Knowledge Gaps on the Effectiveness of Seed Dispersal by Mammals and the Effect of Human Disturbances: A Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Data Compilation
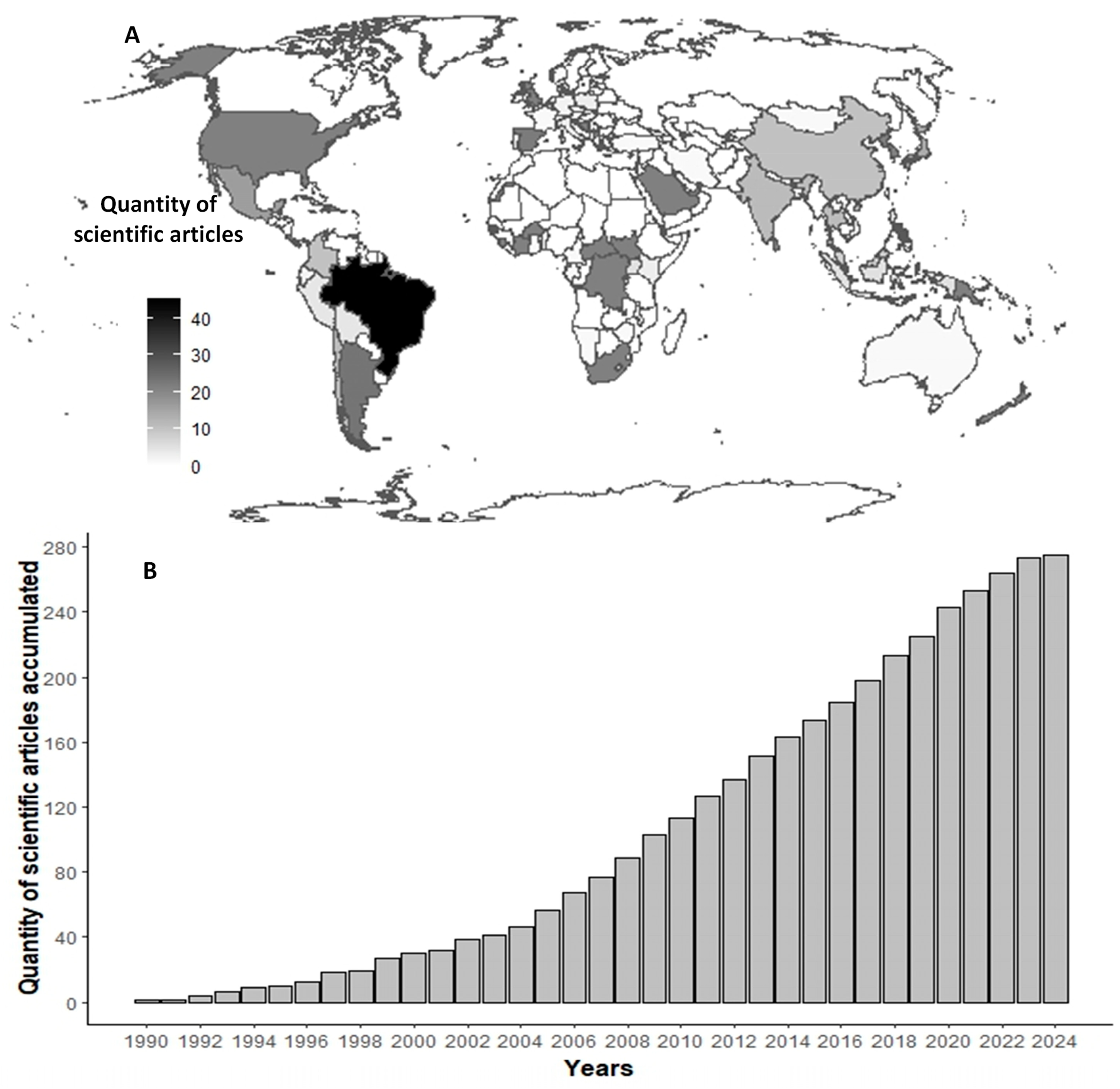
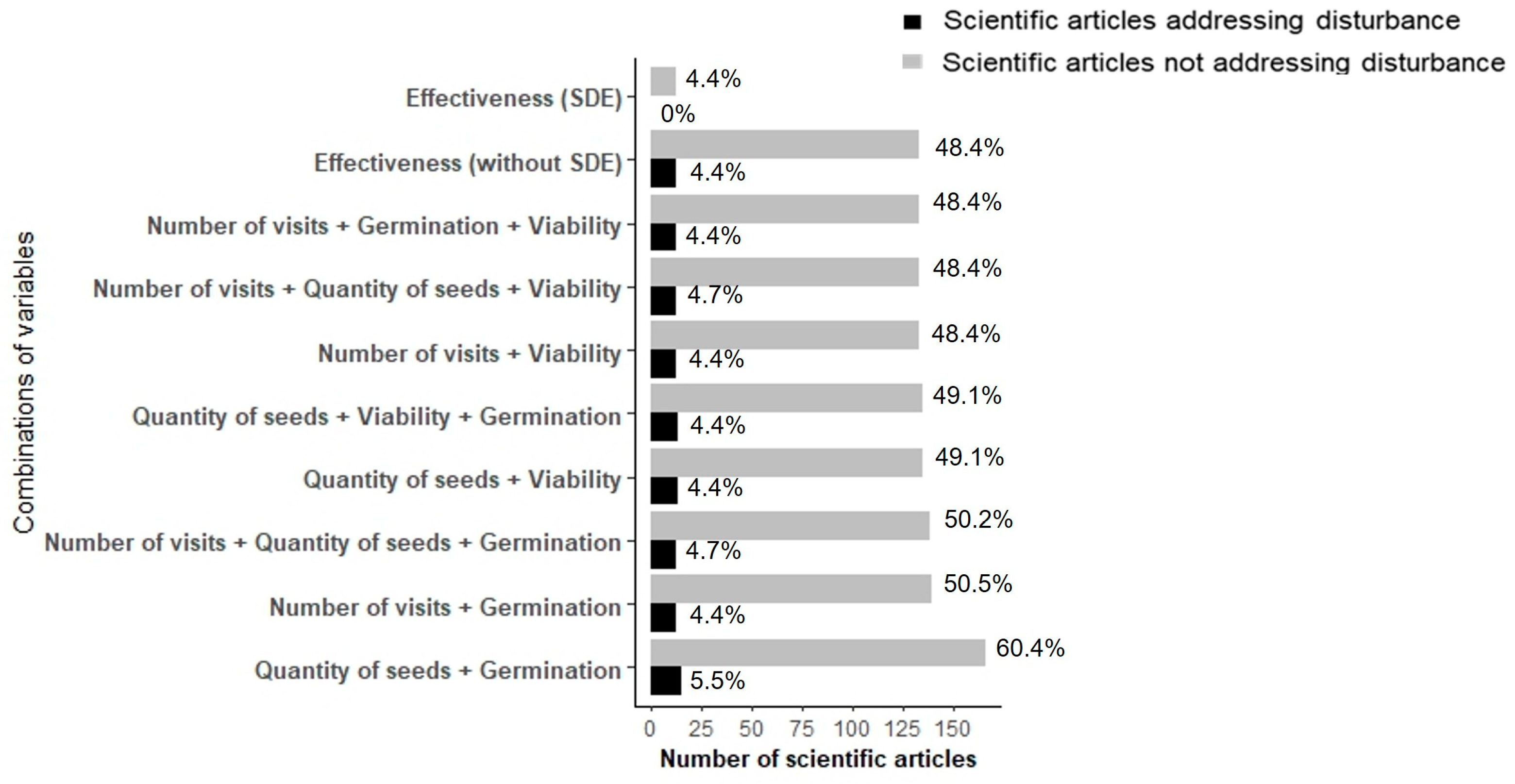
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bobrietsov, A.B. Population dynamics of red-backed voles (Clethrionomys rutilus, Rodentia) in the northern Cis-Urals over half a century. Zool. J. 2009, 88, 1115–1129. [Google Scholar]
- Valiente-Banuet, A.; Aizen, M.A.; Alcántara, J.M.; Arroyo, J.; Cocucci, A.; Galetti, M.; García, M.B.; García, D.; Gómez, J.M.; Jordano, P.; et al. Beyond species loss: The extinction of ecological interactions in a changing world. Ecology 2015, 29, 299–307. [Google Scholar] [CrossRef]
- Gomes, L.G.L.; Oostra, V.; Nijman, V.; Cleef, A.M.; Kappelle, M. Tolerance of frugivorous birds to habitat disturbance in a tropical cloud forest. Biol. Conserv. 2008, 141, 860–871. [Google Scholar] [CrossRef]
- Howe, H.F.; Smallwood, J. Ecology of seed dispersal. Annu. Rev. Ecol. Syst. 1982, 13, 201–228. [Google Scholar] [CrossRef]
- Ollerton, J.; Stott, A.; Allnutt, E.; Shove, S.; Taylor, C.; Lamborn, E. Pollination niche overlap between a parasitic plant and its host. Oecologia 2007, 151, 473–485. [Google Scholar] [CrossRef] [PubMed]
- Nathan, R.; Muller-Landau, H.C. Spatial patterns of seed dispersal, their determinants and consequences for recruitment. Trends Ecol. Evol. 2000, 15, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Levin, S.A.; Muller-Landau, H.; Nathan, R.; Chave, J. The ecology and evolution of seed dispersal: A Theoretical Perspective. Rev. Ecol. Evol. Syst. 2003, 34, 575–604. [Google Scholar] [CrossRef]
- Levine, J.M.; Murrell, D.J. The community–level consequences of seed dispersal patterns. Annu. Rev. Ecol. Evol. Syst. 2003, 34, 549–574. [Google Scholar] [CrossRef]
- Lundberg, J.; Moberg, F. Mobile link organisms and ecosystem functioning: Implications for ecosystem resilience and management. Ecosystems 2003, 6, 87–98. [Google Scholar] [CrossRef]
- Montoya, D.; Zavala, M.A.; Rodríguez, M.A.; Purves, D.W. Animal versus wind dispersal and the robustness of tree species to deforestation. Science 2008, 320, 1502–1504. [Google Scholar] [CrossRef] [PubMed]
- Schupp, E.W. Quantity, quality and the effectiveness of seed dispersal by animals. Vegetation 1993, 107–108, 15–29. [Google Scholar] [CrossRef]
- Schupp, E.W.; Jordano, P.; Gómez, J.M. Seed dispersal effectiveness revisited: A conceptual review. New Phytol. 2010, 188, 333–353. [Google Scholar] [CrossRef]
- Schupp, E.W.; Jordano, P.; Gómez, J.M. A general framework for effectiveness concepts in mutualisms. Ecol. Lett. 2017, 20, 577–590. [Google Scholar] [CrossRef]
- Sala, O.E.; Chapin, F.S., 3rd; Armesto, J.J.; Berlow, E.; Bloomfield, J.; Dirzo, R.; Huber-Sanwald, E.; Huenneke, L.F.; Jackson, R.B.; Kinzig, A.; et al. Global biodiversity scenarios for the year 2100. Science 2000, 287, 1770–1774. [Google Scholar] [CrossRef] [PubMed]
- Tabeni, S.; Ojeda, R.A. Ecology of the Monte Desert small mammals in disturbed and undisturbed habitats. J. Arid Environ. 2005, 63, 244–255. [Google Scholar] [CrossRef]
- Dirzo, R.; Mendoza, E. Size-Related Differential Seed Predation in a Heavily Defaunated Neotropical Rain Forest. Biotropica 2007, 39, 355–362. [Google Scholar] [CrossRef]
- Oliveira, V.H.F.; Barlow, J.; Gardner, T.; Louzada, J. Are we selecting appropriate metrics to assess human impacts on biodiversity? Basic Appl. Ecol. 2017, 21, 85–93. [Google Scholar] [CrossRef][Green Version]
- Janzen, D.H. The deflowering of Central America. Nat. Hist. 1974, 83, 48–53. [Google Scholar]
- Miguel, F.; Cona, M.I.; Campos, C.M. Seed removal by different functional mammal groups in a protected and grazed landscape of the Monte, Argentina. Seed Sci. Res. 2017, 27, 174–182. [Google Scholar] [CrossRef]
- Morán-López, T.; Fernández, M.; Alonso, C.L.; Flores-Rentería, D.; Valladares, F.; Díaz, M. Effects of forest fragmentation on the oak-rodent mutualism. Oikos 2015, 124, 1482–1491. [Google Scholar] [CrossRef]
- Markl, J.S.; Schleuning, M.; Forget, P.M.; Jordano, P.; Lambert, J.E.; Traveset, A.S.; Wright, S.J.; Böhning-Gaese, K. Analysis of the Effects of Human Disturbance on Seed Dispersal by Animals. Conserv. Biol. 2012, 26, 1072–1081. [Google Scholar] [CrossRef] [PubMed]
- Asquith, N.M.; Wright, S.J.; Clauss, M.J. Does Mammal Community Composition Control Recruitment in Neotropical Forests? Ecology 1997, 78, 941–946. [Google Scholar] [CrossRef]
- Guariguata, M.R.; Arias, L.C.H.; Jones, G. Tree seed fate in a logged and fragmented forest landscape, northeastern Costa Rica. Biotropica 2002, 34, 405–415. [Google Scholar] [CrossRef]
- Leonard, R.J.; Hochuli, D.F. Exhausting all avenues: Why impacts of air pollution should be part of road ecology. Front. Ecol. Environ. 2017, 15, 443–449. [Google Scholar] [CrossRef]
