Habitat Selection to Reintroduce Iris bismarckiana in Semi-Arid Environments
Abstract
1. Introduction
2. Materials and Methods
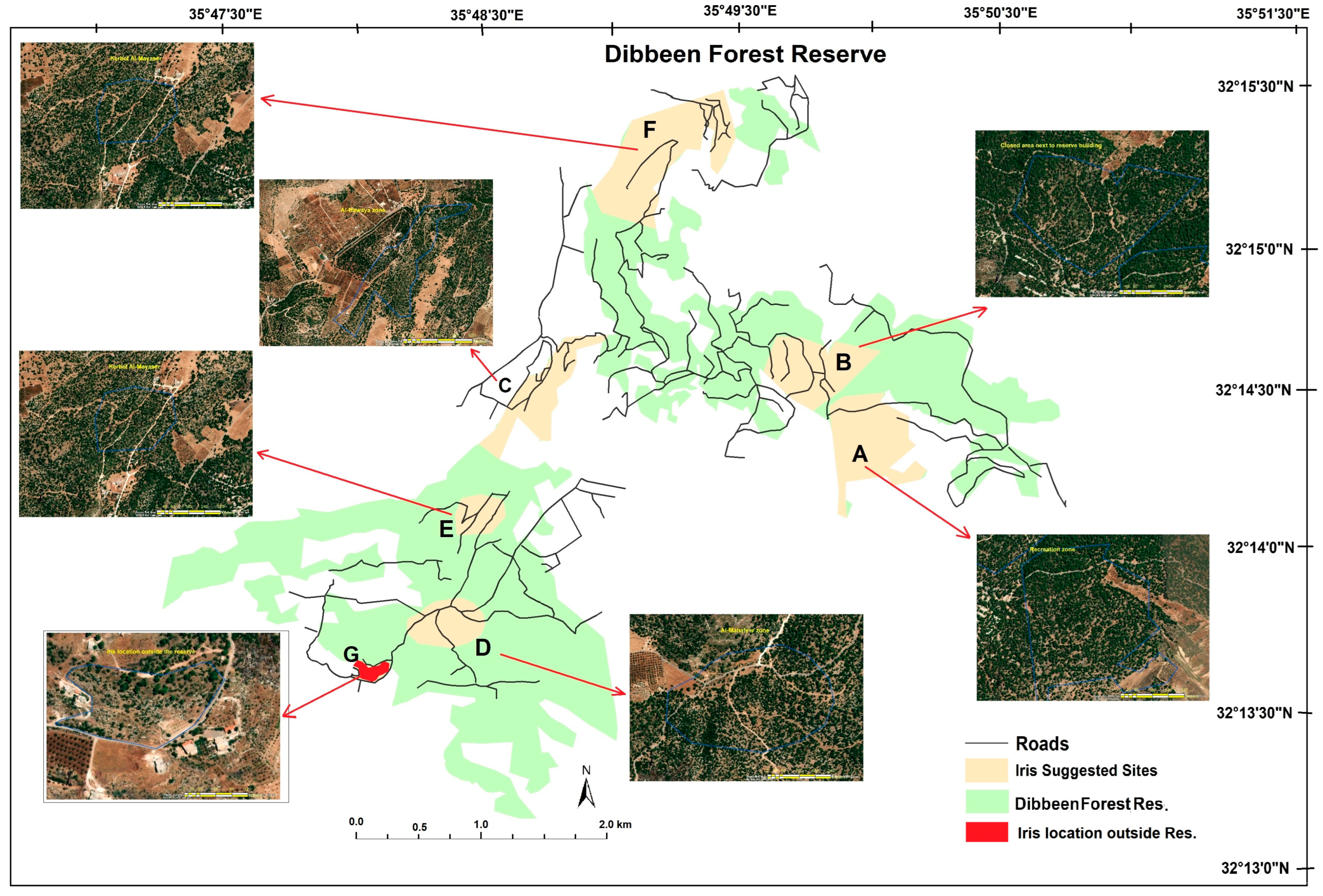
2.1. Study Site
2.2. A Brief History of Nazareth Iris
2.3. Measurements
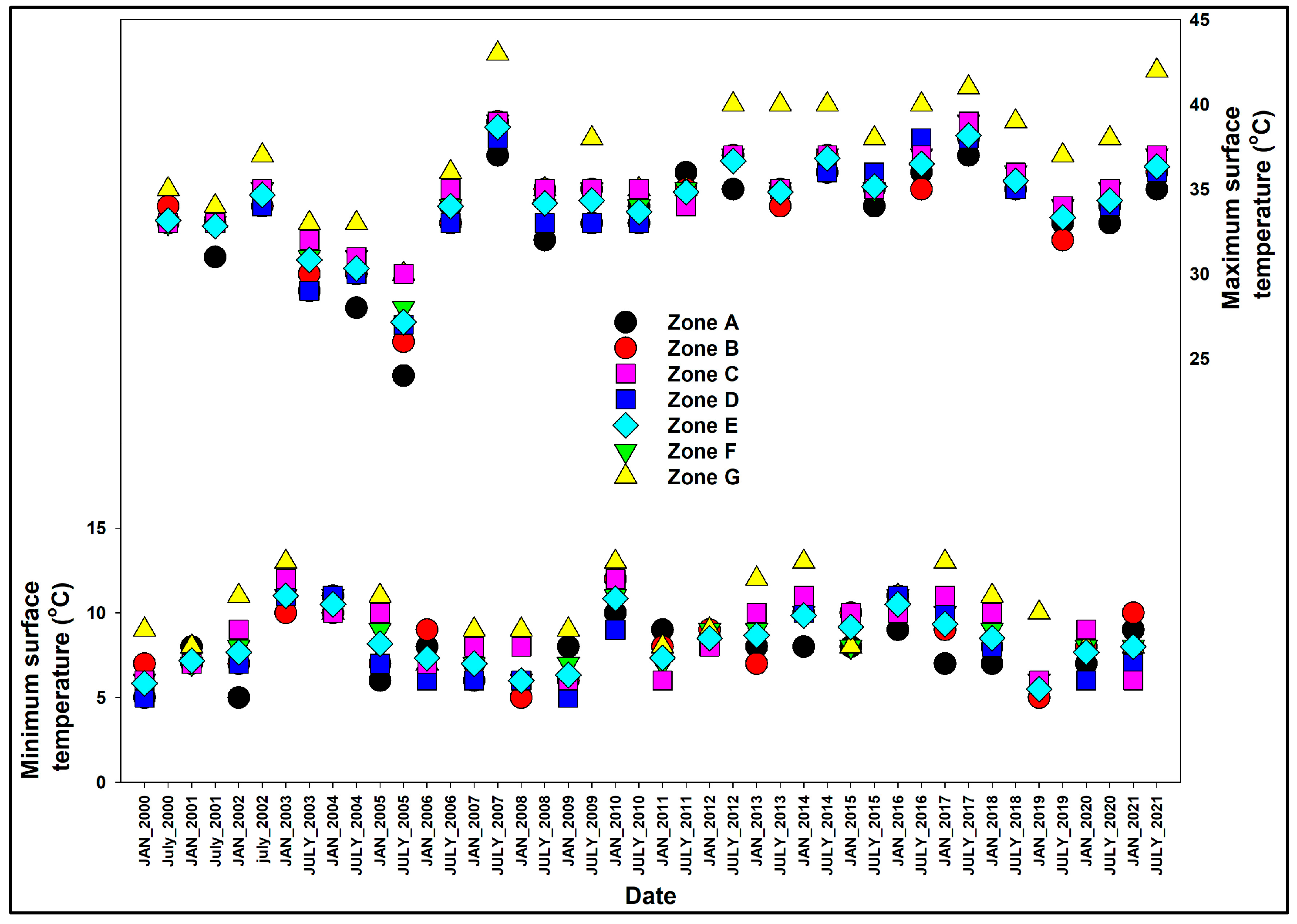
2.3.1. Surface Temperature and Precipitation
2.3.2. Human Disturbance
2.3.3. Soil Edaphic, Vegetation, and Topographic Variables
3. Results and Discussion
3.1. Climatic Conditions
3.2. Human Disturbance
3.3. Soil Edaphic Factors
3.4. Site Topography and Tree Canopy Cover
3.5. Successful Reintroduction Component
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ren, H.; Jian, S.; Liu, H.; Zhang, Q.; Lu, H. Advances in the reintroduction of rare and endangered wild plant species. Sci. China Life Sci. 2014, 27, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Tsaliki, M.; Diekmann, M. Effects of habitat fragmentation and soil quality on reproduction in two heathland Genista species. Plant Biol. 2010, 12, 622–629. [Google Scholar] [PubMed]
- Heywood, V. An overview of in situ conservation of plant species in the Mediterranean. Flora Mediterr. 2014, 24, 5–24. [Google Scholar] [CrossRef]
- Volis, S. How to conserve threatened Chinese plant species with extremely small populations? Plant Divers. 2016, 38, 45–52. [Google Scholar] [CrossRef] [PubMed]
- IUCN. The IUCN Red List of Threatened Species. Available online: https://www.iucnredlist.org/search/stats?query=Vascular%20Plants&searchType=species (accessed on 22 February 2023).
