Bill Shape Variation in African Penguin (Spheniscus demersus) Held Captive in Two Zoos
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Molecular Sex Verification
2.3. Geometric Morphometry
2.4. Statistical Analysis
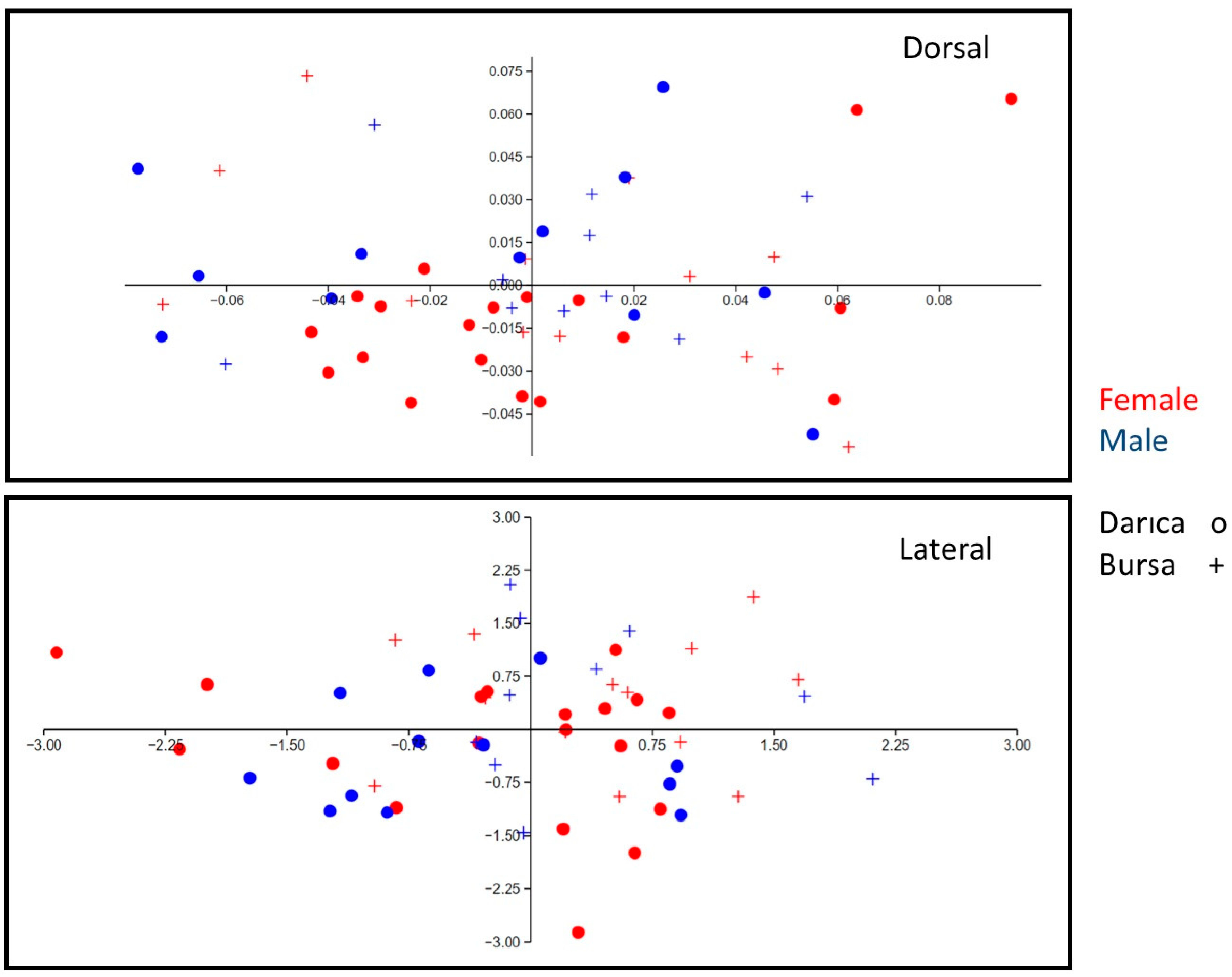
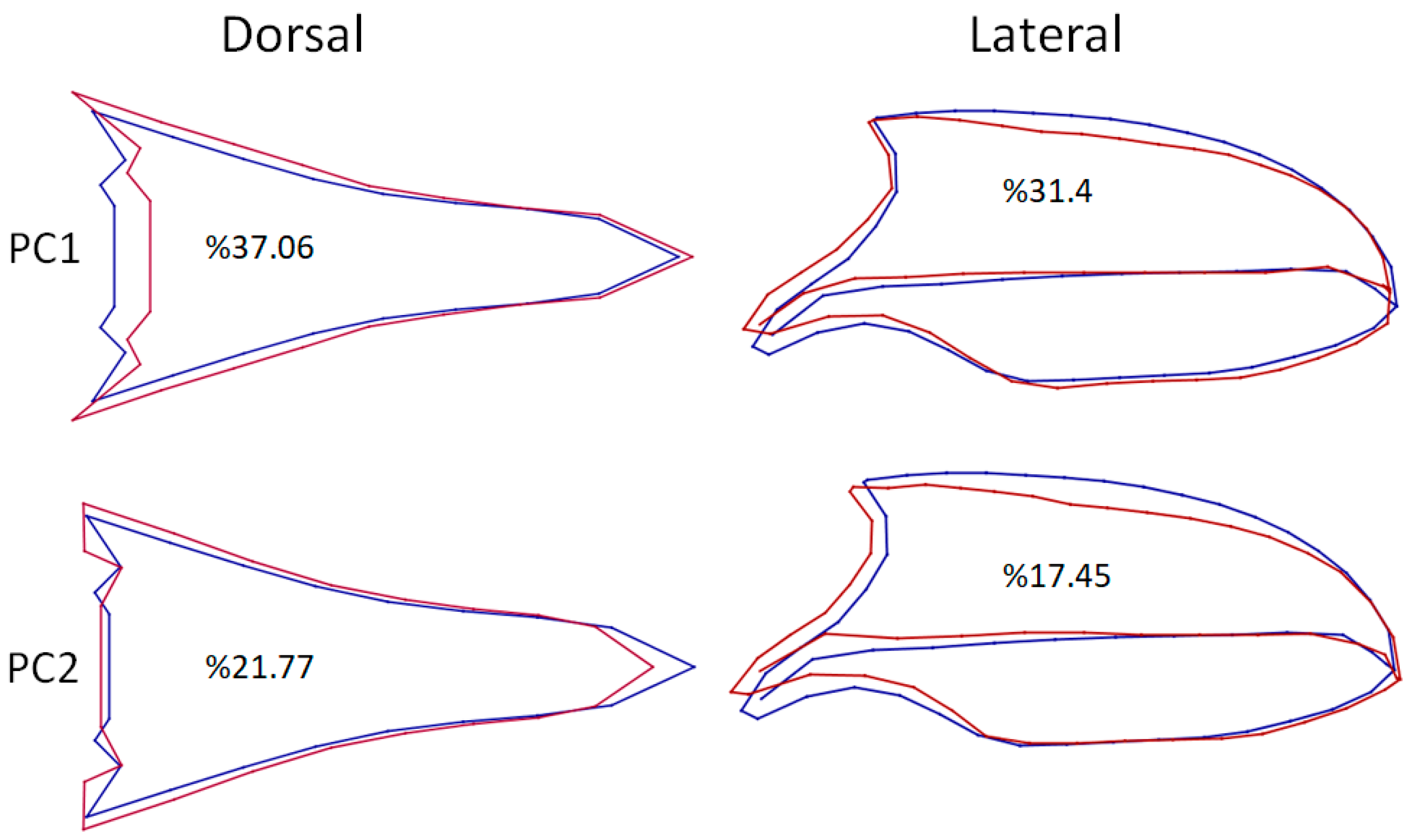
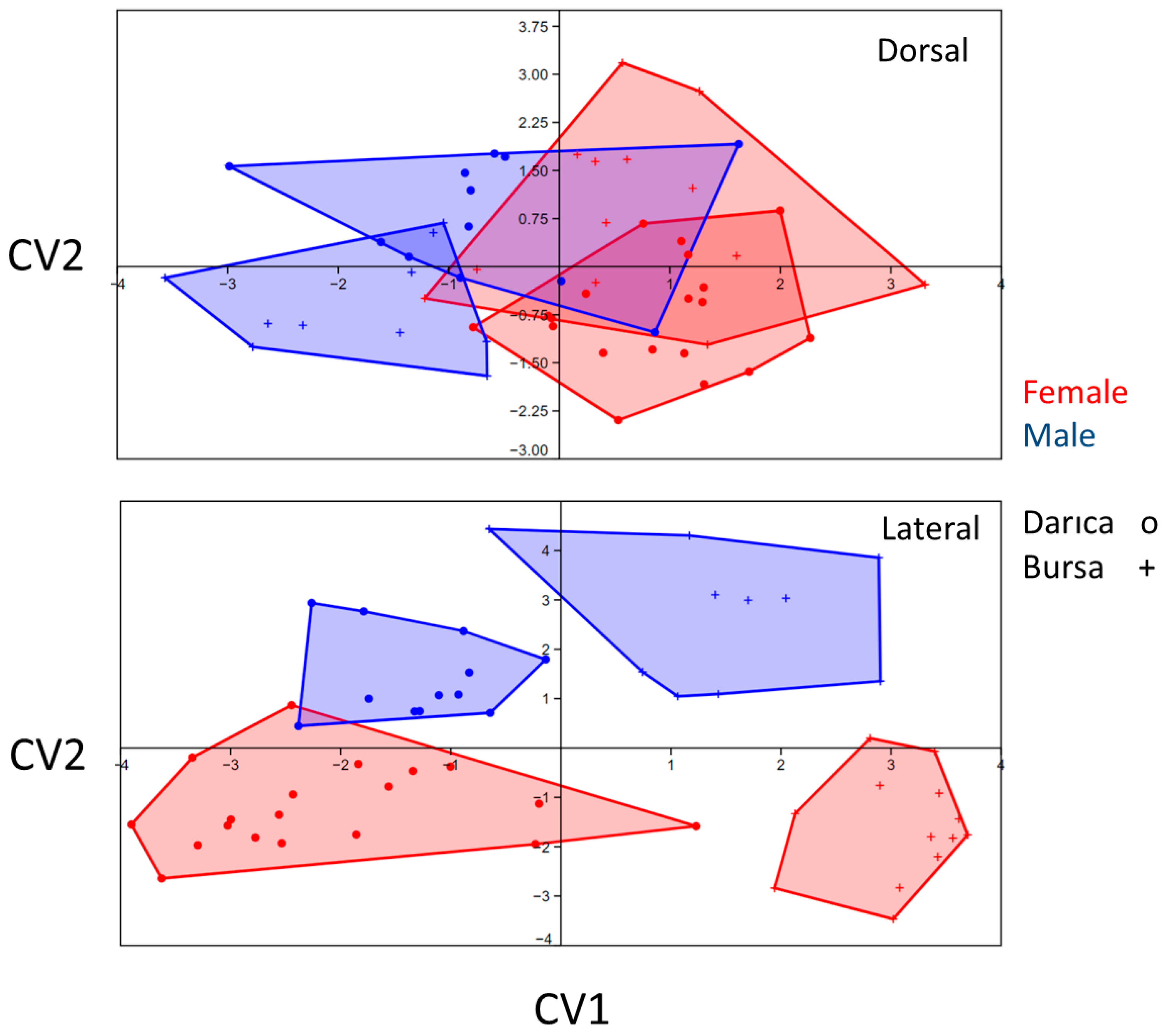
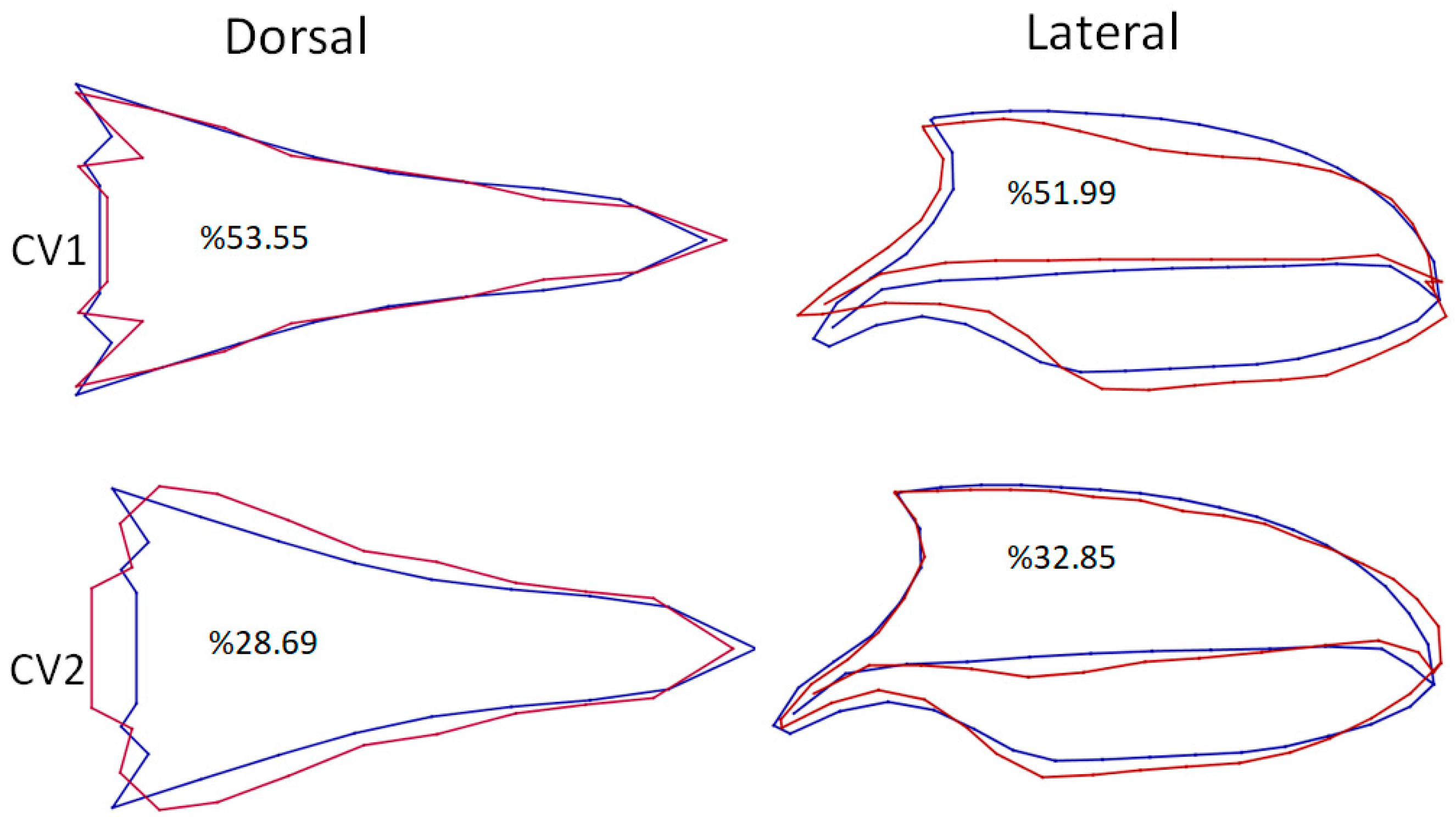
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shelton, P.A.; Crawford, R.J.M.; Cooper, J.; Brooke, R.K. Distribution, population size and conservation of the Jackass Penguin Spheniscus demersus. S. Afr. J. Mar. Sci. 1984, 2, 217–257. [Google Scholar] [CrossRef]
- Sherley, R.B.; Crawford, R.J.; de Blocq, A.D.; Dyer, B.M.; Geldenhuys, D.; Hagen, C.; Kemper, J.; Makhado, A.; Pichegru, L.; Tom, D.; et al. The conservation status and population decline of the African penguin deconstructed in space and time. Ecol. Evol. 2020, 10, 8506–8516. [Google Scholar] [CrossRef]
- Ozella, L.; Favaro, L.; Carnovale, I.; Pessani, D. Pond use by captive African penguins (Spheniscus demersus) in an immersive exhibit adjacent to human bathers. J. Appl. Anim. Welf. Sci. 2015, 18, 303–309. [Google Scholar] [CrossRef]
- Campbell, K.J.; Farah, D.; Collins, S.; Parsons, N.J. Sex determination of African Penguins Spheniscus demersus using bill measurements: Method comparisons and implications for use. Ostrich 2016, 87, 47–55. [Google Scholar] [CrossRef]
