Morphological Variation and New Description of the Subcutaneous Gland of Sepiella inermis (Van Hasselt, 1835) in Thai Waters
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Analysis of Morphological Indices
2.3. External and Internal Structure Characteristics
2.3.1. Scanning Electron Microscopy (SEM)
2.3.2. Histological Techniques
3. Results
3.1. Morphological Characteristics of Sepiella inermis
3.2. The Subcutaneous Gland’s Structure
3.2.1. Internal Structure Subcutaneous Gland at Hatching Stage
- The wall of the subcutaneous sac consists of four layers: (1) The epidermis is the outermost layer protected by an external membrane. (2)The middle layer comprises connective tissue and chromatophores pigments. (3) The muscularis is a thick layer of muscle fibers. (4) The sac’s interior is coated by mucosa and cuboidal cells. Melanin pigment is spread along the margin of the wall, as depicted in Figure 8b.
- Within the sac of the wall is a large cavity containing a high quantity of perforate lamellae resembling nerve nets from connective tissue. The basal subcutaneous gland is a production source melanin glandule, comprising four different trabeculae branches of connective tissue in the cavity Figure 8c. These branches consist of two lengthy ones extending from the center basal gland to the lumen, as shown in Figure 8d. All branches from the basal subcutaneous gland are interconnected. Each branch of trabeculae contains melanin granule, as referred to in Figure 8f.
- The gland pore is controlled by a muscle that regulates ink release. The mantle musculature and ventral fins regulate the release and transmission of liquid ink refer to Figure 8e.
3.2.2. Internal Structure of Subcutaneous Gland at Adult Stage
3.3. Morphological Variations Analysis and Length–Weight Relationship
3.3.1. Morphological Variations of S. inermis
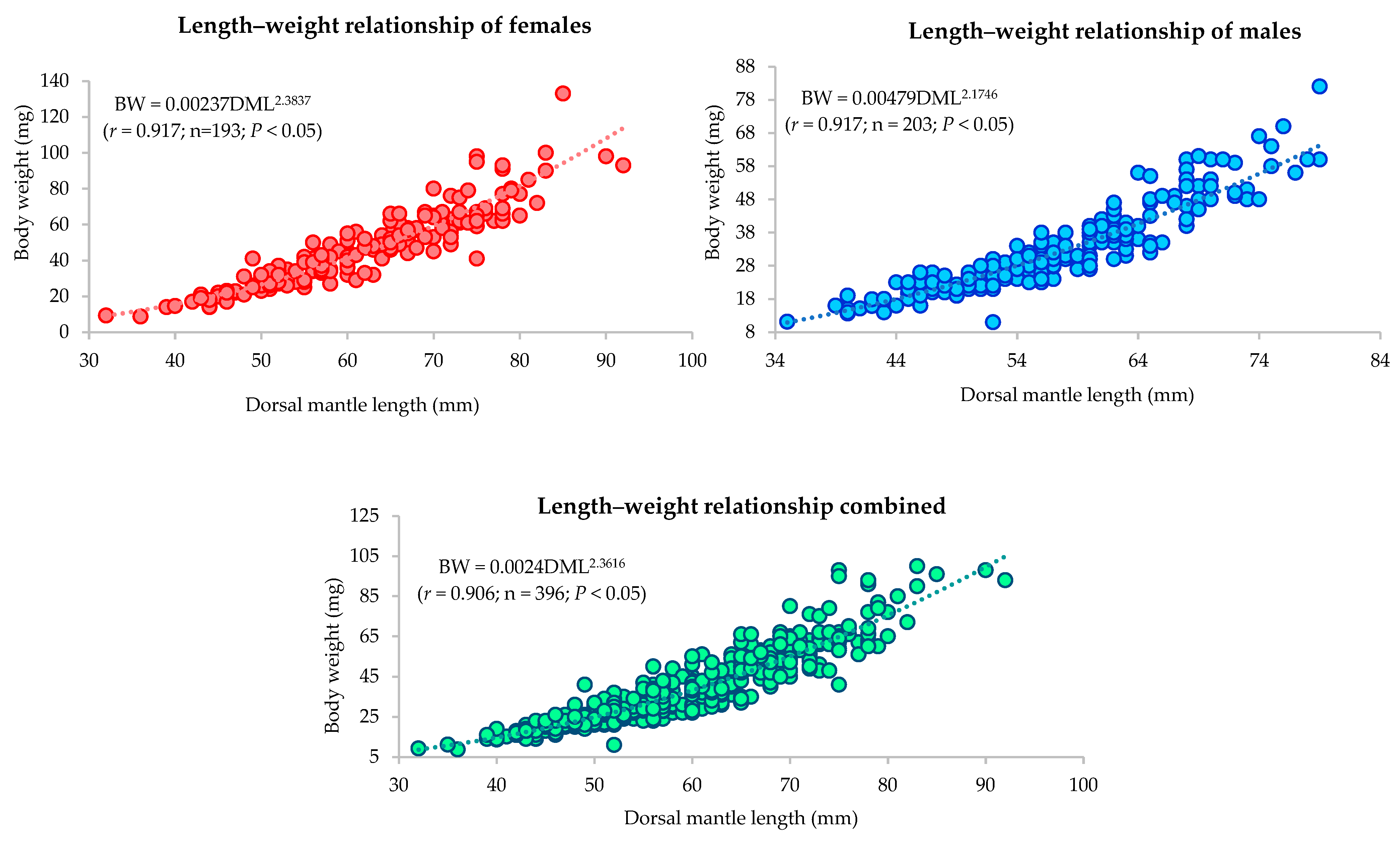
3.3.2. Length–Weight Relationship
4. Discussion
4.1. Morphological Characteristics of S. inermis
4.2. The Subcutaneous Gland’s Structure
4.3. Morphological Variations, Population Analysis, and Length–Weight Relationship
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Morphological Index | Gentle | Number of Samples | (mm) Mean ± SD | Rage | Percentage of Length | |
|---|---|---|---|---|---|---|
| Min | Max | |||||
| BW | Female | 193 | 46.18 ± 20.76 | 8.74 | 133.00 | |
| Male | 203 | 32.89 ± 12.87 | 11.00 | 82.00 | ||
| Total | 396 | 39.37 ± 18.40 | 8.74 | 133.00 | ||
| DML | Female | 193 | 61.34 ± 11.35 | 32.00 | 92.00 | |
| Male | 203 | 56.91 ± 9.16 | 35.00 | 79.00 | ||
| Total | 396 | 59.07 ± 10.51 | 32.00 | 92.00 | ||
| TL | Female | 193 | 111.11 ± 22.46 | 58.00 | 166.00 | 55.21% of TL |
| Male | 203 | 104.71 ± 19.79 | 64.00 | 154.00 | 54.35% of TL | |
| Total | 396 | 107.83 ± 21.35 | 58.00 | 166.00 | 54.78% of TL | |
| DMW | Female | 193 | 47.94 ± 8.00 | 24.27 | 73.82 | 78.15% of DML |
| Male | 203 | 43.49 ± 6.36 | 23.41 | 60.16 | 76.42% of DML | |
| Total | 396 | 45.66 ± 7.53 | 23.41 | 73.82 | 77.29% of DML | |
| CL | Female | 193 | 58.77 ± 11.17 | 30.10 | 85.00 | 95.81% of DML |
