Species Composition and Population Dynamics of Culicidae during their Peak Abundance Period in Three Peri-Urban Aquatic Ecosystems in Northern Spain
Abstract
1. Introduction
2. Materials and Methods
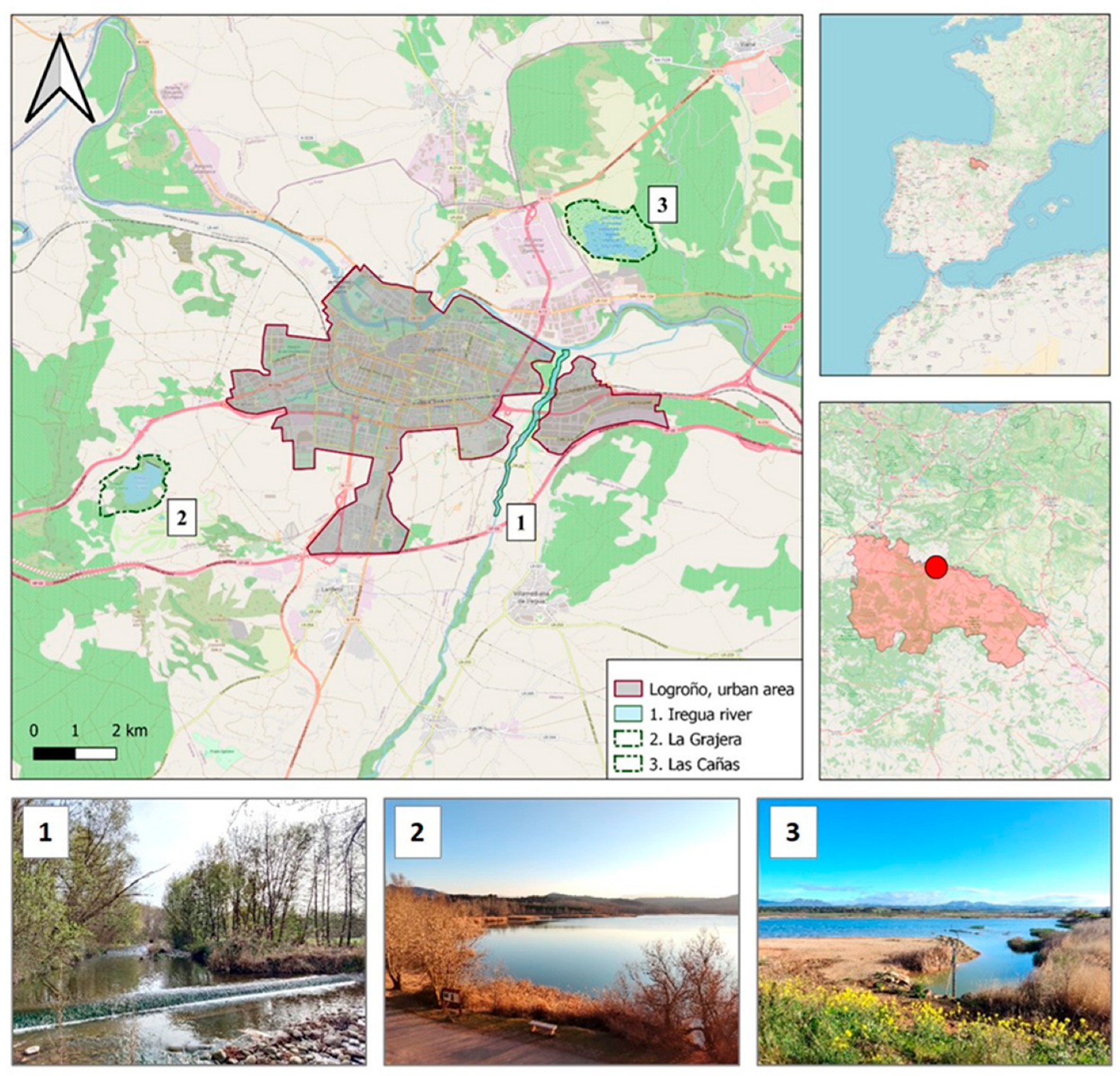
2.1. Study Area
2.2. Mosquito Collection and Identification
2.3. Data Analysis
2.4. Ethics Approval and Consent to Participate
3. Results
3.1. Molecular Identification of the Claviger Complex
3.2. Species Composition and Abundance
3.3. Population Dynamics in the Peak Abundance Period
3.4. Human Landing Collection
4. Discussion
4.1. Molecular Identification of the Claviger Complex
4.2. Species Composition and Abundance
4.3. Population Dynamics in the Peak Abundance Period
4.4. Human Landing Collection: Epidemiological Implications of the Identified Species
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Benelli, G.; Senthil-Nathan, S. Together in the Fight against Arthropod-Borne Diseases: A One Health Perspective. Int. J. Environ. Res. Public Health 2019, 16, 4876. [Google Scholar] [CrossRef] [PubMed]
- Portillo, A.; Ruiz-Arrondo, I.; Oteo, J.A. Artrópodos vectores en España y sus enfermedades transmisibles. Med. Clin. 2018, 151, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Lucientes, J.; Alarcón-Elbal, P.M. Culicoides biting midges in Spain: A brief overview. Small Rum. Res. 2016, 142, 69–71. [Google Scholar] [CrossRef][Green Version]
- Bravo-Barriga, D.; Ruiz-Arrondo, I.; Peña, R.E.; Lucientes, J.; Delacour-Estrella, S. Phlebotomine sand flies (Diptera, Psychodidae) from Spain: An updated checklist and extended distributions. ZooKeys 2022, 1106, 81–99. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Arrondo, I.; Alarcón-Elbal, P.M.; Figueras, L.; Delacour-Estrella, S.; Muñoz, A.; Kotter, H.; Pinal, R.; Lucientes, J. Expansión de los simúlidos (Diptera: Simuliidae) en España: Un nuevo reto para la salud pública y la sanidad animal. Bol. SEA 2014, 54, 193–200. Available online: http://sea-entomologia.org/PDF/Boletin54/193200BSEA54ExpansionsimulidosenEspa%C3%B1a.pdf (accessed on 15 May 2023).
