The Study of Biting Midges Culicoides Latreille, 1809 (Diptera: Ceratopogonidae) and the Prevalence of Haemoproteus Kruse, 1890 (Haemosporida: Haemoproteidae) on the Curonian Spit of the Baltic Sea
Abstract
1. Introduction
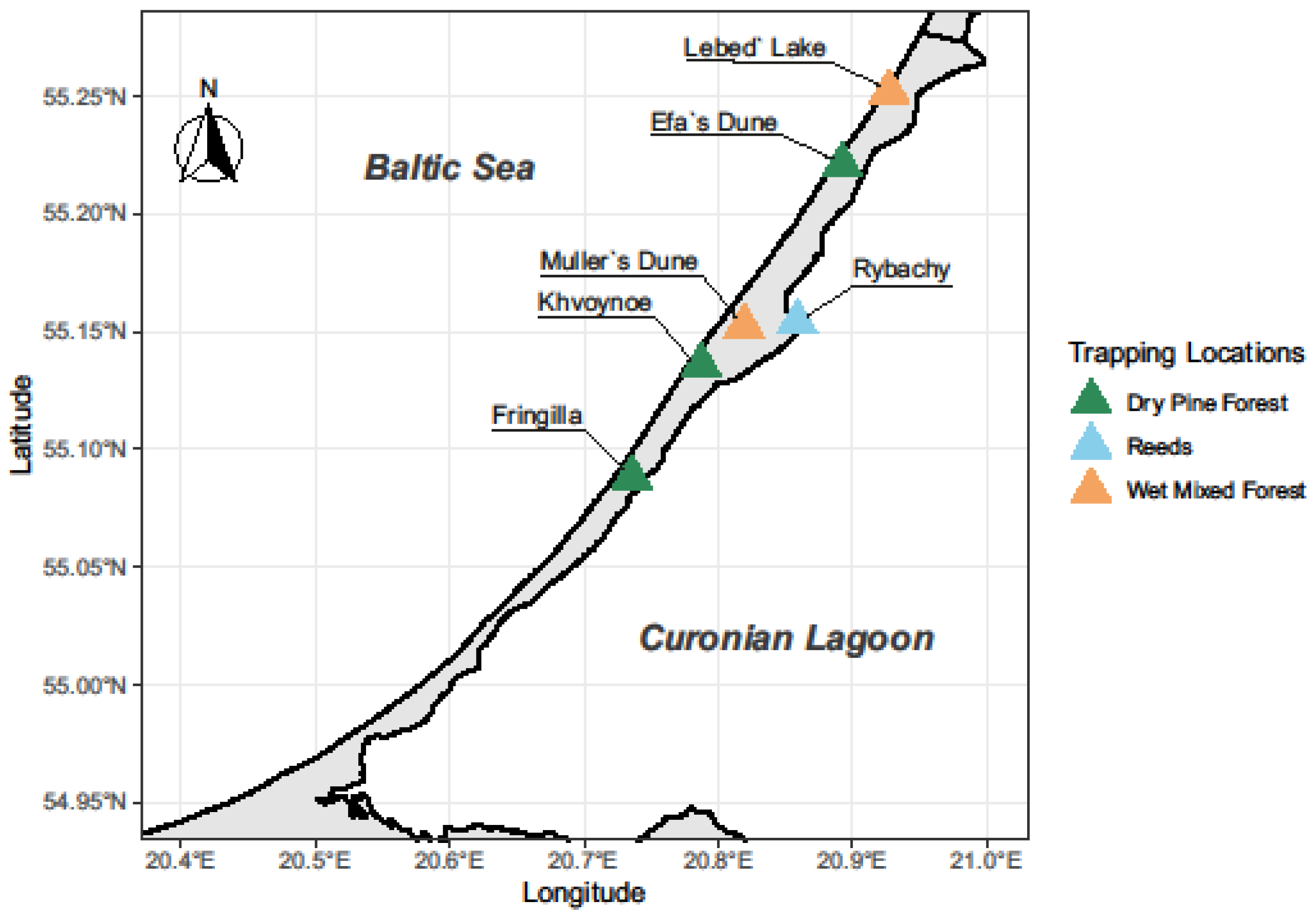
2. Material and Methods
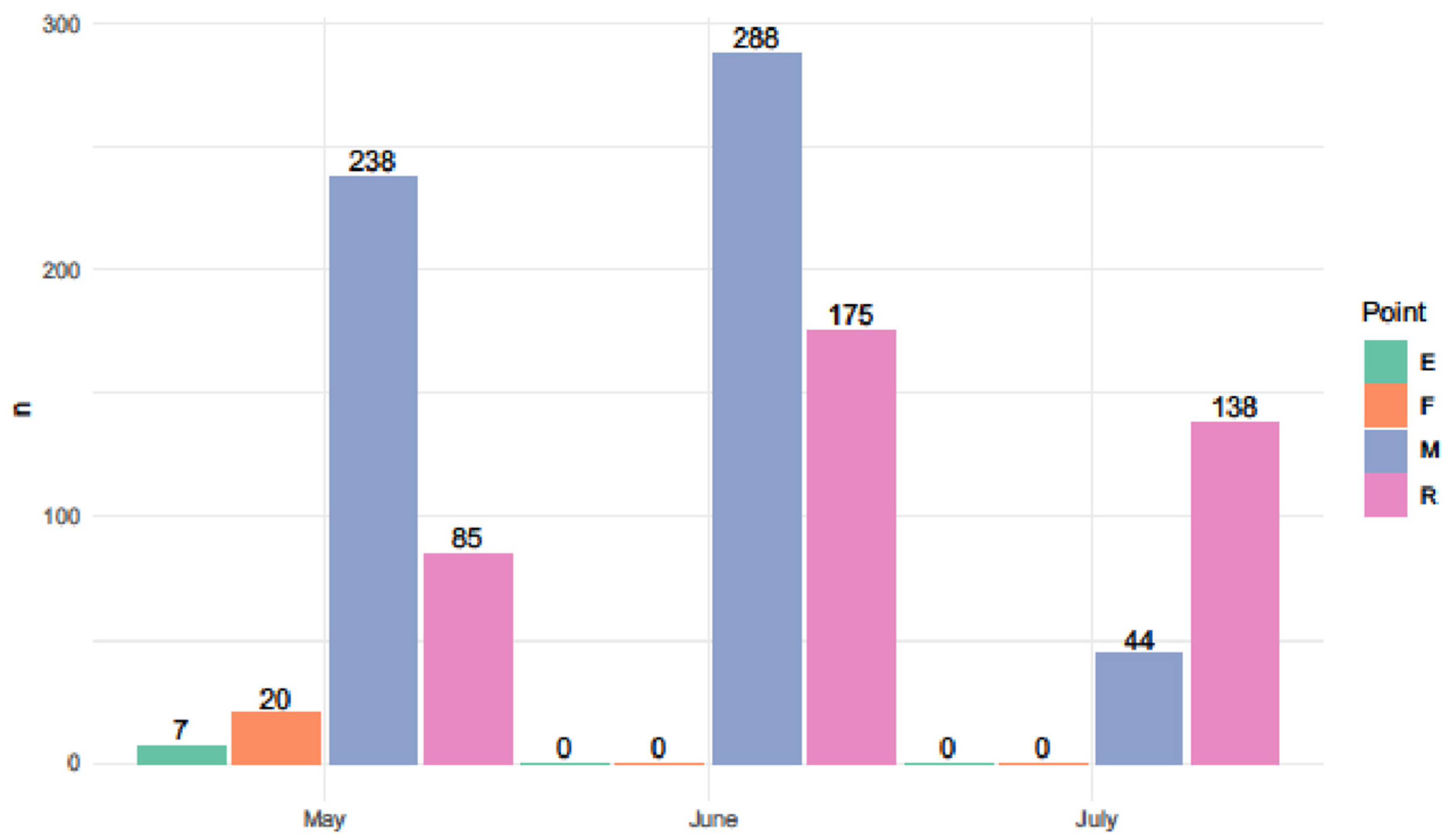
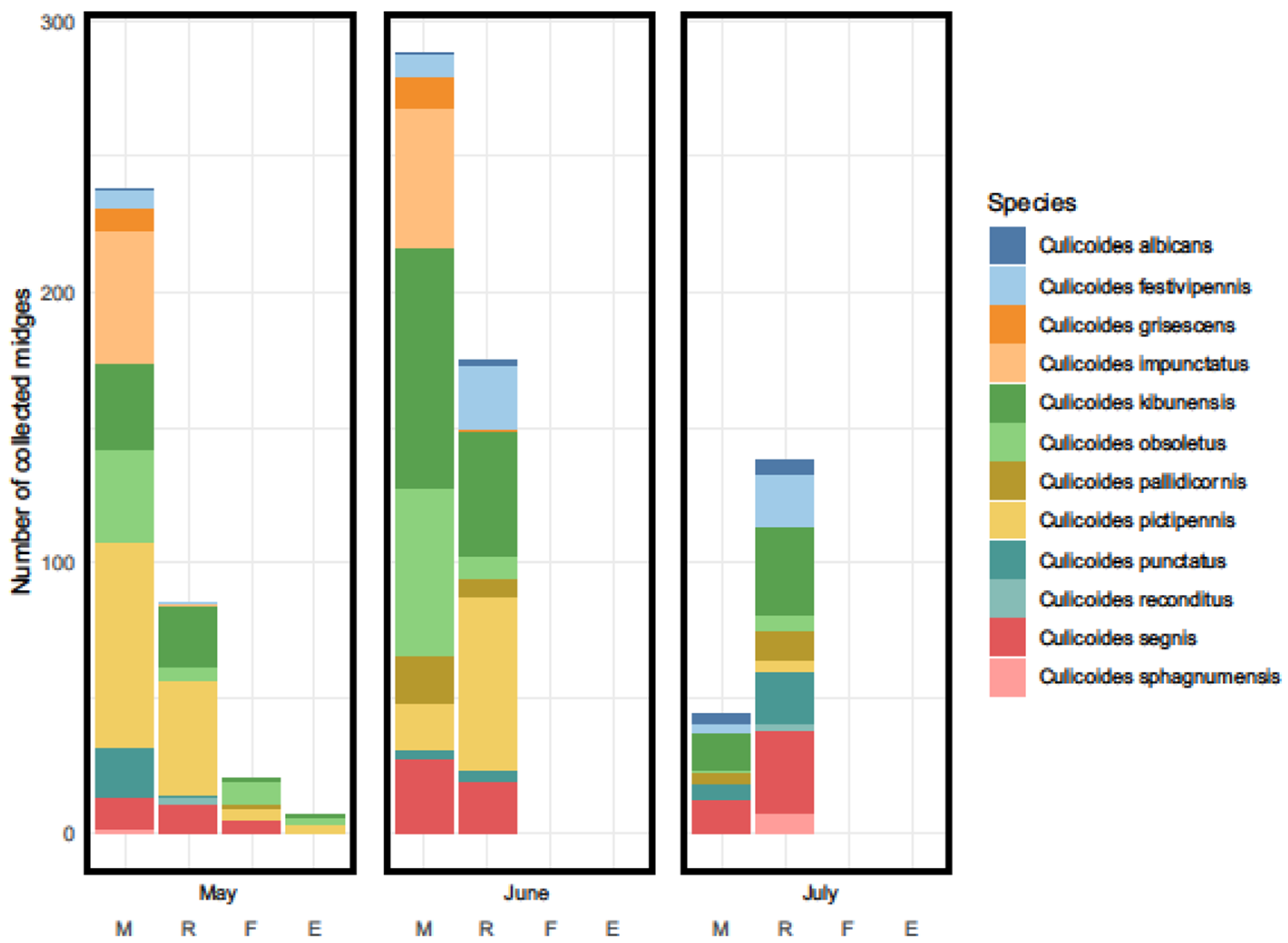
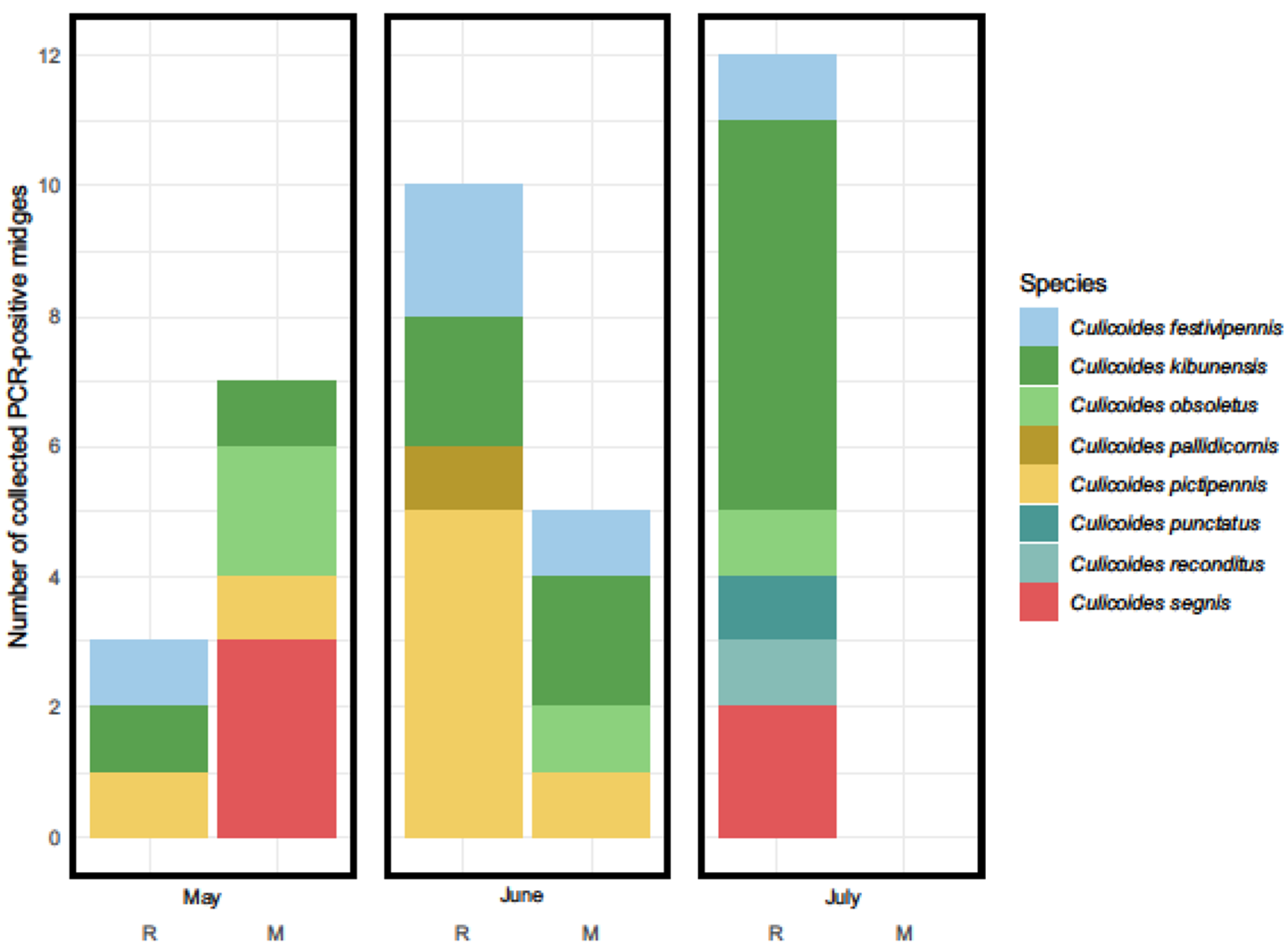
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Valkiūnas, G. Avian Malaria Parasites and Other Haemosporidia; CRC Press: Boca Raton, FL, USA, 2005; ISBN 978-0415300971. [Google Scholar]
- Muriel, J.; González-Blázquez, M.; Matta Cahacho, N.E.; Vargas-León, C.M.; Marzal, A. Parasitas Sanguíneos de Anfíbios; Editora da Universidade Federal do Piauí: Teresina, Brazil, 2021. [Google Scholar]
- Bensch, S.; Hellgren, O.; Pérez-Tris, J. MalAvi: A public database of malaria parasites and related haemosporidians in avian hosts based on mitochondrial cytochrome b lineages. Mol. Ecol. Resour. 2009, 9, 1353–1358. [Google Scholar] [CrossRef] [PubMed]
- Valkiūnas, G.; Iezhova, T.A. Keys to the avian Haemoproteus parasites (Haemosporida, Haemoproteidae). Malar. J. 2022, 21, 269. [Google Scholar] [CrossRef] [PubMed]
- Clark, N.J. Phylogenetic uniqueness, not latitude, explains the diversity of avian blood parasite communities worldwide. Glob. Ecol. Biogeogr. 2018, 27, 744–755. [Google Scholar] [CrossRef]
- Bennett, G.F.; Peirce, M.A.; Ashford, R.W. Avian haematozoa: Mortality and pathogenicity. J. Nat. Hist. 1993, 27, 993–1001. [Google Scholar] [CrossRef]
- Palinauskas, V.; Iezhova, T.A.; Križanauskienė, A.; Markovets, M.Y.; Bensch, S.; Valkiūnas, G. Molecular characterization and distribution of Haemoproteus minutus (Haemosporida, Haemoproteidae): A pathogenic avian parasite. Parasitol. Int. 2013, 62, 358–363. [Google Scholar] [CrossRef]
