Changes in Bacterial Community Structure in Reservoir Sediments before and after the Flood Season
Abstract
:1. Introduction
2. Materials and Method
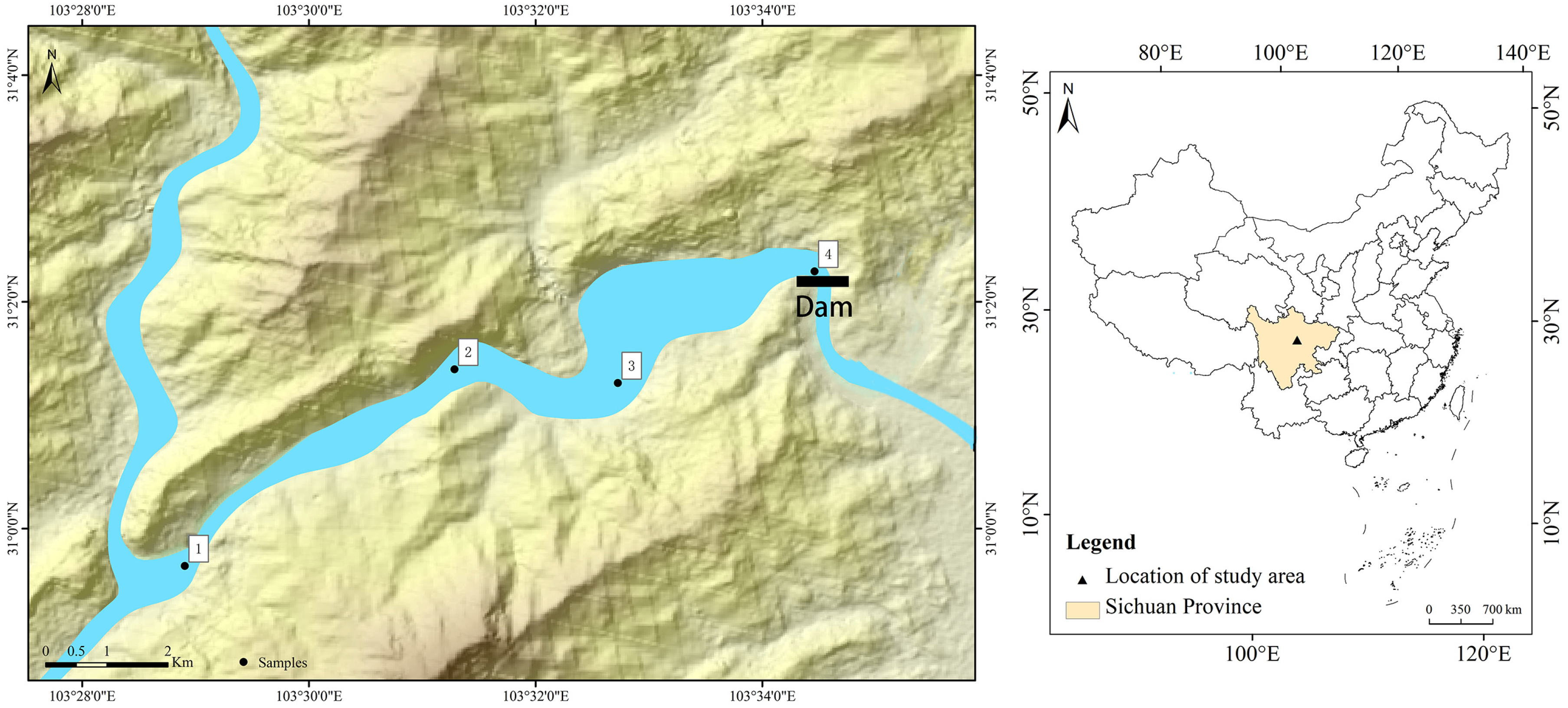
2.1. Study Site and Sample Collection
2.2. DNA Extraction and PCR Amplification
2.3. Illumina NovaSeq Sequencing and Bioinformatics Data Processing
2.4. Statistical Analysis
3. Results
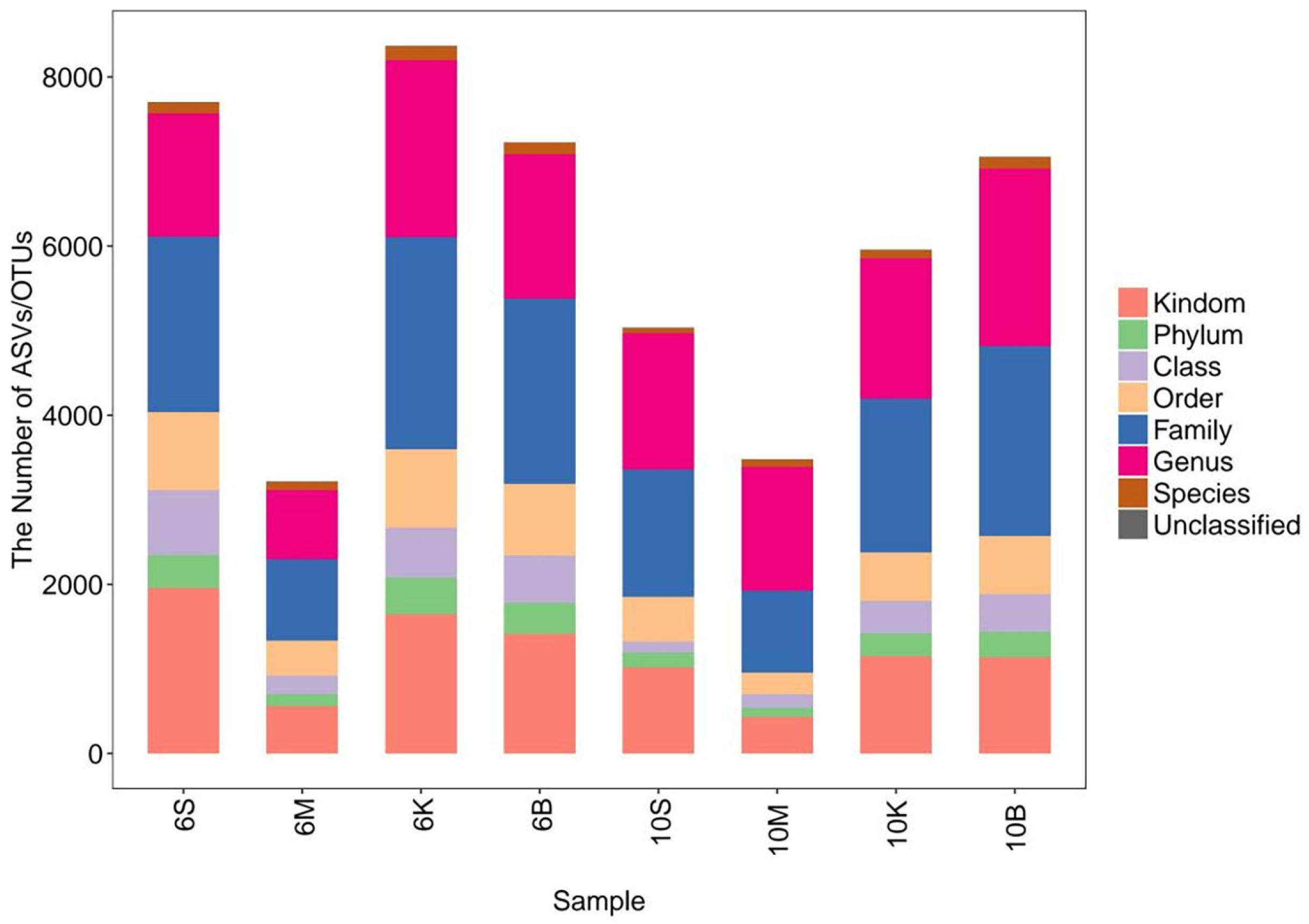
3.1. Taxonomic Classification Unit Count
3.2. Diversity of Bacterial Community
3.2.1. Indices of Bacterial Diversity in Sediment at Different Locations
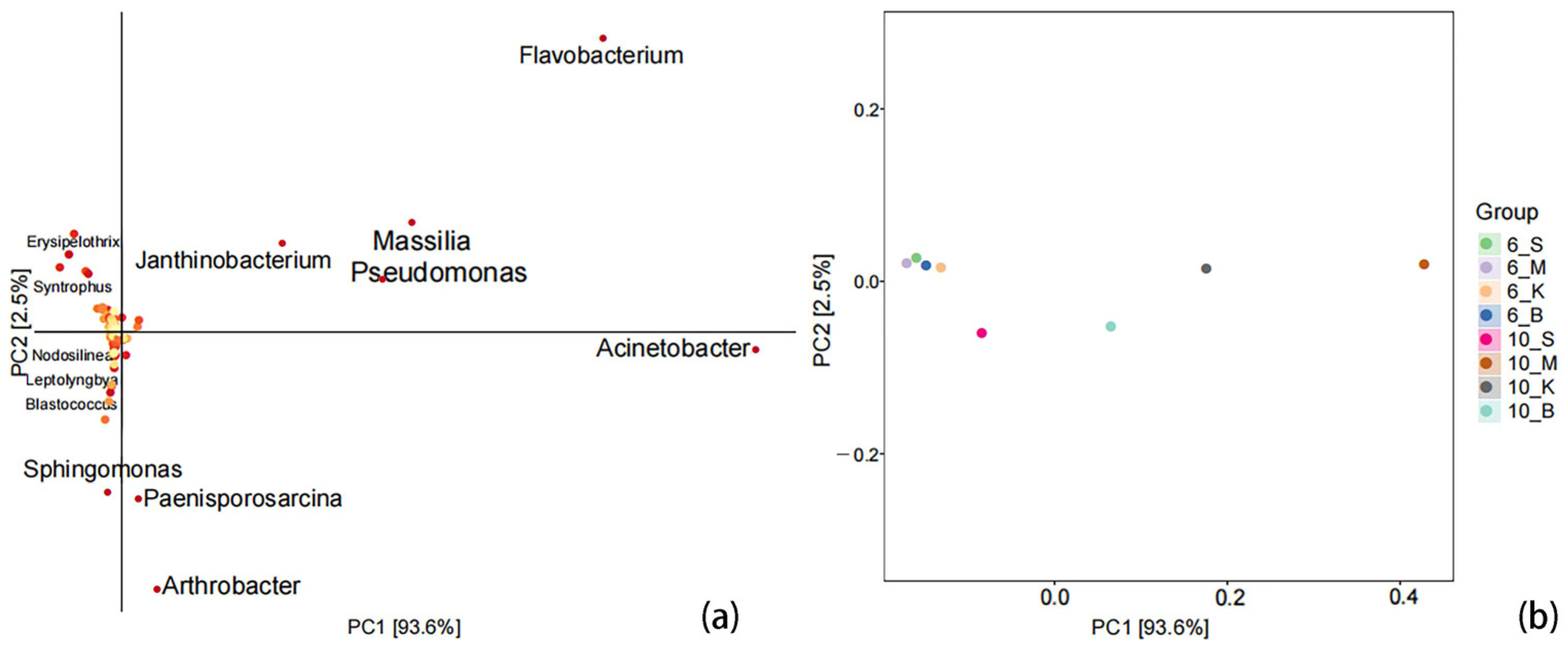
3.2.2. Comparison of Similarity between Samples
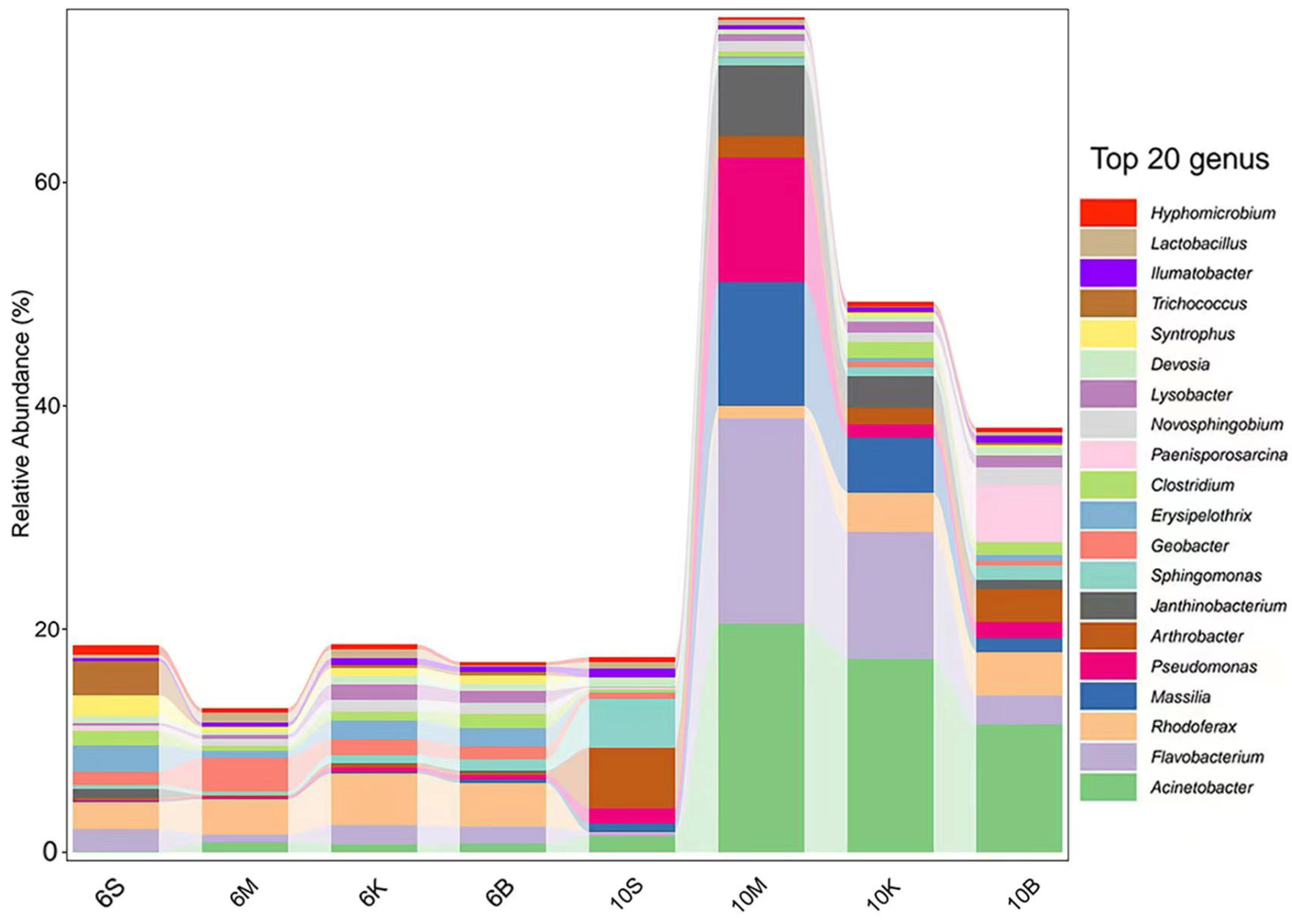
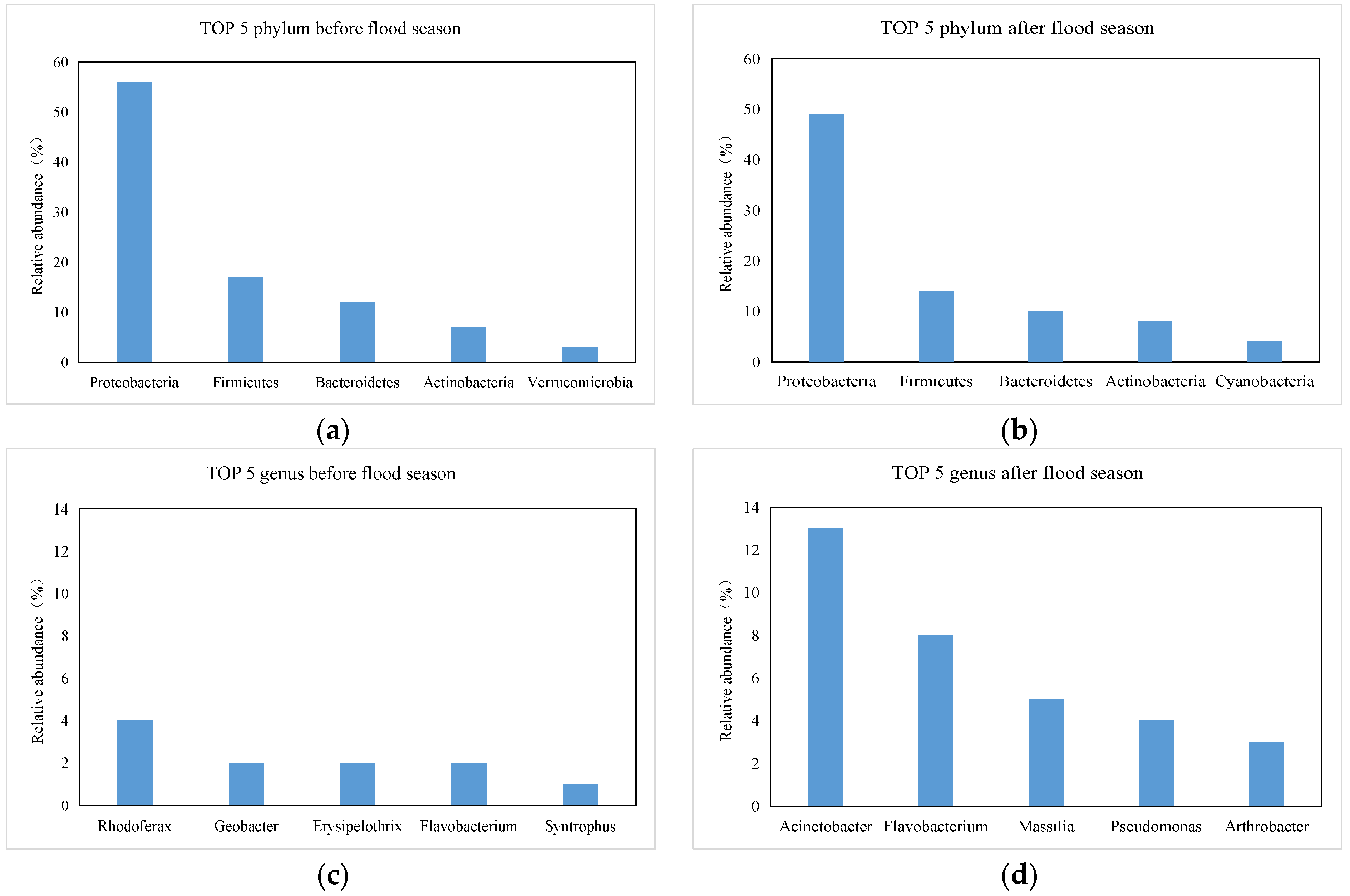
3.3. Relative Abundance Distribution Trend
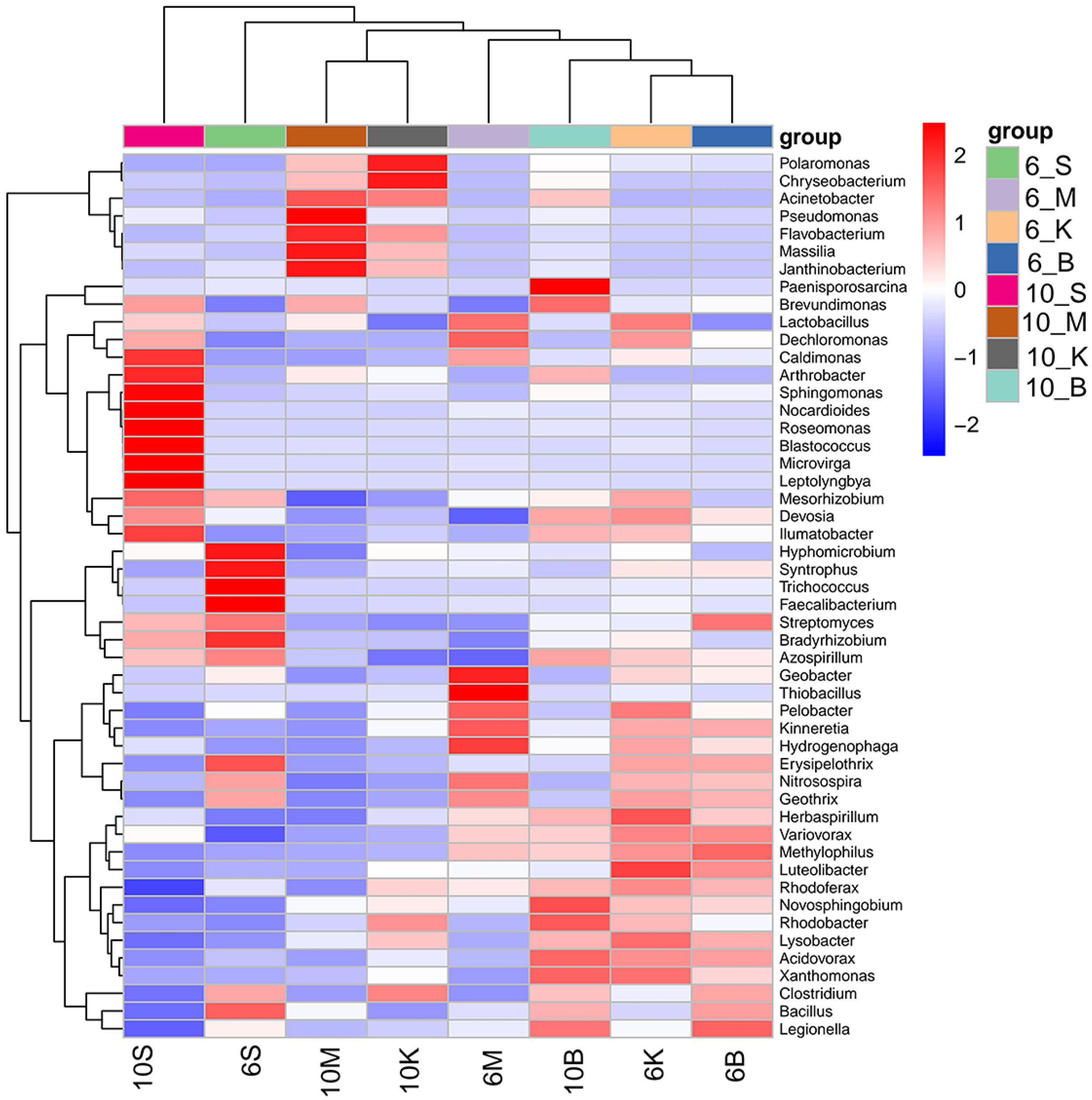
3.4. Identification of Key Differential Bacteria at the Genus Level
3.4.1. Quantitative Analysis of the Degree of Difference between Each Sample
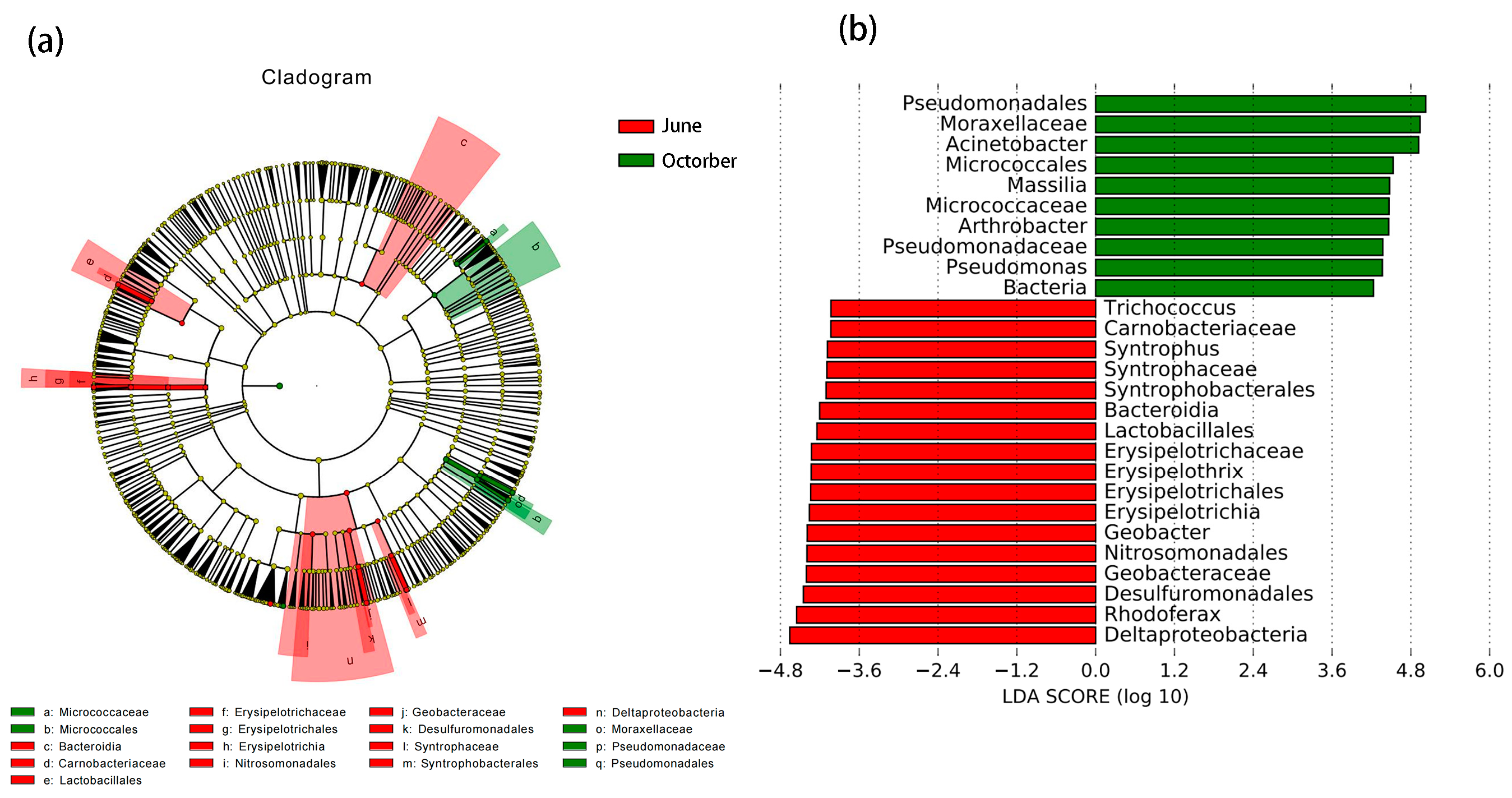
3.4.2. Comparison of Changes in Significantly Enriched Biomarkers before and after the Flood Season
3.4.3. Determination of the Significantly Divergent Genera
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Drummond, J.D.; Aquino, T.; Davies-Colley, R.J.; Stott, R.; Krause, S. Modeling contaminant microbes in rivers during both baseflow and stormflow. Geophys. Res. Lett. 2022, 49, e2021GL096514. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Pang, Y.; Yi, Y.; Yang, S.; Wang, Y.; He, C.; Shi, Q.; He, D. Response of dissolved organic matter chemistry to flood control of a large river reservoir during an extreme storm event. Water Res. 2023, 230, 119565. [Google Scholar] [CrossRef] [PubMed]
- Raymond, P.A.; Saiers, J.E.; Sobczak, W.V. Hydrological and biogeochemical controls on watershed dissolved organic matter transport: Pulse-shunt concept. Ecology 2016, 97, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Doering, M.; Freimann, R.; Antenen, N.; Roschi, A.; Robinson, C.T.; Rezzonico, F.; Smits, T.H.M.; Tonolla, D. Microbial communities in floodplain ecosystems in relation to altered flow regimes and experimental flooding. Sci. Total Environ. 2021, 788, 147497. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Hu, A.; Wang, F.; Hong, Y.; Krom, M.D.; Chen, N. Impacts of a subtropical storm on nitrogen functional microbes and associated cycling processes in a river-estuary continuum. Sci. Total Environ. 2023, 861, 160698. [Google Scholar] [CrossRef] [PubMed]