| Male | 203 | 53.00 ± 8.36 | 28.00 | 76.00 | 93.14% of DML | |
| Total | 396 | 55.82 ± 10.23 | 28.00 | 85.00 | 94.49% of DML | |
| CW | Female | 193 | 25.10 ± 4.99 | 12.00 | 38.00 | 40.93% of DML |
| Male | 203 | 21.25 ± 3.18 | 14.00 | 33.00 | 37.33% of DML | |
| Total | 396 | 23.13 ± 4.58 | 12.00 | 38.00 | 39.15% of DML | |
| CWt | Female | 193 | 1.57 ± 0.76 | 0.27 | 4.81 | 3.40% of BW |
| Male | 203 | 1.13 ± 0.60 | 0.18 | 3.87 | 3.45% of BW | |
| Total | 396 | 1.35 ± 0.72 | 0.18 | 4.81 | 3.42% of BW | |
| HL | Female | 193 | 30.31 ± 5.03 | 17.35 | 43.17 | 49.41% of DML |
| Male | 203 | 28.87 ± 6.64 | 19.36 | 83.74 | 50.73% of DML | |
| Total | 396 | 19.80 ± 4.37 | 7.78 | 32.91 | 66.95% of DML | |
| HW | Female | 193 | 20.62 ± 4.50 | 11.95 | 32.91 | 68.05% of HL |
| Male | 203 | 19.01 ± 4.09 | 7.78 | 29.84 | 65.85% of HL | |
| Total | 396 | 29.57 ± 5.95 | 17.35 | 83.74 | 50.05% of HL | |
| ED | Female | 193 | 8.83 ± 1.92 | 4.63 | 16.80 | 29.15% of HL |
| Male | 203 | 8.68 ± 1.85 | 4.77 | 19.09 | 30.05% of HL | |
| Total | 396 | 8.75 ± 1.88 | 4.63 | 19.09 | 29.59% of HL | |
| FL | Female | 193 | 32.36 ± 6.04 | 11.38 | 47.30 | 52.75% of DML |
| Male | 203 | 29.29 ± 5.25 | 10.54 | 45.19 | 51.46% of DML | |
| Total | 396 | 19.80 ± 4.37 | 7.78 | 32.91 | 66.95% of DML | |
| FW | Female | 193 | 11.21 ± 2.63 | 5.41 | 19.97 | 18.28% of DML |
| Male | 203 | 10.10 ± 2.19 | 5.34 | 15.67 | 17.74% of DML | |
| Total | 396 | 10.64 ± 2.48 | 5.34 | 19.97 | 18.01% of DML | |
| LI | Female | 193 | 23.01 ± 6.93 | 10.01 | 40.68 | 37.50% of DML |
| Male | 203 | 25.47 ± 8.37 | 10.80 | 49.13 | 44.76% of DML | |
| Total | 396 | 24.27 ± 7.79 | 10.01 | 49.13 | 41.08% of DML | |
| LII | Female | 193 | 23.22 ± 6.89 | 10.33 | 42.21 | 37.85% of DML |
| Male | 203 | 24.73 ± 8.05 | 11.67 | 50.97 | 43.46% of DML | |
| Total | 396 | 24.00 ± 7.54 | 10.33 | 50.97 | 40.62% of DML | |
| LIII | Female | 193 | 24.59 ± 6.83 | 11.40 | 41.73 | 40.09% of DML |
| Male | 203 | 27.33 ± 7.41 | 12.09 | 49.24 | 48.02% of DML | |
| Total | 396 | 25.99 ± 7.25 | 11.40 | 49.24 | 44.00% of DML | |
| LIV | Female | 193 | 32.64 ± 8.26 | 15.33 | 59.91 | 53.20% of DML |
| Male | 203 | 32.82 ± 9.06 | 16.69 | 58.46 | 57.66% of DML | |
| Total | 396 | 32.73 ± 8.67 | 15.33 | 59.91 | 55.40% of DML | |
| RI | Female | 193 | 23.07 ± 7.26 | 9.93 | 39.35 | 37.61% of DML |
| Male | 203 | 25.51 ± 8.39 | 11.10 | 50.64 | 44.82% of DML | |
| Total | 396 | 24.32 ± 7.95 | 9.93 | 50.64 | 41.17% of DML | |
| RII | Female | 193 | 23.51 ± 7.13 | 10.27 | 41.72 | 38.33% of DML |
| Male | 203 | 25.27 ± 8.19 | 11.15 | 48.10 | 44.40% of DML | |
| Total | 396 | 24.41 ± 7.73 | 10.27 | 48.10 | 41.32% of DML | |
| RIII | Female | 193 | 24.86 ± 7.16 | 12.21 | 42.54 | 40.53% of DML |
| Male | 203 | 27.44 ± 8.01 | 12.37 | 49.19 | 48.22% of DML | |
| Total | 396 | 26.18 ± 7.71 | 12.21 | 49.19 | 44.32% of DML | |
| RIV | Female | 193 | 31.98 ± 8.96 | 16.82 | 64.52 | 52.14% of DML |
| Male | 203 | 32.67 ± 9.06 | 16.63 | 56.61 | 57.41% of DML | |
| Total | 396 | 32.34 ± 9.01 | 16.63 | 64.52 | 54.74% of DML | |
