Abstract
Dam construction disrupts river continuity, and alters hydrological dynamics and the distributional composition of aquatic organisms. Understanding the spatial distribution of aquatic communities following dam construction is crucial for the effective management and restoration of riverine ecosystems. This study focused on the macroinvertebrate community of the Hanjiang River during the low-flow period, and explored the relationship between water quality indices and bioindicators. The results revealed significant changes in both the composition and functional feeding groups (FFGs) of macroinvertebrate communities from the upper to the lower reaches of the river. Compared to the natural reach, the dam-affected reaches showed a decrease in the number of sensitive taxa of Ephemeroptera, Plecoptera, and Trichoptera (EPT), and an increase in the number of moderate tolerant taxa of Gastropoda and tolerant taxa of Oligochaeta. The collector-gatherers (CGs) dominated in the Hanjiang River. In the dam-affected reaches, the relative abundance of collector-filterers (CFs) and shredders (SHs) appeared to decrease, while that of scrapers (SCs) and CGs increased. Principal component analysis (PCA) revealed that latitude and dissolved oxygen (DO) played a crucial role in the spatial pattern of macroinvertebrates, and the biotic index (BI) and family biotic index (FBI) more accurately reflected the level of organic pollution in the Hanjiang River. The findings of this study are valuable for ecological management and biodiversity conservation following dam construction.
1. Introduction
Rivers are significant ecosystems that underpin the structure and function of the Earth’s biosphere. Despite the fact that the volume of freshwater in rivers constitutes 0.3% of the global freshwater total [1], their water resources are of vital importance for maintaining the structure, function, and material cycles of ecosystem [2]. With the rapid expansion of the population and the speedy development of the economy, the construction of water conservancy projects has substantially boosted the utilization of water resources, enhanced flood-control capabilities, and satisfied the requirements of social development such as water storage and irrigation, water diversion, power generation, and landscape tourism [3,4]. Nevertheless, dams and similar water-related infrastructure have increasingly emerged as the principal threats to the health of river ecosystems and biodiversity [5]. The fragmentation effect of dams disrupts the continuity of rivers, altering hydrological parameters including flow velocity, water depth, and discharge, and thereby modifying the original hydrological characteristics of the rivers. This also impacts the physicochemical properties of rivers (e.g., the migration of particulate matter, organic matter, and nutrients) as well as the migratory patterns of aquatic organisms both upstream and downstream (e.g., the migratory behavior of fish and the drift and dispersal of macroinvertebrates). As a result, changes occur in the spatial pattern of aquatic organisms, affecting the composition, abundance, and biomass of aquatic life in the rivers [6,7].
Macroinvertebrates are long-lived organisms within river ecosystems. They possess a great variety of species, and are widely distributed, being highly sensitive to environmental alterations. Their existence serves as an evident sign of the impact of the external environmental on rivers, mirroring the overall health conditions of river ecosystem. From past research, macroinvertebrates are frequently utilized as bioindicators for evaluating the effect of dams on aquatic ecosystems [8]. Because of factors like water thermal stratification, variations in river water levels, flow velocities, and sediment retention, dams have a negative influence on the community and functional structure of downstream macroinvertebrates [1,9,10]. The biomass of downstream macroinvertebrate increases while the abundance decreases. The number of tolerant taxa (e.g., Diptera, Oligochaeta, and Mollusca) rises, while that of sensitive taxa like Ephemeroptera, Plecoptera, Trichoptera (EPT) decreases markedly [11]. For instance, species richness and the Shannon-Wiener diversity index of macroinvertebrates downstream of small hydropower plants in the Yangtze River basin were lower compared to natural rivers [12]. Moreover, the functional morphology and diversity of macroinvertebrates also respond significantly to environmental changes resulting from hydropower development. For example, a study on small reservoirs in Spain reveals that the drying-up of the downstream area caused by water storage in summer leads to a substantial reduction in functional diversity. Among them, collector-gatherers and shredders are especially affected [13]. Compared with a single hydropower plant, the development of multiple consecutive hydropower plants along the same river poses a greater threat to the macroinvertebrate community.
The biotic indices, which are values derived from the responsibility of macroinvertebrates for environmental disturbances, are widely employed to evaluate ecological status [14,15,16]. Commonly used methods include the BMWP [17], SIGNAL [18], BI [19], and FBI [20]. Additionally, community structure indicators such as diversity [21], EPT ratio [22], functional feeding groups (FFGs) ratio [23] are more frequently applied. The influence of environmental factors on macroinvertebrates is highly intricate because different environmental variables signify different forms of water pollution (e.g., NH3-N and CODMn signal organic pollution, while TP and TN indicate eutrophication), and their impacts on different macroinvertebrate taxa are not always consistent [24]. Given that various water quality indicators represent different types of pollution, such as the BMWP and BI indices for organic pollution, an interaction analysis between these indicators and physio-chemical parameters can offer a comprehensive understanding of the influence of pollution or human activities on water quality, aquatic organisms, and aquatic ecosystems.