- Steichen, J.L.; Labonté, J.M.; Windham, R.; Hala, D.; Kaiser, K.; Setta, S.; Faulkner, P.C.; Bacosa, H.; Yan, G.; Kamalanathan, M.; et al. Microbial, physical, and chemical changes in Galveston Bay following an extreme flooding event, Hurricane Harvey. Front. Mar. Sci. 2020, 7, 186. [Google Scholar] [CrossRef]
- Cardoso, S.; Vidal, L.; Mendonça, R.; Tranvik, L.; Sobek, S.; Roland, F. Spatial variation of sediment mineralization supports differential CO2 emissions from a tropical hydroelectric reservoir. Front. Microbiol. 2013, 4, 101. [Google Scholar] [CrossRef]
- Wang, Y.; Sheng, H.F.; He, Y.; Wu, J.Y.; Jiang, Y.X.; Tam, N.F.; Zhou, H.W. Comparison of the levels of bacterial diversity in freshwater, intertidal wetland, and marine sediments by using millions of illumina tags. Appl. Environ. Microbiol. 2012, 78, 8264–8271. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, S.; Zhao, H.; Deng, L.; Wang, C.; Zhao, Q.; Dong, S. The phosphorus speciations in the sediments up- and down-stream of cascade dams along the middle Lancang River. Chemosphere 2015, 120, 653–659. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, H.; Zhang, Q.; Chai, B.; Lei, X.; Ye, M.; Chen, B. Effects of dissolved oxygen on phosphorus transformation in reservoir sediments: Novel insights on bacterial community and functional genes. J. Soils Sediments 2022, 22, 2094–2104. [Google Scholar] [CrossRef]
- Chen, Z.; Xu, S.; Yu, L.; Liu, J.; Su, Y.; Shao, X.; Ruan, Y.; Wang, D. Bacterial diversity of surface sediment at Lake Dian in summer and winter seasons. Int. J. Agric. Biol. 2020, 23, 559–565. [Google Scholar]
- Mihale, M.J.; Tungaraza, C.; Baeyens, W.; Brion, N. Distribution and sources of carbon, nitrogen and their isotopic compositions in tropical estuarine sediments of Mtoni, Tanzania. Ocean Sci. J. 2021, 56, 241–255. [Google Scholar] [CrossRef]