- Wagensommer, R.P.; Fröhlich, T.; Fröhlich, M. First record of the southeast European species Cerinthe retorta Sibth. & Sm. (Boraginaceae) in Italy and considerations on its distribution and conservation status. Acta Bot. Gall. 2014, 161, 111–115. [Google Scholar]
- Taifour, H.; El-Oqlah, A. Jordan Plant Red List, 1st ed.; Royal Botanic Garden: Amman, Jordan, 2014; Volume 1. [Google Scholar]
- Taifour, H. Jordan Plant Red List, 1st ed.; Royal Botanic Garden: Amman, Jordan, 2017; Volume 2. [Google Scholar]
- Al-Kofahi, S.D.; Gharaibeh, A.A.; Bsoul, E.Y.; Othman, Y.A.; Hilaire, R.S. Investigating domestic gardens’ densities, spatial distribution and types among city districts. Urban Ecosyst. 2019, 22, 567–581. [Google Scholar] [CrossRef]
- Perrino, E.V.; Wagensommer, R.P.; Silletti, G.N.; Signorile, G.; Angiulli, F. Nuovi dati distributivi e relazione con la Direttiva 92/43/CEE di taxa critici pugliesi dalla Provincia di Bari. Inform. Bot. Ital. 2013, 45, 53–62. [Google Scholar]
- Perrino, E.V.; Tomaselli, V.; Wagensommer, R.P.; Silletti, G.N.; Esposito, A.; Stinca, A. Ophioglossum lusitanicum L.: New Records of Plant Community and 92/43/EEC Habitat in Italy. Agronomy 2022, 12, 3188. [Google Scholar] [CrossRef]
- Abeli, T.; Cauzzi, P.; Rossi, G.; Adorni, M.; Vagge, I.; Parolo, G.; Orsenigo, S. Restoring population structure and dynamics in translocated species: Learning from wild populations. Plant Ecol. 2016, 217, 183–192. [Google Scholar] [CrossRef]
- Volis, S.; Blecher, M. Translocation success in Iris atrofusca: Importance of replicating sites and long-term monitoring. Restor. Ecol. 2022, 30, e13502. [Google Scholar] [CrossRef]
- IUCN/SSC. Guidelines for Reintroductions and Other Conservation Translocations, Version 1.0; IUCN Species Survival Commission: Gland, Switzerland, 2013; 57p. [Google Scholar]
- IUCN. Guidelines for Re-introductions. In Prepared by the IUCN/SSC Re-Introduction Specialist Group; IUCN: Gland, Switzerland; Cambridge, UK, 1998. [Google Scholar]
- Maschinski, J.; Albrecht, M. Center for Plant Conservation’s Best Practice Guidelines for the reintroduction of rare plants. Plant Divers. 2017, 39, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Godefroid, S.; Le Pajolec, S.; Van Rossum, F. Pre-translocation considerations in rare plant reintroductions: Implications for designing protocols. Plant Ecol. 2016, 217, 169–182. [Google Scholar] [CrossRef]
- Munt, D.; Marques, I.; Iriondo, J. Acquiring baseline information for successful plant translocations when there is no time to lose: The case of the neglected Critically Endangered Narcissus cavanillesii (Amaryllidaceae). Plant Ecol. 2016, 217, 193–206. [Google Scholar] [CrossRef]
- Alananbeh, K.M.; Othman, Y.A.; Tahat, M.M.; Al-Dakil, H.; Yahya, A.A.; Ayasrah, B.; Al-Share, T.; Alkhatatbeh, S.; Al-Zoubi, R.; Alnaanah, M.; et al. Forest health assessment in four Jordanian reserves located in semi-arid environments. Forests 2023, 14, 918. [Google Scholar] [CrossRef]
- Oran, S.; Al-Gabbiesh, A. Conservation of Iris bismarckiana Regel (Iridaceae) using plant regeneration via somatic embryogenesis. J. Agric. Sci. Technol. 2014, 4, 606–611. [Google Scholar]
- IUCN. The IUCN Red List of Threatened Species: Iris bismarckiana. 2016. Available online: https://www.iucnredlist.org/species/13161523/112203841#habitat-ecology (accessed on 21 June 2023).