The Hanjiang River, being the largest tributary of the Yangtze River and an essential water source for the South-to-North Water Diversion Project, plays a vital role in guaranteeing national water security and supporting socio-economic development. A large number of hydraulic projects have been constructed along the Hanjiang River from upstream to downstream. These projects have directly altered the vertical connectivity and flow pattern, thereby leading to subsequent changes in the habitats of aquatic organisms. This study, with the Hanjiang River as the research object, investigates the characteristics of macroinvertebrates and the water environment at sites in the upper, middle and lower reaches. The specific aims are as follows: (1) to elucidate the current state of the community structure and functional characteristics of macroinvertebrates in the Hanjiang River under cascade dam construction; (2) to explore the response relationship between water quality parameters and macroinvertebrate indicators in the Hanjiang River. The findings of this research offer valuable insights for river management and restoration in the Hanjiang River, providing guidance for ecological management and biodiversity conservation in the context of dam construction.
2. Material and Methods
2.1. Study Area and Distribution of Sampling Sites
The Hanjiang River originates at the Qinling Mountains and flows into the Yangtze River at the city of Wuhan. The total length is 1577 km, with elevations ranging from 13 to 3525 m. The Hanjiang River basin is located in the subtropical monsoon climate zone, with an average annual precipitation of 700 to 1400 mm and an average annual temperature of 12 to 16 °C. The Danjiangkou Reservoir serves as the source for the South-to-North Water Diversion Project, and is also the division point between upstream canyon section and the middle and lower plain section.
A total of 12 sampling sites were established along the Hanjiang River (Figure 1). Site HJ-1 was situated in a natural reach (R1) that was not affected by dams. The remaining sites were located downstream of different dams. Site HJ-2, site HJ-3 and site HJ-4 were located on the upstream affected reach (R2), while site HJ-5 to site HJ-12 were located on the midstream affected reach (R3).
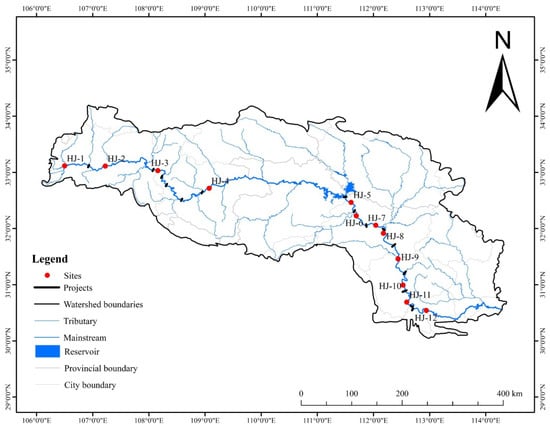
Figure 1.
Location of sampling sites in the Hanjiang River.
2.2. Sampling and Analyses
To minimize the impact of hydrological variations caused by precipitation, all samples were collected during the low-flow period (April 2023). Macroinvertebrates were sampled and identified following the Chinese industry standard [25]. A D-frame net with a mesh size of 40 microns was used, with the straight edge (0.3 m) pressed firmly against the riverbed. The net was moved upstream for a distance of 5 m, covering an area of approximately 1.5 m2. Three sample replicates were taken at each site. The specimens were sorted and preserved in a 75% ethanol solution. In the laboratory, samples were further categorized morphologically and identified to the species level using a stereomicroscope. The classification and identification process relied on references, including “Outline of Chinese Mayflies”, “Aquatic Insects of China Useful for Monitoring Water Quality”, and other relevant sources [26,27,28,29].
Water quality parameters, including water temperature (T, °C), pH, dissolved oxygen (DO, mg/L), and electrical conductivity (EC, μS/cm), were measured by using portable multiparameter analyzers (Hach, Loveland, CO, USA). Turbidity (NTU) was measured with Hach 2100Q turbidimeter (Hach, United States). Other parameters, including total nitrogen (TN, mg/L), total phosphorus (TP, mg/L), ammonia nitrogen (NH3-N, mg/L), and permanganate index (CODMn, mg/L), were determined in the laboratory after water samples collection. The measured values of each water quality parameter are shown on Table S1.
2.3. Index Calculations
The macroinvertebrates community structure indices including the family-level EPT ratio, Shannon–Wiener diversity index (H), Margalef richness index (d), functional feeding groups (FFGs) ratio and biological indices including the stream invertebrate grade number—average level (SIGNAL), biological monitoring working party (BMWP), biotic index (BI) and family biotic index (FBI). FFGs are classified as gatherer-collector group (GC), filterer-collector group (FC), predator group (PR), scraper group (SC) and shredder group (SH). The FFGs ratio reflects the relative abundance of each group within the total abundance at each sampling site. The formulas for calculating the bioindicators are provided below:
where Oj is the order-level sensitivity value of taxon j in the sample [21], m is the total number of order-level taxa in the sample, and wj is the weight of the order-level taxa, which is determined by the number of individuals in the taxon (Table S2).
where ti is the family-level sensitivity value of family i and N is the total number of family-level taxa in the sample.
where Ni is the number of individuals of taxon i, ti is the pollution tolerance value of taxon i, ni is the number of individuals of family i, pi is the pollution tolerance value of family i, and N is the total number of individuals.
where SE, SP and ST represent the number of family-level taxa of EPT.
where S represents the number of taxa, Ni refers to the individual count of the taxon i and N is the total number of individuals.