- Lu, S.; Sun, Y.; Lu, B.; Zheng, D.; Xu, S. Change of abundance and correlation of Nitrospira inopinata-like comammox and populations in nitrogen cycle during different seasons. Chemosphere 2020, 241, 125098. [Google Scholar] [CrossRef] [PubMed]
- Schwanen, C.A.; Müller, J.; Schulte, P.; Schwarzbauer, J. Distribution, remobilization and accumulation of organic contaminants by flood events in a Meso-scaled catchment system. Environ. Sci. Eur. 2023, 35, 15. [Google Scholar] [CrossRef]
- Tang, X.Q.; Li, R.; Wang, D.Y.; Jing, Z.; Zhang, W. Reservoir flood regulation affects nutrient transport through altering water and sediment condition. Water Res. 2023, 233, 119728. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, F.; Zheng, S.; Chen, L.; Zhang, X.; Gong, J. The differentiation of iron-reducing bacterial community and iron-reduction activity between riverine and marine sediments in the Yellow River estuary. Mar. Life Sci. Technol. 2020, 2, 87–96. [Google Scholar] [CrossRef]
- Liu, Y.; Lin, Q.; Feng, J.; Yang, F.; Du, H.; Hu, Z.; Wang, H. Differences in metabolic potential between particle-associated and free-living bacteria along Pearl River Estuary. Sci. Total Environ. 2020, 728, 138856. [Google Scholar] [CrossRef] [PubMed]
- Zinger, L.; Amaral-Zettler, L.A.; Fuhrman, J.A.; Horner-Devine, M.C.; Huse, S.M.; Welch, D.B.; Martiny, J.B.; Sogin, M.; Boetius, A.; Ramette, A. Global patterns of bacterial beta-diversity in seafloor and seawater ecosystems. PLoS ONE 2011, 6, e24570. [Google Scholar] [CrossRef]
- Ren, Z.; Wang, F.; Qu, X.; Elser, J.J.; Liu, Y.; Chu, L. Taxonomic and functional differences between microbial communities in Qinghai Lake and its input streams. Front. Microbiol. 2017, 8, 2319. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Gao, H.; Elser, J.J.; Zhao, Q. Microbial functional genes elucidate environmental drivers of biofilm metabolism in glacier-fed streams. Sci. Rep. 2017, 7, 12668. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-B.; Yao, J.; Zhang, H.-Y.; Wang, X.-G.; Li, K.-H.; Lü, X.-T.; Wang, Z.-W.; Zhou, J.-Z.; Han, X.-G. Environmental and spatial variables determine the taxonomic but not functional structure patterns of microbial communities in Alpine Grasslands. Sci. Total Environ. 2019, 654, 960–968. