Plant-Parasitic and Free-Living Nematode Community Associated with Oak Tree of Magoebaskloof Mountains, Limpopo Province, South Africa
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Sampling
2.2. Nematode Diagnosing
2.3. Soil Properties Analysis
2.4. Statistical Analysis
3. Results
3.1. Diversity of Nematodes
3.1.1. Plant-Parasitic Nematodes
3.1.2. Free-Living Nematodes
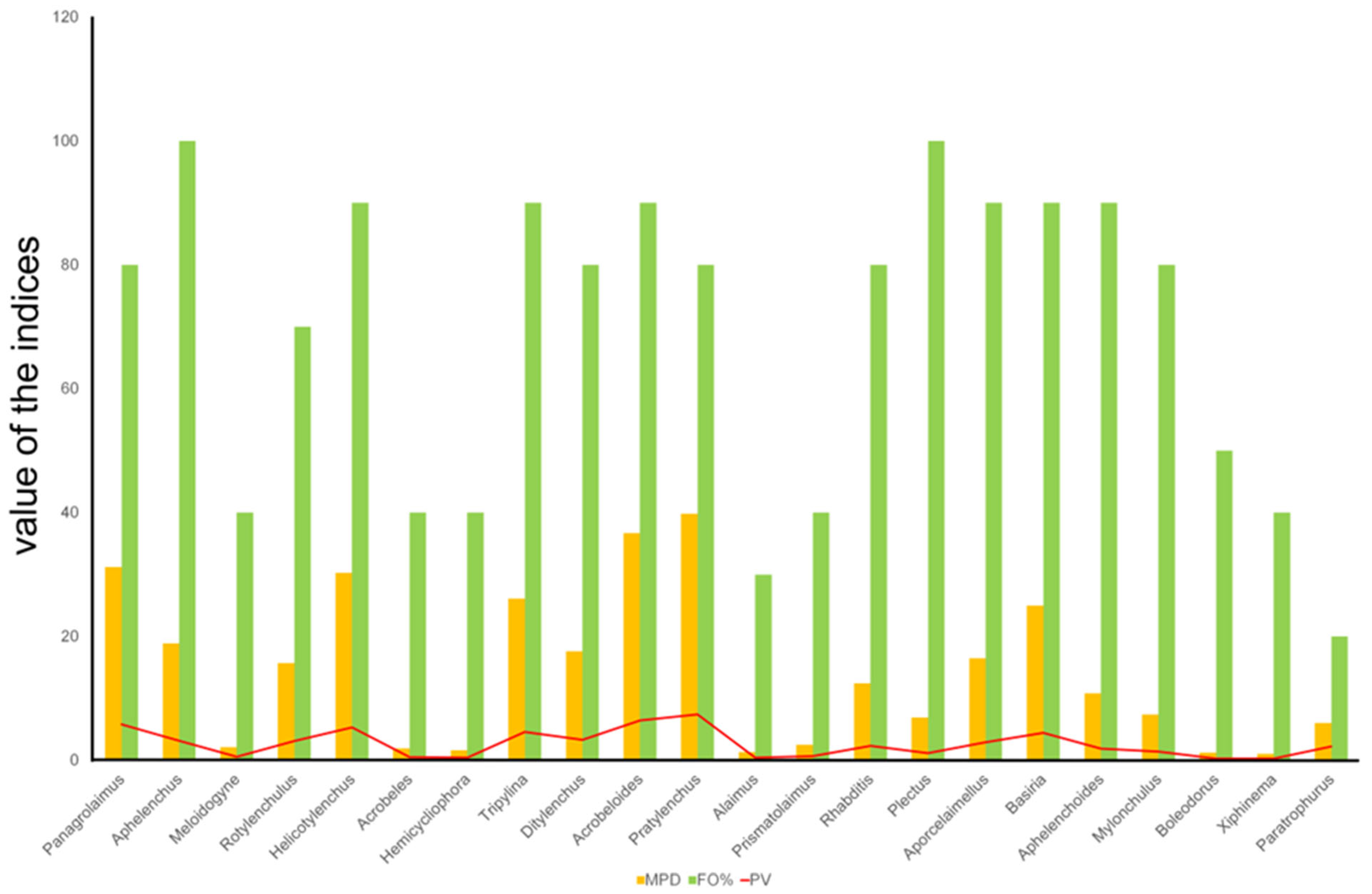
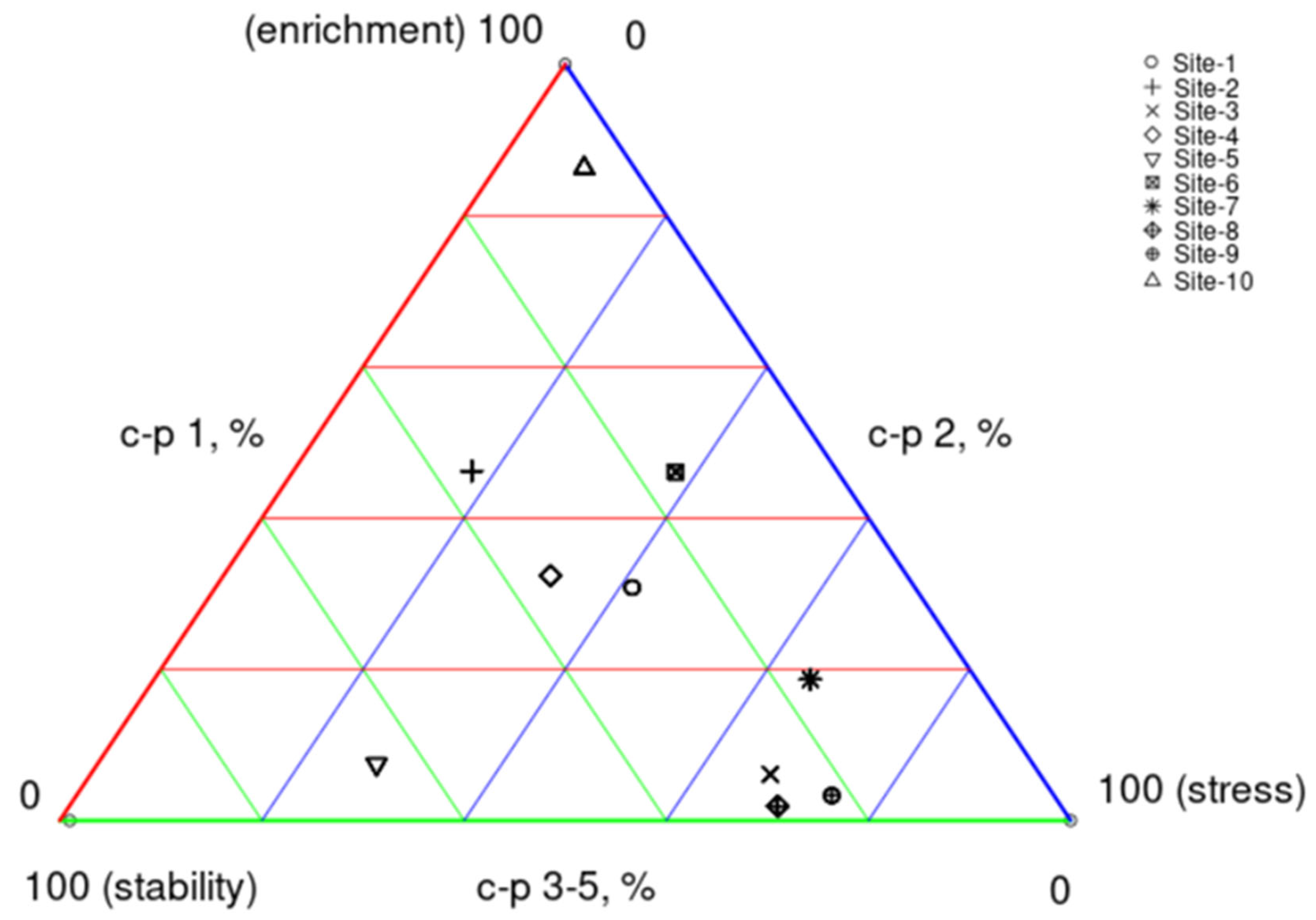
3.2. Indices of the Nematode Communities
3.2.1. Plant-Parasitic Nematodes
3.2.2. Free-Living Nematodes
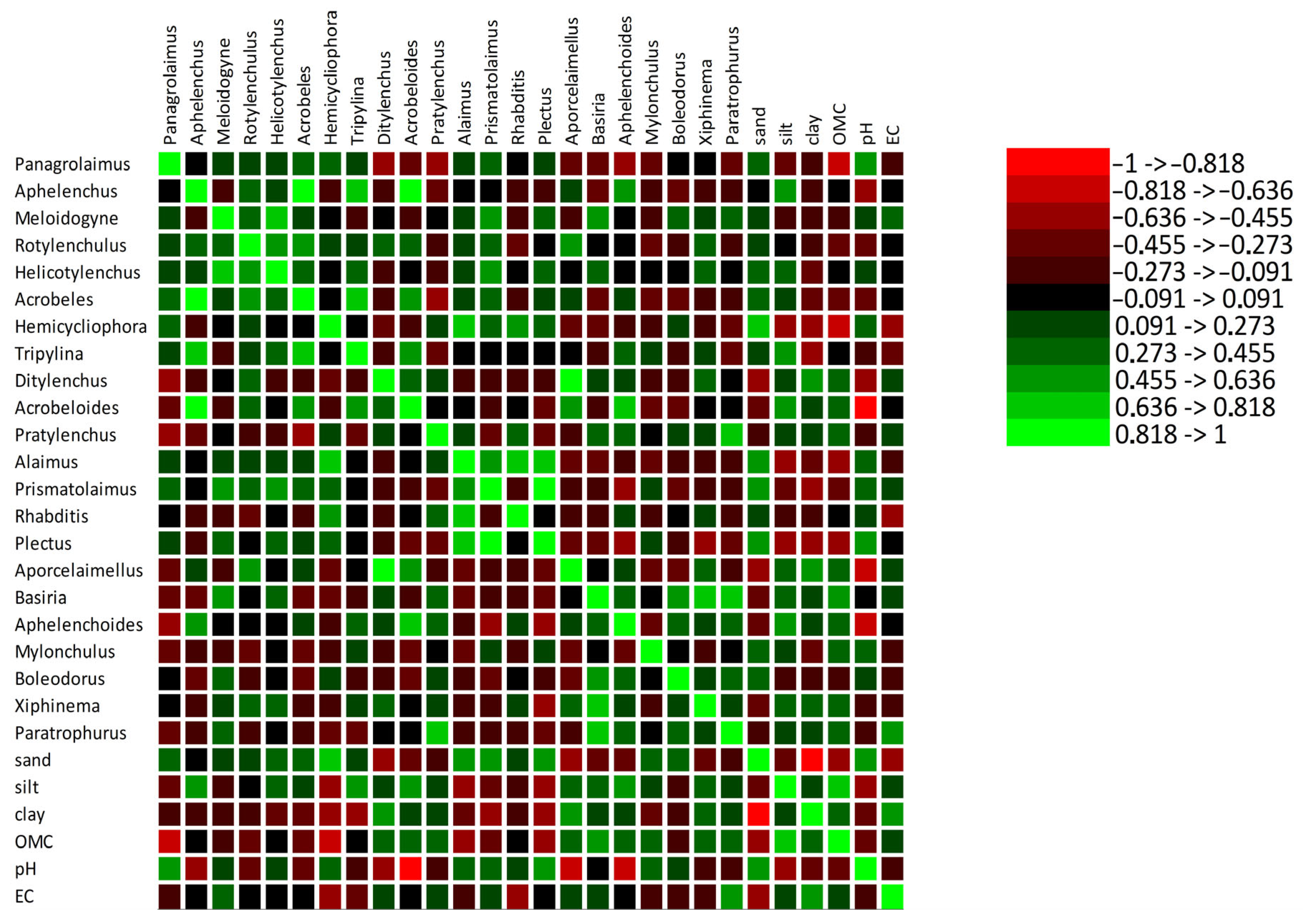
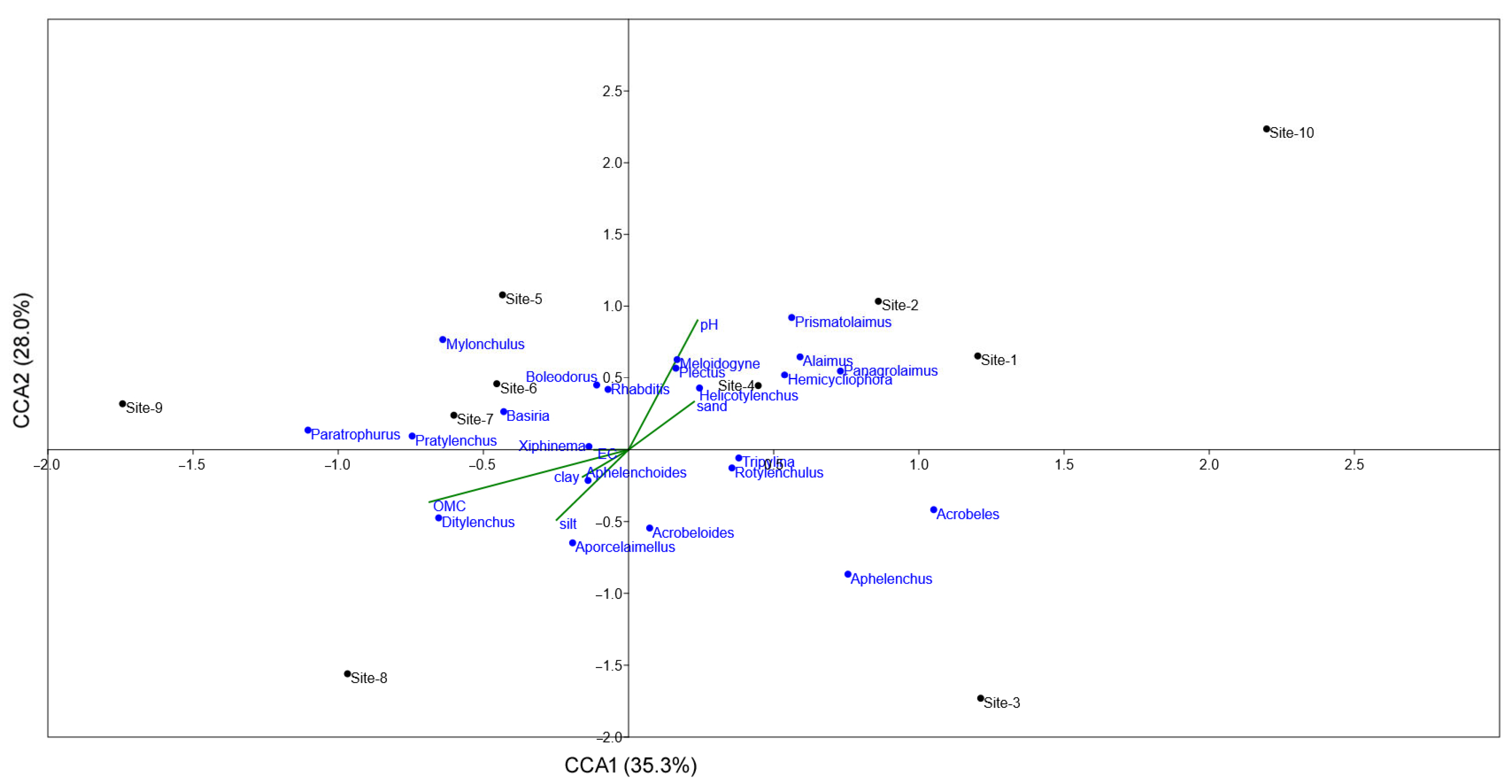
3.3. Correlation Between Soil Parameters and Nematodes
3.3.1. Plant-Parasitic Nematodes
3.3.2. Free-Living Nematodes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pimentel, C.; Khan, M.R.; Zheng, Y.; Quintanilla, M. Nematode problems in forests and their sustainable management. In Nematode Diseases of Crops and Their Sustainable Management; Khan, M.R., Quintanilla, M., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 457–493. [Google Scholar]
- Hogan, C.M. Oak. In Encyclopedia of Earth; National Council for Science and the Environment: Washington, DC, USA, 2012; Available online: https://en.wikipedia.org/wiki/Oak (accessed on 1 January 2024).
- Dianne, D.J. What Are Oak Trees Used for? 2019. Available online: https://sciencing.com/oak-trees-used-for-7222170.html (accessed on 9 October 2023).
- Bakonyi, G.; Nagy, P.; Kovacs-Lang, E.; Kovacs, E.; Barabas, S.; Repasi, V.; Seres, A. Soil nematode community structure as affected by temperature and moisture in a temperate semiarid shrubland soil nematode community structure as affected by temperature. Appl. Soil Ecol. 2007, 37, 31–40. [Google Scholar] [CrossRef]
