A New Species and Four New Recorded Species of Naididae (Annelida: Oligochaeta) from Korea †
Abstract
1. Introduction
2. Materials and Methods
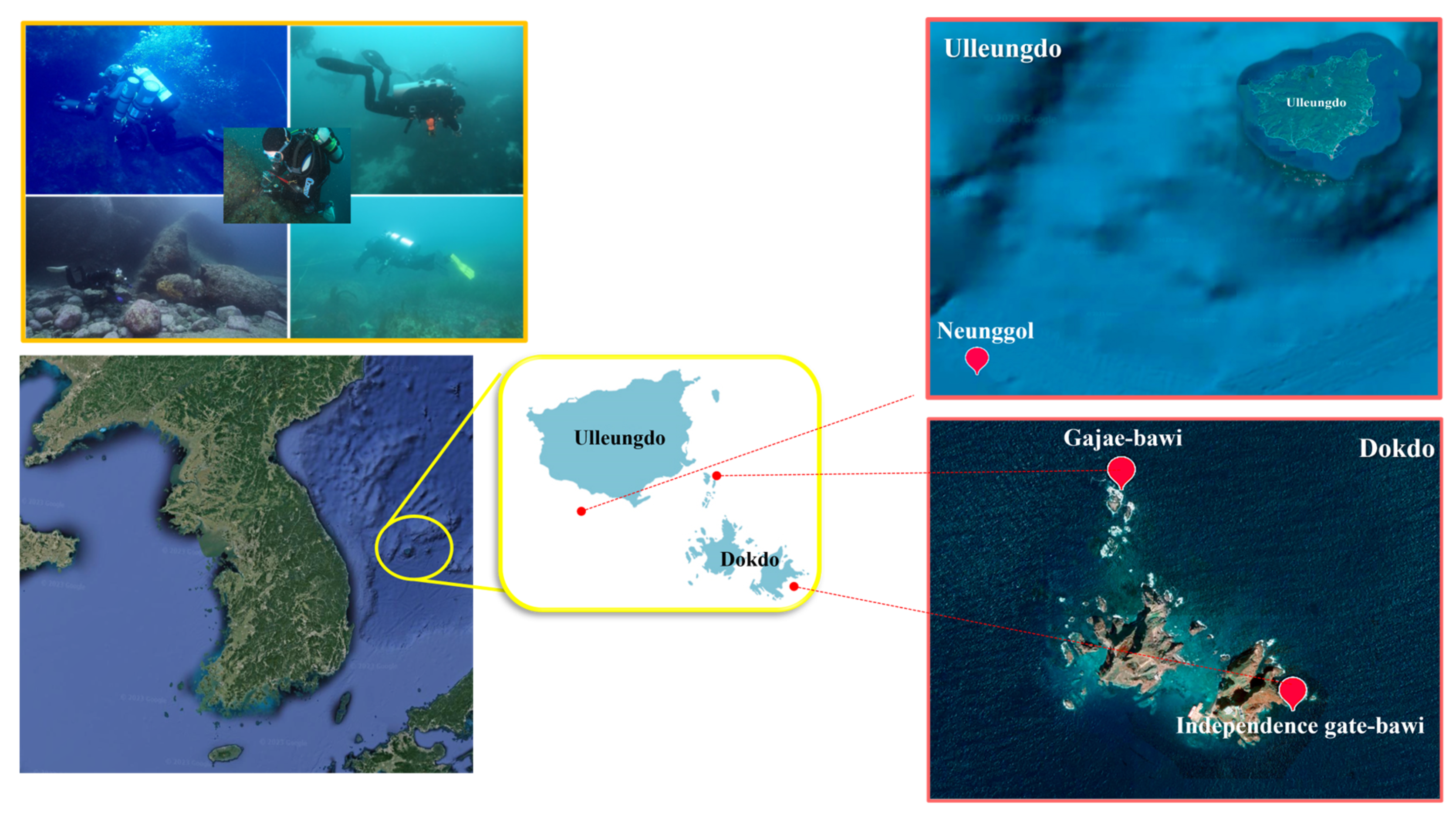
Specimen Sampling and Collection of New Species
- -
- Neunggol is a huge underwater rock located in the south of Ulleungdo Island. It is famous for its strong currents. The bottom is coarse and made of sand and gravel.
- -
- Independence gate-bawi is a sea arch located in the easternmost part of Dongdo. It is home to a large amount of seaweed, Undaria, and Saccharina, and Eisenia. The bottom is medium to coarse and made of gravel and rubble.
- -
- Gajae Rock is located in the north of Seodo Island. It has good currents with many rocks and valleys. The large Crayfish Rock in the northernmost part has two valleys. The northern valley is made up of torn vertical walls. The bottom is coarse and made of sand. Specimens were kept alive and transported to the laboratory. Anesthesia and washing were carried out as previously described [73]. Specimens were sorted from sieved mixtures using a stereomicroscope (SMZ-168, Motic, Hong Kong). They were then fixed in an 8% formalin solution or 80% ethanol. Fixed worms were mounted on microscope slides with Canada balsam following the protocol of Erséus, 1994 [74]. Taxonomic identifications were carried out using a BX41 research microscope fitted with an Olympus DP22 camera system (Olympus, Tokyo, Japan). InnerViewTM-i series software (Innerview Co. Ltd., Seongnam, Republic of Korea) was used to measure and edit photographs. Voucher specimens of species described here have been deposited in the Marine Biological Resource Institute, Sahmyook University, Seoul, Republic of Korea, and the National Institute of Biological Resources in Korea.
3. Molecular Analysis
3.1. Extraction, PCR Amplification, and Sequencing
3.2. GenBank Data
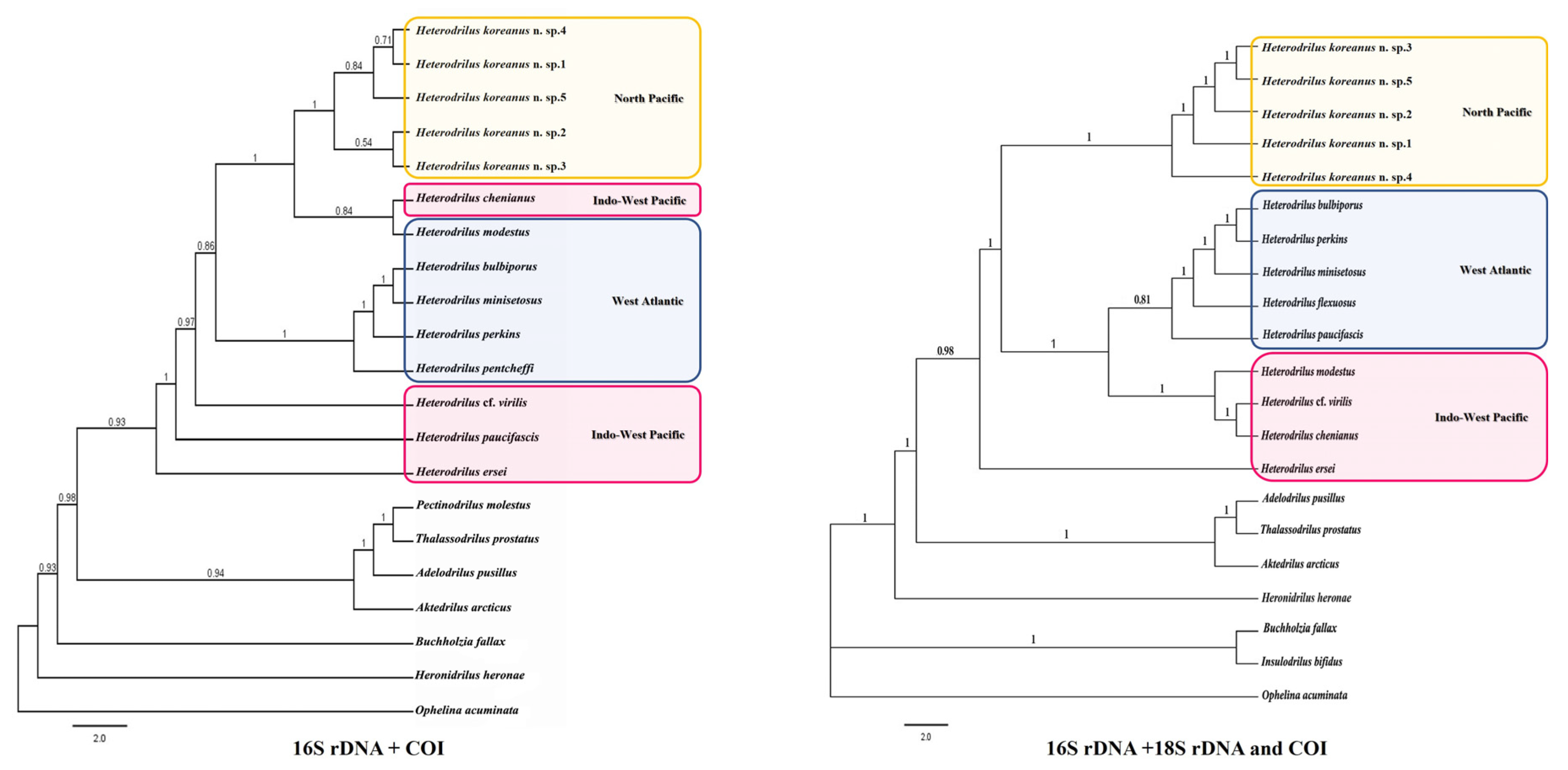
4. Phylogenetic Analysis
5. Results
- Taxonomy account
- Phylum Annelida
- Class Clitellata
- Subclass Oligochaeta
- Order Tubificida
- Family Naididae Ehrenberg, 1831
- Subfamily Limnodriloidinae Erséus, 1982
- Genus Limnodriloides Pierantoni, 1903
- Type species. Limnodriloides appendiculatus Pierantoni, 1903.
