Responses of Natural Microorganisms to Land Reclamation and Applications of Functional Microorganisms in Biorestoration of Coal Mining Area
Abstract
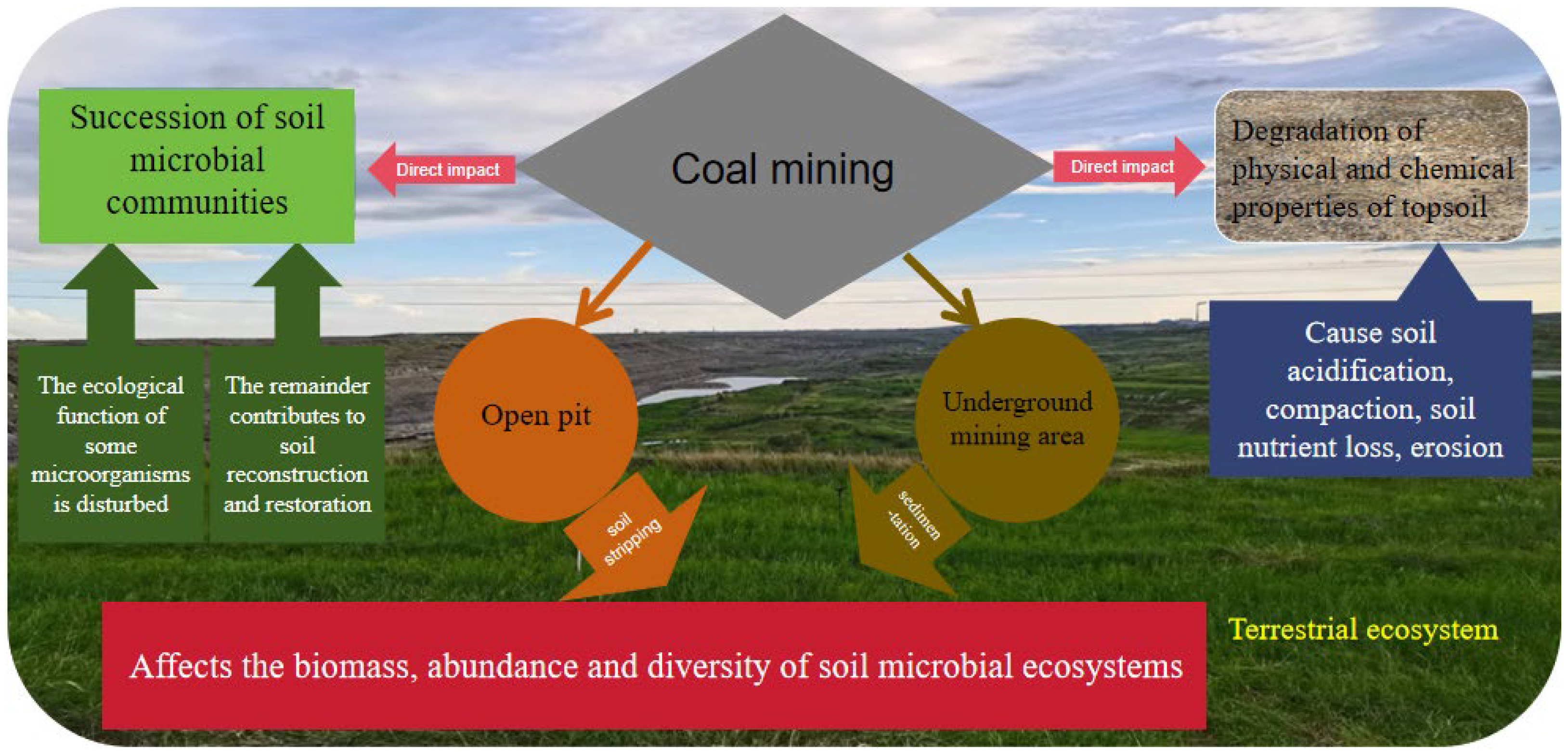
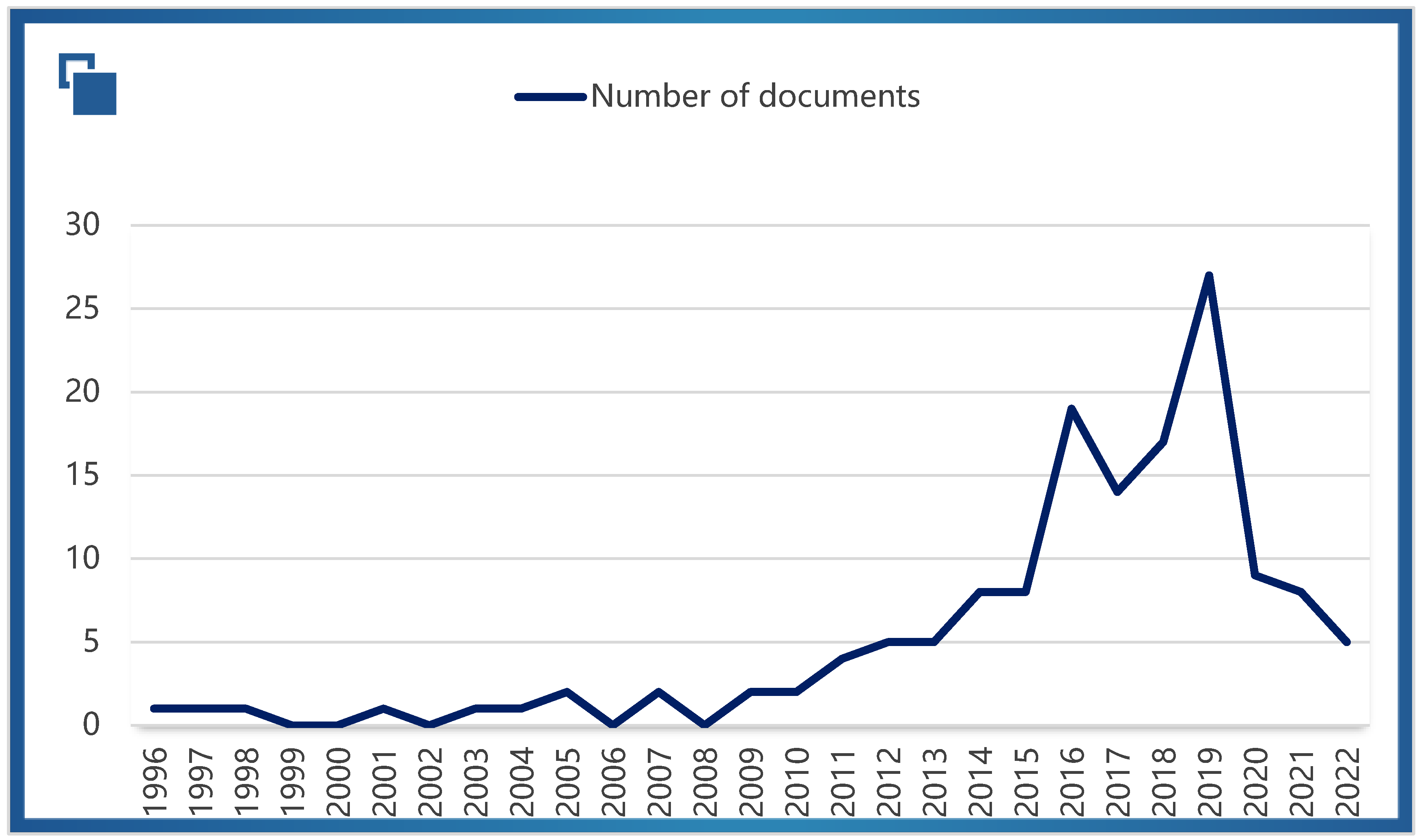
1. Introduction
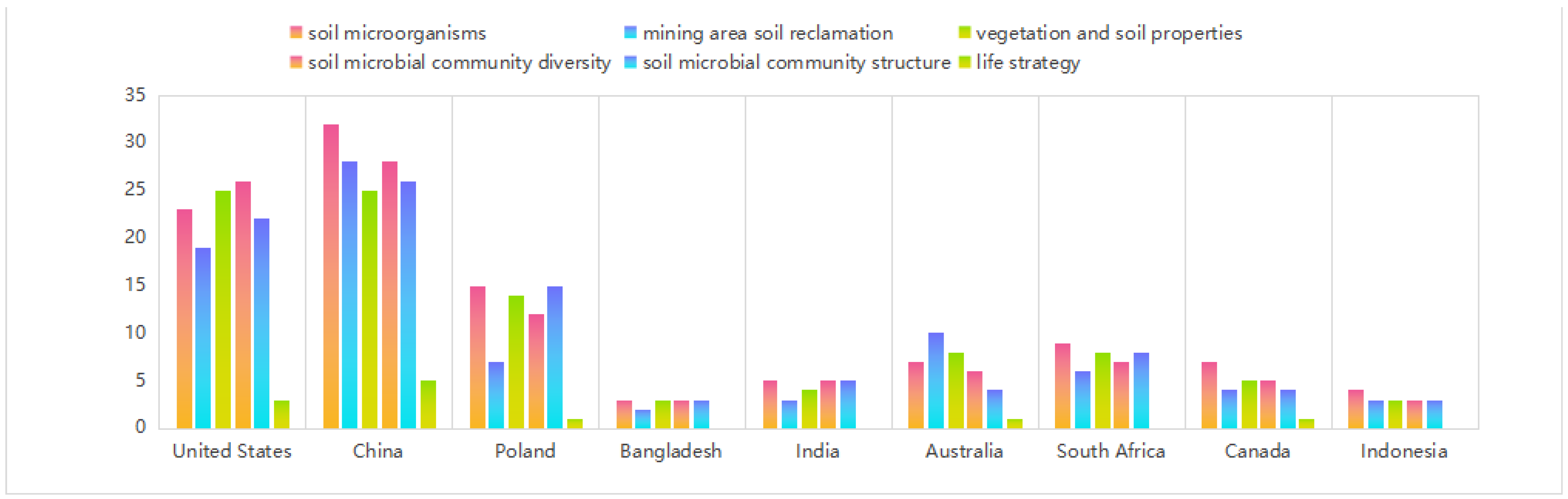
2. Literature Review
3. Responses of Natural Microorganisms to Land Reclamation
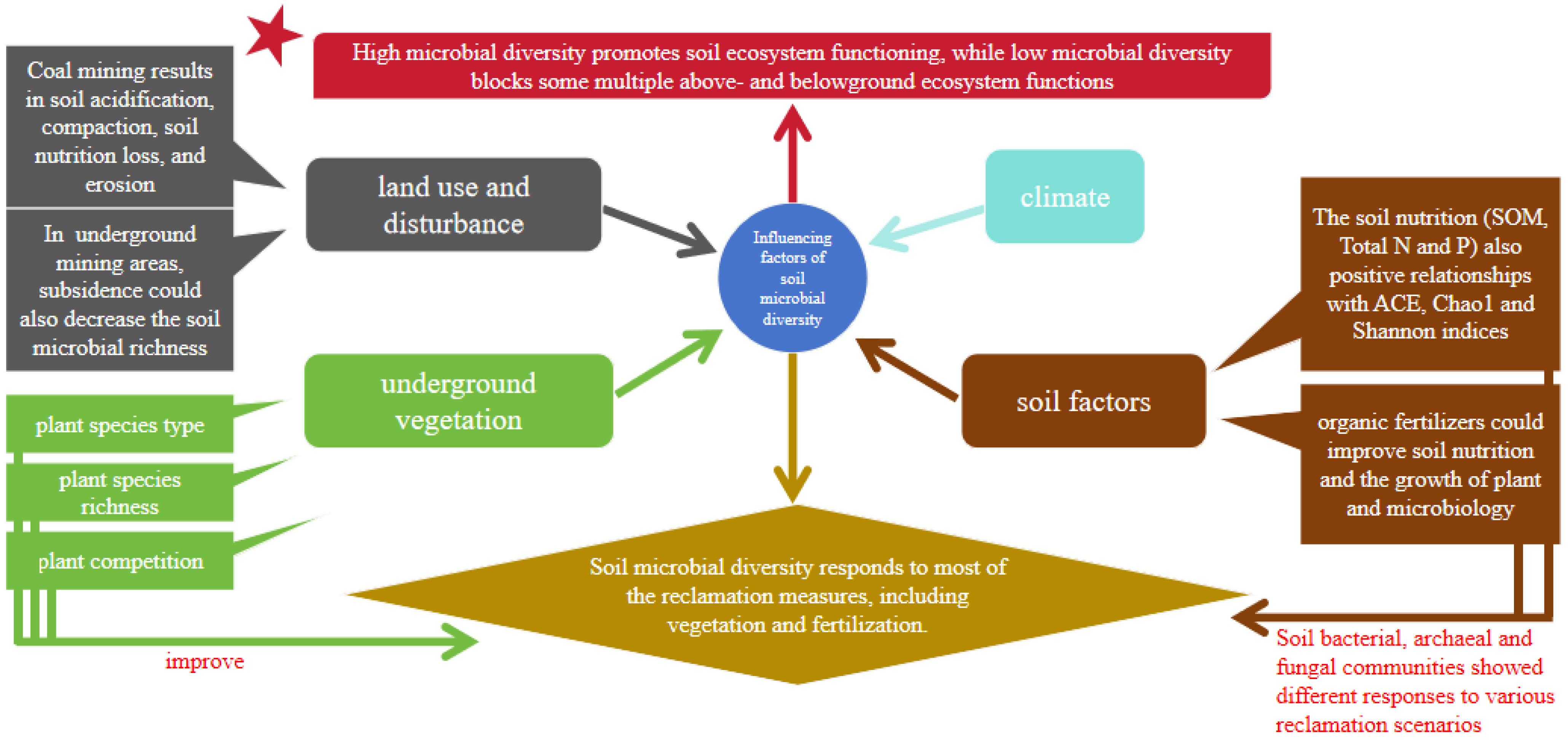
3.1. Biodiversity Changes of Microbial Community after Land Reclamation
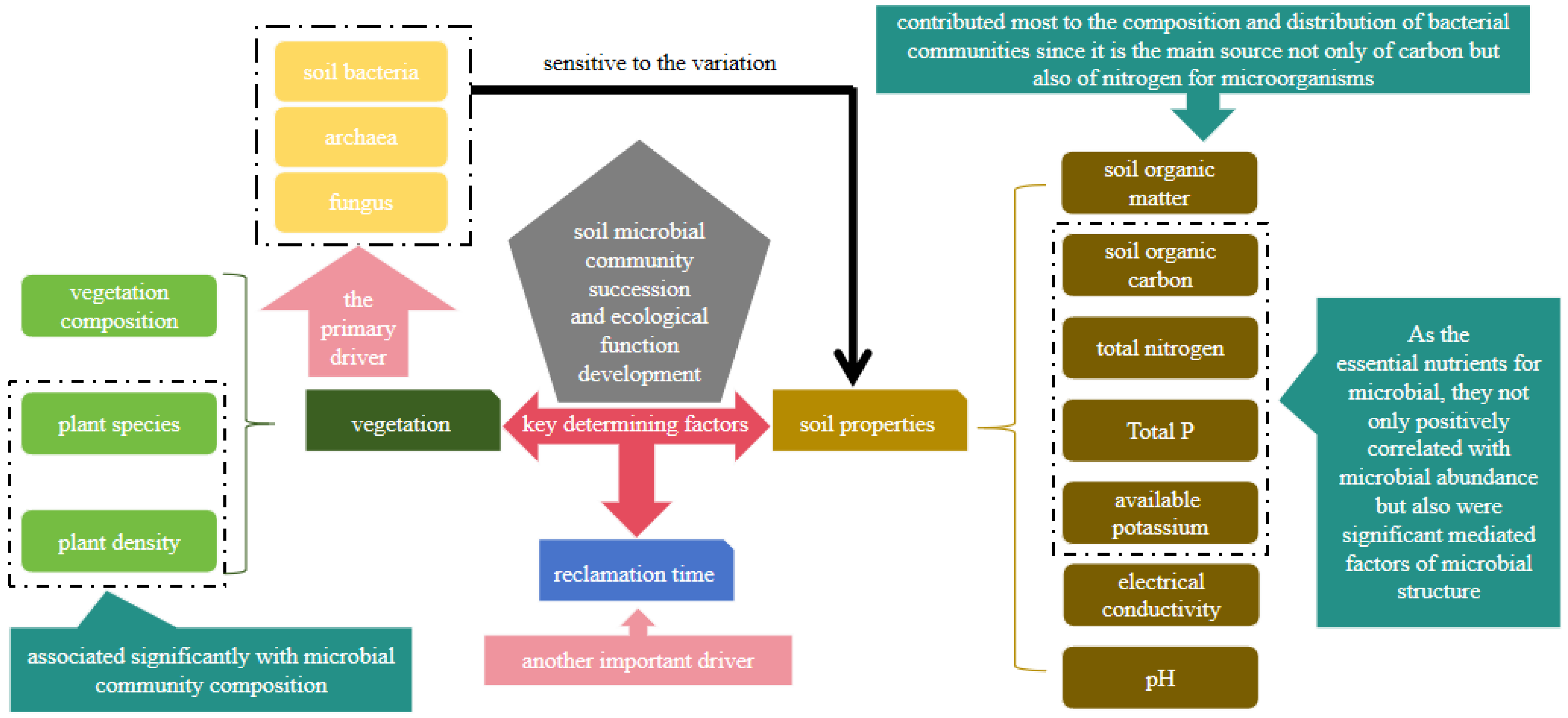
3.2. Changes of Microbial Community Structure after Land Reclamation
3.3. Main Driven Factors of the Microbial Community in Reclamation
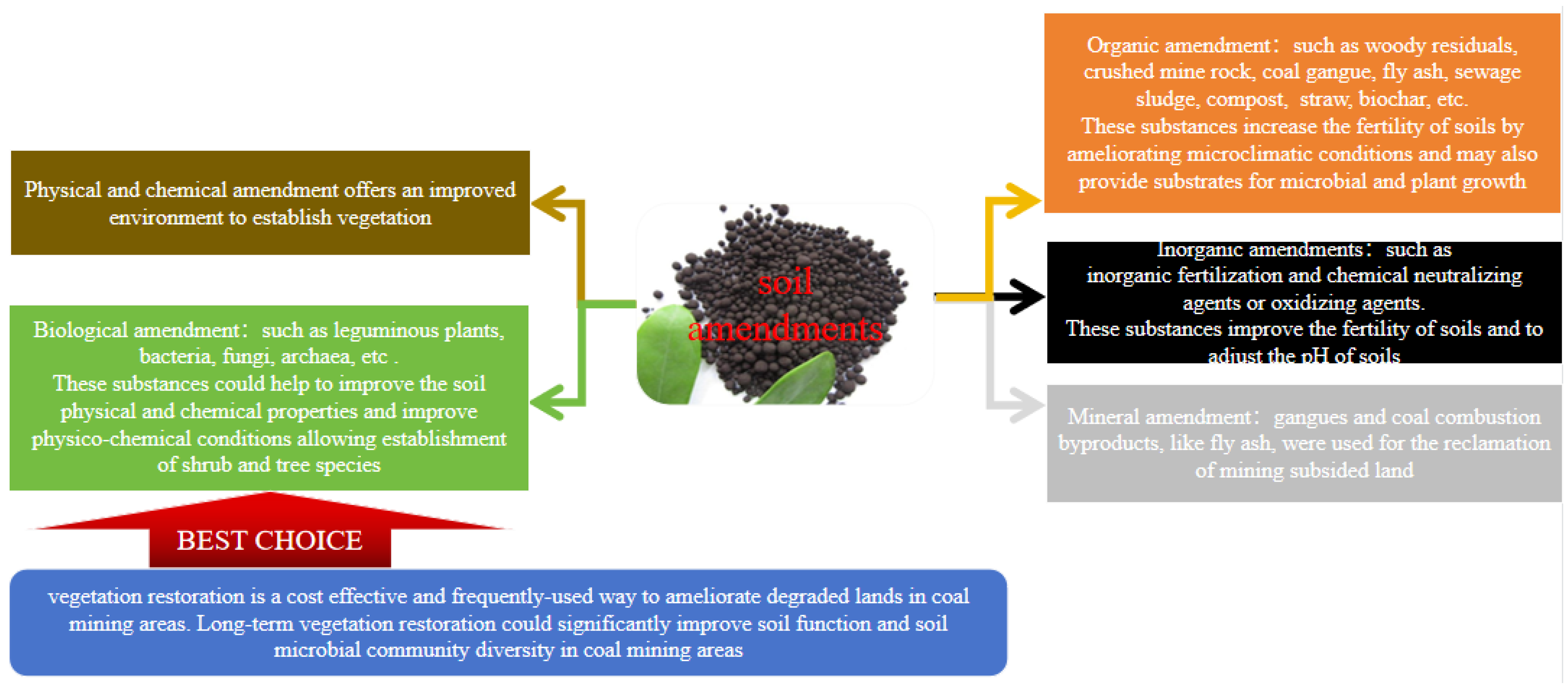
4. Applications of Functional Microorganisms on Biorestoration
4.1. Application of Microbial Inoculation on Biorestoration in the Coal Mining Area
4.2. Applications of Microorganisms in Assessing Reclaimed Soils
4.3. Applications of Microorganisms to Remediate Pollution in Mining Areas
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Palmer, M.A.; Bernhardt, E.S.; Schlesinger, W.H.; Eshleman, K.N.; Foufoula-Georgiou, E.; Hendryx, M.S.; Lemly, A.D.; Likens, G.E.; Loucks, O.L.; Power, M.E.; et al. Mountaintop Mining Consequences. Science 2010, 327, 148–149. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Zhang, Y.X.; Ruan, M.Y.; Guo, J.; Chai, T.Y. Land Subsidence in a Coal Mining Area Reduced Soil Fertility and Led to Soil Degradation in Arid and Semi-Arid Regions. Int. J. Environ. Res. Public Health 2019, 16, 3929. [Google Scholar] [CrossRef] [PubMed]
- Ciesielczuk, J.; Misz-Kennan, M.; Hower, J.C.; Fabianska, M.J. Mineralogy and geochemistry of coal wastes from the Starzykowiec coal-waste dump (Upper Silesia, Poland). Int. J. Coal Geol. 2014, 127, 42–55. [Google Scholar] [CrossRef]
- Howladar, M.F. Environmental impacts of subsidence around the Barapukuria Coal Mining area in Bangladesh. Energy Ecol. Environ. 2016, 1, 370–385. [Google Scholar] [CrossRef]
- Bian, Z.; Inyang, H.I.; Daniels, J.L.; Otto, F.; Struthers, S. Environmental issues from coal mining and their solutions. Min. Sci. Technol. 2010, 20, 215–223. [Google Scholar] [CrossRef]
- Zhang, G.X.; Yuan, X.Z.; Wang, K.H. Biodiversity and temporal patterns of macrozoobenthos in a coal mining subsidence area in North China. PeerJ 2019, 7, 22. [Google Scholar] [CrossRef] [PubMed]
- Schmid, C.A.O.; Reichel, R.; Schroeder, P.; Brueggemann, N.; Schloter, M. 52 years of ecological restoration following a major disturbance by opencast lignite mining does not reassemble microbiome structures of the original arable soils. Sci. Total Environ. 2020, 745, 140955. [Google Scholar] [CrossRef] [PubMed]
- de Quadros, P.D.; Zhalnina, K.; Davis-Richardson, A.G.; Drew, J.C.; Menezes, F.B.; Camargo, F.A.D.; Triplett, E.W. Coal mining practices reduce the microbial biomass, richness and diversity of soil. Appl. Soil Ecol. 2016, 98, 195–203. [Google Scholar] [CrossRef]
- Shi, P.L.; Zhang, Y.X.; Hu, Z.Q.; Ma, K.; Wang, H.; Chai, T.Y. The response of soil bacterial communities to mining subsidence in the west China aeolian sand area. Appl. Soil Ecol. 2017, 121, 1–10. [Google Scholar] [CrossRef]
- Hobbs, R.J.; Norton, D.A. Towards a conceptual framework for restoration ecology. Restor. Ecol. 1996, 4, 93–110. [Google Scholar] [CrossRef]
