Global Subterranean Biodiversity: A Unique Pattern
1. Introduction
2. Goals
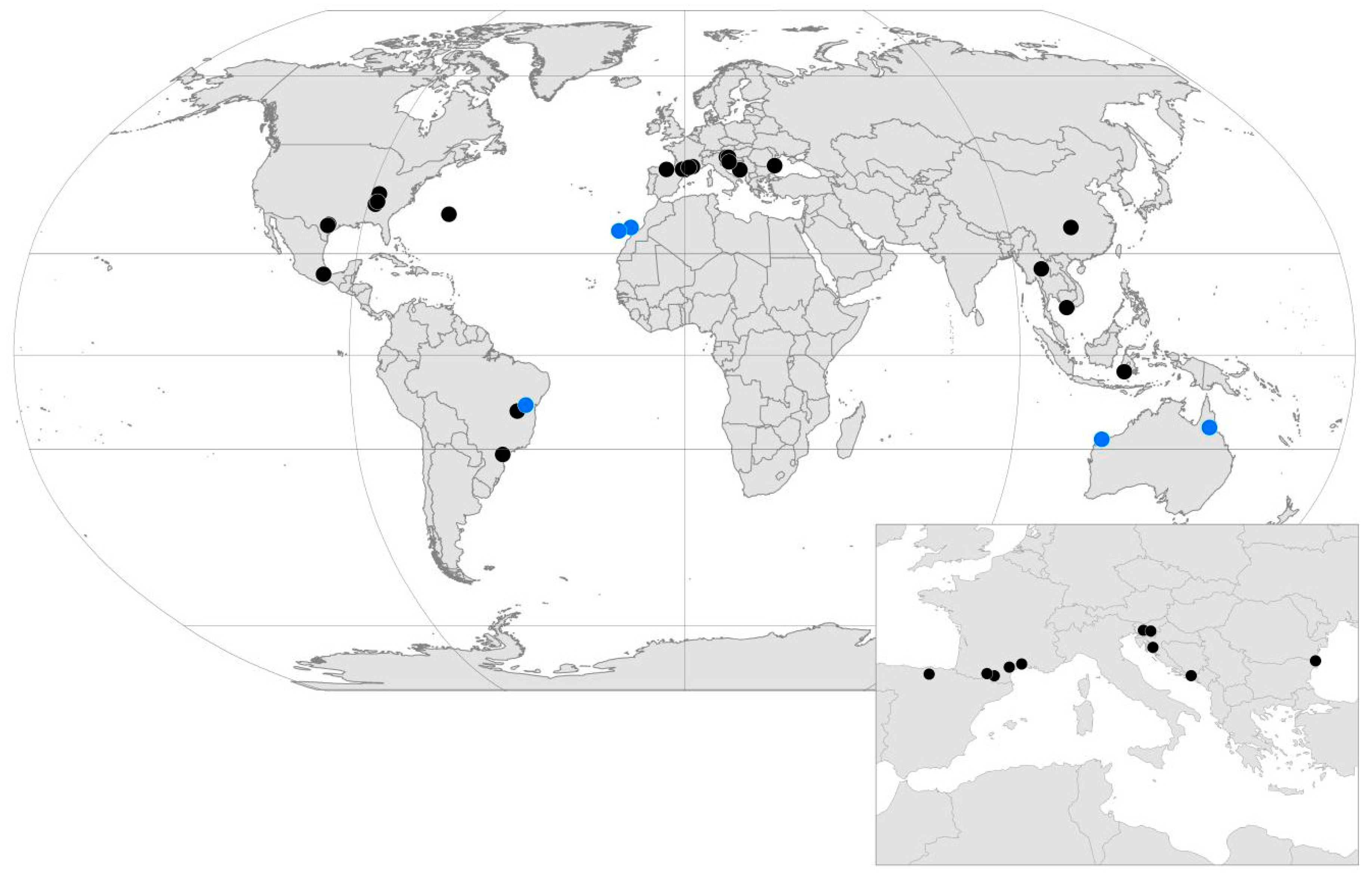
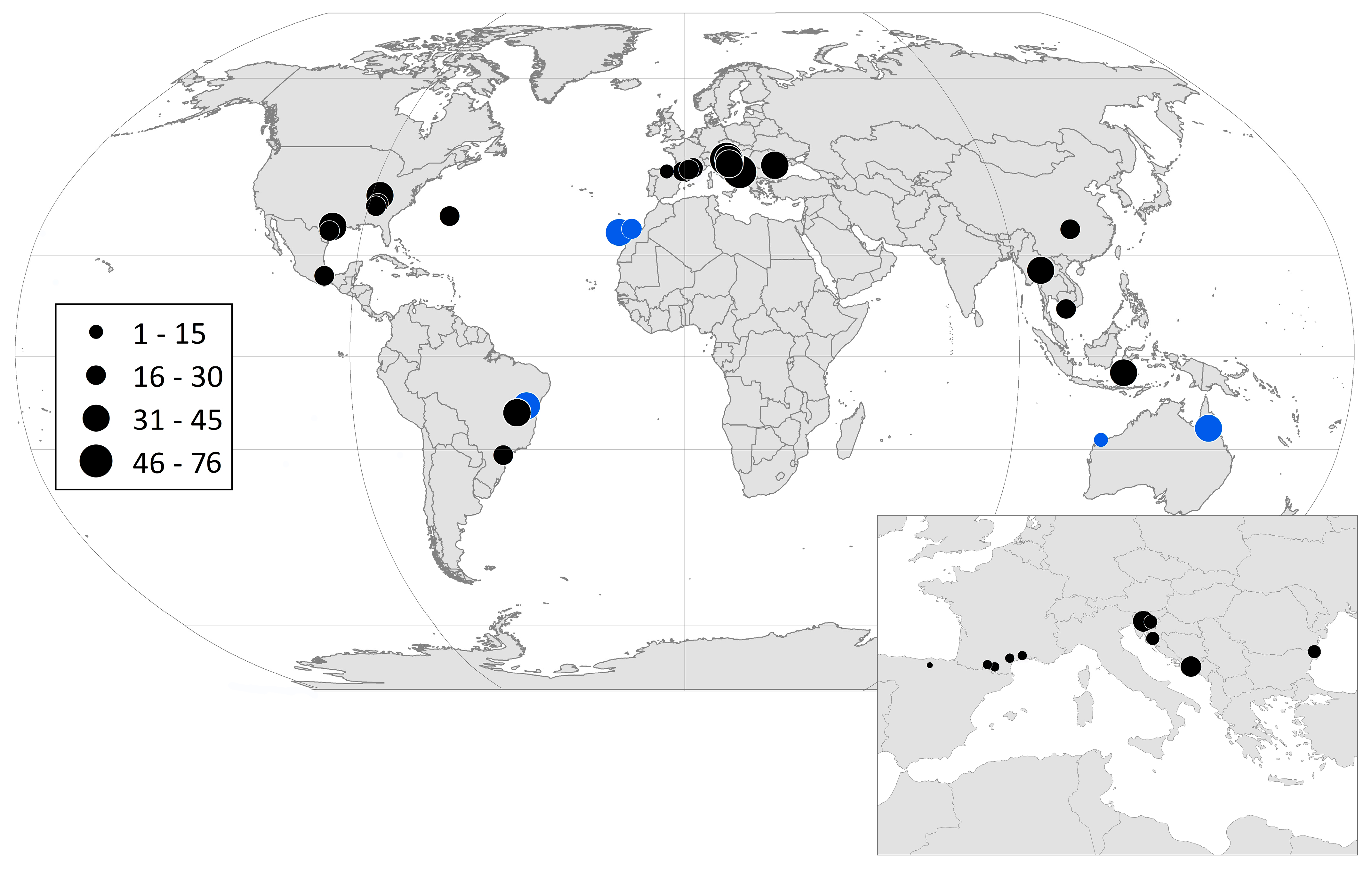
3. Results
- Postojna Planina Cave System, Slovenia (105);
- Vjetrenica Cave System, Bosnia and Hercegovina (93);
- Walsingham Caves, Bermuda (63);
- Križna Jama, Slovenia (59);
- Baget System, France (57);
- San Marcos Artesian Well, Texas (55);
- Ojo Guareña System, Spain (54).