- Limnodriloides monothecus: Erséus 1987: 274.
- Limnodriloides anxius: Erséus, 1990 [3] (pp. 243–303), Figs. 26I–O, 27A–D.
- Material examined. NIBRIV0000901382, NIBRIV0000901503, NIBRIV0000901504. Independence gate-bawi at Dokdo-ri, Ulleung-eup, Ulleung-gun, Gyeongsangbuk-do, Republic of Korea, (37.239363° N 131.871777° E), 1 May 2022, in medium-coarse sediment of 20 m in depth at 14 °C through scuba diving.
- Description. Length 4.7–6.9 mm long (three complete specimens)
- Prostomium small conical shape (Figure 2A). Clitellum poorly developed. Chaetae bifid, upper tooth thinner and shorter than lower (Figure 2B,C). Chaetae 30–64 μm long, 2(1)–4 per bundle anteriorly, absent from (X)XI. Pharyngeal glands in IV–V. Esophageal diverticula present in IX. Male genitalia paired, vas deferens shorter than atrium, entering atrium subapically. Atrial ampulla oblong, prostatic pad elongate, ventral in ampulla (Figure 2E). Prostate gland lobed, large. Atrial duct slender, 56–71 μm long, convoluted. Ental part atrial duct thick inner epithelium. Duct terminating in pseudopenial papilla inside copulatory sac; sac 20–38 μm long (Figure 2E). Spermathecal pore unpaired, mid-dorsal in middle of X (Figure 2D). Spermatheca elongate pear-shaped, 95–150 μm long, inner epithelium thicker in ectal than in ental part. Sperm arranged as 1–2 spindle-shaped spermatozeugmata in spermatheca. Spermatozeugmata 80–95 μm long; length about 6–8 × width (Figure 2D).
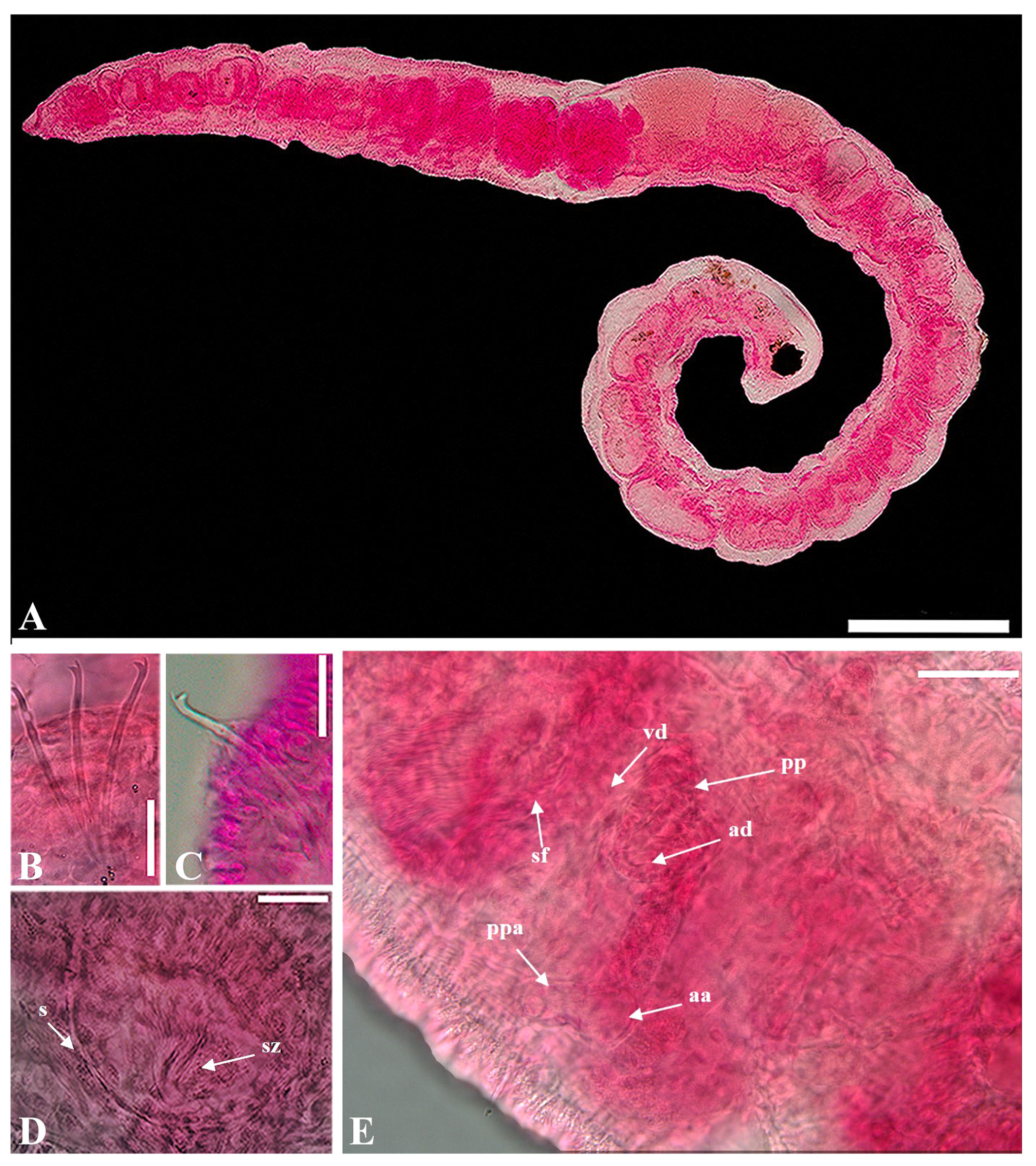
- Distribution. West Atlantic (Belize, Bonaire, Venezuela, Bravados, Florida through Virginia) and Korea.
- Habitat. Lower intertidal and subtidal, generally muddy sand to at least 20 m in depth.
- Remarks. According to Erséus (1990) [27], both the chaetal arrangement and male genitalia exhibit significant variability in Limnodriloides monothecus and L. anxius. As a result, previous literature often reported this species as L. monothecus. However, a key distinguishing feature between these two species is the shape of the spermatozeugmata within sperm, with L. monothecus having cylindrical sperm, setting it apart from L. anxius. This character offers reliable means for accurate distinguishing the two species. Our specimens (4.7–6.9 mm) were smaller than the those in the original description (7.8–8.8 mm). Despite the size difference, the atrial ampullae in our specimens closely resembled those of the type series. Additionally, while the shape of the spermatheca was not precisely an enlarged pear-shape, it bore a strong resemblance. Furthermore, the sperm within the spermatheca was organized as 1–2 spindle-shaped spermatozeugmata. Therefore, our samples were confirmed to share key characteristics with type species. This species was reported for the first time in Korea key characteristics with type species.
- Genus Smithsonidrilus Brinkhurst, 1966
- Type species. Smithsonidrilus marinus Brinkhurst, 1966.
- Smithsonidrilus exspectatus Erséus, 1993 (Figure 3A–D)
- Material examined. MABIKNA00157827, Neunggeol at Ulleung-eup, Ulleung-gun, Gyeongsangbuk-do, Republic of Korea, (37.26586° N, 130.52299° E), 5 June 2022, in medium-coarse sediment from 52 m in depth at 11 °C, obtained through scuba diving. Specimens were deposited at the National Marine Biodiversity Institute of Korea (MABIK).
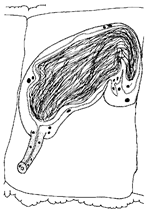
- Description. Prostomium pointed triangular (Figure 3A). Clitellum extending over 2/3X–2/3XII. All chaetae bifid, upper tooth thinner and shorter than lower (Figure 3B). Chaetae 42–86 μm long, 2–3 per bundle anteriorly, 1(2) per bundle posteriorly, absent from XI, two per bundle thereafter. Male pore unpaired, located mid-ventrally, posterior to middle of XI. Spermathecal pore unpaired, mid-ventral, near middle of X. Male genitalia complex, with ejaculatory duct and pseudopenis unpaired. Spermathecal ampulla unpaired with oval-shape (Figure 3C), Male and spermathecal pores unpaired, located mid-ventrally posterior of XI and middle of X, respectively. Sperm funnel conspicuous and deep (Figure 3D). Vas deferens thin-walled, ciliated, as long as atrial ampulla, not clearly set off from latter. Atrial ampulla 100–170 μm long, ectally dilated, without cilia inside. Ectal part of atrial ampulla narrow. Prostate gland large and lobed, attached to diverticulum of atrial duct (Figure 3D). Atrial ampulla ectally terminating and slender, with non-granulated atrial duct. Atrial ducts ectally transiting into conspicuous, unpaired ejaculatory duct. Ejaculatory duct heavily muscular, often folded. Spermatheca unpaired with inconspicuous vestibula, a large, thin-walled, oval ampulla, and a filiform diverticulum attached to inner end of ampulla (Figure 3C). Sperm in random mass, denser in diverticulum than in ampulla throughout spermatheca.