- Duc, M.; Ilgūnas, M.; Kubiliūnaitė, M.; Valkiūnas, G. First report of Haemoproteus (Haemosporida, Haemoproteidae) megalomeronts in the brain of an avian host, with description of megalomerogony of Haemoproteus pastoris, the blood parasite of the common starling. Animals 2021, 11, 2824. [Google Scholar] [CrossRef]
- Ortiz-Catedral, L.; Brunton, D.; Stidworth, M.F.; Elsheikha, H.M.; Pennycott, T.; Schulze, C.; Braun, M.; Wink, M.; Gerlach, H.; Pendl, H.; et al. Haemoproteus minutus is highly virulent for Australasian and South American parrots. Parasites Vectors 2019, 12, 40. [Google Scholar] [CrossRef]
- Fallis, A.M.; Wood, D.M. Biting midges (Diptera: Ceratopogonidae) as intermediate hosts for Haemoproteus of ducks. Ibidem 1957, 35, 425–435. [Google Scholar] [CrossRef]
- Žiegytė, R.; Valkiūnas, G. Recent advances in vector studies of avian haemosporidian parasites. Ekologija 2014, 60, 73–83. [Google Scholar] [CrossRef]
- Bukauskaitė, D.; Iezhova, T.A.; Ilgūnas, M.; Valkiūnas, G. High susceptibility of the laboratory-reared biting midges Culicoides nubeculosus to Haemoproteus infections, with review on Culicoides species that transmit avian haemoproteids. Parasitology 2019, 146, 333–341. [Google Scholar] [CrossRef]
- Köchling, K.; Schaub, G.A.; Werner, D.; Kampen, H. Avian Plasmodium spp. and Haemoproteus spp. parasites in mosquitoes in Germany. Parasites Vectors 2023, 16, 369. [Google Scholar] [CrossRef] [PubMed]
- Bobeva, A.; Zehtindjiev, P.; Bensch, S.; Radrova, J. A survey of biting midges of the genus Culicoides Latreille, 1809 (Diptera: Ceratopogonidae) in NE Bulgaria, with respect to transmission of avian haemosporidians. Acta Parasitol. 2013, 58, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Bobeva, A.; Ilieva, M.; Dimitrov, D.; Zehtindjiev, P. Degree of associations among vectors of the genus Culicoides (Diptera: Ceratopogonidae) and host bird species with respect to haemosporidian parasites in NE Bulgaria. Parasitol. Res. 2014, 113, 4505–4511. [Google Scholar] [CrossRef] [PubMed]