| Station | |||||||
|---|---|---|---|---|---|---|---|
| Location | TT | CB | SSM | PT | SNI | SK | RN |
| Number of Samples | 30 | 61 | 80 | 75 | 30 | 45 | 75 |
| Morphological Index (mm mean ± SD) | |||||||
| BW | 58.7 ± 21.93 | 21.61 ± 6.07 | 41.05 ± 14.91 | 39.49 ± 14.07 | 47.43 ± 20.47 | 54.93 ± 18.14 | 31.60 ± 12.00 |
| TL | 133.53 ± 17.08 | 80.48 ± 9.37 | 111.76 ± 9.38 | 112.12 ± 11.61 | 122.5 ± 15.69 | 124.2 ± 16.30 | 95.62 ± 13.37 |
| DML | 69.53 ± 10.16 | 48.07 ± 6.22 | 61.3 ± 9.10 | 62.43 ± 7.95 | 61.17 ± 7.87 | 68.02 ± 7.15 | 51.89 ± 6.89 |
| DMW | 54.02 ± 6.39 | 36.99 ± 4.57 | 46.51 ± 5.17 | 44.43 ± 4.30 | 52.81 ± 7.05 | 52.17 ± 5.66 | 42.91 ± 6.04 |
| CL | 65.07 ± 10.11 | 43.35 ± 5.10 | 57.38 ± 9.02 | 59.69 ± 8.64 | 56.17 ± 7.82 | 64.38 ± 6.55 | 51.43 ± 6.46 |
| CW | 27.00 ± 5.41 | 18.44 ± 2.56 | 24.11 ± 4.37 | 23.92 ± 3.97 | 24.17 ± 3.70 | 26.24 ± 3.32 | 21.25 ± 3.39 |
| CWt | 2.16 ± 1.07 | 0.68 ± 0.22 | 1.24 ± 0.46 | 1.40 ± 0.62 | 1.69 ± 0.68 | 2.07 ± 0.52 | 1.04 ± 0.45 |
| HL | 33.48 ± 4.02 | 24.21 ± 2.95 | 28.62 ± 4.19 | 31.53 ± 3.67 | 30.80 ± 3.61 | 36.46 ± 9.84 | 26.80 ± 2.93 |
| HW | 24.19 ± 5.05 | 15.27 ± 2.07 | 20.57 ± 3.26 | 19.74 ± 3.80 | 20.03 ± 2.50 | 24.78 ± 3.00 | 17.88 ± 3.23 |
| ED | 11.06 ± 2.62 | 6.33 ± 0.72 | 8.53 ± 1.47 | 9.52 ± 0.96 | 10.16 ± 1.25 | 9.98 ± 1.53 | 7.97 ± 0.79 |
| FL | 37.28 ± 4.46 | 23.42 ± 3.03 | 31.30 ± 4.82 | 31.52 ± 3.75 | 34.12 ± 4.96 | 35.97 ± 3.28 | 28.43 ± 4.84 |
| FW | 14.09 ± 1.85 | 7.72 ± 1.32 | 10.98 ± 2.05 | 1.48 ± 7.42 | 12.16 ± 2.48 | 12.25 ± 1.87 | 9.38 ± 1.64 |
| LI | 30.33 ± 4.54 | 15.58 ± 2.65 | 24.83 ± 6.89 | 26.01 ± 4.95 | 29.20 ± 5.32 | 32.71 ± 9.20 | 19.54 ± 4.13 |
| LII | 30.96 ± 4.52 | 16.00 ± 2.54 | 24.80 ± 6.69 | 24.36 ± 4.75 | 27.80 ± 6.08 | 32.89 ± 8.38 | 19.63 ± 4.12 |
| LIII | 32.64 ± 5.08 | 18.01 ± 3.15 | 26.40 ± 6.29 | 27.08 ± 4.00 | 30.11 ± 6.88 | 33.72 ± 7.83 | 22.03 ± 4.28 |
| LIV | 46.74 ± 7.72 | 22.88 ± 4.06 | 33.05 ± 7.23 | 33.19 ± 4.32 | 36.90 ± 5.28 | 39.72 ± 8.29 | 28.48 ± 4.84 |
| RI | 30.39 ± 4.96 | 15.39 ± 2.74 | 25.13 ± 7.12 | 26.08 ± 5.28 | 29.23 ± 5.07 | 32.91 ± 9.09 | 19.42 ± 4.06 |
| RII | 30.95 ± 6.00 | 15.60 ± 2.75 | 25.38 ± 6.79 | 25.72 ± 5.03 | 28.74 ± 5.12 | 32.69 ± 8.57 | 19.93 ± 3.74 |
| RIII | 33.53 ± 5.54 | 17.38 ± 2.70 | 26.77 ± 6.91 | 27.52 ± 4.74 | 31.22 ± 5.92 | 34.13 ± 8.17 | 21.66 ± 3.88 |
| RIV | 45.87 ± 10.31 | 22.74 ± 3.76 | 32.57 ± 7.73 | 33.67 ± 4.65 | 35.65 ± 6.55 | 38.81 ± 9.17 | 27.93 ± 5.11 |
References
- Jereb, P.; Roper, C.F.E. Cephalopods of the World. In An Annotated and Illustrated Catalogue of Cephalopod Species Known to Date. Volume 1. Chambered Nautiluses and Sepioids (Nautilidae, Sepiidae, Sepiolidae, Sepiadariidae, Idiosepiidae and Spirulidae), FAO Species Catalogue for Fishery Purposes; FAO: Rome, Italy, 2005; pp. 130–131. [Google Scholar]
- Nateewathana, A. The Sepiidae (Cephalopoda) of Thailand. Phuket Mar. Biol. Cent. Res. Bull. 2008, 69, 25–41. [Google Scholar]
- Montague, T.G.; Rieth, I.J.; Axel, R. Embryonic development of the camouflaging dwarf cuttlefish, Sepia bandensis. Dev. Dyn. 2021, 250, 1688–1703. [Google Scholar] [CrossRef]
