Abstract
Two new species of copepods of the genus Elaphoidella Chappuis, 1929 were discovered in a cave and a spring in northeastern Thailand. The first species, E. phuphamanensis sp. nov., belongs to species-group VII sensu Lang. It is most similar to E. turgisetosa Petkovski, 1980 in the armament of the male third exopod of the fourth swimming leg and the shape and armament of the fifth swimming leg in both sexes. However, it is easily distinguished from other congeners by the segmentation of the first swimming leg, the endopod of the fourth swimming leg, and the armature of the third exopod of swimming legs 2–4 in both sexes. The second species, E. propecabezasi sp. nov., is located in species-group I sensu Lang, where the male does not have a transformed seta on the third exopod of the fourth swimming leg and the female fifth swimming leg has four baseoendopodal robust setae, unequal in length. It is most similar to E. cabezasi Petkovski, 1982 and E. paraaffinis Watiroyram, Sanoamuang and Brancelj, 2017 in having the same armature formula as endopods 1–2 of female swimming legs 1–4. However, the ornamentation of the anal operculum, the shape of the caudal ramus, and the armature of the fifth swimming leg in both sexes distinguish them from each other. A rare gynandromorphic specimen of E. propecabezasi sp. nov. was recorded, and a revised key to Elaphoidella species in Southeast Asia is provided.
1. Introduction
The family Canthocamptidae comprises 58 valid genera. Among these, Elaphoidella Chappuis, 1929, is the largest genus, with 217 species and subspecies [] that are divided into species groups [,]. The genus has members that occur in a wide range of freshwater habitats around the world []. Some of them live on the bottom of rivers and lakes [], while other species have been found in semi-terrestrial and terrestrial environments like leaf litter, mosses, moist soils, and phytotelmata [,,]. However, the majority of them, more than 134 species, were reported to inhabit exclusively groundwater habitats [], colonizing alluvial or karstic aquifers [,].
Thirty-four species have previously been reported from Southeast Asia (SEA), with about 75% of them (actually 26 taxa) having been known from a single country and several of them from a single locality []. The first record of the genus Elaphoidella in Thailand was from Daday, who reported E. grandidieri (Guerne and Richard, 1893) from two ponds at Wat Sabatome Palace in Bangkok []. The second species, E. margaritae Pesce and Apostolov, 1985 [], was recorded from a man-made well in Phuket Island, Southern Thailand. The next record of the genus, E. cf. grandidieri, was from Chiang Dao Cave, northern Thailand []. However, E. cf. grandidieri was not reported recently from Chiang Dao Cave [], but another species, E. namnaoensis Brancelj, Watiroyram and Sanoamuang, 2010 [], has been recorded there.
The number of Elaphoidella species recorded from Thailand significantly increased after 2010, when intensive studies on the diversity of cave-dwelling copepods started within a program coordinated by the Applied Taxonomic Research Center, Department of Biology, Faculty of Science, Khon Kaen University. As a result, the first confirmed cave-dwelling species, E. namnaoensis, was discovered in a cave in northeastern Thailand []. The number of Elaphoidella species, either new species for science or species already known from other countries, has thereafter increased rapidly, with a total of 15 species having been recorded in Thailand [].
During our continuing investigation of cave-dwelling copepods from Thailand, we have come across two more undescribed Elaphoidella species. Thus, this contribution provides illustrated descriptions of E. phuphamanensis sp. nov. and E. propecabezasi sp. nov.
2. Materials and Methods
Groundwater-dwelling organisms were collected from a cave and a spring in Khon Kaen and Loei Provinces in northeastern Thailand (Figure 1). Samples were collected using a hand net with a mesh size of 60 µm. Immediately after sampling, samples were fixed in 96% ethanol. Before the morphological examination, some specimens were put in a mixture of glycerol and 70% ethanol (1/10 v/v) for 30 min and were subsequently transferred into a drop of pure glycerol on a glass slide and dissected under a stereomicroscope. The body parts were covered with a coverslip, sealed with nail polish, and examined under a Nikon Eclipse E200 compound microscope at 1000 times magnification.
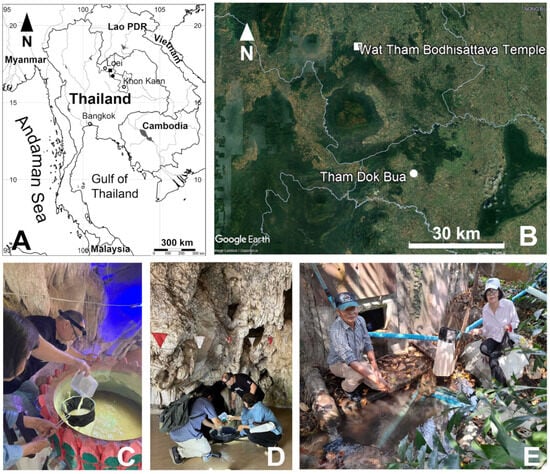
Figure 1.
Geographical location and details on sampling sites. (A) map of Thailand and location of sampling sites in Khon Kaen and Loei provinces (indicated by black circle and square, respectively); (B) geographical details of sampling sites (Google Earth map); (C,D) sampling sites in which Elaphoidella phuphamanensis sp. nov. was collected; (E) sampling site in which Elaphoidella propecabezasi sp. nov. was collected.
Habitus and appendages were drawn using a drawing tube attached to a compound microscope at 400 and 1000 times magnification, respectively, and afterward were digitally inked using the CorelDraw 19.0 graphic program.
Abbreviations used for the morphological description are as follows: ae = aesthetasc; Enp = endopod; Exp = exopod; Enp-1(2) = proximal (distal) segment of the Enp; Exp-1 (2, 3) = proximal (middle, distal) segment of the Exp; P1–P6 = first to sixth swimming legs.
Seta I–VII = first to seventh caudal ramus seta; seta I = anterolateral accessory seta; seta II = anterolateral seta; seta III = postereolateral seta; seta IV = outer terminal seta; seta V = inner terminal seta; seta VI = terminal accessory seta; seta VII = dorsal seta. The descriptive terminology follows Huys and Boxshall [].
Refractile points are spots on the body surface of specimens, which under microscope observation reflect/absorb light.
The type material of both new species was deposited at the Thailand Natural History Museum, Natural Science Museum Thailand (THNHM), and at the Applied Taxonomic Research Center, Khon Kaen University, Thailand (KKU).
3. Results
Harpacticoida Sars G.O., 1903
Canthocamptidae Brady, 1880
Canthocamptinae Brady, 1880
3.1. Elaphoidella phuphamanensis sp. nov.
Material examined. Holotype: 1 ♀ (adult), length 485 μm; completely dissected and mounted on a slide in glycerol, covered with a cover slip, and sealed with nail polish; access number: THNHM-IV-20352. Allotype: 1 ♂ (adult), length 428 μm; completely dissected and mounted on a slide in glycerol, covered with a cover slip, and sealed with nail polish; access number: THNHM-IV-20353. Paratypes: 1 ♀ (adult), 1 ♂ (adult), length 502 μm and 441 μm, respectively; completely dissected and mounted on a slide in glycerol, and sealed with nail polish; access numbers: THNHM-IV-20354–20355, respectively. Additional material: several adult females and males; preserved in 70% ethanol; retained in the collection of the first author (CB). All specimens were collected at the type locality on 2 April 2023, by A. Brancelj, L. Sanoamuang, and N. Sanoamuang.
Type locality. Tham Dok Bua Cave (=Lotus Flower Cave), Phuphaman District, Khon Kaen Province, Thailand; coordinates of the cave entrance: 16°45′41.74″ N, 101°56′49.41″ E, 302 m a.s.l.; a small concrete pond and a bucket next to it in the entrance zone of the cave (Figure 1A–D).
Etymology. The specific epithet is derived from Phuphaman, reflecting the name of the area (i.e., district) in which the type locality is located. The name is an adjective in the nominative singular, gender feminine.
Description of adult female. Total body length, excluding caudal setae, 485–502 µm (mean = 495; N = 3) (Figure 2A). Preserved specimens colorless. Habitus cylindrical, slightly tapering posteriorly, with numerous transverse rows of spinules and integumental refractile points dorsally (Figure 2C–E). Rostrum short, completely fused to cephalothorax. Prosome as long as urosome (including caudal rami), comprising cephalothorax and three free pedigerous somites.
Cephalothorax as wide as the rest of prosome, about 1.3 times as long as wide and about half as long as the prosome; integumental window (nuchal organ) well developed; hyaline free posterior margin serrated; sensilla distributed as illustrated (Figure 2A).
Three subsequent pedigerous somites each with integumental pore middorsally, near anterior margin; three pairs of sensilla on each segment; hyaline free posterior margin of each segment serrated; without conspicuous boundary between prosome and urosome (Figure 2A).
Urosome comprising P5 bearing somite, genital double somite, two free somites and anal somite with caudal rami (Figure 2A and Figure 3A). All urosomites, except anal somite, with hyaline free posterior margin serrated. P5-bearing somite wider than long; with pair of sensilla dorsally.
Genital double somite about 0.7 times as long as wide, with two pairs of sensilla dorsally; one pore middorsally (Figure 2A); ventral surface with fused plate bearing P6 proximally; one lateroventral pore on each side of genital double somite posteriorventrally; four sensilla along posterior margin ventrally (Figure 3B). Genital complex (Figure 3B) with large copulatory pore, bell-shaped, sclerotized; seminal receptacle small, sclerotized.
Urosomites 4 and 5 with pair of sensilla dorsolaterally, near posterior margin; middorsal pore present on urosomite 4 but absent on subsequent segments; row of rather robust spinules midventrally, near posterior margins (Figure 3A).
Anal somite (Figure 2A and Figure 3A,C,D) with oblique row of strong spinules laterally; rows of 3–5 spinules ventrally, near base of caudal rami. Anal operculum slightly convex; free margin with 10–12 robust spinules; pair of sensilla at base of operculum.
Caudal rami (Figure 2A and Figure 3A,C–E) elliptical, slightly divergent, each ramus about 1.7 times as long as wide; dorsal keel present, with long acute extension surpassing insertion of dorsal seta VII (Figure 3C–E); few spinules at base of seta II; longitudinal row of spinules between insertion of seta II and seta III on outer margin, row of robust spinules on inner distal margin.
Seta I minute, inserted just below insertion of seta II (Figure 3C–E). Seta II at 1/3 ramus length; seta III at 2/3 ramus length; setae II and III subequal in length. Seta IV swollen at base, with no breaking plane, about twice as long as seta II and III. Seta V well developed, about 0.9 times as long as body length and about three times as long as seta IV. Seta VI about 2/3 length of seta IV. Seta VII articulate, inserted at 2/3 ramus length, as long as seta II and III.
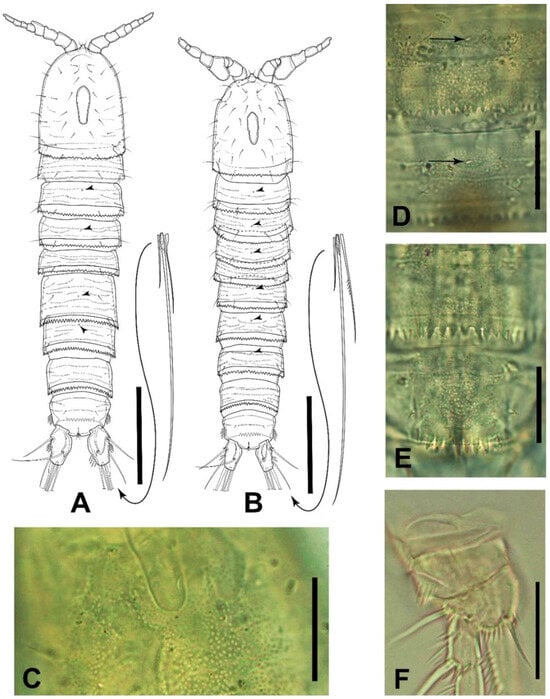
Figure 2.
Elaphoidella phuphamanensis sp. nov., paratypes (A,B), holotype (C–E), and allotype (F). (A) female habitus, dorsal view; (B) male habitus, dorsal view; (C) surface of female cephalothorax with refractile points and the distal part of the nuchal organ, dorsal view; (D) female P2- and P3-bearing somites, dorsal view; (E) female urosomite 4 and anal somite, dorsal view; (F) male P1, anterior view. Arrowheads on plates A, B, and D indicate middorsal pores. Scale bars: (A,B) 100 μm; (C–F) 25 μm.
Antennule (Figure 1A and Figure 4A,B) short, 8-segmented. Armature formula: I-[1], II-[9], III-[5], IV-[1 + (1 + ae)], V-[1], VI-[3], VII-[2], VIII-[6 + (1 + ae)]. Segment I with short row of spinules medially. Aesthetasc on segments IV and VIII fused at base to nearby seta, forming acrotheck; all aesthetascs shorter than accompanying seta; aesthetasc on segment IV surpassing tip of ultimate segment.