- Griffiths, R.; Double, M.C.; Orr, K.; Dawson, R.J. A DNA test to sex most birds. Mol. Ecol. 1998, 7, 1071–1075. [Google Scholar] [CrossRef]
- Klingenberg, C.P. Size, shape, and form: Concepts of allometry in geometric morphometrics. Dev. Genes Evol. 2016, 226, 113–137. [Google Scholar] [CrossRef]
- Bookstein, F.L. A newly noticed formula enforces fundamental limits on geometric morphometric analyses. Evol. Biol. 2017, 44, 522–541. [Google Scholar] [CrossRef]
- Gonzalez, P.N.; Bernal, V.; Perez, S.I. Geometric morphometric approach to sex estimation of human pelvis. Forensic Sci. Int. 2009, 189, 68–74. [Google Scholar] [CrossRef]
- Boz, İ.; Manuta, N.; Özkan, E.; Kahvecioğlu, O.; Pazvant, G.; Ince, N.G.; Hadziomerovic, N.; Szara, T.; Altundağ, Y.; Gundemir, O. Geometric morphometry in veterinary anatomy. Veterinaria 2023, 72, 15–27. [Google Scholar]
- Gündemir, O.; Duro, S.; Szara, T.; Koungoulos, L.; Jashari, T.; Demircioğlu, İ.; Hadžiomerović, N.; Ilieski, V.; Melnyk, O.P.; Melnyk, O.O. Skull variation in different breeds sheep from Balkan countries. Ann. Anat.-Anat. Anz. 2023, 249, 152083. [Google Scholar] [CrossRef]