- Derby, D.C. Cephalopod ink: Production, chemistry, functions and applications. Mar. Drugs 2014, 12, 2700–2730. [Google Scholar] [CrossRef]
- Nabhitabhata, J.; Nilaphat, P.; Promboon, P.; Jaroongpattananon, C.; Nilaphat, G.; Reunreng, A. Performance of simpler large-scale cephalopod culture system in Thailand. Phuket Mar. Biol. Cent. Res. Bull. 2005, 66, 337–350. [Google Scholar]
- Roper, C.F.E.; Voss, G.L. Guidelines for taxonomic descriptions of cephalopod species. The Biology and Resource Potential of Cephalopods. Mem. Natl. Mus. Vic. 1983, 44, 48–63. [Google Scholar] [CrossRef]
- Sukhsangchan, C.; Sunthornket, P. Species identification of commercially important cephalopods of Thailand. Fish People SEAFDEC 2015, 13, 37–42. [Google Scholar]
- Sukhsangchan, C.; Sunthornket, P.; Phuynoi, S. Morphological characteristics of paralarvae of cephalopods found in Thai waters. Mar. Biodivers. 2017, 47, 639–645. [Google Scholar] [CrossRef]
- Norman, M.J.; Reid, A. A Guide to Squid, Cuttlefish and Octopuses of Australia; CSIRO Publication: Melbourne, Australia, 2000; p. 96. [Google Scholar]
- Nabhitabhata, J.; Kbinrum, S. Biological Studies on Economic Cephalopods—III; Note on Survival of Cephalopods Hatchling during Continuous Change of Salinity. Technical Paper; Rayong Brackishwater Fisheries Section, Brackishwater Fisheries Division, Department of Fisheries: Rayong Province, Thailand, 1984; Volume 6, p. 15. [Google Scholar]
- Willmore, K.E.; Young, M.N.; Richtsmeier, T.J. Phenotypic Variability: Its Components, Measurement and Underlying Developmental Processes. Evol. Biol. 2007, 34, 99–120. [Google Scholar] [CrossRef]
- Muchisin, Z.A.; Zulkarnaini, B.; Pirnawan, S.; Muhadjier, A.; Fadli, N.; Cheng, S.H. Morphometric variations of three species of harvested cephalopods found in northern sea of Aceh Province, Indonesia. Biodivers. J. Biol. Divers. 2014, 15, 142–146. [Google Scholar]
- Guerra-Marrero, A.; Bartolomé, A.; Couce-Montero, L.; Espino-Ruano, A.; Jiménez-Alvarado, D.; Castro, J.J.; Perales-Ray, C. Age, growth, and population structure of the African cuttlefish Sepia bertheloti based on beak microstructure. Mar. Biol. 2023, 170, 118. [Google Scholar] [CrossRef]
- Boyle, P.R.; Boletzky, S.V. Cephalopod populations: Definition and dynamics. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 1996, 351, 985–1002. [Google Scholar]
- Sundaram, S.; Zafar, K.M. Morphometric relationship of spineless cuttlefish, Sepiella inermis (Orbigny, 1848) from Mumbai waters. J. Indian Fish. Assoc. 2010, 37, 51–55. [Google Scholar]
- Huxley, J.S.; Teissier, G. Terminology of relative growth. Nature 1936, 137, 780–781. [Google Scholar] [CrossRef]
- Vasconcelos, P.; Pereira, F.; Carvalho, A.N.; Gaspar, M.B. Weight-Length Relationships and Relative Growth of the Cuttlefish (Sepia officinalis): Causes and Effects of Hypoallometry. Thalass. Int. J. Mar. Sci. 2018, 34, 323–331. [Google Scholar] [CrossRef]
