What Is in the Bank? Assessing Persistent Soil Seed Bank Density of Sclerocactus wrightiae (Cactaceae)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Soil Sampling
2.3. Analysis
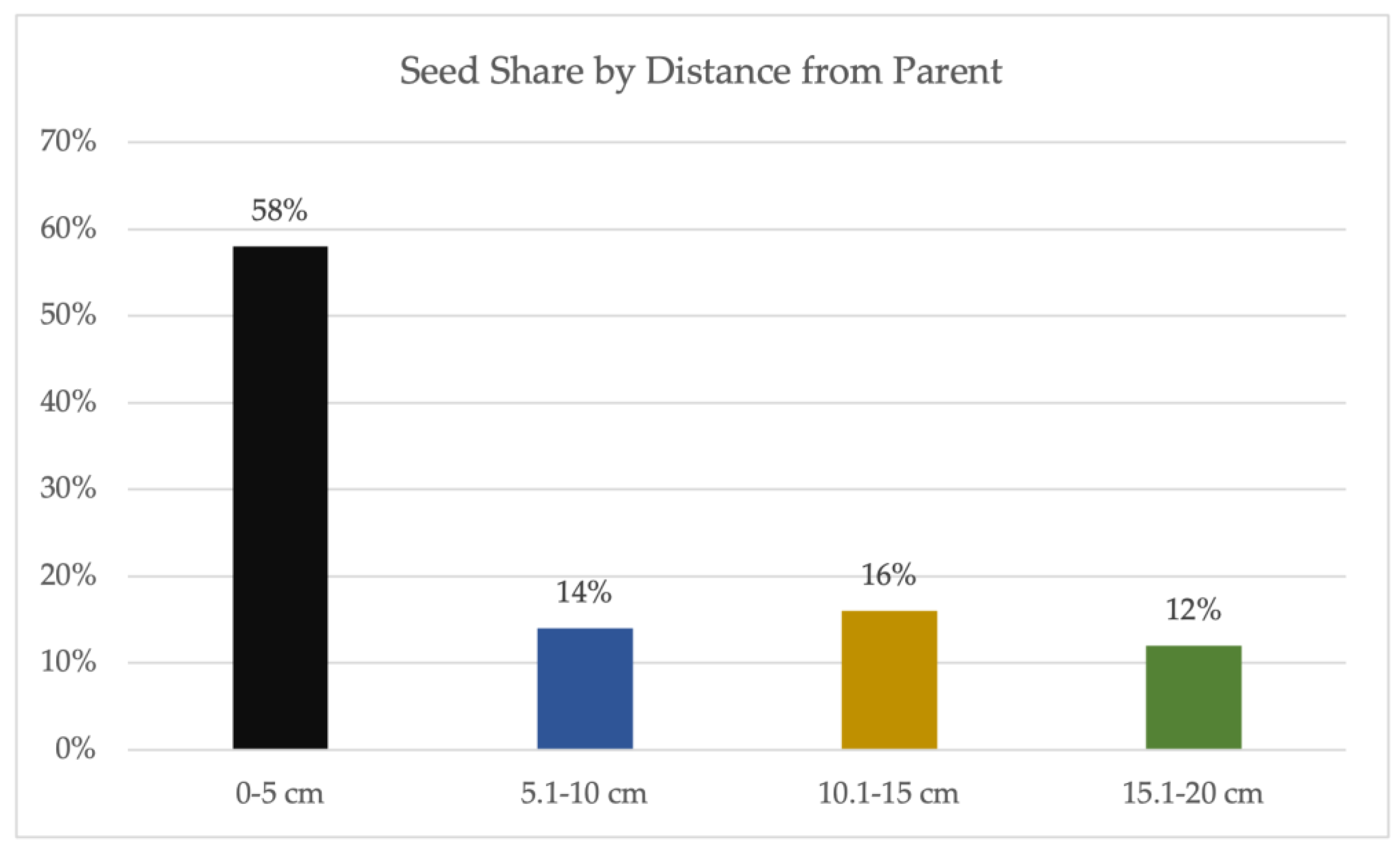
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Britton, N.L.; Rose, J.N. The Cactaceae 3; The Carnegie Institution: Washington, DC, USA, 1922. [Google Scholar]
- Porter, M.; Clifford, A. Genetic Diversity within Sclerocactus Cloverae Heil & Porter Based on Ddrad-Seq: The Genetic Basis for Subspecies Recognition. 2018; unpublished report. [Google Scholar]
- Fritz, H.; Holland, C. The Genus Sclerocactus (Cactaceae)—Part 1. Br. Cactus Succul. J. 1995, 13, 73–79. [Google Scholar]
- USFWS. FWS-Listed U.S. Species by Taxonomic Group—All Flowering Plants; ECOS, US Fish and Wildlife Service: Bailey’s Crossroads, VA, USA, 2021.
