Abstract
A new cyprinid fish, Opsariichthys rubriventris sp. nov., is described from the Xizhijiang River, a tributary of the Pearl River basin in Huizhou City, Guangdong Province, southern China. The species is distinguished from all other congeners by the following combination of characters: predorsal scales 13–14; lower jaw projecting slightly beyond upper jaw; cheek with two mainly longitudinal rows of tubercles; and lower jaw, belly, pectoral fin, and anterior margin of anal fin in adult males being reddish-orange. The principal component analysis result of the morphological data indicated that O. rubriventris sp. nov. could be clearly distinguished fromfour other congeners. The phylogenetic analysis conducted in this study, utilizing both Maximum Likelihood (ML) and Bayesian Inference (BI) methods, supported the monophyly of the novel species O. rubriventris sp. nov. at the species level. Additionally, the genetic distance analysis revealed that O. rubriventris sp. nov. exhibits a genetic distance ranging from 0.14 to 0.16 with its congeneric species, further affirming its taxonomic status.
1. Introduction
Since the establishment of the genus Opsariichthys by Bleeker in 1863, over a span of more than a century, extensive phylogenetic and morphological investigations have yielded a comprehensive depiction of 14 species within this genus [1,2]. Advancements in taxonomic research have led to a refinement of the traditional boundaries of the genus, resulting in its subdivision into distinct, yet closely related, genera, namely Opsariichthys Bleeker, 1863, Zacco Jordan & Evermann, 1902, Candidia Jordan & Richardson, 1909, Parazacco Chen 1982, and Nipponocypris Chen, Wu & Hsu, 2008 [3,4]. Most species of the opsariichthine group are known to have color dimorphism, especially Opsariichthys [2]. Opsariichthys is characterized by a large and elongate anal fin, as well as a series of nuptial tubercles (pearl organs) on the jaws in adults [5]. The genus Opsariichthys has a wide distribution, with species occurring in different regions. For example, O. bidens is distributed in China’s main drainages (Yangtze and Pearl) [6]; O. uncirostris can be found in Japan, Korea, and Russia [7]; O. evolans can be found in southern China; while O. pachycephalus and O. kaopingensis are only in Taiwan [8]; and O. duchuunguyeni is also found in northern Vietnam [9] and southern China (this study). This reflects the species diversity of the genus Opsariichthys and the rich distribution in different geographical regions. Up to now, the commonly reported species of the genus Opsariichthys in various river systems in southern China include O. evolans, O. bidens, O. hainanensis, and O. duchuunguyeni.
Phylogenetic definitions, by delineating ancestral lineages tied to nomenclatural designations, meticulously clarify taxonomic group boundaries. This precision illuminates evolutionary phenomena, including temporal origins and lineage persistence [10,11]. The methodology is key in unraveling fish species’ taxonomic status and phylogenetic relationships within the broader evolutionary context [12,13]. Among East Asian fish species, members of the Danioninae subfamily have undergone phylogenetic analyses, resulting in clearer classification and phylogenetic positioning of the various genera [14,15,16,17]. Similarly, the opsariichthine group, which belongs to the Danioninae subfamily, has also benefited from phylogenetic studies, enabling a more definitive delineation of the taxonomic relationships between the genus Opsariichthys and its related genera [3,4,5,6,8]. The cytochrome b (CYTB) gene serves as a versatile tool in biology, being extensively employed for the classification of novel species [18,19] and the assessment of phylogenetic relationships and population genetic structures [20], and notably, it exhibits greater ease of amplification from highly processed or degraded tissues compared to nuclear DNA [12], making it a preferred choice in certain experimental contexts.
During a field trip in April 2024, we collected 39 specimens of Opsariichthys spp. from streams in Huidong County, Huizhou City, Guangdong Province, China. These specimens exhibit significant morphological differences from common relatives of the genus and are confirmed by mitochondrial genetic evidence to be significantly different from all known species of Opsariichthys. Therefore, we are describing a unique species found in Huizhou, Guangdong.
2. Materials and Methods
2.1. Sample Collection and Storage
A total of 39 specimens of Opsariichthys spp. were collected from the Pearl River System (Huizhou), Guangdong Province, in order to clarify their taxonomic status. Caudal fin tissue from 30 juvenile specimens was cut and fixed with 95% ethanol for subsequent DNA extraction for molecular biological analysis. The sexually mature specimens of the new species (6 males, 3 females), O. evolans (3 males, 2 females), O. duchuunguyeni (3 males, 2 females), O. bidens (3 males, 2 females), and O. hainanensis (3 males, 2 females) were fixed in a neutral buffered formalin (10%) for morphological trait analysis before being transferred to a 75% ethanol solution for long-term storage. All specimens were captured using brail and fishing tackle, and they were preserved in the fish ichthyological collection at South China Normal University (SCNU).
2.2. Morphological Description
For morphological analysis, nine specimens of O. rubriventris sp. nov. (SCNU202404001-SCNU202404009, 54.3–81.7 mm SL), five specimens of O. evolans (SCNU202404010-SCNU202404014, 69.3–92.9 mm SL), five specimens of O. duchuunguyeni (SCNU202404015-SCNU202404019, 66.8–86.8 mm SL), five specimens of O. bidens (SCNU202404020-SCNU202404024, 96.6–156.9 mm SL), and five specimens of O. hainanensis (SCNU202404025-SCNU202404029, 67.9–85.6 mm SL) were examined.
Morphometric measurements were conducted by a single observer on the left side of the fish using a digital vernier caliper (AIRAJ, Qingdao, China), with data accuracy to 0.1 mm. The statistical software SPSS 26.0 [21] was utilized for data processing and calculation of mean values. Morphological reference counting index and measurement followed the methods outlined by Huynh et al. [9], Hosoya et al. [22] and Chen et al. [8].
2.3. Morphological Analysis
Based on the morphological data, principal component analysis (PCA) [23] was performed on the five Opsariichthys species using R 4.3.1 software [24]. From the cumulative contribution of the principal components, the scores of the first principal component (PC1) and the second principal component (PC2) were plotted.