Antenna (Figure 4C) biramous, comprising coxa, allobasis, 1-segmented Exp, and 1-segmented Enp. Coxa short, unornamented. Allobasis with row of long spinules at 1/2 length on inner margin of segment. Exp short, with two bipinnate setae apically, two unipinnate setae laterally. Enp with two rows of spinules and two robust spines on medial inner margin; six elements apically: three longest apical ones geniculate, two medial ones spiniform, outermost apical one shortest, slender.
Mandible (Figure 4D) comprising coxa with well developed gnathobase and 1-segmented Enp. Gnathobase sclerotized, cutting edge with two bicuspidate and four multicuspidate teeth, accompanied by bipinnate lateral seta. Enp with one seta laterally and four setae apically on distal segment.
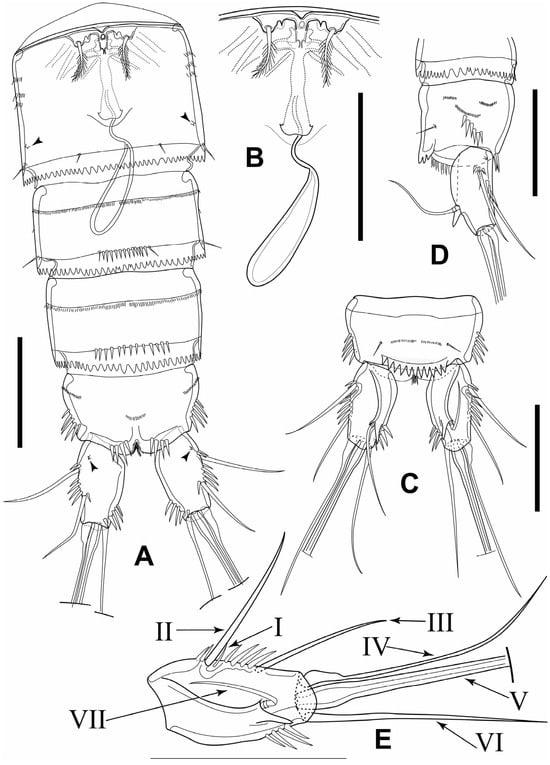
Figure 3.
Elaphoidella phuphamanensis sp. nov., female, holotype. (A) urosome, ventral view (arrowheads indicate integumental pores); (B) genital complex and P6, ventral view; (C) anal somite and caudal rami, dorsal view; (D) anal somite and caudal rami, lateral view; (E) caudal ramus, dorsal view (Roman numerals: setae numbers after Huys and Boxshall []). Scale bars: 50 μm.
Maxillule (Figure 4E) comprising praecoxa, coxa, and basis with Enp and Exp completely incorporated into basis. Praecoxal arthrite robust, with seta on anterior surface and six elements apically: five of them strong curved spines; lateral one setiform, bipinnate. Coxa with cylindrical endite with two setae apically: one robust, bipinnate; other smooth. Basis with three setae apically: one robust, bipinnate; two smooth, slim. Enp and Exp completely incorporated into basis, represented by four setae.
Maxilla (Figure 4F) comprising syncoxa, allobasis, and Enp fully incorporated to the latter. Syncoxa with two rows of spinules on outer margin, with two endites, each with three elements of which proximalmost robust, spinulose; other two weak, setiform. Allobasis drawn out into strong claw, with proximal seta on posterior surface. Enp completely incorporated into allobasis, represented by one smooth seta.
Maxilliped (Figure 4G) subchelate, 3-segmented, comprising syncoxa, basis, and Enp. Syncoxa with few spinules on inner distal corner. Basis with longitudinal row of spinules on anterior and posterior surfaces; two or three groups of spinules on outer margin. Enp represented by strong claw-like spine, with proximal slender seta at its base.
P1–P4 (Figure 5A–D) comprising praecoxa, coxa, basis; 3-segmented Exp; Enp P1 3-segmented, P2–P3 2-segmented, P4 1-segmented, respectively (for details see Table 1). Intercoxal plates concave; coxa rectangular (P1) or trapezoidal (P2–P4) with row of strong spinules on outer distal corner; basis with integumental pore on anterior surface, with strong spinules at base of outer element and row of spinules at base of Enp. Armature formula of P1–P4 as in Table 1.

Table 1.
Armature formula of P1–P4 of Elaphoidella phuphamanensis sp. nov. (female and male combined). (Legend: outer–inner element; outer–apical–inner element; Arabic numerals: number of setae; Roman numerals: number of spines).
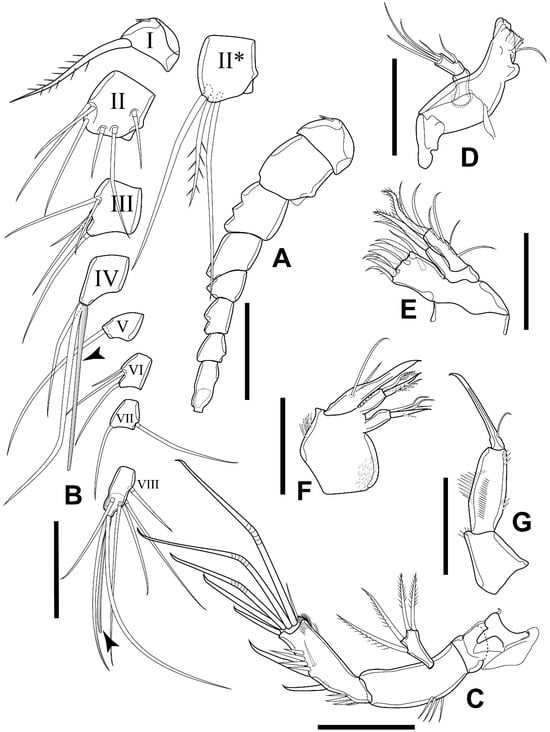
Figure 4.
Elaphoidella phuphamanensis sp. nov., female, holotype. (A) A1 without setae; (B) A1 with setae and aesthetascs (arrowheads indicate aesthetascs; II*, ventral view); (C) A2; (D) mandible; (E) maxillule; (F) maxilla; (G) maxilliped, posterior view. Scale bars: 50 μm.
P1 (Figure 5A) intercoxal plate bare, concave, relatively wide. Coxa short, rectangular, with row of robust spinules near outer margin. Basis with inner spine as long as 3/4 of Enp-1; outer seta unipinnate. Segments of Exp 1–3 about 1.5 times as long as wide; Exp-3 with one unipinnate spine on outer margin; three elements apically: outer one unipinnate spine, inner two geniculate setae. Enp slightly surpassing tip of Exp; Enp-1 about three times as long as wide, reaching distal margin of Exp-2; Enp-2 twice as long as wide, with unilateral brush-like seta on inner distal corner; Enp-3 about 1.5 times as long as wide; outer margin bare; three elements apically: outer unipinnate spine, median geniculate seta, inner short slender seta.
P2 (Figure 5B) intercoxal plate with arcuate row of spinules on each side. Basis like P1 but without inner spine; outer margin with short seta. Outer spine of Exp-1 and Exp-2 smooth, inner seta on Exp-2 with unilateral brush-like tip. Exp-3 about 2.5 times as long as wide, with five elements: outer lateral and sub-apical spine, both unipinnate; two setae unequal in length apically of which innermost longer; inner lateral seta at about 1/2 length of segment, with unilateral brush-like tip. Enp-1 short, wider than long, bare. Enp-2 about twice as long as wide, with two elements apically: outer one spiniform, shorter than supporting segment, inner one bipinnate seta slightly longer than supporting segment.
P3 (Figure 5C) intercoxal plate bare. Coxa, basis and Exp as in P2, but outer seta of basis comparatively longer. Enp-1 bare, short. Enp-2 about 2.5 times as long as wide, with five elements: outer spine subapically; bipinnate spiniform medial seta as long as supporting segment, long distal inner seta with unilateral brush-like tip apically; two short bare setae inserted close to each other at 1/2 length of inner margin.
P4 (Figure 5D) intercoxal plate, coxa, basis and Exp as in P3, but without spinules at base of Enp. Exp-1 about 1.5 as long as wide, with few strong spinules on outer margin. Exp-2 with few strong spinules on outer margin; Exp-3 about 1.5 times as long as wide, more robust than that of P2 and P3. Enp 1-segmented, small, as long as wide, with one bipinnate spiniform seta apically.
P5 (Figure 5E) with left and right legs distinctly separated. Baseoendopod moderately developed, with long bare outer seta; endopodal lobe with four slender bipinnate setae of which outermost shortest, inner apical longest; innermost and outer apical seta subequal in length. Exp separated from baseoendopod, with bare margins, as long as wide, reaching tip of baseoendopodal lobe, with three smooth setae apically; outermost shortest, innermost longest; innermost about three times as long as outermost.
P6 (Figure 3A,B) reduced to minute plate on anterior margin of genital double somite ventrally, each leg with one plumose seta on peduncle.
Description of adult male. Total body length, excluding caudal setae, 428–441 µm (mean = 432; N = 3). Preserved specimens colorless. Prosome about 1.2 times as long as urosome (Figure 2B). Cephalothorax longer than wide, as long as rest of prosome. Rostrum and surface ornamentation of prosome as in female.
Urosome (Figure 2B and Figure 6A,B) comprising P5-bearing somite, genital somite with P6, three free abdominal somites and anal somite with caudal rami. All somites, except for anal somite, with hyaline free posterior margin serrated. Anal somite with a group of 3–4 spinules at the base of each furcal ramus ventrally. P5-bearing somite short. Genital somite bearing reduced plate ventrally, representing remnants of P6; unarmed, unornamented. First abdominal somite with pair of sensilla dorsally and single pore middorsally (Figure 2B). Anal somite and operculum as in female.
Caudal ramus (Figure 6B) about 1.8 times as long as wide, similar to that of female, but: (1) without spinules on medial margin distally, (2) row of spinules between seta II and seta III with smaller number of spinules compared to female, (3) seta IV longer than in female, about 2.4 times as long as seta II and seta III. Seta V about 0.7 times as long as body length and about 2.6 times as long as seta IV. Seta VII articulated, inserted at 2/3 ramus length, as long as seta II and III.
Antennule (Figure 6C,D) subchirocerate, 9-segmented, with geniculated articulation between segments VI and VII. Segment V bulbous. Armature formula: I-[1], II-[9], III-[3], IV-[2], V-[5 + (1 + ae)], VI-[2], VII-[0], VIII-[1], IX-[8 + (1 + ae)]. Aesthetascs on segments V and IX at base fused with seta (forming acrotheck).
The rostrum, antenna, mouthparts, and P1 (Figure 7A) as in females.
The P2 (Figure 7B) Exp as in females. Enp-1 is half as long as wide and unarmed. Enp-2 subeliptical with a sharp distal part, unarmed. There four strong spinules along the outer margin increasing in length toward the tip.
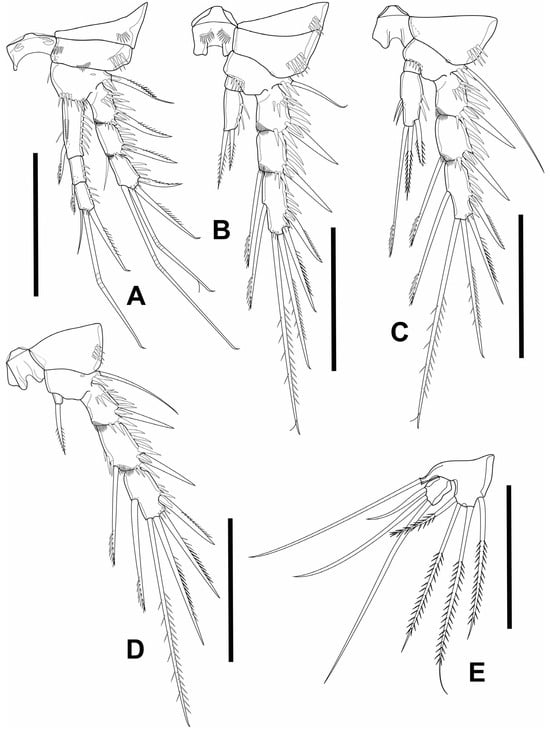
Figure 5.
Elaphoidella phuphamanensis sp. nov., female, holotype. (A) P1; (B) P2; (C) P3; (D) P4; (E) P5. Scale bars: 50 μm.
P3 (Figure 7C) coxa, basis and Exp as in female; but Exp-3 with cuticular pore near insertion of inner seta with unilateral brush-like tip. Enp 3-segmented; Enp-1 short, unarmed; Enp-2 drawn out into long apophysis with harpoon-like tip; Enp-3 with long sword-like spine apically.
P4 (Figure 7D) similar to female, but Exp-3 shorter, about 1.2 times as long as wide, robust, with one subapical and one apical element transformed into antler-like spines.
P5 (Figure 6A) baseoendopods fused into a simple plate, distinctly separated from somite, with integumental pore and long smooth seta on outer margin of each lobe. Exp small, well separated from baseoendopod, as long as wide, with two smooth long setae apically; inner about twice as long as outer.