- Kerkow, A.; Wieland, R.; Früh, L.; Hölker, F.; Jeschke, J.M.; Werner, D.; Kampen, H. Can data from native mosquitoes support determining invasive species habitats? Modelling the climatic niche of Aedes japonicus japonicus (Diptera, Culicidae) in Germany. Parasitol. Res. 2020, 119, 31–42. [Google Scholar] [CrossRef]
- Collantes, F.; Delgado, J.A.; Alarcón-Elbal, P.M.; Delacour, S.; Lucientes, J. First confirmed outdoor winter reproductive activity of Asian tiger mosquito (Aedes albopictus) in Europe. An. Biol. 2014, 36, 71–76. [Google Scholar] [CrossRef]
- Delacour, S.; Barandika, J.F.; García-Pérez, A.L.; Collantes, F.; Ruiz Arrondo, I.; Alarcón-Elbal, P.M.; Bengoa, M.; Delgado, J.A.; Juste, R.A.; Molina, R.; et al. Detección temprana del mosquito tigre (Aedes albopictus Skuse, 1894) en el País Vasco (España). An. Biol. 2015, 37, 25–30. [Google Scholar] [CrossRef]
- Eritja, R.; Ruiz-Arrondo, I.; Delacour-Estrella, S.; Schaffner, F.; Álvarez-Chachero, J.; Bengoa, M.; Puig, M.Á.; Melero-Alcíbar, R.; Oltra, A.; Bartumeus, F. First detection of Aedes japonicus in Spain: An unexpected finding triggered by citizen science. Parasites Vectors 2019, 12, 53. [Google Scholar] [CrossRef]
- Eritja, R.; Delacour-Estrella, S.; Ruiz-Arrondo, I.; González, M.A.; Barceló, C.; García-Pérez, A.L.; Lucientes, J.; Miranda, M.Á.; Bartumeus, F. At the tip of an iceberg: Citizen science and active surveillance collaborating to broaden the known distribution of Aedes japonicus in Spain. Parasites Vectors 2021, 14, 375. [Google Scholar] [CrossRef]
- Martínez-Barciela, Y.; González, A.P.; Rial, D.G.; González, J.G. First records of five species of mosquitoes (Diptera: Culicidae) in Galicia, including the first evidence of the genus Coquillettidia in northwestern Spain. J. Vector Ecol. 2021, 46, 96–102. [Google Scholar] [CrossRef] [PubMed]
- González, M.A.; Cevidanes, A.; Goiri, F.; Barandika, J.F.; García-Pérez, A.L. Diversity and distribution of larval habitats of mosquitoes (Diptera: Culicidae) in northern Spain: From urban to natural areas. J. Vector Ecol. 2021, 46, 173–185. [Google Scholar] [CrossRef]