- Spedicato, A.; Zeppilli, D.; Thouzeau, G.; Michaud, E. Nematode diversity patterns in mangroves: A review of environmental drivers at different spatial scales. Biodivers. Conserv. 2023, 32, 1451–1471. [Google Scholar] [CrossRef]
- Zullini, A.; Semprucci, F. Morphological differences between free-living soil and freshwater nematodes in relation to their environments. Nematology 2020, 22, 125–132. [Google Scholar] [CrossRef]
- Nickle, W.R. A Taxonomic Review of the Genera of the Aphelenchoidea (Fuchs, 1937) Thorne, 1949 (Nematoda: Tylenchida). J. Nematol. 1970, 2, 375–392. [Google Scholar]
- Baldwin, J.G.; Nadler, S.A.; Adams, B.J. Evolution of plant parasitism among nematodes. Ann. Rev. Phytopathol. 2004, 42, 83–105. [Google Scholar] [CrossRef]
- Ferris, H.; Venette, R.C.; van der Meulen, H.R.; Lau, S.S. Nitrogen mineralization by bacterial feeding nematodes: Verification and measurement. Plant Soil 1998, 203, 159–171. [Google Scholar] [CrossRef]
- Ruehle, J.L. Nematodes and forest trees—Types of damage to tree roots. Annu. Rev. Phytopathol. 1973, 11, 99–118. [Google Scholar] [CrossRef]
- Marais, M.; Swart, A. Plant nematodes in South Africa. 11. Checklist of plant nematodes of the protected areas of KwaZuluNatal. Koedoe 2013, 55, 1086. [Google Scholar] [CrossRef]
- Marais, M.; Swart, A. Plant nematodes in South Africa. 6. Tzaneen area, Limpopo Province. Afr. Plant Prot. 2003, 9, 99–107. [Google Scholar]
- van Capelle, C.; Schrader, S.; Brunotte, J. Tillage-induced changes in the functional diversity of soil biota—A review with a focus on German data. Eur. J. Soil Biol. 2012, 50, 165–181. [Google Scholar] [CrossRef]
- Whitehead, A.G.; Hemming, J.R. A Comparison of some quantitative methods extracting small vermiform nematodes from the soil. Ann. Appl. Biol. 1965, 55, 25–38. [Google Scholar] [CrossRef]