P6 (Figure 6A) reduced, forming a simple plate with smooth free margin; without armature or ornamentation.
Variability. The median spinule row is present on the ventral surface of urosomite 4 in three individuals, but is absent in the allotype.
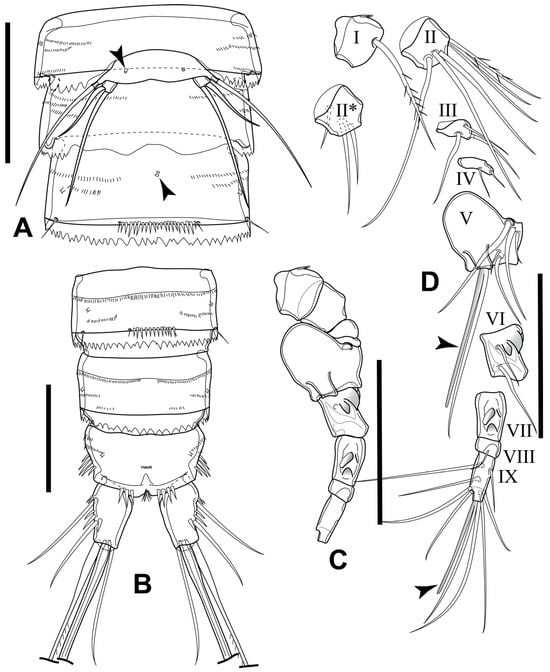
Figure 6.
Elaphoidella phuphamanensis sp. nov., male, allotype. (A) urosomites 1–3, ventral view (arrowheads indicate integumental pores); (B) urosomites 4–6, ventral view; (C) A1, without setae; (D) A1, with setae and aesthetascs (arrowheads indicate aesthetascs, II*, ventral view). Scale bars: 50 μm.
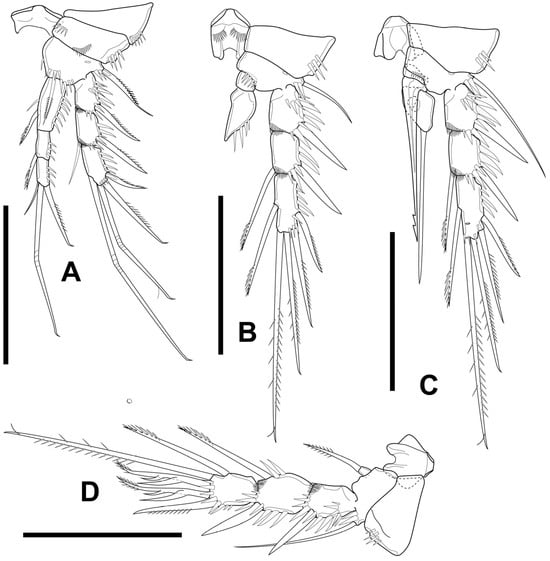
Figure 7.
Elaphoidella phuphamanensis sp. nov., male, allotype. (A) P1; (B) P2; (C) P3; (D) P4. Scale bars: 50 μm.
Distribution. The new species is known from the type locality only. Specimens of the new species were collected in two of the three sampling sites separated by 10 m at the entrance of the cave (Figure 1C,D).
Ecology. The cave is a simple horizontal gallery, about 150 m long, with a Buddhist temple in the entrance hall. A concrete pool with a volume of about 1 m3 (with about 100 L of water during sampling) and a black plastic bucket (with about 10 L of water) are filled intensively during the rainy season with dripping water from the ceiling, but a small amount of permanent dripping water is present during the dry season. On the sampling date, the water in both locations was colorless, with a temperature of 25.2 °C, a pH of 7.9, and an electric conductivity of 668 µS cm−1. A small amount of guano (bat excrement) was present on both sites. Another species, E. isana Watiroyram, Sanoamuang and Brancelj, 2021, was collected in the concrete pool, too. The abundance of the new species was higher. One hundred and eighty out of about two hundred individuals of Elaphoidella belong to the new species, and about twenty individuals belong to E. isana. Deeper into the cave, i.e., at the third sampling point, Atthyella thailandica Watiroyram, 2021, was collected from several small pools (with a volume of a few milliliters) on the rocks and concrete floor filled with dripping water. The third sampling point was located under a large opening at the top of a gallery with walls covered with wet moss and shrubs, about 50 m from the type locality of the new species.
Differential diagnosis and remarks. Based on Lang [], the new species belongs to species-group VII (bromeliaecola group) with the following characteristics: (1) 3-segmented P1 Enp in both sexes; (2) P3–P4 Exp-3 with inner marginal seta; (3) armature of P2–P4 Exp-3 in both sexes as 5.5.5.; (4) the male P4 Exp-3 with two antler-like transformed spines, one positioned subapically and one apically; (5) the baseoendopodal lobe of the female P5 being relatively long and bearing relatively long setae. Within known species of Elaphoidella, 16 species and subspecies are included in this group, excluding the new species (Table 2).

Table 2.
List of copepod taxa included in Elaphoidella group VII (bromeliaecola group), with their habitats and distribution worldwide.
Among the representatives of this group, the new species is most similar to Elaphoidella turgisetosa Petkovski, 1980 in having: (1) the male with five elements on P4 Exp-3; (2) the female with two and one apical elements on P2 and P4 Enp, respectively; and (3) the male with two apical setae on P5 Exp and the female with three and four marginal setae on P5 Exp and the baseoendopodal lobe, respectively. Furthermore, the shape of P5 and the caudal rami look similar between both species. The lack of armature element(s) on the terminal segment of the male P2 Enp, as observed in the new species, was found also in Elaphoidella crenobia []. However, they are easily distinguished from each other based on the configuration of the female P4 and P5. P4 Enp is 1-segmented with one apical element in the new species, whereas it is 2-segmented in E. crenobia. In the new species, the baseoendopodal lobe and Exp of the female P5 have four marginal and three robust setae, respectively, but there are two marginal setae and four setae in E. crenobia, respectively. Furthermore, the new species has P1 with a 3-segmented Enp, but it is 2-segmented in E. crenobia.
Five species of the bromeliaecola group have been recorded so far from Southeast Asia: E. bromeliaecola [], E. thienemanni [], E. laboni [], E. sanoamuangae [], and E. phuphamanensis sp. nov. The new species is distinct from the other four species by having 1-segmented instead of 2-segmented P4 Enp in both sexes (Table 3). Comparing the key for the males of the species of Elaphoidella [] and original descriptions [,,,], the armature element(s) on the terminal segment of the male P2 Enp, the presence of one element on Enp-3 with a sword-like spine, and the armament and shape of P5 in both sexes are unique to the new species (Table 3). Based on the mentioned characteristics, the new species differs from the other four species in (1) the absence of the armature element on the male P2 Enp, which is present in other species; (2) the male P5 Exp with two setae, while there are three in most other species and two or three in E. laboni; (3) the female P5 Exp with three elements, while there are four elements in other species; and (4) the absence of prominence at the tip of the female P5 baseoendopod where seta II is inserted, which is present in other species.

Table 3.
Morphological comparison of five Southeast Asian species of Elaphoidella group VII (bromeliaecola group) sensu Lang [].
To date, a 1-segmented P4 Enp is known to be present in members of species-group X (armata group) sensu Lang [] and Stygoelaphoidella sensu Apostolov []. However, members of the latter groups lack the transformed spine in the male P4 Exp-3 []. Furthermore, the P1 Enp of the new species is 3-segmented and the P4 Enp possesses only one apical seta, making it different from other members of the armata group and Stygoelaphoidella, which possess 2-segmented P1 Enp and three armature elements on P4 Enp, respectively.
Besides the structure of P4 Enp, the armature complement of P2–P4 Exp-3 as 5.5.5, and the chaetotaxy of P5 in both sexes, the new species also shows a close affinity to the Romanian cave-dwelling species E. winkleri (Chappuis, 1928) [], previously defined as a member of the genus Neoelaphoidella []. The new species differs from E. winkleri in having the following characteristics: (1) P1 with a 3-segmented Enp in the new species, but 2-segmented in E. winkleri; (2) the unarmed male P2 Enp in the new species, while there are two setae in E. winkleri; and (3) the shape of the outermost seta on the female P5 Exp being the shortest in the new species, but the median is the shortest in E. winkleri. Furthermore, the endopodal lobe and Exp of the female P5 are relatively shorter in the new species compared to E. winkleri.
3.2. Elaphoidella propecabezasi sp. nov.
Material examined. Holotype: 1 ♀ (adult), length 654 μm; completely dissected and mounted on a slide in glycerol, covered with a cover slip, and sealed with nail polish; access number: THNHM-IV-20356. Allotype: 1 ♂ (adult), length 593 μm; completely dissected and mounted on a slide in glycerol, covered with a cover slip, and sealed with nail polish; access number: THNHM-IV-20357. Paratypes: 1 ♀ (adult), 1 ♂♀ (adult intersex), length 649 μm and 605 μm, respectively; completely dissected and mounted on a slide in glycerol, covered with a cover slip, and sealed with nail polish; access numbers: THNHM-IV-20358–20359, respectively. Additional material: 3 ♀♀ (adults), 2 ♂♂ (adults); preserved in 70% ethanol; retained in the collection of the first author (CB). All specimens were collected at the type locality on 2 April 2023, by A. Brancelj, L. Sanoamuang, and N. Sanoamuang.
Type locality. A captated karstic spring in Wat Tham Bodhisattva Temple, used for water supply for the temple; Nong Hin District, Loei Province, northeast Thailand; coordinates of the spring: 17°05′13.19″ N, 101°46′55.59″ E, 377 m a.s.l. (Figure 1A,B,E).
Etymology. The specific epithet is formed from the Latin adverb prope (i.e., nearly, close to), referring to the close affinity to E. cabezasi. The name is a noun in the genitive case.
Description of adult female. Total body length, excluding caudal setae, 649–666 µm (mean = 659; N = 4). Preserved specimens colorless. Habitus cylindrical, slightly tapering posteriorly, surface with numerous transverse rows of minute spinules dorsally and ventrally (Figure 8A,D and Figure 9A). Rostrum short, completely fused to cephalothorax. Prosome as long as urosome (including caudal rami), comprising cephalothorax and three free pedigerous somites (Figure 8A). Cephalothorax, as wide as the rest of prosome, about 1.3 times as long as wide and about half-length of prosome; integumental window (nuchal organ) well developed; hyaline free posterior margin serrated; sensilla distributed as illustrated (Figure 8A). Three subsequent pedigerous somites with two pairs of sensilla at posterior margin; hyaline free posterior margin of each segment serrated; with inconspicuous boundary between prosome and urosome (Figure 8A).
Urosome comprising P5-bearing somite, genital double somite, two free abdominal somites, and anal somite with caudal rami (Figure 8A and Figure 9A). All urosomites, except for anal somite, with hyaline free posterior margin serrated; P5-bearing somite wider than long, pair of sensilla dorsally. Urosomites 2 and 3 completely fused, forming genital double somite, about 0.8 times as long as wide, with a pair of sensilla dorsally (Figure 8A); ventral surface with fused plate bearing P6 anteriorly; pair of integumental pores next to copulatory pore posteriorly (Figure 9A,B).
Genital complex bell-shaped, sclerotized, with large copulatory pore; seminal receptacle small, sclerotized (Figure 9A,B). Urosomites 4 and 5 with row of spinules near posterior margin ventrally; dorsal surface without spinules. Anal somite (Figure 8D and Figure 9A,C) with pair of sensilla at base of operculum, with several transverse rows of minute spinules dorsally and ventrally; row of 3–5 spinules ventrally, near base of each caudal ramus. Anal operculum broadly convex; free margin with 22–25 short spinules.
Caudal rami (Figure 8A,D and Figure 9A,C) slightly conical, parallel, about 1.6 times as long as wide; dorsal keel present, with short, acute extension, slightly surpassing the insertion of seta VII; few spinules at base of seta III. Seta I minute, inserted below seta II (Figure 9C; see an asterisk). Seta II and seta III subequal in length, inserted at 1/2 and 2/3 length of ramus, respectively. Seta IV about 2.7 times as long as seta II and seta III. Seta V well developed, about 0.6 times as long as body length, without breaking plane. Seta VI bare, short. Seta VII articulated, inserted at 2/3 length of ramus, as long as seta II and seta III.
Antennule (Figure 10A) short, 8-segmented. Armature formula: I-[1], II-[9], III-[5], IV-[1 + (1 + ae)], V-[1], VI-[3], VII-[2], VIII-[6 + (1 + ae)]. Segment I with row of spinules at 1/2 segment length. Aesthetascs on segment IV and VIII fused with seta arising nearby (forming acrotheck); all aesthetascks slightly shorter than accompanying seta; aesthetasc on segment IV surpassing tip of ultimate segment.