- Benson, L. Cactaceae Emery Co., San Rafael Ridge (A Revision of Sclerocactus). Cactus Succul. J. 1966, 38, 55. [Google Scholar]
- Welsh, S.L.; Atwood, N.D.; Goodrich, S.; Higgins, L.C. A Utah Flora, 3rd ed.; Brigham Young University: Provo, UT, USA, 2003. [Google Scholar]
- U.S. Fish and Wildlife Service. Rules and Regulations Department of the Interior Fish and Wildlife Service 50 CPFI Part 17 Endangered and Threatened Wildlife and Plants. In Determination That the Purple-Spined Hedgehog Cactus and Wright Fishhook Cactus Are Endangered Species; Federal Register; U.S. Fish and Wildlife Service: Washington, DC, USA, 1979; Volume 44, pp. 58866–58868. [Google Scholar]
- Kass, R.J. Mortality of the Endangered Wright Fishhook Cactus (Sclerocactus wrightiae) by an Opuntia-Borer Beetle (Cerambycidae: Moneilema semipunctatum). West. N. Am. Nat. 2001, 61, 495–497. [Google Scholar]
- Coles, J.J.; Decker, K.L.; Naumann, T.S. Ecology and Population Dynamics of Sclerocactus Mesae-Verdae (Boissev. & C. Davidson) LD Benson. West. N. Am. Nat. 2012, 72, 311–322. [Google Scholar]
- Spector, T. Impacts to Federally Listed Cacti Species from Livestock on the Colorado Plateau in Utah: A Review and Summary; Utah Field Office, Ecological Services, U.S. Fish and Wildlife Service: West Valley City, UT, USA, 2013. [Google Scholar]
- Bates, T.H.; Anderson, V.J.; Johnson, R.L.; Petersen, S.L.; Allphin, L.; Rooks, D.L. Effects of Cattle Disturbance on Change in Population Densities and Flowering of Wright Fishhook Cactus (Scleropogon wrightiae L.D. Bensen). Southwest. Nat. 2022, 66, 304–308. [Google Scholar] [CrossRef]
- Lariviere, D.; Anderson, V.; Johnson, R.; Terry, T.; Bates, T. Effects of Cattle Traffic on Sclerocactus wrightiae. Land 2023, 12, 853. [Google Scholar] [CrossRef]
- Harper, J.L. Population Biology of Plants; Academic Press: London, UK, 1977. [Google Scholar]
- Gurvich, D.E.; Lorenzati, M.A.; Sosa-Pivatto, M.; Bauk, K.; Barroso, F.L. Effects of Long-Term Seed Storage on Germination of 13 Cactus Species from Central Argentina. J. Arid Environ. 2021, 185, 104382. [Google Scholar] [CrossRef]
- Rojas-Aréchiga, M.; Vázquez-Yanes, C. Cactus Seed Germination: A Review. J. Arid Environ. 2000, 44, 85–104. [Google Scholar] [CrossRef]
- Ricardo, A.-E.; Godínez-Álvarez, H.; De la Torre-Almaráz, R. Seed Banking in the Columnar Cactus Stenocereus Stellatus: Distribution, Density, and Longevity of Seeds. Seed Sci. Res. 2014, 24, 315–320. [Google Scholar]
- Holland, J.N.; Molina-Freaner, F. Hierarchical Effects of Rainfall, Nurse Plants, Granivory and Seed Banks on Cactus Recruitment. J. Veg. Sci. 2013, 24, 1053–1061. [Google Scholar] [CrossRef]
- Bowers Janice, E. New Evidence for Persistent or Transient Seed Banks in Three Sonoran Desert Cacti. Southwest. Nat. 2005, 50, 482–487. [Google Scholar] [CrossRef]
- Ordoñez-Salanueva, C.A.; Orozco-Segovia, A.; Canales-Martínez, M.; Seal, C.E.; Pritchard, H.W.; Flores-Ortiz, C.M. Ecological Longevity of Polaskia Chende (Cactaceae) Seeds in the Soil Seed Bank, Seedling Emergence and Survival. Plant Biol. 2017, 19, 973–982. [Google Scholar] [CrossRef]
- Lindow-López, L.; Galíndez, G.; Sühring, S.; Pastrana-Ignes, V.; Gorostiague, P.; Gutiérrez, A.; Ortega-Baes, P. Do Cacti Form Soil Seed Banks? An Evaluation Using Species from the Southern Central Andes. Plant Biol. 2018, 20, 1053–1058. [Google Scholar] [CrossRef]
- Angel, M.-R.M.; Sosa, V.J. Nurse Plants vs. Nurse Objects: Effects of Woody Plants and Rocky Cavities on the Recruitment of the Pilosocereus Leucocephalus Columnar Cactus. Ann. Bot. 2008, 101, 175–185. [Google Scholar]
- Arroyo-Cosultchi, G.; Golubov, J.; Mandujano, M.C.; Salguero-Gómez, R.; Martínez, A.J. What Are the Demographic Consequences of a Seed Bank Stage for Columnar Cacti? Popul. Ecol. 2022, 64, 35–46. [Google Scholar] [CrossRef]