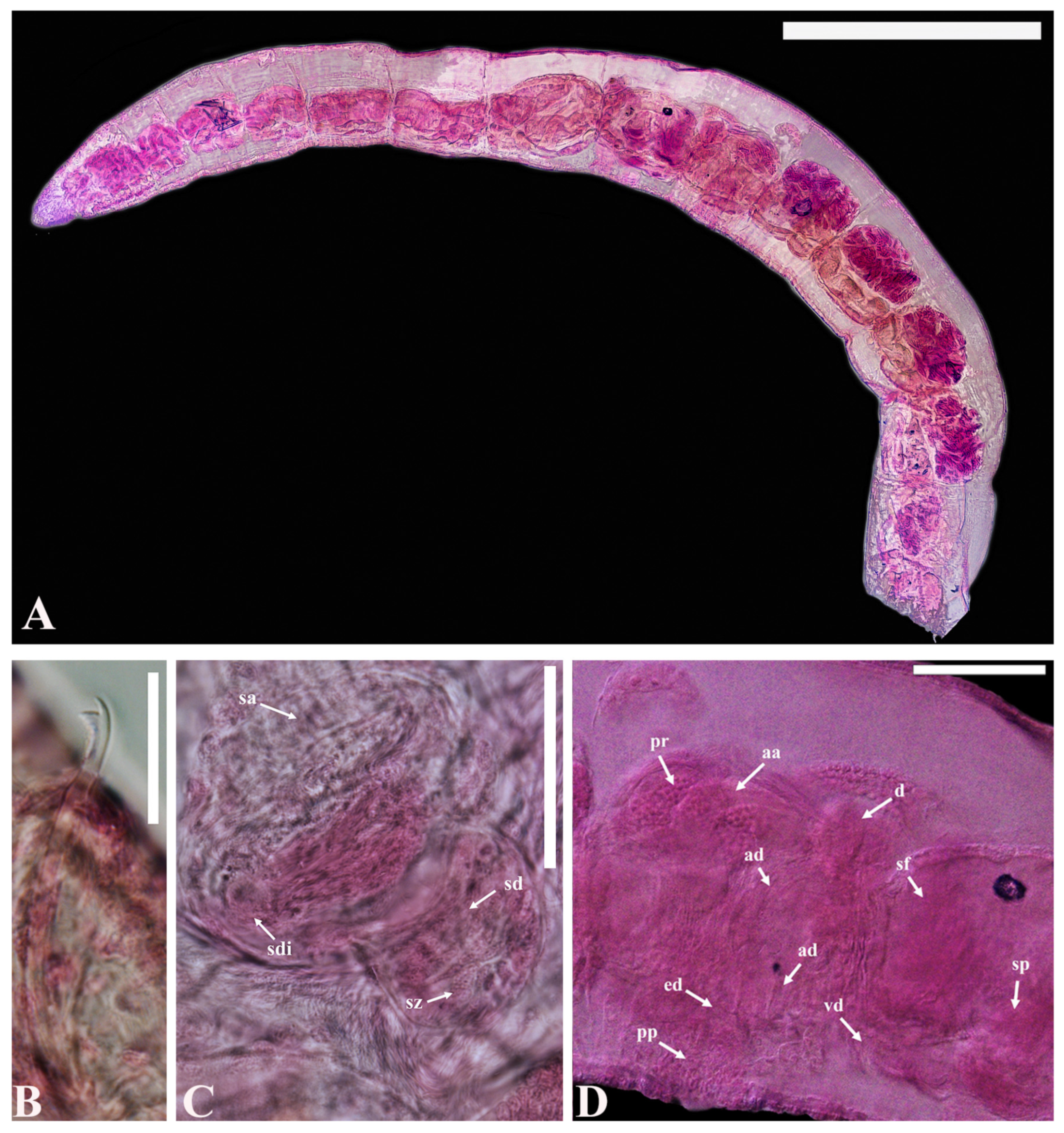
- Distribution. Florida Keys (Atlantic coast of southern Florida), and the East Sea of Korea
- Habitat. Barely subtidal (known from 0.1–0.6 m in depth), medium to coarse sand.
- Remarks. According to the original description [31], Smithsonidrilus exspectatus, belonging to the S. marinus complex, is characterized by unpaired male and spermathecal pores. However, it distinguishes itself from other species within this group in terms of spermathecal structure and copulatory apparatus. In contrast to other members of the complex, this species lacks a distinct filiform diverticulum at the inner end of the spermathecal ampulla. While maintaining a complex copulatory apparatus, it possesses a much simpler configuration than other species in the S. marinus complex. These differences in spermathecal structure and copulatory apparatus provide clear distinctions between this species and its counterparts within the S. marinus complex.
- Subfamily Tubificidae d’Udekem, 1855
- Genus Tubificoides Lastočkin, 1937
- Type species. Tubificoides heterochaetus Lastočkin, 1937 = T. swirencowi Jaroschenko, 1948 (non T. heterochaetus (Michaelsen, 1926)).
- Tubificoides pollex Milligan, 1991 (Figure 4A–D)
- Material examined. 1 May 2022 NIBRIV0000901383, NIBRIV0000901505, NIBRIV0000901506. Independence gate-bawi at Dokdo-ri, Ulleung-eup, Ulleung-gun, Gyeongsangbuk-do, Republic of Korea, (37.239363° N, 131.871777° E), 1 May 2022, in medium-coarse sediment of 20 m in depth at 14 °C through scuba diving. Specimen has been deposited at the National Institute of Biological Resources (NIBR).
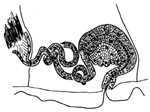
- Description. Prostomium not retractable, short, bluntly triangular. Anterior segments distinctly wider than postclitellar segments (Figure 4A). Body wall slightly papillate posteriorly with foreign material adhering. Clitellum weak, extending over X–XII. Ventral chaetae bifid, upper tooth slightly thinner than lower, 3–4 per bundle (Figure 4B). Ventral chaetae absent in XI; thereafter, two per bundle, upper tooth shorter and much thinner than lower. Posterior segments with one chaeta in both dorsal and ventral bundles. Anterior dorsal bundles of II–VIII with 1–2 bifid needle, upper thinner and shorter than lower, and 1(2) hair chaetae, short, 1(2) per bundle (Figure 4C). Pharyngeal glands in IV and V. Esophagus appearing slightly enlarged in IX. Male genitalia paired. Sperm funnel large. Vas deferens thin-walled, about twice as long as atrium, internal ciliation fine and dense. Prostate gland large, attached to atrium opposite entrance of vas deferens. Penis cuticularized, thumb-shaped, a large, oval lateral opening bearing a flap-like projection (Figure 4D). Spermathecae with narrow, elongate, and large oval ampullae. Sperm traps not well developed.
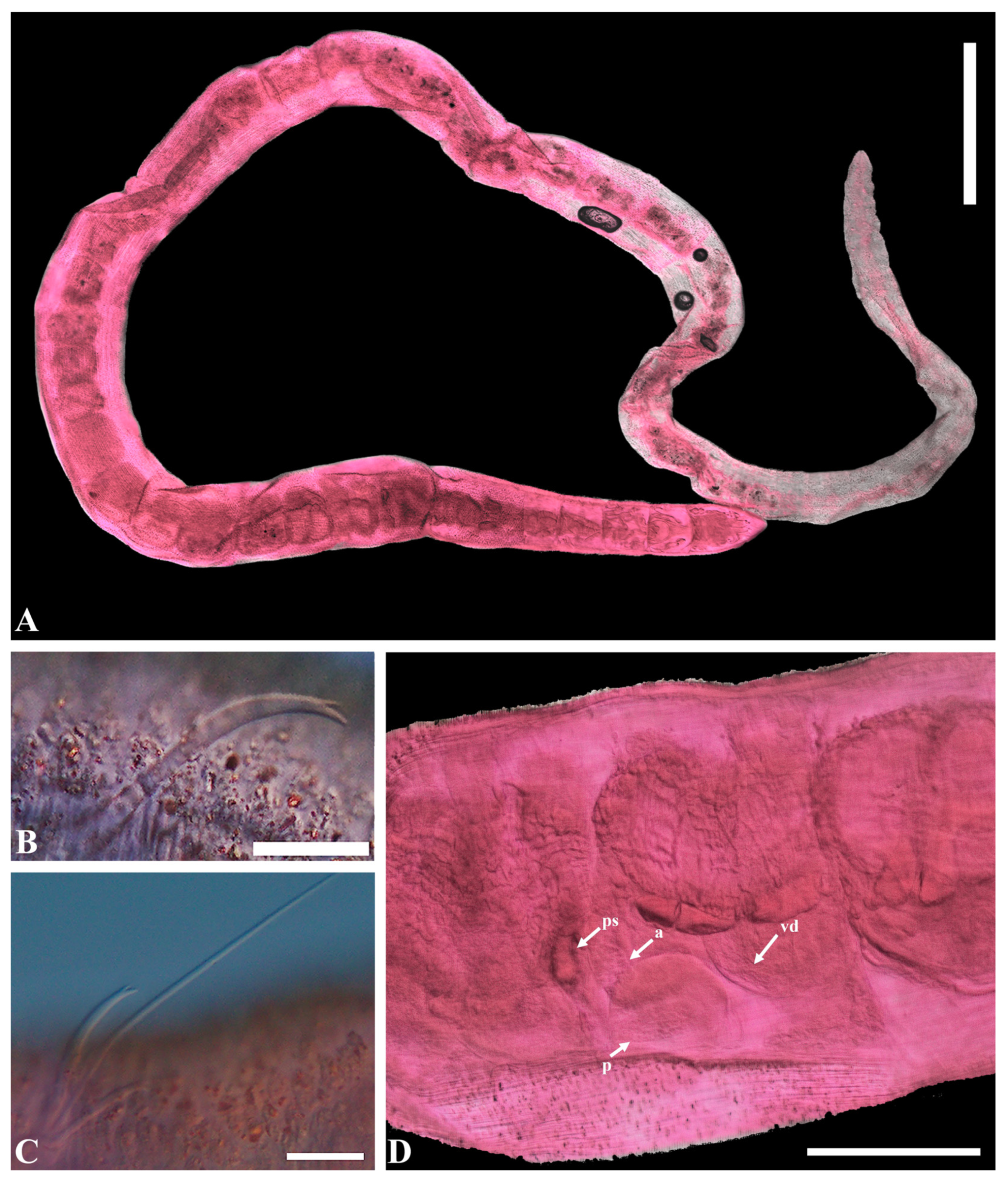
- Distribution. Europe (North Sea, Danube estuary), North America (Virginia, North Carolina, Louisiana, Florida), Northern Gulf of Mexico, and the East Sea of Korea.
