Aspects of Breeding Performance of Scopoli’s Shearwater (Calonectris diomedea): The Case of the Largest Colony in Greece
Abstract
1. Introduction
2. Materials and Methods
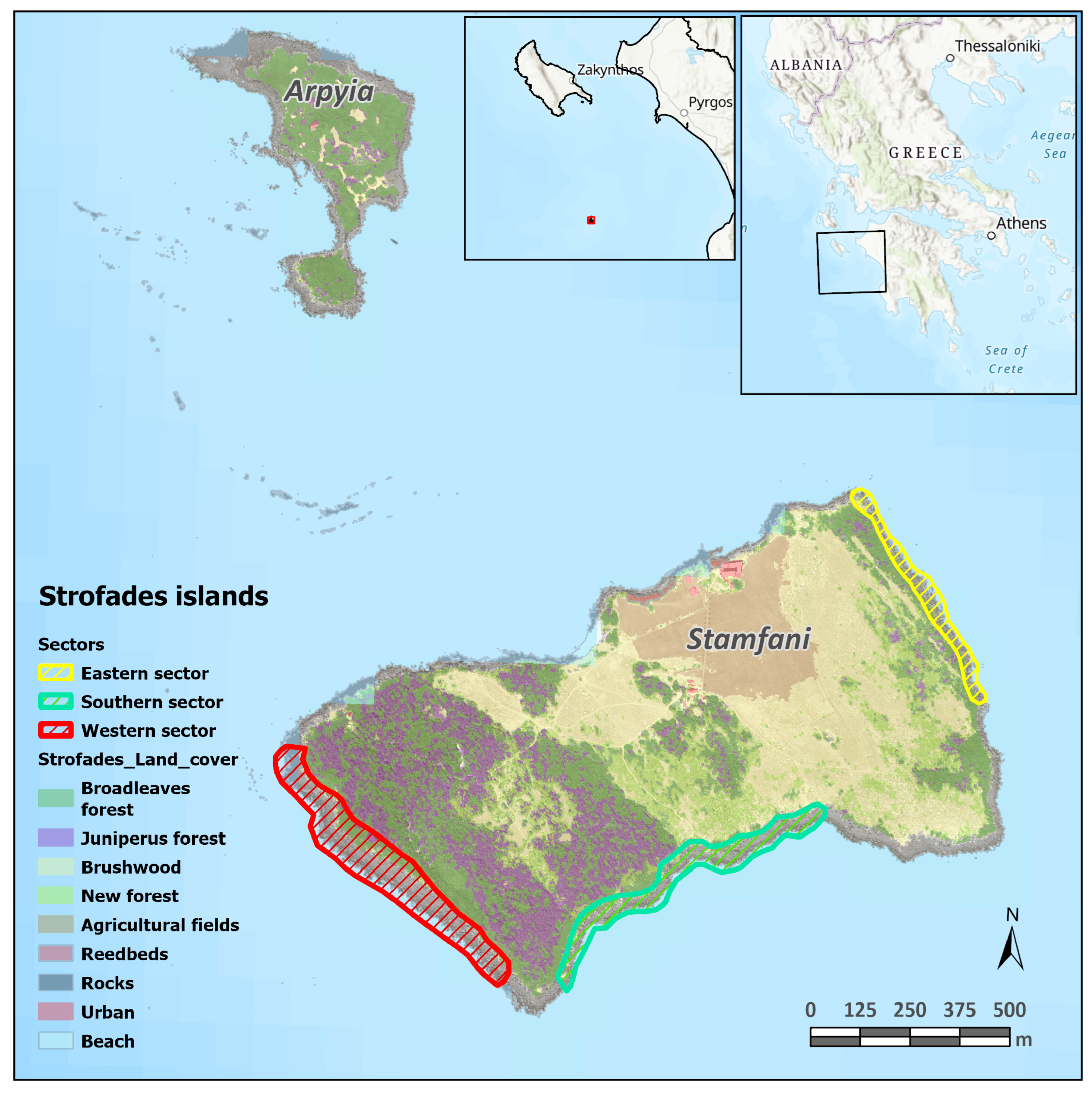
2.1. Study Area
2.2. Breeding Performance
2.3. Factors Influencing Breeding Success
2.4. Statistical Analysis
3. Results
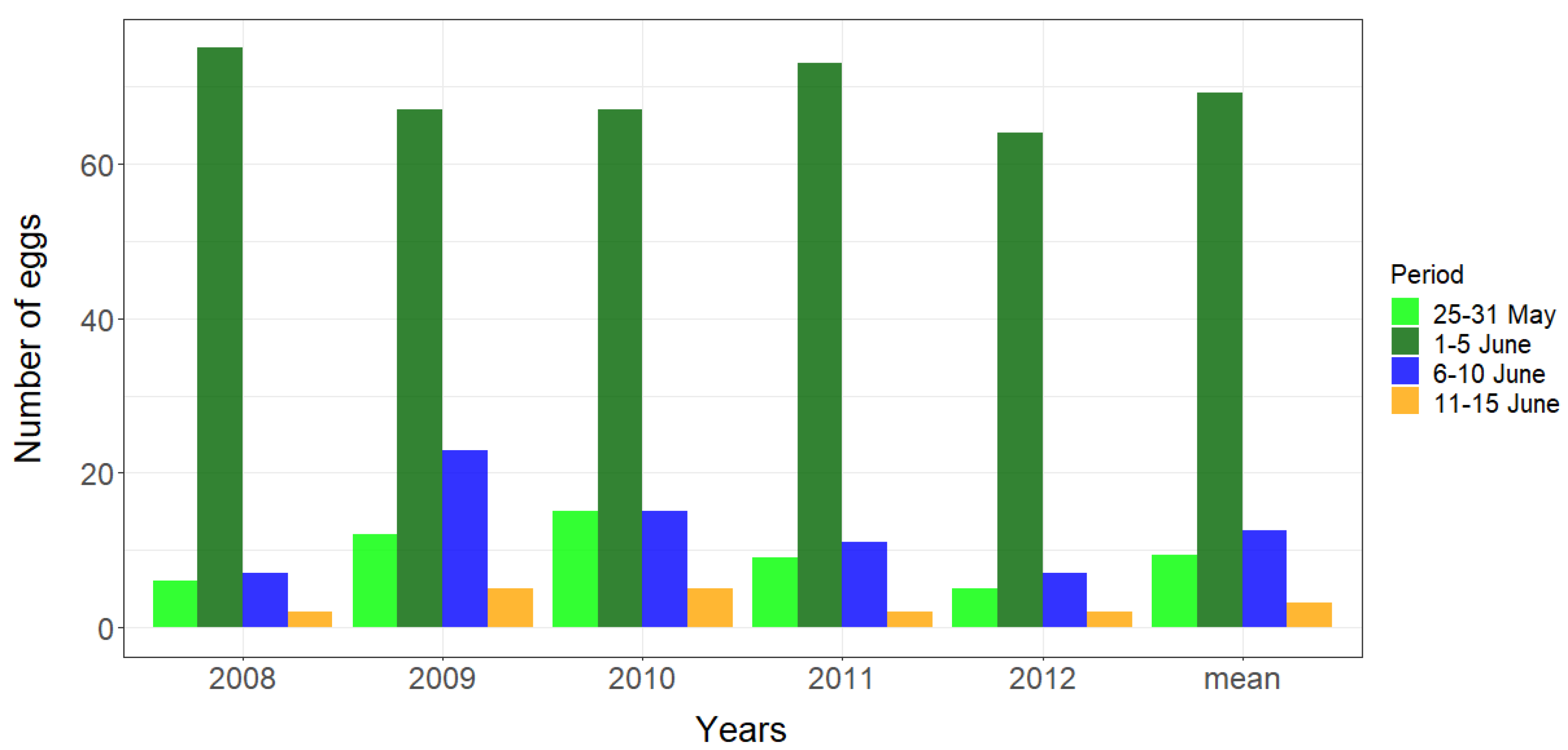
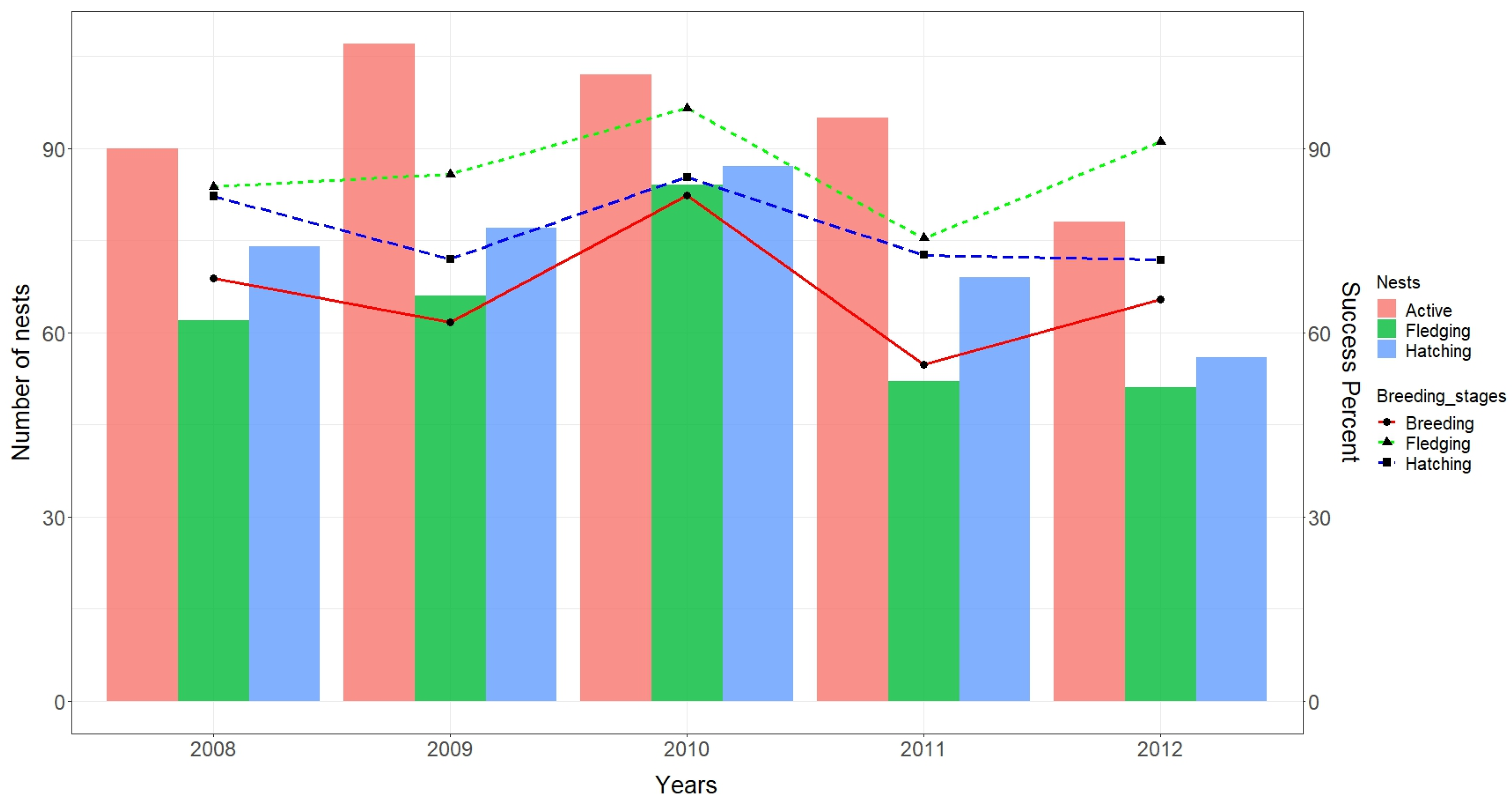
3.1. Breeding Phenology
3.2. Breeding Performance
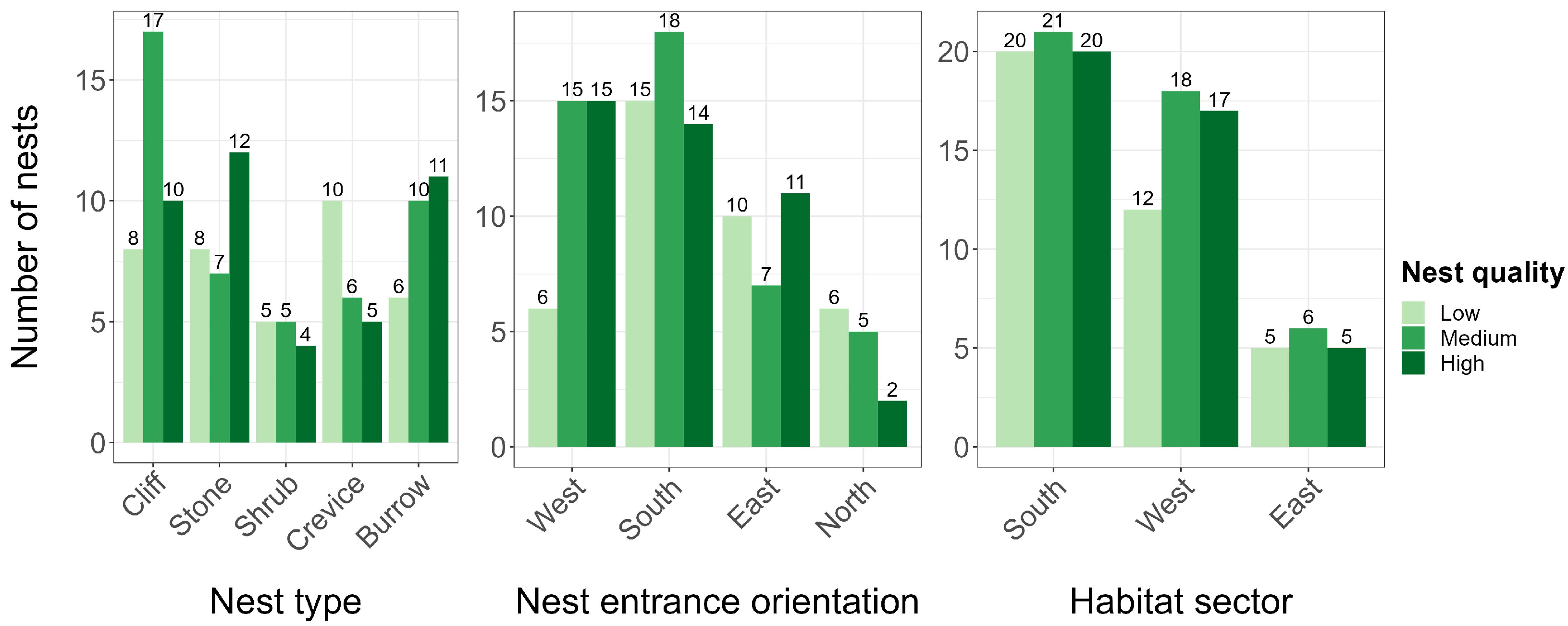
3.3. Factors Influencing Breeding Success
4. Discussion
4.1. Breeding Phenology
4.2. Breeding Performance
4.3. Factors Influencing Breeding Success
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harrison, P.; Perrow, M.R.; Larsson, H. Seabirds. The New Identification Guide, 1st ed.; Lynx Edicions: Barcelona, Spain, 2021. [Google Scholar]
- Croxall, J.P.; Butchart, S.M.; Lascelles, B.; Stattersfield, A.J.; Sullivan, B.; Symes, A.; Taylor, P. Seabird conservation status, threats and priority actions: A global assessment. Bird Conserv. Int. 2012, 22, 1–34. [Google Scholar] [CrossRef]
- BirdLife International. State of the World’s Birds: Taking the Pulse of the Planet; BirdLife International: Cambridge, UK, 2018. [Google Scholar]
- Dias, M.P.; Martin, R.; Pearmain, E.J.; Burfield, I.J.; Small, C.; Phillips, R.A.; Yates, O.; Lascelles, B.; Borboroglu, P.G.; Croxall, J.P. Threats to seabirds: A global assessment. Biol. Conserv. 2019, 237, 525–537. [Google Scholar] [CrossRef]
- Hamer, K.C.; Schreiber, E.A.; Burger, J. Breeding Biology, Life Histories, and Life History–Environment Interactions in Seabirds. In Biology of Marine Birds; Schreiber, E.A., Burger, J., Eds.; Chapter 8; CRC Press LLC: Boca Raton, FL, USA, 2002; pp. 217–262. [Google Scholar]
- Parsons, M.; Mitchell, I.; Butler, A.; Ratcliffe, N.; Frederiksen, M.; Foster, S.; Reid, J.B. Seabirds as indicators of the marine environment. ICES J. Mar. Sci. 2008, 65, 1520–1526. [Google Scholar] [CrossRef]