2.4. Statistical Analysis
Non-metric multidimensional scaling (NMDS) analysis was used to evaluate the differences in macroinvertebrate taxa composition among three groups of R1, R2 and R3. To reduce the influence of rare species, the biological data were converted to relative abundance prior to the NMDS analysis. This analysis was conducted using the metaMD function from the vegan package [30], with a stress value computed as an indicator of the model’s goodness of fit. The interpretation of stress values was as follows: 0–0.05 (ideal), 0.05–0.15 (reasonably good), 0.15–0.20 (acceptable), and >0.20 (suggesting the need to reconsider the number of ordination axes) [31]. The NMDS analysis and graphical outputs were generated using R version 4.2.3. Subsequently, differences in FFGs between R2 and R1, R3 and R1 were compared, followed by statistical analysis of the disparities of FFGs between affected reaches and natural reach. To investigate the natural and environmental factors influencing sampling sites, principal component analysis (PCA) was employed to reduce dimensionality. This analysis focused on spatial factors (latitude, longitude, and altitude), physicochemical parameters, and biological indicators to minimize data redundancy. Biplots were generated from the scores and loadings of the first two principal components to examine the relationships between biological indicators and physicochemical parameters [32]. All statistical and PCA were performed using Origin Pro 2021 software.
3. Results
3.1. Differences in Species Composition and Community Structure of Macroinvertebrates
A total of 79 macroinvertebrate taxa were collected in the Hanjiang River, representing four phyla, nine classes, 17 orders and 40 families. Of these, 57 taxa belong to the phylum Arthropoda, comprising 72.2% of the total, with 53 taxa from Insecta (67.1%), three taxa from Malacostraca (3.8%), and one taxon from Arachnida (1.3%). The phylum Mollusca accounted for 13.9%, with 11 taxa, including seven taxa of Gastropoda (8.9%), two taxa of Bivalvia (2.5%), and two taxa of Lamellibranchia (2.5%). The phylum Annelida represented 12.7%, with 10 taxa, including eight taxa of Oligochaeta (10.1%) and two taxa of Hirudinea (2.5%). Lastly, one taxon of Nematoda was recorded, contributing 1.3% (Figure 2a). The natural reach (R1) was inhabited exclusively by taxa from Arthropoda and Nematoda, with a greater number of Arthropoda taxa than Nematoda. Arthropoda dominated in R2, followed by Annelida, while Mollusca was the least distributed. R3 was dominated by Arthropoda, followed by Mollusca, with Annelida being the lowest proportion (Figure 2b). The species composition at each sampling site is provided in Table S3.
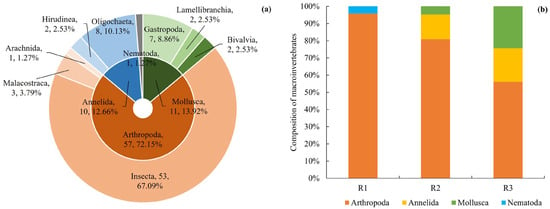
Figure 2.
The compositions of macroinvertebrate communities in the Hanjiang River (a) and in various sampling reaches (b) are presented.
The NMDS analysis revealed a clear distinction in the macroinvertebrate communities among R1, R2, and R3 (Figure 3). R1 was predominantly characterized by EPT taxa, with no Gastropoda or Oligochaeta taxa present (Table S3). Additionally, several species were exclusive to this site, including Simuliidae, Tabanidae, Tipulidae, and Chrysomelidae. The abundance of EPT in R2 was lower than in R1, and no Gastropoda taxa were present in R2. Oligochaeta were limited to two taxa of Naididae and one taxon of Tubificidae. R3 was primarily composed of Gastropoda, Diptera (Insecta), and Tubificidae, with fewer EPT taxa.
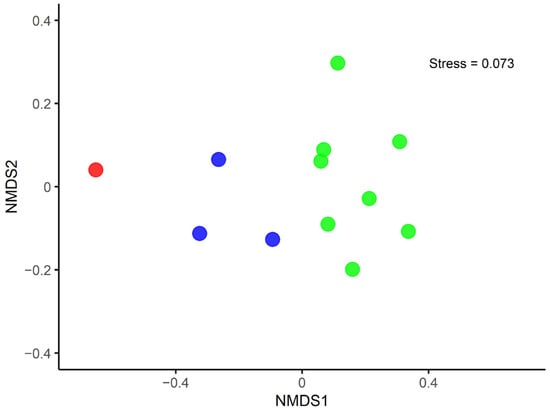
Figure 3.
NMDS between the three groups of the natural reach and the affected reaches. R1 is represented in red, R2 in blue, and R3 in green.
3.2. Functional Feeding Groups of Macroinvertebrates and Differences Between Groups
All taxa of macroinvertebrates were categorized into five FFGs: six taxa of FCs, seven taxa of SCs, 47 taxa of GCs, 15 taxa of PRs, and three taxa of SHs (Table S3). In the Hanjiang River as a whole, GCs were the most dominant group, comprising 65.1% of the total abundance, followed by PRs (13.9%), SHs (8.3%), FCs (7.9%), and SCs (4.8%). As for R1, the relative abundances of FFGs were as follows: GCs (62%), FCs (18.1%), SHs (17.6%), and PRs (2.4%). As for R2, GCs were also the dominant group (84.8%), followed by SHs (8%) and FCs (4.5%), with PRs contributing the least (2.7%). Both R1 and R2 lacked SCs. As for R3, the most abundant group was again GCs (64.5%), followed by PRs (19.9%) and SCs (7.4%), while SHs (5.2%) and FCs (5%) contributed less (Figure 4).