- Shokoohi, E. Observation on Hemicriconemoides brachyurus (Loof, 1949) Chitwood & Birchfield, 1957 Associated with Grass in South Africa. Helminthologia 2022, 59, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Andrássy, I. Free-Living Nematodes of Hungary (Nematoda errantia); Hungarian Natural History Museum, Systematic Zoology Research Group of the Hungarian Academy of Sciences: Budapest, Hungary, 2005; Volume 1. [Google Scholar]
- Geraert, E. The Tylenchidae of the World: Identification of the Family Tylenchidae (Nematoda); Academia Press: Ghent, Belgium, 2008. [Google Scholar]
- Shokoohi, E.; Abolafia, J. Soil and Freshwater Rhabditid Nematodes (Nematoda, Rhabditida) from Iran: A Compendium; University of Jaen (UJA) Publishing: Jaén, Spain, 2019; 226p. [Google Scholar]
- Schulte, E.E.; Hopkins, B.G. Estimation of organic matter by weight loss-on-ignition. In Soil Organic Matter: Analysis and Interpretation; SSSA Spec. Publ. 46; Magdoff, F.R., Tabatabai, M.A., Hanlon, E.A., Jr., Eds.; SSSA: Madison, WI, USA, 1996; pp. 21–31. [Google Scholar]
- Agri Laboratory Association of South Africa. Handbook on Feeds and Plant Analyses; Agri Laboratory Association of South Africa: Cape Town, South Africa, 2007. [Google Scholar]
- Norton, D.C.; Schmitt, D.P. Community analyses of plant-parasitic nematodes in the Kalsow Prairie, Iowa. J. Nematol. 1978, 10, 171–176. [Google Scholar]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. Past: Paleontological Statistics Software Package for Education and Data Analysis. Palaeont. Electr. 2001, 4, 9. [Google Scholar]
- Addinsoft. Data Analysis and Statistical Solution for Microsoft Excel; Addinsoft SARL: Paris, France, 2021; Available online: https://www.xlstat.com (accessed on 1 January 2020).