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Y.; Wei, L.; Li, M.; Zhu, W.; Zhu, L. Spatiotemporal correlations between water quality and microbial community of typical inflow river into Taihu Lake, China. Environ. Sci. Pollut. Res. Int. 2022, 29, 63722–63734. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zeng, G.; Liang, D.; Xu, Y.; Li, Y.; Huang, X.; Ma, Y.; Wang, F.; Liao, C.; Tang, C.; et al. An analysis of the colony structure of Prokaryotes in the Jialing River waters in Chongqing. Int. J. Environ. Res. Public Health 2022, 19, 5525. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, P.; Thomaz, S.M.; Bini, L.M. Effects of water level, abiotic and biotic factors on bacterioplankton abundance in lagoons of a tropical floodplain (Paraná River, Brazil). Hydrobiologia 2003, 510, 67–74. [Google Scholar] [CrossRef]
- Yue, Y.; Cai, L.; Tang, Y.; Zhang, Y.; Yang, M.; Wang, F. Vertical distribution of bacterial community in water columns of reservoirs with different trophic conditions during thermal stratification. Front. Environ. Sci. 2021, 9, 632089. [Google Scholar] [CrossRef]
- Carney, R.; Mitrovic, S.; Jeffries, T.; Westhorpe, D.; Curlevski, N.; Seymour, J. River bacterioplankton community responses to a high inflow event. Aquat. Microb. Ecol. 2015, 75, 187–205. [Google Scholar] [CrossRef]
- Farjalla, V.F.; Marinho, C.C.; Faria, B.M.; Amado, A.M.; Esteves, F.A.; Bozelli, R.L.; Giroldo, D. Synergy of fresh and accumulated organic matter to bacterial growth. Microb. Ecol. 2009, 57, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Liao, N.; Li, H.; Zhang, L.; Chen, M.; Li, J.; You, L. Coupled effects of environmental conditions on the spatio-temporal variability of Phytoplankton in canyon-shaped reservoirs. J. Clean. Prod. 2023, 386, 135797. [Google Scholar] [CrossRef]
- Chen, M.; You, L.H.; Zhang, L.L.; Liao, N.; Song, Y.; Wang, H.W.; Li, J. Mixing processes in a reservoir corresponding to different water level operations caused spatial differences during two Phytoplankton bloom events. J. Hydrol. 2022, 612, 128139. [Google Scholar] [CrossRef]
