figueredo, C.C.; Petrucci, G.; Lemaire, B.; Von Sperling, E.; Tassin, B.; Seidl, M.; Khac, V.T.; et al. Modelagem da Lagoa da Pampulha: Uma ferramenta para avaliar o impacto da bacia hidrográfica na dinâmica do fitoplâncton. Eng. Sanitária Ambient. 2016, 21, 95–108. [Google Scholar] [CrossRef]
- Olivier, L.; Schneiderman, M.A. method for estimating the density of aquatic snail populations. Exp. Parasitol. 1956, 5, 109–117. [Google Scholar] [CrossRef]
- Ministério da saúde (MS). Vigilância e Controle de Moluscos de Importância Epidemiológica; Ministério da saúde (MS): Brasília, Brasil, 2008; ISBN 9788533414389.
- Lobato, P. Lymnaea viatrix: A Study of Tropotypic Specimens (Mollusca: Lymnaeidae). Rev. Bras. Biol. 1976, 36, 419–428. [Google Scholar]
- Molluscabase.org. Molluscabase—Lymnaea columella Say. 1817. Available online: https://www.molluscabase.org/aphia.php?p=taxdetails&id=724461 (accessed on 30 November 2023).
- Simone, L.R.L. Land and Freshwater Mollusks of Brazil: An Illustrated Inventory on the Brazilian Malacofauna, Including Neighbor Regions of the South America, Respect to the Terrestrial and Freshwater Ecosystem; Museu de Zoologia, Universidade de São Paulo: São Paulo, Brazil, 2006. [Google Scholar]
- Deslandes, N. Técnica de dissecação e exame de planorbídeos. Rev. Serv. Espec. Saude Publica 1951, 4, 371–382. [Google Scholar]
- Cuezzo, M.G.; Gregoric, D.E.G.; Pointier, J.P.; Vázquez, A.A.; Ituarte, C.; Mansur, M.C.D.; Oliveira Arruda, J.; Barker, G.M.; dos Santos, S.B.; Ovando, X.M.C.; et al. Phylum Mollusca. In Thorp and Covich’s Freshwater Invertebrates; Academic Press: Cambridge, MA, USA, 2020; pp. 261–430. [Google Scholar]
- Thiengo, S.C.; Hayes, K.A.; Matos, A.C.; Fernandez, M.A.; Cowie, R.H. A Família Ampullariidae No Brasil: Aspectos Morfológicos, Biológicos e Taxonômicos; Fernandez, M.A., Santos, S.B., Pimenta, A.D., Thiengo, S.C., Eds.; Tópicos em Malacologia: Ecos Do XIX Encontro Brasileiro de Malacologia; Sociedade Brasileira de Malacologia: Rio de Janeiro, Brazil, 2011; pp. 95–111. [Google Scholar]
- Arruda, J.O.; Gomes, S.R.; Ramírez, R.; Thomé, J.W. Morfoanatomia de duas espécies do gênero Omalonyx (Mollusca, Pulmonata, Succineidae) com novo registro para Minas Gerais, Brasil. Biociências 2006, 14, 61–70. [Google Scholar]
- Vidigal, T.H.; Caldeira, R.L.; Simpson, A.J.; Carvalho, O.S. Further studies on the molecular systematics of Biomphalaria snails from Brazil. Mem. Inst. Oswaldo Cruz 2000, 95, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Caldeira, R.L.; Teodoro, T.M.; Jannotti-Passos, L.K.; Lira-Moreira, P.M.; Goveia, C.D.O.; Carvalho, O.D.S. Characterization of South American Snails of the Genus Biomphalaria (Basommatophora: Planorbidae) and Schistosoma mansoni (Platyhelminthes: Trematoda) in Molluscs by PCR-RFLP. Biomed. Res. Int. 2016, 2016, 1045391. [Google Scholar] [CrossRef] [PubMed]
- Harper, D.A.T. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. Available online: http://palaeo-electronica.org/2001_1/past/issue1_01.htm (accessed on 1 December 2023).