- Szara, T.; Duro, S.; Gündemir, O.; Demircioğlu, İ. Sex determination in Japanese Quails (Coturnix japonica) using geometric morphometrics of the skull. Animals 2022, 12, 302. [Google Scholar] [CrossRef]
- Jashari, T.; Kahvecioğlu, O.; Duro, S.; Gündemir, O. Morphometric analysis for the sex determination of the skull of the Deltari Ilir dog (Canis lupus familiaris) of Kosovo. Anat. Histol. Embryol. 2022, 51, 443–451. [Google Scholar] [CrossRef]
- Boersma, P.D.; Davies, E.M. Sexing monomorphic birds by vent measurements. Auk 1987, 104, 779–783. [Google Scholar] [CrossRef]
- Volodin, I.; Kaiser, M.; Matrosova, V.; Volodina, E.; Klenova, A.; Filatova, O.; Kholodova, M. The technique of noninvasive distant sexing for four monomorphic dendrocygna whistling duck species by their loud whistles. Bioacoustics 2009, 18, 277–290. [Google Scholar] [CrossRef]
- Pazvant, G.; İnce, N.G.; Özkan, E.; Gündemir, O.; Avanus, K.; Szara, T. Sex determination based on morphometric measurements in yellow-legged gulls (Larus michahellis) around Istanbul. BMC Zool. 2022, 7, 35. [Google Scholar] [CrossRef] [PubMed]
- Fridolfsson, A.K.; Ellegren, H. A simple and universal method for molecular sexing of non-ratite birds. J. Avian Biol. 1999, 30, 116–121. [Google Scholar] [CrossRef]