- Hobbs, R.J.; Harris, J.A. Restoration ecology: Repairing the Earth’s ecosystems in the new millennium. Restor. Ecol. 2001, 9, 239–246. [Google Scholar] [CrossRef]
- Corlett, R.T. Restoration, Reintroduction, and Rewilding in a Changing World. Trends Ecol. Evol. 2016, 31, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Watkinson, A.D.; Lock, A.S.; Beckett, P.J.; Spiers, G. Developing manufactured soils from industrial by-products for use as growth substrates in mine reclamation. Restor. Ecol. 2017, 25, 587–594. [Google Scholar] [CrossRef]
- Li, S.Q.; Di, X.Y.; Wu, D.M.; Zhang, J.T. Effects of sewage sludge and nitrogen fertilizer on herbage growth and soil fertility improvement in restoration of the abandoned opencast mining areas in Shanxi, China. Environ. Earth Sci. 2013, 70, 3323–3333. [Google Scholar] [CrossRef]
- Du, T.; Wang, D.M.; Bai, Y.J.; Zhang, Z.Z. Optimizing the formulation of coal gangue planting substrate using wastes: The sustainability of coal mine ecological restoration. Ecol. Eng. 2020, 143, 105669. [Google Scholar] [CrossRef]
- Paramashivam, D.; Dickinson, N.M.; Clough, T.J.; Horswell, J.; Robinson, B.H. Potential Environmental Benefits from Blending Biosolids with Other Organic Amendments before Application to Land. J. Environ. Qual. 2017, 46, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Fellet, G.; Marchiol, L.; Delle Vedove, G.; Peressotti, A. Application of biochar on mine tailings: Effects and perspectives for land reclamation. Chemosphere 2011, 83, 1262–1267. [Google Scholar] [CrossRef]
- Maiti, S.K.; Ahirwal, J. Ecological Restoration of Coal Mine Degraded Lands: Topsoil Management, Pedogenesis, Carbon Sequestration, and Mine Pit Limnology. In Phytomanagement of Polluted Sites; Pandey, V.C., Bauddh, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; Chapter 3; pp. 83–111. [Google Scholar]
- Li, J.J.; Zheng, Y.M.; Yan, J.X.; Li, H.J.; Wang, X.; He, J.Z.; Ding, G.W. Effects of Different Regeneration Scenarios and Fertilizer Treatments on Soil Microbial Ecology in Reclaimed Opencast Mining Areas on the Loess Plateau, China. PLoS ONE 2013, 8, e63275. [Google Scholar] [CrossRef]
- Saha, S.; Sinha, A. A review on treatment of acid mine drainage with waste materials: A novel approach. Glob. NEST J. 2018, 20, 512–528. [Google Scholar] [CrossRef]
- RoyChowdhury, A.; Sarkar, D.; Datta, R. A combined chemical and phytoremediation method for reclamation of acid mine drainage-impacted soils. Environ. Sci. Pollut. Res. 2019, 26, 14414–14425. [Google Scholar] [CrossRef]
- Huang, J.; Wang, P.; Xu, C.R.; Zhu, Z.Z. Fly Ash Modified Coalmine Solid Wastes for Stabilization of Trace Metals in Mining Damaged Land Reclamation: A Case Study in Xuzhou Coalmine Area. Int. J. Environ. Res. Public Health 2018, 15, 2317. [Google Scholar] [CrossRef]
- Kumari, S.; Maiti, S.K. Reclamation of coalmine spoils with topsoil, grass, and legume: A case study from India. Environ. Earth Sci. 2019, 78, 429. [Google Scholar] [CrossRef]
- Ruzek, L.; Ruzkova, M.; Vorisek, K.; Vrablikova, J.; Vrablik, P. Slit seeded grass-legume mixture improves coal mine reclamation. Plant. Soil Environ. 2012, 58, 68–75. [Google Scholar] [CrossRef]
- Li, P.F.; Zhang, X.C.; Hao, M.D.; Cui, Y.X.; Zhu, S.L.; Zhang, Y.J. Effects of Vegetation Restoration on Soil Bacterial Communities, Enzyme Activities, and Nutrients of Reconstructed Soil in a Mining Area on the Loess Plateau, China. Sustainability 2019, 11, 2295. [Google Scholar] [CrossRef]