- Primack, R.B.; Rodrigues, E. Biologia da Conservação. Planta editora. 2006. vii-327. Available online: https://scholar.google.com.br/citations?view_op=view_citation&hl=en&user=vv5869sAAAAJ&citation_for_view=vv5869sAAAAJ:u5HHmVD_uO8C (accessed on 1 December 2023).
- Kuntz, R.E. Effect of light and temperature on shedding of Schistosoma mansoni cercariae. Eff. Light. Temp. Shedding Schistosoma Mansom Cercariae 1946, 7, 7–16. [Google Scholar]
- Pinto, H.A.; De Melo, A.L. Larvas de trematódeos em moluscos do Brasil: Panorama e perpectivas após um século de estudos. Rev. Patol. Trop. 2014, 42, 369–386. [Google Scholar] [CrossRef]
- IGAM—Boletim Trimestral da Densidade de Cianobactérias na Bacia Hidrográfica do Ribeirão Pampulha: Dezembro de 2021. Belo Horizonte: Igam, Dez, 2021. Available online: http://www.repositorioigam.meioambiente.mg.gov.br/jspui/handle/123456789/3983 (accessed on 1 December 2023).
- IGAM—Boletim de Qualidade da Água 2018 a 2022—Sub Bacia do Ribeirão Pampulha: Belo Horizonte: Igam, Mar. 2023. Available online: http://www.repositorioigam.meioambiente.mg.gov.br/jspui/handle/123456789/4298 (accessed on 1 December 2023).
- Frissell, C.A.; Bayles, D. Ecosystem Management and The Conservation of Aquatic Biodiversity and Ecological Integrity 1. JAWRA J. Am. Water Resour. Assoc. 1996, 32, 229–240. [Google Scholar] [CrossRef]
- Sax, D.F.; Stachowicz, J.J.; Brown, J.H.; Bruno, J.F.; Dawson, M.N.; Gaines, S.D.; Grosberg, R.K.; Hastings, A.; Holt, R.D.; Mayfield, M.M.; et al. Ecological and evolutionary insights from species invasions. Trends Ecol. Evol. 2007, 22, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Didham, R.K.; Tylianakis, J.M.; Gemmell, N.J.; Rand, T.A.; Ewers, R.M. Interactive effects of habitat modification and species invasion on native species decline. Trends Ecol. Evol. 2007, 22, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Wilcove, D.S.; Rothstein, D.; Dubow, J.; Phillips, A.; Losos, E. Quantifying threats to imperiled species in the United States. BioScience 1998, 48, 607–615. [Google Scholar] [CrossRef]
- Simberloff, D. Biological invasions: What’s worth fighting and what can be won? Ecol. Eng. 2014, 65, 112–121. [Google Scholar] [CrossRef]
- Mansur, M.C.D.; Callil, C.T.; Cardoso, F.R.; Santos, C.P.; Ibarra, J.A.A. Uma retrospectiva e mapeamento da invasão de espécies de Corbicula (Mollusca, Bivalvia, Veneroida, Corbiculidae) oriundas do sudeste asiático, na América do Sul. In Interrciências: Água de lastro e bioinvasão; Silva, J.S.V., Souza, R.C.C.L., Eds.; Interciência: Rio de Janeiro, Brazil, 2004; pp. 39–58. [Google Scholar]
- Callil, C.T.; Mansur, M.C.D. Corbiculidae in the Pantanal: History of invasion in souther and South America and biometrical data. Amazonian 2002, 17, 153–167. [Google Scholar]
- Silva, E.C.; Barros, F. Macrofauna bentônica introduzida no Brasil: Lista de espécies marinhas e dulcícolas e distribuição atual. Oecol. Austral. 2011, 15, 326–344. [Google Scholar] [CrossRef]
- Miyahira, I.C.; Carneiro, J.B.; Gonçalves, I.C.B.; Lacerda, L.E.M.; Oliveira, J.L.; Vasconcelos, M.C.; Santos, S.B. Freshwater mollusks and environmental assessment of Guandu River, Rio de Janeiro, Brazil. Biota Neotrop. 2017, 17, e20170342. [Google Scholar] [CrossRef]