- Berns, C.M.; Adams, D.C. Bill shape and sexual shape dimorphism between two species of temperate hummingbirds: Black-Chinned hummingbird (Archilochus alexandri) and Ruby-Throated hummingbird (A. colubris). Auk 2010, 127, 626–635. [Google Scholar] [CrossRef]
- Navarro, J.; Kaliontzopoulou, A.; González-Solís, J. Sexual dimorphism in bill morphology and feeding ecology in Cory’s Shearwater (Calonectris diomedea). Zoology 2009, 112, 128–138. [Google Scholar] [CrossRef]
- Babbitt, G.A.; Frederick, P.C. Selection for sexual bill dimorphism in ibises: An evaluation of hypotheses. Waterbirds 2007, 30, 199–206. [Google Scholar] [CrossRef]
- Ingolfsson, A. Sexual dimorphism of large gulls (Larus spp.). Auk 1969, 86, 732–737. [Google Scholar] [CrossRef]
- Hocken, A.G.; Russell, J.J. A method for determination of gender from bill measurements in Otago blue penguins (Eudyptula minor). N. Z. J. Zool. 2002, 29, 63–69. [Google Scholar] [CrossRef]
- Polito, M.J.; Clucas, G.V.; Hart, T.O.M.; Trivelpiece, W.Z. A simplified method of determining the sex of Pygoscelis penguins using bill measurements. Mar. Ornithol. 2012, 40, 89–94. [Google Scholar]
- Overeem, R.; Wallis, R.; Salzman, S. Sexing Little Penguins Eudyptula Minor Using Bill Measurements. Victorian Nat. 2006, 123, 390–395. [Google Scholar]
- Albayrak, T.; Aytek, A.İ. Bill variation of captive and wild Chukar partridge populations: Shape or size. Diversity 2022, 14, 48. [Google Scholar] [CrossRef]