- Watts, R.D.; Compton, R.W.; McCammon, J.H.; Rich, C.L.; Wright, S.M.; Owens, T.; Ouren, D.S. Roadless Space of the Conterminous United States. Science 2007, 316, 736–738. [Google Scholar] [CrossRef] [PubMed]
- Ibisch, P.L.; Hoffmann, M.T.; Kreft, S.; Pe’er, G.; Kati, V.; Biber-Freudenberger, L.; DellaSala, D.A.; Vale, M.M.; Hobson, P.R.; Selva, N. A global map of roadless areas and their conservation status. Science 2016, 354, 1423–1427. [Google Scholar] [CrossRef]
- Chen, W.; Xie, Z.; Zhou, Y. Proximity to roads reduces acorn dispersal effectiveness by rodents: Implication for forest regeneration and management. For. Ecol. Manag. 2019, 433, 625–632. [Google Scholar] [CrossRef]
- Niu, H.-Y.; Xing, J.-J.; Zhang, H.-M.; Wang, D.; Wang, X.-R. Roads limit of seed dispersal and seedling recruitment of Quercus chenii in an urban hillside forest. Urban For. Urban Green. 2018, 30, 307–314. [Google Scholar] [CrossRef]
- Traveset, A.; Escribano-Avila, G.; Gómez, J.M.; Valido, A. Conflicting selection on Cneorum tricoccon (Rutaceae) seed size caused by native and alien seed dispersers. Evol. Int. J. Org. Evol. 2019, 73, 2204–2215. [Google Scholar] [CrossRef]
- Amodeo, M.R.; Vázquez, M.B.; Zalba, S.M. Generalist dispersers promote germination of an alien fleshy-fruited tree invading natural grasslands. PLoS ONE 2017, 12, e0172423. [Google Scholar] [CrossRef] [PubMed]
- Naoe, S.; Tayasu, I.; Sakai, Y.; Masaki, T.; Kobayashi, K.; Nakajima, A.; Sato, Y.; Yamazaki, K.; Kiyokawa, H.; Koike, S. Mountain-climbing bears protect cherry species from global warming through vertical seed dispersal. Curr. Biol. 2016, 26, R315–R316. [Google Scholar] [CrossRef] [PubMed]
- Naoe, S.; Tayasu, I.; Sakai, Y.; Masaki, T.; Kobayashi, K.; Nakajima, A.; Sato, Y.; Yamazaki, K.; Kiyokawa, H.; Koike, S. Downhill seed dispersal by temperate mammals: A potential threat to plant escape from global warming. Sci. Rep. 2019, 9, 14932. [Google Scholar] [CrossRef]
- González-Varo, J.P.; López-Bao, J.V.; Guitián, J. Seed dispersers help plants to escape global warming. Oikos 2017, 126, 1600–1606. [Google Scholar] [CrossRef]
- Corrêa, M.C.; Uriarte, M. Integrating frugivory and animal movement: A review of the evidence and implications for scaling seed dispersal. Biol. Rev. 2012, 88, 255–272. [Google Scholar]
- Cordeiro, N.J.; Howe, H.F. Forest fragmentation severs mutualism between seed dispersers and an endemic African tree. Proc. Natl. Acad. Sci. USA 2003, 100, 14052–14056. [Google Scholar] [CrossRef]
- Galetti, M.; Donatti, C.I.; Pires, A.S.; Guimaraes, P.R.; Jordano, P. Seed survival and dispersal of an endemic Atlantic forest palm: The combined effects of defaunation and forest fragmentation. Bot. J. Linn. Soc. 2006, 151, 141–149. [Google Scholar] [CrossRef]
- Jordano, P.; Schupp, E.W. Seed disperser effectiveness: The quantity component and patterns of seed rain for Prunus mahaleb. Ecol. Monogr. 2000, 70, 591–615. [Google Scholar] [CrossRef]
- Lidicker, W.Z.; Peterson, J.A. Responses of small mammals to habitat edges. In Landscape Ecology of Small Mammals; Barrett, G.W., Peles, J.D., Eds.; Springer: New York, NY, USA, 1999; pp. 211–227. [Google Scholar]
- Fahrig, L. Non optimal animal movement in human altered landscapes. Funct. Ecol. 2007, 21, 1003–1015. [Google Scholar] [CrossRef]
- López-Bao, J.V.; González-Varo, J.P. Frugivory and spatial patterns of seed deposition by carnivorous mammals in anthropogenic landscapes: A multi-scale approach. PLoS ONE 2011, 6, e14569. [Google Scholar] [CrossRef]
- Suárez-Esteban, A.; Delibes, M.; Fedriani, J.M. Unpaved road verges as hotspots of fleshy-fruited shrub recruitment and establishment. Biol. Conserv. 2013, 167, 50–56. [Google Scholar] [CrossRef]
- Hodara, K.; Busch, M. Return to preferred habitats (edges) as a function of distance in Akodon azarae (Rodentia, Muridae) in cropfield-edge systems of central Argentina. J. Ethol. 2006, 24, 141–145. [Google Scholar] [CrossRef]
- Gómez, D.; Steinmann, A.; Chiappero, M.; Priotto, J. Movement distances of two species of sympatric rodents in linear habitats of Central Argentina agroecosystems. Mamm. Biol. 2011, 76, 58–63. [Google Scholar] [CrossRef]
- Kollmann, J.; Buschor, M. Edge effects on seed predation by rodents in deciduous forests of northern Switzerland. Plant Ecol. 2002, 164, 249–261. [Google Scholar] [CrossRef]
- Jacob, H.S.; Minkey, D.M.; Gallagher, R.S.; Borger, C.P. Variation in postdispersal weed seed predation in a crop field. Weed Sci. 2006, 54, 148–155. [Google Scholar] [CrossRef]
- Baraibar, B.; Westerman, P.R.; Carrión, E.; Recasens, J. Effects of tillage and irrigation in cereal fields on weed seed removal by seed predators. J. Appl. Ecol. 2009, 46, 380–387. [Google Scholar] [CrossRef]
- Fischer, J.; Lindenmayer, D.B.; Montague-Drake, R. The role of landscape textura in conservation biogeography: A case study on birds in south-eastern Australia. Divers. Distrib. 2008, 14, 38–46. [Google Scholar] [CrossRef]
- Galetti, M.; Alves-Costa, C.P.; Cazetta, E. Effects of forest fragmentation, anthropogenic edges and fruit colour on the consumption of ornithocoric fruits. Biol. Conserv. 2003, 111, 269–273. [Google Scholar] [CrossRef]