- Miyahira, I.C.; Pereira, L.S.; Santos, L.N. Non-native freshwater molluscs in the Neotropics: What can be learned from Brazilian reservoirs? Aquat. Invasions 2020, 15, 455–472. [Google Scholar] [CrossRef]
- Coelho, P.N.; Fernandez, M.A.; César, D.A.S.; Ruocco, A.M.C.; Henry, R. Updated distribution and range expansion of the gastropod invader Melanoides tuberculata (Müller, 1774) in Brazilian waters. BioInvasions Rec. 2018, 7, 405–409. [Google Scholar] [CrossRef]
- Dillon, R.T.; Wethington, A.R.; Rhett, J.M.; Smith, T.P. Populations of the european freshwater pulmonate Physa acuta are not reproductively isolated from american Physa heterostropha or Physa integra. Invertebr. Biol. 2002, 121, 226–234. [Google Scholar] [CrossRef]
- Van Leeuwen, C.H.A.; Van der Velde, G.; Van Lith, B.; Klaassen, M. Experimental quantification of long distance dispersal potential of aquatic snails in the gut of migratory birds. PLoS ONE 2012, 7, e32292. [Google Scholar] [CrossRef]
- Paraense, W.L.; Pointier, J.P. Physa acuta Draparnaud. 1805 (Gastropoda: Physidae): A study of topotypic specimens. MemóRias Inst. Oswaldo Cruz 2003, 98, 513–517. [Google Scholar] [CrossRef]
- Bernot, R.J.; Kennedy, E.E.; Lamberti, G.A. Effects of ionic liquids on the survival, movement, and feeding behavior of the freshwater snail Physa acuta. Environ. Toxicol. Chem. 2005, 24, 1759–1765. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, C.; Kroll, O.; Terrazas, E.M.; Wilke, T. Invasion of ancient Lake Titicaca by the globally invasive Physa acuta (Gastropoda: Pulmonata: Hygrophila). Biol. Invasions 2009, 11, 1821–1826. [Google Scholar] [CrossRef]
- Tietze, E.; Francesco, C. Environmental significance of freshwater mollusks in the Southern Pampas, Argentina: To what detail can local environments be inferred from mollusk composition? Hydrobiologia 2010, 641, 133–143. [Google Scholar] [CrossRef]
- Fernandez, M.A.; Thiengo, S.C.; Simone, L.R.L. Distribution of the introduced freshwater snail Melanoides tuberculatus (Gastropoda: Thiaridae) in Brazil. Nautilus 2003, 117, 78–82. [Google Scholar]
- Carvalho, O.S. Ocorrência de um tiarídeo (Mollusca) no lago da Pampulha, Belo Horizonte, MG, Brasil. Rev. Soc. Bras. Med. Trop. 1986, 19, 57. [Google Scholar] [CrossRef][Green Version]
- Bedê, L.C. Dinâmica Populacional de Melanoides Tuberculata (Prosobranchia: Thiaridae) No Reservatório da Pampulha; Dissertação de Mestrado, Universidade Federal de Minas Gerais: Belo Horizonte, Brasil, 1992; 112p. [Google Scholar]
- Freitas, J.R.; Santos, M.B.L. Current advances on the study of snail-snail interactions, with special emphasis on competition process. Mem. Inst. Oswaldo Cruz 1995, 90, 261–265. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Silva, R.E.; Melo, A.L.; Pereira, L.H.; Frederico, L.F. Levantamento malacológico da bacia hidrográfica do lago Soledade, Ouro Branco (Minas Gerais, Brasil). Rev. Inst. Med. Trop. São Paulo 1994, 36, 437–444. [Google Scholar] [CrossRef][Green Version]
- Paz, R.J.; Watanabe, T.; Dijek, M.P.M.; Abílio, F.J.P. First record of Melanoides tuberculata (Müller, 1774) (Gastropoda: Prosobranchia: Thiaridae) in the state of Paraíba (Brazil) and its possible ecological implications. Rev. Nord. Biol. 1995, 10, 79–84. [Google Scholar]