- Habitat. Silt mixed with calcareous sand, subtidal to 25 m in depth.
- Remarks. Tubificoides pollex exhibits several distinctive morphological features that set it apart from other species within the genus Tubificoides. The most prominent feature is the conical shape of the cuticularized penis sheath, a characteristic not observed in any other Tubificoides species. Additionally, T. pollex features a lateral opening on the penis sheath, which is marked by a distinctive flap-like projection along its proximal margin. This unique combination of morphological characteristics distinguishes T. pollex from its closest relatives within the genus Tubificoides, including T. diazi Brinkhurst & Baker, 1979, T. crenicoleus Baker, 1983, T. longipenis (Brinkhurst, 1965), T. uncinatus Helgason & Erskus, 1987, and T. lunatus Milligan, 1991. Furthermore, none of the other species in the Tubificoides genus exhibit both a conical shape of the penis sheath and the presence of lateral openings with flap-like projections. These distinctive characteristics make T. pollex a unique and easily identifiable member of the Tubificoides genus.
- Subfamily Rhyancodrilinae Hrabe, 1963
- Genus Heterodrilus, 1902
- Type species. Heterodrilus arenicolus Pierantoni, 1902.
- Clitellio arenicolus (partim) Giere, 1979 [12] (p. 304).
- Material examined. NIBRIV0000910381, Gajae-bawi at Dokdo-ri, Ulleung-eup, Ulleung-gun, Gyeongsangbuk-do, Republic of Korea, (37.247542° N 131.862955° E), 21 June 2022, in medium-coarse sediment from 24.1 m in depth at 16 °C, obtained through scuba diving. Specimens were deposited at the National Institute of Biological Resources (NIBR).
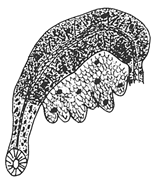
- Description. Prostomium round (Figure 5A). Clitellum extending over 1/2X–XII. Chaetae trifid, two per bundle, with upper tooth thin and short, middle tooth long and basally wide, 40–110 μm long, two per bundle, one per bundle thereafter. Posterior chaetae lower tooth generally becomes reduced and straight, single-pointed (Figure 5C). Penial chaetae simple-pointed, erect expanded, with two per bundle (Figure 5D). Male pore paired in posterior of XI. Spermathecal pore paired in anterior of X, with small epidermal pad located mid-ventrally between spermathecal pores. Coelomocytes granulated, very abundant. Male genitalia paired, vas deferens very long and tightly coiled in a spiral, narrowing entally. Atrium very long and slender, M-shaped, with slender outer muscular layer and densely granulated inner epithelium, bulbous pseudopenis. Prostate gland with large lobes broadly attached to most of the length of the atrium. Spermathecae paired, each with a narrow distinct duct and voluminous sacciform ampulla with an irregular thick wall containing large granules of secretion. Sperm arranged as large bundles.
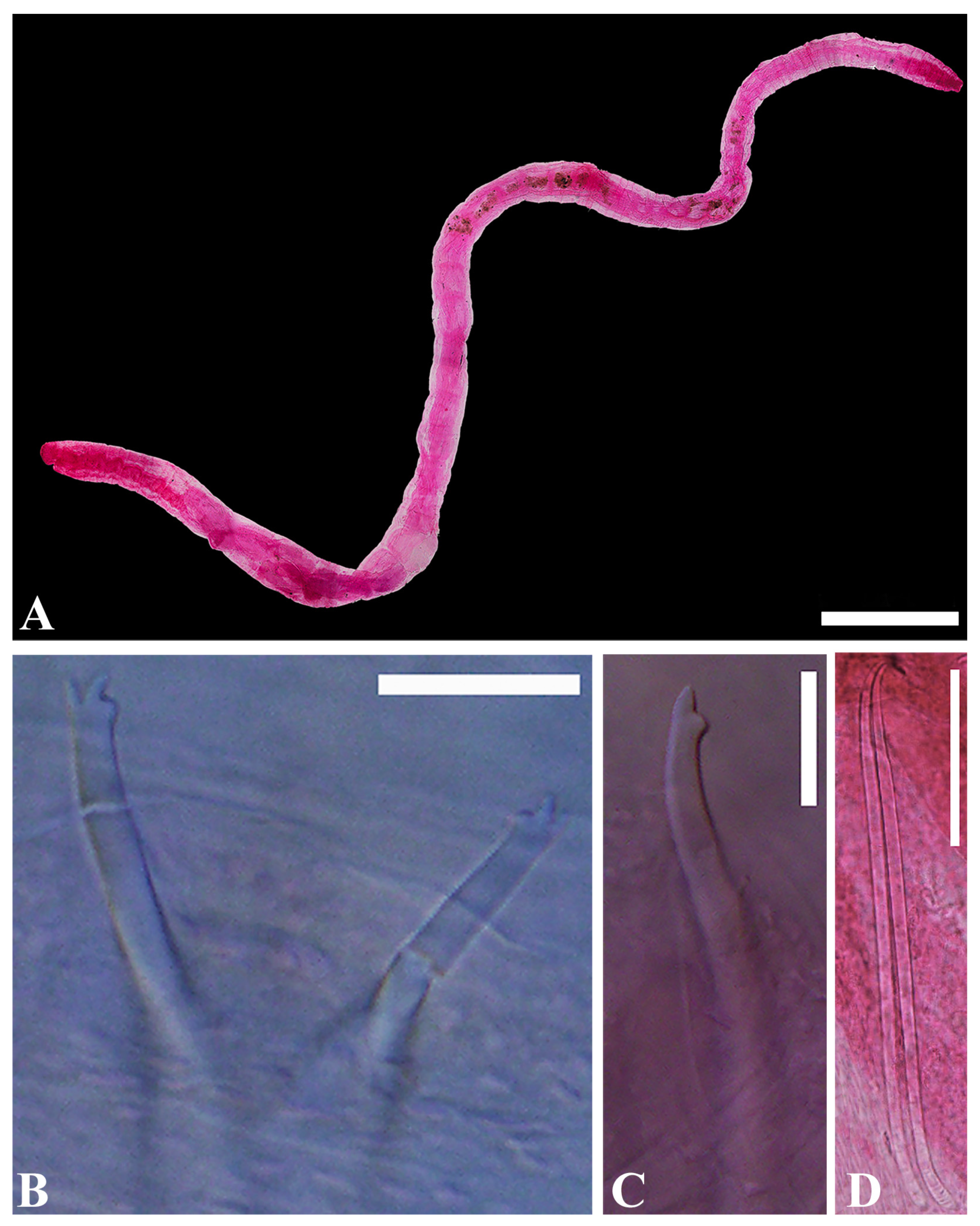
- Distribution. Caribbean (Belize, Panama), East coast of North America (Florida, North Carolina, New Jersey), Bermuda, Galapagos Islands, and the East Sea of Korea.
- Habitat. Coarse sand, subtidal from 0.5 m to 39 m in depth.
- Remarks. Heterodrilus pentcheffi has chaetae similar to H. minisetosus, H. bulbiporus, H. perkinsi, and H. koreanus n. sp. Among these, H. minisetosus, H. bulbiporus, H. perkinsi are common in Florida waters [32]. Most H. minisetosus do not have penial chaetae. If they do, they are very small. This worm has distinctly bifid chaetae in posterior segments and vas deferens that are uncoiled. Other species, such as H. bulbiporus and H. perkinsi, have distinctly bifid posterior chaeta, two per bundle. H. pentcheffi differs from H. koreanus n. sp. in terms of the shape of spermathecae and prostate gland attachment part of atrium.
- Holotype. NIBRIV0000901381. Mature, complete individual, stained in alcoholic paracarmine and whole-mounted in Canada Balsam. This specimen has been deposited at the National Institute of Biological Resources (NIBR)
- Paratype. NIBRIV0000901501, NIBRIV00009015012. Mature, complete individuals, stained in alcoholic paracarmine and whole-mounted in Canada Balsam. Specimens have been deposited at the National Institute of Biological Resources (NIBR).
- Other Material. Collected from the type locality. Ten specimens were fixed on 80% ethanol. Specimens have been deposited in the Marine Biological Resource Institute, Sahmyook University, Seoul, Republic of Korea.