- Piatt, J.F.; Sydeman, W.J. Seabirds as indicators of marine ecosystems. Mar. Ecol. Prog. Ser. 2007, 352, 199–204. [Google Scholar] [CrossRef]
- Iverson, S.J.; Springer, A.M.; Kitaysky, A.S. Seabirds as indicators of food web structure and ecosystem variability: Qualitative and quantitative diet analyses using fatty acids. Mar. Ecol. Prog. Ser. 2007, 352, 235–244. [Google Scholar] [CrossRef]
- Rodríguez, A.; Arcos, J.M.; Bretagnolle, V.; Dias, M.P.; Holmes, N.D.; Louzao, M.; Provencher, J.; Raine, A.F.; Ramírez, F.; Rodríguez, B.; et al. Future Directions in Conservation Research on Petrels and Shearwaters. Front. Mar. Sci. 2019, 6, 94. [Google Scholar] [CrossRef]
- Mougin, J.-L.; Jouanin, C.; Roux, F. The attendance cycle of the Cory’s Shearwater Calonectris diomedea borealis on Selvagem Grande. C. R. Acad. Sci. III 2000, 323, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Mougin, J.-L.; Jouanin, C.; Roux, F. Inexperienced birds and breeding in the Cory’s Shearwater (Calonectris diomedea). J. Ornithol. 2002, 143, 57–63. [Google Scholar] [CrossRef]
- Catry, P.; Dias, M.P.; Phillips, R.A.; Granadeiro, J.P. Carry-over effects from breeding modulate the annual cycle of a long-distance migrant: An experimental demonstration. Ecology 2013, 94, 1230–1235. [Google Scholar] [CrossRef]
- Ristow, D.; Swatschek, I.; Wink, M. Does Cory’s Shearwater breed every year or is there evidence for a sabbatical? Avocetta 1992, 16, 105–107. [Google Scholar]
- Ristow, D.; Feldmann, F.; Scharlau, W.; Wink, M. Population structure, philopatry and mortality of Cory’s Shearwater Calonectris d. diomedea. Vogelwelt 1990, 111, 172–181. [Google Scholar]
- Swatschek, I.; Ristow, D.; Wink, M. Mate fidelity and parentage in Cory’s shearwater Calonectris diomedea—Field studies and DNA fingerprinting. Mol. Ecol. 1994, 3, 259–262. [Google Scholar] [CrossRef]