- Santos, C.M.; Eskinazi-Sant’Anna, E.M. The introduced snail Melanoides tuberculatus (Muller, 1774) (Mollusca: Thiaridae) in aquatic ecosystems of the Brazilian Semiarid Northeast (Piranhas-Assu River basin, State of Rio Grande do Norte). Braz. J. Biol. 2010, 70, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Milward-de-Andrade, R.; Carvalho, O.S.; Guimarães, C.T. Alguns dados bioecológicos de Pomacea haustrum (Reeve, 1856), predador-competidor de hospedeiros intermediários de Schistosoma mansoni Sambon, 1907. Rev. Saúde Pública 1978, 12, 78–89. [Google Scholar] [CrossRef][Green Version]
- Souza, C.P.; Pereira, J.P.; Rodrigues, M.S. Atual distribuição geográfica dos moluscos hospedeiros intermediários do Schistosoma mansoni em Belo Horizonte, MG, Brasil. Mem. Inst. Oswaldo Cruz 1981, 76, 383–391. [Google Scholar] [CrossRef][Green Version]
- Guimarães, C.T. Algumas observações de campo sobre biologia e ecologia de Pomacea haustrum (Reeve, 1856) (Mollusca, Pilidae). Mem. Inst. Oswaldo Cruz 1981, 76, 343–351. [Google Scholar] [CrossRef]
- Paulinyi, H.M.; Paulini, E. Laboratory observations on the biological control of Biomphalaria glabrata by a species of Pomacea (Ampullariidae). Bull. World Health Organ. 1972, 46, 243–247. [Google Scholar]
- Mozzer, L.R.; Coaglio, A.L.; Dracz, R.M.; Ribeiro, V.M.A.; Lima, W.S. The development of Angiostrongylus vasorum (Baillet, 1866) in the freshwater snail Pomacea canaliculata (Lamarck, 1822). J. Helminthol. 2015, 89, 755–759. [Google Scholar] [CrossRef]
- Montresor, L.C. Implicações Ecotoxicológicas do Controle Químico de Limnoperna fortunei (Dunker, 1857) (Bivalvia: Mytilidae). 2015, p. 68. Available online: https://gia.org.br/portal/acoes-e-impactos-dos-principais-metodos-quimicos-utilizados-no-controle-de-moluscos-bivalves-incrustantes-no-mundo/ (accessed on 1 December 2023).
- Hayes, K.A.; Cowie, R.H.; Thiengo, S.C.; Strong, E.E. Comparing apples with apples: Clarifying the identities of two highly invasive neotropical Ampullariidae (Caenogastropoda). Zool. J. Linn. Soc. 2012, 166, 723–753. [Google Scholar] [CrossRef]
- Coelho, P.R.S.; Ker, F.T.; Araujo, A.D.; Pinto, H.A.; Negrão-Corrêa, D.A.; Caldeira, R.L.; Geiger, S.M. Survey on Limnic Gastropods: Relationships between Human Health and Conservation. Pathogens 2022, 11, 1533. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, O.D.S.; Mendonça, C.L.F.D.; Marcelino, J.M.D.R.; Passos, L.K.J.; Fernandez, M.A.; Leal, R.D.S.; Caldeira, R.L.; Scholte, R.G.C.; Carmo, E.H.; Mesquita, S.G.; et al. Distribuição geográfica dos hospedeiros intermediários do Schistosoma mansoni nos estados do Paraná, Minas Gerais, Bahia, Pernambuco e Rio Grande do Norte, 2012–2014. Epidemiol. Serviços Saúde 2018, 27, e2017343. [Google Scholar] [CrossRef]
- Paraense, W.L. Lymnaea viatrix and Lymnaea columella in the neotropical region: A distributional outline. Mem. Inst. Oswaldo Cruz 1982, 77, 181–188. [Google Scholar] [CrossRef]
- Souza, C.P.; Lima, L.C.; Jannotti-Passos, L.K.; Ferreira, S.S.; Guimarães, C.T.; Vieira, I.B.F.; Mariani, R., Jr. Moluscos límnicos da microrregião de Belo Horizonte, MG, com ênfase nos vetores de parasitoses. Rev. Soc. Bras. Med. Trop. 1998, 31, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Neves, D.P.; Melo, A.L.M.; Linardi, P.M.; Vitor, R.W.A. Parasitologia Humana, 14th ed.; Editora Atheneu; Brazil; 2022; ISBN 978-6555865196. Available online: https://www.amazon.com.br/Parasitologia-Humana-David-Pereira-Neves/dp/6555865199 (accessed on 1 December 2023).