2.4. Molecular Phylogenetic Analysis
2.4.1. DNA Extraction, PCR Amplification, and Sequencing
The fin DNA extraction of the new species followed the instructions of the Ezup column animal genomic DNA purification kit (Sangon Biotech Co., Ltd., Shanghai, China). Primers FishcytB-F (5′-ACCACCGTTGTTATTCAACTACAAGAA-3′) and TruccytB-R (5′-CCGACTTCCGGATTACAAGACCG-3′) [25] were used to amplify and sequence the cytochrome b gene. The PCR protocols were as follows: predenaturation step at 95 °C for 5 min; then 33 cycles at 92 °C for 45 s, 50 °C for 90 s, 72 °C for 90 s; extension at 72 °C for 10 min. The PCR products were run on a 1.0% agarose gel (TransGen Biotech Co., Ltd., Beijing, China) stained with ethidium bromide (Sangon Biotech Co., Ltd., Shanghai, China) for band characterization under ultraviolet transillumination (Zhuhai Hema medical instrument Co., Ltd, Zhuhai, China). PCR products were cycle sequenced using the BigDye Terminator Kit (Thermo Fisher Scientific, Waltham, MA, USA) and purified and read on an ABI PRISM 3730XL sequencer (Applied Biosystems, Foster City, CA, USA) with the BigDye Terminator Kit (Applied Biosystems, Waltham, MA, USA). Chromatograms were checked using CHROMAS 2.5.1 software (Technelysium Pty Ltd., Helensvale, Australia). A total of 30 sequences of O. rubriventris sp. nov. were obtained and submitted to GenBank to obtain their accession number (Table A1).
2.4.2. Phylogenetic Analysis
After the paired-end sequencing sequences were spliced in BioEdit v.7.2.5 [26], the sequences of the new species were aligned with other opsariichthine fish, as well as the outgroup Aphyocypris normalis (Table A1), using the MUSCLE algorithm on MEGA XI [27], and phylogenetic trees were constructed using PhyML 3.0 [28] and MrBayes 3.2.7 [29], respectively.
3. Results
3.1. Morphological Analysis
Principal component analysis (PCA) was conducted on five Opsariichthys species based on morphological data. The cumulative contribution of PC1 and PC2 was 92%, representing most of the information in the original data. The contribution rate of PC1 was 84.3%, and it contributes the most to the model. The contribution rate of PC2 was 7.7%. In the principal component score plot, O. bidens was mainly clustered on the negative side of the PC1 axis, O. evolans and O. duchuunguyeni were mainly distributed on the origin of the PC1 axis, O. hainanesis was mainly distributed on the positive side of the PC1 axis and the positive side of the PC2 axis, while the O. rubriventris sp. nov. was mainly distributed on the positive side of the PC1 axis, so that O. rubriventris sp. nov. could be clearly distinguished from the other four species (Figure 1).
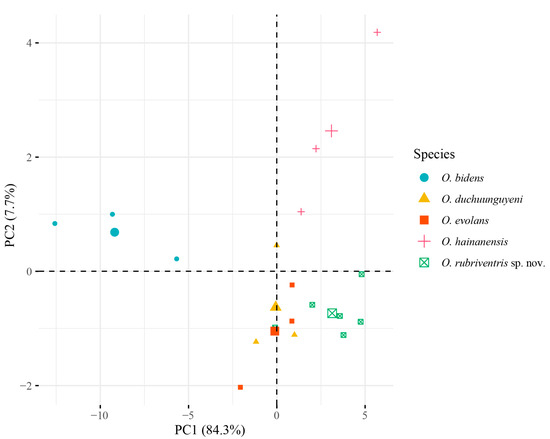
Figure 1.
PCA score plots for five Opsariichthys species derived from morphological data.
3.2. Molecular Phylogenetic Analysis
The complete CYTB sequences were amplified and sequenced for the 30 individuals from Xizhijiang River, Huizhou City, China. The average base content of A, T, G, and C were 26.7%, 26.6%, 15.8%, and 30.9%. The A + T content (57.6%) was higher than that of G + C (42.4%).
Based on the outgroup assignment of Aphyocypris normalis, the phylogenetic analysis of both ML and BI methods provided consistent results at the species level (Figure 2). In ML analyses, PhyML [28] resulted in most likely trees with the best model of TN93 + R. In the BI analysis, the phylogenetic tree reconstructed by using the GTR model and the majority rule consensus Bayesian tree (mean lnL = −7705.98) had the same topology as the ML tree, which supports the monophyly of O. rubriventris sp. nov., forming a sister group with other Opsariichthys species in a clade (Figure 2).
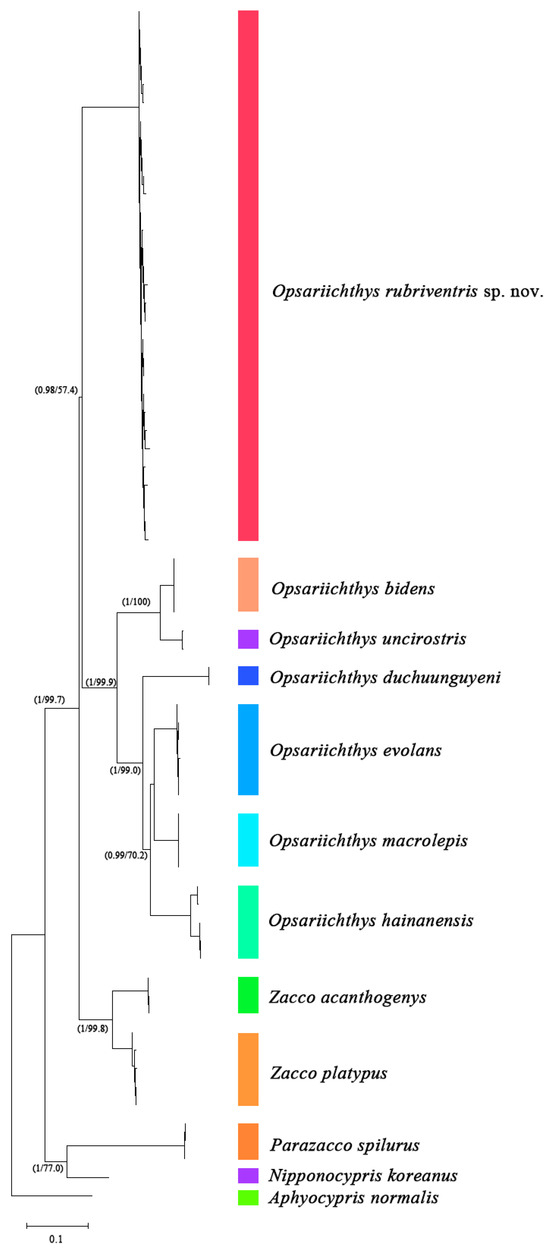
Figure 2.