- Karris, G.; Xirouchakis, S.; Maina, I.; Grivas, K.; Kavadas, S. Home range and foraging habitat preference of Scopoli’s Shearwater Calonectris diomedea during the early chick-rearing phase in the eastern Mediterranean. Wildl. Biol. 2018, 2018, wlb-00388. [Google Scholar] [CrossRef]
- Rubolini, D.; Maggini, I.; Ambrosini, R.; Imperio, S.; Paiva, V.H.; Gaibani, G.; Saino, N.; Cecere, J.C. The effect of moonlight on Scopoli’s shearwater Calonectris diomedea colony attendance patterns and nocturnal foraging: A test of the foraging efficiency hypothesis. Ethology 2015, 121, 284–299. [Google Scholar] [CrossRef]
- Morera-Pujol, V.; Catry, P.; Magalhães, M.; Péron, C.; Reyes-González, J.M.; Granadeiro, J.P.; Militão, T.; Dias, M.P.; Oro, D.; Dell’Omo, G.; et al. Methods to detect spatial biases in tracking studies caused by differential representativeness of individuals, populations and time. Divers. Distrib. 2023, 29, 19–38. [Google Scholar] [CrossRef]
- Karris, G.; Fric, J.; Kitsou, Z.; Kalfopoulou, J.; Giokas, S.; Sfenthourakis, S.; Poirazidis, K. Does by-catch pose a threat for the conservation of seabird populations in the southern Ionian Sea (eastern Mediterranean)? A questionnaire-based survey of local fisheries. Mediterr. Mar. Sci. 2013, 14, 19–25. [Google Scholar] [CrossRef]
- Karris, G.; Ketsilis-Rinis, V.; Kalogeropoulou, A.; Xirouchakis, S.; Machias, A.; Maina, I.; Kavadas, S. The use of demersal trawling discards as a food source for two scavenging seabird species: A case study of an eastern Mediterranean oligotrophic marine ecosystem. Avian Res. 2018, 9, 26. [Google Scholar] [CrossRef]
- Keller, V.; Herrando, S.; Voříšek, P.; Franch, M.; Kipson, M.; Milanesi, P.; Martí, D.; Anton, M.; Klvaňová, A.; Kalyakin, M.V.; et al. European Breeding Bird Atlas 2: Distribution, Abundance and Change; European Bird Census Council & Lynx Edicions: Barcelona, Spain, 2020. [Google Scholar]