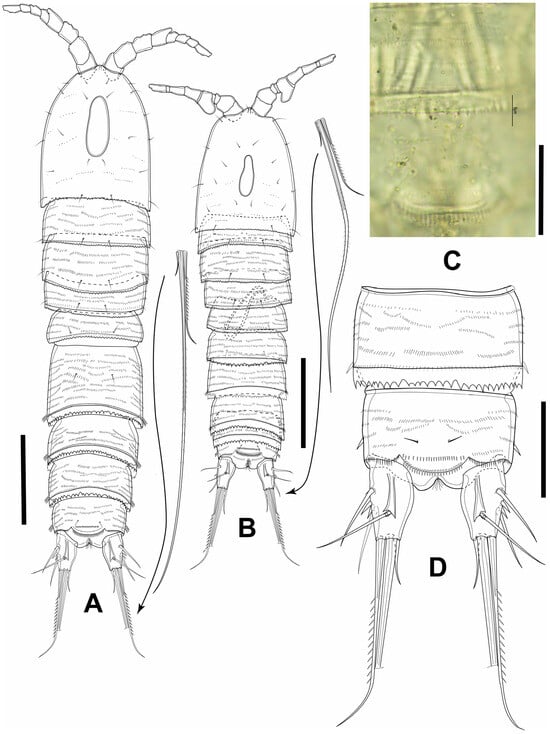
Figure 8.
Elaphoidella propecabezasi sp. nov. paratypes (A,B) and holotype (C,D). (A) female habitus, dorsal view; (B) male habitus, dorsal view; (C) female anal somite, dorsal view; (D) urosomite 4 and anal somite, dorsal view. Scale bars: (A,B): 100 μm; (C): 25 μm; (D): 50 μm.
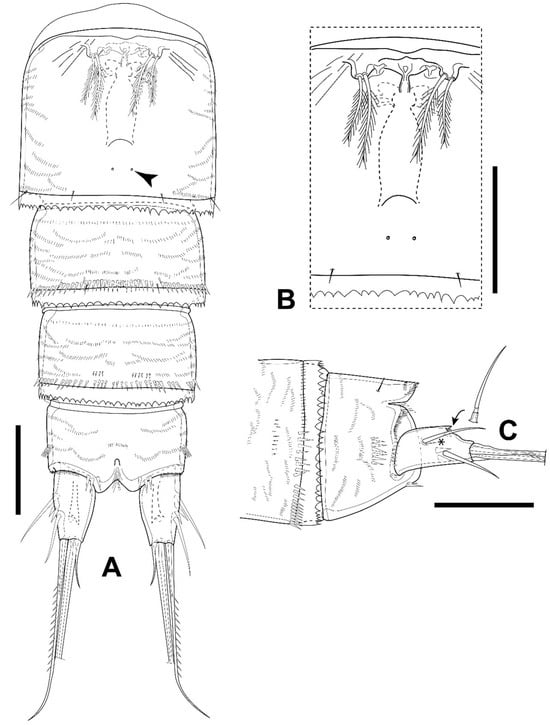
Figure 9.
Elaphoidella propecabezasi sp. nov., female, holotype. (A) urosomites 2–5 and anal somite, ventral view (arrowhead indicates integumental pore); (B) P6 and genital complex, ventral view; (C) anal somite and caudal ramus, lateral view (an asterisk indicates seta I and an arrow indicates the posterior tip of the dorsal keel). Scale bars: 50 μm.
Antenna (Figure 10B) biramous, comprising coxa, allobasis, 1-segmented Exp and 1-segmented Enp. Coxa short, unornamented. Allobasis with row of long spinules at 1/2 length of segment. Exp about three times as long as wide, with two unipinnate setae apically and two bipinnate ones laterally. Enp with two rows of strong spinules proximally and subdistantly, two robust spines on inner margin; six elements apically: three longest one geniculate, bare; two medial ones spiniform, unequal in length; outermost one shortest, slender. Inner geniculate seta with two spinules inserted medially.
Mandible (Figure 10C) comprising coxa with well developed gnathobase, basis and 1-segmented Enp. Gnathobase sclerotized; cutting edge with one bicuspidate, one tricuspidate and four multicuspidate teeth, accompanied by pinnate seta laterally. Basis about four times as long as wide; Enp with one bare lateral seta, accompanied by few setules laterally; four bare setae apically.
Maxillule (Figure 10D) comprising praecoxa, coxa, and basis, with Enp and Exp completely incorporated into the latter. Praecoxal arthrite robust, with surface seta on anterior surface and six robust spines apically. Coxa with cylindrical endite with two setae apically, one robust and pinnate; fused with basis. Basis with two setae apically, one laterally, of which inner apical one robust and bipinnate. Enp and Exp completely incorporated into basis, represented by four setae.
Maxilla (Figure 10E) comprising syncoxa, allobasis, and Enp. Syncoxa with two rows of spinules on outer margin, with two endites, each with three elements apically of which proximal one robust and spinulose, other two weak and setiform. Allobasis drawn-out into robust claw, with one accompanying seta. Enp completely incorporated into allobasis, represented by smooth seta.
Maxilliped (Figure 10F) subchelate, 3-segmented, comprising syncoxa, basis, and Enp. Syncoxa with transwerse row of spinules on anterior surface. Basis about three times as long as wide, with long longitudinal row of inner spinules; with one proximal and one subdistal groups of spinules on outer margin. Enp 1-segmented, drawn-out into robust claw, with minute seta near its base.
P1–P4 (Figure 11A–D) comprising praecoxa, coxa, basis, 3-segmented Exp and 3, 2, 2, 2-segmented Enp, respectively (for details see Table 4). Intercoxal sclerites bare, concave. Praecoxae triangular, each with row of spinules on outer distal corner; coxae rectangular, each with row of strong spinules on outer distal corner and three rows of minute spinules on anterior surface; bases with integumental pore on anterior surface, with strong spinules at base of outer element and a row of spinules at base of Enp. Armature formula of P1–P4 as in Table 4.

Table 4.
Armature formula of P1–P4 of Elaphoidella propecabezasi sp. nov. (female and male combined). (Legend: outer–inner element; outer–apical–inner element; Arabic numerals: number of setae; Roman numerals: number of spines).
P1 (Figure 11A) intercoxal plate bare, concave, relatively short. Coxa about 0.5 times as long as wide. Basis with row of long spinules on medial margin; outer element spiniform, bipinnate; inner spiniform seta slim, bipinnate, about 2/3 length of Enp-1. Exp segments 1–3 about 1.5 times as long as wide; outer spine of Exp-1 and Exp-2 unipinnate, slightly longer than supporting segment; Exp-3 with four elements: outer and outer apical spine, two geniculate setae apically. Enp well surpassing tip of Exp; Enp-1 about four times as long as wide, reaching distal margin of Exp-2, seta on inner margin with unilateral brush-like tip; Enp-2 about three times as long as wide, with several robust spines along inner and outer margin; Enp-3 about three times as long as wide, with three elements apically: outer unipinnate spine, median geniculate seta, inner short slender seta.
P2 (Figure 11B) coxa similar to that of P1, but distal margin without row of spinules. Basis with row of long spinules on median margin; outer element spiniform. Exp-1 about 1.5 times as long as wide, with one spine on outer margin. Exp-2 about 1.5 times as long as wide, with one spine on outer margin and one seta on inner margin. Exp-3 about 3.5 times as long as wide, with five elements: two robust spines along outer margin; apically one spiniform seta, one unipinnate median seta, one plumose inner seta, as long as Exp; seta on inner margin with unilateral brush-like tip. Enp-1 short, wider than long, bare. Enp-2 about 3 times as long as wide, with five elements: one outer spine, slightly shorter than segment; two plumose setae equal in length apically; inner two setae with unilateral brush-like tips.
P3 (Figure 11C) similar to P2, but basis lacks inner row of long spinules, with long bare seta on outer margin.
P4 (Figure 11D) Exp similar to P3, except distal seta on inner margin with unilateral brush-like tip, which is plumose on P3. Enp-2 about three times as long as wide, with four elements: short bipinnate spine subapically on outer margin; long plumose seta apically; two spiniform setae with unilateral brush-like tips on inner margin.
P5 (Figure 11E) with left and right leg distinctly separated. Baseoendopod well developed, with outer basal seta long and bare; endopodal lobe with four robust pinnate setae: outermost one shortest, other three long, equal in length. Exp about twice as long as wide, well overreaching tip of baseoendopod, with three robust bipinnate setae: outermost shortest; outer seta apically as long as supporting segment, inner seta apically longest, about three times as long as outer one.
P6 (Figure 9A,B) reduced to minute prominence on anterior margin of genital double somite ventrally, each leg with two plumose setae on peduncle.
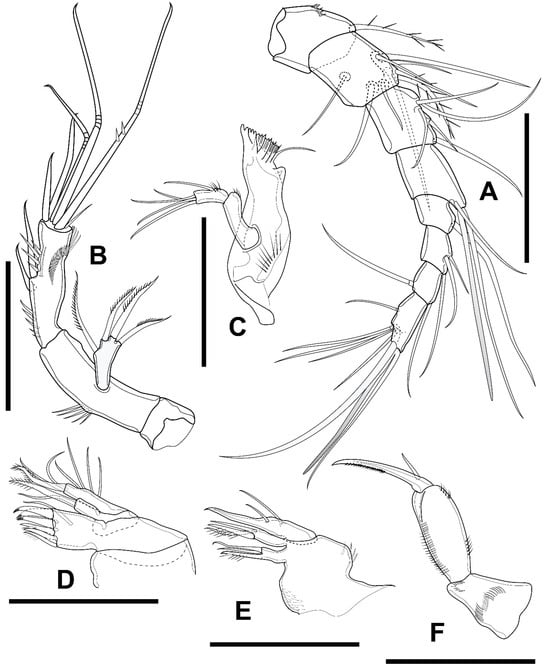
Figure 10.
Elaphoidella propecabezasi sp. nov., female, holotype. (A) A1; (B) A2; (C) mandible; (D) maxillule; (E) maxilla; (F) maxilliped. Scale bars: 50 μm.
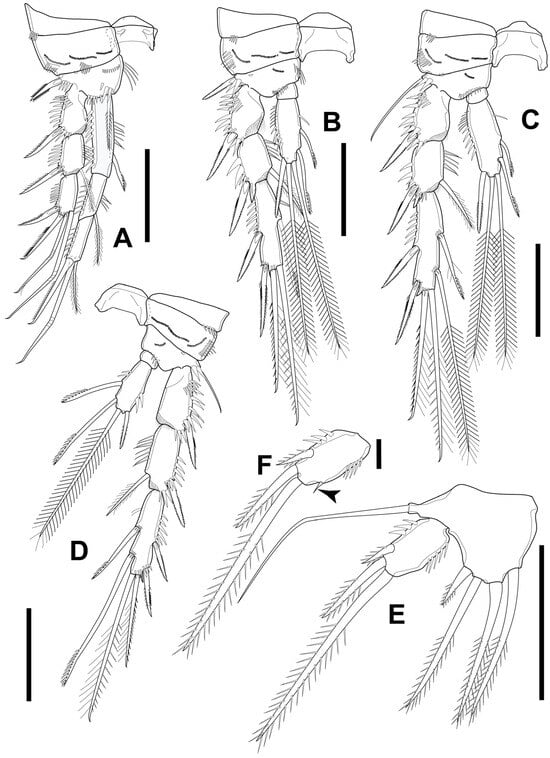
Figure 11.
Elaphoidella propecabezasi sp. nov., female, holotype (A–E) and paratype (F). (A) P1; (B) P2; (C) P3; (D) P4; (E) P5; (F) P5 Exp (arrowhead indicates additional seta). Scale bars: (A–E): 50 μm; (F): 5 μm.
Description of adult male. Total body length, excluding caudal setae, 590–605 µm (mean = 598; N = 4). Preserved specimens colorless. Prosome as long as urosome (Figure 8B). Cephalothorax longer than wide, about half length of prosome. Rostrum and surface ornamentation of prosome as in female.
Urosome (Figure 8B and Figure 12A) 6-segmented, comprising P5-bearing somite, genital somite (i.e., P6-bearing somite), three free somites and anal somite with caudal rami. All somites, except anal somite, with serrated hyaline free posterior margin. Genital somite ventrally with reduced plate representing P6; several rows of spinules near posterior margin laterally and ventrolaterally. Urosomites 3 and 4 with continuous row of spinules near posterior margin laterally and ventrally. Urosomite 5 and anal somite as in female (Figure 9A and Figure 12A).
Caudal rami (Figure 12A) as in female, but slightly longer in male, about 1.7 times as long as wide. Seta IV about 4.3 times as long as seta II and seta III. Seta V about 0.6 times as long as body length and about 2.8 times as long as seta IV.
Antennule (Figure 12B, C) subchirocerate, 9-segmented; geniculated articulation between segments VI and VII. Segment V bulbous, with acrotheck on peduncle. Armature formula: I-[1], II-[9], III-[3], IV-[2], V-[5 + (1 + ae)], VI-[2], VII-[0], VIII-[1], IX-[8 + (1 + ae)]. Aesthetascs on segments V and IX at base fused with seta, forming acrotheck. Aesthetasc on segment V surpassing ultimate segment.
Rostrum, antenna, mouthparts, and P1 as in female.
P2 (Figure 13A) as in female, but Enp-2 without spine on outer distal margin.