- Type Locality. Independence gate-bawi at Dokdo-ri, Ulleung-eup, Ulleung-gun, Gyeongsangbuk-do, Republic of Korea (37.239363° N 131.871777° E), 1 May 2022, in medium-coarse sediment from 20 m in depth at 14 °C, obtained through scuba diving.
| Species | H. keenani | H. chenianus | H. koreanus n. sp. | H. claviatriatus | H. mediopapillosus | |
|---|---|---|---|---|---|---|
| Chaetae | Anterior chaeta (with subdental liganment) | trifid | trifid | trifid | trifid | trifid |
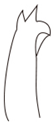 |  |  | 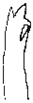 | 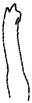 | ||
| Posterior chaeta | bifid with subdental liganment | bifid without subdental liganment | bifid with subdental liganment | bifid with subdental liganment | bifid with subdental liganment | |
 |  |  | 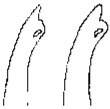 |  | ||
| Penial chaeta | relatively small, straight, single-point, ectal tips slightly curved, inconspicuous ectal node, ectal tips slightly curved | abesnt | stout, simple-pointed, relatively straight with blunt tip, slightly curved at the end | straight, single-point, ectal node followed by tapered, slightly curved tip | straight, single-pointed | |
| Spermathecae | oblong ampulla with distinct narrow duct | absent | large oval ampulla with indistinct duct | very large, sacciform ampulla with distinct narrow duct | large ampullae of varying shape with narrow lumen | |
 |  |  |  |  | ||
| Male genitalia | Atrium | C-shaped with slender | Club-shaped with more or less curved | M-shaped with cylindrical, slender, very long | Club-shaped with slender | M-shaped with tubular |
 |  | 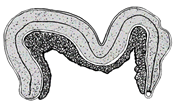 | 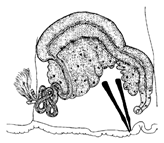 | 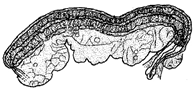 | ||
| Prostate glands | ventral surface of atrium | broadly attached ventral surface of ental part of atrium | ventral surface of atrium | ventral surface of atrium | ventral surface of atrium | |
| Pseudopenis | very in inconspicuous | not mention | small | Small, sometimes protruded | pear-shaped | |
| Vas deferens | very long, coiled | longer than atrium with irregularly coiled | long and tightly coiled, not spiral | some tight coiled, not spiral | long and irregularly coiled | |
| locality | Heron Island in Great Barrier Reef of S. Australia | Wuzhi Island in South Hainan of China | Dokdo in the East Sea of Korea | Heron Island in Great Barrier, Reef of S. Australia | Wakayama Prefecture in S. Japan | |
| reference | Erséus, 1981 [16] | Wang and Erséus, 2003 [43] | This study | Erséus, 1981 [16] | Takashima & Mawatari, 1997 [88] | |
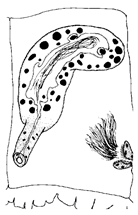
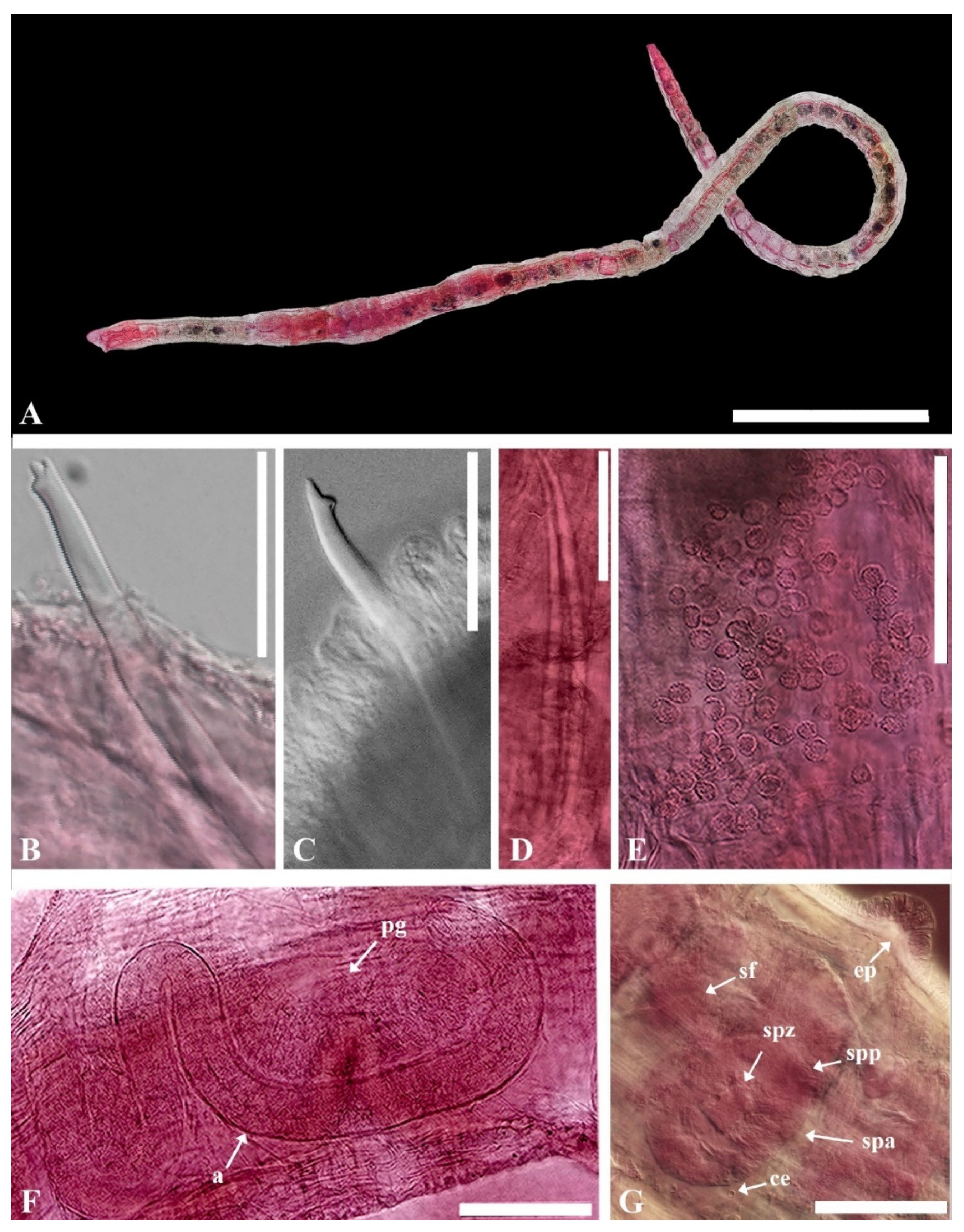
- Description. Length 1.3–1.9 mm (holotype 1.3 mm), width at X 4.33–4.85 μm (holotype 4.85 μm). Hair chaetae absent. Prostomium short, conical shape (Figure 1A). Chaetae with thin subdental liganment, 53.98–97.56 μm long, two per bundles in segments anterior to clitellum, only one chaeta representing each bundle posterior to clitellum, reversed, large, and stout. Anterior chaetae trifid with upper and middle teeth same length, pointed and lower tooth shaft present, posterior chaetae bifid with wide diverging broad teeth, upper tooth longer and thicker than lower (Figure 6B,C). Clitellum extending over 1/2X–1/2XII, without ventral chaetae in XI. Pharyngeal glands present in IV–V. Coelomocyte abundant with granulated round shape (Figure 6E). Penial chaetae, two per bundle, 269.31–321.32 μm, stout, simple-pointed, relatively straight with blunt tip and slightly curved at the end in XI (Figure 6D). Atrium cylindrical, slender, very long, 636–721 μm long, 34–63 μm wide, M-shaped with thick muscular outer layer and granulated inner epithelium in XI, sometimes extending posterior to X or anterior to XII. Prostate glands multi-lobed, attached along most of the ventral surface of the atrium (Figure 6F). Male pores paired, located slightly ventral to line of ventral somatic chaetae, posteriorly in XI. Unpaired epidermal papilla present ventrally to line in X (Figure 6G). Pseudopenis small. Vas deferens long and tightly coiled, not forming a proper spiral in anterior part of XI. Sperm funnel small in X. Spermathecae paired in X, indistinct duct and large oval ampulla with thick wall containing large granules of secretion. Spermathecal chaetae absent. Sperm in spermathecal ampulla as thimble-shaped spermatozoa (Figure 6G).
- Etymology. The specific epithet ‘koreanus’ refers to ‘Korea’, the type locality.
- Remarks. The genus Heterodrilus is large and exclusively marine. It is distributed globally in tropical and subtropical zones [16,33,46,84]. Its species are common in sandy sediments. The morphology of Heterodrilus koreanus n. sp. appears similar to that of H. claviatriatus (Erséus 1981) or H. mediopapillosus (Takashima & Mawatari, 1997) (Table 1).
- The atrium of the latter two species is very long and slender, with prostate glands attached to the ventral surface of the atrium and the vas deferens being long and irregularly coiled. Anterior chaetae are trifid, with the middle tooth being larger than the other two, containing a thin subdental ligament. The new species differs from H. claviatriatus in having spermathecae with distinct narrow ducts. The penial chaetae and atrium are shorter in our specimens. H. koreanus n. sp. more closely resembles the Japanese species H. mediopapillosus in that they share an M-shaped atrium and unpaired epidermal papilla (Table 1). However, the spermathecal ampulla of H. mediopapillosus is oval-shaped, with a faint outline of the duct (Figure 6F), while the penial chaetae of our species are shorter and slightly curved at the end (Figure 6E).