- González, M.A.; Delacour-Estrella, S.; Bengoa, M.; Barceló, C.; Bueno-Marí, R.; Eritja, R.; Ruiz-Arrondo, I. A Survey on Native and Invasive Mosquitoes and Other Biting Dipterans in Northern Spain. Acta Parasitol. 2022, 67, 867–877. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Arrondo, I.; McMahon, B.J.; Hernández-Triana, L.M.; Santibañez, P.; Portillo, A.; Oteo, J.A. Surveillance of Mosquitoes (Diptera, Culicidae) in a Northern Central Region of Spain: Implications for the Medical Community. Front. Vet. Sci. 2019, 6, 86. [Google Scholar] [CrossRef]
- Ruiz-Arrondo, I.; Hernández-Triana, L.M.; Nikolova, N.I.; Fooks, A.R.; Oteo, J.A. Integrated Approaches in Support of Taxonomic Identification of Mosquitoes (Diptera: Culicidae) in Vector Surveillance in Spain. Vector-Borne Zoonotic Dis. 2020, 20, 831–842. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Arrondo, I.; Delacour-Estrella, S.; Santibáñez, P.; Oteo, J.A. Primera detección del mosquito tigre, Aedes albopictus (Diptera: Culicidae), en La Rioja: Implicaciones en salud pública. An. Biol. 2021, 43, 117–122. [Google Scholar] [CrossRef]
- Heym, E.C.; Kampen, H.; Walther, D. Mosquito species composition and phenology (Diptera, Culicidae) in two German zoological gardens imply different risks of mosquito-borne pathogen transmission. J. Vector Ecol. 2018, 43, 80–88. [Google Scholar] [CrossRef] [PubMed]
- González, M.A.; Goiri, F.; Cevidanes, A.; Hernández-Triana, L.M.; Barandika, J.F.; García-Pérez, A.L. Mosquito community composition in two major stopover aquatic ecosystems used by migratory birds in northern Spain. Med. Vet. Entomol. 2023, 37, 616–629. [Google Scholar] [CrossRef]
- González, M.; López, S.; Alarcón-Elbal, P.M. Blood-feeding Diptera (Culicidae and Ceratopogonidae) in an urban park of the city of Vitoria-Gasteiz (Basque Country, Spain). J. Eur. Mosq. Control Assoc. 2015, 33, 10–14. Available online: https://e-m-b.myspecies.info/sites/e-m-b.org/files/JEMCA%2833%2910-14.pdf (accessed on 10 April 2023).