- Friedman, N.R.; Remeš, V. Global geographic patterns of sexual size dimorphism in birds: Support for a latitudinal trend? Ecography 2016, 39, 17–25. [Google Scholar] [CrossRef]
- Alonso, J.C.; Magaña, M.; Alonso, J.A.; Palacín, C.; Martín, C.A.; Martín, B. The most extreme sexual size dimorphism among birds: Allometry, selection, and early juvenile development in the great bustard (Otis tarda). Auk 2009, 126, 657–665. [Google Scholar] [CrossRef]
- Widén, P. Reversed sexual size dimorphism in birds of prey: Revival of an old hypothesis. Oikos 1984, 43, 259–263. [Google Scholar] [CrossRef]
- Jehl, J.R., Jr.; Murray, B.G., Jr. The evolution of normal and reverse sexual size dimorphism in shorebirds and other birds. In Current Ornithology; Springer: Boston, MA, USA, 1986; Volume 3, pp. 1–86. [Google Scholar]
- Bock, W.J. An approach to the functional analysis of bill shape. Auk 1966, 83, 10–51. [Google Scholar] [CrossRef]
- Nebel, S.; Jackson, D.L.; Elner, R.W. Functional association of bill morphology and foraging behaviour in calidrid sandpipers. Anim. Biol. 2005, 55, 235–243. [Google Scholar] [CrossRef]
- Yusuf, L.; Heatley, M.C.; Palmer, J.P.; Barton, H.J.; Cooney, C.R.; Gossmann, T.I. Noncoding regions underpin avian bill shape diversification at macroevolutionary scales. Genome Res. 2020, 30, 553–565. [Google Scholar] [CrossRef]