- International Council for the Exploration of the Sea (ICES). Report of the Working Group on Cephalopod Fisheries and Life History (WGCEPH). In Proceedings of the International Council for the Exploration of the Sea (ICES) 2013/SSGEF:13, Caen, France, 11–14 June 2013. [Google Scholar]
- Mahadwala, P.L.; Nirmale, V.H.; Metar, S.Y.; Bhosale, B.P.; Pawar, R.A.; Chogale, N.D. Diet composition and reproductive biology of spineless cuttlefish Sepiella inermis (Orbigny, 1848) from Ratnagiri (Arabian Sea, North-West coast of India). Indian J. Fish. 2023, 70, 44–51. [Google Scholar] [CrossRef]
- Ye, Y.; Yan, C.; Xu, K.; Li, J.; Miao, J.; Lü, Z.; Guo, B. Genetic Diversity and Population Genetics of Cuttlefish Sepiella japonica based on Newly Developed SSR Markers. Pak. J. Zool. 2023, 55, 1501–2000. [Google Scholar] [CrossRef]
- Arkhipkin, A.I.; Hendrickson, L.C.; Payá, I.; Pierce, G.J.; Roa-Ureta, R.H.; Robin, J.P.; Winter, A. Stock assessment and management of cephalopods: Advances and challenges for short-lived fishery resources. ICES J. Mar. Sci. 2021, 78, 714–730. [Google Scholar] [CrossRef]
- Li, J.; Ye, Y.; Wu, C.; Guo, B.; Gul, Y. Genetic diversity and population structure of Sepiella japonica (Mollusca: Cephalopoda: Decapoda) inferred by mitochondrial DNA (COI) variations. Biochem. Syst. Ecol. 2014, 56, 8–15. [Google Scholar] [CrossRef]
- Carrasco, S.A.; Varela, A.I.; Ibáñez, C.M.; Sellanes, J.; Thiel, M. Paralarval and juvenile stages as a proxy for cephalopod diversity in the Juan Fernández and Desventutadas ecoregion, southeast Pacific Ocean. Bull. Mar. Sci. 2020, 96, 263–279. [Google Scholar] [CrossRef]
- López-Galán, A.; Alejo-Plata, M.C. Sexual Differentiation of Octopus hubbsorum (Mollusca: Cephalopoda) Using Body Dimensions and the Shape and Size of the Stylets: A Traditional and Geometric Morphometric Analysis. Amer. Malac. Bull. 2016, 33, 177–184. [Google Scholar] [CrossRef]
- Li, J.H.; Chen, S.; Chen, W.D.; Xie, S.W.; Ni, X.P.; Zheng, X.D. Morphological and genetic diversity analysis of Octopus sinensis in Nanji Island. Oceanol. Limnol. Sin. 2022, 53, 486–495. [Google Scholar]
- Vega, M.A.; Rocha, F.J.; Guerra, A.; Osorio, C. Morphological differences between the Patagonia squid Loligo gohi populations from the Pacific and Atlantic Oceans. Bull. Mar. Sci. 2002, 71, 903–913. [Google Scholar]
- Neige, P. Morphometrics of hard structure in cuttlefish. Vie Milieu 2006, 56, 121–127. [Google Scholar]
- Reid, A.; Lu, C.C. A new Sepiella Gray, 1849 (Cephalopoda: Sepiidae) from Northern Australia, with a redescription of Sepiella weberi Adam, 1939. Beagle 1998, 14, 71–102. [Google Scholar] [CrossRef]
- Neige, P.; Dommergues, J.L. Disparity of beaks and statoliths of some coleoids: A morphometric approach to depict shape differentiation. Abh. Geol. 2002, 57, 393–399. [Google Scholar]
- Samuel, D.V.; Patterson, J. A comparative study on the radula of three coleoid cephalopods. S. Pac. J. Mission Stud. 2003, 24, 33–38. [Google Scholar]
- Yoshida, M.; Tsuneki, K.; Furuya, H. Phylogeny of selected Sepiidae (Mollusca, Cephalopoda) based on 12S, 16S, and COI sequence, with comments on the taxonomic reliability of several morphological characters. Zool. Sci. 2006, 23, 341–351. [Google Scholar] [CrossRef]