- Sun, S.Y.; Sun, H.; Zhang, D.S.; Zhang, J.F.; Cai, Z.Y.; Qin, G.H.; Song, Y.M. Response of Soil Microbes to Vegetation Restoration in Coal Mining Subsidence Areas at Huaibei Coal Mine, China. Int. J. Environ. Res. Public Health 2019, 16, 1757. [Google Scholar] [CrossRef] [PubMed]
- Ohsowski, B.M.; Klironomos, J.N.; Dunfield, K.E.; Hart, M.M. The potential of soil amendments for restoring severely disturbed grasslands. Appl. Soil Ecol. 2012, 60, 77–83. [Google Scholar] [CrossRef]
- Montes, K.R.; Pino, N.J.; Penuela, G.A.; Mendoza, A. Response of Rhizobacterial Community to Biochar Amendment in Coal Mining Soils with Brachiaria Decumbens as Pioneer Plant. Soil Sediment Contam. 2019, 29, 26–42. [Google Scholar] [CrossRef]
- Li, Y.Y.; Wen, H.Y.; Chen, L.Q.; Yin, T.T. Succession of Bacterial Community Structure and Diversity in Soil along a Chronosequence of Reclamation and Re-Vegetation on Coal Mine Spoils in China. PLoS ONE 2014, 9, e115024. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.P.; Wang, C.; Ding, Z.Y.; Zhang, S.L.; Yang, Y.J.; Ma, J.; Chen, F.; Li, J.R. Variation in the Soil Microbial Community of Reclaimed Land over Different Reclamation Periods. Sustainability 2018, 10, 2286. [Google Scholar] [CrossRef]
- Thavamani, P.; Samkumar, R.A.; Satheesh, V.; Subashchandrabose, S.R.; Ramadass, K.; Naidu, R.; Venkateswarlu, K.; Megharaj, M. Microbes from mined sites: Harnessing their potential for reclamation of derelict mine sites. Environ. Pollut. 2017, 230, 495–505. [Google Scholar] [CrossRef]
- Shrestha, P.; Gautam, R.; Ashwath, N. Effects of agronomic treatments on functional diversity of soil microbial community and microbial activity in a revegetated coal mine spoil. Geoderma 2019, 338, 40–47. [Google Scholar] [CrossRef]
- Li, J.J.; Liu, F.; Chen, J.W. The Effects of Various Land Reclamation Scenarios on the Succession of Soil Bacteria, Archaea, and Fungi over the Short and Long Term. Front. Ecol. Evol. 2016, 4, 32. [Google Scholar] [CrossRef]
- Ngugi, M.R.; Dennis, P.G.; Neldner, V.J.; Doley, D.; Fechner, N.; McElnea, A. Open-cut mining impacts on soil abiotic and bacterial community properties as shown by restoration chronosequence. Restor. Ecol. 2018, 26, 839–850. [Google Scholar] [CrossRef]
- Dangi, S.R.; Stahl, P.D.; Wick, A.F.; Ingram, L.J.; Buyer, J.S. Soil Microbial Community Recovery in Reclaimed Soils on a Surface Coal Mine Site. Soil Sci. Soc. Am. J. 2012, 76, 915–924. [Google Scholar] [CrossRef]
- Benayas, J.M.R.; Newton, A.C.; Diaz, A.; Bullock, J.M. Enhancement of Biodiversity and Ecosystem Services by Ecological Restoration: A Meta-Analysis. Science 2009, 325, 1121–1124. [Google Scholar] [CrossRef] [PubMed]
- Atlas, R.M.; Bartha, R. Microbial Ecology, 4th ed.; Addison-Wesley: Reading, UK, 1998. [Google Scholar]
- Schloss, P.D.; Handelsman, J. Introducing DOTUR, a computer program for defining operational taxonomic units and estimating species richness. Appl. Environ. Microb. 2005, 71, 1501–1506. [Google Scholar] [CrossRef] [PubMed]