- Fontúrbel, F.E.; Candia, A.B.; Malebrán, J.; Salazar, D.A.; González-Browne, C.; Medel, R. Meta-analysis of anthropogenic habitat disturbance effects on animal-mediated seed dispersal. Glob. Change Biol. 2015, 21, 3951–3960. [Google Scholar] [CrossRef] [PubMed]
- Bermejo, T.; Guitian, J. Fruit consumption by foxes and martens in NW Spain in autumn: A comparison of natural and agricultural areas. Folia Zool. 2000, 49, 89–92. [Google Scholar]
- Perea, R.; Delibes, M.; Polko, M.; Suárez-Esteban, A.; Fedriani, J.M. Context-dependent fruit–frugivore interactions: Partner identities and spatio-temporal variations. Oikos 2013, 122, 943–951. [Google Scholar] [CrossRef]
- Peredo, A.; Martínez, D.; Rodríguez–Pérez, J.; García, D. Mammalian seed dispersal in Cantabrian woodland pastures: Network structure and response to forest loss. Basic Appl. Ecol. 2013, 14, 378–386. [Google Scholar] [CrossRef]
- Lindenmayer, D.B.; Cunningham, R.B.; Pope, M.L.; Donnely, C.F. The response of arboreal marsupials to landscape context: A large-scale fragmentation study. Ecol. Appl. 1999, 9, 594–611. [Google Scholar] [CrossRef]
- Fedriani, J.M.; Delibes, M. Seed dispersal in the Iberian pear, Pyrus bourgaeana: A role for infrequent mutualists. Ecoscience 2009, 16, 311–321. [Google Scholar] [CrossRef]
- Rodríguez-Cabal, M.A.; Aizen, M.A.; Novaro, A.J. Habitat fragmentation disrupts a plant-disperser mutualism in the temperate forest of South America. Biol. Conserv. 2007, 139, 195–202. [Google Scholar] [CrossRef]
- Lancaster, M.L.; Taylor, A.C.; Cooper, S.J.B.; Carthew, S.M. Limited ecological connectivity of an arboreal marsupial across a forest/plantation landscape despite apparent resilience to fragmentation. Mol. Ecol. 2011, 20, 2258–2271. [Google Scholar] [CrossRef] [PubMed]
- Boyle, S.A.; Zartman, C.E.; Spironello, W.R.; Smith, A.T. Implications of habitat fragmentation on the diet of bearded saki monkeys in central Amazonian forest. J. Mammal. 2012, 93, 959–976. [Google Scholar] [CrossRef]
- Gray, M.A.; Baldauf, S.L.; Mayhew, P.J.; Hill, J.K. The response of avian feeding guilds to tropical forest disturbance. Conserv. Biol. 2007, 21, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Vargas, R.R.; Fontúrbel, F.E.; Bonaccorso, E.; Simonetti, J.A. Variation in reproductive life-history traits of birds in fragmented habitats: A review and meta-analysis. Bird Conserv. Int. 2012, 22, 462–467. [Google Scholar] [CrossRef]
- Cancio, I.; González-Robles, A.; Bastida, J.M.; Manzaneda, A.J.; Salido, T.; Rey, P.J. Habitat loss exacerbates regional extinction risk of the keystone semiarid shrub Ziziphus lotus through collapsing the seed dispersal service by foxes (Vulpes vulpes). Biodivers. Conserv. 2016, 25, 693–709. [Google Scholar] [CrossRef]
- Franco-Quimbay, J.; Rojas-Robles, R. Frugivoría y dispersión de semillas de la palma Oenocarpus bataua en dos regiones con diferente estado de conservación. Actual. Biol. 2014, 37, 273–285. [Google Scholar] [CrossRef]
- Serio-Silva, J.C.; Rico-Gray, V. Interacting effects of forest fragmentation and howler monkey foraging on germination and disperal of fig seeds. Oryx 2002, 36, 266–271. [Google Scholar] [CrossRef]
- Miguel, M.F.; Tabeni, S.; Cona, M.I.; Campos, C.M. Secondary seed dispersal by mammals between protected and grazed semiarid woodland. For. Ecol. Manag. 2018, 422, 41–48. [Google Scholar] [CrossRef]
- Colon, C.P.; Sugau, J.B. Notes on the Diet of the Malay Civet (Viverra tangalunga) and other Civets in Logged and Unlogged Lowland Dipterocarp Rain Forests in Sabah, Borneo. Malay. Nat. J. 2012, 64, 69–74. [Google Scholar]
- Chaves, O.M.; Stoner, K.E.; Arroyo-Rodríguez, V.; Estrada, A. Effectiveness of Spider Monkeys (Ateles geoffroyi vellerosus) as Seed Dispersers in Continuous and Fragmented Rain Forests in Southern Mexico. Int. J. Primatol. 2011, 32, 177–192. [Google Scholar] [CrossRef]
- Periago, M.E.; Tamburini, D.M.; Ojeda, R.A.; Cáceres, D.M.; Díaz, S. Combining ecological aspects and local knowledge for the conservation of two native mammals in the Gran Chaco. J. Arid Environ. 2017, 147, 54–62. [Google Scholar] [CrossRef]
- Zhou, Y.B.; Zhang, L.; Kaneko, Y.; Newman, C.; Wang, X.M. Frugivory and seed dispersal by a small carnivore, the Chinese ferret-badger, Melogale moschata, in a fragmented subtropical forest of central China. For. Ecol. Manag. 2008, 255, 1595–1603. [Google Scholar] [CrossRef]
- Farwig, N.; Böhning-Gaese, K.; Bleher, B. Enhanced seed dispersal of Prunus africana in fragmented and disturbed forests? Oecologia 2006, 147, 238–252. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Shi, X.; Zhang, X.; Yi, X.; Wang, D.; Ma, J. Effects of selective logging on rodent-mediated seed dispersal. For. Ecol. Manag. 2017, 406, 147–154. [Google Scholar] [CrossRef]
- Paolucci, L.N.; Pereira, R.L.; Rattis, L.; Silvério, D.V.; Marques, N.C.S.; Macedo, M.N.; Brando, P.M. Lowland tapirs facilitate seed dispersal in degraded Amazonian forests. Biotropica 2019, 51, 245–252. [Google Scholar] [CrossRef]