4. Challenges
4.1. How to Define Stygobionts, Troglobionts, etc.
- (1)
- Morphological inference is based on presence or absence of troglomorphic traits. The presence of a set of convergent troglomorphic traits in most arthropods (eye and pigment reduction combined with appendage and size increases compared to surface relatives) points to obligate cave life. Depigmentation and eye reduction are trends shared by many soil and cave arthropods, and Brignoli [60] stressed that the equation “blind = troglobite” has a limited value. When they are combined with appendage shortening and a decrease in size, they qualify a species as euedaphomorphic [59]. It is only when eye and pigment regression are combined with appendage elongation and an increase in size (or other characters recognized as troglomorphic), that they qualify a species as troglomorphic. Statistically, the correlation of troglomorphic and euedaphomorphic life forms with the ecological categories of troglobiont and edaphobiont is one-way and robust. Where the set of troglomorphic traits is not present, such as in many guano-associated and tropical species, we have to rely on other inferences [61].
- (2)
- Parallel sampling inference is based on the absence of species outside subterranean habitats, and allows to assign a status of troglobionts to species that do not exhibit troglomorphy (“obligate troglophiles” of Howarth and Wynne [51]). Statistically meaningful data on the occurrence of species, both inside and outside caves, can be extracted from the literature for well-investigated regions. In lesser known areas such as the tropics, sampling in parallel cave and non-cave habitats may allow us to reasonably assess the ecological status of a species, the strength of such an inference being dependent on sampling efforts and on the rarity of the species.
- (3)
- Taxonomic inference is based on the ecological status of related taxa. Certain groups are known to greatly diversify in subterranean habitats [62,63,64], while others never colonize such habitats. A species from a group which is not prone to underground diversification and lack troglomorphic traits is less likely to be a troglobiont.
- (4)
- Barcoding inference is based on genetic divergence between populations and species. Within a troglophilic or stygophilic species, molecular analyses may characterize populations that live in caves as different from those that live outside [65], leading to split the original species into cave-restricted and non-cave-restricted lineages or species. Barcoding may conversely lead a species to lose its ecological status of cave-restricted if it is shown to be molecularly inseparable from another species which is not cave-restricted.
4.2. Taxonomic Completeness
5. Coldspots, Low-Diversity Spots and Undersampled Spots
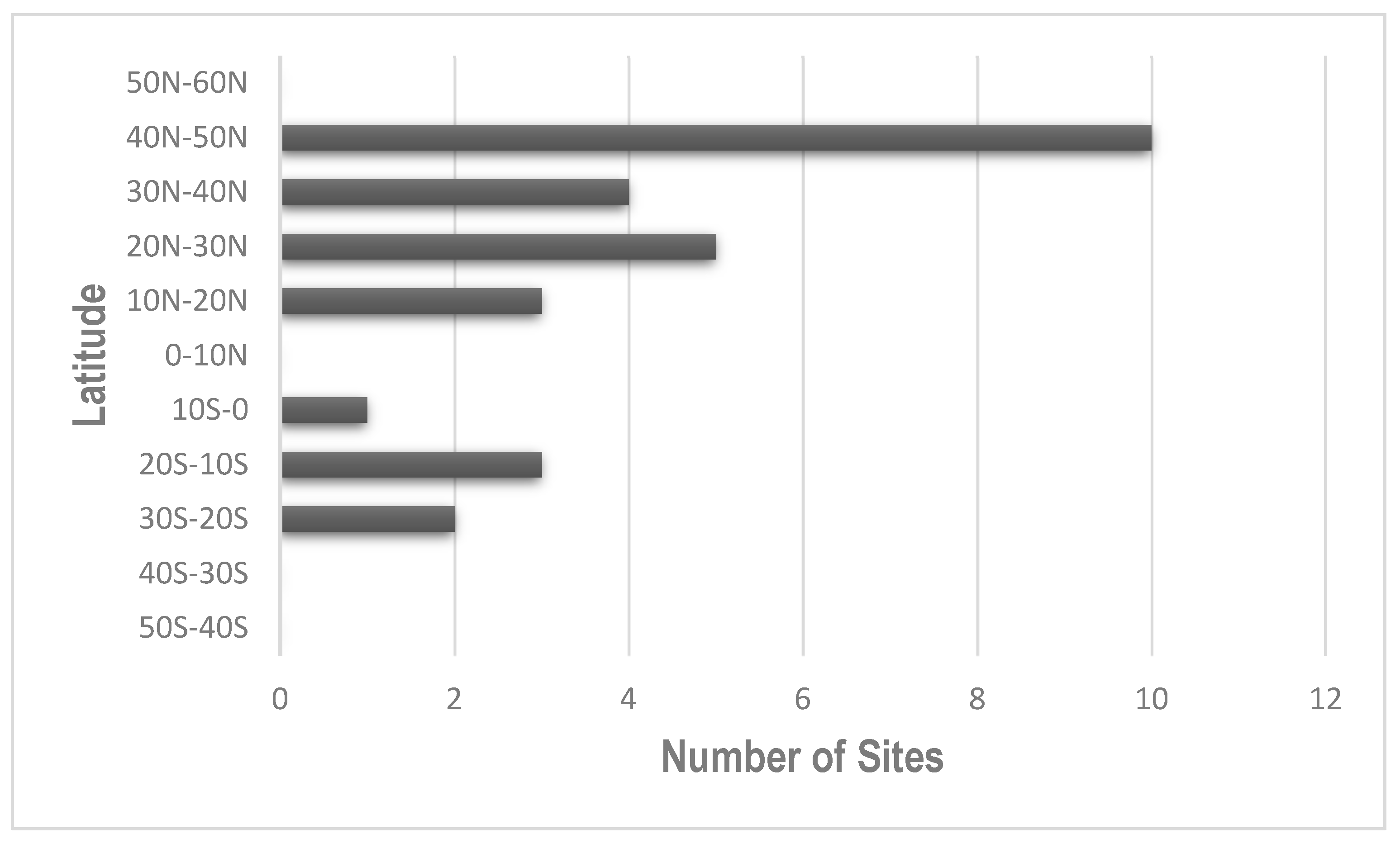
5.1. Where Are the Coldspots?
5.2. Lower Biodiversity Habitats
5.3. Lower Biodiversity Sites and Regions
5.3.1. Lower-Biodiversity Spots in Africa
5.3.2. Lower-Biodiversity Spots in Southern Tropical Asia and the Pacific
5.3.3. Low-Biodiversity Spots in the Temperate Zone
6. Discussion
6.1. The Emerging Global Pattern and Its Causes
- Phreatic aquifers. Relatively few aquifers have been sampled, usually in wells or springs. Five of these sites are on the hotspot list—San Marcos Artesian Well (Texas), Comal Springs (Texas), Robe River (Australia), Lez aquifer (France), Cent Fonts (France), and Baget System (France). The first three sites are also sites of chemoautotrophy, which acts to increase the resource base of subterranean communities.
- Sites with known chemoautotrophy, including Movile Cave and Walsingham Caves.
- Lava tubes. Canarian lava tubes and Australian lava tubes, which occur very close to the surface, have a high species richness once again possibly due to increased resources, including tree roots [137].
- It is next to the Mediterranean Sea, and the marine fauna of the Mediterranean was a source of colonists of subterranean sites, particularly during the Messinian Salinity Crisis.
- It is a region of high annual rainfall, relative to the rest of Europe. Additionally, temperatures are high for that latitude of the Dinarides. Therefore, productivity is higher.
6.2. Weighting Species Value in Conservation of Subterranean Sites
6.3. Vulnerabilities and Threats
6.4. Protection Strategies
- Fern Cave (National Wildlife Refuge);
- Igatu Cave System (Chapada Diamantina National Park);
- Lukina Jama–Trojama Cave System (Velebit National Park);
- Mammoth Cave (National Park);
- Ojo Guareña System (National Monument);
- Movile Cave (owned by the municipality of Mangalia);
- Tham Chiang Dao (Chiang Dao Wildlife Sanctuary);
- Towakkalak System (Bantimurung-Bulu Saraung National Park);
- Vjetrenica Cave System (owned by the municipality of Ravno).