ML tree of genetic relationships based on the mitochondrial CYTB gene among 12 species including O. rubriventris sp. nov. and closely related species. Outgroup is Aphyocypris normalis. The values on the branches are the posterior probabilities of bootstrap values for the Bayesian and ML analyses, respectively.
Based on the p-distance between the new species and other opsariichthine species re-examined here, the genetic distance between O. rubriventris sp. nov. and other Opsariichthys species ranges from 0.14 to 0.16, and between other Zacco species ranges from 0.12 to 0.15 (Table 1).

Table 1.
Nucleotide distances based on p-distance model between O. rubriventris sp. nov. from the Pearl River, Southern China, and other closely related species, as revealed by CYTB analysis.
3.3. Taxonomic Description
Opsariichthys rubriventris sp. nov. J.-B. Chen, Y.-T. Li, J.-J. Zhou & J.-J. Wang, new species (Figure 3).

Figure 3.
Opsariichthys rubriventris sp. nov.: (a) freshly preserved male; (b) male, preserved specimen, SCNU202404001, holotype, 67.9 mm SL, Huidong County, Huizhou City, Guangdong Province, China; (c) female, SCNU202404002, paratype, 60.7 mm SL, Huidong County, Huizhou City, Guangdong Province, China.
Holotype: SCNU202404001, 67.9 mm SL, Huizhou City, Guangdong Province, China, Guo-xi Weng, 23 April 2024.
Paratypes: SCNU202404002, 003, 004, 005, 006, 007, 008, and 009, 8 specimens, 54.3–81.7 mm SL, Huizhou City, Guangdong Province, China, Guo-xi Weng, 23 April 2024.
3.3.1. Diagnosis
Opsariichthys rubriventris sp. nov. can be distinguished from the congeneric species by the following combination of morphological characters: (1) no maxillary barbels; (2) no anterior notch at the tip of the upper lip; (3) lateral line scales 39–43; (4) predorsal scales 13–14; (5) narrow body width; (6) lower jaw protrudes slightly beyond the front of the snout; (7) 2 rows of pearl organs of the cheek, and the pearl organs are cone-shaped; (8) the pectoral fins of adult males do not extend to the origin of the pelvic fins; (9) adult males have significant nuptial coloration; the lower jaw and ventral side are orange-red; (10) the envelope of the first six rays of the anal fin of adult males, females, and juveniles is orange-red, and the remaining rays gradually become colorless.
Morphologically, O. rubriventris sp. nov. can be clearly distinguished from its congeners O. bidens and O. hainanesis based on these characteristics: O. bidens and O. hainanesis have a concave notch at the front of the upper jaw in which the anterior end of the mandible fits, while the O. rubriventris sp. nov. does not have a deep notch at the front of the upper jaw (Figure 4a,b,g,h–j), similar to O. evolans and O. duchuunguyeni (Figure 4a–f).
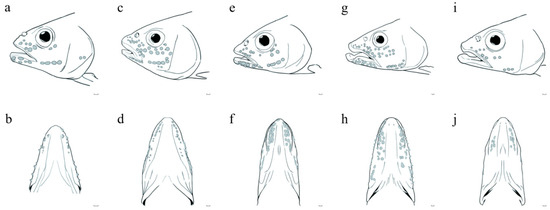
Figure 4.
Head illustrations of O. rubriventris sp. nov. and congeneric species: (a,b) O. rubriventris sp. nov., Huizhou City, Guangdong Province, China; (c,d), O. evolans, Guangzhou City, Guangdong Province, China; (e,f), O. duchuunguyeni, Guangxi Province, China; (g,h), O. bidens, Chaozhou City, Guangdong Province, China; (i,j) O. hainanensis, Qionghai City, Hainan Province, China. Bar = 1 mm.
Furthermore, O. rubriventris sp. nov. can be clearly distinguished from O. evolans by the following characteristics: (1) predorsal fin scales, 13–14 (vs. 15–16); (2) scales around the caudal peduncle, 14–15 (vs. 16); (3) scales below the lateral line, three (vs. four); (4) the lower jaw of adult males protrudes slightly beyond the front of the snout (vs. the lower jaw being shorter than the front of the snout); (5) the lower orbital margin and cheek of adult males have two rows of pearl organs, which are thick and conical (vs. three rows of pearl organs at the lower edge of the eye socket, and the pearl organ being in the shape of small cones); (6) the membrane between the first and last ray of the dorsal fin of adult males gradually changes from green to black (vs. the dorsal fin membrane of adult males being pink); (7) the pelvic fin of adult males is white (vs. the pelvic fin of adult males being pink); (8) the membrane of the first six rays of the anal fin of adult male, juvenile, and female individuals is orange-red, and the remaining rays gradually change to colorless (vs. the anal fin of adult males being pink, and the anal fin of juvenile and female individuals is colorless); (9) the color of lower jaw to the anal fin base is orange-red in the adult male (vs. the lower jaw of adult male fish being yellow and black) (Table 2).

Table 2.
Frequency distribution of meristic features of O. rubriventris sp. nov., O. evolans, O. duchuunguyeni, O. bidens, and O. hainanensis from southern China.