- Ferraguti, M.; Martínez-de la Puente, J.; Ruiz, S.; Soriguer, R.; Figuerola, J. On the study of the transmission networks of blood parasites from SW Spain: Diversity of avian haemosporidians in the biting midge Culicoides circumscriptus and wild birds. Parasites Vectors 2013, 6, 208. [Google Scholar] [CrossRef]
- Veiga, J.; Martinez-de la Pueante, J.; Vaclav, R.; Figuerola, J.; Valera, F. Culicoides paolae and C. circumscriptus as potential vectors of avian haemosporidians in an arid ecosystem. Parasites Vectors 2018, 11, 524. [Google Scholar] [CrossRef]
- Inumaru, M.; Nakamura, K.; Odagawa, T.; Suzuki, M.; Murata, K.; Sato, Y. The first detection of avian haemosporidia from Culicoides biting midges in Japan, with notes on potential vector species and the transmission cycle. Vet. Parasitol. 2023, 39, 100840. [Google Scholar] [CrossRef]
- Bernotienė, R.; Žiegytė, R.; Vaitkutė, G.; Valkiūnas, G. Identification of a new vector species of avian haemoproteids, with a description of methodology for the determination of natural vectors of haemosporidian parasites. Parasites Vectors 2019, 12, 307. [Google Scholar] [CrossRef]
- Njabo, K.Y.; Cornel, A.J.; Sehgal, R.N.; Loiseau, C.; Buermann, W.; Harrigan, R.J.; Pollinger, J.; Valkiūnas, G.; Smith, T.B. Coquillettidia (Culicidae, Diptera) mosquitoes are natural vectors of avian malaria in Africa. Malar. J. 2009, 8, 193. [Google Scholar] [CrossRef]
- Žiegytė, R.; Bernotiene, R. Contribution to the knowledge on black flies (Diptera: Simuliidae) as vectors of Leucocytozoon (Haemosporida) parasites in Lithuania. Parasitol. Int. 2022, 87, 102515. [Google Scholar] [CrossRef]
- Žiegyte, R.; Bernotienė, R.; Palinauskas, V. Culicoides segnis and Culicoides pictipennis Biting Midges (Diptera, Ceratopogonidae), new reported vectors of Haemoproteus parasites. Microorganisms 2022, 10, 898. [Google Scholar] [CrossRef]
- Žiegytė, R.; Palinauskas, V.; Bernotienė, R. Natural vector of avian Haemoproteus asymmetricus parasite and factors altering the spread of infection. Insects 2023, 14, 926. [Google Scholar] [CrossRef] [PubMed]