- Houngbégnon, F.G.A.; Gillet, J.F.; Michaux, J.; Brostaux, Y.; Zébazé, D.; Lhoest, S.; Vermeulen, C.; Sonké, B.; Doucet, J.L. Seed dispersal by duikers in selectively logged rainforests: Overlooked dispersal of an important animal community. For. Ecol. Manag. 2023, 529, 120650. [Google Scholar] [CrossRef]
- Aroca, A.K.; González, L.A.; Hurtado, M.A.; Oscar, E.; Murillo, O.E.G. Diet Preference in Frugivorous Bats (Phyllostomidae) within a Fragment of Dry Tropical Forest. Rev. Cienc. 2016, 20, 139–146. [Google Scholar]
- Kleber, A.M.O.; Pulchério-Leite, A.; Ribeiro, J.W.F.; Fernandes, V. Can Carollia perspicillata (Chiroptera: Phyllostomidae) induce seed germination of Cecropia pachystachya? Mastozoología Neotrop. 2020, 27, 194–199. [Google Scholar]
- Mahesha, P.; Rajnish, V. Some aspects of seed dispersal by Asian elephants (Elephas maximus) in Kaudulla National Park, Sri Lanka. Curr. Sci. 2020, 118, 648–654. [Google Scholar] [CrossRef]
- Pérez-Flores, G.A.; Sánchez-Sánchez, M.; Sánchez-Alarcón, J.; García-de Jesús, S.; Flores-Morales, M. Dispersión endozoocórica de plantas en un bosque de encino de Tlaxcala por el cacomixtle. Ecosistema Recur. Agropecu. 2021, 8, e2793. [Google Scholar]
- Murdoch, J.D.; Buyandelger, S.; Cypher, B.L. Patterns of seed occurrence in corsac and red fox diets in Mongolia. J. Arid Environ. 2009, 73, 381–384. [Google Scholar] [CrossRef]
- Rost, J.; Pons, P.; Bas, J.M. Seed dispersal by carnivorous mammals into burnt forests: An opportunity for non-indigenous and cultivated plant species. Basic Appl. Ecol. 2012, 13, 623–630. [Google Scholar] [CrossRef]
- Torres, D.A.; Castaño, J.H.; Carranza-Quiceno, J.A. Global patterns in seed germination after ingestion by mammals. Mammal. Rev. 2020, 50, 278–290. [Google Scholar] [CrossRef]
- Corlett, R.T. Frugivory and seed dispersal by vertebrates in the Oriental (Indomalayan) Region. Biol. Rev. 1998, 73, 413–448. [Google Scholar] [CrossRef] [PubMed]
- Corlett, R.T. How to be a frugivore (in a changing world). Acta Oecologica 2011, 37, 674–681. [Google Scholar] [CrossRef]
- Fuzessy, L.F.; Cornelissen, T.G.; Janson, C.; Silveira, F.A.O. How do primates affect seed germination? A meta-analysis of gut passage effects on neotropical plants. Oikos 2016, 125, 1069–1080. [Google Scholar] [CrossRef]
- Fuzessy, L.F.; Janson, C.H.; Silveira, F.A.O. How far do Neotropical primates disperse seeds? Am. J. Primatol. 2017, 79, e22659. [Google Scholar] [CrossRef] [PubMed]
- Jordano, P. What is long-distance dispersal? And a taxonomy of dispersal events. J. Ecol. 2017, 105, 75–84. [Google Scholar] [CrossRef]
- Marques, C.D.; Kissling, W.D. The mutualism–antagonism continuum in Neotropical palm–frugivore interactions: From interaction outcomes to ecosystem dynamics. Biol. Rev. 2022, 97, 527–553. [Google Scholar] [CrossRef] [PubMed]
- McConkey, K.R.; Prasad, S.; Corlett, R.T.; Campos-Arceiz, A.; Brodie, J.F.; Rogers, H.; Santamaria, L. Seed dispersal in changing landscapes. Biol. Conserv. 2012, 146, 1–13. [Google Scholar] [CrossRef]
- Messeder, J.V.S.; Silveira, F.A.O.; Cornelissen, T.G.; Fuzessy, L.F.; Guerra, T.J. Frugivory and seed dispersal in a hyperdiverse plant clade and its role as a keystone resource for the Neotropical fauna. Ann. Bot. 2021, 127, 577–595. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, A. Animal-mediated seed dispersal in India: Implications for conservation of India’s biodiversity. Biotropica 2022, 54, 1320–1330. [Google Scholar] [CrossRef]
- Draper, J.P.; Young, J.K.; Schupp, E.W.; Beckman, N.G.; Atwood, T.B. Frugivory and Seed Dispersal by Carnivorans. Front. Ecol. Evol. 2022, 10, 864864. [Google Scholar] [CrossRef]
- Silva, S.I.; Bozinovic, F.; Jaksic, F.M. Frugivory and seed dispersal by foxes in relation to mammalian prey abundance in a semiarid thornscrub. Austral Ecol. 2005, 30, 739–746. [Google Scholar] [CrossRef]
- Takahashi, K.; Shiota, T.; Tamatani, H.; Koyama, M.; Washitani, I. Seasonal variation in fleshy fruit use and seed dispersal by the Japanese black bear (Ursus thibetanus japonicus). Ecol. Res. 2008, 23, 471–478. [Google Scholar] [CrossRef]
- Jordano, P.; Galetti, M.; Pizo, M.A.; Silva, W.R. Ligando frugivoria e dispersão de sementes à biologia da conservação. In Biologia da Conservação: Essências; Duarte, C.F., Bergallo, H.G., Santos, M.A., Va, A.E., Eds.; Editora Rima: São Paulo, Brazil, 2006; pp. 411–436. [Google Scholar]
- Fleming, T.H.; Geiselman, C.; Kress, W.J. The evolution of bat pollination: A phylogenetic perspective. Ann. Bot. 2009, 104, 1017–1043. [Google Scholar] [CrossRef] [PubMed]
- Muscarella, R.; Fleming, T.H. The role of frugivorous bats in tropical forest succession. Biol. Rev. 2007, 82, 573–590. [Google Scholar] [CrossRef]
- Fleming, T.H.; Kress, W.J. The Frugivory Mutualism. In The Ornaments of Life: Coevolution and Conservation in the Tropics; The University of Chicago Press: London, UK, 2013. [Google Scholar]