- Pinto, H.A.; Mati, V.L.T.; Melo, A.L. The Pampulha reservoir remains a potential urban focus of schistosomiasis mansoni in Brazil: Changes in the occurrence patterns of Biomphalaria species and a new record of the parasite. Rev. Soc. Bras. Med. Trop. 2013, 46, 478–483. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pinto, H.A.; Melo, A.L. Melanoides tuberculata (Mollusca: Thiaridae) as an intermediate host of Centrocestus formosanus (Trematoda: Heterophyidae) in Brazil. Rev. Inst. Med. Trop. São Paulo 2010, 52, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Souza, C.P.; Araújo, N.; Carvalho, O.S.; Freitas, J.R. Potencialidade de Biomphalaria tenagophila do Lago da Pampulha, Belo Horizonte, MG, como hospedeira do Schistosoma mansoni. Mem. Inst. Oswaldo Cruz 1987, 82, 67–70. [Google Scholar] [CrossRef]
- Lopez-Hernandez, D.; Locke, S.A.; de Assis, J.C.A.; Drago, F.B.; de Melo, A.L.; Rabelo, É.M.L.; Pinto, H.A. Molecular, morphological and experimental-infection studies of cercariae of five species in the superfamily Diplostomoidea (Trematoda: Digenea) infecting Biomphalaria straminea (Mollusca: Planorbidae) in Brazil. Acta Trop. 2019, 199, 105082. [Google Scholar] [CrossRef]
- Pinto, H.A. Biologia e Taxonomia de Trematódeos Transmitidos Por Moluscos Dulciaquícolas na Represa da Pampulha. Belo Horizonte, Brasil. 2013, p. 299. Available online: https://repositorio.ufmg.br/handle/1843/BUBD-9EFHCC (accessed on 1 December 2023).
- Giovanelli, A.; Silva, C.L.P.A.C.; Leal, G.B.E.; Baptista, D.F. Habitat preference of freshwater snails in relation to environmental factors and the presence of the competitor snail Melanoides tuberculatus (Müller, 1774). Mem. Inst. Oswaldo Cruz 2005, 100, 169–176. [Google Scholar] [CrossRef]
- Pointier, J.P.; David, P. The Biological Control of the Snail Hosts of Schistosomes: The Role of Competitor Snails and Biological Invasions. In Biomphalaria Snails and Larval Trematodes; Toledo, R., Fried, B., Eds.; Springer: New York, NY, USA, 2011; pp. 215–238. [Google Scholar]
- Paraense, W.L.; Santos, J.M. Um ano de observações sobre esquistossomose em planorbídeos da Lagoa Santa. Rev. Bras. Malariol. Doenças Trop. 1953, 3, 253–269. [Google Scholar]
- Giovanelli, A.; Soares, M.S.; D’Andréa, P.S.; Gonçalves, M.M.L.; Rey, L. Abundância e infecção do molusco Biomphalaria glabrata pelo Schistosoma mansoni no Estado do Rio de Janeiro, Brasil. Rev. Saúde Pública 2001, 35, 523–530. [Google Scholar] [CrossRef]
- Teles, H.M.S. Distribuição geográfica das espécies dos caramujos transmissores de Schistosoma mansoni no Estado de São Paulo. Rev. Soc. Bras. Med. Trop. 2005, 38, 426–432. [Google Scholar] [CrossRef]
- Rumi, A.; Bechara, J.A.; Hamann, M.I.; Ostrowski, M.N. Ecology of potential hosts of schistosomiasis in anthropic environments of Chaco, Argentina. Malacologia 2002, 44, 273–288. [Google Scholar]
- Kloos, H.; Passos, L.K.; Loverde, P.; Oliveira, R.C.; Gazzinelli, A. Distribution and Schistosoma mansoni infection of Biomphalaria glabrata in different habitats in a rural area in the Jequitinhonha Valley, Minas Gerais, Brazil: Environmental and epidemiological aspects. Mem. Inst. Oswaldo Cruz 2004, 99, 673–681. [Google Scholar] [CrossRef]