P3 (Figure 13B) coxa, basis and Exp as in female, but Exp-3 with cuticular pore near insertion of outer proximal spine. Enp 3-segmented; Enp-1 short, unarmed; Enp-2 drawn-out into apophysis, reaching 1/2 length of Exp-3, with harpoon-like tip; Enp-3 with one seta subapically and one seta apically.
P4 (Figure 13C) Exp-3 relatively shorter than in female. Enp-2 with three elements: outer pinnate spine and long plumose seta apically, spiniform seta subapically on inner margin, bare, slightly longer than outer spine.
P5 (Figure 12A) left and right baseoendopod fused into uniform plate, distinctly separated from somite. Baseoendopod with long smooth outer basal seta, with no armature on inner margin. Exp small, well separated from baseondopod, as long as wide, with three elements: outermost as short thin bare seta subapically; two robust bipinnate spines apically, of which inner one about twice as long as outer.
P6 (Figure 12A) reduced to simple plate, unarmed and unornamented; fused to somite.
Variability. A minute seta was observed on the inner margin of the right P5 Exp of the paratype (Figure 11F). One individual, recognized as intersex, was found with prevailing male characters. For details, see the next paragraph.
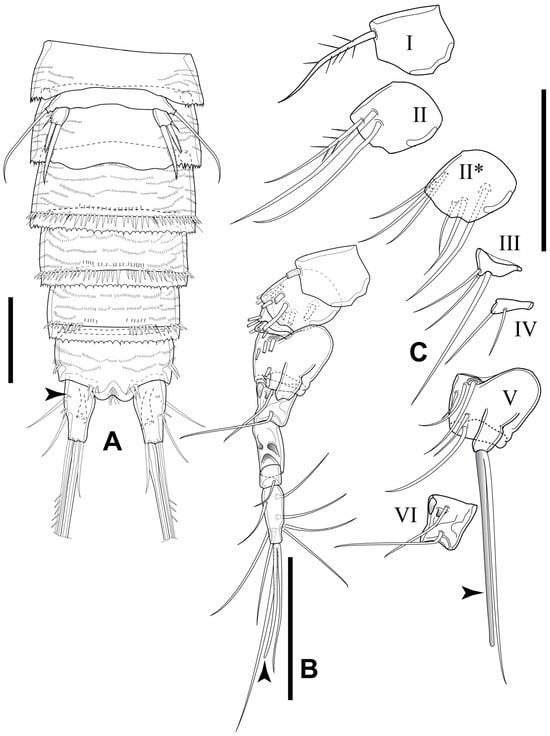
Figure 12.
Elaphoidella propecabezasi sp. nov., male, allotype. (A) urosome, ventral view (arrowhead indicates integumental pore); (B) A1, left side, complete appendage with partial figured armature (arrowhead indicates aesthetasc on segment IX); (C) details of setation on segments I–VI (arrowhead indicates aesthetasc on segment V; II*, ventral view). Scale bars: 50 μm.
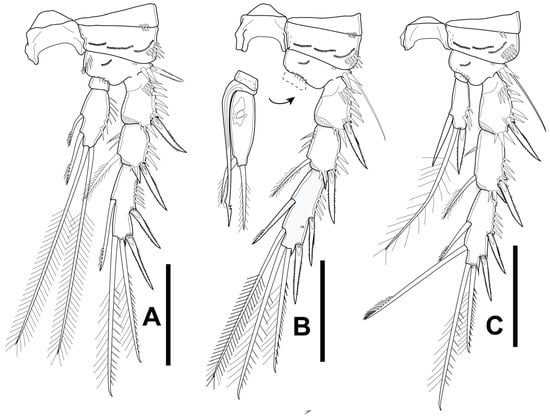
Figure 13.
Elaphoidella propecabezasi sp. nov. male, allotype. (A) P2; (B) P3; (C) P4. Scale bars: 50 μm.
3.3. Gynandromorphic Specimen
One individual displayed characteristics of both sexes, with the majority of them resembling the male, while parts of A1, P5, P6, and the reproductive system resembled those of the female. The body size and appendages are slightly larger than in the allotype. The sixth thoracic somite and the first abdominal somite in the gynandromorphic specimen are completely separated dorsally, as in the normal male (Figure 14A), but fused ventrally as in the normal female (Figure 14B). The seminal receptacle, characteristic of the normal female, is present but deformed (Figure 14B). The left and right P5 baseoendopodal lobes of the gynandromorphic specimen have one pinnate and two smooth spiniform setae, respectively (Figure 14B), but four robust pinnate setae, unequal in length, are present in the normal female (Figure 11E) and are absent in the normal male (Figure 12A). The P5 Exp in the gynandromorphic specimen has a setiform and smooth outer seta (Figure 14B), as in the normal male (Figure 12A), compared to a spiniform and pinnate seta in the normal female (Figure 11E).
The left and right P6 in the gynandromorphic specimen are armed with one and two setae, two smooth and one with robust spinules on the inner side, respectively, inserted on a deformed peduncle (Figure 14B), which is represented by two plumose setae in the normal female and absent in the normal male. The P4 Enp-2 is as in the male, but the inner element in the gynandromorphic specimen is represented by a long seta, with a unilateral brush-like tip characteristic of the female (Figure 14D), while the male has a bare and straight spine there (Figure 13C).
The antennule in the gynandromorphic specimen is eight-segmented, with a bulbous V segment characteristic of the normal male, but this is nine-segmented in the normal male, while an eight-segmented antennule is characteristic of the normal female (Figure 12B).
Antenna, mouthparts, and P1–P3 are normally developed, resembling those of the male.
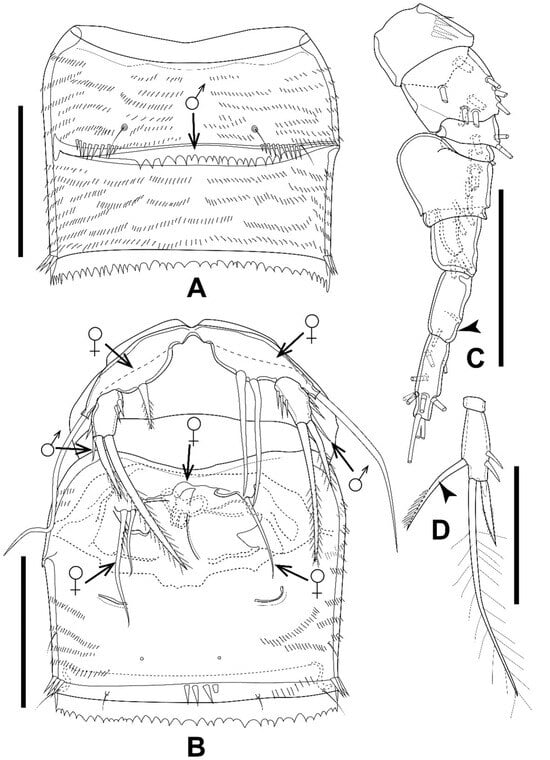
Figure 14.
Elaphoidella propecabezasi sp. nov. gynandromorphic specimen. (A) genital double somite, dorsal view (arrow indicates the separation in the normal male); (B) genital double somite, ventral view (arrows indicate characteristics different from the normal male (♂) or female (♀)); (C) A1; left side, complete appendage with a partially figured armature (arrowhead indicates partly fused segment 8); (D) P4 Enp-2 (arrowhead indicates the spine, different from that of the normal male). Scale bars: 50 μm.
Distribution. The new species is known only from the type locality.
Ecology. The new species was collected from a karstic spring just below a cave entrance. The mouth of the spring is located in a concrete reservoir (2 × 2 × 1 m) with a solid roof and a service entrance of 60 × 60 cm on one side. The bottom of the reservoir is covered with fine sand (grain size > 1 mm). No environmental parameters (i.e., temperature, pH, conductivity) were measured during sampling. Together with the new species of Elaphoidella, three species of Nitrocrella and one of Acanthocyclops sp. were collected.
Differential diagnosis and remarks. According to the absence of a transformed spine in the male P4 Exp-3, the female P5 bearing a relatively short baseoendopodal lobe with four marginal robust setae, and the configuration of the female P5, E. propecabezasi sp. nov. belongs to species-group I (similis group) sensu Lang []. In total, the group includes 32 species worldwide, three of them described from Thailand: E. margaritae Pesce and Apostolov, 1985 [], E. paraaffinis Watiroyram, Sanoamuang and Brancelj, 2017 [], and E. ligorae Watiroyram, Sanoamuang, and Brancelj, 2017 []. Based on elements on P2–P4, including the Enp-1 armament (0.0.0), Exp-3 elements (5.6.6), Enp-2 elements of the female (5.5.4), and the number of setae on the distal segment in the male P2–P4 Enp (4, 2, 3), the new species most closely resembles E. cabezasi Petkovski, 1982 [], from Cuba, and E. paraaffinis from Thailand. The combination of the armature of P2–P4 Enp-1 and Enp-2 is shared by these three species, distinguishing them from all other Elaphoidella species in the key of Harpacticoida [] and all 16 recently described species from Borneo (Indonesia), Colombia, Slovenia, Taiwan, and Thailand [,,,,]. The new species is distinct from the Cuban and Thai Elaphoidella in the characteristics of P5 in both sexes, the ornamentation of body segments, the anal operculum, and the shape of caudal rami (Table 4).
The new species resembles E. ligorae in the following characteristics in the female: (1) the relative length of the P1 Enp, (2) the armature of Exp-3 and Enp-2 of the female P1–P4, and (3) the shape of the caudal rami. However, the presence of an inner seta on P2 Enp-1 in both sexes and the armature of P2–P4 Enp-1 (1.0.0) in E. ligorae differ from the new species (0.0.0). Furthermore, the armature of the male P2 Enp-2 and the configuration of the male P5 Exp are different between these two species. The male of the new species bears four elements on P2 End-1 (five in E. ligorae) and three elements on P5 Exp (four in E. ligorae) (Table 4).
The first described groundwater-dwelling Thai Elaphoidella, E. margaritae, and the new species share characteristics of P2–P4 Enp-1 armature in the female and the armature of P5 in both sexes, but the armature of P2–P4 Enp-2 and the ornamentation of the anal operculum in the female are different in these two Thai representatives. Table 5 shows the morphological differences among the most closely related species of E. propecabezasi sp. nov.

Table 5.
Morphological characters of four Thai species and E. cabezasi from Cuba of Elaphoidella group I (similis group) sensu Lang [].
Elaphoidella margaritae differs from E. cabezasi in that (1) the female bears three elements on P4 End-2 but four in E. cabezasi; (2) the female and male each bear two elements on P5 Exp but four in both female and male in E. cabezasi; and (3) the male bears three elements on P2 Enp-2 but four in E. cabezasi. Furthermore, the free margin of the anal operculum is ornamented with strong spinules in E. margaritae but smooth in E. cabezasi.
4. Discussion
4.1. Presence of the Genus Elaphoidella in Thailand
The first groundwater-dwelling copepod described from Thailand was Elaphoidella margaritae (Pesce and Apostolov, 1985) [] found in a man-made well on Phuket Island. Afterward, there was a long-term gap with no records of groundwater-dwelling species. The break-through happened in 2010, when two new species, Elaphoidella namnaoensis Brancelj, Watiroyram, and Sanoamuang, 2010 [] and Asiacaris dispar Cottarelli, Bruno, and Berera, 2010 [], were described. Since then, the number of new Copepoda species has increased significantly—up to 36 at the moment. Among the new taxa, three new genera, Asiacaris Cottarelli, Bruno, and Berera, 2010 (Harpacticoida) [], Siamcyclops Boonyanusith, Sanoamuang, and Brancelj, 2018 (Cyclopoida) [], and Pseudohesperocyclops Brancelj, Boonyanusith, and Sanoamuang, 2024 (Cyclopoida) [] have been established so far.
Most of the new species originate from the epikarst zone. Actually, they were collected from pools filled with dripping water from the epikarst in the vadose zone, including “Budha pots” in the temples at the entrances of the caves, which also receive dripping water []. Only a few were collected from other types of groundwater, like karstic springs or hyporheic zone along the rivers.
Among groundwater-dwelling genera in Thailand (with an area of 513,000 km2), the genus Elaphoidella is the most copious, represented by 13 taxa (including two described in this paper), and it represents more than 1/3 of known stygobitic species (13 out of 34 species). In comparison, in Europe (with an area of about 10,180,000 km2), about 95 species and subspecies of the genus Elaphoidella are known at the moment []. In total, about 219 taxa are known worldwide [].
Most subterranean Copepoda species worldwide, including representatives of the genus Elaphoidella, show a narrow geographical distribution, i.e., they are narrow endemics. There are a few exceptions with a wider geographical distribution, but they could actually be cryptic species, i.e., similar in morphology but different in molecular fingerprint, supporting their endemic nature []. Frequently, subterranean species are recorded from a single location, actually only from the type locality. The reasons for limited knowledge of their distribution are (1) the low frequency of sampling; (2) the low number of specialists; (3) the specific sampling techniques that are required; and (4) the fragmented/patchy distribution of habitats.