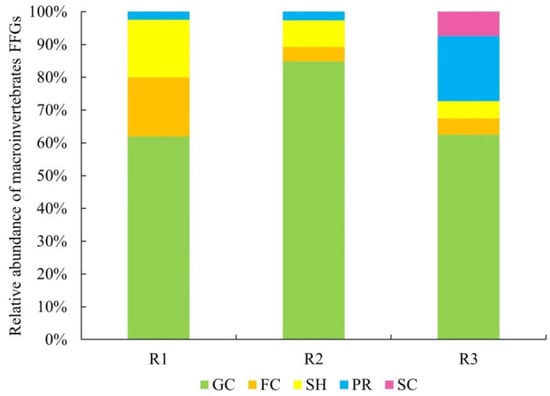
Figure 4.
Relative abundance of macroinvertebrate FFGs in the natural and affected reaches.
The FFGs of macroinvertebrates in the Hanjiang River were compared by examining the difference in the proportion of each FFG between R1 and R2 or R3. A positive value indicated an increase in the proportion of a group in the natural reach, while a negative value indicated a decrease. The results, shown in Figure 5, revealed the following patterns. (1) FCs: In R2, the proportion of HJ-3 increased, while this group was absent at all other sites. In R3, only HJ-11 showed an increased proportion, while all other sites exhibited a decrease. The group was completely absent at HJ-5, HJ-10, and HJ-12. (2) SCs: This group was absent in R1 and in all sites of R2. In R3, except for HJ-11, which lacked SCs, all other sites showed an increase in their proportion. (3) GCs: The proportion increased at all sites in R2. In R3, however, the proportion decreased at HJ-7 and HJ-9, while all other sites showed an increase. (4) PRs: Only HJ-3 in R2 exhibited an increase in the proportion of PR, with the group absent at all other sites. In R3, the proportion of PRs decreased at HJ-8, HJ-10, and HJ-12, with HJ-12 completely lacking this group. All other sites showed an increase in predator proportions. (5) SHs: In R2, the proportion at HJ-2 increased, while the group was absent at all other sites. In R3, the proportion at HJ-7 increased, but all other sites showed a decrease, with HJ-5, HJ-6, HJ-8, HJ-10, and HJ-11 entirely lacking this group.
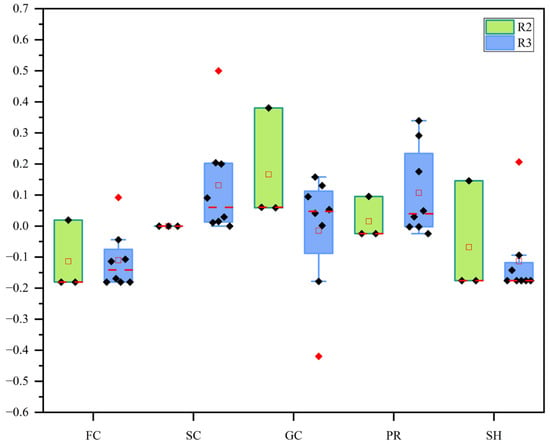
Figure 5.
Comparison of FFGs between R2 and R3. R2 is represented in green, R3 is shown in purple. The red line indicates the median, and the red points represent outliers.
3.3. Response Relationship Between Physicochemical Parameters and Bioindicators
The calculated values of bioindicators at each site are presented in Table S4. PCA results (Figure 6) indicate that the first two principal components accounted for 61.3% of the total variance, with the first component explaining 45.8% and the second 15.5%. Under the influence of longitude, the biological indices BI and FBI, as well as the FFGs ratio for FCs and SCs, showed positive correlations with EC, NH3-N, TP, pH and turbidity, particularly with T and CODMn. Conversely, these indices were negatively correlated with DO and TN. In contrast, the biological index SIGNAL, the FFGs ratio for SHs, and the community structure indices (EPT, H’, and d) were positively correlated with DO and TN, with a notably stronger correlation with DO, and were influenced by latitude and altitude. GCs and PRs were positively correlated with DO and CODMn, while the BMWP was positively correlated with EC and TN. The first principal component (PCA Axis 1) was negatively correlated with SIGNAL, EPT, H’, d, SHs, TN, latitude and altitude, distinguishing the reference site (HJ-1) from other sites. Axis 1 also exhibited a positive correlation with NH3-N, T, TP, pH, turbidity, CODMn, BI, FBI, FCs and longitude, effectively separating the midstream affected reach (R3: HJ-10, HJ-11, HJ-12) from other sites in this reach. Axis 2 was positively correlated with EC, BMWP and SCs, but negatively correlated with DO, GCs and PRs, distinguishing the upstream affected reach (R2) from R3. In summary, spatial factors, environmental parameters and biological indicators clearly differentiate the reference reach (R1) from the affected reaches (R2 and R3). PCA results highlight significant relationships between physicochemical parameters and bioindicators, emphasizing the water quality differences between the natural and affected reaches of the Hanjiang River.