6. Molecular Analysis
7. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Brinkhurst, R.O. British and other marine and estuarine oligochaetes. In Synopses of the British Fauna (New Series); Kermack, D.M., Barnes, R.S.K., Eds.; Cambridge University Press: Cambridge, UK, 1982; Volume 21, p. 127. [Google Scholar]
- Diaz, R.J.; Erséus, C. Habitat preferences and species associations of shallow-water marine Tubificidae (Oligochaeta) from the barrier reef ecosystems off Belize, Central America. Hydrobiologia 1994, 278, 93–105. [Google Scholar] [CrossRef]
- Erséus, C. Marine Oligochaeta of Hong Kong. In Proceedings of the Second International Marine Biological Workshop, The Marine Flora and Fauna of Hong Kong and Southern China, Hong Kong, China, 2–24 April 1986; Morton, B., Ed.; Hong Kong University Press: Hong Kong, China, 1990; pp. 259–335. [Google Scholar]
- Erséus, C. Phylogeny of oligochaetous Clitellata. Hydrobiologia 2005, 535, 357–372. [Google Scholar] [CrossRef]
- Finogenova, N.P. New species of Oligochaeta from Dnieper and Bug Firth and Black Sea and revision of some species. Nauka Leningr. Tr. Zool. Instituta 1972, 52, 65–74. (In Russian) [Google Scholar]
- Hartzell, P.L.; Nghiem, J.V.; Richio, K.J.; Shain, D.H. Distribution and phylogeny of glacier ice worms (Mesenchytraeus solifugus and Mesenchytraeus solifugus rainierensis). Can. J. Zool. 2005, 83, 1206–1213. [Google Scholar] [CrossRef]
- Healy, B.; Coates, K.A. Finding enchytraeid oligochaetes (Clitellata) in hot climates: Species occurence on the shores of Bermuda. Hydrobiologia 1999, 406, 111–117. [Google Scholar] [CrossRef]
- Prantoni, A.L.; Di Domênico, M.; da Cunha Lana, P. A taxonomic overview of marine and estuarine oligochaetes from Brazil. Mar. Biodivers. 2014, 44, 275–278. [Google Scholar] [CrossRef]
- Wang, H.; Erséus, C. Marine Phallodrilinae (Oligochaeta, Tubificidae) of Hainan Island in southern China. Hydrobiologia 2001, 462, 199–204. [Google Scholar] [CrossRef]
- Lee, J.H. Taxonomic Study on the Aquatic Oligochaetes (Annelida: Clitellata) from Korea. Ph.D. Thesis, Ewha Womans University, Seoul, Republic of Korea, 2021; pp. 1–322. [Google Scholar]
- Righi, G.; Kanner, E. Marine Oligochaeta (Tubificidae and Enchytraeidae) from the Caribbean Sea. Stud. Fauna Curaçao Other Caribb. Isl. 1979, 58, 44–68. [Google Scholar]
- Giere, O. Studies on marine Oligochaeta from Bermuda, with emphasis on new Phallodrilus species (Tubificidae). Cah. Biol. Mar. 1979, 3, 301–314. [Google Scholar]
- Erséus, C. Taxonomic revision of the Marine Genus Phallodrilus Pierantoni (Oligochaeta, Tubificidae), with description of thirteen new species. Zool. Scr. 1979, 8, 187–208. [Google Scholar] [CrossRef]
- Brinkhurst, R.O.; Baker, H.R. A review of the marine Tubificidae (Oligochaeta) of North America. Can. J. Zool. 1979, 57, 1553–1569. [Google Scholar] [CrossRef]
- Erséus, C. Taxonomic Studies on the Marine Genera Aktedrilus Knöllner and Bacescuella Hrabě (Oligochaeta, Tubificidae), with with descriptions of seven new species. Zool. Scr. 1980, 9, 97–111. [Google Scholar] [CrossRef]
- Erséus, C. Taxonomic Revision of the marine genus Heterodrilus Pierantoni (Oligochaeta, Tubificidae). Zool. Scr. 1981, 10, 111–132. [Google Scholar] [CrossRef]
- Erséus, C. Taxonomic Revision of the marine genus Limnodriloides (Oligochaeta: Tubificidae). Verhandlungen Des Naturwissenschaftlichen Ver. Hambg. 1982, 25, 207–277. [Google Scholar]
- Erséus, C. Duridrilus tardus gen. et sp.n., a marine tubificid (Oligochaeta) from Bermuda and Barbados. Sarsia 1983, 68, 29–32. [Google Scholar] [CrossRef]
- Erséus, C. The marine Tubificidae (Oligochaeta) of Hong Kong and southern China. Asian Mar. Biol. 1984, 1, 135–175. [Google Scholar]
- Erséus, C. Aspects of the phylogeny of the marine Tubificidae. Hydrobiologia 1984, 115, 37–44. [Google Scholar] [CrossRef]
- Bonomi, G.; Erséus, C. A taxonomic and faunistic survey of the marine Tubificidae and Enchytraeidae (Oligochaeta) of Italy. Introduction and preliminary results. Hydrobiologia 1984, 115, 207–210. [Google Scholar] [CrossRef]
- Erséus, C. Annelida of Saudi Arabia. Marine Tubificidae (Oligochaeta) of the Arabian Gulf coast of Saudi Arabia. Fauna Saudi Arab. 1985, 6, 130–154. [Google Scholar]
- Erséus, C. Marine tubificidae (Oligochaeta) at Hutchinson Island, Florida. Proc. Biol. Soc. Wash. 1986, 99, 286–315. [Google Scholar]
- Erséus, C. A new species of Phallodrilus (Oligochaeta, Tubificidae) from a limestone cave on Bermuda. Sarsia 1986, 71, 7–9. [Google Scholar] [CrossRef]
- Milligan, M.R. Marine Tubificidae (Oligochaeta) from Puerto Rico with descriptions of two new species, Tubificoides aguadillensis and Heterodrilus paucifascis. Proc. Biol. Soc. Wash. 1987, 100, 480–489. [Google Scholar]
- Erséus, C. Two new species of the marine genus Limnodriloides and a record of Tubificoides fraseri Brinkhurst (Oligochaeta: Tubificidae) from New Zealand. N. Z. J. Mar. Freshw. Res. 1989, 23, 557–561. [Google Scholar] [CrossRef]
- Erséus, C. The marine Tubificidae (Oligochaeta) of the barrier reef ecosystems at Carrie Bow Cay, Belize, and other parts of the Caribbean Sea, with descriptions of twenty-seven new species and revision of Heterodrilus, Thalassodrilides and Smithsonidrilus. Zool. Scr. 1990, 19, 243–303. [Google Scholar] [CrossRef]
- Erséus, C. Records of the marine genus Bathydrilus (Oligochaeta: Tubificidae) from California, with descriptions of two new species. Proc. Biol. Soc. Wash. 1991, 104, 622–626. [Google Scholar]
- Erséus, C. Groundwater and marine intertidal Tubificidae (Oligochaeta) from the Canary and Cabo Verde Islands, with descriptions of two new species. Bijdr. Tot Dierkd. 1992, 62, 63–70. [Google Scholar] [CrossRef]
- Erséus, C. The marine Tubificidae (Oligochaeta) of Rottnest Island, Western Australia. In Proceedings of the Fifth International Marine Biological Workshop: The Marine Flora and Fauna of Rottnest Island, Western Australia, Rottnest Island, Australia, 5 January 1991; Wells, F.E., Walker, D.I., Kirkman, H., Lethbridge, R., Eds.; Western Australian Museum: Perth, Australia, 1993; pp. 331–390. [Google Scholar]
- Erséus, C. A new marine species of Smithsonidrilus (Oligochaeta: Tubificidae) from the Florida Keys. Proc. Biol. Soc. Wash. 1993, 106, 587–590. [Google Scholar]
- Milligan, M.R. Identification Manual for the Aquatic Oligochaeta of Florida. Volume II. Estuarine and Nearshore Marine Oligochaetes; Center for Systematics and Taxonomy: Sarasota, FL, USA, 1996; pp. 239+214. [Google Scholar]
- Morton, B. The Marine Flora and Fauna of Hong Kong and Southern China IV; Hong Kong University Press: Hong Kong, China, 1997; Volume 4. [Google Scholar]
- Erséus, C. The marine tubificidae (Oligochaeta) of Darwin Harbour, Northern Territory, Australia, with descriptions of fifteen new species. In The Marine Flora and Fauna of Darwin Harbour, Northern Territory, Australia; Hamley, R.H., Caswell, G., Megirian, D., Larson, H.K., Eds.; Museums and Art Galleries of the Northern Territory and the Australian Marine Sciences Association: Darwin, Australia, 1997; pp. 99–132. [Google Scholar]
- Erséus, C. Marine Tubificidae (Oligochaeta) from the Montebello and Houtman Abrolhos Islands, Western Australia, with descriptions of twenty-three new species. In The Marine Flora and Fauna of the Houtman Abrolhos Islands, Western Australia; Wells, F.E., Ed.; Western Australian Museum: Perth, Australia, 1997; pp. 389–458. [Google Scholar]