- Ristow, D.; Hädrich, J.; Baum, F.; Wink, M. Pesticide residues in Cory’s Shearwater eggs. Avocetta 1992, 16, 102–104. [Google Scholar]
- Thibault, J.C. Effect of predation by Black Rat Rattus rattus on the breeding success of Cory’s Shearwater in Corsica. Mar. Ornithol. 1995, 23, 1–10. [Google Scholar]
- Igual, J.M.; Forero, M.G.; Gomez, T.; Orueta, J.F.; Oro, D. Rat control and breeding performance in Cory’s shearwater (Calonectris diomedea): Effects of poisoning effort and habitat features. Anim. Conserv. 2006, 9, 59–65. [Google Scholar] [CrossRef]
- Zino, F.; Hounsome, M.V.; Buckle, A.P.; Biscoito, M. Was the removal of rabbits and house mice from Selvagem Grande beneficial to the breeding of Cory’s shearwaters Calonectris diomedea borealis? Oryx 2008, 42, 151–154. [Google Scholar] [CrossRef]
- Martinis, A.; Chaideftou, E.; Minotou, C.; Poirazidis, K. Ecological assessment of Juniperus turbinata Guss. Forest on the Strofades Islands, Ionian Sea, Greece. J. For. Sci. 2018, 64, 345–352. [Google Scholar] [CrossRef]
- Karris, G.; Xirouchakis, S.; Grivas, C.; Voulgaris, M.D.; Sfenthourakis, S.; Giokas, S. Estimating the population size of Scopoli’s Shearwaters (Calonectris diomedea) frequenting the Strofades islands (Ionian Sea, western Greece) by raft counts and surveys of breeding pairs. N. West. J. Zool. 2017, 13, 101–108. [Google Scholar]
- Barboutis, C.; Navarrete, E.; Karris, G.; Xirouchakis, S.; Fransson, T.; Bounas, A. Arriving depleted after crossing of the Mediterranean: Obligatory stopover patterns underline the importance of Mediterranean islands for migrating birds. Anim. Migr. 2022, 9, 27–36. [Google Scholar]