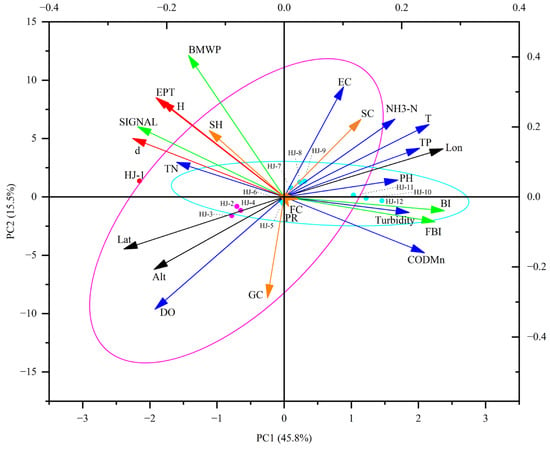
Figure 6.
The PCA biplot of spatial factors, physicochemical parameters, and bioindicators at the sampling sites. Red circular points represent the natural reach (R1), while pink circular points correspond to the upstream affected reach (R2), with the pink ellipse indicating the 95% confidence interval for R2. Light blue circular points denote the midstream affected reach (R3), with the light blue ellipse representing the 95% confidence interval for R3. Black arrows denote spatial factors, blue arrows indicate physicochemical parameters, green arrows represent biological indices, orange arrows FFGs of various types, and red arrows represent community structure indices.
4. Discussion
The structural characteristics of the macroinvertebrate community in the Hanjiang River have significant changes from the upper to the lower reaches, shaped by the combined influences of spatial processes and environmental filtering. In the natural reach (R1), the macroinvertebrate community is predominantly composed of organisms from the EPT taxa (Ephemeroptera, Plecoptera, and Trichoptera) within the Insecta class. In the upstream affected reach (R2), the number of EPT has decreased, with only Ephemeridae and Caenidae remaining, while Oligochaeta have increased. In the midstream affected reach (R3), the community is primarily composed of species from Mollusca and Annelida, with Ephemeridae found only at select sites (Table S3). This pattern is consistent with Abernethy’s study, which found that dam-induced peak flow negatively impacts the richness, abundance, and biomass of Insecta and EPT taxa. The study showed that, downstream of hydropower stations with weak peak flow, the macroinvertebrate community is dominated by Insecta, while species of non-Insecta predominate in areas with strong peak flow [33]. The results of this study indicate that, from upstream to downstream, EPT taxa gradually diminish, while Oligochaeta and Gastropoda steadily increase. Additionally, macroinvertebrate abundance and biodiversity indices (Shannon–Wiener and Margalef) are highest in the natural reach (R1) and significantly decrease in the affected reaches (R2 and R3) (Table S4). These changes can likely be attributed to the varying adaptive capacities of macroinvertebrates to fluctuations in water flow and temperature [34]. For example, species from Bivalvia and Gastropoda can thrive under diverse flow conditions, including periodic droughts, making these groups dominant in the affected reaches downstream of the dam [33]. Furthermore, as the Hanjiang River flows from its upper to lower reaches, the altitude gradually decreases, and, due to the construction of cascade dams, river velocity decreases, and the macroinvertebrate community transitions from a fast-flowing to a slow-flowing type [35,36,37]. EPT taxa prefer substrates dominated by cobbles and gravel, but dam construction alters the river habitat, leading to still waters where fine sediments prevail. These fine particles can clog the branched gills of EPT taxa, impairing their respiration. Since EPT are sensitive to environmental changes [38], they dominate in high-flow reaches, while Oligochaeta are more common in low-flow areas [39,40]. This pattern is consistent with the findings of this study.
The types and distribution of food resources available to macroinvertebrates downstream of the dam differ significantly from those upstream, accompanied by a notable shift in the FFGs. The composition of these groups is influenced by various factors, including the geographical scale of the river, nutrient inputs, and seasonal variations, each with its own distinct characteristics [41]. In this study, the natural reach (R1) and the upstream affected reach (R2) both lack SCs, while the midstream affected reach (R3) shows a notable increase in their proportion. SCs primarily feed on algae, microorganisms, protozoans and various invertebrates. The deterioration of water quality and the reduction in flow velocity resulting from the dam’s construction in the Hanjiang River have created favorable conditions for the persistence of SCs, as they are more resilient to pollution and adaptable to varying hydrological conditions. Consequently, species within this group thrive in the dam-affected habitats [33]. In contrast, the abundance of SHs follows an inverse trend to that of SCs, being higher in R1 than in R2 and R3. This is primarily due to SHs feeding on detritus and coarse organic matter. The dam’s construction in the affected reaches has led to a reduction in forested areas, a decrease in riparian vegetation coverage, and a significant decline in available food sources, such as fallen leaves, which are crucial for SHs, thereby limiting their survival and reproduction [42]. In the Hanjiang River, GCs dominate the functional feeding groups. Compared to the R1, the affected reaches downstream of the dam (R2 and R3) show greater diversity in these groups, consistent with findings from other studies [43].
The spatial processes at sampling sites, along with the physicochemical parameters of aquatic environments, play a crucial role in shaping macroinvertebrate communities [44,45]. The construction of dams has significantly altered the hydrological characteristics of the Hanjiang River [46]. Studies show that dam construction and operation can lead to substantial shifts in water temperature, especially during spring and autumn [47]. This finding aligns with the results of this study, where downstream temperatures are generally higher than those at the source site, HJ-1 (Table S1). PCA reveals that latitude and dissolved oxygen (DO) significantly influences the spatial distribution of macroinvertebrates, while the first PCA axis strongly correlates with pH, turbidity, CODMn, and TN. These four environmental variables are therefore key parameters affecting both water quality and macroinvertebrate species composition in the Hanjiang River. Indicators such as TN and CODMn have primarily driven the decline of EPT taxa and the increase of species like Gastropoda and Oligochaeta, consistent with findings from other studies [35,48]. Biological indicators (Table S4) demonstrate that these parameters effectively reflect river health, with the BI and FBI offering particularly reliable indications of organic pollution, given their significant positive correlation with CODMn and NH3-N.