- Erséus, C. Additional notes on the taxonomy of the marine Oligochaeta of Hong Kong, with a description of a new species of Tubificidae. In The Marine Flora and Fauna of Hong Kong and Southern China; Morton, B., Ed.; Hong Kong University Press: Hong Kong, China, 1997; pp. 37–52. [Google Scholar]
- Erséus, C. A record of Randiella from New Caledonia, the first known occurrence of the marine interstitial family Randiellidae (Annelida; Oligochaeta) in the South Pacific Ocean. J. Nat. Hist. 1997, 31, 1745–1750. [Google Scholar] [CrossRef]
- Erséus, C. Marine Tubificidae (Oligochaeta) from a mangrove habitat in Kenya. Trop. Zool. 1999, 12, 137–143. [Google Scholar] [CrossRef][Green Version]
- Huang, Z. Marine Species and Their Distribution in China’s Seas; Krieger Pub Co.: Malabar, FL, USA, 2001. [Google Scholar]
- Giere, O. Taxonomy and new bacterial symbioses of gutless marine Tubificidae (Annelida, Oligochaeta) from the Island of Elba (Italy). Org. Divers. Evol. 2002, 2, 289–297. [Google Scholar] [CrossRef]
- Erséus, C. Mangroves and marine oligochaete diversity. Wetl. Ecol. Manag. 2002, 10, 197–202. [Google Scholar] [CrossRef]
- Wang, H.; Erséus, C. Marine species of Ainudrilus and Heterodrilus (Oligochaeta: Tubificidae: Rhyacodrilinae) from Hainan Island in southern China. N. Z. J. Mar. Freshw. Res. 2003, 37, 205–217. [Google Scholar] [CrossRef]
- Erséus, C.; Wang, H. Marine Tubificidae (Oligochaeta) of the Dampier area, Western Australia. In The Marine Flora and Fauna of Dampier, Western Australia; Wells, F.E., Walker, D.I., Jones, D.S., Eds.; Western Australian Museum: Perth, Australia, 2003; pp. 362–393. [Google Scholar]
- Erséus, C.; Giere, O.; Dreyer, J.; Bailey-Brock, J.H. A new marine species of Tubificoides (Annelida: Oligochaeta: Tubificidae) from Hawaii, U.S.A. Proc. Biol. Soc. Wash. 2005, 118, 264–269. [Google Scholar] [CrossRef]
- Mejlon, E.; De Wit, P.; Matamoros, L.; Erséus, C. DNA-based phylogeny of the marine genus Heterodrilus (Annelida, Clitellata, Naididae). J. Zool. Syst. Evol. Res. 2015, 53, 194–199. [Google Scholar] [CrossRef]
- Cai, L.; Lin, X. Quantitative distribution of marine and brackish-water Oligochaeta on the intertidal zone in Deep Bay, China. In Proceedings of the 10th International Symposium on Aquatic Oligochaeta, Wuhan, China, 16–21 October 2006; Wang, H.Z., Wetzel, M.J., Pinder, A.M., Verdonschot, P.F.M., Arslan, N., Eds.; State Key Laboratory of Freshwater Ecology and Biotechnology, Institute of Hydrobiology, Chinese Academy of Sciences: Wuhan, China, 2006; pp. 1–21. [Google Scholar]
- Chen, X.; Cai, L.; Zhou, X.; Rao, Y. Geographical variation in oligochaete density and biomass in subtropical mangrove wetlands of China. J. Ocean Univ. China 2017, 16, 925–931. [Google Scholar] [CrossRef]
- Erséus, C.; Daoyuan, S.; Yanling, L.; Bin, S. Marine Oligochaeta of Jiaozhou Bay, Yellow Sea coast of China. Hydrobiologia 1990, 202, 107–124. [Google Scholar] [CrossRef]
- Erséus, C.; Diaz, R.J. The Oligochaeta of the Cape d’Aguilar Marine Reserve, Hong Kong. In The Marine Flora and Fauna of Hong Kong and Southern China IV; Morton, B., Ed.; Hong Kong University Press: Hong Kong, China, 1997; pp. 189–204. [Google Scholar]
- Erséus, C.; Qi, S. Two aberrant Tubificidae (Oligochaeta) from Pearl River in the People’s Republic of China. Hydrobiologia 1985, 127, 193–196. [Google Scholar] [CrossRef]
- Hallett, S.; Erséus, C.; Lester, R. Actinosporea from Hong Kong marine Oligochaeta. In The Marine Flora and Fauna of Hong Kong and Southern China IV; Morton, B., Ed.; Hong Kong University Press: Hong Kong, China, 1997; pp. 1–7. [Google Scholar]
- Ohtaka, A. New record of Tubificoide brevicoleus Baker (Oligochaeta, Tubificidae) from the Pacific coasts of Hokkaido, Northern Japan. J. Fac. Sci. Hokkaido Univ. Ser. VI Zool. 1987, 25, 1–8. Available online: http://hdl.handle.net/2115/27700 (accessed on 8 November 2023).
- Takashima, Y.; Mawatari, S.F. Marine Tubificidae (Oligochaeta) from Hokkaido, northern Japan, with descriptions of two new species. Species Divers. 1996, 1, 55–70. [Google Scholar] [CrossRef]
- Takashima, Y.; Mawatari, S.F. Mitinokuidrilus excavatus n. sp., a marine tubificid (Oligochaeta) with a unique mode of reproduction. Zool. Sci. 1998, 15, 593–597. [Google Scholar] [CrossRef]
- Torii, T.; Erséus, C.; Martinsson, S.; Ito, M. Morphological and genetic characterization of the first species of Thalassodrilides (Annelida: Clitellata: Naididae: Limnodriloidinae) from Japan. Species Divers. 2016, 21, 117–125. [Google Scholar] [CrossRef]
- Takashima, Y. Taxonomy of marine Tubificidae of Japan (6) Subfamily Limnodriloidinae. Aquabiology 2000, 22, 398–402. [Google Scholar]
- Takashima, Y. Taxonomy of the marine Tubificidae of Japan (8) Subfamily Phallodrilinae. Aquabiology 2001, 23, 70–76. [Google Scholar]
- Choi, H.K.; Jung, T.W.; Yoon, S.M. A new species of Lagis (Annelida: Polychaeta: Pectinariidae) from Korean waters. Zootaxa 2017, 4227, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Jeong, M.-K.; Wi, J.H.; Suh, H.-L. A reassessment of Capitella species (Polychaeta: Capitellidae) from Korean coastal waters, with morphological and molecular evidence. Mar. Biodivers. 2018, 48, 1969–1978. [Google Scholar] [CrossRef]
- Jong, W.L. Systematic studies on Syllidae (Annelida, Polychaeta) from the South Sea and the East Sea in Korea. Anim. Syst. Evol. Divers. 1994, 10, 131–144. [Google Scholar]
- Lee, J.H.; Je, J.G.; Choi, J.W. Taxonomical review of Perinereis aibuhitensis Grube, 1878 (Nereidae: Polychaeta) in Korea. Korean J. Syst. Zool. 1992, 8, 1–10. [Google Scholar]
- Park, T.-S.; Kim, W. A taxonomic study on Perinereis nuntia species group (Polychaeta: Nereididae) of Korea. Anim. Syst. Evol. Divers. 2007, 23, 75–85. [Google Scholar] [CrossRef]
- Rho, B.J.; Lee, J.W. A systematic study on the errantiate Polychaeta in Korea. Anim. Syst. Evol. Divers. 1987, 3, 74–90. [Google Scholar]
- Rho, B.J.; Song, K.H. A study on the classification of the Korean Polychaeta (I). Jous. Kor. Res. Inst. Bet 1974, 54, 73–85. [Google Scholar]
- Lee, J.H.; Jae, J.G. Polychaetous annelids from the Yellow Sea. Ocean Polar Res. 1983, 5, 19–27. [Google Scholar]
- Paik, E.-I. New records of five polychaetous Annelida species in Korea. Korean J. Fish. Aquat. Sci. 1979, 12, 35–39. [Google Scholar]
- Lee, J.H.; Jung, J.W. The First Record of Marine Oligochaete, Marionina coatesae (Annelida; Oligochaeta; Enchytraeidae) from Korean Waters. Anim. Syst. Evol. Divers. 2021, 37, 171–176. [Google Scholar] [CrossRef]
- Dózsa-Farkas, K.; Ortmann-Ajkai, A.; Horváth, F. New enchytraeid species (Oligochaeta: Enchytraeidae) from the Danube–Dráva National Park. Acta Zool. Acad. Sci. Hung. 2015, 61, 305–327. [Google Scholar] [CrossRef]
- Cho, J.-S.R.; Kim, Y.-M.; Sagong, J.; Lee, J.-H.; Yeo, M.-Y.; Bahn, S.-Y.; Kim, H.-M.; Lee, G.-S.; Lee, D.-H.; Choo, Y.-S. Biodiversity of marine invertebrates on rocky shores of Dokdo, Korea. Zool. Stud. 2012, 51, 710–726. [Google Scholar]
- Lee, S.H.; Kang, C.-K.; Lee, C.I.; Kwak, J.H. Current status of the East Sea ecosystem in a changing world. Deep Sea Res. Part II Top. Stud. Oceanogr. 2017, 146, 101–103. [Google Scholar] [CrossRef]
- Jo, Y.-H.; Choi, J.-G.; Park, J. Physical boundaries of intrathermocline Ulleung eddies in the East/Japan Sea. Deep Sea Res. Part II Top. Stud. Oceanogr. 2017, 143, 15–23. [Google Scholar] [CrossRef]
- Higgins, R.P.; Thiel, H. Introduction to the Study of Meiofauna; Smithsonian Institution Press: Washington, DC, USA, 1988. [Google Scholar]