- Petrella, D. Impacts of Rat Predation on the Breeding Performance of the Mediterranean Cory’s Shearwater (Calonectris diomedea diomedea) Colony on Strofades Island Complex (Ionian Sea, Western Greece). Master’s Thesis, University of Liege, Liege, Belgium, 2011. [Google Scholar]
- Carey, M.J. The effects of investigator disturbance on procellariiform seabirds: A review. N. Z. J. Zool. 2009, 36, 367–377. [Google Scholar] [CrossRef]
- Round, P.D.; Swann, R.L. Aspects of the breeding of Cory’s Shearwater Calonectris diomedea in Crete. Ibis 1977, 19, 350–353. [Google Scholar] [CrossRef]
- Zammit, R.C.; Borg, J. On the breeding biology of the Cory’s Shearwater in the Maltese Islands. Merill 1987, 24, 1–9. [Google Scholar]
- Ristow, D.; Feldmann, F.; Scharlau, W.; Wink, C.; Wink, M. Population dynamics of Cory’s Shearwater (Calonectris diomedea) and Eleonora’s falcon (Falco eleonorae) in Eastern Mediterranean. In Species Conservation: A Population-Biological Approach; Seitz, A., Loeschcke, V., Eds.; Birkhauser: Basel, Switzerland, 1991; pp. 199–212. [Google Scholar]
- Thibault, J.C.; Rabouam, C.; Bretagnolle, V. Calonectris diomedea Cory’s shearwater. BWP Update 1997, 1, 75–98. [Google Scholar]
- Levy, P.S.; Lemeshow, S. Sampling of Populations: Methods and Applications, 4th ed.; J. Wiley & Sons, Inc.: Hoboken, NJ, USA, 2008. [Google Scholar]
- Thibault, J.C. Nest-site tenacity and mate fidelity in relation to breeding success in Cory’s Shearwater Calonectris diomedea. Bird Study 1994, 41, 25–28. [Google Scholar] [CrossRef]
- Mougin, J.-L. Factors affecting egg dimensions and breeding success in the Cory’s Shearwater (Calonectris diomedea) of Selvagem Grande. J. Ornithol. 1998, 139, 179–184. [Google Scholar] [CrossRef]
- Guerreiro, C.B.B.; Foltescu, V.; de Leeuw, F. Air Quality Status and Trends in Europe. Atmos. Environ. 2014, 98, 376–384. [Google Scholar] [CrossRef]
- McLeod, A. R Package “Kendall”. 2015. Available online: http://www.stats.uwo.ca/faculty/aim (accessed on 29 November 2023).