Acknowledgments
Conflicts of Interest
References
- Wilson, E.O. (Ed.) Biodiversity; National Academy Press: Washington, DC, USA, 1988. [Google Scholar]
- Olson, D.M.; Dinerstein, E.; Wikramanayake, E.D.; Burgess, N.D.; Powell, G.V.N.; Underwood, J.A.; D’Amico, J.; Itoua, I.; Strand, H.E.; Morrison, J.C.; et al. Terrestrial ecoregions of the world: A new map of life on earth. Bioscience 1988, 51, 933–938. [Google Scholar] [CrossRef]
- Culver, D.C.; Pipan, T. Shallow Subterranean Habitats. Ecology, Evolution, and Conservation; Oxford University Press: Oxford, UK, 2014. [Google Scholar]
- Gibert, J.; Deharveng, L. Subterranean ecosystems: A truncated functional diversity. Bioscience 2002, 52, 473–481. [Google Scholar] [CrossRef]
- Malard, F.; Boutin, C.; Camacho, A.I.; Ferreira, D.; Michel, G.; Sket, B.; Stoch, F. Diversity patterns of stygobiotic crustaceans across multiple spatial scales in western Europe. Freshw. Biol. 2009, 54, 756–776. [Google Scholar] [CrossRef]
- Culver, D.C.; Sket, B. Hotspots of subterranean biodiversity in caves and wells. J. Cave Karst Stud. 2000, 62, 11–17. [Google Scholar]
- Culver, D.C.; Pipan, T. Subterranean ecosystems. In Encyclopedia of Biodiversity, 7th ed.; Levin, S.A., Ed.; Academic Press: Waltham, MA, USA, 2013; Volume 7, pp. 49–62. [Google Scholar]
- Culver, D.C.; Pipan, T. Biology of Caves and Other Subterranean Habitats, 2nd ed.; Oxford University Press: Oxford, UK, 2019. [Google Scholar]
- Culver, D.C.; Deharveng, L.; Pipan, T.; Bedos, A. An overview of subterranean biodiversity hotspots. Diversity 2021, 13, 487. [Google Scholar] [CrossRef]
- Souza-Silva, M.; Cerqueira, R.F.V.; Pellegrini, T.G.; Ferreira, R.L. Habitat selection of cave-restricted fauna in a new hotspot of subterranean biodiversity in Neotropics. Biodiv. Conserv. 2021, 30, 4223–4250. [Google Scholar] [CrossRef]
- Martínez, A.; Gonzalez, B.C. Volcanic Anchialine Habitats of Lanzarote. In Cave Ecology; Moldovan, O.T., Kováč, L., Halse, S., Eds.; Springer Nature: Cham, Switzerland, 2018; pp. 399–414. [Google Scholar]
- Prié, V.; Alonzo, C.; Bou, C.; Galassi, D.M.P.; Marmonier, P.; Dole-Olivier, M.-J. The Cent Fonts aquifer: An overlooked subterranean biodiversity hotspot in a stygobiont-rich region. Diversity 2024, 16, 50. [Google Scholar] [CrossRef]
- Danielopol, D.L.; Pospisil, P. Hidden biodiversity in the groundwater of the Danube Flood Plain National Park, Austria. Biodiv. Conserv. 2001, 10, 1711–1721. [Google Scholar] [CrossRef]
- Dole, M.-J. La domaine aquatique souterrain de la plaine alluviale du Rhône à l’est de Lyon. I Diversité hydrologique et biocénotique de trois stations representatives de la dynamique fluviale. Vie Et Milieu 1984, 33, 219–229. [Google Scholar]
- Culver, D.C.; Deharveng, L.; Bedos, A.; Lewis, J.J.; Madden, M.; Reddell, J.R.; Sket, B.; Trontelj, P.; White, D. The mid-latitude biodiversity ridge in terrestrial cave fauna. Ecography 2006, 29, 120–128. [Google Scholar] [CrossRef]
- Klimchouk, A. Types and settings of hypogene karst. In Hypogene Karst Regions and Caves of the World; Klimchouk, A., Palmer, A.N., De Waele, J., Auler, A.S., Audra, P., Eds.; Springer Nature: Cham, Switzerland, 2017; pp. 1–42. [Google Scholar]
- De Waele, J.; Gutiérrez, F. Karst Hydrogeology, Geomorphology, and Caves; Wiley Blackwell: Hoboken, NJ, USA, 2022. [Google Scholar]
- Jones, W.K. Water tracing in karst aquifers. In Encyclopedia of Caves, 3rd ed.; White, W.B., Culver, D.C., Pipan, T., Eds.; Elsevier: London, UK, 2019; pp. 1144–1155. [Google Scholar]
- Rouch, R. Considérations sur l’écosystème karstique. Compte Rendu Acad. Sci. 1977, 284, 1101–1103. [Google Scholar]
- Doctor, D.H.; Orndorff, W. Hypogene caves of the central Appalachian Shenandoah Valley. In Hypogene Karst Regions and Caves of the World; Klimchouk, A., Palmer, A.N., De Waele, J., Auler, A.S., Audra, P., Eds.; Springer Nature: Cham, Switzerland, 2017; pp. 691–708. [Google Scholar]
- Brad, T.; Iepure, S.; Sarbu, S.M. The Chemoautotrophically Based Movile Cave Groundwater Ecosystem, a Hotspot of Subterranean Biodiversity. Diversity 2021, 13, 128, Erratum in Diversity 2021, 13, 461. [Google Scholar] [CrossRef]
- Iliffe, T.M.; Calderón-Gutiérrez, F. Bermuda’s Walsingham Caves: A global hotspot for anchialine stygobionts. Diversity 2021, 13, 352. [Google Scholar] [CrossRef]
- Clark, H.L.; Buzatto, B.A.; Halse, S.A. A hotspot of arid zone subterranean biodiversity: The Robe Valley in Western Australia. Diversity 2021, 13, 482. [Google Scholar] [CrossRef]
- Sket, B. The nature of biodiversity in subterranean waters and how it is endangered. Biodiv. Conserv. 1999, 8, 1319–1338. [Google Scholar] [CrossRef]
- Eberhard, S.M.; Howarth, F.G. Undara Lava Cave Fauna in Tropical Queensland with an Annotated List of Australian Subterranean Biodiversity Hotspots. Diversity 2021, 13, 326. [Google Scholar] [CrossRef]
- Delić, T.; Pipan, T.; Ozimec, R.; Culver, D.C.; Zagmajster, M. The subterranean species of the Vjetrenica Cave System in Bosnia and Hercegovina. Diversity 2023, 15, 912. [Google Scholar] [CrossRef]
- Ferreira, R.L.; Berbert-Born, M.; Souza-Silva, M. The Água Clara Cave System in northeastern Brazil: The richest hotspot of subterranean biodiversity in South America. Diversity 2023, 15, 761. [Google Scholar] [CrossRef]
- Ferreira, R.L.; Souza-Silva, M. Beyond expectations: Recent discovery of new cave-restricted species elevates Água Clara Cave System to the richest hotspot of subterranean biodiversity in the Neotropics. Diversity 2023, 15, 1215. [Google Scholar] [CrossRef]
- Gallão, J.E.; Ribeiro, D.B.; Bichuette, M.E. There and back again, the siliciclastic Igatu hotspot caves: Expanding the data for the subterranean fauna in Brazil, Chapada Diamantina region. Diversity 2023, 15, 991. [Google Scholar] [CrossRef]
- Huang, S.; Zhao, M.; Luo, X.; Bedos, A.; Wang, Y.; Chocat, M.; Tian, M.; Liu, W. Feihu Dong, a new hotspot cave of subterranean biodiversity from China. Diversity 2023, 15, 902. [Google Scholar] [CrossRef]
- Oromí, P.; Socorro, S. Biodiversity in the Cueva del Viento lava tube system (Tenerife, Canary Islands). Diversity 2021, 13, 226. [Google Scholar] [CrossRef]