- Ferraguti, M.; Martínez-de la Puente, J.; Roiz, D.; Ruiz, S.; Soriguer, R.C.; Figuerola, J. Effects of landscape anthropization on mosquito community composition and abundance. Sci. Rep. 2016, 6, 29002. [Google Scholar] [CrossRef]
- Rueda, J.; Hernández, R.; Alarcón-Elbal, P.M. Nuevas aportaciones al conocimiento de dos especies de culícidos agrestes de España: Aedes (Rusticoidus) refiki Medschid, 1928 y Aedes (Ochlerotatus) pullatus (Coquillett, 1904) (Diptera, Culicidae). An. Biol. 2017, 39, 191–197. [Google Scholar] [CrossRef]
- Honnen, A.C.; Monaghan, M.T. City-Dwellers and Country Folks: Lack of Population Differentiation Along an Urban–Rural Gradient in the Mosquito Culex pipiens (Diptera: Culicidae). J. Insect Sci. 2017, 17, 107. [Google Scholar] [CrossRef]
- Medlock, J.M.; Vaux, A.G.C. Seasonal dynamics and habitat specificity of mosquitoes in an English wetland: Implications for UK wetland management and restoration. J. Vector Ecol. 2015, 40, 90–106. [Google Scholar] [CrossRef] [PubMed]
- Bravo-Barriga, D.; Gomes, B.; Almeida, A.P.G.; Serrano-Aguilera, F.J.; Pérez-Martín, J.E.; Calero-Bernal, R.; Reina, D.; Frontera, E.; Pinto, J. The mosquito fauna of the western region of Spain with emphasis on ecological factors and the characterization of Culex pipiens forms. J. Vector Ecol. 2017, 42, 136–147. [Google Scholar] [CrossRef] [PubMed]
- González, M.A.; Prosser, S.W.; Hernández-Triana, L.M.; Alarcón-Elbal, P.M.; López, S.; Ruiz-Arrondo, I.; Hebert, P.D.; García-Pérez, A.L. Avian feeding preferences of Culex pipiens and Culiseta spp. along an urban-to-wild gradient in northern Spain. Front. Ecol. Evol. 2020, 8, 568835. [Google Scholar] [CrossRef]
- Casades-Martí, L.; Frías, M.; Delacour, S.; Ruiz-Fons, F. Confirmed presence of Aedes (Rusticoidus) refiki Medschid, 1928 in a continental dry Mediterranean peri-urban environment in south-central Spain. BMC Zool. 2022, 7, 21. [Google Scholar] [CrossRef]
- Climate Data. Website. Available online: https://es.climate-data.org/europe/espana/la-rioja/logrono-3148/ (accessed on 10 April 2023).
- Confederación Hidrográfica del Ebro. Río Iregua. 2023. Available online: https://www.chebro.es/en/rio-iregua (accessed on 15 May 2023).
- La Rioja Turismo. Parque de La Grajera. 2023. Available online: https://lariojaturismo.com/lugar-de-interes/parque-de-la-grajera/9e375360-9c84-6db4-aa4f-0697115b3439 (accessed on 15 May 2023).
- Navarra Espacios Naturales. Embalse de Las Cañas. 2018. Available online: https://espaciosnaturales.navarra.es/es/embalse-de-las-canas (accessed on 10 April 2023).
- Schaffner, F.; Angel, G.; Geoffroy, B.; Hervy, J.O.; Rhaeim, A. The Mosquitoes of Europe; CD-Rom; IRD Éditions and EID Méditerranée: Montpellier, France, 2001. [Google Scholar]
- Becker, N.; Petric, D.; Zgomba, M.; Boase, C.; Madon, M.; Dahl, C.; Kaiser, A. Mosquitoes and Their Control, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Kampen, H.; Sternberg, A.; Proft, J.; Bastian, S.; Schaffner, F.; Maier, W.A.; Seitz, H.M. Polymerase chain reaction-based differentiation of the mosquito sibling species Anopheles claviger S.S. and Anopheles petragnani (Diptera: Culicidae). Am. J. Trop. Med. Hyg. 2003, 69, 195–199. [Google Scholar] [CrossRef][Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 10 April 2023).
- Bueno-Marí, R.; Bernués-Bañeres, A.; Jiménez-Peydró, R. Updated checklist and distribution maps of mosquitoes (Diptera: Culicidae) of Spain. J. Eur. Mosq. Control Assoc. 2012, 30, 91–126. Available online: https://e-m-b.myspecies.info/sites/e-m-b.org/files/EMB%2830%2991-126-REVISED.pdf (accessed on 10 April 2023).