- Pohlert, T. Package Trend: Non-Parametric Trend Tests and Change-Point Detection. 2020. Available online: https://CRAN.R-project.org/package=trend (accessed on 29 November 2023).
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing: Vienna, Austria. 2017. Available online: https://www.R-project.org/ (accessed on 29 November 2023).
- Sen, P. Estimated of the regression coefficient based on Kendall’s Tau. J. Am. Stat. Assoc. 1968, 39, 1379–1389. [Google Scholar] [CrossRef]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S, 4th ed.; Springer: New York, NY, USA, 2002. [Google Scholar]
- Signorell, A.; Aho, K.; Alfons, A.; Anderregg, N.; Aragon, T.; Arachchige, C.; Arppe, A.; Baddeley, A.; Barton, K.; Bolker, B.; et al. DescTools: Tools for Descriptive Statistics. R Package Version 0.99.23. 2017. Available online: https://github.com/AndriSignorell/DescTools/ (accessed on 29 November 2023).
- Matthew, J. Generalhoslem: Goodness of Fit Tests for Logistic Regression Models. R Package Version 1.3.4. 2019. Available online: https://CRAN.R-project.org/package=generalhoslem (accessed on 29 November 2023).
- Karris, G. Breeding Ecology of Calonectris diomedea (Aves, Procellariiformes) on Strofades Island group. Ph.D Thesis, University of Patras, Patras, Greece, 2014. [Google Scholar]
- Triay, R.; Capó, J. Biometria del Virot Calonectris diomedea a l’illa de Menorca (illes Balears-Mediterrani Occidental). Butll. Grup Catala d’Anellament. 1996, 13, 9–14. [Google Scholar]
- Borg, J.J. Philopatry in Cory’s Shearwater Calonectris diomedea in Malta. I1-Merill 1999, 29, 11–15. [Google Scholar]
- Fernandez, O. Etude synoptique des observations relatives au nid du Puffin cendre Calonectris diomedea sur les isles de Marseille. Alauda 1985, 53, 147–148. [Google Scholar]
- Borg, J.J.; Sultana, J. Aspects on the breeding biology of Cory’s Shearwater (Calonectris diomedea) in the Maltese Islands. Die Vogelwarte 2000, 40, 258–264. [Google Scholar]
- Baccetti, N.; Capizzi, D.; Corbi, F.; Massa, B.; Nissardi, S.; Spano, G.; Sposimo, P. Breeding shearwaters on Italian Islands: Population size, Island selection and co-existence with the main alien predator, the Black Rat. Riv. Ital. Orn. 2009, 78, 83–100. [Google Scholar]
- Mougin, J.-L. The influence of colony characteristics on some breeding parameters in the Cory’s Shearwater Calonectris diomedea borealis. Ardeola 1999, 46, 45–51. [Google Scholar]
- Sacchi, M.; Zenatello, M.; Pezzo, F.; Cozzo, M.; Pollonara, E.; Gotti, C.; De Faveri, A.; Baccetti, N. Nest Change and Individual Fitness in a Scopoli’s Shearwater Population: A Capture-Recapture Multistate Analysis. Diversity 2023, 15, 718. [Google Scholar] [CrossRef]
- Ristow, D.; Wink, M. Sexual dimorphism of Cory’s Shearwater. I1-Merill 1980, 21, 9–12. [Google Scholar]
- Iapichino, C.; Lo Valvo, F.; Massa, B. Biometria della Berta maggiore (Calonectris diomedea) dell’isola di Linosa (Pelagie). Riv. Ital. Orn. 1983, 53, 145–152. [Google Scholar]
- Gaultier, T. Contribution à l’étude de Calonectris diomedea de l’île de Zembra (Tunisie); Association «Les Amis des Oiseaux» et Institut de Recherche Scientifique et Technique de Tunis: Tunis, Tunisia, 1978; 86p. [Google Scholar]
- Jouanin, C. Note sur la biométrie des Puffins cendrés de Tunisie. L’Oiseau et R.F.O. 1976, 46, 97–102. [Google Scholar]
- Araujo, J.; Munoz Cobos, J.; Purroy, F.J. Los rapaces y aves marinas del archipelago de Cabrera. Nat. Hisp. 1977, 12, 1–94. [Google Scholar]