In addition, O. rubriventris sp. nov. can be clearly distinguished from O. duchuunguyeni by the following characteristics: (1) scales around the caudal peduncle 14–15 (vs. 16–17); (2) two rows of pearl organs on the lower edge of the eye socket and cheeks of adult males (vs. three rows); (3) the capsule between the first and last ray of the dorsal fin of adult males changes from green to black (vs. the dorsal fin capsule of adult males being pink); (4) the pelvic fins of adult males are white (vs. the pelvic fins of adult males being orange); (5) the membrane between the first six rays of the anal fin of adult males, juveniles, and females is orange-red, and the remaining rays gradually become colorless (vs. the anal fin of adult males being pink, and the anal fin of juveniles and females being colorless); (6) the area from the lower jaw to the anal fin base of adult males is orange-red (vs. the lower jaw of adult males being yellow and orange) (Table 2).
3.3.2. Description
Body proportions listed in Table 3. Body depth longer than head length; body width rather narrow; no maxillary barbels; head width rather wide (55.4–55.6% in HL); eyes are fairly large (29.4–30.6% in HL); the predorsal length (46.1–47.8% in SL) shorter than in O. evolans, O. bidens, O. hainanensis, and O. duchuunguyeni; the mouth fairly wide (29.9–30.6% in HL); and the anal fin a shorter length at 18.2–34.5%.

Table 3.
Morphometric measurements of O. rubriventris sp. nov., O. evolans, O. duchuunguyeni, O. bidens, and O. hainanensis.
3.3.3. Fin
Dorsal fin iii, 7; pectoral fin i, 12–14; pelvic fin i, 8–9; anal fin iii, 9–11. Dorsal fin with no spines, located in the middle of the body, with nearly straight outer edge. Pectoral fins are pointed at the end, not reaching the base of the pelvic fins, and pectoral fins are shorter in male juveniles and females. Pelvic fins are small, with arc-shaped outer edges, which can reach but not exceed the anus in mature males, and their starting point is opposite to the starting point of the dorsal fin. The first four branched fin rays of the anal fin are extended, and the second one is the longest, which can exceed the end of the caudal peduncle. The anus is close to the starting point of the anal fin. Frequency distribution of meristic counts is provided in Table 2.
3.3.4. Scales
Body is covered with moderately cycloid scales. The lateral line is complete, concave above the pectoral fin, and extends along the lower part of the body to the middle of the caudal peduncle. As indicated in Table 2, lateral line scales 39–43 (nine specimens); scales above lateral line 7–8; scales below lateral line 3; predorsal scales 13–14; scales around the caudal peduncle 14–15. The abdomen is finely scaled.
3.3.5. Coloration in Life
The new species has a silver-gray body on both sides, turning silvery-white from below the lateral line. In juveniles, there are three irregular blue-green stripes on one side of the body; in sexually mature individuals, there are 5–7 irregular blue-green stripes on the body, of which two are wider stripes on the caudal peduncle. There are two fused black spots on the scales at the end of the caudal peduncle. The head is gray-black above and on the sides, and shiny on the sides. Adult males have significant nuptial coloration during the breeding season. The color of the lower jaw to the anal fin base is orange-red in the adult male. The edge of the iris is yellow-green (Figure 3a). The dorsal fin is gray-green to black from the first unbranched ray to the last branched ray, with dark black stripes on the membrane between each ray. The first three unbranched rays of the anal fin to the first three branched rays of juveniles, adult males, and females are orange-red, and the remaining anal fin rays are gradually lighter in color. The base of the pectoral fin starts out orange-red and turns yellow-white at the end. The pelvic fins are orange and white, with no markings on the membrane. The caudal fin is pale yellow with some dark markings between the fin rays.
3.3.6. Head Structure
As shown in Figure 4a, unlike O. evolans (Figure 4c) and O. duchuunguyeni (Figure 4e), the lower jaw of adult male fish of O. rubriventris sp. nov. protrudes slightly beyond the front of the snout. During the breeding season, males will present well-developed keratinous protrusions (pearl organs) on the head and anal fins, especially from the snout to the bottom of the eyes and cheeks. Two rows of pearl organs can be observed from the lower edge of the eye socket to the cheek of adult male individuals, 5–6 pearl organs on the lower edge of the eye socket, about 12 around the upper jaw, about 10 around the lower jaw, and four on the cheeks arranged in a straight line. The pearl organ is cone-shaped (Figure 4b).
3.3.7. Etymology
The specific epithet rubriventris is constructed from the Latin words ruber, meaning red, and venter, meaning belly, an adjective, referring to the red belly of the mature males. In addition, since O. rubriventris sp. nov. is only distributed in Guangdong Province, China, we suggest its Chinese vernacular to be “广东马口鱼”.
3.3.8. Distribution and Habitat
This new species was discovered in Xizhijiang River in Huizhou City, Guangdong Province, which belongs to the Pearl River basin (Figure 5). This species lives in the upper reaches of clear water with moderate velocity, in streams with a bottom composed of small to medium-sized granite and boulders or sand and gravel (Figure 6). The water temperature within the stream varies within a range of 22 °C to 23 °C, while its elevation falls between 200 m and 220 m.
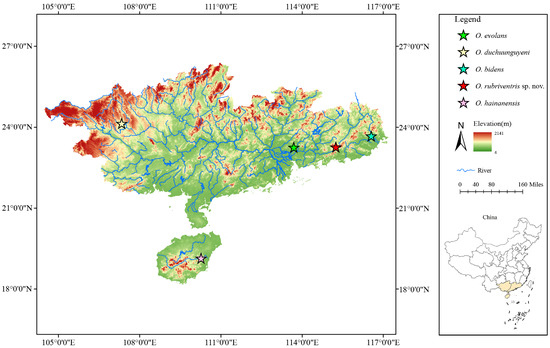
Figure 5.
Map showing sampling sites of O. rubriventris sp. nov. and its four congeners in the present study. Opsariichthys evolans were collected in Xifu River, Guangzhou City, Guangdong Province. Opsariichthys duchunguyeni were collected in Cifu Lake, Hechi City, Guangxi Province. Opsariichthys bidens were collected in Fengjiang River, Chaozhou City, Guangdong Province. Opsariichthys rubriventris sp. nov. were collected in Xizhijiang River, Huizhou City, Guangdong Province. Opsariichthys hainanensis were collected in Wanquan River, Qionghai City, Hainan Province.