- Camacho, A.I.; Puch, C. Ojo Guareña, a hotspot of subterranean biodiversity in Spain. Diversity 2021, 13, 199. [Google Scholar] [CrossRef]
- Bréhier, F.; Defave, D.; Faille, A.; Bedos, A. The Baget-karstic system and the interstitial environment of Lachein, a hotspot of subterranean biodiversity in the Pyrenees (France). Diversity 2024, 16, 62. [Google Scholar] [CrossRef]
- Faille, A.; Deharveng, L. The Coume Ouarnède system, a hotspot of subterranean biodiversity in Pyrenees (France). Diversity 2021, 13, 419. [Google Scholar] [CrossRef]
- Jourde, H.; Dörfliger, N.; Maréchal, J.-C.; Batiot-Guilhe, C.; Bouvier, C.; Courrioux, G.; Desprats, J.-F.; Fullgraf, T.; Ladouche, B.; Léonardi, V.; et al. Project Gestion Multi-Usages de l’Hydrosystème Karstique du Lez—Synthèse des Connaissances Récentes et Passées—Rapport Final; BRGM, Hydrosciences Montpellier, G-eau, Agence de l’eau Rhône-Méditerranée-Corse: Montpellier, France, 2011. [Google Scholar]
- Lukić, M.; Fišer, C.; Delić, T.; Bilandžija, H.; Pavlek, M.; Komerički, A.; Dražina, T.; Jalžić, B.; Ozimec, R.; Slapnik, R.; et al. Subterranean fauna of the Lukina Jama-Trojama cave system in Croatia: The deepest cave of the Dinaric karst. Diversity 2023, 15, 726. [Google Scholar] [CrossRef]
- Deharveng, L.; Rahmadi, C.; Suhardjono, Y.R.; Bedos, A. The Towakkalak System, a hotspot of subterranean biodiversity in Sulawesi, Indonesia. Diversity 2021, 13, 199. [Google Scholar] [CrossRef]
- Francke, O.F.; Monjaraz-Ruedas, R.; Cruz-López, J. Biodiversity of the Huautla Cave System, Oaxaca, Mexico. Diversity 2021, 13, 429. [Google Scholar] [CrossRef]
- Polak, S.; Pipan, T. The subterranean fauna of Križna jama, Slovenia. Diversity 2021, 13, 210. [Google Scholar] [CrossRef]
- Zagmajster, M.; Polak, S.; Fišer, C. Postojna Planina Cave System in Slovenia, a hotspot of subterranean biodiversity and a cradle of speleobiology. Diversity 2021, 13, 271. [Google Scholar] [CrossRef]
- Deharveng, L.; Ellis, M.; Bedos, A.; Jantarit, S. Tham Chiang Dao: A hotspot of subterranean biodiversity in Northern Thailand. Diversity 2023, 15, 1076. [Google Scholar] [CrossRef]
- Hutchins, B.T.; Gibson, J.R.; Diaz, P.H.; Schwartz, B.F. Stygobiont diversity in the San Marcos Artesian Well and Edwards Aquifer groundwater ecosystem, Texas, USA. Diversity 2021, 13, 234. [Google Scholar] [CrossRef]
- Niemiller, M.L.; Zigler, K.S.; Hinkle, A.; Stephen, C.D.R.; Cramphorn, B.; Higgs, J.; Mann, N.; Miller, B.T.; Kendall Niemiller, K.D.; Smallwood, K.; et al. The Crystal-Wonder Cave System: A new hotspot o In subterranean biodiversity in the southern Cumberland Plateau of south-central Tennessee. Diversity 2023, 15, 801. [Google Scholar] [CrossRef]
- Niemiller, M.L.; Slay, M.E.; Inebnit, T.; Miller, B.; Tobin, B.; Cramphorn, B.; Hinkle, A.; Jones, B.D.; Mann, N.; Kendall Niemiller, K.D.; et al. Fern Cave: A hotspot of subterranean biodiversity in the Interior Low Plateau karst region of Alabama in the southeastern United States. Diversity 2023, 15, 633. [Google Scholar] [CrossRef]
- Niemiller, M.L.; Helf, K.; Toomey, R.S. Mammoth Cave: A hotspot of subterranean biodiversity in the United States. Diversity 2021, 13, 199. [Google Scholar] [CrossRef]
- Deharveng, L.; Le, C.K.; Bedos, A.; Judson, M.L.I.; Le, C.M.; Lukić, M.; Luu, H.T.; Ly, N.S.; Nguyen, T.Q.T.; Truong, Q.T.; et al. A hotspot of subterranean biodiversity on the brink: Mo So cave and the Hon Chong karst of Vietnam. Diversity 2023, 15, 1058. [Google Scholar] [CrossRef]
- Sket, B. Can we agree on an ecological classification of subterranean animals? J. Nat. Hist. 2008, 42, 1549–1563. [Google Scholar] [CrossRef]
- Racovitza, E.G. Essai sur les problèmes biospéologiques. Arch. Zool. Expér. Gen. 1907, 4, 371–488. [Google Scholar]
- Barr, T.C. Cave ecology and the evolution of troglobites. Evol. Biol. 1968, 2, 35–102. [Google Scholar]
- Trajano, E.; de Carvalho, M.R. Towards a biologically meaningful classification of subterranean organisms: A critical analysis of the Schiner-Racovitza system from a historical perspective, difficulties of its application and implications for conservation. Subterr. Biol. 2017, 22, 1–26. [Google Scholar] [CrossRef]
- Howarth, F.G.; Wynne, J.J. Influence of the physical environment on terrestrial cave diversity. In Cave Biodiversity. Speciation and Diversity of Subterranean Fauna of Caves; Wynne, J.J., Ed.; Johns Hopkins University Press: Baltimore, MD, USA; Elsevier: London, UK, 2022; pp. 1–56. [Google Scholar]
- Romero, A. Cave Biology: Life in Darkness; Cambridge University Press: Cambridge, UK, 2009. [Google Scholar]
- Christiansen, K.A. Proposition pour la classification des animaux cavernicoles. Spelunca Mémoires 1962, 2, 75–78. [Google Scholar]
- Culver, D.C.; Pipan, T. Shifting paradigms of the evolution of cave life. Acta Carsologica 2015, 44, 415–425. [Google Scholar] [CrossRef]
- Ormelas-García, C.P.; Pedraza-Lara, C. Phylogeny and evolutionary history of Astyanax mexicanus. In Biology and Evolution of the Mexican Cavefish; Keene, A., Yoshizawa, M., McGaugh, S.E., Eds.; Elsevier; Academic Press: Amsterdam, The Netherland, 2016; pp. 77–92. [Google Scholar]
- Wilkens, H.; Strecker, U. Evolution in the Dark; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Culver, D.C.; Pipan, T.; Fišer, Ž. Ecological and evolutionary jargon in subterranean biology. In Groundwater Ecology and Evolution, 2nd ed.; Malard, F., Griebler, C., Rétaux, S., Eds.; Elsevier; Academic Press: London, UK, 2019; pp. 308–319. [Google Scholar]
- Martinez, A.; Mammola, S. Specialized terminology reduces the number of citations of scientific papers. Proc. Royal Soc. Ser. B 2021, 288, 20202581. [Google Scholar] [CrossRef]
- Deharveng, L.; Bedos, A. Diversity of terrestrial invertebrates in subterranean habitats. In Cave Ecology; Moldovan, O.T., Kováč, L., Halse, S., Eds.; Springer Nature: Cham, Switzerland, 2018; pp. 107–172. [Google Scholar]
- Brignoli, P.M. On some cave spiders from Papua, New Guinea. In Proceedings of the Eight International Congress of Speleology, Bowling Green, KY, USA, 18–24 July 1981; Volume 1, pp. 110–112. [Google Scholar]
- Chapman, P. The invertebrate fauna of caves of the Serrania de San Luis, Edo. Falcon, Venezuela. Trans. Br. Cave Res. Assoc. 1980, 7, 179–199. [Google Scholar]