- He, W.; You, L.; Chen, M.; Tuo, Y.; Liao, N.; Wang, H.; Li, J. Varied sediment archive of Fe and Mn contents under changing reservoir mixing patterns, oxygenation regimes, and runoff inputs. Ecol. Indic. 2023, 147, 109967. [Google Scholar] [CrossRef]
- Martin, M. CUTADAPT removes adapter sequences from high-throughput sequencing reads. EMBnet. J. 2011, 17, 10. [Google Scholar] [CrossRef]
- Kemp, P.F.; Aller, J.Y. Bacterial diversity in aquatic and other environments: What 16S rDNA libraries can tell us. FEMS Microbiol. Ecol. 2004, 47, 161–177. [Google Scholar] [CrossRef] [PubMed]
- Paradis, E.; Claude, J.; Strimmer, K. Ape: Analyses of phylogenetics and evolution in R language. Bioinformatics 2004, 20, 289–290. [Google Scholar] [CrossRef] [PubMed]
- Metsalu, T.; Vilo, J. Clustvis: A web tool for visualizing clustering of multivariate data using principal component analysis and heatmap. Nucleic Acids Res. 2015, 43, W566–W570. [Google Scholar] [CrossRef] [PubMed]
- Simpson, G.L.; Solymos, P.; Stevens, M.; Wagner, H. Vegan: Community ecology package. time international. Time Int. 2018, 14, 927. [Google Scholar]
- Ramette, A. Multivariate analyses in microbial ecology. FEMS Microbiol. Ecol. 2007, 62, 142–160. [Google Scholar] [CrossRef]
- Chang, F.; He, S.; Dang, C. Assisted selection of biomarkerss by linear discriminant analysis effect size (LEfSe) in microbiome data. J. Vis. Exp. 2022, 183, e61715. [Google Scholar] [CrossRef]
- Farh, M.E.; Kim, Y.J.; Singh, P.; Jung, S.Y.; Kang, J.P.; Yang, D.C. Rhodoferax koreense sp. nov, an obligately aerobic bacterium within the family Comamonadaceae, and emended description of the genus Rhodoferax. J. Microbiol. 2017, 55, 767–774. [Google Scholar] [CrossRef]
- Yang, L.; Jiang, M.; Zou, Y.; Qin, L.; Chen, Y. Geographical distribution of iron redox cycling bacterial community in peatlands: Distinct assemble mechanism across environmental gradient. Front. Microbiol. 2021, 12, 674411. [Google Scholar] [CrossRef]
- Holmes, D.E.; O’Neil, R.A.; Vrionis, H.A.; N’Guessan, L.A.; Ortiz-Bernad, I.; Larrahondo, M.J.; Adams, L.A.; Ward, J.A.; Nicoll, J.S.; Nevin, K.P.; et al. Subsurface clade of Geobacteraceae that predominates in a diversity of Fe(III)-reducing subsurface environments. ISME J. 2007, 1, 663–677. [Google Scholar] [CrossRef]