- Johnson, P.T.J.; Lunde, K.B.; Thurman, E.M.; Ritchie, E.G.; Wray, S.W.; Sutherland, D.R.; Kapfer, J.M.; Frest, T.J.; Bowerman, J.; Blaustein, A.R. Parasite (Ribeiroia ondatrae) infection linked to amphibian malformations in the western United States. Ecol. Monogr. 2002, 72, 151–168. [Google Scholar] [CrossRef]
- Johnson, P.T.J.; Chase, J.M. Parasites in the food web: Linking amphibian malformations and aquatic eutrophication. Ecol. Lett. 2004, 7, 521–526. [Google Scholar] [CrossRef]
- Johnson, P.T.J.; Sutherland, D.R.; Kinsella, J.M.; Lunde, K.B. Review of the trematode genus Ribeiroia (Psilostomidae): Ecology, life history and pathogenesis with special emphasis on the amphibian malformation problem. Adv. Parasitol. 2004, 57, 191–253. [Google Scholar] [CrossRef] [PubMed]
- Rollinson, D. Biomphalaria: Natural history, ecology and schistosome transmission. In Biomphalaria Snails and Larval Trematodes; Toledo, R., Fried, B., Eds.; Springer: New York, NY, USA, 2011; pp. 57–80. [Google Scholar] [CrossRef]
- Souza, M.A.A.; Melo, A.L. Ecological aspects of Biomphalaria in endemic areas for schistosomiasis in Brazil. In Schistosomiasis; Rokni, M.B., Ed.; Intech Tehran, 2012; pp. 193–208. ISBN 978-953-307-852-6. Available online: https://www.intechopen.com/chapters/25974 (accessed on 1 December 2023). [CrossRef]
- Wells, S.M.; Chatfi, J.E. Threatened Non-marine Molluscs of Europe. In Nature and Environment; No. 64; Council of Europe Press: Strasbourg, France, 1992. [Google Scholar]
- Caixeta, M.B.; Araujo, P.S.; Rodrigues, C.C.; Goncalves, B.B.; Araujo, O.A.; Bevilaqua, G.B.; Malafaia, G.; Silva, L.D.; Rocha, T.L. Risk assessment of iron oxide nanoparticles in an aquatic ecosystem: A case study on Biomphalaria glabrata. J. Hazard. Mater. 2021, 401, 123398. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Baynes, A.; Renner, K.O.; Zhang, M.; Scrimshaw, M.D.; Routledge, E.J. Uptake, elimination and efects of cosmetic microbeads on the freshwater gastropod Biomphalaria glabrata. Toxics 2022, 10, 87. [Google Scholar] [CrossRef] [PubMed]
- de Siqueira, W.N.; de França, E.J.; Pereira, D.R.; Lima, M.D.V.; Silva, H.A.M.F.; Sá, J.L.F.; de Araújo, H.D.A.; de Albuquerque Melo, A.M.M. Toxicity and genotoxicity of domestic sewage sludge in the freshwater snail Biomphalaria glabrata (Say, 1818). Environ. Sci. Pollut. Res. 2021, 28, 69343–69353. [Google Scholar] [CrossRef] [PubMed]
- Martins, S.E.; Bianchini, A. Toxicity tests aiming to protect Brazilian aquatic systems: Current status and implications for management. J. Environ. Monit. 2011, 13, 1866–1875. [Google Scholar] [CrossRef] [PubMed]
- de Freitas Tallarico, L. Freshwater gastropods as a tool for ecotoxicology assessments in Latin America. Am. Malacol. Bull. 2015, 33, 330–336. [Google Scholar] [CrossRef]
- USEPA. U.S. Environmental Protection Agency, Draft Update of Ambient Water Quality Criteria for Copper; U.S. Environmental Protection Agency: Washington, DC, USA, 2003; p. 86.