- Zhang, Z.; Zhang, X.; Mahamood, M.; Zhang, S.; Huang, S.; Liang, W. Effect of long-term combined application of organic and inorganic fertilizers on soil nematode communities within aggregates. Sci. Rep. 2016, 6, 31118. [Google Scholar] [CrossRef] [PubMed]
- Sieriebriennikov, B.; Ferris, H.; de Goede, R.G.M. NINJA: An automated calculation system for nematode-based biological monitoring. Eur. J. Soil Biol. 2014, 61, 90–93. [Google Scholar] [CrossRef]
- Ferris, H. Form and function: Metabolic footprints of nematodes in the soil food web. Eur. J. Soil Biol. 2010, 46, 97–104. [Google Scholar] [CrossRef]
- Santamour, F.S. Susceptibility of Oaks to Root-Knot Nematodes. Arboric. Urban For. 1992, 18, 216–219. [Google Scholar] [CrossRef]
- Villain, L. Economic importance, epidemiology and management of Pratylenchus sp. in coffee plantations. In Plant-Parasitic Nematodes of Coffee; Souza, R.M., Ed.; Springer: Dordrecht, The Netherlands, 2009. [Google Scholar] [CrossRef]
- Shokoohi, E. Impact of agricultural land use on nematode diversity and soil quality in Dalmada, South Africa. Horticulturae 2023, 9, 749. [Google Scholar] [CrossRef]
- Shokoohi, E.; Moyo, N.; Gouveia, F. Relationship of nematodes in natural and disturbed land with physicochemical properties in Magoebaskloof, Limpopo Province, South Africa. Biologia 2023, 78, 3223–3233. [Google Scholar] [CrossRef]
- Escalante, O.L.; Brye, K. Relationships among soil properties, nematode densities, and soybean yield in a long-term, double-crop system in eastern Arkansas. Agric. Sci. 2023, 14, 1605–1623. [Google Scholar] [CrossRef]
- McSorley, R.; Frederick, J.J. Effect of subsurface clay on nematode communities in a sandy soil. Appl. Soil Ecol. 2002, 19, 1–11. [Google Scholar] [CrossRef]