- Asimaki, E.; Nolte, O.; Overesch, G.; Strahm, C. A dangerous hobby? Erysipelothrix rhusiopathiae bacteremia most probably acquired from freshwater aquarium fish handling. Infection 2016, 45, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Nhung, N.T.; Chansiripornchai, N.; Carrique-Mas, J.J. Antimicrobial resistance in bacterial poultry pathogens: A review. Front. Vet. Sci. 2017, 4, 126. [Google Scholar] [CrossRef] [PubMed]
- Breton-Deval, L.; Sanchez-Flores, A.; Juárez, K.; Vera-Estrella, R. Integrative study of microbial community dynamics and water quality along the Apatlaco River. Environ. Pollut. 2019, 255, 113158. [Google Scholar] [CrossRef] [PubMed]
- Chang, N.; Zhang, Z.; Mamuti, A.; Du, F.; Dilihuma, A.; Ma, Z. Diversity of the bacterial community in reservoirs in the north part of Tianshan Mountain. Acta Ecol. Sin. 2022, 42, 2843–2852. [Google Scholar] [CrossRef]
- Sousa, J.A.D.; Silva-Souza, Â.T. Bacterial community associated with fish and water from Congonhas River, Sertaneja, Paraná, Brazil. Braz. Arch. Biol. Technol. 2001, 44, 373–381. [Google Scholar] [CrossRef]
- Li, B.; Wei, Z.; Huang, Z.; Xiao, X.; Ming, S.; Jiao, H.; Cheng, X. Removal of toluene from synthetic waste gas through aerobic denitrification in biotrickling reactor. Environ. Eng. Sci. 2020, 37, 769–781. [Google Scholar] [CrossRef]
- Hu, E.; Hu, L.; Zheng, Y.; Wu, Y.; Wang, X.; Sun, C.; Su, Y. Bacterial abundance and community structure in response to nutrients and photodegraded terrestrial humic acids in a eutrophic lake. Environ. Sci. Pollut. Res. 2021, 29, 8218–8231. [Google Scholar] [CrossRef]
- Kim, M.; Lee, J.; Yang, D.; Park, H.Y.; Park, W. Seasonal dynamics of the bacterial communities associated with cyanobacterial blooms in the Han River. Environ. Pollut. 2020, 266, 115198. [Google Scholar] [CrossRef]
- Huang, Z.; Xie, B.; Yuan, Q.; Xu, W.; Lu, J. Microbial community study in newly established Qingcaosha Reservoir of Shanghai, China. Appl. Microbiol. Biotechnol. 2014, 98, 9849–9858. [Google Scholar] [CrossRef]
- Zhu, J.S.; Qin, H.L.; Sun, Q.Y.; Wang, B.Z.; Gao, R.X.; Guo, R.L.; Li, W.B. Microbial diversity and influencing factors in a small watershed in winter. Huan Jing Ke Xue 2020, 41, 5016–5026. [Google Scholar] [CrossRef]