In Thailand, about 170 caves have been sampled for Copepoda since 2010, where the same pattern was observed, i.e., most species were recorded in a single or only a few locations, with few exceptions []. This reflects the effect of the fragmented nature of the cave habitat []. While the fragmented nature of the cave habitat could be defined as an intrinsic factor causing the rapid increase in the number of species of groundwater-dwelling Copepoda, the extrinsic factor is an increased research interest in the diversity of subterranean fauna in Thailand.
Data show that the richness of Elaphoidella in SEA is equal to that of the Neotropical region, in which 36 species of Elaphoidella have been reported []. However, it is still lower than that of Europe, which is considered a hot spot for groundwater fauna []. More than 70 species of Elaphoidella have been reported there [,]. The present situation is not a real picture of the diversity of Elaphoidella in Thailand, or in SEA, as most of the areas and many groundwater-dependent habitats have not been investigated yet [].
Even though many new species have been described from Thailand, only four species of Copepoda have been found in the saturated karstic aquifer (from springs; i.e., Fierscyclops solaris Boonyanusith, Brancelj and Sanoamuang, 2013) or porous aquifers (from hyporheic zone and wells; i.e., Asiacaris dispar Cottarelli, Bruno, and Berera, 2010; Kinnecaris iulianae Bruno and Cotarelli, 2015, and Elaphoidella margaritae Pesce and Apostolov, 1985) [,,,]. The discovery of a new species, E. propecabezasi sp. nov., is the second record of the species from the saturated karstic aquifer. Although, the number of new species recorded from saturated karstic aquifers is presently low compared with that of other subterranean habitats, like epikarst, where a high biodiversity of copepods has been found. The high potential for new species from the phreatic zone is supported by the fact that an additional three new species of the genus Nitrocrella and one of the genus Acanthocyclops were also observed in the type locality of the new species (CB’s personal observation).
4.2. Gynandromorphic Specimen
In sexually reproducing animals, two types of male and female secondary characteristics in a single individual have been recognized: (1) hermaphrodite and (2) gynandromorph (also called intersex) []. Hermaphroditism is the normal condition of an individual in which both the male and female reproductive organs occur, both the male and female gametes can be produced, and, after fertilization, fertile offspring are produced. On the other hand, gynandromorphism is, either in a bilateral symmetric or haphazard pattern, defined as an abnormal condition of an individual in which a mix of male and female phenotypes occurs [].
Gynandromorphism has been reported in several Copepoda groups, most of them from the well-studied order Calanoida and less from the orders Cyclopoida or Harpacticoida []. However, the gynandromorphic individuals have been frequently overlooked as they are (1) small and thus difficult to record during routine work or (2) some researchers might consider them as a natural variation of the species. For that reason, relatively few cases of detailed studies of gynandromorphic copepods have been reported in comparison with other large-bodied crustacean groups, where the gynandromorphic individuals are easily recognized without special equipment (i.e., optical devices) []. So far, knowledge of gynandromorphism in copepods is known primarily from marine habitats [,]. Our finding is among the few records of gynandromorphic specimens in freshwater harpacticoids.
Harpacticoid species that have been reported with the gynandromorphic individuals in their populations include predominantly marine species like Paramphiascella hyperborea (Scott T., 1903), Stenhelia gibba Boeck, 1865, and Halectinosoma similidistinctum Lang, 1965. The species were collected from the Edinburgh coastline (Scotland). Amphiascoides debilis (Giesbrecht, 1881) was collected from Helgoland (Germany), Archisenia sibirica (Sars G. O., 1898) from East Greenland, and Lonchoeidestenhelia prote Gómez, 2020, from Sinaloa State (Mexico) [,,,,]. Among them, the morphological characteristics of the gynandromorphs can vary in the degree of expression. Few individuals possess the male characteristics in the antennule and P5, while most of the gynandromorphic harpacticoids display the female form on the prosome, a P5 intermediate between the male and female, and male characteristics on the P6 and urosome. In the case of A. debilis, the gynandromorph resembles the intersex material of L. prote []. It has a geniculated A1 and modified P3 Enp, which are characteristics of the male, while the P5 and genital double somites are typical for the female []. In the case of the gynandromorphic specimen of A. sibirica, it is more masculinized, with well developed male reproductive structures and urosome, while the prosome has the female antennule and an intermediate nature of swimming legs [].
4.3. Amended Key to Elaphoidella Species from Southeast Asia
To update the key to the species of Elaphoidella from SEA, the characteristics of species recorded from the region were checked based on Lang [] and Wells [], together with the original descriptions of species of the region and those from Thailand published after 2010.
In the past, some species were described insufficiently, with a lack of details that would unanimously define the species. Existing descriptions of several Elaphoidella are inadequate []; thus, the taxonomic revision of the species, as well as the genus, is urgently needed to make a precise definition (range of variability) of the species. An example is E. grandedieri, reported from many places around the world. According to the high degree of morphological variability (for details, see the next paragraph), some authors suggest that E. grandidieri could belong to a species complex or that the species could represent distinct sub-specific taxa [,,]. As an example, in the original description, no seta was recorded on the female P4 Enp-1 (p. 239, Figure 8) [], the female P5 Exp has three long setae and one spiniform element (p. 241, Figure 9) [], while the Japanese (p. 50, Plate 3, Figure e,) [] and Chinese (p. 249, Figure 137h, []) specimens bear an inner seta on the P4 Enp-1 and five well developed setae on the female P5 Exp.
Another variable characteristic is the serration of the hyaline frill, which has been reported as serrated in the Mexican population (p. 231) [], but smooth in the Thai population (p. 185) []. In the original description, the posteroventral spinule(s) were not indicated near the base of a caudal ramus (Figure 1) [], but other authors reported 1–2 spinules there (p. 1136; p. 185; p. 237, Figure 1C), respectively [,,]. Great variability has been observed in the female P5 Exp, where four, five, or six elements have been reported on the outer margin [,,], while the elements have been regarded as setae, spines, or large accessory spinules.
In the key to the species of Harpacticoida [] and the key to the species of Elaphoidella recorded in SEA [] for E. grandidieri, the armature of P2–P4 Enp-1 in the female was set as 1.1.1. The seta is present on the female P4 Enp-1 in some populations, such as Chinese, Japanese, and Hawaiian populations [,,], but absent in other populations, such as those from Madagascar (type locality), Colombia, Mexico, Australia, Ethiopia, Japan, Sumatra (Indonesia), and Thailand [,,,,,,,]. Although it seems likely that the seta on P4 Enp-1 is absent in SEA populations, the trait of not having an inner seta in the female P4 Enp-1 was used in the present amended key (instead of having the seta present).
Furthermore, after comparing the characteristics of Elaphoidella recorded in SEA, the characteristics of E. namnaoensis are most similar to those of E. grandidieri. They share characteristics of the configuration of the caudal ramus (with outgrowth on the distal tip) and the armature of P2–P4 Enp-2 and P1–P4 Exp-3 in the females. Based on the comments in the previous paragraph, E. namnaoensis and E. grandidieri share the same armature of P2–P4 Enp-1 in the females (1.1.0). The only considerable difference between these two species is the armament of the anal operculum. The anal operculum of E. namnaoensis has about 20 spinules on the free margin [], resembling that of E. isana, in which the free margin of the anal operculum has about 20 oblong indentures (p. 556, Figure 4D, []). In contrast, the free hyaline margin of the anal operculum of E. grandidieri is furnished with about 40 fine spinules (p. 237, Figure 4D), []; (p. 1581, Figure 1G) []. So far, the only considerable difference between E. namnaoensis and E. grandidieri is the ornamentation of the anal operculum. However, this could be an indication of a cryptic species complex and the relevance of microcharacters in species delimitation. However, the revision of E. grandidieri should be carried out to trace the phylogenetic relationship between these two species (E. namnaoensis vs. E. grandidieri) because there are records of the latter from nearby countries, including Sumatra, Java, and Vietnam. It is possible that it will be recorded in Thailand, too. For that reason, a detailed morphological and molecular study of both species would be highly welcome. At the same time, more environmental data (habitat type, physical and chemical parameters of water) are needed to position the species in the system and to detect potential cryptic species.
In the following key, five recently described species—E. fatimae Fefilova and Alekseev, 2018, E. isana Watiroyram, Sanoamuang, and Brancelj, 2021; E. brancelji Sanoamuang, and Watiroyram, 2021; E. longiramus Watiroyram, Janpong, and Sanoamuang, 2022; and E. stygobiota Watiroyram and Koompoot, 2023—are included, too.
4.4. Key to the Females of Elaphoidella Chappuis, 1929, Recorded in Southeast Asia
| 1. - P1 Enp two-segmented …………………………….……..……………………………......….. 2 |
| - P1 Enp three-segmented ………………………….……………………………........................ 9 |
| 2. - P4 Enp completely reduced …………………………….……………...……………………… 3 |
| - P4 Enp two-segmented ……………………………….......…….……..…..………………….. 4 |
| 3. - P2–P3 Enp-2 each with two elements apically; inner seta reaching tip of Exp-3 at most …………………...........................................................….……………… E. thailandensis |
| - P2–P3 Enp-2 each with two and three elements apically, respectively: inner seta considerably surpassing tip of Exp-3 …............................……………….….. E. longiramus |
| 4. - P5 Enp lobe small, tip of Enp lobe reaching the middle of Exp at most; armature of P2–P4 Enp-1: 1.1.0 …………………………………………………………….......…. E. bidens bidens |
| - P5 Enp lobe, surpassing tip of Exp; armature of P2–P4 Enp-1: 0.0.0 ………………………………………….………………………………………………….. 5 |
| 5. - P5 Exp with three setae …………………….…………………..…………………….….….. 6 |
| - P5 Exp with four setae ………………………………….……………………………..…….. 7 |
| 6. - Caudal seta VI spiniform, curved inwards; seta IV (outermost lateral seta) on P5 Enp lobe minute, shorter than length of P5 Exp……………………..................................... E. cornuta |
| - Caudal seta VI normally developed; seta IV (outermost lateral seta) on P5 Enp lobe as long as or longer than the length of P5 Exp............................................................ E. javaensis |
| 7. - P1 Enp-2 with two elements apically: one outer spine and one geniculate, inner seta …...…….………………………………………………..……………………. E. stygobiota |
| - P1 Enp-2 with three elements apically …………………………………………………….. 8 |
| 8. - All seta on caudal ramus normally developed; anal operculum with several short and small spinules on its free margin ………………………….……………………… E. elegans |
| - Caudal seta V and VI modified, swollen or bulbous at base; anal operculum with strong spinules on its free margin ………………………….…....……...………… E. sanoamuangae |
| 9. - P4 with one-segmented Enp ………..………………………… E. phuphamanensis sp. nov. |
| - P4 with two-segmented Enp …………………………………………………….…..…….. 10 |
| 10. - Armature of P2–P4 Enp-1: 1.1.1 or 1.0.0 ………………………………………..….……… 11 |
| - Armature of P2–P4 Enp-1: 1.1.0 or 0.1.0 ……………………………………..…..……….. 21 |
| - Armature of P2–P4 Enp-1: 0.0.0 …………………………….………………….……..…… 30 |
| 11. - Armature of P2–P4 Enp-1: 1.0.0 ………………………………………………….. E. ligorae |
| - Armature of P2–P4 Enp-1: 1.1.1 …………………………………………………………… 12 |
| 12. - Free margin of anal operculum with 10–14 strong spinules…………..………………… 13 |