- Chagas, C.R.F.; Duc, M.; Kazak, M.; Valavičiūtė-Pocienė, K.; Bukauskaitė, D.; Hernández-Lara, C.; Bernotienė, R. High Abundance of Haemoproteus parasites in Culicoides (Diptera, Ceratopogonidae), with a confirmation of Culicoides reconditus as a new vector of these avian blood parasites. Insects 2024, 15, 157. [Google Scholar] [CrossRef] [PubMed]
- Žiegytė, R.; Platonova, E.; Kinderis, E.; Mukhin, A.; Palinauskas, V.; Bernotienė, R. Culicoides biting midges involved in transmission of haemoproteids. Parasites Vectors 2021, 14, 27. [Google Scholar] [CrossRef]
- Gutsevich, A.V. Blood-sucking midges (Ceratopogonidae). In Fauna of the USSR, 1st ed.; Nauka Press: Leningrad, Russia, 1973; Volume 3. [Google Scholar]
- Glukhova, V.M. Blood-sucking midges of the genera Culicoides and Forcipomyia (Ceratopogonidae). In Fauna of the USSR. Dipteran Insects; Nauka: Leningradskoe Otdelenie: Leningrad, Russia, 1989; Volume 3. [Google Scholar]
- Mathieu, B.; Ceêtre-Sossah, C.; Garros, C.; Chavernac, D.; Balenghien, T.; Carpenter, S.; Setier-Rio, M.L.; Vignes-Lebbe, R.; Ung, V.; Candolfi, E.; et al. Development and validation of IIKC: An interactive identification key for Culicoides (Diptera: Ceratopogonidae) females from the Western Palaearctic region. Parasites Vectors 2012, 5, 137. [Google Scholar] [CrossRef]
- Sambrook, J.; Fritsch, E.F.; Maniatis, T. Molecular Cloning: A Laboratory Manual, 2nd ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 1989. [Google Scholar]
- Hellgren, O.; Waldenstrom, J.; Bensch, S. A new PCR assay for simultaneous studies of Leucocytozoon, Plasmodium, and Haemoproteus from avian blood. J. Parasitol. 2004, 90, 797–802. [Google Scholar] [CrossRef]
- Drovetski, S.V.; Aghayan, S.A.; Mata, V.A.; Lopes, R.J.; Mode, N.A.; Harvey, J.A.; Voelker, G. Does the niche breadth or trade–off hypothesis explain the abundance–occupancy relationship in avian Haemosporidia? Mol. Ecol. 2014, 23, 3322–3329. [Google Scholar] [CrossRef]