- Kemp, P.F.; Aller, J.Y. Bacterial diversity in aquatic and other environments: What 16S rDNA libraries can tell us. FEMS Microbiol. Ecol. 2004, 47, 161–177. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Maestre, F.T.; Reich, P.B.; Jeffries, T.C.; Gaitan, J.J.; Encinar, D.; Berdugo, M.; Campbell, C.D.; Singh, B.K. Microbial diversity drives multifunctionality in terrestrial ecosystems. Nat. Commun. 2016, 7, 10541. [Google Scholar] [CrossRef]
- Maron, P.A.; Sarr, A.; Kaisermann, A.; Leveque, J.; Mathieu, O.; Guigue, J.; Karimi, B.; Bernard, L.; Dequiedt, S.; Terrat, S.; et al. High Microbial Diversity Promotes Soil Ecosystem Functioning. Appl. Environ. Microb. 2018, 84, e02738-17. [Google Scholar] [CrossRef]
- Ngugi, M.R.; Fechner, N.; Neldner, V.J.; Dennis, P.G. Successional dynamics of soil fungal diversity along a restoration chronosequence post-coal mining. Restor. Ecol. 2020, 28, 543–552. [Google Scholar] [CrossRef]
- Chen, J.; Nan, J.; Xu, D.L.; Mo, L.; Zheng, Y.X.; Chao, L.M.; Qu, H.T.; Guo, Y.Q.; Li, F.S.; Bao, Y.Y. Response differences between soil fungal and bacterial communities under opencast coal mining disturbance conditions. Catena 2020, 194, 104779. [Google Scholar] [CrossRef]
- Zhang, J.; Xie, Y.H.; Wei, Y.; Meng, H.S.; Cao, Y.Z.; Qin, J.M.; Hong, J.P. Effects of fertilisation on microbial communities in short-term coal mine reclamation. Soil Res. 2020, 58, 779–789. [Google Scholar] [CrossRef]
- Brooks, J.P.; Adeli, A.; Smith, R.K.; McGrew, R.; Lang, D.J.; Read, J.J. Bacterial Community Structure Recovery in Reclaimed Coal Mined Soil under Two Vegetative Regimes. J. Environ. Qual. 2019, 48, 1029–1037. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Ji, L.; Salahuddin; Yang, Y.C.; Yang, L.X. The influence of tree species on soil properties and microbial communities following afforestation of abandoned land in northeast China. Eur. J. Soil Biol. 2018, 85, 73–78. [Google Scholar] [CrossRef]
- Li, Y.Y.; Chen, L.Q.; Wen, H.Y. Changes in the composition and diversity of bacterial communities 13 years after soil reclamation of abandoned mine land in eastern China. Ecol. Res. 2015, 30, 357–366. [Google Scholar] [CrossRef]
- Wang, X.Y.; Li, Y.; Wei, Y.; Meng, H.S.; Cao, Y.Z.; Lead, J.R.; Hong, J.P. Effects of fertilization and reclamation time on soil bacterial communities in coal mining subsidence areas. Sci. Total Environ. 2020, 739, 9. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.H.; Liu, B.R.; Chen, Z.F.; Mao, D.C.; Jiang, X.S. Re-vegetation Improves Soil Quality by Decreasing Soil Conductivity and Altering Soil Microbial Communities: A Case Study of an Opencast Coal Mine in the Helan Mountains. Front. Microbiol. 2022, 13, 11. [Google Scholar] [CrossRef]
- Li, J.J.; Zhou, X.M.; Yan, J.X.; Li, H.J.; He, J.Z. Effects of regenerating vegetation on soil enzyme activity and microbial structure in reclaimed soils on a surface coal mine site. Appl. Soil Ecol. 2015, 87, 56–62. [Google Scholar] [CrossRef]
- Wang, Y.J.; Zheng, G.D.; Zhao, Y.K.; Bo, H.Z.; Li, C.C.; Dong, J.Y.; Wang, Y.; Yan, S.W.; Zhang, F.L.; Liu, J. Different bacterial and fungal community patterns in restored habitats in coal-mining subsidence areas. Environ. Sci. Pollut. Res. 2023, 30, 104304–104318. [Google Scholar] [CrossRef]
- Thomson, B.C.; Tisserant, E.; Plassart, P.; Uroz, S.; Griffiths, R.I.; Hannula, S.E.; Buee, M.; Mougel, C.; Ranjard, L.; Van Veen, J.A.; et al. Soil conditions and land use intensification effects on soil microbial communities across a range of European field sites. Soil Biol. Biochem. 2015, 88, 403–413. [Google Scholar] [CrossRef]