- Mavrodiev, E.; Martínez-Azorín, M.; Dranishnikov, P.; Crespo, M. At least 23 genera instead of one: The case of Iris L. s.l. (Iridaceae). PLoS ONE 2014, 9, e106459. [Google Scholar] [CrossRef]
- Zito, P.; Rosselli, S.; Bruno, M.; Maggio, A.; Sajeva, M. Floral scent in Iris planifolia (Iridaceae) suggests food reward. Phytochemistry 2019, 158, 86–90. [Google Scholar] [CrossRef]
- Wilson, C.A.; Padiernos, J.; Sapir, Y. The royal irises (Iris subg. Iris sect. Oncocyclus): Plastid and low-copy nuclear data contribute to an understanding of their phylogenetic relationships. Taxon 2016, 65, 35–46. [Google Scholar]
- Eid, E.; Katbeh-Bader, A.; Al-Otoom, M.; Othman, Y. Contribution to the entomofauna of Dibeen Forest Reserve in Jordan. Cesa News 2009, 49, 19–45. [Google Scholar]
- GBIF. Iris bismarckiana Damm. And Spreng. ex Regell. Global Biodiversity Information Facility. Available online: https://www.gbif.org/species/165438025 (accessed on 22 February 2023).
- Ayasrah, B.; Meemy, Q. Important plants area in Jordan. In Critical Ecosystem Partnership Fund and Birdlife International, 1st ed.; Royal Society for Conservation of Nature Press: Amman, Jordan, 2021; pp. 40–41. [Google Scholar]
- FAO. Soil Texture. The Food and Agriculture Organization of the United Nations (FAO) Training. Available online: https://www.fao.org/fishery/docs/CDrom/FAO_Training/FAO_Training/General/x6706e/x6706e06.htm (accessed on 25 June 2023).
- Qarallah, B.; Al-Ajlouni, M.; Al-Awasi, A.; Alkarmy, M.; Al-Qudah, E.; Naser, A.B.; Al-Assaf, A.; Gevaert, C.M.; Al Asmar, Y.; Belgiu, M.; et al. Evaluating post-fire recovery of Latroon dry forest using Landsat ETM+, unmanned aerial vehicle and field survey data. J. Arid Environ. 2021, 193, 104587. [Google Scholar] [CrossRef]
- Qarallah, B.; Othman, Y.; Al-Ajlouni, M.; Alheyari, H.; Qoqazeh, B. Assessment of small-extent forest fires in semi-arid environment in Jordan using Sentinel-2 and Landsat sensors data. Forests 2023, 14, 41. [Google Scholar] [CrossRef]
- Sawalhaha, M.; Al-Kofahi, S.; Othman, Y.; Cibils, A. Assessing rangeland cover conversion in Jordan after the Arab spring using a remote sensing approach. J. Arid Environ. 2018, 157, 97–102. [Google Scholar] [CrossRef]
- Sawalhaha, M.; Othman, Y.; Abu Yahya, A.; Al-Kofahi, S.; Al-Lataifeh, F.; Cibils, A. Evaluating the influence of COVID-19 pandemic lockdown on Jordan Badia rangelands. Arid Land Res. Manag. 2021, 35, 483–495. [Google Scholar] [CrossRef]
- Schlesinger, W.H.; Andrews, J.A. Soil respiration and the global carbon cycle. Biogeochemistry 2000, 48, 7–20. [Google Scholar] [CrossRef]
- ESRI. An Overview of the Raster Surface Toolset-How Slope Works. ArcGIS Pro. Available online: https://pro.arcgis.com/en/pro-app/latest/tool-reference/3d-analyst/an-overview-of-the-raster-surface-toolset.htm (accessed on 25 April 2023).