- vonHoldt, B.M.; Kartzinel, R.Y.; Huber, C.D.; Le Underwood, V.; Zhen, Y.; Ruegg, K.; Lohmueller, K.E.; Smith, T.B. Growth factor gene IGF1 is associated with bill size in the black-bellied seedcracker Pyrenestes ostrinus. Nat. Commun. 2018, 9, 4855. [Google Scholar] [CrossRef] [PubMed]
- Chhaya, V.; Reddy, S.; Krishnan, A. Climate influences bill shape diversification in cavity-excavating birds. bioRxiv 2022. [Google Scholar] [CrossRef]
- Symonds, M.R.; Tattersall, G.J. Geographical variation in bill size across bird species provides evidence for Allen’s rule. Am. Nat. 2010, 176, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, R.; Danner, R.; Olsen, B.; Luther, D. High summer temperature explains bill size variation in salt marsh sparrows. Ecography 2012, 35, 146–152. [Google Scholar] [CrossRef]
- Peterson, A.T. Adaptive geographical variation in bill shape of scrub jays (Aphelocoma coerulescens). Am. Nat. 1993, 142, 508–527. [Google Scholar] [CrossRef]
- Wallace, R.S.; Dubach, J.; Michaels, M.G.; Keuler, N.S.; Diebold, E.D.; Grzybowski, K.; Teare, J.A.; Willis, M.J. Morphometric determination of gender in adult Humboldt Penguins (Spheniscus humboldti). Waterbirds 2008, 31, 448–453. [Google Scholar] [CrossRef]