- Faille, A. Beetles. In Encyclopedia of Caves, 3rd ed.; White, W.B., Culver, D.C., Pipan, T., Eds.; Elsevier: London, UK, 2019; pp. 102–108. [Google Scholar]
- Lukić, M. Collembola. In Encyclopedia of Caves, 3rd ed.; White, W.B., Culver, D.C., Pipan, T., Eds.; Elsevier: London, UK, 2019; pp. 308–319. [Google Scholar]
- Fišer, Z. Niphargus—A model system for evolution and ecology. In Encyclopedia of Caves, 3rd ed.; White, W.B., Culver, D.C., Pipan, T., Eds.; Elsevier: London, UK, 2019; pp. 746–755. [Google Scholar]
- Lukić, M.; Delić, T.; Pavlek, M.; Deharveng, L.; Zagmajster, M. Distribution pattern and radiation of the European subterranean genus Verhoeffiella (Collembola, Entomobryidae). Zool Scr. 2019, 49, 86–100. [Google Scholar] [CrossRef]
- Gittleson, S.M.; Hoover, R.L. Cavernicolous protozoa: Review of the literature and new studies in Mammoth Cave, Kentucky. Ann. Speleol. 1969, 24, 737–776. [Google Scholar]
- Olivier, M.J.; Martin, D.; Bou, C.; Prié, V. Interprétation du Suivi Hydrobiologique de la Faune Stygobie, Réalisé sur le Système Karstique des Cent Fonts Lors du Pompage d’essai. In Système karstique des Cent Fonts. Simulation de scénarios de Gestion de la Ressource; BRGM/RP+54865-FR: Montpellier, France, 2006; pp. 235–275. [Google Scholar]
- Pipan, T. Epikarst—A Promising Habitat; Založba ZRC: Ljubljana, Slovenia, 2005. [Google Scholar]
- Pipan, T.; Culver, D.C. Regional species richness in an obligate subterranean dwelling fauna—Epikarst copepods. J. Biogeoggraph. 2007, 34, 854–861. [Google Scholar] [CrossRef]
- Camacho, A.I.; Valdecasas, A.G.; Rodriguez, J.; Cuezva, S.; Lario, J.; Sánchez-Moral, S. Habitats constraints in epikarstic waters of an Iberian Peninsula cave system. Int. J. Limnol. 2006, 42, 127–140. [Google Scholar] [CrossRef][Green Version]
- Deharveng, L.; Bedos, A. Biodiversity in the tropics. In Encyclopedia of Caves, 3rd ed.; White, W.B., Culver, D.C., Pipan, T., Eds.; Elsevier: London, UK, 2019; pp. 146–162. [Google Scholar]
- Peck, S.B. A review of the cave fauna of Canada, and the composition and ecology of the invertebrate fauna of caves and mines in Ontario. Canad. J. Zool. 1988, 66, 1197–1213. [Google Scholar] [CrossRef]
- Weber, D.; Binder, H.; Pust, J. Germany. In Encyclopaedia Biospeologica Tome 3; Juberthie, C., Decu, V., Eds.; Société de Biospéologie: Moulis, France; Bucarest, Romania, 2001; pp. 693–702. [Google Scholar]
- Dumnicka, E.; Galas, J.; Najberek, K.; Urban, J. The influence of Pleistocene glaciations on the distribution of obligate subterranean aquatic invertebrate fauna in Poland. Zool. Anz. 2020, 286, 90–99. [Google Scholar] [CrossRef]
- Christian, E.; Spötl, C. Karst geology and cave fauna of Austria: A concise review. Int. J. Speleol. 2010, 39, 3. [Google Scholar] [CrossRef]
- Culver, D.C.; Pipan, T.; Gottstein, S. Hypotelminorheic—A unique freshwater habitat. Subterr. Biol. 2006, 4, 1–8. [Google Scholar]
- Gerovasileiou, V.; Nike Bianchi, C. Mediterranean marine caves: A synthesis of current knowledge. Oceanogr. Mar. Biol. Ann. Rev. 2021, 59, 1–88. [Google Scholar]
- Iliffe, T.M.; Alvarez, F. Research in anchialine caves. In Cave Ecology; Moldovan, O.T., Kováč, V., Halse, S., Eds.; Springer: Cham, Switzerland, 2018; pp. 383–398. [Google Scholar]
- Delamare Deboutteville, C. Biologie des Eaux Souterraines Littorales et Continentales; Hermann: Paris, France, 1960. [Google Scholar]
- Svedmark, B. The interstitial fauna of marine sand. Biol. Rev. 1964, 39, 1–42. [Google Scholar] [CrossRef]
- Danielopol, D.L.; Marmonier, P. Aspects of research on groundwater along the Rhône, Rhine, and Danube. Reg. Rivers Res. Manag. 1992, 7, 5–16. [Google Scholar] [CrossRef]
- Růžička, V.; Klimeš, L. Spider (Araneae) communities of scree slopes in the Czech Republic. J. Arachnol. 2005, 33, 280–289. [Google Scholar] [CrossRef]
- Jaume, D.; Boxshall, G.A. Life in extreme ocean environments: Anchialine caves. In Marine Ecology; UNESCO-EOLSS: Oxford, UK, 2009; pp. 230–251. [Google Scholar]
- Vandel, A. Biospeleology: The Biology of Cavernicolous Animals; Freeman, B.F., Translator; Pergamon Press: New York, NY, USA, 1965. [Google Scholar]
- Prevorčnik, S.; Remškar, A.; Fišer, C.; Sket, B.; Bračko, G.; Delić, T.; Mori, N.; Brancelj, N.; Zagmajster, M. Interstitial fauna of the Sava River in eastern Slovenia. Natura Sloveniae 2019, 21, 13–23. [Google Scholar] [CrossRef]
- Ward, J.V.; Stanford, J.A.; Voelz, N.J. Spatial distribution patterns of Crustacea in the floodplain aquifer of an alluvial river. Hydrobiologia 1994, 287, 11–17. [Google Scholar] [CrossRef]
- Juberthie, C.; Delay, B.; Bouillon, M. Extension du milieu souterrain en zone non calcaire: Description d’un nouveau milieu et de son peuplement par les Coléoptères troglobies. Mém. Biospéol. 1980, 7, 19–52. [Google Scholar]
- Jiménez-Valverde, A.; Gilgado, J.D.; Sendra, A.; Pérez-Suárez, G.; Herrero-Borgoñón, J.J.; Ortuño, V.M. Exceptional invertebrate diversity in a scree slope in Eastern Spain. J. Insect Conserv. 2015, 19, 713–728. [Google Scholar] [CrossRef]
- Jureková, N.; Raschmanová, N.; Miklisová, D.; Kováč, L. Mesofauna at the soil-scree interface in a deep karst environment. Diversity 2021, 13, 242. [Google Scholar] [CrossRef]
- Gouze, A. Données thermiques sur le milieu souterrain superficiel et les horizons du sol sus-jacents. Mém. Biospéol. 1988, 15, 175–187. [Google Scholar]
- Pipan, T.; Culver, D.C.; Papi, F.; Kozel, P. Partitioning diversity in subterranean invertebrates: The epikarst fauna of Slovenia. PLoS ONE 2018, 13, e0185991. [Google Scholar] [CrossRef]
- Pipan, T.; Culver, D.C. Epikarst: An important aquatic and terrestrial habitat. In Encyclopedia of Inland Waters, 2nd ed.; Mehner, T., Tockner, K., Eds.; Elsevier: Amsterdam, The Netherland, 2022; pp. 437–438. [Google Scholar]
- Culver, D.C.; Holsinger, J.R.; Feller, D.J. The fauna of seepage springs and other shallow subterranean habitats in the mid-Atlantic Piedmont and Coastal Plain, U.S.A. Northeast. Nat. 2012, 19, 1–42. [Google Scholar] [CrossRef]
- Burch, E.; Culver, D.C.; Alonzo, M.; Malloy, E.J. Landscape features and forest maturity promote the occurrence of macroinvertebrates specialized for seepage springs in urban forests in Washington, DC. Aquat. Conserv. Mar. Freshw. Ecosyst. 2022, 32, 922–929. [Google Scholar] [CrossRef]