Figure 6.
Habitat of O. rubriventris sp. nov. in Huizhou City, Guangdong Province, China.
4. Discussion
The description of O. rubriventris herein brings the number of valid species of Opsariichthys known to 15, of which nine species are recorded from China [5,8,9,30,31,32]. Recently, based on morphological comparison and molecular analysis, the Opsariichthys group were studied to be a monophyletic group and sister to the Zacco group [4,9]. However, based on the phylogenetic analysis of the CYTB gene, O. rubriventris forms a sister group with other Opsariichthys species in a clade, which shows the evolutionary diversity of the genus Opsariichthys.
With the clarification of the taxonomic status of the genus Opsariichthys and its closely related genus Zacco, morphological traits, particularly the presence of the pearl organ (tuberculate structure) on the cheeks of male individuals and the distinct lateral cross-bars along the body, have emerged as pivotal characteristics for distinguishing between the two genera [4,8]. Opsariichthys rubriventris demonstrates a striking resemblance in these morphological characteristics, specifically the lateral cross-bars and the pearl organ features (Figure 3). Furthermore, molecular analyses offer additional support, confirming its association with the genus Opsariichthys and clearly distinguishing it from Zacco (Figure 2).
Opsariichthys rubriventris, which was first identified in the upstream reaches of the Xizhi River (Figure 5), retains the characteristic notched-mouth morphology but displays a less pronounced development compared to O. bidens. This adaptive morphological variation is likely an adaptation to its specialized habitat in the upper reaches of mountain streams, enhancing its prey-capture capabilities while maintaining a streamlined body for agile maneuverability within stream confines. Notably, O. rubriventris demonstrates a strong habitat preference for upstream regions, in contrast to the broader distribution patterns observed for related species such as O. bidens and O. evolans in downstream regions and lakes [6,8]. This habitat specificity is likely to be interwoven with unique niche requirements and reproductive behavior.
Despite compelling evidence supporting the distinctiveness of O. rubriventris as a new species, its coexistence with species like O. evolans and P. spilurus within the same habitat may suggest incomplete allopatric speciation. The intricacies of freshwater fish speciation are rooted in the continuous fluctuations of environmental factors, including variations in water depth and substrate composition, which can serve as triggers for genetic and phenotypic mutations [33]. In fact, freshwater fish exhibit a remarkable capacity for speciation through natural selection on relatively small spatial scales [34], as exemplified by the numerous sympatrically occurring, closely related species found within individual lakes [35].
In conclusion, both morphological and molecular evidence converge to support the validation of O. rubriventris as a unique species. However, a more comprehensive investigation is warranted to elucidate the specific mechanisms underlying its speciation process.
5. Comparative Materials
O. evolans: SCNU202404010-014, five specimens, 69.3–92.9 mm SL, Xifu River, Zengcheng County, Guangzhou City, Guangdong Province.
O. duchunguyeni: SCNU202404015-019, five specimens, 66.8–86.8 mm SL, Cifu Lake, Bama Yao Autonomous County, Hechi City, Guangxi Province.
O. bidens: SCNU202404020-024, five specimens, 96.6–156.9 mm SL, Fengjiang River, Xiangqiao County, Chaozhou City, Guangdong Province.
O. hainanensis: SCNU202404025-029, five specimens, 67.9–85.6 mm SL, Wanquan River, Qionghai City, Hainan Province.
6. Nomenclatural Acts Registration
This published work and the nomenclatural acts it contains have been registered in ZooBank LSIDs (Life Science Identifiers).
Publication LSID: urn:lsid:zoobank.org:pub:CEC5CCF2-AE8B-4ABE-BEC6-B1FB798A5EB0.
Opsariichthys rubriventris LSID: urn:lsid:zoobank.org:act:A2EA2B62-6514-4E64-8568-DCFECED3D52D.
Author Contributions
Conceptualization, J.-J.W.; Methodology, J.-B.C. and Y.-T.L.; Software, J.-B.C.; Validation, J.-J.Z. and J.-J.W.; Formal Analysis, J.-B.C. and Y.-T.L.; Investigation, J.-B.C. and Y.-T.L.; Resources, Y.-T.L., G.-X.W. and C.L.; Data Curation, J.-B.C. and Y.-T.L.; Writing—Original Draft Preparation, J.-B.C. and Y.-T.L.; Writing—Review & Editing, J.-J.Z. and J.-J.W.; Visualization, J.-B.C. and Y.-T.L.; Supervision, J.-J.W. and H.-D.L.; Project Administration, J.-J.W.; Funding Acquisition, J.-J.W. All authors have read and agreed to the published version of the manuscript.
Funding
The China-ASEAN Maritime Cooperation Fund, Grant/Award Number: CAMC-2018F.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
All newly produced sequences were deposited in GenBank.
Acknowledgments
We are grateful to Jun Wang (School of Life Science, Guangzhou University) for providing the head illustrations of the specimens. We are grateful to Jia-Rui Lin for providing the specimens of the new species.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Appendix A

Table A1.
List of data used for the phylogenetic analyses and sampling sites.
Table A1.
List of data used for the phylogenetic analyses and sampling sites.