- Wallace, H.R. Nematode Ecology and Plant Disease; Arnold, E., Ed.; University of Michigan: Ann Arbor, MI, USA, 1973; 226p. [Google Scholar]
- Dias-Arieira, C.R.; Ceccato, F.J.; Marinelli, E.Z.; Boregio, V.J.L.; de Oliveira, A.G.; Santana-Gomes, S. Correlations between nematode numbers, chemical and physical soil properties, and soybean yield under different cropping systems. Rhizosphere 2021, 19, 100386. [Google Scholar] [CrossRef]
- Schmitt, D.P.; Barker, K.R. Damage and reproductive potentials of Pratylenchus brachyurus and P. penetrans on soybean. J. Nematol. 1981, 13, 327–332. [Google Scholar]
- Quist, C.W.; Gort, G.; Mooijman, P.; Brus, D.J.; van den Elsen, S.; Kostenko, O.; Vervoort, M.; Bakker, J.; van der Putten, W.H.; Helder, J. Spatial distribution of soil nematodes relates to soil organic matter and life strategy. Soil Biol. Biochem. 2019, 136, 107542. [Google Scholar] [CrossRef]
- Griffiths, B.; Neilson, R.; Bengough, A.G. Soil factors determined nematode community composition in a two year pot experiment. Nematology 2003, 5, 889–897. [Google Scholar] [CrossRef]
- Landesman, W.J.; Treonis, A.M.; Dighton, J. Effects of a one-year rainfall manipulation on soil nematode abundances and community composition. Pedobiologia 2011, 54, 87–91. [Google Scholar] [CrossRef]
- Sánchez-Moreno, S.; Cano, M.; Lopez-Perez, A.; Benayas, J.M.R. Microfaunal soil food webs in Mediterranean semi-arid agroecosystems. Does organic management improve soil health? Appl. Soil Ecol. 2018, 125, 138–147. [Google Scholar] [CrossRef]
- Ney, L.; Franklin, D.; Mahmud, K.; Cabrera, M.; Hancock, D.; Habteselassie, M.; Newcomer, Q.; Dahal, S.; Subedi, A. Sensitivity of nematode community analysis to agricultural management practices and inoculation with local effective microorganisms in the southeastern United States. Soil Syst. 2019, 3, 41. [Google Scholar] [CrossRef]