| - Free margin of anal operculum with fine hairs or fine spinules or comb-like, (more than 18 hairs, spinules or indentations) ......................…………………………………………… 14 |
| 13. - Caudal ramus subquadrate, with acute extension dorsally ………….. E. bidens decorata |
| - Caudal ramus conical, without acute extension dorsally .……………..…......... E. fatimae |
| 14. - P5 Enp lobe with three elements ……………………....................................... E. trisaetosa |
| - P5 Enp lobe with four elements …………………………………………………..………. 15 |
| 15. - P5 Exp as long as or more than 3.0 times as long as wide ………..……..…..…………. 16 |
| - P5 Exp less than 2.5 times as long as wide ……………….…….…………….………….. 17 |
| 16. - P5 Exp less than 4.0 times as long as wide …….……………………………. E. longipedis |
| - P5 Exp more than 4.6 times as long as wide ……………………………… E. superpedalis |
| 17. - P5 Exp with three or four setae…………………………………….…….……….. E. affinis |
| - P5 Exp with six setae ………………….............................................................. E. bryophila |
| - P5 Exp with five setae …………………………………………………….….…………… 18 |
| 18. - Caudal seta IV and VI modified; caudal seta VI short, wavy; caudal seta IV short, bulbous, pear-shaped …………………………………………………………………...…. E. cuspidata |
| - Caudal seta IV normally developed, setiform ……………..…………….…………….. 19 |
| 19. - All setae on P5 Exp normally developed, seta IV on P5 Exp as long as or longer than supporting segment ………………………………………..………………………………… 20 |
| - Two outer seta on P5 Exp short, slim, bare, seta IV much shorter than supporting segment ………………………………………………………………………………… E. isana |
| 20. - Caudal seta III inserted near seta IV, below the insertion of caudal seta IV; spinule on lateral surface of urosomite 4 relatively large and strong……..…………...……. E. similis |
| - Caudal seta III inserted on lateral surface, below the insertion of caudal seta II; spinule on lateral surface of urosomite 4 slim .....……..………………………………. E. intermedia |
| 21. - Armature of P2–P4 Enp-1: 0.1.0 ……………………………………………….………..… 22 |
| - Armature of P2–P4 Enp-1: 1.1.0 ……………….……………………………..……………. 23 |
| 22. - Inner marginal and apical setae on P4 Enp-2 less than three times as long as supporting segment; caudal rami without spinules at base of caudal seta V and VI ventrally………… E. malayica |
| - Inner marginal and apical setae on P4 Enp-2 relatively long, more than three times as long as the segment bearing them; caudal rami with or without spinules at base of caudal seta V and VI ventrally…...…..………………………………………………………………... E. labani |
| 23. - P5 Enp lobe relatively short, insertion of seta II on P5 Enp lobe reaching the middle of Exp at most ……………………………………………………………………..………...…………. 24 |
| - P5 Enp lobe relatively long, insertion of seta II on P5 Enp lobe reaching or surpassing tip of Exp ……………………………………………………..…………………………..………… 26 |
| 24. - Anal operculum short, reaching distal margin of anal somite, free margin with strong spinules; dorsal keel on caudal ramus with acute extension distally …... E. bidens coronata |
| - Anal operculum surpassing distal margin of anal somite, semicircular or semi-oval in shape, free margin comb-like; dorsal keel on caudal ramus without acute extension distally …………….……………………………………………………………..……………… 25 |
| 25. - Free margin of anal operculum with about 40 spinules…….…………….. E. grandidieri |
| - Free margin of anal operculum with about 20 oblong spinules ………… E. namnaoensis |
| 26. - P4 Enp-2 with three (exceptionally two) setae and spines …………..…..…...………… 27 |
| - P4 Enp-2 with four setae and spines ….………...………………………..…..…………… 28 |
| 27. - P3 Enp-2 with four elements; inner lateral seta on P4 Enp-2 relatively short, less than three times as long as supporting segment; caudal rami without spinules at base of caudal seta V and VI ventrally……..……….…………………………………………………. E. sewellis. str. |
| - P3 Enp-2 with five elements; inner lateral seta on P4 Enp-2 relatively long, more than three times as long as supporting segment; caudal rami with or without spinule at base of caudal seta V and VI ventrally .….…… ………………………………………………………. E. labani |
| 28. - P3 Enp-2 with three elements ……………………………………………. E. bromeliaecola |
| - P3 Enp-2 with five elements ……….……………………….…...……… E. thienemanni (29) |
| 29. - - Two apical elements on P4 Enp-2 spiniform setae ………...................... E. t. thienemanni |
| - Two apical elements apically on P4 Enp-2 comprisingg one spiniform and one longer seta……………………………………………………………..…….…………….. E. t. serrulata |
| 30. - P5 Exp with two–four elements; P3 Enp-2 with four–five elements ………………….. 31 |
| - P5 Exp with five elements; P3 Enp-2 with six elements …………….… ….. E. jaesornensis |
| 31. - P3 Enp-2 with four setae and spines ……………………………………………………… 32 |
| - P3 Enp-2 with five setae and spines ……………………………………….……………… 33 |
| 32. - Armature formula of P2–P4 Enp-2: 5.4.3………………………………………. E. brancelji |
| - Armature formula of P2–P4 Enp-2: 4.4.3………...…………………………… E. vietnamica |
| 33. - Armature formula of P2–P4 Enp-2: 5.5.3; P5 Exp with two setae …………. E. margaritae |
| - Armature formula of P2–P4 Enp-2: 5.5.4; P5 Exp with three or four setae……………. 34 |
| 34. - P5 Exp with three elements ……………………………………. E. propecabezasi sp. nov. |
| - P5 Exp with four elements ………………..…………………….……………E. paraaffinis |
4.5. Key to the Males of Elaphoidella Chappuis, 1929, Recorded in Southeast Asia
The males of the following species are not included in this key, as they have not been recorded so far: E. affinis, E. bidens, E. cornuta, E. elegans, E. isana, E. jaesornensis, E. longipedis, E. namnoaensis, E. superpedalis, and E. trisaetosa.
| 1. - P1 Enp two-segmented ……………….……………………………………..………………. 2 |
| - P1 Enp three-segmented ……………………..……………………………………………… 6 |
| 2. - P4 Enp completely reduced; outer apical element of P4 Exp-3 transformed, antler-like spine ……………………………………………..……………………...……..….…………….. 3 |
| - P4 Enp two-segmented ………………………………….………….…………….…………. 4 |
| 3. - P2 Enp-2 with two elements: outer short and spiniform, inner one seta; segment 5 of A1 with spinous process in median anterior margin and accompanied by one seta…………………...................................................................……………... E. thailandensis |
| - P2 Enp-2 with one, long seta; A1 normally developed as characteristic of the genus ………………………………...…………………….……………………. E. longiramus |
| 4. - P4 Exp-3 with distal outer and outer apical antler-like, transformed spines; anal operculum with about nine strong spinules on free margin ………….. E. sanoamuangae |
| - P4 Exp-3 without transformed spines …………………….…………….…………………. 5 |
| 5. - P5 Exp with two elements; P3 Enp-3 with two elements; P2 Enp-2 with two or three elements ……….………………………….………………………………...……... E. javaensis |
| - P5 Exp with three elements; P3 Enp-2 with one element; P2 Enp-2 with one apical element ……………………….………………….………………………………. E. stygobiota |
| 6. - P4 Exp-3 normally developed, more than 1.5 times as long as wide, without transformed elements; P3 Enp-3 with two elements………………………………………….................... 7 |
| - P4 Exp-3 normally developed, more than 1.5 times as long as wide; proximal outer spine transformed and shorter than distal outer one…...…..…....….…………………………… 11 |
| - P4 Exp-3 shortened; one or two elements on outer half of P4 Exp-3 transformed into antler-like spine …...…..............................................................................................………… 13 |
| 7. - P5 Exp with two elements ………………….…….………...…..……..……… E. margaritae |
| - P5 Exp with three elements, two inner ones spiniform, outermost one short, slender seta …………………………………………...…………………….. E. propecabezasi sp. nov. |
| - P5 Exp with four elements ……………………………….…………….………….……….. 8 |
| 8. - P4 Enp-2 with two elements ………………………………..…………….….……. E. similis |
| - P4 Enp-2 with three elements ……………………………….…………….……………….. 9 |
| 9. - P2 Enp-2 with five elements ……………………………………………......…….. E. ligorae |
| - P2 Enp-2 with four elements ………………………………………..…………………….. 10 |
| 10. - Inner apical element on P3 Enp-3 modified, obtuse, with long hairs……… E. cuspidata |
| - Inner apical element on P3 Enp-3 normally developed, setiform ……….. E. paraaffinis |
| 11. - P5 Exp with three setae; anal operculum reaching mid of caudal ramus, free margin with about 40 spinules……………….……………………………………………….. E. grandidieri |
| - P5 Exp with four setae ……………………………………..………………..…….………. 12 |
| 12. - Caudal ramus without dorsal keel, about 1.5 times as long as wide; P5 Exp shorter than wide, with three spiniform elements on inner margin; length of seta II (inner to outer) less than two times of innermost one …………………….……..………………….. E. bryophila |
| - Caudal ramus with dorsal keel, about twice as long as wide; P5 Exp as long as wide, three inner elements setiform, length of seta II more than two times the innermost one …………………………….……………………….……..…………………... E. intermedia |
| 13. - Only outer apical element of P4 Exp-3 transformed …...……………………………….. 14 |
| - Both distal outer and outer apical elements of P4 Exp-3 transformed…….................... 16 |
| 14. - P2 and P4 Enp-2 with four and three elements, respectively…………… E. sewellis. str. |
| - P2 and P4 Enp-2 each with three elements………………………………...…………….. 15 |
| 15. - Caudal ramus subquadrate; free margin of anal operculum with about six strong spinules; apical seta on P2 Enp-2 short and slim, as long as or slightly longer than supporting segment; distal inner seta on P2 Enp-2 reaching the tip of the apical seta.………................................................................................................................. E. malayica |
| - Caudal ramus slightly longer than wide, conical; free margin of anal operculum with 18–20 short spinules ………………………..……………..……………………….. E. vietnamica |
| - Caudal ramus about twice as long as wide, crescent, outer margin strongly concaved; free margin of anal operculum with more than 25 spinules; apical seta on P2 Enp-2 more than twice as long as distal inner seta ………………………………………………… E. brancelji |
| 16. - P4 Enp-2 one-segmented; P4 Exp-3 subquadrate; P2 Enp-2 without armature ………………………………………..…… E. phuphamanensis sp. nov. |
| - P4 Enp-2 two-segmented ……………..…………………………..……………………….. 17 |
| 17. - P2 and P4 Enp-1 each without inner seta ………………..………………………. E. labani |
| - P2 and P4 Enp-1 with and without inner seta, respectively …………………………… 18 |
| 18. - P3 Enp-3 with two short setae apically, subequal in length; P5 Exp wider than long; length of apical seta on P2 Enp-2 less than twice length of distal inner seta; outer spine on P3 Exp-2 just reaching insertion of proximal spine of P3 Exp-3 on outer margin ………………………….………………………..…………………… E. bromeliaecola |
| - P3 Enp-3 with thick blunt spine and much longer seta; P5 Exp as long as wide; length of apical seta on P2 Enp-2 more than twice length of distal inner seta; outer spine on P3 Exp-2 reaching tip of P3 Exp-3 …………..………………….…………………..… E. thienemanni |
Author Contributions
A.B. and L.S. performed field sampling; A.B. performed the sorting of biological material and its preliminary determination; C.B. produced graphics and the final determination of the material; L.S. and C.B. produced the first draft of the manuscript. A.B. formulated the final version of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a grant (RP67-2-Research Center-001) from Research and Graduate Studies at Khon Kaen University, Thailand. Part of this research was also supported by the Slovenian Research Agency (ARRS/ARIS) through the P1-0255 program.
Institutional Review Board Statement
The animal study protocol was approved by the Ethics Committee of Nakhon Ratchasima Rajabhat University (protocol code AE-RDI-NRRU 01/2567) for studies involving animals.
Data Availability Statement
Availability on material is evident through access numbers next to the description of each species.
Acknowledgments
The second author (A.B.) received a grant for his stay and research on groundwater fauna in Thailand from the Khon Kaen University Inbound Visiting Scholar of the 2023 fiscal year, for which he would like to thank the authorities of the KKU. The authors are greatly thankful to Niwat Sanoamuang and Prapatsorn Dabseepai for their help during fieldwork. We would like to thank the three anonymous reviewers who carefully read the manuscript and added valuable comments and corrections, which improved the final version of the manuscript.
Conflicts of Interest
The authors have no conflicts of interest.
References
- Walter, T.C.; Boxshall, G. World of Copepods Database. Available online: https://marinespecies.org/aphia.php?p=taxdetails&id=1080 (accessed on 28 February 2024).
