Abstract
Genetic diversity and ecology are two important aspects of vector species crucial to a full understanding of disease epidemiology. In this study, we examined genetic diversity, genetic structure and the physiochemical parameters of the breeding habitats of the two significant black fly vector species, Simulium chumpornense Takaoka and Kuvangkadilok and S. nodosum Puri, from Laos. Genetic diversity of S. chumpornense in Laos was relatively high with maximum genetic divergence of 3.05% but no significant genetic differentiation between populations. Comparisons with conspecific populations from Thailand also found no genetic differentiation between the two countries. This possibly related to the recent history of this species, as a very recent (31,000 years ago) historical population expansion was detected. Physicochemical parameters of the breeding habitats suggest the ability to utilize diverse stream sizes from small flows (1 m wide) to huge rivers (290 m or more wide). Populations of S. nodosum from Laos had low genetic diversity with maximum genetic divergence of 2.56% and no genetic structuring among populations. Comparisons with those reported from other countries revealed five genetically divergent lineages (I–V) with minimum genetic divergence of 1.36%. The majority (42 of 52) of specimens from Laos belonged to lineage I and the remaining (10) comprised lineage II. Lineage I is the largest and representative of specimens from Thailand, Myanmar, Vietnam, Taiwan and Laos. Population history analysis revealed that lineage I had undergone recent demographic expansion dating back to 7000 years ago. This very recent population expansion resembles others reported from Thailand and possibly indicates a response to increasing human and domestic animals following the agricultural revolution. Breeding sites of S. nodosum are diverse in respect to elevation, velocity, water conductivity and streambed particle size. The ability to utilize a wide variety of breeding sites could promote the production of a large adult population, which can be a pest for humans and other animals.
1. Introduction
Understanding the genetic diversity of species is fundamental in biodiversity assessment [1]. This information is particularly important for the organisms involved in disease epidemiology, such as vector species. Levels of genetic diversity can be related to vector competence or involve resistance to a particular control method such as insecticide [2]. Application of genetic markers (e.g., DNA barcode) has uncovered hidden diversity in many vector species such as mosquitoes [3]), sand flies [4] and black flies [5] that are possibly related to vector competency [6]. On the other hand, genetic markers could also be used to solve the uncertainty often encountered in traditional morphological taxonomy, such as that synonymized with morphologically similar but geographically isolated species [7]. In addition to the genetic characterization, ecological aspects of the vector species are also important as they can be used to design appropriate control strategy [8,9].
Black flies (Diptera: Simuliidae) are small (adult wingspan typically <10 mm) [10] and have limited diagnostic morphological characters particularly among closely related species. Therefore, fully understanding biodiversity and accurate species identification often require integration of morphology, and genetic and ecological information [7]. Mitochondrial cytochrome oxidase I (COI) has been extensively employed to elucidate genetic diversity and species identification within several black fly species [11]. This genetic marker has proven particularly effective in uncovering cryptic diversity and determining the species status of geographically isolated populations [12]. For example, DNA barcoding can unequivocally differentiate S. yvonneae Takaoka from and Low S. siamense Takaoka and Suzuki complex. However, morphology of the adults is nearly indistinguishable [13], leading to incorrect identification if reliant only on this character [14].
There are more than 2400 black fly species recorded [15]. Many species are significant pests and vectors of the pathogens causing diseases in human and other animals [10,16,17]. The most significant human disease for which black fly species are vectors is human onchocerciasis, caused by the filarial nematode Onchocerca volvulus. Black flies also transmit the filarial nematode Mansonella ozzardi, the causative agent of mansonellosis in humans. Furthermore, there are at least 13 other filarial species for which black fly species are vectors involved in transmitting the organism to wild and domestic animals [17,18]. Black flies can also transmit other pathogens, including viruses, protozoa and bacteria that can cause diseases in domestic and wild animals such as leucocytozonosis cause by Leucocytozoon spp. [17].
Simulium chumpornense Takaoka and Kuvangkadilok belong to the S. varicorne species group of the subgenus Gomphostilbia [19]. This species was described from Chumporn Province in southern Thailand [20]. Simulium chumpornense is geographically widespread in Thailand but thus far has not been reported in other countries [15]. This species is a chicken biter and a potential vector of an unidentified Leucocytozoon and Trypanosoma avium [21,22,23]. Despite adults being found in many areas at relatively high abundance compared to other species, knowledge of the immature habitats is limited. As of 2024, larvae and pupae of S. chumpornense had only been collected from eight locations in Thailand [24]. The immature stages of S. chumpornense occupy diverse stream sizes from small flows (3 m wide) to huge (400 m wide) rivers, e.g., the Mekong [24].
Genetic diversity has been examined in various populations of S. chumpornense in Thailand and it was found that this species possessed relatively high intraspecific genetic divergence with a maximum value of 4.20%, suggesting the possibility of cryptic diversity [25]. Population genetic study indicated that the high genetic variation is due to the existent of two genetically divergent lineages [26]. Later, one lineage was formally described as a different but morphologically very similar to the species S. khelangense Takaoka, Srisuka and Saeung [27]. This is an example of the necessity of integration of genetic data with morphology for fully understanding black fly biodiversity.
Simulium nodosum Puri belong to the S. nobile species group of the subgenus Simulium. This species was described from India [28] and has been recorded in other countries, namely Bhutan, Myanmar, southern China, Taiwan (=S. shirakii), Thailand and Vietnam. Simulium nodosum is a human and bovid biter [14,29,30,31] and has been implicated as vector of Onchocerca sp. transmitted among ruminants [30]. Ecological study of the preimaginal habitats in Thailand revealed that S. nodosum occurs in streams at various elevations up to 800 m above sea level [32,33,34]. However, this species has also been recorded at higher elevation in Myanmar (958 m) and Vietnam (1439 m) [7]. Immature stages of the S. nodosum also utilize diverse stream sizes (from <0.5 m to 15 m wide) and current velocities (0.11–1.56 m/s) [32]. In contrast to its wide range of ecological niches, cytogenetic study found low genetic diversity, with only two polymorphic inversions found [32]. Low diversity at the cytogenetic level also resembled the low genetic diversity with no genetic structuring that occurs within Thai populations [35].