- Andresen, E.; Arroyo-Rodríguez, V.; Ramos-Robles, M. Primate Seed Dispersal: Old and New Challenges. Int. J. Primatol. 2018, 39, 443–465. [Google Scholar] [CrossRef]
- Fuzessy, L.F.; Sobra, G.; Culot, L. Linking howler monkey ranging and defecation patterns to primary and secondary seed dispersal. Am. J. Primatol. 2022, 84, e23354. [Google Scholar] [CrossRef] [PubMed]
- Fuzessy, L.F.; Janson, C.H.; Silveira, F.A.O. Effects of seed size and frugivory degree on dispersal by Neotropical frugivores. Acta Oecologica 2018, 93, 41–47. [Google Scholar] [CrossRef]
- Chen, Y.; Kim, R.; McConkey, K.R.; Fan, P. Sympatric primate seed dispersers and predators jointly contribute to plant diversity in a subtropical forest. Oecología 2023, 202, 715–727. [Google Scholar] [CrossRef]
- Hawes, J.E.; Peres, C.A. Fruit–frugivore interactions in Amazonian seasonally flooded and unflooded forests. J. Trop. Ecol. 2014, 30, 381–399. [Google Scholar] [CrossRef]
- Bodmer, R.E. Strategies of Seed Dispersal and Seed Predation in Amazonian Ungulates. Biotropica 1991, 23, 225–261. [Google Scholar] [CrossRef]
- Janzen, D.H. Dispersal of small seedsby big herbivores: Foliage is the fruit. Am. Nat. 1984, 123, 338–353. [Google Scholar] [CrossRef]
- Myers, J.A.; Vellend, M.; Gardescu, S. Seed dispersal by white-tailed deer: Implications for long-distance dispersal, invasion, and migration of plants in eastern North America. Oecologia 2004, 139, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Dellafiore, C.M. ¿Afecta el Zorro (Lycalopex gymnocercus) la Germinación de Piracanta (Pyracantha atalantoides) Rosaceae? Mastozoología Neotrop. 2018, 25, 53–58. [Google Scholar] [CrossRef]
- Yasumoto, Y.; Takatsuki, S. The Japanese Marten Favors Actinidia arguta, a Forest Edge Liane as a Directed Seed Disperser. Zool. Sci. 2015, 32, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Fedriani, J.M.; Zywiec, M.; Delibes, M. Thieves or mutualists? Pulp feeders enhance endozoochore local recruitment. Ecology 2012, 93, 575–587. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.B.; Newman, C.; Xie, Z.; Macdonald, D.W. Peduncles elicit large–mammal endozoochory in a dry–fruited plant. Ann. Bot. 2013, 112, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Campos, C.M.; Velez, S.; Miguel, M.F.; Papú, S.; Cona, M.I. Studying the quantity component of seed dispersal effectiveness from exclosure treatments and camera trapping. Ecol. Evol. 2018, 8, 5470–5479. [Google Scholar] [CrossRef] [PubMed]
- Campos, C.M.; Campos, V.; Giannoni, S.M.; Rodríguez, D.; Albanese, S.; Cona, M.I. Role of small rodents in the seed dispersal process: Microcavia australis consuming Prosopis flexuosa fruits. Austral Ecol. 2017, 42, 113–119. [Google Scholar] [CrossRef]
- Campos, C.M.; Campos, V.; Mongeaud, A.; Borghi, C.E.; Ríos, C.; Giannoni, S.M. Relationships between Prosopis flexuosa (Fabaceae) and cattle in the Monte desert: Seeds, seedlings and saplings on cattle-use site classes. Rev. Chil. Hist. Nat. 2011, 84, 289–299. [Google Scholar] [CrossRef]
- Campos, C.M.; Ojeda, R.A. Dispersal and germination of Prosopis flexuosa (Fabaceae) seeds by desert mammals in Argentina. J. Arid Environ. 1997, 35, 707–714. [Google Scholar] [CrossRef]
- Maldonado, D.E.; Pacheco, L.F.; Saavedra, L.V. Legitimidad en la dispersión de semillas de algarrobo (Prosopis flexuosa, Fabaceae) por zorro andino (Lycalopex culpaeus, Canidae) en el Valle de La Paz (Bolivia). Ecol. Boliv. 2014, 49, 93–97. [Google Scholar]
- Maldonado, D.E.; Loayza, A.P.; Garcia, E.; Pacheco, L.F. Qualitative aspects of the effectiveness of Culpeo foxes (Lycalopex culpaeus) as dispersers of Prosopis alba (Fabaceae) in a Bolivian dry valley. Acta Oecologica 2018, 87, 29–33. [Google Scholar] [CrossRef]
- Kamler, J.F.; Klare, U.; Macdonald, D.W. Seed dispersal potential of jackals and foxes in semi-arid habitats of South Africa. J. Arid Environ. 2020, 183, 104284. [Google Scholar] [CrossRef]
- Silverstein, R.P. Germination of Native and Exotic Plant Seeds Dispersed by Coyotes (Canis latrans) in Southern California. Southwest. Nat. 2005, 50, 472–478. [Google Scholar] [CrossRef]
- Anzures-Dadda, A.; Manson, R.H.; Andresen, E.; Martínez, M.L. Possible implications of seed dispersal by the howler monkey for the early recruitment of a legume tree in small rain-forest fragments in Mexico. J. Trop. Ecol. 2016, 32, 340–343. [Google Scholar] [CrossRef]
- Tavşanoğlu, Ç.; Kazanci, D.D.; Soyumert, A.; Ertürk, A.; Değirmenci, C.Ü. Seed dispersal by the brown bear in a mixed temperate forest: Fruit type matters. Mammal. Res. 2021, 66, 137–147. [Google Scholar] [CrossRef]
- Koike, S.; Tochigi, K.; Yamazaki, K. Are seeds of trees with higher fruit production dispersed farther by frugivorous mammals? J. For. Res. 2023, 28, 64–72. [Google Scholar] [CrossRef]
- Fredriksson, G.M.; Wich, S.A. Trisno Frugivory in sun bears (Helarctos malayanus) is linked to El Niño-related fluctuations in fruiting phenology, East Kalimantan, Indonesia. Biol. J. Linn. Soc. 2006, 89, 489–508. [Google Scholar] [CrossRef]