- Gage, E.A.; Cooper, D.J. Urban forest structure and land cover composition effects on land surface temperature in a semi-arid suburban area. Urban For. Urban Green 2017, 28, 28–35. [Google Scholar] [CrossRef]
- Furtak, K.; Gałązka, A. Edaphic factors and their influence on the microbiological biodiversity of the soil environment. Advanc. Microbiol. 2019, 58, 375–384. [Google Scholar] [CrossRef]
- Lehmann, J.; Bossio, D.A.; Kögel-Knabner, I.; Rilling, M. The concept and future prospects of soil health. Nat. Rev. Earth Environ. 2020, 1, 544–553. [Google Scholar] [CrossRef]
- Haney, R.; Haney, E.; Smith, D.; White, M. Estimating potential nitrogen mineralisation using the Solvita soil respiration system. Open J. Soil Sci. 2015, 5, 319–323. [Google Scholar] [CrossRef]
- Morandage, S.; Vanderborght, J.; Zörner, M.; Cai, G.; Leitner, D.; Vereecken, H.; Schnepf, A. Root architecture development in stony soils. Vadose Zone J. 2021, 20, e20133. [Google Scholar] [CrossRef]
- Drew, M.C. Soil aeration and plant root metabolism. Soil Sci. 1992, 154, 259–268. [Google Scholar] [CrossRef]
- A’saf, T.; Al-Ajlouni, M.; Ayad, J.; Othman, Y.; Hilaire, R.S. Performance of six different soilless green roof substrates for the Mediterranean region. Sci. Total Environ. 2020, 730, 139182. [Google Scholar] [CrossRef] [PubMed]
- Defersha, M.B.; Quraishi, S.; Melesse, A. The effect of slope steepness and antecedent moisture content on interrill erosion, runoff and sediment size distribution in the highlands of Ethiopia. Hydrol. Earth Syst. Sci. 2011, 15, 2367–2375. [Google Scholar] [CrossRef]
- Kosmas, C.; Kirkby, M.; Geeson, N. Manual on: Key Indicators of Desertification and Mapping Environmentally Sensitive Areas to Desertification; EUR 18882; European Commission, Energy, Environment and Sustainable Development: Brussels, Belgium, 1999; p. 87. [Google Scholar]
- Jourgholami, M.; Karami, S.; Tavankar, F.; Lo Monaco, A.; Picchio, R. Effects of Slope Gradient on Runoff and Sediment Yield on Machine-Induced Compacted Soil in Temperate Forests. Forests 2021, 12, 49. [Google Scholar] [CrossRef]
- Zhang, G. Characteristics of runoff nutrient loss and particle size distribution of eroded sediment under varied rainfall intensities. In Proceedings of the 4th International Conference on Machinery, Materials and Computing Technology, Hangzhou, China, 23–24 January 2016. [Google Scholar] [CrossRef]
- Jones, K.; Kaye, T. Factors influencing germination of a functionally important grassland plant. Iris tenax. PLoS ONE 2014, 9, e90084. [Google Scholar] [CrossRef]
- Morgan, M. Seed germination characteristics of Iris virginica. Am. Midl. Nat. 1990, 124, 209–213. [Google Scholar] [CrossRef]