- Hall, T.A. A user-friendly biological sequence alignment editor and analysis program for Windows 98/98/NT. Nucleic. Acid. Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar]
- Glukhova, V.M.; Valkiūnas, G. On the fauna and ecology of biting midges (Ceratopogonidae: Culicoides) in the Curonian spit, the methods of their collection from the birds and experimental infection with haemoproteids (Haemosporidia: Haemoproteidae). Ekologija 1993, 2, 68–73. [Google Scholar]
- Liutkevičius, G. The new data on the epidemiology of bird haemoproteids (Haemosporida: Haemoproteidae) on the Curonian Spit. Acta Zool. Lithuan. 2000, 2, 72–77. [Google Scholar] [CrossRef]
- Bernotienė, R. New data on the fauna of biting midges (Diptera, Ceratopogonidae) from Lithuania. Acta Zool. Lithuan. 2002, 12, 288–293. [Google Scholar] [CrossRef]
- Kazak, M.; Valavičiūtė-Pocienė, K.; Kondrotaitė, S.; Duc, M.; Bukauskaitė, D.; Hernández-Lara, C.; Bernotienė, R.; Chagas, C.R.F. Culicoides biting midges feeding behaviour as a key for understanding avian Haemoproteus transmission in Lithuania. J. Med. Entomol. 2024, 38, 530–541. [Google Scholar] [CrossRef] [PubMed]
- Chagas, C.R.F.; Hernández-Lara, C.; Duc, M.; Valavičiūtė-Pocienė, K.; Bernotienė, R. What can haemosporidian lineages found in Culicoides biting midges tell us about their feeding preferences? Diversity 2022, 14, 957. [Google Scholar] [CrossRef]
- Bernotienė, R. Peculiarities of biting midges (Ceratopogonidae) distribution and biodiversity in forest habitats. Miškininkystė 2006, 1, 35–42. [Google Scholar]
- Mands, V.; Kline, D.L.; Blackwell, A. Culicoides midge trap enhancement with animal odour baits in Scotland. Med. Vet. Entomol. 2004, 18, 336–342. [Google Scholar] [CrossRef]
- Ander, M.; Meiswinkel, R.; Chirico, J. Seasonal dynamics of biting midges (Diptera: Ceratopogonidae: Culicoides), the potential vectors of bluetongue virus, in Sweden. Vet. Parasitol. 2012, 184, 59–67. [Google Scholar] [CrossRef]