- Alarcón-Elbal, P.M.; Delacour-Estrella, S.; Ruiz-Arrondo, I.; Pinal, R.; Muñoz, A.; Oropeza, V.; Carmona-Salido, V.J.; Estrada, R.; Lucientes, J. Los culícidos (Diptera Culicidae) del valle medio del Ebro I: La Rioja (Norte de España). Bol. SEA 2012, 50, 359–365. Available online: http://sea-entomologia.org/Publicaciones/PDF/BOLN_50/359365BSEA50CulicidaeEbroI.pdf (accessed on 15 May 2023).
- Bueno Marí, R. Estudio faunístico y eco-epidemiológico de los mosquitos (Diptera, Culicidae) de La Rioja. Zubía 2012, 30, 141–161. Available online: https://dialnet.unirioja.es/descarga/articulo/6868149.pdf (accessed on 15 May 2023).
- Ruiz-Arrondo, I.; Hernández Triana, L.; Oteo Revuelta, J.A. Fauna de mosquitos (Diptera, Culicidae) presentes en el humedal de La Grajera (Logroño) y sus implicaciones en Salud Pública. Zubía 2017, 35, 123–140. Available online: https://dialnet.unirioja.es/descarga/articulo/6338493.pdf (accessed on 10 April 2023).
- Zamburlini, R.; Cargnus, E. Anofelismo residuo nel litorale altoadriatico a 50 anni dalla scomparsa della malaria [Residual mosquitoes in the northern Adriatic seacoast 50 years after the disappearance of malaria]. Parassitologia 1998, 40, 431–437. [Google Scholar] [PubMed]
- Walter Reed Biosystematics Unit. Anopheles claviger Species Page. Walter Reed Biosystematics Unit. 2023. Available online: https://wrbu.si.edu/vectorspecies/mosquitoes/claviger (accessed on 15 May 2023).
- Alarcón-Elbal, P.M.; Sánchez Murillo, J.M.; Delacour Estrella, S.; Ruiz Arrondo, I.; Pinal Prieto, R.; Lucientes Curdi, J. Asociación de vector del VNO e hidrófito invasor: Culex pipiens Linnaeus, 1758 y Ludwigia grandiflora (Michaux) Greuter and Burdet en el marjal de Xeraco-Xeresa, Valencia. An. Biol. 2013, 35, 17–27. [Google Scholar] [CrossRef]
- Gangoso, L.; Aragonés, D.; Martínez-de la Puente, J.; Lucientes, J.; Delacour-Estrella, S.; Estrada Peña, R.; Montalvo, T.; Bueno-Marí, R.; Bravo-Barriga, D.; Frontera, E.; et al. Determinants of the current and future distribution of the West Nile virus mosquito vector Culex pipiens in Spain. Environ. Res. 2020, 188, 109837. [Google Scholar] [CrossRef]
- Bueno Marí, R.; Bernués Bañeres, A.; Chordá Olmos, F.A.; Jiménez Peydró, R. Aportes al conocimiento de la distribución y biología de Anopheles algeriensis Theobald, 1903 en España. Bol. Mal. Salud. Amb. 2011, 51, 93–96. [Google Scholar]
- Observatorio de Mosquitos Del Guadalquivir. Website. Available online: https://mosquitos.ebd.csic.es/ (accessed on 15 May 2023).
- Alarcón-Elbal, P.M.; Rodríguez-Sosa, M.A.; Ruiz-Matuk, C.; Tapia, L.; Arredondo Abreu, C.A.; Fernández González, A.A.; Rodríguez Lauzurique, R.M.; Paulino-Ramírez, R. Breeding Sites of Synanthropic Mosquitoes in Zika-Affected Areas of the Dominican Republic. J. Am. Mosq. Control. Assoc. 2021, 37, 10–19. [Google Scholar] [CrossRef]
- Metge, G.; Hassaïne, K. Study of the environmental factors associated with oviposition by Aedes caspius and Aedes detritus along a transect in Algeria. J. Am. Mosq. Control. Assoc. 1998, 14, 283–288. Available online: https://www.biodiversitylibrary.org/content/part/JAMCA/JAMCA_V14_N3_P283-288.pdf (accessed on 10 April 2023).