- Howarth, F.G. Why the delay in recognizing terrestrial obligate cave species in the tropics? Int. J. Speleol. 2023, 52, 23–43. [Google Scholar] [CrossRef]
- Souza-Silva, M.; Ferreira, R.L. The first two hotspots of subterranean biodiversity in South America. Subterr. Biol. 2016, 19, 1–21. [Google Scholar] [CrossRef]
- Gallão, J.E.; Bichuette, M.E. Taxonomic distinctness and conservation of a new high biodiversity subterranean area in Brazil. An. Acad. Bras. Cienc. 2015, 87, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Beron, P. Comparative study of the invertebrate cave faunas of Southeast Asia and New Guinea. Hist. Nat. Bulg. 2015, 21, 169–210. [Google Scholar]
- Deharveng, L.; Stoch, F.; Gibert, J.; Bedos, A.; Galassi, D.; Zagmajster, M.; Brancelj, A.; Camacho, A.; Fiers, F.; Martin, P.; et al. Groundwater biodiversity in Europe. Freshw. Biol. 2009, 54, 709–728. [Google Scholar] [CrossRef]
- Zagmajster, M.; Culver, D.C.; Christman, M.C.; Sket, B. Evaluating the sampling bias in pattern of subterranean species richness—Combining approaches. Biodiv. Conserv. 2010, 19, 3035–3048. [Google Scholar] [CrossRef]
- Tian, M.; Huang, S.; Wang, X.; Tang, M. Contributions to the knowledge of subterranean trechine beetles in southern China’s karsts: Five new genera (Insecta, Coleoptera, Carabidae, Trechinae). ZooKeys 2016, 564, 121. [Google Scholar] [CrossRef]
- Jeannel, R.; Racovitza, E. Biospeologica XXXIX—Enumérations des grottes visitées 1913-1917 (sixième série). Arch. Zool. Exp. Gén. 1918, 57, 203–470. [Google Scholar]
- Juberthie, C.; Decu, V. Africa, généralités. In Encyclopaedia Biospeologica Tome 3; Juberthie, C., Decu, V., Eds.; Société de Biospéologie: Moulis, France; Bucarest, Romania, 2001; pp. 1461–1462. [Google Scholar]
- Messana, G.; Chelazzi, L.; Baccetti, N. Biospeleology of Somalia. Mugdile and Showli Berdi Caves. Monitore Zoologico Italiano Supplemento 1985, 20, 325–340. [Google Scholar] [CrossRef]
- Ferreira, R.L.; Giribet, G.; Du Preez, G.; Ventouras, O.; Janion, C.; Silva, M.S. The Wynberg cave system, the most important site for cave fauna in South Africa at risk. Subterr. Biol. 2010, 36, 73–81. [Google Scholar] [CrossRef]
- Juberthie, C.; Decu, V. Tanzanie. In Encyclopaedia Biospeologica Tome 3; Juberthie, C., Decu, V., Eds.; Société de Biospéologie: Moulis, France; Bucarest, Romania, 2001; pp. 1703–1712. [Google Scholar]
- Moseley, M.; Lim, T.W.; Lim, T.T. Fauna reported from Batu Caves, Selangor, Malaysia: Annotated checklist and bibliography. Cave Karst Sci. 2012, 39, 77–92. [Google Scholar]
- Chapman, P. The invertebrate fauna of the caves of Gunung Mulu National Park. Sarawak Mus. J. 1984, 30, 1–8. [Google Scholar]
- Harries, D.B.; Kharkongor, I.J.; Saikia, U. The biota of Siju Cave, Meghalaya, India: A comparison of biological records from 1922 and from 2019. Cave Karst Sci. 2020, 47, 119–130. [Google Scholar]
- Howarth, F.G. Cavernicoles in lava tubes in the island of Hawaii. Science 1972, 175, 325–326. [Google Scholar] [CrossRef] [PubMed]
- Wessel, A.; Hoch, H.; Asche, M.; von Rintelen, T.; Stelbrink, B.; Heck, U.; Stone, F.D.; Howarth, F.G. Founder effects initiated rapid species radiation in Hawaiian cave planthoppers. Proc. Nat. Acad. Sci. USA 2013, 110, 9391–9396. [Google Scholar] [CrossRef] [PubMed]
- Oromí, P. Researches in lava tubes. In Cave Ecology; Moldovan, O.T., Kováč, Ľ., Halse, S., Eds.; Springer Nature: Cham, Switzerland, 2018; pp. 369–382. [Google Scholar]
- Deharveng, L.; Lips, J.; Rahmadi, C. Focus on guano. In The Natural History of Santo: Caves and Soils; Bouchet, P., Le Guyader, H., Pascal, O., Eds.; Patrimoines Naturels; Muséum National d’Histoire Naturelle: Paris, France; IRD: Marseille, France; Pro-Natura International: Paris, France, 2011; Volume 70, pp. 300–305. [Google Scholar]
- Decu, V. Bat guano ecosystem a new classifications and some considerations with special references to Neotropical data. Res. Exp. Biol. 1981, 1981, 9–15. [Google Scholar]
- Smith, G.B. Biospeleology. In Report of the 1978 Speleological Expedition to the Atea Kananda, Southern Highlands, Papua New Guinea; James, J.M., Dyson, H.J., Eds.; Speleological Research Council: Sydney, Australia, 1980; pp. 121–129. [Google Scholar]
- Chapman, P. Speleobiology. Trans. Brit. Cave Res. Assoc. 1975, 3, 192–203. [Google Scholar]
- Beron, P. Interim zoological results from the British Speleological Expedition to Papua New Guinea, 1975. Cave Karst Sci. 2018, 45, 77–82. [Google Scholar]
- Martin, P.; De Broyer, C.; Fiers, F.; Michel, G.; Sablon, R.; Wouters, K. Biodiversity of Belgian groundwater fauna in relation to environmental conditions. Freshw. Biol. 2009, 54, 814–829. [Google Scholar] [CrossRef]
- Kocot-Zalewska, J.; Domagała, P.J. Terrestrial invertebrate fauna of Polish caves–A summary of 100 years of research. Subterr. Biol. 2020, 33, 45–69. [Google Scholar] [CrossRef]
- May, B.M.; Decu, V.; Juberthie, C. New Zealand. In Encyclopaedia Biospeologica Tome 3; Juberthie, C., Decu, V., Eds.; Société de Biospéologie: Moulis, France; Bucarest, Romania, 2001; pp. 2079–2091. [Google Scholar]
- Culver, D.C.; Christman, M.C.; Elliott, W.R.; Hobbs, H.H., III; Reddell, J.R. The North American obligate cave fauna: Regional patterns. Biodiv. Conserv. 2003, 12, 441–468. [Google Scholar] [CrossRef]
- Knight, L.R.F.D. The aquatic macro-invertebrate fauna of Swildon’s Hole, Mendip Hills, Somerset, UK. Cave Karst Sci. 2011, 38, 81–92. [Google Scholar]
- Holsinger, J.R. The cave fauna of Pennsylvania. In Geology and Biology of Pennsylvania Caves; White, W.B., Ed.; Pennsylvania Geological Survey: Harrisburg, PA, USA, 1976; pp. 72–87. [Google Scholar]
- Barr, T.C. A synopsis of cave beetles of the genus Pseudanophthalmus of the Mitchell Plain in southern Indiana (Coleoptera, Carabidae). Ame. Midland Nat. 1960, 63, 307–320. [Google Scholar] [CrossRef]
- Coutterand, S.; Schoeneich, P.; Nicoud, G. Le Lobe Glaciaire Lyonnais au Maximum Würmien: Glacier du Rhône ou/et Glaciers Savoyard? HAL, 2011. pp. 11–22. Available online: https://shs.hal.science/halshs-00389085 (accessed on 15 December 2023).