- Coll, M.; Piroddi, C.; Steenbeek, J.; Kaschner, K.; Ben Rais Lasram, F.; Aguzzi, J.; Ballesteros, E.; Bianchi, C.N.; Corbera, J.; Dailianis, T.; et al. The Biodiversity of the Mediterranean Sea: Estimates, Patterns, and Threats. PLoS ONE 2010, 5, 11842. [Google Scholar] [CrossRef]
- Hipfner, J.M.; Gaston, A.J.; de Forest, L.N. The role of female age in determining egg size and laying date of Thick-billed Murres. J. Avian Biol. 1997, 28, 271–278. [Google Scholar] [CrossRef]
- Massaro, M.; Darby, J.T.; Davis, L.S.; Edge, K.-A.; Hazel, M.J. Investigation of interacting effects of female age, laying dates, and egg size in Yellow-eyed Penguins (Megadyptes antipodes). Auk 2002, 119, 1137–1141. [Google Scholar] [CrossRef]
- Ollason, J.C.; Dunnet, G.M. Relative effects of parental performance and egg quality on breeding success of Fulmars Fulmarus glacialis. Ibis 1986, 128, 290–296. [Google Scholar] [CrossRef]
- Bourgeois, K.; Vidal, É. Υelkouan shearwater nest-cavity selection and breeding success. C. R. Biol. 2007, 330, 205–214. [Google Scholar]
- Michielsen, R.J.; Ausems, A.N.M.A.; Jakubas, D.; Pętlicki, M.; Plenzler, J.; Shamoun-Baranes, J.; Wojczulanis-Jakubas, K. Nest characteristics determine nest microclimate and affect breeding output in an Antarctic seabird, the Wilson’s storm-petrel. PLoS ONE 2019, 14, e0217708. [Google Scholar] [CrossRef] [PubMed]
- Stenhouse, I.J.; Montevecchi, W. Habitat utilization and breeding success in Leach’s Storm-Petrel: The importance of sociality. Can. J. Zool. 2000, 78, 1267–1274. [Google Scholar] [CrossRef][Green Version]
- Pagenaud, A.; Ravache, A.; Bourgeois, K.; Mathivet, M.; Bourguet, E.; Vidal, E.; Thibault, M. Nest site selection and its influence on breeding success in a poorly-known and declining seabird: The Tahiti petrel Pseudobulweria rostrata. PLoS ONE 2022, 17, e0267408. [Google Scholar] [CrossRef] [PubMed]
- Warham, J. The Petrels: Their Ecology and Breeding Systems; Academic Press: London, UK, 1990. [Google Scholar]
- Ramos, J.A.; Monteiro, L.R.; Sola, E.; Moniz, Z. Characteristics and competition for nest cavities in burrowing Procellariiformes. Condor 1997, 99, 634–641. [Google Scholar] [CrossRef]
- Martin, J.-L.; Thibault, J.-C.; Bretagnolle, V. Black Rats, Island Characteristics, and Colonial Nesting Birds in the Mediterranean: Consequences of an Ancient Introduction. Conserv. Biol. 2000, 14, 1452–1466. [Google Scholar] [CrossRef]

| Model Breeding Success Classes~Nest Type | |||||
|---|---|---|---|---|---|
| Estimate | Std. Error | t Value | p-Value | Odds Ratio (95% CI) | |
| Intercept | |||||
| Low-quality nests/Medium-quality nests | −0.381 | 0.304 | −1.252 | 0.211 | 0.682 |
| Medium-quality nests/High-quality nests | 0.583 | 0.306 | 1.904 | 0.056 | 1.792 |
| Coefficient | |||||
| Cliff cover nests | 0.179 | 0.352 | 0.508 | 0.611 | 1.196 (0.599–2.391) |
| Stone cover nests | 0.316 | 0.371 | 0.852 | 0.393 | 1.372 (0.663–2.844) |
| Burrow nests | 0.363 | 0.369 | 0.983 | 0.325 | 1.438 (0.697–2.973) |
| Crevice nests | −0.245 | 0.389 | −0.628 | 0.529 | 0.782 (0.364–1.679) |
| Model Breeding Success Classes~Nest-Entrance Orientation | |||||
|---|---|---|---|---|---|
| Estimate | Std. Error | t Value | p-Value | Odds Ratio (95% CI) | |
| Intercept | |||||
| Low-quality nests/Medium-quality nests | −0.045 | 0.317 | −0.144 | 0.885 | 0.955 |
| Medium-quality nests/High-quality nests | 0.925 ** | 0.325 | 2.847 | 0.004 | 2.523 |
| Coefficient | |||||
| Eastern orientation | 0.491 | 0.381 | 1.289 | 0.197 | 1.635 (0.777–3.471) |
| Southern orientation | 0.409 | 0.354 | 1.155 | 0.248 | 1.506 (0.754–3.038) |
| Western orientation | 0.792 * | 0.367 | 2.153 | 0.031 | 2.208 (1.079–4.569) |
