The Velamen Radicum Is Common in the Genus Anthurium, Both in the Epiphytic and Terrestrial Species
Abstract
:1. Introduction
2. Materials and Methods
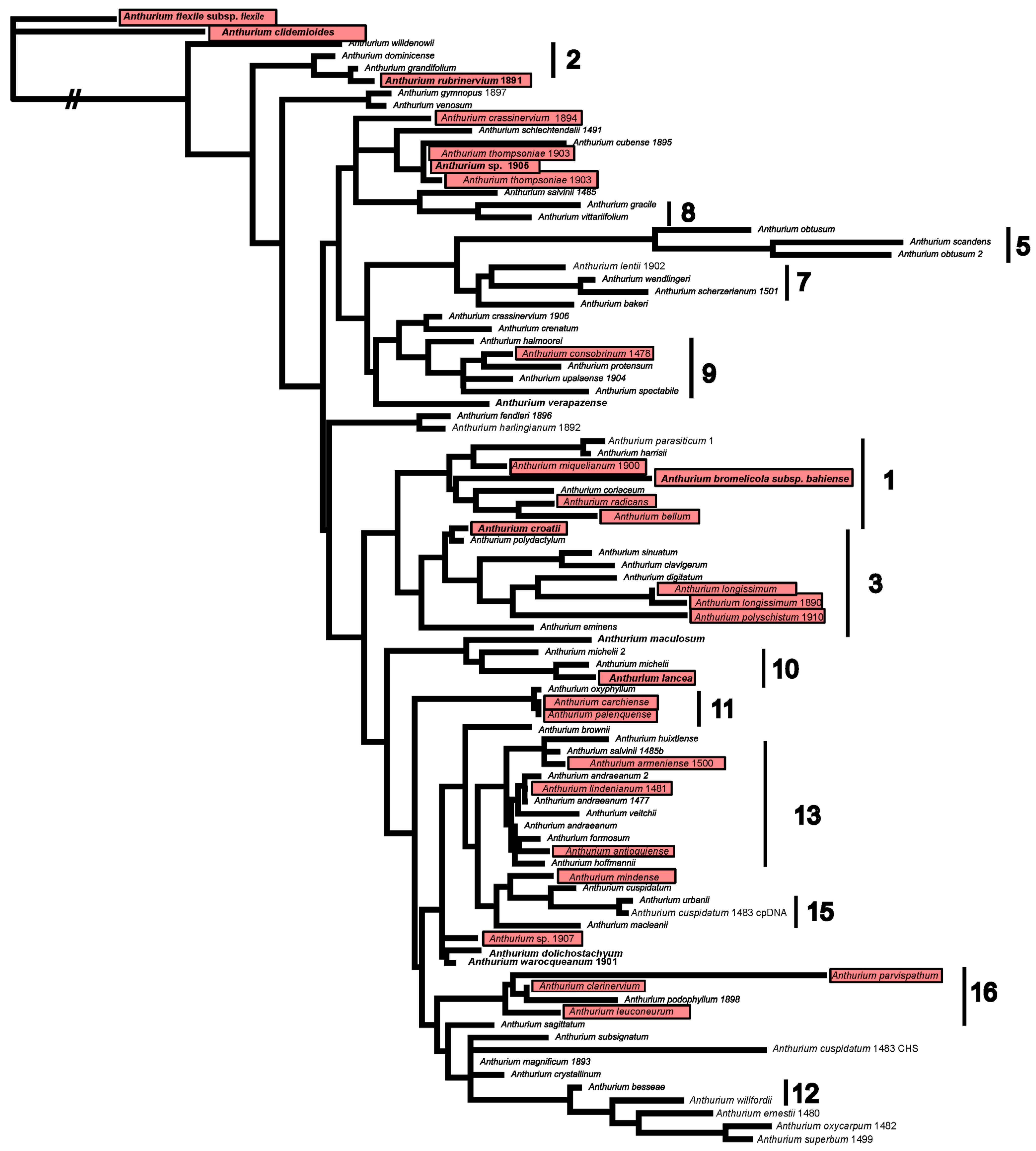
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pridgeon, A.M. The velamen and exodermis of orchid roots. In Orchid Biology. Reviews and Perspectives IV; Arditti, J., Ed.; Cornell University Press: Ithaca, NY, USA, 1987; pp. 139–192. [Google Scholar]
- Link, H.F. Elementa Philosophiae Botanicae; Haude & Spenersche: Berlin, Germany, 1824. [Google Scholar]
- Schleiden, M.J. Grundzüge der Wissenschaftlichen Botanik; Engelmann: Leipzig, Germany, 1842. [Google Scholar]
- Lüttge, U. Physiological Ecology of Tropical Plants, 2nd ed.; Springer: Berlin, Germany, 2008; p. 458. [Google Scholar]
- Benzing, D.H. Vascular Epiphytes. General Biology and Related Biota; Cambridge University Press: Cambridge, UK, 1990; p. 354. [Google Scholar]
- Zotz, G.; Winkler, U. Aerial roots of epiphytic orchids: The velamen radicum and its role in water and nutrient uptake. Oecologia 2013, 171, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Chomicki, G.; Bidel, L.P.R.; Ming, F.; Coiro, M.; Zhang, X.; Wang, Y.; Baissac, Y.; Jay-Allemand, C.; Renner, S.S. The velamen protects photosynthetic orchid roots against UV-B damage, and a large dated phylogeny implies multiple gains and losses of this function during the Cenozoic. New Phytol. 2015, 205, 1330–1341. [Google Scholar] [CrossRef] [PubMed]
- Mulay, B.N.; Panikkar, T.K.B. Origin, development and structure of velamen in the roots of some species of terrestrial orchids. Proc. Rajasthan Acad. Sci. 1956, 6, 31–48. [Google Scholar]
- Mulay, B.N.; Deshpande, B.D. Velamen in terrestrial monocots. I. Ontogeny and morphology of velamen in the Liliaceae. J. Indian Bot. Soc. 1959, 38, 383–390. [Google Scholar]
- Deshpande, B.D. Velamen in terrestrial monocots. II. Ontogeny and morphology of velamen in the Amaryllidaceae with a discussion on the exodermis in the Liliaceae and Amaryllidaceae. J. Indian Bot. Soc. 1960, 39, 593–600. [Google Scholar]
- Zotz, G.; Schickenberg, N.; Albach, D. The velamen radicum is common among terrestrial monocotyledons. Ann. Bot. 2017, 120, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Bowes, B.G.; Mauseth, J.D. Plant Structure, 2nd ed.; Manson Publishing: London, UK, 2008. [Google Scholar]