In this study, we examined genetic diversity, genetic structure and physicochemical parameters of breeding sites of two vector species, S. chumpornense and S. nodosum from Laos. Because these species have not yet been reported in Laos, assessment of the specific status using genetic data in addition to morphology is necessary. Knowledge of physicochemical conditions of the breeding habitats is very important because it can be integrated with morphological and genetic data for determining species status [7]. In addition, ecological conditions of the breeding habitats can also be used to design an appropriate control strategy [8,9].
2. Materials and Methods
2.1. Specimen Collection and Identification
Black fly specimens were collected from 12 sampling sites in three provinces of Laos (Table 1 and Figure 1) between April and June 2024. Larvae and pupae were collected by hand from substrates such as fallen leaves and trailing grasses using fine forceps. Larvae and pupae were placed in plastic vials (1.5 mL) containing 80% ethanol and stored at –20 °C until use. Physicochemical parameters of the stream that previous studies [36] found useful for predicting black fly species distribution were measured during specimen collections. These stream variables were width, depth, velocity, pH, water temperature and conductivity, elevation, streambed particle size, canopy cover and riparian vegetation. Measurements of pH, temperature and water conductivity were performed using a ProQuatro handheld multiparameter meter (YSI, Yellow Springs, OH, USA). Current velocity calculation, streambed particle size, canopy cover and riparian vegetation classification followed the method described in McCreadie et al. [37]. Larvae and pupae were identified morphologically using the key features of black flies in nearby countries, Thailand [19] and Vietnam [38].

Table 1.
Sampling location, number of specimens collected, number of specimens used for molecular study, haplotype diversity (h) and nucleotide diversity (π) of Simulium chumpornense and S. nodosum from Laos used in this study.

Figure 1.
Sampling locations of larvae and pupae of Simulium chumpornense and S. nodosum in Laos. Details of sampling sites are given in Table 1.
2.2. Molecular Study
Representative specimens for each sampling location were used for molecular study. However, specimens of S. chumpornense from Meuang Feuang, Vientiane Province (CP657) and S. nodosum from Ban Tha Si, Xaisomboun Province (ND662) and Thathom, Xaisomboun Province (ND664)) were not included because there was a limited number of specimens (<7) and they were geographically very close (<9 km) to adjacent locations. DNA was extracted from the whole individuals using the GF-1 Nucleic Acid Extraction Kit (Vivantis Technologies Sdn. Bhn, Shah Alam, Malaysia). The primers LCO1490 and HCO2198 [39] were used to amplify a 658 bp fragment of the cytochrome c oxidase I (COI) gene. PCR reaction condition analysis followed Tangkawanit et al. [40] using TaKaRa Ex Taq® (Takara Bio Inc., Kusatsu, Japan). PCR products were checked using the 1% agarose gel electrophoresis staining with 1X Novel Juice Loading Dye (GenDirex®, Taiwan, China) and were purified using PureDirex PCR CleanUp & Gel Extraction Kit (Bio-Helix, New Taipei City, Taiwan). Purified PCR products were sent for sequencing at the ATCG Company Limited (Thailand Science Park, Khlong Nueng, Thailand) using the same primers as for PCR.
2.3. Data Analysis
In total, 39 COI sequences (accession nos. PQ386184–PQ386222), 14 from pupa and 25 from larva, were obtained from S. chumpornense. For S. nodosum, 52 COI sequences (accession nos. PQ386229–PQ386280), were obtained from 9 pupae and 43 larvae. Conspecific sequences of S. chumpornense recorded in NCBI GenBank were retrieved and included for data analyses. Three genetic diversity indices, p-distance, haplotype diversity and nucleotide diversity, were calculated. The p-distance values were calculated using TaxonDNA [41]. Haplotype and nucleotide diversities were estimated using Arlequin ver. 3.5 [42]. Genetic relationships between mitochondrial DNA haplotypes were inferred using the median-joining (MJ) network [43] in NETWORK ver. 10.2.0.0 (https://www.fluxus-engineering.com) (accessed on 17 January 2024). Genetic differentiation between populations was calculated based on pairwise FST in Arlequin. Tests of statistical significance were based on 1023 permutations. To reduce the chance of adopting false-positive results from multiple tests, the p-value was adjusted using Bonferroni correction. Mismatch distribution analysis performed in Arlequin was used to test whether the population had undergone recent expansion [44]. Sum-of-square deviation (SSD) and Harpending’s raggedness index [45] were used to test deviation from the sudden population expansion model. If these tests revealed no significant deviation between the observed and simulated data under the sudden expansion model, then historical population expansion is suggested. In addition to the mismatch distribution, the two neutrality tests, Tajima’s D [46] and Fu’s FS [47], were also used. Significant negative values of these tests indicate the signal of population expansion. If the population expansion hypothesis was supported by aforementioned analyses, the expansion time was calculated using equation τ = 2ut (where u = mTμ, mT is the sequences length of the nucleotide under study (623-bp for S. chumpornense and 586-bp for S. nodosum), μ is the mutation rate per nucleotide and t is the generation time [44]). The insect COI gene sequence divergence rate of 3.54% per million years [48] was used. The generation time for tropical black flies was assumed to be 12 generations per year [16,49].
3. Results
3.1. Breeding Habitats
3.1.1. Simulium chumpornense
A total of 217 specimens (51 pupae and 166 larvae) of S. chumpornense (Figure 2) were collected from four locations in Vientiane Province, Laos. All of these sampling sites were at low elevation (<220 m above sea level) but with great variation in width of flow of watercourse, i.e., from small flows (1 m wide) to large rivers (Nam Ngum River, 290 m wide) (Table 2 and Figure 2). Immature stages of S. chumpornense also occurred in a wide range of stream physical conditions. This species was found in current velocities varying from 0.44 m/s to 1.01 m/s with diverse streambed particle characteristics (Table 2).