- Xue, P.P.; Carrillo, Y.; Pino, V.; Minasny, B.; McBratney, A.B. Soil Properties Drive Microbial Community Structure in a Large Scale Transect in South Eastern Australia. Sci. Rep. 2018, 8, 11725. [Google Scholar] [CrossRef] [PubMed]
- Llado, S.; Lopez-Mondejar, R.; Baldrian, P. Drivers of microbial community structure in forest soils. Appl. Microbiol. Biotechnol. 2018, 102, 4331–4338. [Google Scholar] [CrossRef] [PubMed]
- Ezeokoli, O.T.; Mashigo, S.K.; Paterson, D.G.; Bezuidenhout, C.C.; Adeleke, R.A. Microbial community structure and relationship with physicochemical properties of soil stockpiles in selected South African opencast coal mines. Soil Sci. Plant. Nutr. 2019, 65, 332–341. [Google Scholar] [CrossRef]
- Frouz, J.; Novakova, A. Development of soil microbial properties in topsoil layer during spontaneous succession in heaps after brown coal mining in relation to humus microstructure development. Geoderma 2005, 129, 54–64. [Google Scholar] [CrossRef]
- Harantova, L.; Mudrak, O.; Kohout, P.; Elhottova, D.; Frouz, J.; Baldrian, P. Development of microbial community during primary succession in areas degraded by mining activities. Land Degrad. Dev. 2017, 28, 2574–2584. [Google Scholar] [CrossRef]
- Snajdr, J.; Dobiasova, P.; Urbanova, M.; Petrankova, M.; Cajthaml, T.; Frouz, J.; Baldrian, P. Dominant trees affect microbial community composition and activity in post-mining afforested soils. Soil Biol. Biochem. 2013, 56, 105–115. [Google Scholar] [CrossRef]
- Fierer, N.; Bradford, M.A.; Jackson, R.B. Toward an ecological classification of soil bacteria. Ecology 2007, 88, 1354–1364. [Google Scholar] [CrossRef]
- Trivedi, P.; Anderson, I.C.; Singh, B.K. Microbial modulators of soil carbon storage: Integrating genomic and metabolic knowledge for global prediction. Trends Microbiol. 2013, 21, 641–651. [Google Scholar] [CrossRef]
- Buelow, H.N.; Winter, A.S.; Van Horn, D.J.; Barrett, J.E.; Gooseff, M.N.; Schwartz, E.; Takacs-Vesbach, C.D. Microbial Community Responses to Increased Water and Organic Matter in the Arid Soils of the McMurdo Dry Valleys, Antarctica. Front. Microbiol. 2016, 7, 1040. [Google Scholar] [CrossRef]
- Das, S.; Jeong, S.T.; Das, S.; Kim, P.J. Composted Cattle Manure Increases Microbial Activity and Soil Fertility More than Composted Swine Manure in a Submerged Rice Paddy. Front. Microbiol. 2017, 8, 543–552. [Google Scholar] [CrossRef]
- Fierer, N.; Lauber, C.L.; Ramirez, K.S.; Zaneveld, J.; Bradford, M.A.; Knight, R. Comparative metagenomic, phylogenetic and physiological analyses of soil microbial communities across nitrogen gradients. ISME J. 2012, 6, 1007–1017. [Google Scholar] [CrossRef] [PubMed]
- Morrissey, E.M.; Mau, R.L.; Schwartz, E.; Caporaso, J.G.; Dijkstra, P.; van Gestel, N.; Koch, B.J.; Liu, C.M.; Hayer, M.; McHugh, T.A.; et al. Phylogenetic organization of bacterial activity. ISME J. 2016, 10, 2336–2340. [Google Scholar] [CrossRef] [PubMed]
- Leff, J.W.; Jones, S.E.; Prober, S.M.; Barberan, A.; Borer, E.T.; Firn, J.L.; Harpole, W.S.; Hobbie, S.E.; Hofmockel, K.S.; Knops, J.M.H.; et al. Consistent responses of soil microbial communities to elevated nutrient inputs in grasslands across the globe. Proc. Natl. Acad. Sci. USA 2015, 112, 10967–10972. [Google Scholar] [CrossRef] [PubMed]