| Accession | Species | Locality | Source |
|---|---|---|---|
| PP975436 | Opsariichthys rubriventris sp. nov. | Xizhijiang River, Huizhou, Guangdong, China | This Study |
| PP975437 | Opsariichthys rubriventris sp. nov. | Xizhijiang River, Huizhou, Guangdong, China | This Study |
| PP975438 | Opsariichthys rubriventris sp. nov. | Xizhijiang River, Huizhou, Guangdong, China | This Study |
| PP975439 | Opsariichthys rubriventris sp. nov. | Xizhijiang River, Huizhou, Guangdong, China | This Study |
| PP975440 | Opsariichthys rubriventris sp. nov. | Xizhijiang River, Huizhou, Guangdong, China | This Study |
| PP975441 | Opsariichthys rubriventris sp. nov. | Xizhijiang River, Huizhou, Guangdong, China | This Study |
| PP975442 | Opsariichthys rubriventris sp. nov. | Xizhijiang River, Huizhou, Guangdong, China | This Study |
| PP975443 | Opsariichthys rubriventris sp. nov. | Xizhijiang River, Huizhou, Guangdong, China | This Study |
| PP975444 | Opsariichthys rubriventris sp. nov. | Xizhijiang River, Huizhou, Guangdong, China | This Study |
| PP975445 | Opsariichthys rubriventris sp. nov. | Xizhijiang River, Huizhou, Guangdong, China | This Study |
| PP975446 | Opsariichthys rubriventris sp. nov. | Xizhijiang River, Huizhou, Guangdong, China | This Study |
| PP975447 | Opsariichthys rubriventris sp. nov. | Xizhijiang River, Huizhou, Guangdong, China | This Study |
| PP975448 | Opsariichthys rubriventris sp. nov. | Xizhijiang River, Huizhou, Guangdong, China | This Study |
| PP975449 | Opsariichthys rubriventris sp. nov. | Xizhijiang River, Huizhou, Guangdong, China | This Study |
| PP975450 | Opsariichthys rubriventris sp. nov. | Xizhijiang River, Huizhou, Guangdong, China | This Study |
| PP975451 | Opsariichthys rubriventris sp. nov. | Xizhijiang River, Huizhou, Guangdong, China | This Study |
| PP975452 | Opsariichthys rubriventris sp. nov. | Xizhijiang River, Huizhou, Guangdong, China | This Study |
| PP975453 | Opsariichthys rubriventris sp. nov. | Xizhijiang River, Huizhou, Guangdong, China | This Study |
| PP975454 | Opsariichthys rubriventris sp. nov. | Xizhijiang River, Huizhou, Guangdong, China | This Study |
| PP975455 | Opsariichthys rubriventris sp. nov. | Xizhijiang River, Huizhou, Guangdong, China | This Study |
| PP975456 | Opsariichthys rubriventris sp. nov. | Xizhijiang River, Huizhou, Guangdong, China | This Study |
| PP975457 | Opsariichthys rubriventris sp. nov. | Xizhijiang River, Huizhou, Guangdong, China | This Study |
| PP975458 | Opsariichthys rubriventris sp. nov. | Xizhijiang River, Huizhou, Guangdong, China | This Study |
| PP975459 | Opsariichthys rubriventris sp. nov. | Xizhijiang River, Huizhou, Guangdong, China | This Study |
| PP975460 | Opsariichthys rubriventris sp. nov. | Xizhijiang River, Huizhou, Guangdong, China | This Study |
| PP975461 | Opsariichthys rubriventris sp. nov. | Xizhijiang River, Huizhou, Guangdong, China | This Study |
| PP975462 | Opsariichthys rubriventris sp. nov. | Xizhijiang River, Huizhou, Guangdong, China | This Study |
| PP975463 | Opsariichthys rubriventris sp. nov. | Xizhijiang River, Huizhou, Guangdong, China | This Study |
| PP975464 | Opsariichthys rubriventris sp. nov. | Xizhijiang River, Huizhou, Guangdong, China | This Study |
| PP975465 | Opsariichthys rubriventris sp. nov. | Xizhijiang River, Huizhou, Guangdong, China | This Study |
| KJ940956 | Opsariichthys duchuunguyeni | Ky Cung—Bang Giang River basin, northern Vietnam | [9] |
| KJ940957 | Opsariichthys duchuunguyeni | Ky Cung—Bang Giang River basin, northern Vietnam | [9] |
| MZ507526 | Opsariichthys bidens | Balidian, Huzhou, Zhejiang, China | [36] |
| MZ507527 | Opsariichthys bidens | Balidian, Huzhou, Zhejiang, China | [36] |
| MZ507528 | Opsariichthys bidens | Balidian, Huzhou, Zhejiang, China | [36] |
| MZ507529 | Opsariichthys bidens | Balidian, Huzhou, Zhejiang, China | [36] |
| AY958197 | Opsariichthys uncirostris | L. Biwa, Shiga, Japan | [4] |
| AB218897 | Opsariichthys uncirostris | L. Biwa, Shiga, Japan | [37] |
| MH350625 | Opsariichthys evolans | Rong River, Fengshun, Guangdong, China | [38] |
| MH350626 | Opsariichthys evolans | Rong River, Fengshun, Guangdong, China | [38] |
| MH350627 | Opsariichthys evolans | Rong River, Fengshun, Guangdong, China | [38] |
| MH350628 | Opsariichthys evolans | Rong River, Fengshun, Guangdong, China | [38] |
| MH350629 | Opsariichthys evolans | Rong River, Fengshun, Guangdong, China | [38] |
| MH350630 | Opsariichthys evolans | Rong River, Fengshun, Guangdong, China | [38] |
| MH350779 | Opsariichthys macrolepis | Yangtze River, Zunyi, Guizhou, China | [38] |
| MH350780 | Opsariichthys macrolepis | Yangtze River, Zunyi, Guizhou, China | [38] |
| MH350781 | Opsariichthys macrolepis | Yangtze River, Zunyi, Guizhou, China | [38] |
| MH350782 | Opsariichthys macrolepis | Yangtze River, Zunyi, Guizhou, China | [38] |
| MH350784 | Zacco acanthogenys | Xin’an River, Huangshan, Anhui, China | [38] |
| MH350785 | Zacco acanthogenys | Xin’an River, Huangshan, Anhui, China | [38] |
| MH350786 | Zacco acanthogenys | Xin’an River, Huangshan, Anhui, China | [38] |