5. Conclusions
This study examined the community structure and functional traits of macroinvertebrates in the Hanjiang River following the construction of cascade dams, and explored the relationship between physicochemical parameters and bioindicators. The results revealed significant changes in both the composition and FFGs of macroinvertebrate communities from the upper to the lower reaches of the Hanjiang River. Compared to natural reach, sensitive taxa such as EPT gradually declined downstream of the dams, whereas pollution-tolerant taxa, including Gastropoda and Oligochaeta, steadily increased. The Hanjiang River was primarily inhabited by GCs, with a reduction in the relative abundance of FCs and SHs at downstream sites, while the relative abundance of SCs and GCs increased. Furthermore, PCA showed that latitude and dissolved oxygen (DO) were key factors influencing the spatial distribution of macroinvertebrates, while biological indices (BI and FBI) offered more reliable assessments of organic pollution in the river. These findings offer valuable insights for the management and restoration of rivers in the Hanjiang River and provide guidance for ecological management and biodiversity conservation following the construction of dams.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d16120772/s1, Table S1: Measured values of physical and chemical indexes of water quality at sampling sites; Table S2: Weight of SIGNAL index; Table S3: List of species of macroinvertebrates; Table S4: Calculated values of bioindicators at sampling sites.
Author Contributions
Conceptualization, M.Z. and S.D.; methodology, M.Z.; software, M.Z.; validation, M.Z., G.S. and S.D.; formal analysis, M.Z.; investigation, G.S. and S.D.; resources, S.D.; data curation, M.Z.; writing—original draft preparation, M.Z.; writing—review and editing, G.S. and S.D.; visualization, M.Z.; supervision, S.D.; project administration, S.D.; funding acquisition, S.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key Research and Development Program of China (No. 2021YFC3201003). And the APC was funded by the first phase of the joint research project on ecological protection and high-quality development of the Yellow River Basin (2022-YRUC-01-0603-01).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
All supported data of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, J.; Ding, L.; Tao, J.; Ding, C.; He, D. The Effects of Dams on Macroinvertebrates: Global Trends and Insights. River Res. Appl. 2019, 35, 702–713. [Google Scholar] [CrossRef]
- Vörösmarty, C.J.; McIntyre, P.B.; Gessner, M.O.; Dudgeon, D.; Prusevich, A.; Green, P.; Glidden, S.; Bunn, S.E.; Sullivan, C.A.; Liermann, C.R.; et al. Global Threats to Human Water Security and River Biodiversity. Nature 2010, 467, 555–561. [Google Scholar] [CrossRef]
- Van Looy, K.; Tormos, T.; Souchon, Y. Disentangling Dam Impacts in River Networks. Ecol. Indic. 2014, 37, 10–20. [Google Scholar] [CrossRef]
- Lehner, B.; Liermann, C.R.; Revenga, C.; Vörösmarty, C.; Fekete, B.; Crouzet, P.; Döll, P.; Endejan, M.; Frenken, K.; Magome, J.; et al. High-Resolution Mapping of the World’s Reservoirs and Dams for Sustainable River-Flow Management. Front. Ecol. Environ. 2011, 9, 494–502. [Google Scholar] [CrossRef]
- Zarfl, C.; Lumsdon, A.E.; Berlekamp, J.; Tydecks, L.; Tockner, K. A Global Boom in Hydropower Dam Construction. Aquat. Sci. 2015, 77, 161–170. [Google Scholar] [CrossRef]
- Gillett, N.D.; Pan, Y.; Eli Asarian, J.; Kann, J. Spatial and Temporal Variability of River Periphyton below a Hypereutrophic Lake and a Series of Dams. Sci. Total Environ. 2016, 541, 1382–1392. [Google Scholar] [CrossRef]
- Anderson, E.P.; Jenkins, C.N.; Heilpern, S.A.; Maldonado-Ocampo, J.A.; Carvajal-Vallejos, F.M.; Encalada, A.C.; Rivadeneira, J.F.; Hidalgo, M.; Cañas, C.M.; Ortega, H.; et al. Fragmentation of Andes-to-Amazon Connectivity by Hydropower Dams. Sci. Adv. 2018, 4, eaao1642. [Google Scholar] [CrossRef] [PubMed]