- Bourgeois, K.; Suehs, C.M.; Vidal, E.; Médail, F. Invasional meltdown potential: Facilitation between introduced plants and mammals on French Mediterranean islands. Ecoscience 2005, 12, 248–256. [Google Scholar] [CrossRef]
- Hernández-Montero, J.R.; Rojas-Soto, O.R.; Saldaña-Vázquez, R.A. Consumo y dispersión de semillas de Solanum schlechtendalianum (Solanaceae) por el murciélago frugívoro Sturnira ludovici (Phyllostomidae). Chiropt. Neotrop. 2011, 17, 1017–1021. [Google Scholar]
- Burgos, T.; Fedriani, J.M.; Escribano-Ávila, G.; Seoane, J.; Hernández-Hernández, J.; Virgós, E. Predation risk can modify the foraging behaviour of frugivorous carnivores: Implications of rewilding apex predators for plant–animal mutualisms. J. Anim. Ecol. 2022, 91, 1024–1035. [Google Scholar] [CrossRef] [PubMed]
- Acevedo-Quintero, J.F.; Zamora-Abrego, J.G. Papel de los mamíferos en los procesos de dispersión y depredación de semillas de Mauritia flexuosa (Arecaceae) en la Amazonía colombiana. Rev. Biol. Trop. 2016, 64, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Aguado, W.D.; Rogers, H.S.; Pruetz, J.D. Chimpanzees as ecosystem service providers: Seed dispersal of an economically important plant resource. Biotropica 2022, 54, 656–669. [Google Scholar] [CrossRef]
- Farris, E.; Canopoli, L.; Cucca, E.; Landi, S.; Maccioni, A.; Filigheddu, R. Foxes provide a direct dispersal service to Phoenician junipers in Mediterranean coastal environments: Ecological and evolutionary implications. Plant Ecol. Evol. 2017, 150, 117–128. [Google Scholar] [CrossRef]
- Muñoz-Gallego, R.; Fedriani, J.M.; Traveset, A. Non-native Mammals Are the Main Seed Dispersers of the Ancient Mediterranean Palm Chamaerops humilis L. in the Balearic Islands: Rescuers of a Lost Seed Dispersal Service? Front. Ecol. Evol. 2019, 7, 161. [Google Scholar] [CrossRef]
- Varela, O.; Bucher, E.H. Passage time, viability, and germination of seeds ingested by foxes. J. Arid Environ. 2006, 67, 566–578. [Google Scholar] [CrossRef]
- Rubalcava-Castillo, F.A.; Sosa-Ramírez, J.; Luna-Ruíz, J.J.; Valdivia-Flores, A.G.; Díaz-Núñez, V.; Íñiguez-Dávalos, L.I. Endozoochorous dispersal of forest seeds by carnivorous mammals in Sierra Fría, Aguascalientes, Mexico. Ecol. Evol. 2020, 10, 2991–3003. [Google Scholar] [CrossRef] [PubMed]
- Cáceres, N.C.; Prates, L.Z.; Ghizoni, I.R.; Graipel, M.E. Frugivory by the black-eared opossum Didelphis aurita in the Atlantic Forest of southern Brazil: Roles of sex, season and sympatric species. Biotemas 2009, 22, 203–211. [Google Scholar] [CrossRef][Green Version]
- Koike, S.; Masaki, T.; Nemoto, Y.; Kozakai, C.; Yamazaki, K.; Kasai, S.; Nakajima, A.; Kaji, K. Estimate of the seed shadow created by the Asiatic black bear Ursus thibetanus and its characteristics as a seed disperser in Japanese cool-temperate forest. Oikos 2010, 120, 280–290. [Google Scholar] [CrossRef]
- Tsuji, Y.; Yasumoto, Y.; Takatsuki, S. Multi-annual variation in the diet composition and frugivory of the Japanese marten (Martes melampus) in western Tokyo, central Japan. Acta Theriol. 2014, 59, 479–483. [Google Scholar] [CrossRef]
- Ríos-Blanco, M.C.; Pérez-Torres, J. Dieta de las especies dominantes del ensamblaje de murciélagos frugívoros en un bosque seco tropical (Colombia). Mastozoología Neotrop. 2015, 22, 103–111. [Google Scholar]
- Lanszki, Z.; Purger, J.J.; Bocz, R.; Szép, D.; Lanszki, J. The stone marten and the red fox consumed predominantly fruits all year round: A case study. Acta Zool. Acad. Sci. Hung. 2019, 65, 45–62. [Google Scholar] [CrossRef]
- González-Varo, J.P.; Fedriani, J.M.; López-Bao, J.V.; Guitián, J.; Suárez-Esteban, A. Frugivoría y dispersión de semillas por mamíferos carnívoros: Rasgos funcionales. Ecosistemas 2015, 24, 43–50. [Google Scholar] [CrossRef]
- Herrera, C.M. Frugivory and seed dispersal by carnivorous mammals, and associated fruit characteristics, in undisturbed Mediterranean habitats. Oikos 1989, 55, 250–262. [Google Scholar] [CrossRef]
- Matías, L.; Zamora, R.; Mendoza, I.; Hódar, J.A. Seed dispersal patterns by large frugivorous mammals in a degraded mosaic landscape. Restor. Ecol. 2010, 18, 619–627. [Google Scholar] [CrossRef]
- Reid, N. Dispersal of mistletoes by honeyeaters and flowerpeckers: Components of seed dispersal quality. Ecology 1989, 70, 137–145. [Google Scholar] [CrossRef]
- Rubalcava-Castillo, F.A.; Sosa-Ramírez, J.; Luna-Ruíz, J.J.; Valdivia-Flores, A.G.; Íñiguez-Dávalos, L.I. Seed dispersal by carnivores in temperate and tropical dry forests. For. Ecol. Evol. 2021, 11, 3794–3807. [Google Scholar] [CrossRef]
- Draper, J.P.; Atwood, T.B.; Beckman, N.G.; Kettenring, K.M.; Young, J.K. Mesopredator frugivory has no effect on seed viability and emergence under experimental conditions. Ecosphere 2021, 12, e03702. [Google Scholar] [CrossRef]
- Rubalcava-Castillo, F.A.; Valdivia-Flores, A.G.; Luna-Ruíz, J.J.; Íñiguez-Dávalos, L.I.; Martínez-Calderón, V.M.; Meraz, A.J.J.; Sosa-Ramírez, J. Effects of endozoochory and diploendozoochory by captive wild mammals on Juniperus deppeana seeds. Ecol. Evol. 2023, 13, e10262. [Google Scholar] [CrossRef]