- López Sánchez, S. Control Integral de Mosquitos en Huelva; Junta de Andalucía, Conserjería de Salud y Servicios Sociales: Andalusia, Spain, 1989; p. 340. [Google Scholar]
- Encinas Grandes, A. Taxonomía y Biología de los Mosquitos del Área Salmantina (Diptera, Culicidae); CSIC: Madrid, Spain, 1982; p. 437. [Google Scholar]
- Bueno Marí, R.; Corella, E.; Jiménez Peydró, R. Culícidofauna (Diptera: Culicidae) presente en los distintos enclaves hídricos de la ciudad de Valencia (España). Rev. Colomb. Entomol. 2010, 36, 235–241. [Google Scholar] [CrossRef]
- Alarcón-Elbal, P.M. Incidencia de Ochlerotatus (Ochlerotatus) Caspius (Pallas, 1771) (Diptera: Culicidae) En El Término Municipal de Sagunto (Valencia, EspañA). Master’s Thesis, Facultad de Farmacia, Universidad de Valencia, España, Valencia, Spain, 2007; p. 111. [Google Scholar]
- Camp, J.V.; Kniha, E.; Obwaller, A.G.; Walochnik, J.; Nowotny, N. The transmission ecology of Tahyna orthobunyavirus in Austria as revealed by longitudinal mosquito sampling and blood meal analysis in floodplain habitats. Parasites Vectors 2021, 14, 561. [Google Scholar] [CrossRef]
- Service, M.W. Flight periodicities and vertical distribution of Aedes cantans (Mg.), Ae. geniculatus (Ol.), Anopheles plumbeus Steph. and Culex pipiens L. (Dipt.; Culicidae) in southern England. Bull. Entomol. Res. 1971, 60, 639–651. [Google Scholar] [CrossRef]
- Ruiz-Arrondo, I.; Garza-Hernández, J.A.; Reyes-Villanueva, F.; Lucientes-Curdi, J.; Rodríguez-Pérez, M.A. Human-landing rate, gonotrophic cycle length, survivorship, and public health importance of Simulium erythrocephalum in Zaragoza, northeastern Spain. Parasites Vectors 2017, 10, 175. [Google Scholar] [CrossRef] [PubMed]
- Gutsevich, A.V.; Monchadski, A.S.; Shtakelberg, A. Fauna of the U.S.S.R., Diptera Family Culicidae. Acad. Sci. USSR 1974, III, 408. [Google Scholar]
- Soliman, D.E.; Farid, H.A.; Hammad, R.E.; Gad, A.M.; Bartholomay, L.C. Innate cellular immune responses in Aedes caspius (Diptera: Culicidae) mosquitoes. J. Med. Entomol. 2016, 53, 262–267. [Google Scholar] [CrossRef]
- Napp, S.; Petrić, D.; Busquets, N. West Nile virus and other mosquito-borne viruses present in Eastern Europe. Pathog. Glob. Health 2018, 112, 233–248. [Google Scholar] [CrossRef]
- Chordá Olmos, A. Biología de Mosquitos (Diptera: Culicidae) En Enclaves Representativos de la Comunidad Valenciana. Ph.D. Thesis, Facultat de Farmàcia, Universitat de València, Valencia, Spain, 2014. Available online: https://roderic.uv.es/bitstream/handle/10550/35297/TESIS%20DOCTORAL%20ALBERTO%20CHORD%C3%81%20OLMOS.pdf (accessed on 15 May 2023).