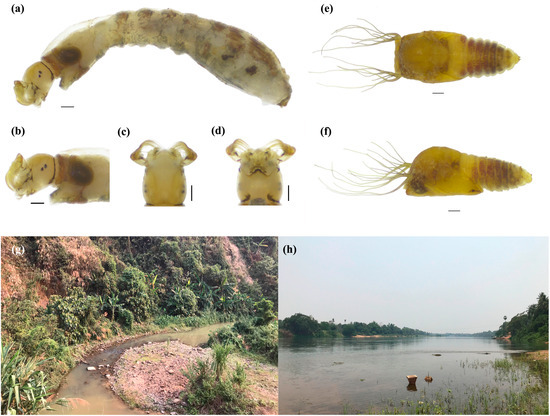
Figure 2.
Larva of Simulium chumpornense (a–d). (a) Whole body (lateral view), (b–d) head capsule (b), lateral view (c) dorsal view, (d) ventral view, (e,f) pupa ((e) dorsal view, (f) lateral view), (g,h) breeding habitats ((g), Huay Kang Chang, Vientiane Province (CP655), (h) Nam Ngum River, Vientiane Province (CP658)). Scale bars 0.2 mm.

Table 2.
Environmental conditions of the breeding habitats of Simulium chumpornense and S. nodosum from Laos collected between April and June 2024.
3.1.2. Simulium nodosum
Immature stages (larvae and pupae) of S. nodosum (Figure 3) were collected from eight sampling sites in two provinces, Xaisomboun and Xiang Khoang. In total, 651 (598 larvae and 53 pupae) were obtained. The larvae and pupae of S. nodosum were found in a wide range of elevations, from 209 m to >1100 m above sea level (Table 2). However, this species was mostly found in small (<10 m) streams, although one sampling site was 30 m wide. Preimaginal stages of S. nodosum utilized a wide range of current velocity values varying from 0.44 m/s to 1.31 m/s. They also occurred in streams with diverse streambed particle characteristics (sand, small stone, rubble, boulder) (Table 2).

Figure 3.
Larva of Simulium nodosum (a–d). (a) Whole body (lateral view), (b–d) head capsule ((b), lateral view, (c), dorsal view, (d) ventral view, (e,f) pupa with cocoon ((e) dorsal view, (f) lateral view), (g,h) breeding habitats (g) Muang Khoune 1, Xiang Khoang Province (ND667), (h) Nam Long, Meuang Thathom, Xaisomboun Province (ND665). Scale bars 0.2 mm.
3.2. Genetic Diversity and Genetic Structure
3.2.1. Simulium chumpornense
In total, 39 specimens from three sampling sites were used for genetic diversity analysis. Intraspecific genetic divergence based on the p-distance model varied between 0% and 3.05%. Comparison with conspecifics from Thailand revealed that the maximum intraspecific genetic divergence slightly increased to 3.21% with two haplotypes shared between S. chumpornense from the two countries. The overall haplotype diversity for S. chumpornense from Laos was 0.9312 and varied between 0.8961 to 1.000 in three sampling sites (Table 1). The overall nucleotide diversity was 0.0075 and varied between 0.0056 to 0.0121 in each sampling site (Table 1). The MJ network (Figure 4) revealed no indication of geographic associations of the genetic cluster for individuals from Thailand and Laos. Haplotypes from the two countries clustered together with two shared haplotypes; one of these was the most common, being shared by 29 individuals (20 from Thailand and 9 from Laos). Population pairwise FST analysis agreed with the MJ network as there was no significant genetic differentiation among three populations of S. chumpornense from Laos (Table 3). Mismatch distribution analysis revealed a bimodal mismatch graph (Figure 5) but there was no statistically significantly deviation from the sudden population expansion model (SSD = 0.0100, p = 0.8500; Harpending’s Raggedness index = 0.0201, p = 0.8700). Historical population expansion was also supported by the significantly negatively Tajima’s D (−2.32626, p = 0.0010) and Fu’s FS (−25.88715, p < 0.0001). The τ value was 8.252 and therefore population expansion time was estimated to be approximately 31,000 years ago.
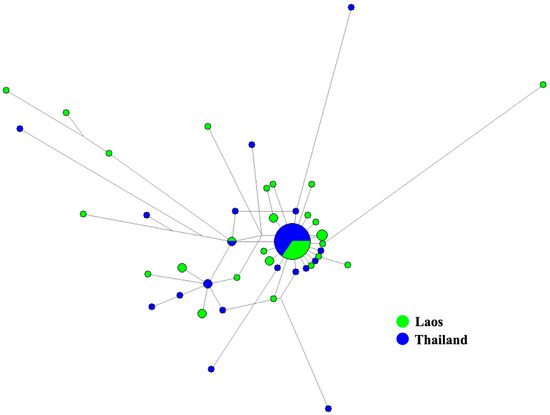
Figure 4.
Median-joining network based on mitochondrial COI sequences of Simulium chumpornense. The COI haplotype is represented by a circle. Sizes of the circle are relative to the number of individuals sharing such a haplotype.

Table 3.
Population pairwise FST values of three populations of Simulium chumpornense from Laos.
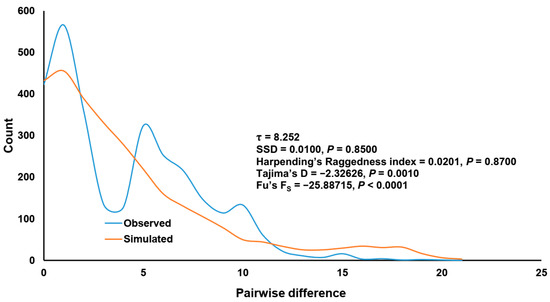
Figure 5.