- Dang, P.; Gao, Y.; Liu, J.L.; Yu, S.C.; Zhao, Z. Effects of thinning intensity on understory vegetation and soil microbial communities of a mature Chinese pine plantation in the Loess Plateau. Sci. Total Environ. 2018, 630, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.H.; Wang, C.K.; Jiang, L.F.; Luo, Y.Q. Trends in soil microbial communities during secondary succession. Soil Biol. Biochem. 2017, 115, 92–99. [Google Scholar] [CrossRef]
- Stone, M.M.; Kan, J.J.; Plante, A.F. Parent material and vegetation influence bacterial community structure and nitrogen functional genes along deep tropical soil profiles at the Luquillo Critical Zone Observatory. Soil Biol. Biochem. 2015, 80, 273–282. [Google Scholar] [CrossRef]
- Swab, R.M.; Lorenz, N.; Byrd, S.; Dick, R. Native vegetation in reclamation: Improving habitat and ecosystem function through using prairie species in mine land reclamation. Ecol. Eng. 2017, 108, 525–536. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Z.; Wu, S.; Yuan, X.; Gao, T. Microbial metal homeostasis of biological soil crusts as a mechanism for promoting soil restoration during desert revegetation. Soil Biol. Biochem. 2022, 169, 108659. [Google Scholar] [CrossRef]
- Sun, C.L.; Liu, G.B.; Xue, S. Interaction Between Plant Competition and Rhizospheric Bacterial Community Influence Secondary Succession of Abandoned Farmland on the Loess Plateau of China. Front. Plant Sci. 2018, 9, 898. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, W.; Zhao, C.; Shi, R.; Xue, R.; Li, X. Revegetation by sowing reduces soil bacterial and fungal diversity. Ecol. Evol. 2020, 10, 431–440. [Google Scholar] [CrossRef]
- Rashid, M.I.; Mujawar, L.H.; Shahzad, T.; Almeelbi, T.; Ismail, I.M.I.; Oves, M. Bacteria and fungi can contribute to nutrients bioavailability and aggregate formation in degraded soils. Microbiol. Res. 2016, 183, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Montesinos-Navarro, A.; Valiente-Banuet, A.; Verdu, M. Mycorrhizal symbiosis increases the benefits of plant facilitative interactions. Ecography 2019, 42, 447–455. [Google Scholar] [CrossRef]
- Asmelash, F.; Bekele, T.; Birhane, E. The Potential Role of Arbuscular Mycorrhizal Fungi in the Restoration of Degraded Lands. Front. Microbiol. 2016, 7, 1095. [Google Scholar] [CrossRef]
- Xiao, L.; Bi, Y.L.; Du, S.Z.; Wang, Y.; Guo, C. Effects of re-vegetation type and arbuscular mycorrhizal fungal inoculation on soil enzyme activities and microbial biomass in coal mining subsidence areas of Northern China. Catena 2019, 177, 202–209. [Google Scholar] [CrossRef]
- Bi, Y.L.; Wang, K.; Wang, J. Effect of different inoculation treatments on AM fungal communities and the sustainability of soil remediation in Daliuta coal mining subsidence area in northwest China. Appl. Soil Ecol. 2018, 132, 107–113. [Google Scholar] [CrossRef]
- Hoeksema, J.D.; Chaudhary, V.B.; Gehring, C.A.; Johnson, N.C.; Karst, J.; Koide, R.T.; Pringle, A.; Zabinski, C.; Bever, J.D.; Moore, J.C.; et al. A meta-analysis of context-dependency in plant response to inoculation with mycorrhizal fungi. Ecol. Lett. 2010, 13, 394–407. [Google Scholar] [CrossRef] [PubMed]