- Zheng, X.D.; Wang, R.C. Morphological study on radula of nine cephalopods in the coastal waters of China. J. Fish. China 2002, 26, 417–422. [Google Scholar]
- Arularasan, S.; Kesavan, K.; Lyla, P.S. Scanning electron microscope (SEM) studies of radula of the Dog conch Strombus canarium (Gastropoda: Prosobranchia: Strombidae). Pelagia Res. Libr. 2011, 1, 122–127. [Google Scholar]
- Abidin, F.; Sulmartiwi, L.; Saputra, E. Characteristics physicochemical of melanin from squid ink (Loligo sp.) extracted by ethanol. In Proceedings of the 1st International Conference on Biotechnology and Food Sciences, INCOBIFS 2020, Surabaya, Indonesia, 11 September 2020. [Google Scholar]
- Lindberg, D.R.; Ponder, W.F. The influence of classification on the evolutionary interpretation of structure a reevaluation of the evolution of the pallial cavity of gastropod molluscs. Org. Divers. Evol. 2001, 1, 273–299. [Google Scholar] [CrossRef]
- Sundaram, S.; Chavan, B.B. Note on the landing of juveniles of Sepiella inermis at Mumbai. J. Environ. Sci. 2005, 186, 18–19. [Google Scholar]
- Nabhitabhata, J.; Polkan, P. Biological Study on Economic Cephalopods: Survival and Growth of Cuttlefish, Sepiella inermis in Various Salinity Conditions; Technical Paper 4; Ranong Brackish Water Fisheries Station, Brackish Water Division Department of Fisheries: Ranong Province, Thailand, 1983; p. 48. [Google Scholar]
- Chacko, D.; Samuel, V.D.; Pattersan, J. Comparative study on the hatching behavior and growth of the cuttlefish Sepiell inermis and Sepia phalaonis. SDMRI 2005, 9, 159–162. [Google Scholar]
- Ali, S.M.; Mohammed, T.A.; Mandour, A.M.; EL-Malek, A.R.A. Structure of ink apparatus and the funnel organ of the squid “Sepioteuthis sepioidea” (cephalopoda: Lologinidae). Egypt. J. Zool. 2017, 67, 35–50. [Google Scholar] [CrossRef]
- Jiang, M.; Xue, R.; Chen, Q.; Zhan, P.; Han, Q.; Peng, R.; Jiang, X. Histology and ultrastructure of ink gland and melanogenesis in the cuttlefish Sepia Pharaonis. Invertebr. Biol. 2020, 139, 1–11. [Google Scholar] [CrossRef]
- Doguzhaeva, L.A.; Mapes, R.H.; Mutvei, H. The shell and ink sac morphology and ultrastructure of the late Pennsylvanian cephalopod Donovaniconus and its phylogenetic significance. Berl. Paläobiol. Abh. 2003, 3, 061–078. [Google Scholar]
- Khanna, D.R.; Yadav, P.R. Digestive system: Biology of Mollusca; Discovery Publishing House: New Delhi, India, 2004; p. 336. [Google Scholar]
- Riad, R.; Atta, M.; Halim, Y.; Elebiary, N. Taxonomical and morphometric studies on Sepia pharaonic Ehrenberg, 1831 (Cephalopoda: Sepioidea) from the Suez Gulf (Red Sea), Egypt. Int. J. Environ. Sci. 2016, 7, 11–22. [Google Scholar]
- Sundaram, S.; Mohammad, Z.K. Biology of the spineless cuttlefish Sepiella inermis (Orbigny, 1848) from Mumbai waters. Indian J. Fish. 2011, 58, 7–13. [Google Scholar]
- Nabhitabhata, J.; Tuanapaya, S.; Tongtherm, K.; Promdam, R. Diversity of Cephalopod Fauna in Peninsular Thailand; Prince of Songkla University: Songkhla, Thailand, 2017; p. 432. [Google Scholar]