- Bernotienė, R.; Bartkevičienė, G.; Bukauskaitė, D. The flying activity of biting midges (Ceratopogonidae: Culicoides) in Verkiai Regional Park, southeastern Lithuania. Parasites Res. 2021, 120, 2323–2332. [Google Scholar] [CrossRef]
- Sokolov, L.V.; Shapoval, A.P. The long-term dynamics of the ratio of adult and young birds in the near and distant migrants during autumn migration on the Curonian Spit, the Baltic Sea. Acta Biol. Sib. 2018, 4, 71–80. [Google Scholar] [CrossRef]
- Milián-García, Y.; Hempel, C.A.; Janke, L.A.A.; Young, R.G.; Furukawa-Stoffer, T.; Ambagala, A.; Steinke, D.; Hanner, R.H. Mitochondrial genome sequencing, mapping, and assembly benchmarking for Culicoides species (Diptera: Ceratopogonidae). BMC Genom. 2022, 23, 584. [Google Scholar] [CrossRef]
- Pacheco, M.A.; Cepeda, A.S.; Bernotienė, R.; Lotta, I.A.; Matta, N.E.; Valkiūnas, G.; Escalante, A.A. Primers targeting mitochondrial genes of avian haemosporidians: PCR detection and differential DNA amplification of parasites belonging to different genera. Int. J. Parasitol. 2018, 48, 657–670. [Google Scholar] [CrossRef]
- Valkiūnas, G.; Ilgūnas, M.; Bukauskaitė, D.; Duc, M.; Iezhova, T.A. Description of Haemoproteus asymmetricus n. sp. (Haemoproteidae), with remarks on predictability of the DNA haplotype networks in haemosporidian parasite taxonomy research. Acta Trop. 2021, 218, 105905. [Google Scholar] [CrossRef] [PubMed]
- Hellgren, O.; Waldenström, J.; Peréz-Tris, J.; Szöll, E.; Si, O.; Hasselquist, D.; Križanauskienė, A.; Ottosson, U.; Bensch, S. Detecting shifts of transmission areas in avian blood parasites: A phylogenetic approach. Mol. Ecol. 2007, 16, 1281–1290. [Google Scholar] [CrossRef] [PubMed]
- Yusupova, D.A.; Schumm, Y.R.; Sokolov, A.A.; Quillfeldt, P. Haemosporidian blood parasites of passerine birds in north-western Siberia. Polar Biol. 2023, 46, 497–511. [Google Scholar] [CrossRef]