- Kumari, P.; Maiti, S.K. Bioassessment in the Aquatic Ecosystems of Highly Urbanized Agglomeration in India: An Application of Physicochemical and Macroinvertebrate-Based Indices. Ecol. Indic. 2020, 111, 106053. [Google Scholar] [CrossRef]
- Wang, F.; Maberly, S.C.; Wang, B.; Liang, X. Effects of Dams on Riverine Biogeochemical Cycling and Ecology. Inland Waters 2018, 8, 130–140. [Google Scholar] [CrossRef]
- Smolar-Žvanut, N.; Mikoš, M. The Impact of Flow Regulation by Hydropower Dams on the Periphyton Community in the Soča River, Slovenia. Hydrol. Sci. J. 2014, 59, 1032–1045. [Google Scholar] [CrossRef]
- Phillips, I.D.; Pollock, M.S.; Bowman, M.F.; MMaster, D.G.; Chivers, D.P. Thermal Alteration and Macroinvertebrate Response below a Large Northern Great Plains Reservoir. J. Great Lakes Res. 2015, 41, 155–163. [Google Scholar] [CrossRef]
- Zhao, W.; Guo, W.; Zhao, L.; Li, Q.; Cao, X.; Tang, X. Influence of Different Types of Small Hydropower Stations on Macroinvertebrate Communities in the Changjiang River Basin, China. Water 2019, 11, 1892. [Google Scholar] [CrossRef]
- Martínez, A.; Larrañaga, A.; Basaguren, A.; Pérez, J.; Mendoza-Lera, C.; Pozo, J. Stream Regulation by Small Dams Affects Benthic Macroinvertebrate Communities: From Structural Changes to Functional Implications. Hydrobiologia 2013, 711, 31–42. [Google Scholar] [CrossRef]
- He, K.; Tang, H.; He, Y.; Feng, X.; Yang, L.; Ji, W. Applicability of Macrobenthos Indexes in Health Assessment Upstream of a Large River: A Case Study in the Babian River of the Red River Basin, China. Ecol. Inform. 2023, 74, 101958. [Google Scholar] [CrossRef]
- Özbek, M.; Aygen, C.; Taşdemir, A.; Yildiz, S.; Topkara, E.T.; Çil, E.A. Determination of the Ecological Status of an Aegean River (Türkiye) Using Benthic Macroinvertebrates as an Indicator of Water Quality. Environ. Monit. Assess. 2023, 195, 1231. [Google Scholar] [CrossRef]
- Vargas-Tierras, T.; Suárez-Cedillo, S.; Morales-León, V.; Vargas-Tierras, Y.; Tinoco-Jaramillo, L.; Viera-Arroyo, W.; Vásquez-Castillo, W. Ecological River Water Quality Based on Macroinvertebrates Present in the Ecuadorian Amazon. Sustainability 2023, 15, 5790. [Google Scholar] [CrossRef]
- Hawkes, H.A. Origin and Development of the Biological Monitoring Working Party Score System. Water Res. 1998, 32, 964–968. [Google Scholar] [CrossRef]
- Chessman, B.C. New Sensitivity Grades for Australian River Macroinvertebrates. Mar. Freshw. Res. 2003, 54, 95–103. [Google Scholar] [CrossRef]
- Chutter, F.M. An Empirical Biotic Index of the Quality of Water in South African Streams and Rivers. Water Res. 1972, 6, 19–30. [Google Scholar] [CrossRef]
- Hilsenhoff, W.L. Rapid Field Assessment of Organic Pollution with a Family-Level Biotic Index. J. N. Am. Benthol. Soc. 1988, 7, 65–68. [Google Scholar] [CrossRef]
- Naqinezhad, A.; Hamzeh’ee, B.; Attar, F. Vegetation–Environment Relationships in the Alderwood Communities of Caspian Lowlands, N. Iran (toward an Ecological Classification). Flora—Morphol. Distrib. Funct. Ecol. Plants 2008, 203, 567–577. [Google Scholar] [CrossRef]
- Kitchin, P.L. Measuring the Amount of Statistical Information in the EPT Index. Environmetrics 2005, 16, 51–59. [Google Scholar] [CrossRef]
- Pellegrini, T.G.; Faria, L.D.B.; Ferreira, R.L. Temporal Diversity Patterns of Benthic Insects in Subterranean Streams: A Case Study in Brazilian Quartzite Caves. Hydrobiologia 2020, 847, 2417–2431. [Google Scholar] [CrossRef]
- Álvarez-Cabria, M.; Barquín, J.; Juanes, J.A. Macroinvertebrate Community Dynamics in a Temperate European Atlantic River. Do They Conform to General Ecological Theory? Hydrobiologia 2011, 658, 277–291. [Google Scholar] [CrossRef]
- HJ 710.8-2014; Technical Guidelines for Biodiversity Monitoring—Freshwater Benthic Macroinvertebrates. Ministry of Ecology and Environment of the People’s Republic of China: Beijing, China, 2014.