- Parmar, H.L.; Desai, A.Y.; Solanki, J.B.; Zofair, S.M.; Bajaniya, V.C. Biology of the spineless cuttlefish, Sepiella inermis (Van Hasselt, 1835) landed along Veraval coast. J. Exp. Zool. India. 2018, 21, 285–1288. [Google Scholar]
- Appannasastry, Y. Length-weight relationship in the cuttlefish Sepiella inermis d’Orbigny of Kakinada Coast. Indian J. Fish 1989, 36, 175–176. [Google Scholar]
- Önsoy, B.; Salman, A. Length weight relationships of coleoid cephalopods from the eastern Mediterranean. Sci. Rep. 2022, 12, 1–7. [Google Scholar] [CrossRef]
- Forsythe, J.W.; Derusha, R.H.; Hanlon, R.T. Growth, reproduction and life span of Sepia officinalis (Cephalopoda, Mollusca) cultured through seven consecutive generations. J. Zool. 1994, 233, 175–192. [Google Scholar] [CrossRef]
- Kassahn, K.S.; Donnellan, S.C.; Fowler, A.J.; Hall, K.C.; Adams, M.; Shaw, P.W. Molecular and morphological analyses of the cuttlefish Sepia apama indicate a complex population structure. Mar. Biol. 2003, 143, 947–962. [Google Scholar] [CrossRef]
- Visweswaran, B. Is Sepiella inermis ‘Spineless’? J. Pharm. Biol. Sci. 2017, 12, 51–60. [Google Scholar]
- Ghofar, A. The biology of the spineless cuttlefish Sepiella inermis d’ Orbingny in the north coastal water of JAVA. J. Coast. Dev. 1998, 1, 179–188. [Google Scholar]
- Unnithan, K.A. Observation on the biology of cuttlefish Sepiella inermis at Mandapam. Indian J. Fish. 1982, 29, 101–111. [Google Scholar]
- Alujević, K.; Ŝegvić-Bubić, T.; Isajlović, I.; Trumbić, Ž.; Petrić, M. Distribution and differentiation patterns of sympatric squids Alloteuthis media and Alloteuthis subulata (Cephalopoda: Loliginidae) using morphological and molecular approaches. Front. Mar. Sci. 2022, 9, 1–12. [Google Scholar] [CrossRef]




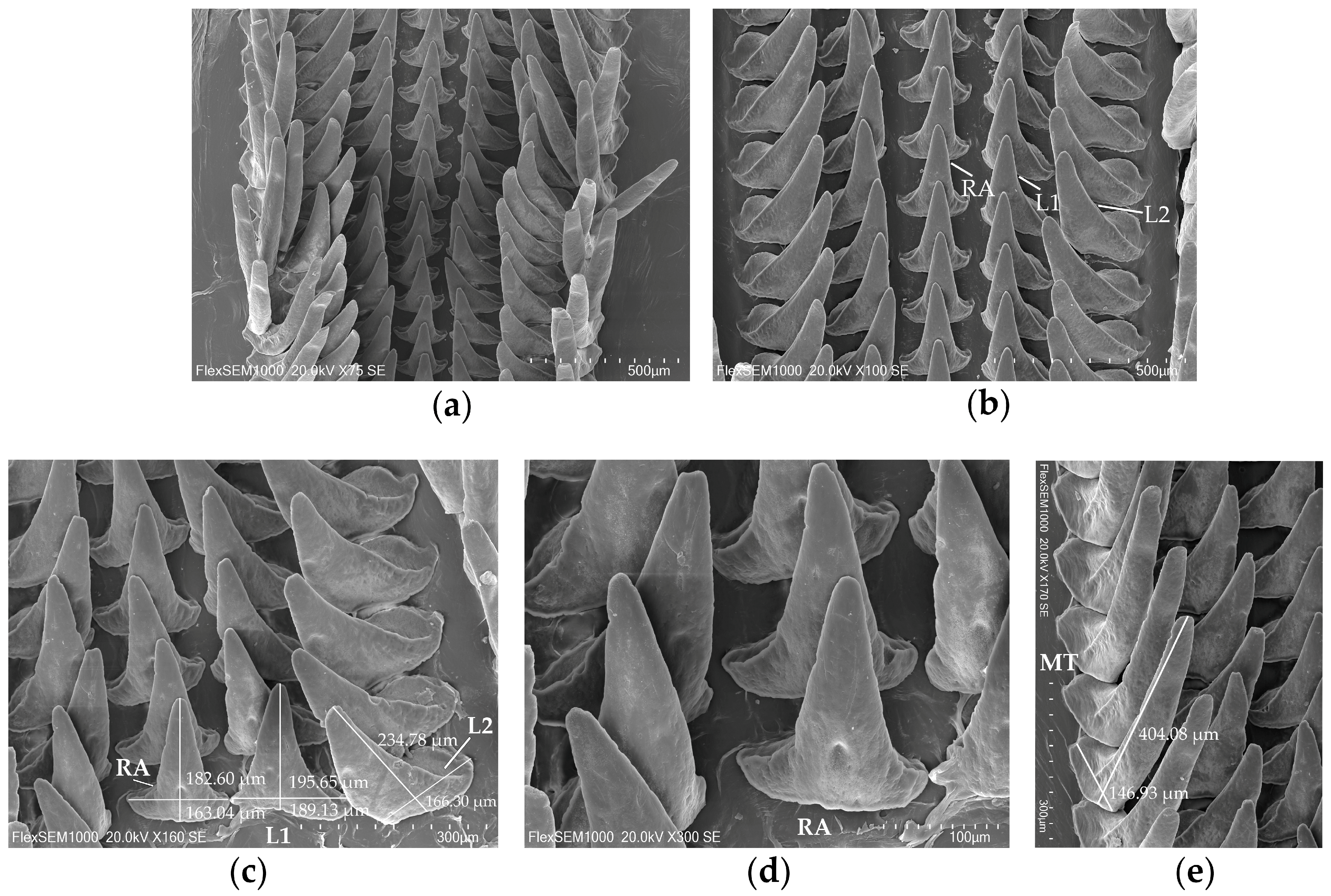

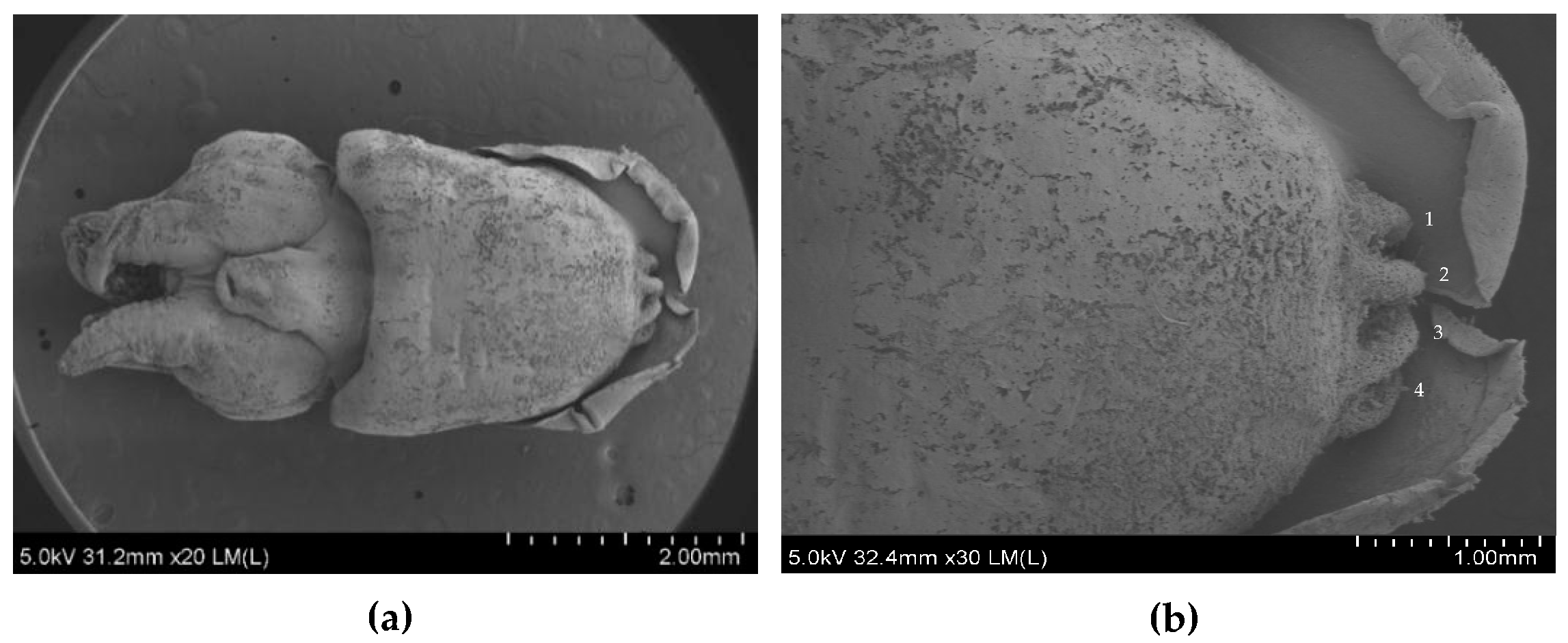

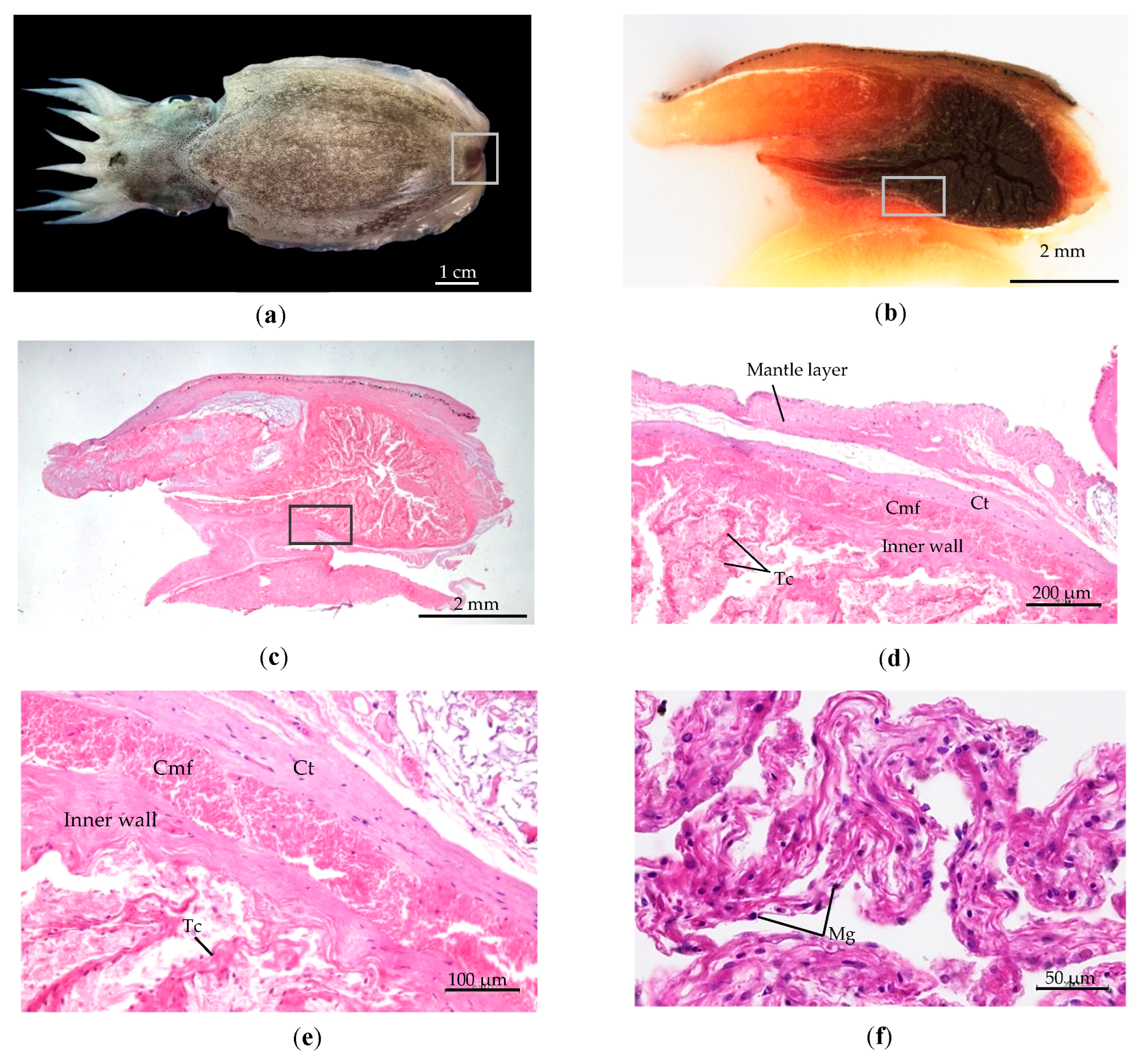



| Stages | Description of the Formation of the Subcutaneous Gland |
|---|---|
| 19 | The subcutaneous gland form has a small knob at the end of the mantle. |
| 20 | The gland is clearly divided into two lobes, light brown, slightly convex, and resembles a cuttlebone. |
| 21 | Two lobes are more convex than in stage 20 and are light orange, and an ink sac is absent. |
| 22 | Two lobes that have grown increasingly convex, starting to take on a darker orange color, and developing an ink sac form. |
| 23 | The gland’s two lobes have grown more convex, and four lines form within the internal structure. |
| 24 | The gland has two larger, dark orange lobes, a longer internal lines structure, and a clear permanent symmetry. Forming the first four lobes. The ink sac and ink duct are visible. |
| 25 | The ventral side of the gland displays four lobes. The gland has changed to dark orange instead of orange in color. Fins, ink duct, and ink sac are formed completely. |
| 26 or Hatching stage | The subcutaneous gland’s structure is clearly visible and the four-line internal structure is located within four lobes on the ventral side. Melanin pigment is contained in the gland. |