| MN325062 | Opsariichthys hainanensis | Changhua River, Qiongzhong County, Hainan, China | [38] |
| MN325063 | Opsariichthys hainanensis | Changhua River, Qiongzhong County, Hainan, China | [38] |
| MN325064 | Opsariichthys hainanensis | Changhua River, Qiongzhong County, Hainan, China | [38] |
| MN325065 | Opsariichthys hainanensis | Changhua River, Qiongzhong County, Hainan, China | [38] |
| MN325066 | Opsariichthys hainanensis | Changhua River, Qiongzhong County, Hainan, China | [38] |
| AB198972 | Zacco platypus | Yoshino River, Kochi, Japan | [39] |
| AB572372 | Zacco platypus | Naka River, Kanto Plain, Japan | [40] |
| AB572384 | Zacco platypus | Naka River, Kanto Plain, Japan | [40] |
| AB572406 | Zacco platypus | Naka River, Kanto Plain, Japan | [40] |
| AB572415 | Zacco platypus | Naka River, Kanto Plain, Japan | [40] |
| PQ283921 | Parazacco spilurus | Xizhijiang River, Huizhou, Guangdong, China | This Study |
| PQ283922 | Parazacco spilurus | Xizhijiang River, Huizhou, Guangdong, China | This Study |
| PQ283923 | Parazacco spilurus | Xizhijiang River, Huizhou, Guangdong, China | This Study |
| NC025286 | Nipponocypris koreanus | Gwangcheon River, South Korea | [41] |
| NC015538 | Aphyocypris normalis | Unknown | [16] |
References
- Chen, Y.Y. A revision of opsariichthine cyprinid fishes. Oceanol. Limnol. Sin. 1982, 13, 293–299. [Google Scholar]
- Chen, I.S.; Chang, Y.C. The Photographic Guide of Inland Water Fishes of Taiwan; Sheichuan Press: Keelung, Taiwan, 2005; Volume I, Cypriniformes. [Google Scholar]
- Liao, N.L.; Huang, S.P.; Wang, T.Y. Interspecific Mating Behavior Between Introduced Zacco platypus and Native Opsariichthys evolans in Taiwan. Zool. Stud. 2020, 59, e6. [Google Scholar] [CrossRef]
- Huang, S.P.; Wang, F.Y.; Wang, T.Y. Molecular Phylogeny of the Opsariichthys Group (Teleostei: Cypriniformes) Based On Complete Mitochondrial Genomes. Zool. Stud. 2017, 56, e40. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, F.; Yu, D.; Liu, H. Mitochondrial divergence suggests unexpected high species diversity in the opsariichthine fishes (Teleostei: Cyprinidae) and the revalidation of Opsariichthys macrolepis. Ecol. Evol. 2019, 9, 2664–2677. [Google Scholar] [CrossRef] [PubMed]
- Perdices, A.; Sayanda, D.; Coelho, M.M. Mitochondrial diversity of Opsariichthys bidens (Teleostei, Cyprinidae) in three Chinese drainages. Mol. Phylogenet Evol. 2005, 37, 920–927. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, T.; Jeon, S.R.; Kitagawa, T. Genetic differentiation of piscivorous chub (genus Opsariichthys) in Japan, Korea and Russia. Zool. Sci. 2002, 19, 601–610. [Google Scholar] [CrossRef]
- Chen, I.; Wu, J.; Huang, S. The taxonomy and phylogeny of the cyprinid genus Opsariichthys Bleeker (Teleostei: Cyprinidae) from Taiwan, with description of a new species. Environ. Biol. Fish. 2009, 86, 165–183. [Google Scholar] [CrossRef]
- Huynh, T.; Chen, I. A new species of cyprinid fish of genus Opsariichthys from Ky Cung—Bang Giang river basin, northern Vietnam with notes on the taxonomic status of the genus from northern Vietnam and southern China. J. Mar. Sci. Tech.-Jpn. 2013, 21, 135–145. [Google Scholar] [CrossRef]
- de Queiroz, K.; Gauthier, J. Phylogeny as a Central Principle in Taxonomy: Phylogenetic Definitions of Taxon Names. Syst. Biol. Biol. 1990, 39, 307–322. [Google Scholar] [CrossRef]
- Dunn, C.W.; Giribet, G.; Edgecombe, G.D.; Hejnol, A. Animal phylogeny and its evolutionary implications. Annu. Rev. Ecol. Evol. Syst. Rev. Ecol. Evol. Syst. 2014, 45, 371–395. [Google Scholar] [CrossRef]
- Briolay, J.; Galtier, N.; Brito, R.M.; Bouvet, Y. Molecular Phylogeny of Cyprinidae Inferred from cytochrome b DNA Sequences. Mol. Phylogenet Evol. 1998, 9, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Cunha, C.; Mesquita, N.; Dowling, T.E.; Gilles, A.; Coelho, M.M. Phylogenetic relationships of Eurasian and American cyprinids using cytochrome b sequences. J. Fish. Biol. 2002, 61, 929–944. [Google Scholar] [CrossRef]
- He, S.; Chen, Y.; Tsuneo, N. Sequences of cytochrome b gene for primitive cyprinid fishes in East Asia and their phylogenetic concerning. Chin. Sci. Bull. 2001, 46, 661–665. [Google Scholar] [CrossRef]
- Tang, K.L.; Agnew, M.K.; Hirt, M.V.; Lumbantobing, D.N.; Raley, M.E.; Sado, T.; Teoh, V.; Yang, L.; Bart, H.L.; Harris, P.M.; et al. Limits and phylogenetic relationships of East Asian fishes in the subfamily Oxygastrinae (Teleostei: Cypriniformes: Cyprinidae). Zootaxa 2013, 3681, 101–135. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.L.; Agnew, M.K.; Hirt, M.V.; Sado, T.; Schneider, L.M.; Freyhof, J.; Sulaiman, Z.; Swartz, E.; Vidthayanon, C.; Miya, M.; et al. Systematics of the subfamily Danioninae (Teleostei: Cypriniformes: Cyprinidae). Mol. Phylogenet Evol. 2010, 57, 189–214. [Google Scholar] [CrossRef]
- Chen, W.; Mayden, R.L. Molecular systematics of the Cyprinoidea (Teleostei: Cypriniformes), the world’s largest clade of freshwater fishes: Further evidence from six nuclear genes. Mol. Phylogenet Evol. 2009, 52, 544–549. [Google Scholar] [CrossRef]