- Van Hemert, C.; Armién, A.G.; Blake, J.E.; Handel, C.M.; O’Hara, T.M. Macroscopic, histologic, and ultrastructural lesions associated with avian keratin disorder in black-capped chickadees (Poecile atricapillus). Vet. Pathol. 2013, 50, 500–513. [Google Scholar] [CrossRef]


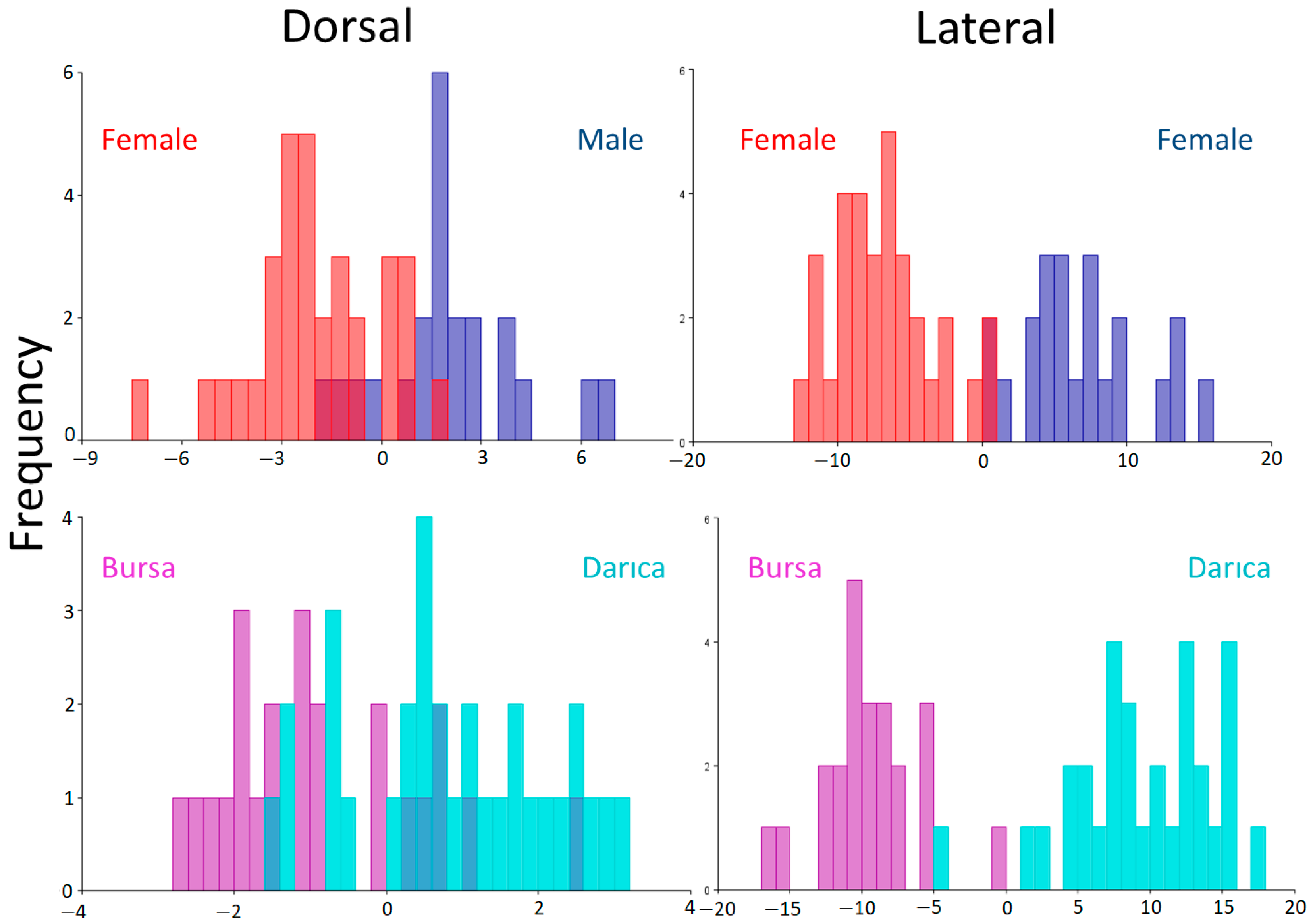
| Dorsal | Lateral | ||||
|---|---|---|---|---|---|
| PC | Eigenvalues | %Variance | PC | Eigenvalues | %Variance |
| PC1 | 0.00161037 | 37.063 | PC1 | 0.00226000 | 31.404 |
| PC2 | 0.00094577 | 21.767 | PC2 | 0.00125549 | 17.446 |
| PC3 | 0.00077402 | 17.814 | PC3 | 0.00095716 | 13.300 |
| Female–Bursa | Female–Darica | Male–Bursa | ||||
|---|---|---|---|---|---|---|
| P-D | p Value | P-D | p Value | P-D | p Value | |
| Female–Darica | 0.0185/0.0444 | 0.7001/0.0499 | ||||
| Male–Bursa | 0.0179/0.0272 | 0.8396/0.7951 | 0.0215/0.0486 | 0.706/0.0387 | ||
| Male–Darica | 0.0200/0.0537 | 0.7619/0.0177 | 0.0281/0.0262 | 0.2695/0.6344 | 0.0231/0.0542 | 0.6484/0.0220 |
| Sex | n | Mean | Sd | p-Value | |
|---|---|---|---|---|---|
| Dorsal | Female | 32 | 1.77591 × 1016 | 1.61886 × 1016 | 0.42 |
| Male | 22 | 2.17361 × 1016 | 1.9654 × 1016 | ||
| Lateral | Female | 32 | 9.10566 × 1015 | 9.48929 × 1015 | 0.01 |
| Male | 22 | 1.65972 × 1016 | 1.14494 × 1016 | ||
| Dorsal | Bursa | 23 | 1.50812 × 1016 | 1.62389 × 1016 | 0.12 |