- Qiao, Z.; Sun, R.; Wu, Y.; Hu, S.; Liu, X.; Chan, J.; Mi, X. Characteristics and metabolic pathway of the bacteria for heterotrophic nitrification and aerobic denitrification in aquatic ecosystems. Environ. Res. 2020, 191, 110069. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.X.; Bi, Q.F.; Hao, X.L.; Zhou, G.W.; Yang, X.R. Massilia phosphatilytica sp. nov., a phosphate solubilizing bacteria isolated from a long-term fertilized soil. Int. J. Syst. Evol. Microbiol. 2017, 67, 2514–2519. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Li, X.; Qi, N.; Gan, M.; Pan, Y.; Han, T.; Hu, X. Massilia neuiana sp. nov., isolated from wet soil. Int. J. Syst. Evol. Microbiol. 2017, 67, 4943–4947. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.-Q.; Cui, Y.-W.; Huang, J.-L.; Sun, F.-L.; Chen, S. A novel pseudomonas aeruginosa strain performs simultaneous heterotrophic nitrification-aerobic denitrification and aerobic phosphate removal. Water Res. 2022, 221, 118823. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Thiri, M.; Wang, H. Simultaneous heterotrophic nitrification and aerobic denitrification by a novel isolated Pseudomonas mendocina X49. Bioresour. Technol. 2021, 319, 124198. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; He, W.; Cai, S. Seasonal variation of dissolved oxygen in the southeast of the Pearl River Estuary. Water 2020, 12, 2475. [Google Scholar] [CrossRef]
- Luo, G.; Xu, G.; Tan, H.; Gao, J.; Liu, W. Effect of dissolved oxygen on denitrification using polycaprolactone as both the organic carbon source and the biofilm carrier. Int. Biodeterior. Biodegrad. 2016, 110, 155–162. [Google Scholar] [CrossRef]
- Zhou, S.; Huang, T.; Zhang, H.; Zeng, M.; Liu, F.; Bai, S.; Shi, J.; Qiu, X.; Yang, X. Nitrogen removal characteristics of enhanced in situ indigenous aerobic denitrification bacteria for micro-polluted reservoir source water. Bioresour. Technol. 2016, 201, 195–207. [Google Scholar] [CrossRef]
- Adame, M.F.; Franklin, H.; Waltham, N.J.; Rodriguez, S.; Kavehei, E.; Turschwell, M.P.; Balcombe, S.R.; Kaniewska, P.; Burford, M.A.; Ronan, M. Nitrogen removal by tropical floodplain wetlands through denitrification. Mar. Freshw. Res. 2019, 70, 1513–1521. [Google Scholar] [CrossRef]