- Zhu, L.; Yu, D.; Liu, H. Zacco sinensis sp. nov. (Cypriniformes: Cyprinidae), a New Fish Species from Northern China. Sichuan J. Zool. 2020, 39, 168–176. [Google Scholar] [CrossRef]
- Yan, Z.; Jiajun, Z.; Jinquan, Y. A new species of genus Zacco from Southern China (Cypriniformes: Cyprinidae). J. Shanghai Ocean Univ. 2023, 32, 544–552. [Google Scholar] [CrossRef]
- Lin, H.; Kuo, P.; Wang, W.; Chiu, Y.; Ju, Y.; Lin, F.; Hsu, K. Speciation and differentiation of the genus Opsariichthys (Teleostei: Cyprinidae) in East Asia. Biochem. Syst. Ecol. 2016, 68, 92–100. [Google Scholar] [CrossRef]
- George, D.; Mallery, P. IBM SPSS Statistics 26 Step by Step: A Simple Guide and Reference, 16th ed.; Routledge: London, UK, 2019. [Google Scholar] [CrossRef]
- Hosoya, K.; Ashiwa, H.; Watanabe, M.; Mizuguchi, K.; Okazaki, T. Zacco sieboldii, a species distinct from Zacco temminckii (Cyprinidae). Ichthyol. Res. 2003, 50, 1–8. [Google Scholar] [CrossRef]
- Abdi, H.; Williams, L.J. Principal component analysis. Wires Comput. Stat. 2010, 2, 433–459. [Google Scholar] [CrossRef]
- R Core Team. R: A language and environment for statistical computing. Msor Connect. 2014, 1. [Google Scholar]
- Sevilla, R.; Diez, A.; Norén, M.; Mouchel, O.; Jérôme, M.; Verrez-Bagnis, V.; Pelt, H.; Favre-Krey, L.; Krey, G.; Con-Sortium, T.; et al. Primers and polymerase chain reaction conditions for DNA barcoding teleost fish based on the mitochondrial cytochrome b and nuclear rhodopsin genes. Mol. Ecol. Notes 2007, 7, 730–734. [Google Scholar] [CrossRef]
- Alzohairy, A. BioEdit: An important software for molecular biology. Gerf Bull. Biosci. 2011, 2, 60–61. [Google Scholar]
- Chen, W.; Li, C.; Chen, F.; Li, Y.; Yang, J.; Li, J.; Li, X. Phylogeographic analyses of a migratory freshwater fish (Megalobrama terminalis) reveal a shallow genetic structure and pronounced effects of sea-level changes. Gene 2020, 737, 144478. [Google Scholar] [CrossRef]
- Guindon, S.; Dufayard, J.F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New algorithms and methods to estimate maximum-likelihood phylogenies: Assessing the performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian Phylogenetic Inference and Model Choice Across a Large Model Space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Kottelat, M. Freshwater Fishes of Northern Vietnam. A Preliminary Check-list of the Fishes Known or Expected to Occur in Northern Vietnam with Comments on Systematics and Nomenclature. In Environment and Social Development Unit, East Asia and Pacific Region; The World Bank: Washington, DC, USA, 2001; pp. 1–73. [Google Scholar]
- Kottelat, M. Fishes of Laos; WHT Publications Ltd.: Colombo, Sri Lanka, 2001; p. 198. [Google Scholar]
- Amaoka, H.K.; Araga, C.; Uyeno, T.; Yoshino, T. The Fishes of the Japanese Archipelago; Tokai University Press: Tokyo, Japan, 1984; Volume 1. [Google Scholar]
- Seehausen, O.; Wagner, C.E. Speciation in Freshwater Fishes. Annu. Rev. Ecol. Evol. Syst. 2014, 45, 621–651. [Google Scholar] [CrossRef]
- Seehausen, O.; Takimoto, G.; Roy, D.; Jokela, J. Speciation reversal and biodiversity dynamics with hybridization in changing environments. Mol. Ecol. 2008, 17, 30–44. [Google Scholar] [CrossRef]
- Barluenga, M.; Stölting, K.N.; Salzburger, W.; Muschick, M.; Meyer, A. Sympatric speciation in Nicaraguan crater lake cichlid fish. Nature 2006, 439, 719–723. [Google Scholar] [CrossRef]
- Liu, S.; Lian, Q.; Jia, Y.; Chi, M.; Li, F.; Jiang, J.; Liu, Y.; Zheng, J.; Cheng, S.; Gu, Z. Genetic diversity analysis of three Opsariichthys bidens populations in Zhejiang Province based on mitochondrial Cyt b gene sequences. Acta Agric. Zhejiangensis 2023, 35, 293–300. [Google Scholar] [CrossRef]
- Saitoh, K.; Sado, T.; Mayden, R.L.; Hanzawa, N.; Nakamura, K.; Nishida, M.; Miya, M. Mitogenomic Evolution and Interrelationships of the Cypriniformes (Actinopterygii: Ostariophysi): The First Evidence Toward Resolution of Higher-Level Relationships of the World’s Largest Freshwater Fish Clade Based on 59 Whole Mitogenome Sequences. J. Mol. Evol. 2006, 63, 826–841. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, J.; Li, C.; Chen, J.; Li, W.; Jiang, S.; Hsu, K.; Zhao, M.; Lin, H.; Zhao, J. Spatial genetic structure of Opsariichthys hainanensis in South China. Mitochondrial Dna A 2020, 31, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Kartavtsev, Y.P.; Chiba, S.N.; Uematsu, T.; Sviridov, V.V.; Hanzawa, N. Genetic divergence and phylogenetic independence of Far Eastern species in subfamily Leuciscinae (Pisces: Cyprinidae) inferred from mitochondrial DNA analyses. Genes Genet. Syst. 2007, 82, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Takamura, K.; Nakahara, M. Intraspecific invasion occurring in geographically isolated populations of the Japanese cyprinid fish Zacco platypus. Limnology 2015, 16, 161–170. [Google Scholar] [CrossRef]
- Chen, I.; Liu, Y.; Huang, S.; Shen, C. The complete mitochondrial genome of the Korean minnow Nipponocypris koreanus (Cypriniformes, Cyprinidae). Mitochondrial Dna A 2016, 27, 708–710. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).