Mismatch distribution of Simulium chumpornense based on 77 COI sequences (39 from Laos obtained in this study and 38 from Thailand recorded in GenBank). Sum-of-square deviation (SSD), Harpending’s Raggedness index, Tajima’s D and Fu’s FS test values are given.
3.2.2. Simulium nodosum
A total of 52 specimens from six sampling sites of S. nodosum were used for genetic diversity analysis. Intraspecific genetic divergence within S. nodosum varied from 0% to 2.56%. Comparisons with the sequences of S. nodosum from other countries (Thailand, Myanmar, Vietnam and Taiwan) reported previously showed genetic divergence varying from 0% to 3.41%. Maximum genetic divergence was found between S. nodosum from Laos (Xaisomboun Province, ND673) and those from Mae Hong Son Province, Northern Thailand. The overall haplotype diversity of S. nodosum from Laos was 0.7587 and varied between 0.5333 and 0.9000 within six sampling sites. The overall nucleotide diversity of Laos populations was 0.0082 and varied from 0.0018 to 0.0085 (Table 1).
The MJ network calculated from 124 sequences (52 obtained in this study and 72 retrieved from NCBI GenBank) revealed five (I–V) genetic lineages (Figure 6) of S. nodosum with a minimum genetic divergence among lineages of 1.36%. Lineage I was the largest with 104 specimens included and represented members of this species from all countries including Laos. The most common haplotype (n = 47) was shared by specimens from all countries included in this study, also within lineage I. Specimens from Laos were resolved into lineages I and II but the majority (42 of 52) belonged to the former. Lineage II comprised 10 specimens from Laos obtained in this study plus one from Thailand and one from Vietnam. Lineages III and IV were each represented by four specimens, and lineage V was represented by a single haplotype; all were from Mae Hong Son Province, northern Thailand. Population pairwise FST analysis comparing six populations in Laos found no genetically significant differentiations (Table 4).
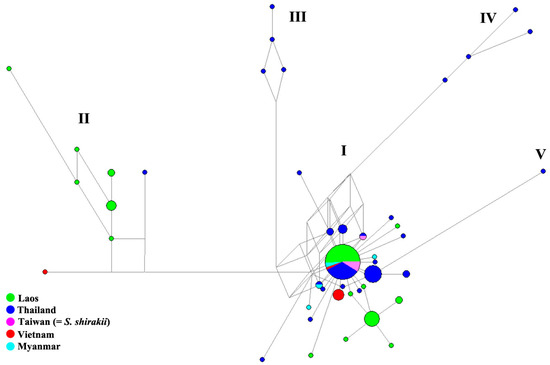
Figure 6.
Median-joining network based on mitochondrial COI sequences of Simulium nodosum. The COI haplotype is represented by a circle. Sizes of the circle are relative to number of individuals sharing such a haplotype.

Table 4.
Population pairwise FST values comparing three populations of Simulium nodosum from Laos.
Mismatch distribution analysis was conducted only for lineage I because other lineages had limited sample sizes (<12). The mismatch graph was unimodal (Figure 7) and there was no statistically significant deviation from simulation under the sudden expansion model (SSD = 0.0002, p = 0.9900; Raggedness index = 0.0275, p = 0.9300). Both neutrality tests, the Tajima’s D (−2.2598, p < 0.00001) and Fu’s FS (−28.0252, p < 0.0001), were significantly negative. Taken together, the evidence indicates that lineage I of S. nodosum has undergone recent population expansion. The population expansion calculated based on a τ value of 1.750 was approximately 7000 years ago.
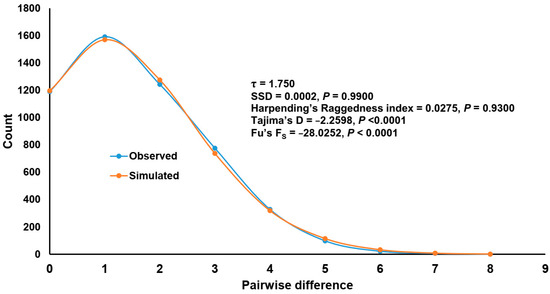
Figure 7.
Mismatch distribution of Simulium nodosum lineage I based on 103 COI sequences (42 from Laos obtained in this study and 61 retrieved from GenBank). Sum-of-square deviation (SSD), Harpending’s Raggedness index, Tajima’s D and Fu’s FS test values are given.
4. Discussion
4.1. Simulium chumpornense
Morphological characteristics of pupa and larva of the specimens identified as S. chumpornense from Laos agree well with the descriptions of this species from Thailand [19]. The mitochondrial COI sequences also supported that they are S. chumpornense, since all of them showed >98.7% sequence similarity to those reported from Thailand. Two haplotypes were shared between specimens from the two countries. One of these shared haplotypes was the most common (shared by 29 of 77 individuals or 38%) including those from the type locality (Kapo waterfall, Chumporn Province, Southern Thailand) with >900 km separation from sampling sites in Laos. Indeed, individuals from all sampling locations in Thailand and Laos shared this haplotype. A geographically widely distribution of the common haplotype with no indication of the genetic isolation in the haplotype network is interpreted as a signal of historical population demographic expansion. Mismatch distribution analysis found no significant deviation from the sudden expansion model and that agreed with the significantly negative neutrality tests. This indicates that populations of S. chumpornense had undergone recent demographic expansion that dated back to near the end of the last glaciations (31,000 years ago). This recent historical expansion was possibly in response to the recovering of climatic conditions from cold and dry during the glaciations to warm and humid, which would have increased the amount of suitable habitats for black flies [49]. Population demographic expansion in SE Asia in response to the Pleistocene climatic change has been reported in many insects including black flies (S. atratum De Meijere) [50], fruit fly (B. correcta) [51], Anopheles mosquitoes [52,53] and the dragonfly (Odonata) Pantala flavescens Fabricius [54].