- Traveset, A.; Verdú, M. Meta-analysis of gut treatment on seed germination. In Frugivores and Seed Dispersal: Ecological; Levey, D.J., Silva, W.R., Galetti, M., Eds.; Evolutionary and Conservation Issues; CABI Publishing: New York, NY, USA, 2002; pp. 339–350. [Google Scholar]
- Leon-Lobos, P.M.; Kalin-Arroyo, M.T. Germinación de semillas de Lithrea caustica (Mol.) H. et A. (Anacardiaceae) dispersadas por Pseudalopex spp. (Canidae) en el bosque esclerófilo de Chile central. Rev. Chil. Hist. Nat. 1994, 67, 59–64. [Google Scholar]
- Traveset, A. Effect of seed passage through vertebrate frugivores’ guts on germination: A review. Perspect. Plant Ecol. Evol. Syst. 1998, 1, 151–190. [Google Scholar] [CrossRef]
- Traveset, A.; Robertson, A.W.; Rodríguez-Pérez, J.A. A review on the role of endozoochory in seed germination. In Seed Dispersal Theory and Its Application in a Changing World; CABI: Wallingford, UK, 2007; pp. 78–103. [Google Scholar]
- Rojas-Martinez, A.E.; Pavon, N.P.; Castillo, J.P. Effects of seed ingestion by the lesser long-nosed bat Leptonycteris yerbabuenae on the germination of the giant cactus Isolatocereus dumortieri. Southwest. Nat. 2015, 60, 85–89. [Google Scholar] [CrossRef]
- Kelm, D.H.; Schaer, J.; Ortmann, S.; Wibbelt, G.; Speakman, J.R.; Voigt, C.C. Efficiency of facultative frugivory in the nectar-feeding bat Glossophaga commissarisi: The quality of fruits as an alternative food source. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 2008, 178, 985–996. [Google Scholar] [CrossRef] [PubMed]
- McGrosky, A.; Navarrete, A.; Isler, K.; Langer, P.; Clauss, M.J. Gross intestinal morphometry and allometry in Carnivora. Eur. J. Wildl. Res. 2016, 62, 395–405. [Google Scholar] [CrossRef]
- Hodgkison, R.; Balding, S.T.; Zubaid, A.; Kunz, T.H. Fruit Bats (Chiroptera: Pteropodidae) as Seed Dispersers and Pollinators in a Lowland Malaysian Rain Forest. Biotropica 2003, 35, 491–502. [Google Scholar] [CrossRef]
- Cypher, B.L.; Cypher, E.A. Germination Rates of Tree Seeds Ingested by Coyotes and Raccoons. Am. Midl. Nat. 1999, 142, 71–76. [Google Scholar] [CrossRef]
- Rivadeneira-Canedo, C. Estudio del oso andino (Tremarctos ornatus) como dispersor legítimo de semillas y elementos de su dieta en la región de Apolobamba-Bolivia. Ecol. Boliv. 2008, 43, 29–39. [Google Scholar]
- García-Rodríguez, A.; Albrecht, J.; Farwig, N.; Frydryszak, D.; Parres, A.; Schabo, D.G.; Selva, N. Functional complementarity of seed dispersal services provided by birds and mammals in an alpine ecosystem. J. Ecol. 2022, 110, 232–247. [Google Scholar] [CrossRef]
- Levey, D.J.; Bolker, B.M.; Tewksbury, J.T.; Sargent, S.; Haddad, N.M. Effects of landscape corridors on seed dispersal by birds. Science 2005, 309, 146–148. [Google Scholar] [CrossRef]
- Estrada, A.; Coates-Estrada, R. Bat species richness in live fences and in corridors of residual rain forest vegetation at Los Tuxtlas, Mexico. Ecography 2001, 24, 94–102. [Google Scholar] [CrossRef]
- Mello, M.A.R.; Marquitti, F.M.D.; Guimarães, P.R., Jr.; Kalko, E.K.V.; Jordano, P.; de Aguiar, M.A.M. The missing part of seed dispersal networks: Structure and robustness of bat–fruit interactions. PLoS ONE 2011, 6, e17395. [Google Scholar] [CrossRef] [PubMed]
- Salafsky, N.; Salzer, D.; Stattersfield, A.J.; Hilton-Taylor, C.; Neugarten, R.; Butchart, S.H.M.; Collen, B.; Cox, N.; Master, L.L.; O’Connor, S.; et al. A Standard Lexicon for Biodiversity Conservation: Unified Classifications of Threats and Actions. Conserv. Biol. 2008, 22, 897–911. [Google Scholar] [CrossRef] [PubMed]
- Hansen, D.M.; Donlan, C.J.; Griffiths, C.J.; Campbell, K.J. Ecological history and latent conservation potential: Large and giant tortoises as a model for taxon substitutions. Ecography 2010, 33, 272–284. [Google Scholar] [CrossRef]
- Murphy, H.; Hardesty, B.; Fletcher, C.; Metcalfe, D.; Westcott, D.; Brooks, S. Predicting dispersal and recruitment of Miconia calvescens (Melastomataceae) in Australian tropical rainforests. Biol. Invasions 2008, 10, 925–936. [Google Scholar] [CrossRef]



| Disturbance | Country | Frugivore | Plant | Quantity Component | Quality Component | Reference | ||
|---|---|---|---|---|---|---|---|---|
| Number of Visits | Quantity of Seeds | Viability | Germination | |||||
| Agriculture and urban expansion | Spain | Vulpes vulpes Martes foina Meles meles Genetta genetta Sus scrofa | Ziziphus lotus | NA | Negative | NA | NA | Cancio et al. (2016) [60] |
| Hunting and logging | Colombia | Dasypus novemcinctus Tayassu pecari Priodontes maximus Eira barbara Didelphis marsupialis Nasua nasua Dasyprocta fuliginosa Leopardus pardalis Proechimys sp. Marmosa robinsoni Dasyprocta punctata Microsciurus mimulus Sciurus granatensis | Oenocarpus bataua | Negative | NA | NA | NA | Franco-Quimbay and Rojas-Robles (2014) [61] |
| Logging, livestock and agriculture | México | Alouatta palliata mexicana | Ficus perforata Ficus lundelli | NA | Positive | NA | Positive | Serio-Silva and Rico-Gray (2002) [62] |