| Stations | Number of Samples | Predict Classification of Female | Discrimination Accuracy% | ||||||
|---|---|---|---|---|---|---|---|---|---|
| T | CB | SSM | PT | SNI | SK | RN | |||
| T | 16 | 12 | 0 | 2 | 0 | 2 | 0 | 0 | 75.0 |
| CB | 26 | 0 | 22 | 0 | 0 | 0 | 0 | 4 | 84.6 |
| SSM | 40 | 3 | 5 | 22 | 1 | 0 | 0 | 9 | 55.0 |
| PT | 25 | 0 | 0 | 1 | 22 | 1 | 1 | 0 | 88.0 |
| SNI | 20 | 0 | 0 | 0 | 0 | 20 | 0 | 0 | 100.0 |
| SK | 18 | 1 | 0 | 3 | 1 | 1 | 10 | 2 | 55.6 |
| RN | 48 | 0 | 4 | 4 | 0 | 0 | 1 | 39 | 81.3 |
| Comprehensive Discrimination Rate: 71.5% | |||||||||
| Stations | Number of Samples | Predict Classification of Male | Discrimination Accuracy% | ||||||
|---|---|---|---|---|---|---|---|---|---|
| T | CB | SSM | PT | SNI | SK | RN | |||
| T | 14 | 13 | 0 | 1 | 0 | 0 | 0 | 0 | 92.9 |
| CB | 35 | 0 | 33 | 2 | 0 | 0 | 0 | 0 | 94.3 |
| SSM | 40 | 0 | 5 | 26 | 4 | 0 | 1 | 4 | 65 |
| PT | 50 | 0 | 0 | 4 | 39 | 2 | 1 | 4 | 78 |
| SNI | 10 | 0 | 0 | 0 | 0 | 10 | 0 | 0 | 100 |
| SK | 27 | 0 | 0 | 2 | 1 | 0 | 23 | 1 | 85.2 |
| RN | 27 | 0 | 2 | 0 | 2 | 1 | 0 | 22 | 81.5 |
| Comprehensive Discrimination Rate: 81.8% | |||||||||
| Sex/ Stations | Length–Weight Relationship Parameters | a | b | r | Growth Relationship |
|---|---|---|---|---|---|
| Female | BW = 0.00237 × DML2.3837 | 0.00237 | 2.3837 | 0.917 | Allometric |
| Male | BW = 0.00479 × DML2.1746 | 0.00479 | 2.1746 | 0.917 | Allometric |
| Combined | BW = 0.00242 × DML2.3616 | 0.00242 | 2.3616 | 0.906 | Allometric |
| TT | BW = 0.00112 × DML2.5507 | 0.00112 | 2.5507 | 0.938 | Allometric |
| CB | BW = 0.00786 × DML2.0382 | 0.00786 | 2.0382 | 0.880 | Allometric |
| SSM | BW = 0.00462 × DML2.1990 | 0.00462 | 2.1990 | 0.904 | Allometric |
| PT | BW = 0.00134 × DML2.4789 | 0.00134 | 2.4789 | 0.909 | Allometric |
| SNI | BW = 0.000174 × DML3.0272 | 0.000174 | 3.0272 | 0.914 | Isometric |
| SK | BW = 0.00171 × DML2.4513 | 0.00171 | 2.4513 | 0.822 | Allometric |
| RN | BW = 0.00121 × DML2.5622 | 0.00121 | 2.5622 | 0.926 | Allometric |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phuynoi, S.; Sukhsangchan, C.; Xu, R.; Zheng, X. Morphological Variation and New Description of the Subcutaneous Gland of Sepiella inermis (Van Hasselt, 1835) in Thai Waters. Diversity 2024, 16, 138. https://doi.org/10.3390/d16030138
Phuynoi S, Sukhsangchan C, Xu R, Zheng X. Morphological Variation and New Description of the Subcutaneous Gland of Sepiella inermis (Van Hasselt, 1835) in Thai Waters. Diversity. 2024; 16(3):138. https://doi.org/10.3390/d16030138
Chicago/Turabian StylePhuynoi, Sonthaya, Charuay Sukhsangchan, Ran Xu, and Xiaodong Zheng. 2024. "Morphological Variation and New Description of the Subcutaneous Gland of Sepiella inermis (Van Hasselt, 1835) in Thai Waters" Diversity 16, no. 3: 138. https://doi.org/10.3390/d16030138
APA StylePhuynoi, S., Sukhsangchan, C., Xu, R., & Zheng, X. (2024). Morphological Variation and New Description of the Subcutaneous Gland of Sepiella inermis (Van Hasselt, 1835) in Thai Waters. Diversity, 16(3), 138. https://doi.org/10.3390/d16030138