- Han, D.; Hu, Z.; Li, D.; Tang, R. Nitrogen removal of water and sediment in grass carp aquaculture ponds by mixed nitrifying and denitrifying bacteria and its effects on bacterial community. Water 2022, 14, 1855. [Google Scholar] [CrossRef]

| Sample Name | Number of Valid Sequences | Coverage | Chao1 | Shannon | Simpson |
|---|---|---|---|---|---|
| 6S | 135,707 | 99% | 7950.37 | 11.67 | 0.99 |
| 6M | 107,840 | 99% | 8575.48 | 11.97 | 0.99 |
| 6K | 121,776 | 99% | 3237.53 | 11.14 | 0.99 |
| 6B | 126,632 | 99% | 7394.85 | 11.94 | 0.99 |
| 10S | 124,842 | 99% | 5041.52 | 10.49 | 0.99 |
| 10M | 109,738 | 99% | 6196.25 | 10.03 | 0.99 |
| 10K | 129,281 | 99% | 3519.53 | 8.72 | 0.99 |
| 10B | 128,026 | 99% | 7269.05 | 10.91 | 0.99 |
| Classification of Differences in Dominant Bacteria p Value | ||
|---|---|---|
| Class Level | Deltaproteobacteria | 0.01 |
| Gammaproteobacteria | 0.01 | |
| Coriobacteriia | 0.02 | |
| Clostridia | 0.09 | |
| Erysipelotrichia | 0.04 | |
| Betaproteobacteria | 0.55 | |
| Flavobacteriia | 0.13 | |
| Genus Level | Acinetobacter | 0.01 |
| Arthrobacter | 0.01 | |
| Flavobacterium | 0.11 | |
| Janthinobacterium | 0.11 | |
| Massilia | 0.12 | |
| Pseudomonas | 0.27 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, X.; Chen, M.; Zhou, L.; He, W.; Liao, N.; Tuo, Y. Changes in Bacterial Community Structure in Reservoir Sediments before and after the Flood Season. Diversity 2023, 15, 946. https://doi.org/10.3390/d15090946
He X, Chen M, Zhou L, He W, Liao N, Tuo Y. Changes in Bacterial Community Structure in Reservoir Sediments before and after the Flood Season. Diversity. 2023; 15(9):946. https://doi.org/10.3390/d15090946
Chicago/Turabian StyleHe, Xianting, Min Chen, Luxin Zhou, Wenyan He, Ning Liao, and Youcai Tuo. 2023. "Changes in Bacterial Community Structure in Reservoir Sediments before and after the Flood Season" Diversity 15, no. 9: 946. https://doi.org/10.3390/d15090946
APA StyleHe, X., Chen, M., Zhou, L., He, W., Liao, N., & Tuo, Y. (2023). Changes in Bacterial Community Structure in Reservoir Sediments before and after the Flood Season. Diversity, 15(9), 946. https://doi.org/10.3390/d15090946