| Breeding Success Classes~Habitat Sector | |||||
|---|---|---|---|---|---|
| Estimate | Std. Error | t Value | p-Value | Odds Ratio (95% CI) | |
| Intercept | |||||
| Low-quality nests/Medium-quality nests | −0.473 | 0.285 | −1.658 | 0.097 | 0.622 |
| Medium-quality nests/High-quality nests | −0.473 | 0.285 | 1.658 | 0.097 | 1.606 |
| Coefficient | |||||
| Western sector | 0.148 | 0.323 | 0.460 | 0.645 | 1.160 (0.615–2.187) |
| Southern sector | 0 | 0.313 | 0 | 0.999 | 0.999 (0.540–1.851) |
| Model Breeding Success Classes~Nest Type + Nest Orientation + Habitat Sector | |||||
|---|---|---|---|---|---|
| Estimate | Std. Error | t Value | p-Value | Odds Ratio (95% CI) | |
| Intercept | |||||
| Low-quality nests/Medium-quality nests | 0.167 | 0.548 | 0.305 | 0.759 | 1.182 |
| Medium-quality nests/High-quality nests | 1.157 * | 0.554 | 2.087 | 0.036 | 3.183 |
| Coefficient | |||||
| Cliff cover nests | 0.144 | 0.363 | 0.398 | 0.690 | 1.155 (0.567–2.358) |
| Stone cover nests | 0.285 | 0.391 | 0.731 | 0.464 | 1.331 (0.619–2.865) |
| Burrow nests | 0.316 | 0.394 | 0.802 | 0.422 | 1.372 (0.633–2.981) |
| Crevice nests | −0.272 | 0.399 | −0.683 | 0.494 | 0.761 (0.347–1.664) |
| Eastern orientation | 0.537 | 0.396 | 1.355 | 0.175 | 1.711 (0.789–3.742) |
| Southern orientation | 0.382 | 0.366 | 1.043 | 0.296 | 1.465 (0.717–3.017) |
| Western orientation | 0.692 | 0.389 | 1.777 | 0.075 | 1.998 (0.935–4.314) |
| Western Sector | 0.168 | 0.404 | 0.417 | 0.676 | 1.184 (0.536–2.626) |
| Southern Sector | 0.127 | 0.346 | 0.367 | 0.713 | 1.135 (0.576–2.246) |
| Models | PseudoR2 | Residual Deviance | AIC | Goodness of Fit | ||||
|---|---|---|---|---|---|---|---|---|
| McFadden | CoxSnell | Nagelkerke | Chi-Squared | df | p-Value | |||
| Breeding success classes~Nest type | 0.015 | 0.034 | 0.038 | 267.323 | 279.323 | 3.764 | 6 | 0.708 |
| Breeding success classes~Nest-entrance orientation | 0.019 | 0.041 | 0.046 | 266.383 | 276.383 | 2.654 | 4 | 0.617 |
| Breeding success classes~Habitat sector | 0.002 | 0.004 | 0.005 | 271,141 | 279.141 | 0.217 | 2 | 0.897 |
| Breeding success classes~A | 0.038 | 0.069 | 0.078 | 262.739 | 284.739 | 74.653 | 74 | 0.456 |
| A = Nest type + Nest-entrance orientation + Habitat sector | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karris, G.; Xirouchakis, S.; Poirazidis, K.; Voulgaris, M.-D.; Tsouroupi, A.; Sfenthourakis, S.; Giokas, S. Aspects of Breeding Performance of Scopoli’s Shearwater (Calonectris diomedea): The Case of the Largest Colony in Greece. Diversity 2024, 16, 150. https://doi.org/10.3390/d16030150
Karris G, Xirouchakis S, Poirazidis K, Voulgaris M-D, Tsouroupi A, Sfenthourakis S, Giokas S. Aspects of Breeding Performance of Scopoli’s Shearwater (Calonectris diomedea): The Case of the Largest Colony in Greece. Diversity. 2024; 16(3):150. https://doi.org/10.3390/d16030150
Chicago/Turabian StyleKarris, Georgios, Stavros Xirouchakis, Konstantinos Poirazidis, Marios-Dimitrios Voulgaris, Anastasia Tsouroupi, Spyros Sfenthourakis, and Sinos Giokas. 2024. "Aspects of Breeding Performance of Scopoli’s Shearwater (Calonectris diomedea): The Case of the Largest Colony in Greece" Diversity 16, no. 3: 150. https://doi.org/10.3390/d16030150
APA StyleKarris, G., Xirouchakis, S., Poirazidis, K., Voulgaris, M.-D., Tsouroupi, A., Sfenthourakis, S., & Giokas, S. (2024). Aspects of Breeding Performance of Scopoli’s Shearwater (Calonectris diomedea): The Case of the Largest Colony in Greece. Diversity, 16(3), 150. https://doi.org/10.3390/d16030150