- Mata, V.A.; da Silva, L.P.; Lopes, R.J.; Drovetski, S.V. The Strait of Gibraltar poses an effective barrier to host-specialised but not to host-generalised lineages of avian Haemosporidia. Int. J. Parasitol. 2015, 45, 711–719. [Google Scholar] [CrossRef]
- Šujanová, A.; Špitalská, E.; Václav, R. Seasonal dynamics and diversity of haemosporidians in a natural woodland bird community in Slovakia. Diversity 2021, 13, 439. [Google Scholar] [CrossRef]
- Himmel, T.; Harl, J.; Matt, J.; Weissenböck, H. A citizen science-based survey of avian mortality focusing on haemosporidian infections in wild passerine birds. Malar. J. 2021, 20, 417. [Google Scholar] [CrossRef]
- Strehmann, F.; Becker, M.; Lindner, K.; Masello, J.F.; Quillfeldt, P.; Schumm, Y.R.; Farwig, N.; Schabo, D.G.; Rösner, S. Half of a forest bird community infected with haemosporidian parasites. Front. Ecol. Evol. 2023, 11, 1107736. [Google Scholar] [CrossRef]
- Santiago-Alarcon, D.; Havelka, P.; Schaefer, H.M.; Segelbacher, G. Bloodmeal analysis reveals avian Plasmodium infections and broad host preferences of Culicoides (Diptera: Ceratopogonidae) vectors. PLoS ONE 2012, 7, e31098. [Google Scholar] [CrossRef]
- Pramual, P.; Jomkumsing, P.; Jumpato, W.; Bunauea, S. Molecular detection of avian haemosporidian parasites in biting midges (Diptera: Ceratopogonidae) from Thailand. Acta Trop. 2021, 224, 106118. [Google Scholar] [CrossRef]

| Species of Biting Midges | Reeds (Rybachy Point, R) | Mixed Wet Forest (Dune Müller Point, M) | ||
|---|---|---|---|---|
| % (N) | Found Genetic Lineages of Parasites | % (N) | Found Genetic Lineages of Parasites | |
| Culicoides albicans | 1.05% (6) | 2.26% (9) | ||
| C. grisescens | 3.51% (20) | 0.25% (1) | ||
| C. festivipennis | 3.33% (19) | hTURDUS2 (1) | 10.8% (43) | H. asymmetricus hTUPHI01 (2), hTURDUS2 (1), lSISKIN2 |
| C. impunctatus | 17.54% (100) | 0.25% (1) | ||
| C. kibunensis | 23.51% (134) | lROFL6 (2), hBRAM1 (1) | 25.38% (101) | hHAWF6 (1), H. parabelopolskyi hSYAT02 (3), H. asymmetricus hTUPHI01 (4), hBRAM1 (1) |