- Rudall, P. Anatomy of Flowering Plants; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Daubenmire, R.F. Plants and the Environment. A Textbook of Plant Autecology; Wiley: New York, NY, USA, 1959; p. 422. [Google Scholar]
- Porembski, S.; Barthlott, W. Velamen radicum micromorphology and classification of Orchidaceae. Nord. J. Bot. 1988, 8, 117–137. [Google Scholar] [CrossRef]
- Leitgeb, H. Die Luftwurzeln der Orchideen. Denkschr. Kaiserl. Akad. Wissensch. Mat.-Nat. Kl. 1865, 24, 179–222. [Google Scholar]
- World Flora Online. 2023. Available online: http://www.worldfloraonline.org (accessed on 7 November 2023).
- Cusimano, N.; Bogner, J.; Mayo, S.J.; Boyce, P.C.; Wong, S.Y.; Hesse, M.; Hetterscheid, W.L.A.; Keating, R.C.; French, J.C. Relationships within the Araceae: Comparison of morphological patterns with molecular phylogenies. Am. J. Bot. 2011, 98, 654–668. [Google Scholar] [CrossRef]
- Carlsen, M.M.; Croat, T.B. A molecular phylogeny of the species-rich Neotropical genus Anthurium (Araceae) based on combined chloroplast and nuclear DNA. Syst. Bot. 2013, 38, 576–588. [Google Scholar] [CrossRef]
- Zotz, G.; Weigelt, P.; Kessler, M.; Kreft, H.; Taylor, A. EpiList 1.0—A global checklist of vascular epiphytes. Ecology 2021, 102, e03326. [Google Scholar] [CrossRef]
- Sanford, W.W.; Adanlawo, I. Velamen and exodermis characters of West African epiphytic orchids in relation to taxonomic grouping and habitat tolerance. Bot. J. Linn. Soc. 1973, 66, 307–321. [Google Scholar] [CrossRef]
- Parrilla Díaz, A.T.; Ackerman, J.D. Epiphyte roots: Anatomical correlates to environmental parameters in Puerto Rican orchids. Orquídea 1990, 12, 105–116. [Google Scholar]
- Zotz, G.; Almeda, F.; Bautista-Bello, A.P.; Eskov, A.K.; Giraldo-Cañas, D.; Hammel, B.E.; Harrison, R.D.; Köster, N.; Krömer, T.; Lowry, P.P., II; et al. Hemiepiphytes revisited. Perspect. Plant Ecol. Evol. Syst. 2021, 51, 125620. [Google Scholar] [CrossRef]
- Ibisch, P.L. Neotropische Epiphytendiversität-das Beispiel Bolivien; Martina Galunder-Verlag: Wiehl, Germany, 1996; Volume 1, p. 357. [Google Scholar]
- Zotz, G.; Einzmann, H.J.R. How epiphytic are filmy ferns? A semi-quantitative approach. Diversity 2023, 15, 270. [Google Scholar] [CrossRef]
- Bautista-Bello, A.P.; Krömer, T.; Acebey, A.R.; Weichgrebe, L.; Zotz, G. Variación biológica en las aráceas trepadoras. Acta Bot. Mex. 2021, 128, e1819. [Google Scholar] [CrossRef]