| Darica | 31 | 2.25684 × 1016 | 1.81664 × 1016 | ||
| Lateral | Bursa | 23 | 1.7753 × 1016 | 1.37855 × 1016 | 0.01 |
| Darica | 31 | 8.00648 × 1016 | 5.29417 × 1015 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szara, T.; Günay, E.; Boz, İ.; Batmankaya, B.; Gencer, H.; Gün, G.; Vatansever Çelik, E.C.; Gündemir, O. Bill Shape Variation in African Penguin (Spheniscus demersus) Held Captive in Two Zoos. Diversity 2023, 15, 945. https://doi.org/10.3390/d15080945
Szara T, Günay E, Boz İ, Batmankaya B, Gencer H, Gün G, Vatansever Çelik EC, Gündemir O. Bill Shape Variation in African Penguin (Spheniscus demersus) Held Captive in Two Zoos. Diversity. 2023; 15(8):945. https://doi.org/10.3390/d15080945
Chicago/Turabian StyleSzara, Tomasz, Ebuderda Günay, İlayda Boz, Berke Batmankaya, Hilal Gencer, Gökhan Gün, Ezgi Can Vatansever Çelik, and Ozan Gündemir. 2023. "Bill Shape Variation in African Penguin (Spheniscus demersus) Held Captive in Two Zoos" Diversity 15, no. 8: 945. https://doi.org/10.3390/d15080945
APA StyleSzara, T., Günay, E., Boz, İ., Batmankaya, B., Gencer, H., Gün, G., Vatansever Çelik, E. C., & Gündemir, O. (2023). Bill Shape Variation in African Penguin (Spheniscus demersus) Held Captive in Two Zoos. Diversity, 15(8), 945. https://doi.org/10.3390/d15080945