| Livestock | Argentina | Microcavia australis Lycalopex griseus Graomys griseoflavus Akodon dolores Conepatus chinga Chaetophractus vellerosus Calomys musculinus Galictis cuja Leopardus geoffroyi Thylamys pallidior Dolichotis patagonum | Neltuma flexuosa | Positive | Positive | NA | NA | Miguel et al. (2018) [63] |
| Logging | Indonesia | Viverra tangalunga | Adenia sp. Alangium sp. Annona sp. Connarus sp. Dialium indium Dimocarpus longan Ficus sp. Garcinia sp. Glochidion sp. Microcos sp. Palaquium sp. Polyalthia sp. Pometia pinnata Psidium sp. | NA | Negative | NA | NA | Colon and Sugau (2012) [64] |
| Urban expansion, agriculture and livestock | México | Ateles geoffroyi | Spondias radlkoferi Spondias mombin Attalea butyracea Bactris balanoidea Sabal mexicana Sapium sp. Acacia farnesiana Dialium guianense Inga punctata Inga sp. Calatola laevigata Nectandra sp. Guarea glabra Guarea grandifoliola Brosimum alicastrum Castilla elastica Ficus sp. Ficus tecolutensis Ficus obtusifolia Faramea occidentalis Paullinia costata Ampelocera hottlei Celtis iguanaea Cissus verticillata | NA | Negative | NA | NA | Chaves et al. (2010) [65] |
| Logging and agriculture | Lycalopex gymnocercus Pecari tajacu | Acacia aroma Acacia gilliesii Bromelia urbaniana Cactaceae Celtis ehrenbergiana Cucurbitella asperata Geoffroea decorticans Prosopis flexuosa Prosopis torquata Ziziphus mistol | Negative | Negative | NA | NA | Periago et al. (2017) [66] | |
| Logging | China | Melogale moschata | Dendrobenthamia japonica Dendrobenthamia capitata Clematoclethra scandens Actinidia chinensis Diospyros lotus Hovenia dulcis Prunus salicina | NA | Negative | NA | NA | Zhou et al. (2008) [67] |
| Agriculture and logging | Kenya | Cercopithecus mitis Cercopithecus ascanius Colobus guereza | Prunus africana | Positive | Positive | NA | NA | Farwig et al. (2006) [68] |
| Logging and hunting | Brazil | Dasyprocta leporina | Astrocaryum aculeatissimum | Negative | Negative | NA | NA | Galetti et al. (2006) [36] |
| Livestock and agriculture | Spain | Canis lupus Genetta genetta Martes foina Martes martes Meles meles Mustela erminea Mustela nivalis Ursus arctos Vulpes vulpes | Crataegus monogyna Ficus carica Frangula alnus Malus domestica Prunus avium Prunus domestica Prunus spinosa Pyrus communis Pyrus cordata Rosa spp. Rubus spp. Sorbus aucuparia Vaccinum myrtillus Vitis vinifera | NA | Positive | NA | NA | López-Bao and González-Varo (2011) [40] |
| Agriculture | Spain | Rodentia | Quercus ilex | NA | Negative | NA | NA | Morán-López et al. (2016) [20] |
| Livestock and logging | Argentina | Dolichotis patagonum Lycalopex griseus | Neltuma flexuosa | Positive | Positive | NA | NA | Miguel et al. (2017) [19] |
| Logging | China | Apodemus peninsulae Apodemus draco Sciurotamias davidianus | Quercus aliena var. acuteserrata | Positive | Positive | NA | NA | Yu et al. (2017) [69] |
| Experimental burns | Brazil | Tapirus terrestris | Bellucia grossularioides Byrsonima crispa Enterolobium schomburgkii Eriotheca globosa Glycine max Guatteria schomburgkiana Humiria balsamifera Hymenaea courbaril Mauritia flexuosa Passiflora sp. Protium guianense Ricinus communis Sacoglottis guianensis Schefflera morototoni Senna sp. Solanum sp. Strychnos xinguensis Terminalia tetraphylla | Positive | Positive | NA | NA | Paolucci et al. (2019) [70] |
| Logging | Cameroon | Cephalophus natalensis Cephalophus silvicultor Philantomba congica | Ageratum conyzoides Axonopus compressus Cardamine sp. Cyperus sp. Ficus sp. Ficus wildemaniana Fimbristylis sp. Kyllinga sp. Laportea aestuans Mitracarpus hirtus Musanga cecropioides Oplismenus burmannii Oxalis barrelieri Oxalis sp. Panicum laxum Paspalum conjugatum Phyllanthus sp. Torenia thouarsii | Positive | Positive | NA | Positive | Houngbégnon et al. (2023) [71] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Triay-Limonta, O.; Hechavarría-García, G.G.; Valdivia, C.E.; Napolitano, C. Knowledge Gaps on the Effectiveness of Seed Dispersal by Mammals and the Effect of Human Disturbances: A Review. Diversity 2024, 16, 780. https://doi.org/10.3390/d16120780
Triay-Limonta O, Hechavarría-García GG, Valdivia CE, Napolitano C. Knowledge Gaps on the Effectiveness of Seed Dispersal by Mammals and the Effect of Human Disturbances: A Review. Diversity. 2024; 16(12):780. https://doi.org/10.3390/d16120780
Chicago/Turabian StyleTriay-Limonta, Onaylis, Gerardo G. Hechavarría-García, Carlos E. Valdivia, and Constanza Napolitano. 2024. "Knowledge Gaps on the Effectiveness of Seed Dispersal by Mammals and the Effect of Human Disturbances: A Review" Diversity 16, no. 12: 780. https://doi.org/10.3390/d16120780
APA StyleTriay-Limonta, O., Hechavarría-García, G. G., Valdivia, C. E., & Napolitano, C. (2024). Knowledge Gaps on the Effectiveness of Seed Dispersal by Mammals and the Effect of Human Disturbances: A Review. Diversity, 16(12), 780. https://doi.org/10.3390/d16120780