- Ferris, H.; Matute, M.M. Structural and functional succession in the nematode fauna of a soil food web. Appl. Soil Ecol. 2003, 23, 93–110. [Google Scholar] [CrossRef]
- Arosa, M.L.; Freitas, H.; Costa, S.R. Temporal effects dominate land use as factors affecting soil nematode communities in mediterranean oak woodlands. Agrofor. Syst. 2016, 90, 127–136. [Google Scholar] [CrossRef]
| Nematode | C-p Class | P-p * Class | Site-1 | Site-2 | Site-3 | Site-4 | Site-5 | Site-6 | Site-7 | Site-8 | Site-9 | Site-10 | Feeding Type |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Plant-Parasitic | |||||||||||||
| Basiria sp. | 0 | 2 | 7 | 6 | 4 | 66 | 22 | 5 | 46 | 23 | 71 | 0 | Root hair feeders |
| Boleodorus sp. | 0 | 2 | 0 | 3 | 0 | 2 | 0 | 1 | 4 | 0 | 2 | 0 | Root hair feeders |
| Helicotylenchus sp. | 0 | 3 | 75 | 5 | 30 | 80 | 40 | 12 | 24 | 2 | 35 | 0 | Semi-endoparasites |
| Hemicycliophora sp. | 0 | 3 | 4 | 5 | 0 | 1 | 0 | 6 | 0 | 0 | 0 | 0 | Ectoparasites |
| Meloidogyne sp. | 0 | 3 | 7 | 0 | 0 | 4 | 0 | 0 | 6 | 0 | 4 | 0 | Sedentary parasites |
| Paratrophurus sp. | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 9 | 0 | 49 | 2 | Ectoparasites |
| Pratylenchus sp. | 0 | 3 | 4 | 15 | 0 | 9 | 22 | 108 | 35 | 50 | 155 | 0 | Migratory endoparasites |
| Rotylenchulus sp. | 0 | 3 | 35 | 25 | 23 | 30 | 0 | 0 | 1 | 28 | 15 | 0 | Sedentary parasites |
| Xiphinema sp. | 0 | 5 | 0 | 0 | 0 | 5 | 0 | 1 | 0 | 2 | 2 | 0 | Ectoparasites |
| Free-Living | |||||||||||||
| Aphelenchoides sp. | 2 | 0 | 21 | 3 | 116 | 15 | 3 | 13 | 6 | 8 | 2 | 2 | Fungivores |
| Aphelenchus sp. | 2 | 0 | 2 | 6 | 22 | 11 | 2 | 15 | 19 | 13 | 18 | 0 | Fungivores |
| Ditylenchus sp. | 2 | 0 | 8 | 0 | 8 | 19 | 12 | 6 | 21 | 88 | 14 | 0 | Fungivores |