- Strinati, P. Faune cavernicole de la Suisse. Ann. Spéléol. 1966, 21, 5–268 and 357–371. [Google Scholar]
- Assmann, T.; Casale, A.; Drees, C.; Habel, J.C.; Matern, A.; Schuldt, A. The dark side of relict species biology: Cave animals as ancient lineages. In Relict Species: Phylogeography and Conservation Biology; Springer: Berlin/Heidelberg, Germany, 2010; pp. 91–103. [Google Scholar]
- Moldovan, O.T.; Iepure, S.; Brad, T.; Kenesz, M.; Mirea, I.C.; Năstase-Bucur, R. Database of Romanian cave invertebrates with a Red List of cave species and a list of hotspot/coldspot caves. Biodivers. Data J. 2020, 8, e53571. [Google Scholar] [CrossRef]
- Gibert, J. Ecologie d’un système karstique jurassien. Hydrogéologie, dérive animale, transits de matières, dynamique de la population de Niphargus (Crustacé Amphipode). Mém. Biospéologie 1986, 13, 1–379. [Google Scholar]
- Šebela, S.; Kogovšek, B.; Kozel, P.; Mulec, J.; Petrič, M.; Pipan, T. Report of ZRK ZRC SAZU on the Performance of the Concession in the Postojna and Predjama Cave Systems in 2022: Climate Monitoring, Biological Monitoring, Expert Supervision, Water Monitoring and Inventory of Research; Znanstvenoraziskovalni Center SAZU, Inštitut za Raziskovanje Krasa: Postojna, Slovenia, 2023; p. 85. [Google Scholar]
- Latella, L. Does size matter? Two subterranean biodiversity hotspots in the Lessini Mountains in the Veneto Prealps in Northern Italy. Diversity 2024, 16, 25. [Google Scholar] [CrossRef]
- Juberthie, C.; Ginet, R. France. In Encyclopaedia Biospeologica Tome 1; Juberthie, C., Decu, V., Eds.; Société de Biospéologie: Moulis, France; Bucarest, Romania, 1994; pp. 665–692. [Google Scholar]
- Zagmajster, M.; Eme, D.; Fišer, C.; Galassi, D.; Marmonier, P.; Stoch, F.; Cornu, J.F.; Malard, F. Geographic variation in range size and beta diversity of groundwater crustaceans: Insights from habitats with low thermal seasonality. Glob. Ecol. Biogeog. 2014, 10, 1135–1145. [Google Scholar] [CrossRef]
- Jeannel, R. Les Fossiles Vivants des Cavernes; Gallimard: Paris, France, 1943. [Google Scholar]
- Barr, T.C. Observations on the ecology of caves. Amer. Natur. 1967, 101, 475–491. [Google Scholar] [CrossRef]
- Saclier, N.; Duchemin, L.; Konecny-Dupré, L.; Grison, P.; Eme, D.; Martin, C.; Callou, C.; Malard, F. A collaborative backbone resource for comparative studies of subterranean evolution: The World Asellidae database. Mol. Ecol. Resour. 2024, 24, e13882. [Google Scholar] [CrossRef] [PubMed]
- Howarth, F.G.; James, S.A.; McDowell, W.; Preston, D.J.; Imada, C.T. Identification of roots in lava tube caves using molecular techniques: Implications for conservation of cave arthropod faunas. J. Insect Conserv. 2007, 11, 251–261. [Google Scholar] [CrossRef]
- Christman, M.C.; Doctor, D.H.; Niemiller, M.L.; Weary, D.J.; Young, J.A.; Zigler, K.S.; Culver, D.C. Predicting the occurrence of cave-inhabiting fauna based on features of the Earth surface environment. PLoS ONE 2016, 11, e0160408. [Google Scholar] [CrossRef] [PubMed]
- Barr, T.C. Ecological studies in the Mammoth Cave system of Kentucky. I. The biota. Int. J. Speleol. 1968, 3, 147–204. [Google Scholar] [CrossRef]
- Christman, M.C.; Culver, D.C.; Madden, M.; White, D. Patterns of endemism of the eastern North American cave fauna. J. Biogeogr. 2005, 32, 1441–1452. [Google Scholar] [CrossRef]
- Golovatch, S.I.; Geoffroy, J.J.; Mauriès, J.P.; Vanden Spiegel, D. New species of the millipede genus Glyphiulus Gervais, 1847 from the granulatus-group (Diplopoda: Spirostreptida: Cambalopsidae). Arthropoda Selecta 2011, 20, 65–114. [Google Scholar] [CrossRef]
- Michel, G.; Malard, F.; Deharveng, L.; Di Lorenzo, T.; Sket, B.; De Broyer, C. Reserve selection for conserving groundwater biodiversity. Freshw. Biol. 2009, 54, 861–876. [Google Scholar] [CrossRef]
- Souza Silva, M.; Martins, R.P.; Ferreira, R.L. Cave conservation priority index to adopt a rapid protection strategy: A case study in Brazilian Atlantic rain forest. Environ. Manag. 2015, 55, 279–295. [Google Scholar] [CrossRef] [PubMed]
- Mammola, S.; Piano, E.; Cardoso, P.; Vernon, P.; Domìnguez-Viller, D.; Culver, D.C.; Pipan, T.; Isaia, M. Climate change going deep: The effects of global climatic alterations on cave ecosystems. Anthr. Rev. 2019, 6, 98–116. [Google Scholar] [CrossRef]
- Fong, D.W. Management of subterranean fauna in karst. In Karst Management; van Beynen, P.E., Ed.; Springer: Cham, Switzerland, 2011; pp. 201–224. [Google Scholar]
- Di Lorenzo, T.; Avramov, M.; Galassi, D.M.P.; Iepure, S.; Mammola, S.; Reboleira, A.S.P.S.; Hervant, F. Physiological tolerance and ecotoxicological constraints on groundwater fauna. In Groundwater Ecology and Evolution, 2nd ed.; Malard, F., Griebler, D., Retaux, S., Eds.; Elsevier; Academic Press: London, UK, 2023; pp. 457–482. [Google Scholar]
- Mammola, S.; Goodacre, S.L.; Isaia, M. Climate change may drive cave spiders to extinction. Ecography 2018, 41, 233–243. [Google Scholar] [CrossRef]
- Medina, M.J.; Antić, D.; Borges, P.A.; Borko, Š.; Fišer, C.; Lauritzen, S.E.; Martín, J.L.; Oromí, P.; Pavlek, M.; Premate, E.; et al. Temperature variation in caves and its significance for subterranean ecosystems. Sci. Rep. 2023, 13, 20735. [Google Scholar] [CrossRef] [PubMed]
- Šebela, S.; Turk, J.; Pipan, T. Cave micro-climate and tourism: Towards 200 years (1819–2015) at Postojnska jama (Slovenia). Cave Karst Sci. 2015, 42, 78–85. [Google Scholar]
- Šebela, S. Natural and Anthropogenic Impacts on Cave Climates: Postojna and Predjama Show Caves (Slovenia); Elsevier: London, UK, 2021. [Google Scholar]
- Vermeulen, J.; Whitten, T. Biodiversity and Cultural Property in the Management of Limestone Resources; World Bank: Washington, DC, USA, 1999. [Google Scholar]
- Thibaud, J.M. Biologie et Écologie des Collemboles Hypogastruridae Édaphiques et Cavernicoles; Éditions du Muséum: Paris, France, 1970. [Google Scholar]
- Wynne, J.J.; Bernard, E.C.; Howarth, F.G.; Sommer, S.; Soto-Adames, F.N.; Taiti, S.; Mockford, E.L.; Horrocks, M.; Pakarati, L.; Pakarti-Hotus, V. Disturbance relicts in a rapidly changing world: The Rapa Nui (Easter Island) factor. Bioscience 2014, 64, 711–718. [Google Scholar] [CrossRef]
- Deharveng, L.; Bedos, A. The cave fauna of southeast Asia. Origin, evolution, and ecology. In Subterranean Ecosystems; Wilkens, H., Culver, D.C., Humphreys, W.F., Eds.; Elsevier: Amsterdam, The Netherland, 2000; pp. 603–632. [Google Scholar]
- Spanjer, G.R.; Fenton, M.B. Behavioral responses of bats to gates at caves and mines. Wildl. Soc. Bull. 2005, 33, 1101–1112. [Google Scholar] [CrossRef]