- Hammel, B.E.; Grayum, M.H.; Herrera, C.; Zamora, N. (Eds.) Manual de Plantas de Costa Rica. Volume II; Missouri Botanical Garden: St. Louis, MO, USA, 2003; p. 694. [Google Scholar]
- Cornejo-Tenorio, G.; Ibarra-Manríquez, G.; Sinaca-Colín, S. Flora de Los Tuxtlas; Universidad Nacional Autónoma de México: Mexico City, Mexico, 2019. [Google Scholar]
- Acebey, A.; Krömer, T. Diversity and distribution of Araceae of the Reserva de la Biosfera Los Tuxtlas, Veracruz, Mexico. Rev. Mex. Biodiv. 2008, 79, 465–471. [Google Scholar] [CrossRef]
- La Selva Florula Digital. Available online: https://sura.ots.ac.cr/florula4/index.php (accessed on 1 August 2023).
- Jørgensen, P.M.; León-Yánez, S. Catalogue of the Vascular Plants of Ecuador; Missouri Botanical Garden: St. Louis, MO, USA, 1999; Volume 75, pp. i–viii, 1182. [Google Scholar]
- Starfinger, U.; Karrer, G. A standard protocol for testing viability with the Triphenyl Tetrazolium Chloride (TTC) Test. Jul.-Kühn-Arch. 2016, 455, 65–66. [Google Scholar] [CrossRef]
- Witty, M. Topographischer Nachweis der Keimfähigkeit der Getreidefrüchte durch Tetrazoliumsalze (Topographic Detection of Germination in Cereal Crops by Tetrazolium Salts—A Translation of Lakon’s 1942 Paper on Tetrazolium Seed Testing). Seed Technol. 2012, 34, 275–282. [Google Scholar]
- Boyce, P.C.; Croat, T.B. The Überlist of Araceae, Totals for Published and Estimated Number of Species in Aroid Genera. 2011. Available online: http://www.aroid.org/genera/20201008Uberlist.pdf (accessed on 1 August 2023).
- Shaw, J.; Lickey, E.B.; Beck, J.T.; Farmer, S.B.; Liu, W.; Miller, J.; Siripun, K.C.; Winder, C.T.; Schilling, E.E.; Small, R.L. The tortoise and the hare II: Relative utility of 21 noncoding chloroplast DNA sequences for phylogenetic analysis. Am. J. Bot. 2005, 92, 142–166. [Google Scholar] [CrossRef]
- Hamilton, M.B. Four primers for the amplification of chloroplast intergenic regions with intraspecific variation. Mol. Ecol. 1999, 8, 513–525. [Google Scholar]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucl. Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [PubMed]
- Kozlov, A.M.; Darriba, D.; Fouri, T.; Morel, B.; Stamatakis, A. RAxML-NG: A fast, scalable, and user-friendly tool for maximum likelihood phylogenetic inference. Bioinformatics 2019, 35, 4453–4455. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: http://www.R-project.org (accessed on 12 September 2023).