| Specimens | Lake Pampulha | ||||
|---|---|---|---|---|---|
| Point 1 | Point 2 | Point 3 | Point 4 | Point 5 | |
| Biomphalaria straminea | X | X | - | - | X |
| Biomphalaria kuhniana | - | - | - | X | - |
| Biomphalaria occidentalis | - | - | X | - | - |
| Drepanotrema cimex | - | - | X | - | - |
| Gundlachia ticaga | X | X | - | - | - |
| Stenophysa marmorata | X | X | X | - | X |
| Physa acuta * | X | X | - | - | X |
| Pseudosuccinea columella | - | X | X | - | X |
| Pomacea maculata * | X | X | X | X | X |
| Melanoides tuberculata * | X | X | X | - | - |
| Omalonyx matheroni | - | X | - | - | X |
| Corbicula largillierti * | - | - | - | X | - |
| Indexes | Point 1 | Point 2 | Point 3 | Point 4 | Point 5 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Shovel | Scoop | Shovel | Scoop | Shovel | Scoop | Shovel | Scoop | Shovel | Scoop | |
| Richness | 6 | 6 | 6 | 7 | 5 | 5 | 2 | 3 | 6 | 4 |
| Dominance | 0.4177 | 0.4484 | 0.4799 | 0.3213 | 0.3015 | 0.4634 | 0.7545 | 0.9158 | 0.338 | 0.6387 |
| Equitability | 0.5787 | 0.5993 | 0.6065 | 0.7505 | 0.8889 | 0.6356 | 0.5971 | 0.2064 | 0.6979 | 0.5109 |
| Simpson 1-D | 0.5823 | 0.5516 | 0.5201 | 0.6787 | 0.6985 | 0.5366 | 0.2455 | 0.08418 | 0.662 | 0.3613 |
| Shannon H | 1.037 | 1.074 | 1.087 | 1.46 | 1.431 | 1.023 | 0.4139 | 0.2267 | 1.25 | 0.7083 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coelho, P.R.S.; Thiengo, S.C.; de Mendonça, C.L.F.; de Oliveira, N.M.T.; dos Santos, S.B.; Caldeira, R.L.; Geiger, S.M. Diversity of Freshwater Mollusks from Lake Pampulha, Municipality of Belo Horizonte, Minas Gerais, Brazil. Diversity 2024, 16, 193. https://doi.org/10.3390/d16040193
Coelho PRS, Thiengo SC, de Mendonça CLF, de Oliveira NMT, dos Santos SB, Caldeira RL, Geiger SM. Diversity of Freshwater Mollusks from Lake Pampulha, Municipality of Belo Horizonte, Minas Gerais, Brazil. Diversity. 2024; 16(4):193. https://doi.org/10.3390/d16040193
Chicago/Turabian StyleCoelho, Paulo Ricardo Silva, Silvana Carvalho Thiengo, Cristiane Lafetá Furtado de Mendonça, Nathália Moreira Teodoro de Oliveira, Sonia Barbosa dos Santos, Roberta Lima Caldeira, and Stefan Michael Geiger. 2024. "Diversity of Freshwater Mollusks from Lake Pampulha, Municipality of Belo Horizonte, Minas Gerais, Brazil" Diversity 16, no. 4: 193. https://doi.org/10.3390/d16040193
APA StyleCoelho, P. R. S., Thiengo, S. C., de Mendonça, C. L. F., de Oliveira, N. M. T., dos Santos, S. B., Caldeira, R. L., & Geiger, S. M. (2024). Diversity of Freshwater Mollusks from Lake Pampulha, Municipality of Belo Horizonte, Minas Gerais, Brazil. Diversity, 16(4), 193. https://doi.org/10.3390/d16040193