- Núñez, A.I.; Talavera, S.; Aranda, C.; Birnberg, L.; Rivas, R.; Pujol, N.; Verdún, M.; Failloux, A.B.; Busquets, N. European Aedes caspius mosquitoes are experimentally unable to transmit Zika virus. Parasites Vectors 2019, 12, 363. [Google Scholar] [CrossRef] [PubMed]
- Ministerio de Sanidad Consumo y Bienestar Social. Primeros Casos de Dengue Autóctono en España, 2018 [Updated 23 November 2018; Cited 20 September 2019]. Available online: https://www.mscbs.gob.es/profesionales/saludPublica/ccayes/alertasActual/docs/ERR_Dengue_autoctono_Espana.pdf (accessed on 15 May 2023).
- Vazeille, M.; Jeannin, C.; Martin, E.; Schaffner, F.; Failloux, A.B. Chikungunya: A risk for Mediterranean countries? Acta Trop. 2008, 105, 200–202. [Google Scholar] [CrossRef]
- Moutailler, S.; Krida, G.; Schaffner, F.; Vazeille, M.; Failloux, A.B. Potential Vectors of Rift Valley Fever Virus in the Mediterranean Region. Vector-Borne Zoonotic Dis. 2008, 8, 749–753. [Google Scholar] [CrossRef]
- Balenghien, T.; Vazeille, M.; Grandadam, M.; Schaffner, F.; Zeller, H.; Reiter, P.; Sabatier, P.; Fouque, F.; Bicout, D.J. Vector competence of some French Culex and Aedes mosquitoes for West Nile virus. Vector-Borne Zoonotic Dis. 2008, 8, 589–596. [Google Scholar] [CrossRef]
- Gutiérrez-López, R.; Bialosuknia, S.M.; Ciota, A.T.; Montalvo, T.; Martínez-de la Puente, J.; Gangoso, L.; Figuerola, J.; Kramere, L.D. Vector competence of Aedes caspius and Ae. albopictus mosquitoes for Zika Virus, Spain. Emerg. Infect. Dis. 2019, 25, 346–348. [Google Scholar] [CrossRef]
- Reiter, P. West Nile virus in Europe: Understanding the present to gauge the future. Eurosurveillance 2010, 15, 19508. [Google Scholar] [CrossRef]