| Obsoletus Complex | 17.01% (97) | lBT5 (1), lSISKIN2 (2) | 5.03% (20) | lBT5 (1) |
| C. pallidicornis | 3.86% (22) | 4.27% (17) | ||
| C. pictipennis | 16.32% (93) | H. belopolskyi hHIICT1 (1), H. parabelopolskyi hSYAT02 (1) | 27.64% (110) | H. parabelopolskyi hSYAT02 (5) |
| C. punctatus | 27 (4.74) | 6.03% (24) | hBRAM1 (1) | |
| C. reconditus | 0 | 1.51% (6) | hBRAM1 (1) | |
| C. segnis | 8.95% (51) | hBRAM1 (3) | 14.8 new | H. belopolskyi hHIICT1 (1), lSISKIN2 (1) |
| 2 (59) | ||||
| C. sphagnumensis | 0.18% (1) | 1.76% (7) | ||
| Total | 100% (570) | 100% (398) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Platonova, E.; Erokhina, M.; Mukhina, A.; Davydov, A.; Mukhin, A. The Study of Biting Midges Culicoides Latreille, 1809 (Diptera: Ceratopogonidae) and the Prevalence of Haemoproteus Kruse, 1890 (Haemosporida: Haemoproteidae) on the Curonian Spit of the Baltic Sea. Diversity 2024, 16, 723. https://doi.org/10.3390/d16120723
Platonova E, Erokhina M, Mukhina A, Davydov A, Mukhin A. The Study of Biting Midges Culicoides Latreille, 1809 (Diptera: Ceratopogonidae) and the Prevalence of Haemoproteus Kruse, 1890 (Haemosporida: Haemoproteidae) on the Curonian Spit of the Baltic Sea. Diversity. 2024; 16(12):723. https://doi.org/10.3390/d16120723
Chicago/Turabian StylePlatonova, Elena, Maria Erokhina, Alexandra Mukhina, Alexander Davydov, and Andrey Mukhin. 2024. "The Study of Biting Midges Culicoides Latreille, 1809 (Diptera: Ceratopogonidae) and the Prevalence of Haemoproteus Kruse, 1890 (Haemosporida: Haemoproteidae) on the Curonian Spit of the Baltic Sea" Diversity 16, no. 12: 723. https://doi.org/10.3390/d16120723
APA StylePlatonova, E., Erokhina, M., Mukhina, A., Davydov, A., & Mukhin, A. (2024). The Study of Biting Midges Culicoides Latreille, 1809 (Diptera: Ceratopogonidae) and the Prevalence of Haemoproteus Kruse, 1890 (Haemosporida: Haemoproteidae) on the Curonian Spit of the Baltic Sea. Diversity, 16(12), 723. https://doi.org/10.3390/d16120723