- Zhou, C.F.; Su, C.R.; Gui, H. Outline of Chinese Mayflies; Science Press: Beijing, China, 2015. [Google Scholar]
- Epler, J.H. Identification Manual for the Larval Chironomidae (Diptera) of North and South Carolina; North Carolina Department of Environmental and Natural Resources, Division of Water Quality: Raleigh, NC, USA, 2001. [Google Scholar]
- Morse, J.C.; Yang, L.; Tian, L. Aquatic Insects of China Useful for Monitoring Water Quality; Hohai University Press: Nanjing, China, 1994. [Google Scholar]
- Liu, Y.Y. Chinese Economic Zoology: Freshwater Mollusca; Science Press: Beijing, China, 1979. [Google Scholar]
- Dixon, P. VEGAN, a Package of R Functions for Community Ecology. J. Veg. Sci. 2003, 14, 927–930. [Google Scholar] [CrossRef]
- Kruskal, J.B. Nonmetric Multidimensional Scaling: A Numerical Method. Psychometrika 1964, 29, 115–129. [Google Scholar] [CrossRef]
- Jabbar, F.K.; Grote, K. Statistical Assessment of Nonpoint Source Pollution in Agricultural Watersheds in the Lower Grand River Watershed, MO, USA. Environ. Sci. Pollut. Res. 2019, 26, 1487–1506. [Google Scholar] [CrossRef]
- Abernethy, E.F.; Muehlbauer, J.D.; Kennedy, T.A.; Tonkin, J.D.; Van Driesche, R.; Lytle, D.A. Hydropeaking Intensity and Dam Proximity Limit Aquatic Invertebrate Diversity in the Colorado River Basin. Ecosphere 2021, 12, e03559. [Google Scholar] [CrossRef]
- Hall, R.O.; Yackulic, C.B.; Kennedy, T.A.; Yard, M.D.; Rosi-Marshall, E.J.; Voichick, N.; Behn, K.E. Turbidity, Light, Temperature, and Hydropeaking Control Primary Productivity in the Colorado River, Grand Canyon. Limnol. Oceanogr. 2015, 60, 512–526. [Google Scholar] [CrossRef]
- Hua, L.; Hu, J.; Li, Y.; Xu, K.; Xu, Z.; You, A.; Yu, G.; Wang, Z.; Chen, Y.; Li, X.; et al. Effects of Cascading Dams on Water Quality and Macroinvertebrates in the Oujiang River, China. Water Resour. 2024, 51, 860–871. [Google Scholar] [CrossRef]
- Mihalicz, J.E.; Jardine, T.D.; Baulch, H.M.; Phillips, I.D. Seasonal Effects of a Hydropeaking Dam on a Downstream Benthic Macroinvertebrate Community. River Res. Appl. 2019, 35, 714–724. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, C.; Mo, K.; Chen, Q.; Tang, L.; Zhang, J.; Li, T.; Wang, J. Dam Cascade Alters Taxonomic Composition of Benthic Macroinvertebrate Community in Upper Yangtze River. River Res. Appl. 2021, 37, 1070–1079. [Google Scholar] [CrossRef]
- Hamid, S.A. Role of Ephemeroptera, Plecoptera and Trichoptera (Insecta) Functional Feeding Groups in Leaf Decomposition in Tropical River. J. Biodivers. Environ. Sci. 2016, 9, 204–213. [Google Scholar]
- Lessmann, J.; Guayasamin, J.M.; Casner, K.L.; Flecker, A.S.; Funk, W.C.; Ghalambor, C.K.; Gill, B.A.; Jácome-Negrete, I.; Kondratieff, B.C.; Poff, L.N.; et al. Freshwater Vertebrate and Invertebrate Diversity Patterns in an Andean-Amazon Basin: Implications for Conservation Efforts. Neotrop. Biodivers. 2016, 2, 99–114. [Google Scholar] [CrossRef]
- Trannum, H.C.; Pedersen, K.B.; Renaud, P.E.; Christensen, G.N.; Evenset, A. Recolonization and Recovery of an Arctic Benthic Community Subject to Mine-Tailings Deposits. J. Sea Res. 2023, 191, 102327. [Google Scholar] [CrossRef]
- Chester, H.; Norris, R. Dams and Flow in the Cotter River, Australia: Effects on Instream Trophic Structure and Benthic Metabolism. Hydrobiologia 2006, 572, 275–286. [Google Scholar] [CrossRef]
- Miserendino, M.L.; Masi, C.I. The Effects of Land Use on Environmental Features and Functional Organization of Macroinvertebrate Communities in Patagonian Low Order Streams. Ecol. Indic. 2010, 10, 311–319. [Google Scholar] [CrossRef]
- Cabrera, S.; Eurie Forio, M.A.; Lock, K.; Vandenbroucke, M.; Oña, T.; Gualoto, M.; Goethals, P.L.M.; Van der heyden, C. Variations in Benthic Macroinvertebrate Communities and Biological Quality in the Aguarico and Coca River Basins in the Ecuadorian Amazon. Water 2021, 13, 1692. [Google Scholar] [CrossRef]
- Shen, Y.; Yang, Y.; Zhou, Y.; Bian, B.; Zhang, L. Unraveling the Nexus of Multi-Environmental Factors and Benthic Macroinvertebrates in Typical Inflow River of Taihu Lake in China. Environ Monit Assess 2020, 192, 137. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, A.; Saha, G.K.; Aditya, G. Macroinvertebrates as Engineers for Bioturbation in Freshwater Ecosystem. Environ. Sci. Pollut. Res. 2022, 29, 64447–64468. [Google Scholar] [CrossRef]
- Chandesris, A.; Van Looy, K.; Diamond, J.S.; Souchon, Y. Small Dams Alter Thermal Regimes of Downstream Water. Hydrol. Earth Syst. Sci. 2019, 23, 4509–4525. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, N.; Wang, D.; Wu, J. Impacts of Cascade Reservoirs on Yangtze River Water Temperature: Assessment and Ecological Implications. J. Hydrol. 2020, 590, 125240. [Google Scholar] [CrossRef]
- Yang, Y.; Yi, Y.; Zhou, Y.; Wang, X.; Zhang, S.; Yang, Z. Spatio-Temporal Variations of Benthic Macroinvertebrates and the Driving Environmental Variables in a Shallow Lake. Ecol. Indic. 2020, 110, 105948. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).