- Anthony, M.A.; Crowther, T.W.; van der Linde, S.; Suz, L.M.; Bidartondo, M.I.; Cox, F.; Schaub, M.; Rautio, P.; Ferretti, M.; Vesterdal, L.; et al. Forest tree growth is linked to mycorrhizal fungal composition and function across Europe. ISME J. 2022, 16, 1327–1336. [Google Scholar] [CrossRef]
- Bashan, Y.; de-Bashan, L.E.; Prabhu, S.R.; Hernandez, J.P. Advances in plant growth-promoting bacterial inoculant technology: Formulations and practical perspectives (1998–2013). Plant Soil 2014, 378, 1–33. [Google Scholar] [CrossRef]
- Singh, J.S. Microbes: The chief ecological engineers in reinstating equilibrium in degraded ecosystems. Agric. Ecosyst. Environ. 2015, 203, 80–82. [Google Scholar] [CrossRef]
- Emam, T. Local soil, but not commercial AMF inoculum, increases native and non-native grass growth at a mine restoration site. Restor. Ecol. 2016, 24, 35–44. [Google Scholar] [CrossRef]
- Benetková, P.; van Diggelen, R.; Hánel, L.; Vicentini, F.; Moradi, R.; Weijters, M.; Bobbink, R.; Harris, J.A.; Frouz, J. Soil fauna development during heathland restoration from arable land: Role of soil modification and material transplant. Ecol. Eng. 2022, 176, 11. [Google Scholar] [CrossRef]
- Radujkovic, D.; van Diggelen, R.; Bobbink, R.; Weijters, M.; Harris, J.; Pawlett, M.; Vicca, S.; Verbruggen, E. Initial soil community drives heathland fungal community trajectory over multiple years through altered plant-soil interactions. New Phytol. 2020, 225, 2140–2151. [Google Scholar] [CrossRef] [PubMed]
- van der Bij, A.U.; Weijters, M.J.; Bobbink, R.; Harris, J.A.; Pawlett, M.; Ritz, K.; Benetková, P.; Moradi, J.; Frouz, J.; van Diggelen, R. Facilitating ecosystem assembly: Plant-soil interactions as a restoration tool. Biol. Conserv. 2018, 220, 272–279. [Google Scholar] [CrossRef]
- van der Bij, A.U.; Pawlett, M.; Harris, J.A.; Ritz, K.; van Diggelen, R. Soil microbial community assembly precedes vegetation development after drastic techniques to mitigate effects of nitrogen deposition. Biol. Conserv. 2017, 212, 476–483. [Google Scholar] [CrossRef]
- Jambhulkar, H.P.; Kumar, M.S. Eco-restoration approach for mine spoil overburden dump through biotechnological route. Environ. Monit. Assess. 2019, 191, 16. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.Y.; Ge, J.L.; Zhang, L.L.; Zhang, C.; Jin, R.; Wang, X.H. A Dibenz [a,h] Anthracene-Degrading Strain Amycolatopsis sp. Y1-2 from Soils in the Coal Mining Areas. Polycycl. Aromat. Compd. 2020, 40, 166–178. [Google Scholar] [CrossRef]
- Wulandari, D.; Saridi; Cheng, W.G.; Tawaraya, K. Arbuscular mycorrhizal fungal inoculation improves Albizia saman and Paraserianthes falcataria growth in post-opencast coal mine field in East Kalimantan, Indonesia. For. Ecol. Manag. 2016, 376, 67–73. [Google Scholar] [CrossRef]
- Zhu, Z.; Fang, Y.; Liang, Y.; Li, Y.; Liu, S.; Li, Y.; Li, B.; Gao, W.; Yuan, H.; Kuzyakov, Y.; et al. Stoichiometric regulation of priming effects and soil carbon balance by microbial life strategies. Soil Biol. Biochem. 2022, 169, 108669. [Google Scholar] [CrossRef]
- Poncelet, D.M.; Cavender, N.; Cutright, T.J.; Senko, J.M. An assessment of microbial communities associated with surface mining-disturbed overburden. Environ. Monit. Assess. 2014, 186, 1917–1929. [Google Scholar] [CrossRef]
- Harris, J. Soil Microbial Communities and Restoration Ecology: Facilitators or Followers? Science 2009, 325, 573–574. [Google Scholar] [CrossRef]