- Harding, K.T. Sclerocactus Wetlandicus: Habitat Characterization, Seed Germination and Mycorrhizal Analysis. Master’s Thesis, Utah State University, Logan, UT, USA, 2017. [Google Scholar]
- Nancy, D.R.; Riley, T.Z. Chipping and Chemical Scarification Effects on Sclerocactus glaucus (K. Schum.) LD Benson (Cactaceae) Seed Germination. Cactus Succul. J. 2018, 90, 216–221. [Google Scholar]
- Gould, F.W. Grassland Systematics; Texas A&M University Press: College Station, TX, USA, 1970. [Google Scholar]
- BLM. Rock Springs Weather Station Data; USDA: Washington, DC, USA, 2017. [Google Scholar]
- Wadman, K.V.; NRCS. Ecological Site R034BY115UT Desert Sandy Loam (Indian Ricegrass); USDA: Washington, DC, USA, 2012.
- Bowers, J.E. Does Ferocactus Wislizeni (Cactaceae) Have a Between-Year Seed Bank? J. Arid Environ. 2000, 45, 197–205. [Google Scholar] [CrossRef]
- Kass, R.J. Final Report of Habitat Inventory of Threatened, Endangered and Candidate Plant Species in the San Rafael Swell, Utah; Bureau of Land Management, Utah State Office: Logan, UT, USA, 1990; unpublished agency report. [Google Scholar]
- Bochet, E. The Fate of Seeds in the Soil: A Review of the Influence of Overland Flow on Seed Removal and Its Consequences for the Vegetation of Arid and Semiarid Patchy Ecosystems. Soil 2015, 1, 131–146. [Google Scholar] [CrossRef]
- Yu, W.; Jiao, J.; Chen, Y.; Wang, D.; Wang, N.; Zhao, H. Seed Removal Due to Overland Flow on Abandoned Slopes in the Chinese Hilly Gullied Loess Plateau Region. Land Degrad. Dev. 2017, 28, 274–282. [Google Scholar] [CrossRef]
- Peters, J.; Lanham, B.; Association of Official Seed Analysts, Tetrazolium Testing Committee. Tetrazolium Testing Handbook: Contribution No. 29 to the Handbook of Seed Testing. Issue 29 of Handbook on Seed Testing; International Seed Testing Association: Washington, DC, USA, 2000. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Bates, D.; Maechler, M.; Bolker, B.M.; Walker, S.C. Fitting Linear Mixed-Effects Models Using Lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. LmerTest Package: Tests in Linear Mixed Effects Models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef]
- Akaike, H. Information Theory as an Extension of the Maximum Likelihood Principle. In Second International Symposium on Information Theory; Petrov, B.N., Csaki, F., Eds.; Akademiai Kiado: Budapest, Hungary, 1973; pp. 267–281. [Google Scholar]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach; Springer: New York, NY, USA, 2002. [Google Scholar]
- Naimi, B.; Hamm, N.A.S.; Groen, T.A.; Skidmore, A.K.; Toxopeus, A.G. Where Is Positional Uncertainty a Problem for Species Distribution Modelling? Ecography 2014, 37, 191–203. [Google Scholar] [CrossRef]
- Arnold, T.W. Uninformative Parameters and Model Selection Using Akaike’s Information Criterion. J. Wildl. Manag. 2010, 74, 1175–1178. [Google Scholar]
- Leroux, S.J. On the Prevalence of Uninformative Parameters in Statistical Models Applying Model Selection in Applied Ecology. PLoS ONE 2019, 14, e0206711. [Google Scholar] [CrossRef]
- Guzmán-Vázquez, I.; Castillo-Argüero, S.; Orozco-Segovia, A.; Collazo-Ortega, M. Spatial and Temporal Dynamics of Two Cacti Seed Banks in a Xerophytic Shrubland in Mexico City. Bot. Sci. 2021, 99, 560–572. [Google Scholar] [CrossRef]
- Montiel, S.; Montaña, C. Seed Bank Dynamics of the Desert Cactus Opuntia Rastrera in Two Habitats from the Chihuahuan Desert. Plant Ecol. 2003, 166, 241–248. [Google Scholar] [CrossRef]
- Godínez-Alvare, H.; Valiente-Banuet, A. Germination and Early Seedling Growth of Tehuacan Valley Cacti Species: The Role of Soils and Seed Ingestion by Dispersers on Seedling Growth. J. Arid Environ. 1998, 39, 21–31. [Google Scholar] [CrossRef]
- Bureau of Land Management. Three-Year Summary Report 2011–2013 Initial and Repeat Inventory for Wright Fishhook Cactus (Sclerocactus wrightiae); Field Office: Richfield, UT, USA, 2013; unpublished agency report.