| Acrobeles sp. | 2 | 0 | 6 | 2 | 9 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | Bacterivores |
| Acrobeloides sp. | 2 | 0 | 31 | 4 | 117 | 28 | 9 | 41 | 33 | 72 | 32 | 0 | Bacterivores |
| Alaimus sp. | 4 | 0 | 6 | 1 | 0 | 0 | 0 | 6 | 0 | 0 | 0 | 0 | Bacterivores |
| Panagrolaimus sp. | 1 | 0 | 59 | 52 | 25 | 61 | 4 | 25 | 17 | 0 | 0 | 69 | Bacterivores |
| Plectus sp. | 2 | 0 | 19 | 6 | 3 | 3 | 11 | 8 | 8 | 4 | 2 | 5 | Bacterivores |
| Prismatolaimus sp. | 3 | 0 | 15 | 2 | 0 | 2 | 6 | 0 | 0 | 0 | 0 | 0 | Bacterivores |
| Rhabditis sp. | 1 | 0 | 6 | 3 | 0 | 17 | 6 | 76 | 8 | 5 | 3 | 0 | Bacterivores |
| Aporcelaimellus sp. | 5 | 0 | 13 | 6 | 30 | 25 | 7 | 0 | 2 | 70 | 10 | 2 | Omnivores |
| Mylonchulus sp. | 4 | 0 | 0 | 13 | 1 | 6 | 38 | 2 | 4 | 0 | 8 | 2 | Predators |
| Tripylina sp. | 3 | 0 | 25 | 21 | 78 | 52 | 36 | 27 | 16 | 4 | 2 | 0 | Predators/Bacterivores |
| Index Name | Site-1 | Site-2 | Site-3 | Site-4 | Site-5 | Site-6 | Site-7 | Site-8 | Site-9 | Site-10 | p Value |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sigma Maturity Index | 2.4 ± 0.1 | 2.4 ± 0.2 | 2.4 ± 0.2 | 2.5 ± 0.3 | 2.9 ± 0.1 | 2.2 ± 0.1 | 2.3 ± 0.1 | 2.7 ± 0.2 | 2.3 ± 0.2 | 1.3 ± 0.2 | <0.001 |
| Plant-Parasitic Index | 2.9 ± 0.1 | 2.9 ± 0.1 | 2.9 ± 0.1 | 2.7 ± 0.2 | 2.7 ± 0.3 | 2.9 ± 0.2 | 2.6 ± 0.1 | 2.6 ± 0.1 | 2.3 ± 0.2 | 3.0 ± 0.1 | <0.001 |
| Channel Index | 10.7 ± 0.2 | 3.9 ± 0.1 | 59.4 ± 0.2 | 12.6 ± 0.1 | 29.8 ± 0.2 | 7.8 ± 0.2 | 31.5 ± 0.1 | 84.5 ± 0.1 | 73.9 ± 0.1 | 0.7 ± 0.1 | <0.001 |
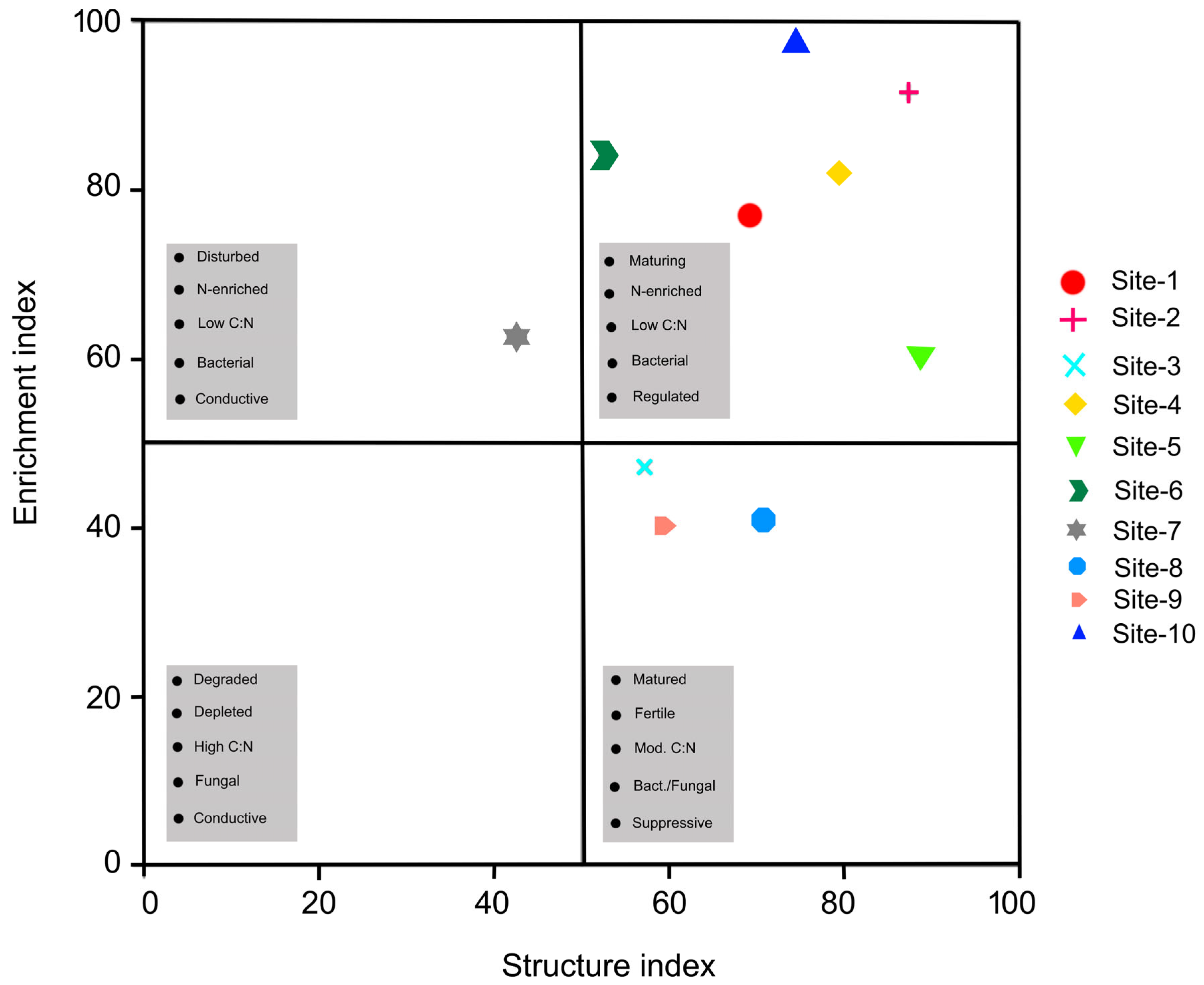
| Enrichment Index | 76.9 ± 0.1 | 91.6 ± 0.3 | 47.2 ± 0.2 | 82.1 ± 0.1 | 60.6 ± 0.3 | 84.1 ± 0.1 | 62.7 ± 0.1 | 41.1 ± 0.1 | 40.4 ± 0.2 | 97.5 ± 0.1 | <0.001 |