- Harris, J.A. Measurements of the soil microbial community for estimating the success of restoration. Eur. J. Soil Sci. 2003, 54, 801–808. [Google Scholar] [CrossRef]
- Gupta, A.; Sar, P. Characterization and application of an anaerobic, iron and sulfate reducing bacterial culture in enhanced bioremediation of acid mine drainage impacted soil. J. Environ. Sci. Health A 2020, 55, 464–482. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, V.; Edrisi, S.A.; Chen, B.; Gupta, V.K.; Vilu, R.; Gathergood, N.; Abhilash, P.C. Biotechnological Advances for Restoring Degraded Land for Sustainable Development. Trends Biotechnol. 2017, 35, 847–859. [Google Scholar] [CrossRef] [PubMed]
- Villegas-Plazas, M.; Sanabria, J.; Junca, H. A composite taxonomical and functional framework of microbiomes under acid mine drainage bioremediation systems. J. Environ. Manag. 2019, 251, 109581. [Google Scholar] [CrossRef] [PubMed]
- Brantner, J.S.; Senko, J.M. Response of Soil-Associated Microbial Communities to Intrusion of Coal Mine-Derived Acid Mine Drainage. Environ. Sci. Technol. 2014, 48, 8556–8563. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Feng, Q.Y.; Liang, H.Q. Effects of long-term discharge of acid mine drainage from abandoned coal mines on soil microorganisms: Microbial community structure, interaction patterns, and metabolic functions. Environ. Sci. Pollut. Res. 2021, 28, 53936–53952. [Google Scholar] [CrossRef] [PubMed]
- Kefeni, K.K.; Msagati, T.A.M.; Mamba, B.B. Acid mine drainage: Prevention, treatment options, and resource recovery: A review. J. Clean. Prod. 2017, 151, 475–493. [Google Scholar] [CrossRef]
- Chen, L.X.; Huang, L.N.; Mendez-Garcia, C.; Kuang, J.L.; Hua, Z.S.; Liu, J.; Shu, W.S. Microbial communities, processes and functions in acid mine drainage ecosystems. Curr. Opin. Biotech. 2016, 38, 150–158. [Google Scholar] [CrossRef]
- Sun, W.M.; Xiao, E.Z.; Krumins, V.; Dong, Y.R.; Li, B.Q.; Deng, J.; Wang, Q.; Xiao, T.F.; Liu, J. Comparative Analyses of the Microbial Communities Inhabiting Coal Mining Waste Dump and an Adjacent Acid Mine Drainage Creek. Microb. Ecol. 2019, 78, 651–664. [Google Scholar] [CrossRef]
- Bhandari, P.; Choudhary, S. Insights on the Role of Sulfur Oxidizing Bacteria in Acid Mine Drainage Biogeochemistry. Geomicrobiol. J. 2022, 39, 270–281. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, Z.; Harris, J.; Zhang, Z. Responses of Natural Microorganisms to Land Reclamation and Applications of Functional Microorganisms in Biorestoration of Coal Mining Area. Diversity 2024, 16, 86. https://doi.org/10.3390/d16020086
Mao Z, Harris J, Zhang Z. Responses of Natural Microorganisms to Land Reclamation and Applications of Functional Microorganisms in Biorestoration of Coal Mining Area. Diversity. 2024; 16(2):86. https://doi.org/10.3390/d16020086
Chicago/Turabian StyleMao, Zhen, Jim Harris, and Zheyu Zhang. 2024. "Responses of Natural Microorganisms to Land Reclamation and Applications of Functional Microorganisms in Biorestoration of Coal Mining Area" Diversity 16, no. 2: 86. https://doi.org/10.3390/d16020086
APA StyleMao, Z., Harris, J., & Zhang, Z. (2024). Responses of Natural Microorganisms to Land Reclamation and Applications of Functional Microorganisms in Biorestoration of Coal Mining Area. Diversity, 16(2), 86. https://doi.org/10.3390/d16020086