- Erseus, C.; Healy, B. Oligochaeta. In Taxonomic Atlas of the Benthic Fauna of the Santa Maria Basin and Western Santa Barbara Channel; US Department of the Interior, Minerals Management Service, Pacific OCS Region: Camarillo, CA, USA, 1994; Volume 4, pp. 5–38. [Google Scholar]
- Norén, M.; Jondelius, U. Phylogeny of the Prolecithophora (Platyhelminthes) inferred from 18S rDNA sequences. Willi Hennig Soc. Cladistics 1999, 15, 103–112. [Google Scholar] [CrossRef]
- Erséus, C.; Kallersjö, M.; Ekman, M.; Hovmöller, R. 18S rDNA phylogeny of the Tubificidae (Clitellata) and its constituent taxa: Dismissal of the Naididae. Mol. Phylogenetics Evol. 2002, 22, 414–422. [Google Scholar] [CrossRef]
- Palumbi, S.R.; Martin, A.; Romano, S.; McMillan, W.O.; Stice, L.; Grabowski, G. The Simple Fool’s Guide to PCR, Version 2.0; University of Hawaii: Honolulu, HI, USA, 1991; pp. 1–48. [Google Scholar]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit 1 from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.F.; von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Huelsenbeck, J.P.; Ronquist, F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 2001, 17, 754–755. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Teslenko, M.; Van Der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Pierantoni, U. Due nuovi generi di Oligocheti marini rinvenuti nel Golfo di Napoli. Boll. Soc. Nat. Napoli 1902, 16, 113–117. [Google Scholar]
- Sjölin, E.; Erséus, C. New species of Heterodrilus (Oligochaeta, Tubificidae) and records of H. maiusculus from the Mediterranean Sea. Ital. J. Zool. 2001, 68, 223–228. [Google Scholar] [CrossRef]
- Cook, D.G. The systematics and distribution of marine Tubificidae (Annelida: Oligochaeta) in the Bahia de San Quintin, Baja California, with descriptions of five new species. Bull. South. Calif. Acad. Sci. 1974, 73, 126–140. [Google Scholar]
- Milligan, M.R. Two new species of Tubificoides (Oligochaeta, Tubificidae) and new records of T. brownae and T. imajimai from the Gulf of Mexico and Caribbean, with a redescription of T. bakeri. Zool. Scr. 1991, 20, 339–345. [Google Scholar] [CrossRef]
- Erséus, C. Interstitial fauna of Galapagos XXXIII. Tubificidae (Annelida, Oligochaeta). Microfauna Mar. 1984, 1, 191–198. [Google Scholar]
- Takashima, Y.; Mawatari, S.F. Marine Tubificidae (Oligochaeta, Annelida) from Shirahama, Western Japan, with a description of a new species. Publ. Seto Mar. Biol. Lab. 1997, 38, 29–36. Available online: http://hdl.handle.net/2433/176274 (accessed on 8 November 2023). [CrossRef][Green Version]
- Erséus, C. Oligochaeta from Hoi Ha Wan. In The Marine Flora and Fauna of Hong Kong and Southern China III, Proceedings of the Fourth International Marine Biological Workshop: Hong Kong and South China, Hong Kong, China, 11–29 April 1989; Morton, B., Ed.; Hong Kong University Press: Hong Kong, China, 1992; pp. 909–917. [Google Scholar]

| Primer Name | Primer Sequence | Reference |
|---|---|---|
| 18S Primer | ||
| TimA | 5′-AMCTGGTTGATCCTGCCAG-3′ | Tim Littlewood (pers. comm. Norén and Jondelius 1999) [75] |
| TimB | 5′-TGATCCATCTGCAGGTTCACCT-3′ | |
| 660F | 5′-GATCTCGGGTCCAGGCT-3′ | Erséus et al., 2002 [76] |
| 1100R | 5′-GATCGTCTTCGAACCTCTG-3′ | Norén and Jondelius, 1999 [77] |
| 16S Primer | ||
| 16S ar-L | 5′-CGC CTG TTT ATC AAA AAC AT-3′ | Palumbi et al., 1999 [78] |
| 16S br-H | 5′-CGC CTG TTT ATC AAA AAC AT-3′ | |
| COI Primer | ||
| LCO1490 | 5′-GGTCAACAAATCATAAAGATATTGG-3′ | Folmer et al., 1994 [79] |
| HCO2198yy | 5′-TAAACTTCAGGGTGACCAAAAAATCA-3′ | |
| Species | Locality | 16S | 18S | COI |
|---|---|---|---|---|
| INGROUP: Clitellata, Naididae | ||||
| Heterodrilus bulbiporus Erséus 1981 | Fort Pierce, Florida, USA | KJ753872 | KJ753886 | KJ753850 |
| Heterodrilus cf. virilis Erséus 1992a | Lizard Island, Australia | KJ753880 | KJ753892 | KJ753853 |
| Heterodrilus chenianus Wang and Erséus 2003 | Hainan, China | AY885601 | AY885574 | KJ753856 |
| Heterodrilus decipiens Erséus 1997a | Rottnest Island, W. Australia | AY885603 | AF209455 | - |
| Heterodrilus devexus Erséus 1997a | Dampier, W. Australia | AY885602 | AY885575 | - |
| Heterodrilus ersei (Giere 1979) | Lee Stocking Island, Bahamas | AY885606 | AY885576 | KJ753857 |
| Heterodrilus flexuosus Erséus 1990 | Carrie Bow Cay, Belize | AY885600 | AY885573 | - |
| Heterodrilus keenani Erséus, 1981 | Heron Island, Australia | - | AY040688 | AY040703 |
| Heterodrilus minisetosus Erséus 1981 | Lee Stocking Island, Bahamas | AY885599 | AF411885 | KJ753859 |
| Heterodrilus modestus Erséus 1990 | Lee Stocking Island, Bahamas | KJ753876 | KJ753888 | KJ753855 |
| Heterodrilus koreanus n. sp.1 | Dokdo, the East Sea, Korea | MW795716 | MW794267 | MT250545 |
| Heterodrilus koreanus n. sp.2 | Dokdo, the East Sea, Korea | MW795717 | MW794268 | MT250546 |
| Heterodrilus koreanus n. sp.3 | Dokdo, the East Sea, Korea | MW795718 | MW794269 | MT250547 |
| Heterodrilus koreanus n. sp.4 | Dokdo, the East Sea, Korea | MW795719 | MW794270 | MT250548 |
| Heterodrilus koreanus n. sp.5 | Dokdo, the East Sea, Korea | MW795720 | MW794271 | MT250549 |
| Heterodrilus occidentalis Erseus 1981 | Fort Pierce, Florida, USA | KJ753877 | KJ753889 | - |
| Heterodrilus paucifascis Milligan 1987 | Carrie Bow Cay, Belize | AY885605 | AF411865 | KJ753858 |
| Heterodrilus pentcheffi Erséus 1981 | Lee Stocking Island, Bahamas | KJ753878 | KJ753890 | KJ753854 |
| Heterodrilus perkinsi Erséus 1986 | Fort Pierce, Florida, USA | KJ753879 | KJ753891 | KJ753849 |
| Heterodrilus queenslandicus (Jamieson, 1977) | Heron Island, Australia | AY885604 | AF411881 | - |
| OUTGROUPS: Clitellata, Naididae | ||||
| Heronidrilus heronae (Erséus and Jamieson, 1981) | Heron Island, Australia | AY885616 | AF209454 | KJ753861 |
| Adelodrilus pusillus Erséus, 1978 | Koster area, SW Sweden | KJ753881 | KJ753894 | KJ753867 |
| Aktedrilus arcticus (Erséus, 1978) | Koster area, SW Sweden | AY885591 | AF209451 | AF064042 |
| Pectinodrilus molestus (Erséus, 1988) | Carrie Bow Cay, Belize | AY885598 | AF209462 | KJ753864 |
| Thalassodrilus prostatus (Knöllner, 1935) | Göteborg, SW Sweden | KJ753883 | KJ753896 | KJ753871 |
| Tubificoides pseudogaster (Dahl, 1960) | Kysing Fjord, Denmark | AY885609 | AF411873 | HM460209 |
| Heronidrilus gravidus Erséus, 1990 | Carrie Bow Cay, Belize | AY885617 | AF411887 | - |
| Clitellata, Phreodrilidae | ||||
| Insulodrilus bifidus Pinder and Brinkhurst, 1997 | Bow River, W. Australia | AY885593 | AF411906 | KJ753862 |
| Clitellata, Enchytraeidae | ||||
| Buchholzia fallax Michaelsen, 1887 | Toscana (soil), Italy | AY885581 | AF411895 | KJ753848 |
| Polychaeta | ||||
| Ophelina acuminata Örsted, 1843 | Øresund Strait, Denmark | HM746716 | AY340439 | MN138411 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Lee, T. A New Species and Four New Recorded Species of Naididae (Annelida: Oligochaeta) from Korea. Diversity 2024, 16, 7. https://doi.org/10.3390/d16010007
Lee J, Lee T. A New Species and Four New Recorded Species of Naididae (Annelida: Oligochaeta) from Korea. Diversity. 2024; 16(1):7. https://doi.org/10.3390/d16010007
Chicago/Turabian StyleLee, Jeounghee, and Taekjun Lee. 2024. "A New Species and Four New Recorded Species of Naididae (Annelida: Oligochaeta) from Korea" Diversity 16, no. 1: 7. https://doi.org/10.3390/d16010007
APA StyleLee, J., & Lee, T. (2024). A New Species and Four New Recorded Species of Naididae (Annelida: Oligochaeta) from Korea. Diversity, 16(1), 7. https://doi.org/10.3390/d16010007