Simulium chumpornense was described in 2000 in Southern Thailand [19]. The adults have been found in several regions throughout the country but the immature stages have only been recorded at eight locations including the type locality [24]. In this study, we collected larvae and pupae of S. chumpornense from four locations in Vientiane Province, Laos; these are new distribution records for this species. Stream variables of the immature habitats of S. chumpornense from Laos were similar to those reported in Thailand. This species occupies low elevation streams with great variation in sizes from very small flows (1 m wide) to a huge river (Nam Ngum River with 290 m wide) with varying strength of current velocity (from 0.44 m/s to 1.01 m/s). Like those reported from Thailand [24], stream habitats of S. chumpornense in Laos also showed relatively high water conductivity (113–301 µS/cm2). The results of this study therefore support previous findings that S. chumpornense is an eurytopic species that can occupy diverse habitats, enabling it to produce a large adult population [55].
4.2. Simulium nodosum
Simulium nodosum is a geographically widespread species that is distributed in India, Bhutan, southern China, Myanmar, Thailand, Vietnam and Taiwan [15]. In this study, we collected larvae and pupae of S. nodosum from Laos. The COI barcoding sequences of these specimens also supported them being S. nodosum. Although there were genetically divergent lineages within specimens from Laos, the majority (42 of 52) belonged to the main lineage (I) of what is most likely the true S. nodosum, since specimens from other countries, namely, Thailand, Myanmar, Vietnam and Taiwan, were included. Additionally, 23 specimens from Laos, representing all sampling sites, shared this most common core haplotype. Therefore, both morphological characteristics and genetic data indicated that S. nodosum occurs in Laos.
Ten specimens collected from three sampling locations (ND665, ND668 and ND673) from Laos belonged to lineage II, with one sequence from northern Thailand reported by Pramual et al. [56] and one from Vietnam reported by Low et al. [7]. Genetic divergence between lineage I and II was 1.36%. The possible explanation for cryptic genetic divergence within S. nodosum is that these lineages represent different but morphologically and genetically very similar species. The level of genetic divergence between lineages (1.36%) was within the range of intraspecific genetic variation of tropical Asian black flies [25,57]. However, low genetic divergence between closely related species of the S. nobile species group have been reported, such as between S. timorense and S. nobile (1.00–1.27%) [7] and S. vanluni (1.10–1.36%) [58]. In addition, lineage I and II also coexist in four populations (ND665, ND667, ND668 and ND673), indicating the possibility of reproductive isolation [59]. Alternatively, all lineages are the same species but represent the relic of ancestral polymorphisms.
The mismatch distribution analysis indicates that the main lineage (I) of S. nodosum has undergone recent population expansion dating back to approximately 7000 years ago. This expansion time is similar to that report for S. nodosum from Thailand (2600–5200 years ago) although using different genetic loci (COI vs. COII) [35]. The very recent population expansion of S. nodosum possibly relates to the increasing host blood sources, such as from human and domestic animals, following the increase of agriculture approximately 5000–10,000 years ago [35,60]. Study in Thailand found that S. nodosum was the most abundant among black fly species attracted to humans and water buffalo [30,56].
Ecological studies conducted in Thailand have revealed that S. nodosum occupies a diverse range of stream habitats, including those with widths varying from less than 0.5 m to 15 m, current velocities ranging from 0.11 m/s to 1.56 m/s, and elevations ranging from low to intermediate levels (<800 m) [32,33,34]. However, this species was also collected from higher elevations in Myanmar (958 m) and Vietnam (1439 m) [7]. Our finding also supports that S. nodosum also occurs at high elevation streams since 3 of 8 locations were at >1000 m above sea level. In addition to the elevation, the immature habitats of S. nodosum in Laos indicate that this species can utilize diverse physical conditions of the stream habitats. This species occurs in small flows (1 m wide) to large streams (30 m wide) with varying velocity from slow (0.44 m/s) to fast (1.31 m/s), with diverse streambed particles (sand or gravel, small stone, rubble and boulders). However, it mostly occurs in streams lacking canopy cover. The ability to utilize diverse habitats suggests that S. nodosum can produce large populations and potentially become a pest for both human and domestic animals.
In conclusion, based on morphology and molecular genetic data, we found that two black fly vector species, S. chumpornense and S. nodosum, are present in Laos. These are new distribution records for these species. The diverse immature habitats of both species can contribute to the production of large adult populations, which may potentially pose significant pest problems for humans and other animals [58]. Cryptic genetic diversity was found in S. nodosum and this warrants further investigation to assess whether the divergent lineages represent different biological species or intraspecific polymorphisms.
Author Contributions
Conceptualization, I.T., K.I. and P.P.; investigation, B.G., W.J., S.N., K.W., W.W., K.I. and B.M.; formal analysis, W.J. and P.P.; resources, B.G., K.I. and B.M.; data curation, I.T., B.G., W.J. and W.W.; writing—original draft preparation, I.T. and P.P.; writing—review and editing, B.G., S.N. and K.W.; visualization, I.T., B.G., W.W. and P.P.; funding acquisition, P.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research project was financially supported by the Faculty of Science, Mahasarakham University, Thailand (grant no. 6702007).
Institutional Review Board Statement
The animal study protocol was approved by the Institu-tional Animal Care and Use Committee, Mahasarakham University (approval number: IACUC-MSU-31/2024) for studies involving animals.
Data Availability Statement
The sequences have been deposited into the NCBI GenBank under the accession numbers PQ386184–PQ386222 and PQ386229–PQ386280. All other data and materials supporting this article are available from the corresponding author, P. P., upon request.