- Nascimento JP, B.; Meiado, M.V. In Situ or Ex Situ Seed Conservation: Which Is the More Effective Way to Maintain Seed Longevity of an Endangered Cactus? Plant Species Biol. 2017, 32, 115–120. [Google Scholar] [CrossRef]




| Model Structure | df a | logLik b | AICc c | Delta d | Weight e |
|---|---|---|---|---|---|
| Presence~Position + Distance | 4 | −106.93 | 221.9 | 0.00 | 0.395 |
| Presence~Depth + Position + Distance | 5 | −106.64 | 223.4 | 1.46 | 0.191 |
| Presence~Distance | 2 | −109.77 | 223.6 | 1.63 | 0.175 |
| Presence~Position | 3 | −109.40 | 224.8 | 2.91 | 0.092 |
| Presence~Depth + Distance | 3 | −109.48 | 225.0 | 3.08 | 0.085 |
| Presence~Depth + Position | 4 | −109.11 | 226.3 | 4.36 | 0.045 |
| Presence~1 | 1 | −113.48 | 229.0 | 7.03 | 0.012 |
| Presence~Depth | 2 | −113.20 | 230.4 | 8.48 | 0.006 |
| Estimate a | Std. Error b | Adj. SEc | z-Value d | LCI e | UCI f | |
|---|---|---|---|---|---|---|
| Intercept | −2.1264 | 0.3437 | 0.3443 | 6.177 | −2.6217 | −1.6312 |
| Position (Nurse plant) | 3.3989 | 884.2836 | 886.4470 | 0.004 | −1271.530 | 1278.3277 |
| Position (Upslope) | −1.8297 | 1.0258 | 1.0283 | 1.779 | −3.3086 | −0.3508 |
| Distance | −0.0772 | 0.0358 | 0.03586 | 2.154 | −0.1288 | −0.0257 |
| Depth | −0.2905 | 0.3823 | 0.3842 | 0.756 | −0.8431 | 0.2620 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lariviere, D.; Anderson, V.; Johnson, R.; Larsen, R. What Is in the Bank? Assessing Persistent Soil Seed Bank Density of Sclerocactus wrightiae (Cactaceae). Diversity 2024, 16, 133. https://doi.org/10.3390/d16030133
Lariviere D, Anderson V, Johnson R, Larsen R. What Is in the Bank? Assessing Persistent Soil Seed Bank Density of Sclerocactus wrightiae (Cactaceae). Diversity. 2024; 16(3):133. https://doi.org/10.3390/d16030133
Chicago/Turabian StyleLariviere, David, Val Anderson, Robert Johnson, and Randy Larsen. 2024. "What Is in the Bank? Assessing Persistent Soil Seed Bank Density of Sclerocactus wrightiae (Cactaceae)" Diversity 16, no. 3: 133. https://doi.org/10.3390/d16030133
APA StyleLariviere, D., Anderson, V., Johnson, R., & Larsen, R. (2024). What Is in the Bank? Assessing Persistent Soil Seed Bank Density of Sclerocactus wrightiae (Cactaceae). Diversity, 16(3), 133. https://doi.org/10.3390/d16030133