- Lang, K. Monographie der Harpacticiden II; Nordiska Bokhandeln: Stockholm, Sweden, 1948. [Google Scholar]
- Gaviria, S. Zwei Canthocamptidae (Copepoda, Harpacticoida) aus kolumbianischen Andengewässern. Ann. Naturhist. Mus. Wien B Bot. Zool. 1993, 94–95, 361–375. [Google Scholar]
- Mori, N.; Brancelj, A. Distribution and habitat preferences of species within the genus Elaphoidella Chappuis, 1929 (Crustacea: Copepoda: Harpacticoida) in Slovenia. Zool. Anz. 2008, 247, 85–94. [Google Scholar] [CrossRef]
- Rundle, S.D.; Bilton, D.T.; Shiozawa, D.K. Global and regional patterns in lotic meiofauna. Freshw. Biol. 2000, 44, 123–134. [Google Scholar] [CrossRef]
- Chappuis, P.A. Neue Harpacticiden aus Java. Treubia 1928, 10, 271–283. [Google Scholar]
- Dumont, H.J.; Maas, S. Five new species of leaf litter harpacticoids (Crustacea, Copepoda) from Nepal. Zool. Scr. 1988, 17, 55–68. [Google Scholar] [CrossRef]
- Reid, J. The harpacticoid and cyclopoid copepod fauna in the Cerrado Region of central Brazil. 1. Species composition, habitats and zoogeography. Acta Limnol. Bras. 1993, 6, 56–68. [Google Scholar]
- Galassi, D.M.P. Groundwater copepods: Diversity patterns over ecological and evolutionary scales. Hydrobiologia 2001, 453/454, 227–253. [Google Scholar] [CrossRef]
- Brancelj, A. Fauna of an unsaturated karstic zone in central Slovenia: Two new species of Harpacticoida (Crustacea: Copepoda), Elaphoidella millenni n. sp. and E. tarmani n. sp., their ecology and morphological adaptations. Hydrobiologia 2009, 621, 85–104. [Google Scholar] [CrossRef]
- Watiroyram, S.; Sanoamuang, L.; Brancelj, A. Two new species of Elaphoidella (Copepoda, Harpacticoida) from caves in southern Thailand and a key to the species of Southeast Asia. Zootaxa 2017, 4282, 501–525. [Google Scholar] [CrossRef]
- Daday, E. Untersuchungen über die Copepodenfauna von Hinerindien, Sumatra und Java, nebst einem Beitrag zur Copepodenkenntnis der Hawaii-Inseln. (Reise von Dr. Walter Volz). Zool. Jahrb. Abt. Syst. Georgr. Biol. Tiere. 1906, 24, 175–206. [Google Scholar]
- Pesce, G.L.; Apostolov, A.M. Elaphoidella margaritae sp. n. a new phreatobitic harpacticoid from subterranean waters of Thailand (Crustacea, Copepoda, Canthocamptidae. Acta Zool. Bulg. 1985, 28, 70–75. [Google Scholar]
- Deharveng, L.; Bedos, A. The cave fauna of Southeast Asia: Origin, evolution and ecology. In Ecosystems of the World; Wilkens, H., Culver, D.C., Humphreys, W.F., Eds.; Elsevier Academic Press: Amsterdam, The Netherlands, 2000; Volume 30, pp. 603–632. [Google Scholar]
- Deharveng, L.; Ellis, M.; Bedos, A.; Jantarit, S. Tham Chiang Dao: A hotspot of subterranean biodiversity in northern Thailand. Diversity 2023, 15, 1076. [Google Scholar] [CrossRef]
- Brancelj, A.; Watiroyram, S.; Sanoamuang, L. The first record of cave-dwelling Copepoda from Thailand and description of a new species: Elaphoidella namnaoensis, sp. nov. (Copepoda, Harpacticoida). Crustaceana 2010, 83, 779–793. [Google Scholar] [CrossRef]
- Watiroyram, S.; Wongduan, J.; Sanoamuang, L. Elaphoidella longiramus sp. nov. (Copepoda: Harpacticoida, Canthocamptidae), a new species of cave-dwelling copepod from central Thailand. Zootaxa 2022, 5138, 152–166. [Google Scholar] [CrossRef]
- Huys, R.; Boxshall, G.A. Copepod Evolution; The Ray Society: London, UK, 1991. [Google Scholar]
- Petkovski, T.K. Subterrane Süßwasser-Harpacticoida von Kuba. In Résultats des Expéditions Biospéologiques Cubano-Roumaines à Cuba; Orghidan, T.N., Ed.; Editura Academiei Republicii Socialiste Romania: Bucharest, Romania, 1973; Volume 1, pp. 125–141. [Google Scholar]
- Chappuis, P.A. Copepoda Harpaticoida der Deutschen Limnologischen Sunda-Expedition. In Tropische Binnengewässer Band 8; Ruttner, F., Ed.; Schweizerbart Science Publishers: Stuttgart, Germany, 1931; pp. 512–584. [Google Scholar]
- Löffler, H. Die Harpacticidenfauna des Mt. Kinabalu (Borneo) mit besonderer Berücksichtigung der Gattung Maraenobiotus nebst Angaben zur Harpacticidenfauna des Gebietes Nuwara (Hochplateau Ceylon). In Hochgebirgsforschung Heft 3: Beiträge zur Kenntnis Einiger Kleinorganismen Tropischer Hochgebirgsseen; Helmich, W., Ed.; Universitatsverlag Wagner: Innsbruck, Austria, 1973; pp. 5–28. [Google Scholar]
- Watiroyram, S.; Brancelj, A. A new species of the genus Elaphoidella Chappuis (Copepoda, Harpacticoida) from a cave in the south of Thailand. Crustaceana 2016, 89, 459–476. [Google Scholar] [CrossRef]
- Wells, J.B.J. An annotated checklist and keys to the species of Copepoda Harpacticoida (Crustacea). Zootaxa 2007, 1568, 1–872. [Google Scholar] [CrossRef]
- Apostolov, A.M. Étude sur quelques copépodes harpacticoïdes du genre Elaphoidella Chappuis, 1929 de Bulgarie avec une révision du genre. Acta Mus. Maced. Sci. Nat. 1985, 17, 133–163. [Google Scholar]
- Petkovski, T.K. Weitere neue Elaphoidella-Arten (Copepoda, Harpacticoida) aus den subterranen Binnengewässern von Kuba. Acta Mus. Maced. Sci. Nat. 1982, 16, 139–174. [Google Scholar]
- Young, S.S. Four new species of freshwater harpacticoid copepods (Canthocamptidae: Copepoda) from mountain lakes of Taiwan. Taiwan J. Biodivers. 2010, 12, 327–340. [Google Scholar]
- Gaviria, S.; Defaye, D. Description of Elaphoidella paramuna n. sp. (Canthocamptidae), a new harpacticoid copepod from Colombia. Crustaceana 2015, 88, 1003–1029. [Google Scholar] [CrossRef]
- Fefilova, E.B.; Alekseev, V.R. A new species and new records of harpacticoids (Crustacea: Copepoda: Harpacticoida) from North-Eastern Borneo. Zoosyst. Ross. 2018, 27, 205–217. [Google Scholar] [CrossRef]
- Cottarelli, V.; Bruno, M.C.; Berera, R. First record of Parastenocarididae from Thailand and description of a new genus (Copepoda: Harpacticoida). J. Crustac. Biol. 2010, 30, 478–494. [Google Scholar] [CrossRef]
- Boonyanusith, C.; Sanoamuang, L.; Brancelj, A. A new genus and two new species of cave-dwelling cyclopoids (Crustacea, Copepoda) from the epikarst zone of Thailand and up-to-date keys to genera and subgenera of the Bryocyclops and Microcyclops groups. Eur. J. Taxon. 2018, 431, 1–30. [Google Scholar] [CrossRef]
- Brancelj, A.; Boonyanusith, C.; Sanoamuang, L. A new cyclopoid genus (Copepoda, Crustacea) from a deep aquifer in northeastern Thailand with comments on peculiar sampling sites and local fauna. Raffles Bull. Zool. 2024, 72, 71–83. Available online: https://lkcnhm.nus.edu.sg/wp-content/uploads/sites/10/2024/02/RBZ-2024-0005.pdf (accessed on 28 February 2024).
- Pesce, G.L. Pesce’s Web Portal. Available online: http://www.luciopesce.net/copepods/arpa/elaph.htm (accessed on 28 December 2023).
- Rocha-Olivares, A.J.; Fleeger, J.W.; Foltz, D.W. Decoupling of molecular and morphological evolution in deep lineages of a meiobenthic harpacticoid copepod. Mol. Biol. Evol. 2001, 18, 1088–1102. [Google Scholar] [CrossRef]
- Gilbert, J.; Deharveng, L. Subterranean ecosystems: A truncated functional biodiversity. BioScience 2002, 52, 473–481. [Google Scholar] [CrossRef]
- Deharveng, L.; Stoch, F.; Gibert, J.; Bedos, A.; Galassi, D.M.P.; Zagmajster, M.; Brancelj, A.; Camacho, A.; Giani, N.; Magniez, G.; et al. Groundwater biodiversity in Europe. Freshw. Biol. 2009, 54, 709–726. [Google Scholar] [CrossRef]
- Boonyanusith, C.; Brancelj, A.; Sanoamuang, L. First representatives of the genus Fierscyclops Karanovic, 2004 (Copepoda, Cyclopidae) from South East Asia. J. Limnol. 2013, 72 (Suppl. 2), 275–289. [Google Scholar] [CrossRef]
- Bruno, M.C.; Cottarelli, V. First record of Kinnecaris (Copepoda: Harpacticoida: Parastenocarididae) from Turkey and Thailand; description of three new species and emended definition of the genus. Ital. J. Zool. 2015, 82, 69–94. [Google Scholar] [CrossRef]
- Narita, S.; Pereira, R.A.S.; Kjellberg, F.; Kageyama, D. Gynandromorphs and intersexes: Potential to understand the mechanism of sex determination in arthropods. Terr. Arthropod Rev. 2010, 3, 63–96. [Google Scholar] [CrossRef]
- Fusco, G.; Minelli, A. Descriptive versus causal morphology: Gynandromorphism and intersexuality. Theory Biosci. 2023, 142, 1–11. [Google Scholar] [CrossRef]
- Gusmão, L.F.M.; McKinnon, A.D. Sex ratios, intersexuality and sex change in copepods. J. Plankton Res. 2009, 31, 1101–1117. [Google Scholar] [CrossRef]
- Moore, C.G.; Stevenson, J.M. The occurrence of intersexuality in harpacticoid copepods and its relationship with pollution. Mar. Pollut. Bull. 1991, 22, 72–74. [Google Scholar] [CrossRef]
- Moore, C.G.; Stevenson, J.M. Intersexuality in benthic harpacticoid copepods in the Firth of Forth, Scotland. J. Nat. Hist. 1994, 28, 1213–1230. [Google Scholar] [CrossRef]
- Klie, W. Ein gynandromorpher Amphiascus (Cop. Harp.) von Helgoland. Zool. Anz. 1944, 145, 77–79. [Google Scholar]
- Huys, R.; Gee, M.G. A revision of Danielssenia Boeck and Psammis Sars with the establishment of two new genera Archisenia and Bathypsammis (Harpacticoida: Paranannopidae). Bull. Br. Mus. Nat. Hist. Zool. 1993, 59, 83–94. [Google Scholar]
- Gómez, S. On some new species of Stenheliinae Brady, 1880 (Copepoda, Harpacticoida, Miraciidae) from north-western Mexico, with the proposal of Lonchoeidestenhelia gen. nov. ZooKeys 2020, 987, 41–79. [Google Scholar] [CrossRef]
- Hamond, R. Non-marine harpacticoid copepods of Auatralia. I. Camthocamptidae of the genus Camthocamptus Westwood s. lat. and Fibulacamtus, gen. nov. and including the description of a related new species of Camthocamptus from New Caledonia. Invertebr. Taxon. 1987, 1, 1023–1247. [Google Scholar] [CrossRef]
- Gutierrez-Aguirre, M.; Suarez-Morales, E.; Cervantes-Martínez, A.; Sarma, N.; Sarma, S.S.S. Morphology of Elaphoidella grandidieri (Guerne and Richard, 1893) (Copepoda, Harpacticoida) from Mexico with notes on fecundity in culture conditions. In Studies on Freshwater Copepoda: A Volume in Honour of Bernard Dussart; Defaye, D., Suarez-Morales, E., Von Vaupel Klein, C., Eds.; Koninklijke Brill: Leiden, The Netherlands, 2011; pp. 227–244. [Google Scholar] [CrossRef]
- Fuentes-Reines, J.M.; de Roa, E.Z. Occurrence of Elaphoidella grandidieri (Guerne and Richard, 1893) (Crustacea: Copepoda: Harpacticoida) in Ciénaga Grande de Santa Marta, Colombia. Check List 2013, 9, 1580–1583. [Google Scholar] [CrossRef][Green Version]
- Guerne, J.; Richard, J. Canthocamptus grandidieri, Alona cambouei, nouveaux Entomostracés d’eau douce de Madagascar. Mém. Soc. Zool. Fr. 1893, 6, 214–224. [Google Scholar]
- Ishida, T. Copepods in the mountain waters of Kyushu, Tsushima and Ryukyu Islands, southwestern Japan. Sci. Rep. Hokkaido Fish Hatch. 1990, 44, 39–51. [Google Scholar]
- Shen, C.J.; Tai, A.Y.; Li, A.Y.; Song, D.X.; Chang, C.Z.; Song, Y.Z.; Chen, G.X. Fauna Sinica, Crustacea: Freshwater Copepoda; Science Press: Beijing, China, 1979. [Google Scholar]
- Defaye, D. Contribution à la connaissance des Crustacés Copépodes d’Ethiopie. Hydrobiologia 1988, 164, 103–147. [Google Scholar] [CrossRef]
- Sars, G.O. Pacifische Plankton-Crustaceen. (Ergebnisse einer Reise nach dem Pacific Schaunsland 1896–1897). Zool. Jahrb. Abt. Syst. Oekol. Geogr. Tiere 1904, 19, 629–646. [Google Scholar]
- Kikuchi, Y. Redescription of a freshwater harpacticoid copepod, Elaphoidella grandidieri (Guerne & Richard, 1893), from a swamp at Itako, central Japan. Publ. Itako Hydrobiol. Stat. 1985, 2, 1–8. [Google Scholar]
- Watiroyram, S.; Sanoamuang, L.; Brancelj, A. A new species of Elaphoidella Chappuis, 1929 and Schizopera Sars, 1905 (Copepoda: Harpacticoida) from two caves in northeastern and southern Thailand. Zootaxa 2021, 5051, 550–569. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).