Acknowledgments
We would like to thank Adrian Plant, of Mahasarakham University, for valuable comments on a previous version of the manuscript. We also would like to thank Chavanut Jaroenchaiwattanachote for assistance with specimen photography.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Mastretta-Yanes, A.; Da Silva, J.M.; Grueber, C.E.; Castillo-Reina, L.; Köppä, V.; Forester, B.R.; Funk, W.C.; Heuertz, M.; Ishihama, F.; Jordan, R.; et al. Multinational evaluation of genetic diversity indicators for the Kunming-Montreal Global Biodiversity Framework. Ecol. Lett. 2024, 27, e14461. [Google Scholar] [CrossRef] [PubMed]
- Powell, J.R. Genetic variation in insect vectors: Death of typology? Insects 2018, 9, 139. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Triana, L.M.; Brugman, V.A.; Nikolova, N.I.; Ruiz-Arrondo, I.; Barrero, E.; Thorne, L.; de Marco, M.F.; Krüger, A.; Lumley, S.; Johnson, N.; et al. DNA barcoding of British mosquitoes (Diptera, Culicidae) to support species identification, discovery of cryptic genetic diversity and monitoring invasive species. ZooKeys 2019, 832, 57–76. [Google Scholar] [CrossRef]
- Rodrigues, B.L.; Carvalho-Costa, L.F.; Pinto, I.D.S.; Rebêlo, J.M.M. DNA barcoding reveals hidden diversity of sand flies (Diptera: Psychodidae) at fine and broad spatial scales in Brazilian endemic regions for leishmaniasis. J. Med. Entomol. 2018, 55, 893–901. [Google Scholar] [CrossRef]
- Jomkumsing, P.; Tangkawanit, U.; Wongpakam, K.; Pramual, P. Who is biting you? DNA barcodes reveal cryptic diversity in human-biting black flies (Diptera: Simuliidae). Acta Trop. 2019, 196, 22–29. [Google Scholar] [CrossRef]
- McCoy, K.D. The population genetic structure of vectors and our understanding of disease epidemiology. Parasite 2008, 15, 444–448. [Google Scholar] [CrossRef]
- Low, V.L.; Adler, P.H.; Sofian-Azirun, M.; Srisuka, W.; Saeung, A.; Huang, Y.T.; Hadi, U.K.; Da Pham, X.; Takaoka, H. Tests of conspecificity for allopatric vectors: Simulium nodosum and Simulium shirakii (Diptera: Simuliidae) in Asia. Parasite Vector 2015, 8, 297. [Google Scholar] [CrossRef]
- Gray, E.W.; Adler, P.H.; Coscaron-Arias, C.; Coscarón, S.; Noblet, R. Development of the first black fly (Diptera: Simuliidae) management program in Argentina and comparison with other programs. J. Am. Mosq. Control Assoc. 1999, 15, 400–406. [Google Scholar]
- Rivers-Moore, N.A.; Hughes, D.A.; De Moor, F.C. A model to predict outbreak periods of the pest blackfly Simulium chutteri Lewis (Simuliidae, Diptera) in the Great Fish River, Eastern Cape Province, South Africa. River Res. Appl. 2008, 24, 132–147. [Google Scholar] [CrossRef]
- Adler, P.H.; Currie, D.C.; Wood, D.M. The Black Flies (Simuliidae) of North America; Cornell University Press: Ithaca, NY, USA, 2004. [Google Scholar]
- Rivera, J.; Currie, D.C. Identification of Nearctic black flies using DNA barcodes (Diptera: Simuliidae). Mol. Ecol. Resour. 2009, 9, 224–236. [Google Scholar] [CrossRef]
- Ruiz-Arrondo, I.; Veiga, J.; Adler, P.H.; Collantes, F.; Oteo, J.A.; Valera, F. Integrated taxonomy of black flies (Diptera: Simuliidae) reveals unexpected diversity in the most arid ecosystem of Europe. PLoS ONE 2023, 18, e0293547. [Google Scholar] [CrossRef] [PubMed]
- Takaoka, H.; Low, V.L.; Tan, T.K.; Ya’cob, Z.; Sofian-Azirun, M.; Dhang Chen, C.; Lau, K.W.; Da Pham, X. A new black fly species of the Simulium (Gomphostilbia) duolongum subgroup (Diptera: Simuliidae) from Vietnam, and molecular comparisons with related species using the COI barcoding gene. J. Med. Entomol. 2019, 56, 432–440. [Google Scholar] [CrossRef]
- Gomontean, B.; Jumpato, W.; Wongpakam, K.; Tangkawanit, U.; Wannasingha, W.; Thanee, I.; Ya’cob, Z.; Pramual, P. Diversity, distribution and host blood meal analysis of adult black flies (Diptera: Simuliidae) from Thailand. Insects 2024, 15, 74. [Google Scholar] [CrossRef] [PubMed]
- Adler, P.H. World Blackflies (Diptera: Simuliidae): A Comprehensive Revision of the Taxonomic and Geographical Inventory [2024]. 2024. Available online: https://biomia.sites.clemson.edu/pdfs/blackflyinventory.pdf (accessed on 31 August 2024).