- Jehan, H.; Courtois, D.; Ehret, C.; Lerch, K.; Petiard, V. Plant regeneration of Iris pallida Lam. and Iris germanica L. via Somatic embryogenesis from leaves apices and young flowers. Plant Cell Rep. 1994, 13, 671–675. [Google Scholar] [CrossRef]
- Shibli, R.; Ajlouni, M. Somatic embryogenesis in the endemic black iris. Plant Cell Tissue Organ Cult. 2000, 61, 15–21. [Google Scholar] [CrossRef]



| Year | Zone | pH | EC (ds m−1) | Organic Matter (%) | CO2-C (mg kg−1) |
|---|---|---|---|---|---|
| 2022 | A | 7.81 a | 0.29 a | 1.03 c | 18.2 d |
| B | 7.53 a | 0.24 a | 4.47 a | 77.1 a | |
| C | 7.59 a | 0.39 a | 4.62 a | 72.3 a | |
| D | 7.49 a | 0.45 a | 3.77 ab | 48.3 b | |
| E | 7.71 a | 0.38 a | 1.93 b | 24.4 cd | |
| F | 7.76 a | 0.29 a | 4.11 a | 55.1 b | |
| G | 7.44 a | 0.47 a | 2.49 b | 29.1 c | |
| p-Value | 0.44 | 0.23 | 0.03 | 0.02 | |
| 2023 | A | 7.73 a | 0.25 a | 1.24 c | 25.7 b |
| B | 7.57 a | 0.30 a | 3.33 ab | 56.8 a | |
| C | 7.70 a | 0.25 a | 3.93 ab | 63.7 a | |
| D | 7.63 a | 0.27 a | 2.87 b | 61.9 a | |
| E | 7.77 a | 0.26 a | 4.43 a | 55.4 a | |
| F | 7.67 a | 0.28 a | 3.38 ab | 60.0 a | |
| G | 7.63 a | 0.34 a | 2.90 b | 60.8 a | |
| p-Value | 0.96 | 0.83 | 0.0037 | 0.0009 |
| Soil Texture (Particle Proportion) | ||||||
|---|---|---|---|---|---|---|
| Zone | Clay | Silt | Sand | Texture | Stone Diameter > 2 mm (% of Stones by Volume) | Soil Depth (cm) |
| A | 20.7 a | 45.7 b | 37.0 a | Loam | 8.4 b | >40 |
| B | 20.0 ab | 43.7 b | 36.2 a | Loam | 8.3 b | >40 |
| C | 18.9 ab | 69.3 a | 15.1 c | Silt-loam | 10.7 b | >40 |
| D | 17.5 ab | 66.1 a | 16.4 c | Silt-loam | 21.0 ab | >40 |
| E | 16.8 ab | 53.7 b | 29.5 ab | Silt-loam | 17.7 ab | >40 |
| F | 15.3 ab | 53.2 b | 28.2 b | Silt-loam | 24.5 a | >40 |
| G | 13.9 b | 68.0 a | 18.1 c | Silt-loam | 13.0 ab | >40 |
| p-Value | 0.037 | 0.0004 | <0.0001 | - | 0.05 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Othman, Y.A.; Ayasrah, B.; Al-Kofahi, S. Habitat Selection to Reintroduce Iris bismarckiana in Semi-Arid Environments. Diversity 2023, 15, 957. https://doi.org/10.3390/d15090957
Othman YA, Ayasrah B, Al-Kofahi S. Habitat Selection to Reintroduce Iris bismarckiana in Semi-Arid Environments. Diversity. 2023; 15(9):957. https://doi.org/10.3390/d15090957
Chicago/Turabian StyleOthman, Yahia A., Bilal Ayasrah, and Salman Al-Kofahi. 2023. "Habitat Selection to Reintroduce Iris bismarckiana in Semi-Arid Environments" Diversity 15, no. 9: 957. https://doi.org/10.3390/d15090957
APA StyleOthman, Y. A., Ayasrah, B., & Al-Kofahi, S. (2023). Habitat Selection to Reintroduce Iris bismarckiana in Semi-Arid Environments. Diversity, 15(9), 957. https://doi.org/10.3390/d15090957