| Structure Index | 69.2 ± 0.1 | 87.4 ± 0.2 | 57.2 ± 0.2 | 79.5 ± 0.4 | 88.7 ± 0.3 | 52.8 ± 0.2 | 42.6 ± 0.1 | 70.7 ± 0.1 | 59.3 ± 0.3 | 74.6 ± 0.1 | <0.001 |
| Shannon Index H’ | 2.5 ± 0.2 | 2.0 ± 0.3 | 2.5 ± 0.1 | 2.3 ± 0.2 | 2.1 ± 0.1 | 2.5 ± 0.1 | 2.0 ± 0.2 | 2.1 ± 0.3 | 0.7 ± 0.1 | 2.4 ± 0.1 | <0.001 |
| Total Biomass, mg | 1.0 ± 0.1 | 0.4 ± 0.1 | 0.7 ± 0.3 | 1.0 ± 0.2 | 0.3 ± 0.1 | 0.7 ± 0.3 | 0.7 ± 0.2 | 1.4 ± 0.1 | 0.7 ± 0.2 | 0.1 ± 0.1 | <0.001 |
| Herbivores, % of total | 38.5 | 33.1 | 12.2 | 45 | 38.5 | 37.8 | 48.3 | 68.2 | 91.4 | 2.4 | - |
| Fungivores, % of total | 9 | 5.1 | 31.3 | 10.3 | 7.8 | 9.7 | 17.8 | 13.1 | 3.2 | 2.4 | - |
| Bacterivores, % of total | 41.4 | 39.3 | 33 | 25.8 | 16.5 | 44.3 | 25.5 | 9.8 | 3.5 | 90.2 | - |
| Predators/Omnivores, % of total | 11.1 | 22.5 | 23.4 | 18.9 | 37.2 | 8.2 | 8.5 | 8.9 | 1.9 | 4.9 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shokoohi, E.; Masoko, P. Plant-Parasitic and Free-Living Nematode Community Associated with Oak Tree of Magoebaskloof Mountains, Limpopo Province, South Africa. Diversity 2024, 16, 673. https://doi.org/10.3390/d16110673
Shokoohi E, Masoko P. Plant-Parasitic and Free-Living Nematode Community Associated with Oak Tree of Magoebaskloof Mountains, Limpopo Province, South Africa. Diversity. 2024; 16(11):673. https://doi.org/10.3390/d16110673
Chicago/Turabian StyleShokoohi, Ebrahim, and Peter Masoko. 2024. "Plant-Parasitic and Free-Living Nematode Community Associated with Oak Tree of Magoebaskloof Mountains, Limpopo Province, South Africa" Diversity 16, no. 11: 673. https://doi.org/10.3390/d16110673
APA StyleShokoohi, E., & Masoko, P. (2024). Plant-Parasitic and Free-Living Nematode Community Associated with Oak Tree of Magoebaskloof Mountains, Limpopo Province, South Africa. Diversity, 16(11), 673. https://doi.org/10.3390/d16110673