- Crosskey, R.W. The Natural History of Blackflies; John Wiley & Sons Ltd.: Chichester, UK, 1990. [Google Scholar]
- Adler, P.H.; McCreadie, J.W. Black flies (Simuliidae). In Medical and Veterinary Entomology; Elsevier: San Diego, CA, USA, 2019; pp. 237–259. [Google Scholar]
- Takaoka, H.; Fukuda, M.; Otsuka, Y.; Aoki, C.; Uni, S.; Bain, O. Blackfly vectors of zoonotic onchocerciasis in Japan. Med. Vet. Entomol. 2012, 26, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Takaoka, H.; Srisuka, W.; Saeung, A. Checklist and keys for the black flies (Diptera: Simuliidae) of Thailand. Med. Entomol. Zool. 2019, 70, 53–77. [Google Scholar] [CrossRef]
- Kuvangkadilok, C.; Takaoka, H. Taxonomic notes on Simuliidae (Diptera) from Thailand: Description of a new species and new distributional records of nine known species. Jpn. J. Trop. Med. Hyg. 2000, 28, 167–175. [Google Scholar] [CrossRef]
- Jumpato, W.; Tangkawanit, U.; Wongpakam, K.; Pramual, P. Molecular detection of Leucocytozoon (Apicomplexa: Haemosporida) in black flies (Diptera: Simuliidae) from Thailand. Acta Trop. 2019, 190, 228–234. [Google Scholar] [CrossRef]
- Thaijarern, J.; Tangkawanit, U.; Wongpakam, K.; Pramual, P. Molecular detection of Trypanosoma (Kinetoplastida: Trypanosomatidae) in black flies (Diptera: Simuliidae) from Thailand. Acta Trop. 2019, 200, 105196. [Google Scholar] [CrossRef]
- Pramual, P.; Tangkawanit, U.; Kunprom, C.; Vaisusuk, K.; Chatan, W.; Wongpakam, K.; Thongboonma, S. Seasonal population dynamics and a role as natural vector of Leucocytozoon of black fly, Simulium chumpornense Takaoka & Kuvangkadilok. Acta Trop. 2020, 211, 105617. [Google Scholar]
- Thanee, I.; Jumpato, W.; Jaroenchaiwattanachote, C.; Gomontean, B.; Wannasingha, W.; Namtaku, S.; Adler, P.H.; Pramual, P. Discovery of the larvae and pupae of the black fly Simulium (Gomphostilbia) khelangense and breeding habitats of potential pest species of the S.(G.) chumpornense subgroup (Simuliidae). Insects 2024, 15, 346. [Google Scholar] [CrossRef]
- Pramual, P.; Jomkumsing, P.; Wongpakam, K.; Wongwian, P. DNA barcoding of tropical black flies (Diptera: Simuliidae) in Thailand: One decade of progress. Acta Trop. 2021, 224, 106116. [Google Scholar] [CrossRef] [PubMed]
- Pramual, P.; Jomkumsing, P.; Wathasith, P.; Wongpakam, K. Population structure and population history of the black fly Simulium chumpornense (Diptera: Simuliidae) from Thailand. Acta Trop. 2022, 227, 106301. [Google Scholar] [CrossRef] [PubMed]
- Srisuka, W.; Aupalee, K.; Otsuka, Y.; Fukuda, M.; Takaoka, H.; Saeung, A. A new species of Simulium (Gomphostilbia) (Diptera, Simuliidae) from Thailand, with a key to identify females of 14 species of the Simulium varicorne species-group. ZooKeys 2022, 1083, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Puri, I.M. Studies on Indian Simuliidae. Part VII. Descriptions of larva, pupa and female of Simulium nodosum sp. nov., with an appendix dealing with S. novolineatum nov. nom. (=S. lineatum Puri). Ind. J. Med. Res. 1933, 20, 813–817. [Google Scholar]
- Datta, M. An overview of the Simuliidae (Diptera) of West Bengal. India J. Bengal Nat. Hist. Soc. 1992, 11, 41–62. [Google Scholar]
- Takaoka, H.; Choochote, W.; Aoki, C.; Fukuda, M.; Bain, O. Black flies (Diptera: Simuliidae) attracted to humans and water buffalos and natural infections with filarial larvae, probably Onchocerca sp., in northern Thailand. Parasite 2003, 10, 3–8. [Google Scholar] [CrossRef][Green Version]
- Choochote, W.; Takaoka, H.; Fukuda, M.; Otsuka, Y.; Aoki, C.; Eshima, N. Seasonal abundance and daily flying activity of black flies (Diptera: Simuliidae) attracted to human baits in Doi Inthanon National Park, northern Thailand. Med. Entomol. Zool. 2005, 56, 335–348. [Google Scholar] [CrossRef]
- Tangkawanit, U.; Kuvangkadilok, C.; Trinachartvanit, W.; Baimai, V. Cytotaxonomy, morphology and ecology of the Simulium nobile species group (Diptera: Simuliidae) in Thailand. Cytogenet. Genome Res. 2011, 134, 308–318. [Google Scholar] [CrossRef]
- Srisuka, W.; Takaoka, H.; Otsuka, Y.; Fukuda, M.; Thongsahuan, S.; Taai, K.; Choochote, W.; Saeung, A. Seasonal biodiversity of black flies (Diptera: Simuliidae) and evaluation of ecological factors influencing species distribution at Doi Pha Hom Pok National Park, Thailand. Acta Trop. 2015, 149, 212–219. [Google Scholar] [CrossRef]
- Jitklang, S.; Sawangproh, W.; Kuvangkadilok, C.; Baimai, V.; Adler, P.H. Ecology of black flies (Diptera: Simuliidae) in streams of northern and southern Thailand: Factors associated with larval and pupal distributions. Acta Trop. 2020, 204, 105357. [Google Scholar] [CrossRef]
- Chaiyasan, P.; Pramual, P. Population genetic structure and demographic history of the black fly vector, Simulium nodosum in Thailand. Med. Vet. Entomol. 2016, 30, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Pramual, P.; Kuvangkadilok, C. Agricultural land use and black fly (Diptera, Simuliidae) species richness and species assemblages in tropical streams, Northeastern Thailand. Hydrobiologia 2009, 625, 173–184. [Google Scholar] [CrossRef]
- McCreadie, J.W.; Adler, P.H.; Grillet, M.E.; Hamada, N. Sampling statistics in understanding distributions of black fly larvae (Diptera: Simuliidae). Acta Entomol. Serbica 2006, 11, 89–96. [Google Scholar]