- Meierhofer, M.B.; Johnson, J.S.; Perez-Jimenez, J.; Ito, F.; Webela, P.W.; Wiantoro, S.; Bernard, E.; Tanalgo, K.C.; Hughes, A.; Cardoso, P.; et al. Effective conservation of subterranean-roosting bats. Conserv. Biol. 2023, 38, e14157. [Google Scholar] [CrossRef]


| Country | Cave | Latitude | Longitude | Features |
|---|---|---|---|---|
| BRA | Areias Cave System | −24.6 | −48.7 | Karstic |
| Tropic of Capricorn | −23.5 | |||
| AUS | Robe River Well 2A | −21.6 | 115.9 | Calcrete/ Hypogene |
| AUS | Undara Lava Tube System | −18.2 | 144.5 | Volcanic |
| BRA | Água Clara System | −13.8 | −44.0 | Karstic |
| BRA | Igatu Cave System | −12.9 | −41.4 | Silici-clastic |
| IDN | Towakkalak System | −5.0 | 119.6 | Karstic |
| Equator | 0 | |||
| VNM | Hang Mo So | 10.2 | 104.6 | Karstic |
| MEX | Sistema Huautla | 18.1 | −96.8 | Karstic |
| THA | Tham Chiang Dao | 19.4 | 98.9 | Karstic |
| Tropic of Cancer | 23.5 | |||
| ESP | Cueva del Viento System | 28.4 | −16.7 | Volcanic |
| ESP | Túnel de la Atlantida | 29.2 | −13.5 | Volcanic |
| CHN | Feihu Dong | 29.2 | 109.3 | Karstic |
| USA | Comal Springs | 29.7 | −98.1 | Hypogene |
| USA | San Marcos Artesian Well | 29.9 | −97.9 | Hypogene |
| BMU | Walsingham Caves | 32.3 | −64.8 | Hypogene |
| USA | Fern Cave | 34.7 | −86.3 | Karstic |
| USA | Crystal-Wonder Cave System | 35.3 | −85.9 | Karstic |
| USA | Mammoth Cave | 37.1 | −86.1 | Karstic |
| BIH | Vjetrenica Cave System | 42.9 | 18.0 | Karstic |
| ESP | Ojo Guareña System | 43.0 | −3.7 | Karstic |
| FRA | Baget System | 43.0 | 1.0 | Karstic |
| FRA | Coume Ouarnède System | 43.0 | 0.9 | Karstic |
| ROU | Movile Cave | 43.8 | 28.6 | Hypogene |
| FRA | Lez Aquifer | 43.8 | 3.8 | Karstic |
| FRA | Cent Fonts | 43.8 | 3.6 | Karstic |
| HRV | Lukina Jama-Trojama Cave System | 44.8 | 15.0 | Karstic |
| SVN | Križna Jama | 45.7 | 14.4 | Karstic |
| SVN | Postojna Planina Cave System | 45.8 | 14.2 | Karstic |
| Country | Cave | S | T | S+T | AOG | MC | S* | TOG | T* | S*+T* | un | un% | Source |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AUS | Robe River Well 2A (a) | 43 | 0 | 43 | 11 | 21 | 11 | 0 | 0 | 11 | 21 | 49 | [23] |
| AUS | Undara Lava Tube System | 1 | 30 | 31 | 0 | 0 | 1 | 0 | 30 | 31 | 25 | 81 | [25] |
| BIH | Vjetrenica Cave System | 48 | 45 | 93 | 8 | 8 | 32 | 1 | 44 | 76 | 6 | 6 | [26] |
| BMU | Walsingham Caves | 63 | 0 | 63 | 8 | 29 | 26 | 0 | 0 | 26 | 0 | 0 | [22] |
| BRA | Água Clara Cave System | 8 | 33 | 41 | 0 | 0 | 8 | 1 | 32 | 40 | 30 | 73 | [27,28] |
| BRA | Areias Cave System | 6 | 22 | 28 | 1 | 0 | 5 | 0 | 22 | 27 | 14 | 50 | [10] |
| BRA | Igatu Cave System | 2 | 35 | 37 | 0 | 0 | 2 | 3 | 32 | 34 | 29 | 78 | [29] |
| CHN | Feihu Dong | 4 | 23 | 27 | 0 | 1 | 3 | 1 | 22 | 25 | 14 | 52 | [30] |
| ESP | Cueva del Viento System (b) | 0 | 42 | 42 | 0 | 0 | 0 | 0 | 42 | 42 | 0 | 0 | [31] |
| ESP | Ojo Guareña System | 46 | 8 | 54 | 14 | 24 | 8 | 2 | 6 | 14 | 23 | 43 | [32] |
| ESP | Túnel de la Atlantida | 34 | 0 | 34 | 0 | 12 | 22 | 0 | 0 | 22 | 4 | 12 | [11] |
| FRA | Baget System | 40 | 17 | 57 | 4 | 27 | 9 | 1 | 16 | 25 | 5 | 9 | [33] |
| FRA | Cent Fonts (c) | 43 | 1 | 44 | 2 | 19 | 22 | 0 | 1 | 23 | 4 | 9 | [12] |
| FRA | Coume Ouarnède System (d) | 17 | 17 | 34 | 1 | 8 | 8 | 2 | 15 | 23 | 1 | 3 | [34] |
| FRA | Lez Aquifer (e) | 39 | 0 | 39 | 2 | 15 | 22 | 0 | 0 | 22 | 7 | 18 | [12,35] |
| HRV | Lukina Jama-Trojama Cave System | 16 | 25 | 41 | 0 | 0 | 16 | 2 | 23 | 39 | 20 | 49 | [36] |
| IDN | Towakkalak System | 10 | 26 | 36 | 0 | 0 | 10 | 1 | 25 | 35 | 18 | 50 | [37] |
| MEX | Sistema Huautla | 0 | 27 | 27 | 0 | 0 | 0 | 0 | 27 | 27 | 10 | 37 | [38] |
| ROU | Movile Cave (f) | 13 | 25 | 38 | 3 | 3 | 7 | 1 | 24 | 31 | 3 | 8 | [21] |
| SVN | Križna Jama (g) | 31 | 28 | 59 | 10 | 10 | 11 | 0 | 28 | 39 | 5 | 8 | [39] |
| SVN | Postojna Planina Cave System (h) | 62 | 43 | 105 | 12 | 29 | 21 | 2 | 41 | 62 | 11 | 10 | [40] |
| THA | Tham Chiang Dao | 4 | 33 | 37 | 2 | 2 | 0 | 1 | 32 | 32 | 17 | 46 | [41] |
| USA | Comal Springs (i, j) | 32 | 0 | 32 | 3 | 2 | 27 | 0 | 0 | 27 | 4 | 13 | [42] |
| USA | Crystal-Wonder Cave System (k) | 8 | 23 | 31 | 0 | 0 | 8 | 1 | 22 | 30 | 3 | 10 | [43] |
| USA | Fern Cave (l) | 8 | 19 | 27 | 2 | 0 | 6 | 1 | 18 | 23 | 7 | 26 | [44] |
| USA | Mammoth Cave (m) | 17 | 32 | 49 | 3 | 2 | 12 | 6 | 26 | 38 | 0 | 0 | [45] |
| USA | San Marcos Artesian Well (j) | 55 | 0 | 55 | 8 | 15 | 32 | 0 | 0 | 32 | 16 | 29 | [42] |
| VNM | Hang Mo So | 0 | 27 | 27 | 0 | 0 | 0 | 1 | 26 | 26 | 20 | 74 | [46] |
| Country | Cave System | Arachnida | Coleoptera | Ratio | ||
|---|---|---|---|---|---|---|
| N | % | N | % | Co/Ar | ||
| FRA | Baget System | 2 | 22.2 | 7 | 77.8 | 3.50 |
| FRA | Coume Ouarnède System | 2 | 25.0 | 6 | 75.0 | 3.00 |
| BIH | Vjetrenica Cave System | 10 | 43.5 | 13 | 56.5 | 1.30 |
| USA | Crystal-Wonder Cave System | 5 | 50.0 | 5 | 50.0 | 1.00 |
| CHN | Feihu Dong | 5 | 50.0 | 5 | 50.0 | 1.00 |
| SVN | Križna Jama | 7 | 50.0 | 7 | 50.0 | 1.00 |
| SVN | Postojna Planina Cave System | 10 | 52.6 | 9 | 47.4 | 0.90 |
| ESP | Cueva del Viento System | 16 | 55.2 | 13 | 44.8 | 0.81 |
| USA | Mammoth Cave | 10 | 55.6 | 8 | 44.4 | 0.80 |
| ROU | Movile Cave | 8 | 61.5 | 5 | 38.5 | 0.63 |
| HRV | Lukina Jama-Trojama Cave System | 5 | 62.5 | 3 | 37.5 | 0.60 |
| USA | Fern Cave | 6 | 66.7 | 3 | 33.3 | 0.50 |
| AUS | Undara Lava Tube System | 10 | 71.4 | 4 | 28.6 | 0.40 |
| BRA | Areias Cave System | 6 | 75.0 | 2 | 25.0 | 0.33 |
| VNM | Hang Mo So | 8 | 80.0 | 2 | 20.0 | 0.25 |
| BRA | Água Clara Cave System | 9 | 81.8 | 2 | 18.2 | 0.22 |
| THA | Tham Chiang Dao | 10 | 83.3 | 2 | 16.7 | 0.20 |
| BRA | Igatu Cave System | 13 | 86.7 | 2 | 13.3 | 0.15 |
| IDN | Towakkalak System | 11 | 91.7 | 1 | 8.3 | 0.09 |
| MEX | Sistema Huautla | 18 | 100.0 | 0 | 0.0 | 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deharveng, L.; Bedos, A.; Pipan, T.; Culver, D.C. Global Subterranean Biodiversity: A Unique Pattern. Diversity 2024, 16, 157. https://doi.org/10.3390/d16030157
Deharveng L, Bedos A, Pipan T, Culver DC. Global Subterranean Biodiversity: A Unique Pattern. Diversity. 2024; 16(3):157. https://doi.org/10.3390/d16030157
Chicago/Turabian StyleDeharveng, Louis, Anne Bedos, Tanja Pipan, and David C. Culver. 2024. "Global Subterranean Biodiversity: A Unique Pattern" Diversity 16, no. 3: 157. https://doi.org/10.3390/d16030157
APA StyleDeharveng, L., Bedos, A., Pipan, T., & Culver, D. C. (2024). Global Subterranean Biodiversity: A Unique Pattern. Diversity, 16(3), 157. https://doi.org/10.3390/d16030157