- Paradis, E.; Claude, J.; Strimmer, K. APE: Analyses of phylogenetics and evolution in R language. Bioinformatics 2004, 20, 289–290. [Google Scholar] [CrossRef] [PubMed]
- Revell, L.J. phytools: An R package for phylogenetic comparative biology (and other things). Methods Ecol. Evol. 2012, 3, 217–223. [Google Scholar] [CrossRef]
- Borges, R.; Machado, J.P.; Gomes, C.; Rocha, A.P.; Antunes, A. Measuring phylogenetic signal between categorical traits and phylogenies. Bioinformatics 2018, 35, 1862–1869. [Google Scholar] [CrossRef]
- Blomberg, S.P.; Garland, T.; Ives, A.R. Testing for phylogenetic signal in comparative data: Behavioral traits are more labile. Evolution 2003, 57, 717–745. [Google Scholar] [CrossRef]
- Kembel, S.W.; Cowan, P.D.; Helmus, M.R.; Cornwell, W.K.; Morlon, H.; Ackerly, D.D.; Blomberg, S.P.; Webb, C.O. Picante: R tools for integrating phylogenies and ecology. Bioinformatics 2010, 26, 1463–1464. [Google Scholar] [CrossRef]
- Govaerts, R.; Frodin, D.G.; Bogner, J. World Checklist and Bibliography of Araceae (and Acoraceae); The Board of Trustees of the Royal Botanic Gardens: Kew, UK, 2002. [Google Scholar]
- Troll, W.; Höhn, K. Allgemeine Botanik: Ein Lehrbuch auf Vergleichend-Biologischer Grundlage; Enke: Stuttgart, Germany, 1973. [Google Scholar]
- Lambers, H.; Chapin III, F.S.; Pons, T.L. Plant Physiological Ecology, 2nd ed.; Springer: New York, NY, USA, 2008; p. 540. [Google Scholar]
- Lösch, R. Wasserhaushalt der Pflanzen; Quelle & Meyer: Wiebelsheim, Germany, 2001; p. 595. [Google Scholar]
- Kowalski, V.K.; Oliveira, F.M.C.d.; Voltolini, C.H.; Tardivo, R.C.; Mourão, K.S.M. Velamen or uniseriate epidermis? Root apices in Bromeliaceae Juss. Flora 2019, 250, 9–17. [Google Scholar] [CrossRef]
- Futuyma, D.J. Evolution; Sinauer: Sunderland, UK, 2005. [Google Scholar]
- de Tombeur, F.; Lambers, H.; Cooke, J.; Hartley, S.; Katz, O.; Schaller, J.; Violle, C. Why do plants silicify? Trends Ecol. Evol. 2023, 38, 275–288. [Google Scholar] [CrossRef]
- Hauber, F.; Konrad, W.; Roth-Nebelsick, A. Aerial roots of orchids: The velamen radicum as a porous material for efficient imbibition of water. Appl. Phys. A 2020, 126, 885. [Google Scholar] [CrossRef]
- Joca, T.A.C.; Oliveira, D.C.d.; Zotz, G.; Winkler, U.; Moreira, A.S.P. The velamen of epiphytic orchids: Variation in structure and correlations with nutrient absorption. Flora 2017, 230, 66–74. [Google Scholar] [CrossRef]
- Rodríguez Quiel, C.; Einzmann, H.J.R.; Zotz, G. Does a velamen radicum effectively protect epiphyte roots against excessive infrared radiation? Plants 2023, 12, 1695. [Google Scholar] [CrossRef] [PubMed]
- Benzing, D.H. Aerial roots and their environments. In Plant Roots: The Hidden Half; Waisel, Y., Eshel, A., Kafkafi, U., Eds.; Marcel Dekker: New York, NY, USA, 1996; pp. 875–894. [Google Scholar]
- Benzing, D.H.; Ott, D.W.; Friedman, W.E. Roots of Sobralia macrantha (Orchidaceae): Structure and function of the velamen-exodermis complex. Am. J. Bot. 1982, 69, 608–614. [Google Scholar] [CrossRef]
- Zotz, G.; Bautista-Bello, A.P.; Kohlstruck, J.; Weichgrebe, T. Life forms in aroids—Natural variability vs. terminological confusion. Aroideana 2020, 43, 315–333. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Werner, J.C.; Albach, D.C.; Can, L.; Zotz, G. The Velamen Radicum Is Common in the Genus Anthurium, Both in the Epiphytic and Terrestrial Species. Diversity 2024, 16, 18. https://doi.org/10.3390/d16010018
Werner JC, Albach DC, Can L, Zotz G. The Velamen Radicum Is Common in the Genus Anthurium, Both in the Epiphytic and Terrestrial Species. Diversity. 2024; 16(1):18. https://doi.org/10.3390/d16010018
Chicago/Turabian StyleWerner, Julia C., Dirk C. Albach, Levent Can, and Gerhard Zotz. 2024. "The Velamen Radicum Is Common in the Genus Anthurium, Both in the Epiphytic and Terrestrial Species" Diversity 16, no. 1: 18. https://doi.org/10.3390/d16010018
APA StyleWerner, J. C., Albach, D. C., Can, L., & Zotz, G. (2024). The Velamen Radicum Is Common in the Genus Anthurium, Both in the Epiphytic and Terrestrial Species. Diversity, 16(1), 18. https://doi.org/10.3390/d16010018