- Takaoka, H.; Sofian-Azirun, M.; Ya’Cob, Z.; Chen, C.D.; Lau, K.W.; Low, V.L.; Da Pham, X.; Adler, P.H. The black flies (Diptera: Simuliidae) of Vietnam. Zootaxa 2017, 4261, 1–165. [Google Scholar] [CrossRef]
- Folmer, O.; Hoeh, W.R.; Black, M.B.; Vrijenhoek, R.C. Conserved primers for PCR amplification of mitochondrial DNA from different invertebrate phyla. Mol. Marine Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar]
- Tangkawanit, U.; Wongpakam, K.; Pramual, P. A new black fly (Diptera: Simuliidae) species of the subgenus Asiosimulium Takaoka Choochote from Thailand. Zootaxa 2018, 4388, 111–122. [Google Scholar] [CrossRef]
- Meier, R.; Shiyang, K.; Vaidya, G.; Ng, P.K. DNA barcoding and taxonomy in Diptera: A tale of high intraspecific variability and low identification success. Syst. Biol. 2006, 55, 715–728. [Google Scholar] [CrossRef]
- Excoffier, L.; Lischer, H.E. Arlequin suite ver 3.5: A new series of programs to perform population genetics analyses under Linux and Windows. Mol. Ecol. Resour. 2010, 10, 564–567. [Google Scholar] [CrossRef]
- Bandelt, H.J.; Forster, P.; Röhl, A. Median-joining networks for inferring intraspecific phylogenies. Mol. Biol. Evol. 1999, 16, 37–48. [Google Scholar] [CrossRef]
- Rogers, A.R.; Harpending, H. Population growth makes waves in the distribution of pairwise genetic differences. Mol. Biol. Evol. 1992, 9, 552–569. [Google Scholar]
- Harpending, H.C. Signature of ancient population growth in a low-resolution mitochondrial DNA mismatch distribution. Hum. Biol. 1994, 66, 591–600. [Google Scholar] [PubMed]
- Tajima, F. Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics 1989, 123, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.X. Statistical tests of neutrality of mutations against population growth, hitchhiking and background selection. Genetics 1997, 147, 915–925. [Google Scholar] [CrossRef]
- Papadopoulou, A.; Anastasiou, I.; Vogler, A.P. Revisiting the insect mitochondrial molecular clock: The mid-Aegean trench calibration. Mol. Biol. Evol. 2010, 27, 1659–1672. [Google Scholar] [CrossRef]
- Pramual, P.; Kuvangkadilok, C.; Baimai, V.; Walton, C. Phylogeography of the black fly Simulium tani (Diptera: Simuliidae) from Thailand as inferred from mtDNA sequences. Mol. Ecol. 2005, 14, 3989–4001. [Google Scholar] [CrossRef]
- Hew, Y.X.; Ya’cob, Z.; Chen, C.D.; Lau, K.W.; Sofian-Azirun, M.; Muhammad-Rasul, A.H.; Putt, Q.Y.; Tan, T.K.; Hadi, U.K.; Suana, I.W.; et al. Co-occurrence of dual lineages within Simulium (Gomphostilbia) atratum De Meijere in the Indonesian Archipelago along Wallace’s Line. Acta Trop. 2024, 250, 107097. [Google Scholar] [CrossRef]
- Kunprom, C.; Sopaladawan, P.N.; Pramual, P. Population genetics and demographic history of guava fruit fly Bactrocera correcta (Diptera: Tephritidae) in northeastern Thailand. Eur. J. Entomol. 2015, 112, 227–234. [Google Scholar] [CrossRef]
- O’loughlin, S.M.; Okabayashi, T.; Honda, M.; Kitazoe, Y.; Kishino, H.; Somboon, P.; Sochantha, T.; Nambanya, S.; Saikia, P.K.; Dev, V.; et al. Complex population history of two Anopheles dirus mosquito species in Southeast Asia suggests the influence of Pleistocene climate change rather than human-mediated effects. J. Evol. Biol. 2008, 21, 555–1569. [Google Scholar] [CrossRef]
- Morgan, K.; O’loughlin, S.M.; Chen, B.; Linton, Y.; Thongwat, D.; Somboon, P.; Fong, M.Y.; Butlin, R.; Verity, R.; Prakash, A.; et al. Comparative phylogeography reveals a shared impact of Pleistocene environmental change in shaping genetic diversity within nine Anopheles mosquito species across the Indo-Burma biodiversity hotspot. Mol. Ecol. 2011, 20, 4533–4549. [Google Scholar] [CrossRef]
- Low, V.L.; Norma-Rashid, Y.; Yusoff, A.; Vinnie-Siow, W.Y.; Prakash, B.K.; Tan, T.K.; Noorhidayah, M.; Chen, C.D.; Sofian-Azirun, M. Pleistocene demographic expansion and high gene flow in the Globe Skimmer dragonfly Pantala flavescens Fabricius (Odonata: Libellulidae) in Peninsular Malaysia. Zool. Anz. 2017, 266, 23–27. [Google Scholar] [CrossRef]
- Adler, P.H.; Kúdelová, T.; Kúdela, M.; Seitz, G.; Ignjatović-Ćupina, A. Cryptic biodiversity and the origins of pest status revealed in the macrogenome of Simulium colombaschense (Diptera: Simuliidae), history’s most destructive black fly. PLoS ONE 2016, 11, e0147673. [Google Scholar] [CrossRef] [PubMed]
- Pramual, P.; Thaijarern, J.; Wongpakam, K. DNA barcoding of human-biting black flies (Diptera: Simuliidae) in Thailand. Acta Trop. 2016, 164, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Putt, Q.Y.; Ya’cob, Z.; Adler, P.H.; Chen, C.D.; Hew, Y.X.; Izwan-Anas, N.; Lau, K.W.; Sofian-Azirun, M.; Pham, X.D.; Takaoka, H.; et al. From bites to barcodes: Uncovering the hidden diversity of black flies (Diptera: Simuliidae) in Vietnam. Parasite Vector 2023, 16, 266. [Google Scholar] [CrossRef] [PubMed]
- Ya’cob, Z.; Takaoka, H.; Low, V.L.; Sofian-Azirun, M. First description of a new cryptic species, Simulium vanluni from Peninsular Malaysia: An integrated morpho-taxonomical and genetic approach for naming cryptic species in the family Simuliidae. Acta Trop. 2017, 167, 31–39. [Google Scholar] [CrossRef]
- Hausdorf, B.; Hennig, C. Species delimitation and geography. Mol. Ecol. Resour. 2020, 20, 950–960. [Google Scholar] [CrossRef]
- Bellwood, P. The origins and dispersals of agricultural communities in Southeast Asia. In Southeast Asia: From Prehistory to History. I; Routledge Curzon: London, UK, 2004; pp. 21–40. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).