- Brugman, V.A.; England, M.E.; Stoner, J.; Tugwell, L.; Harrup, L.E.; Wilson, A.J.; Medlock, J.M.; Logan, J.G.; Fooks, A.R.; Mertens, P.P.C.; et al. How often do mosquitoes bite humans in southern England? A standardised summer trial at four sites reveals spatial, temporal and site-related variation in biting rates. Parasites Vectors 2017, 10, 420. [Google Scholar] [CrossRef] [PubMed]
- García San Miguel Rodríguez-Alarcón, L.; Fernández-Martínez, B.; Sierra Moros, M.J.; Vázquez, A.; Julián Pachés, P.; García Villacieros, E.; Gómez Martín, M.B.; Figuerola Borras, J.; Lorusso, N.; Ramos Aceitero, J.M.; et al. Unprecedented increase of West Nile virus neuroinvasive disease, Spain, summer 2020. Euro Surveill. 2021, 26, 2002010. [Google Scholar] [CrossRef] [PubMed]
- Figuerola, J.; Jiménez-Clavero, M.Á.; Ruíz-López, M.J.; Llorente, F.; Ruiz, S.; Hoefer, A.; Aguilera-Sepúlveda, P.; Jiménez-Peñuela, J.; García-Ruiz, O.; Herrero, L.; et al. A One Health view of the West Nile virus outbreak in Andalusia (Spain) in 2020. Emerg. Microbes Infect. 2022, 11, 2570–2578. [Google Scholar] [CrossRef] [PubMed]
- Petri, D. Seasonal and Daily Activity of Mosquitoes (Diptera: Culicidae) In Vojvodina, 1st ed; University of Novi Sad: Novi Sad, Serbia, 1989. [Google Scholar]
- Santa-Olalla, P.; Vazquez-Torres, M.C.; Latorre-Fandos, E.; Mairal-Claver, P.; Cortina-Solano, P.; Puy-Azon, A.; Adiego Sancho, B.; Leitmeyer, K.; Lucientes-Curdi, J.; Sierra-Moros, M.J. First autochthonous malaria case due to Plasmodium vivax since eradication, Spain, October 2010. Euro Surveill. 2010, 15, 19684. [Google Scholar] [CrossRef]
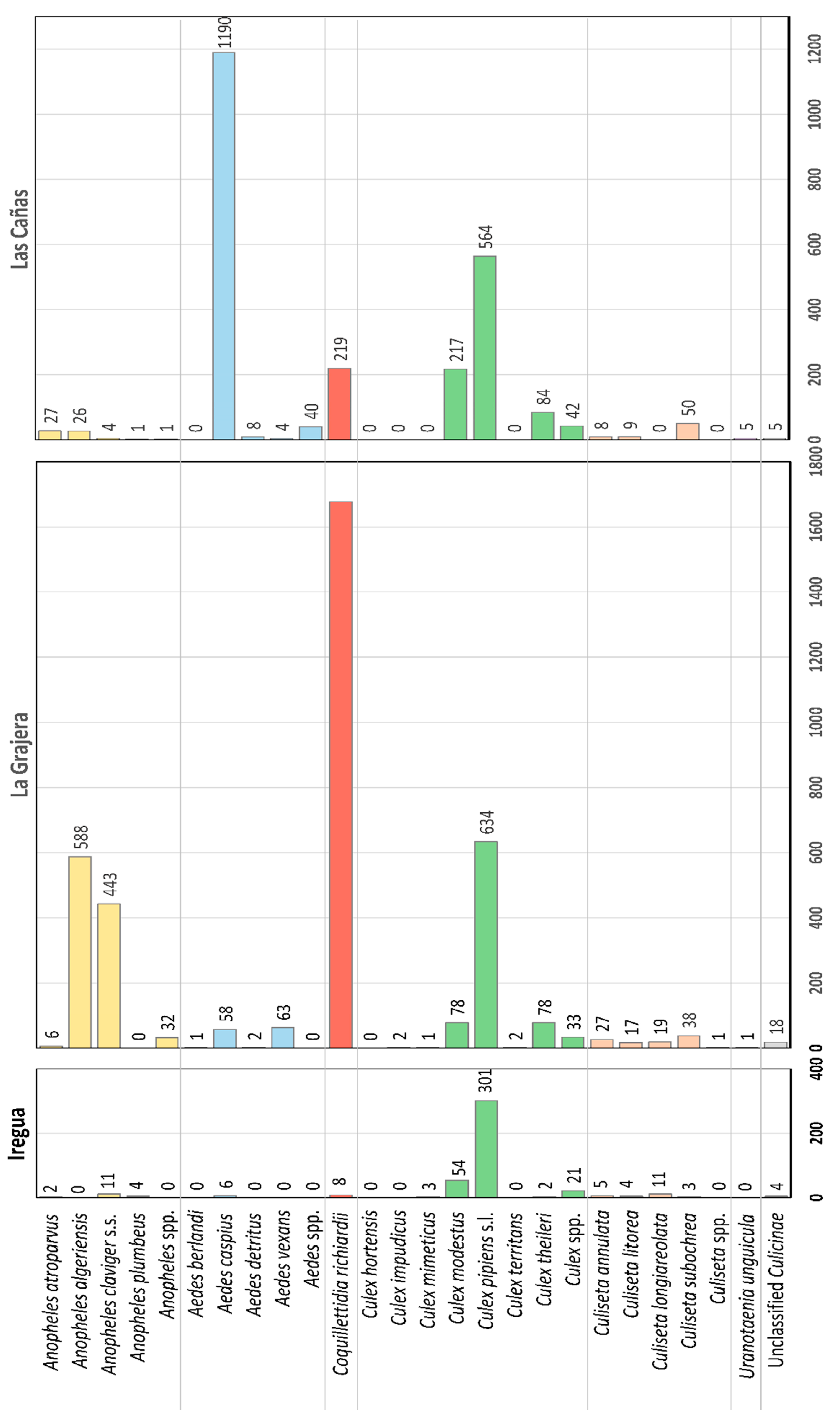
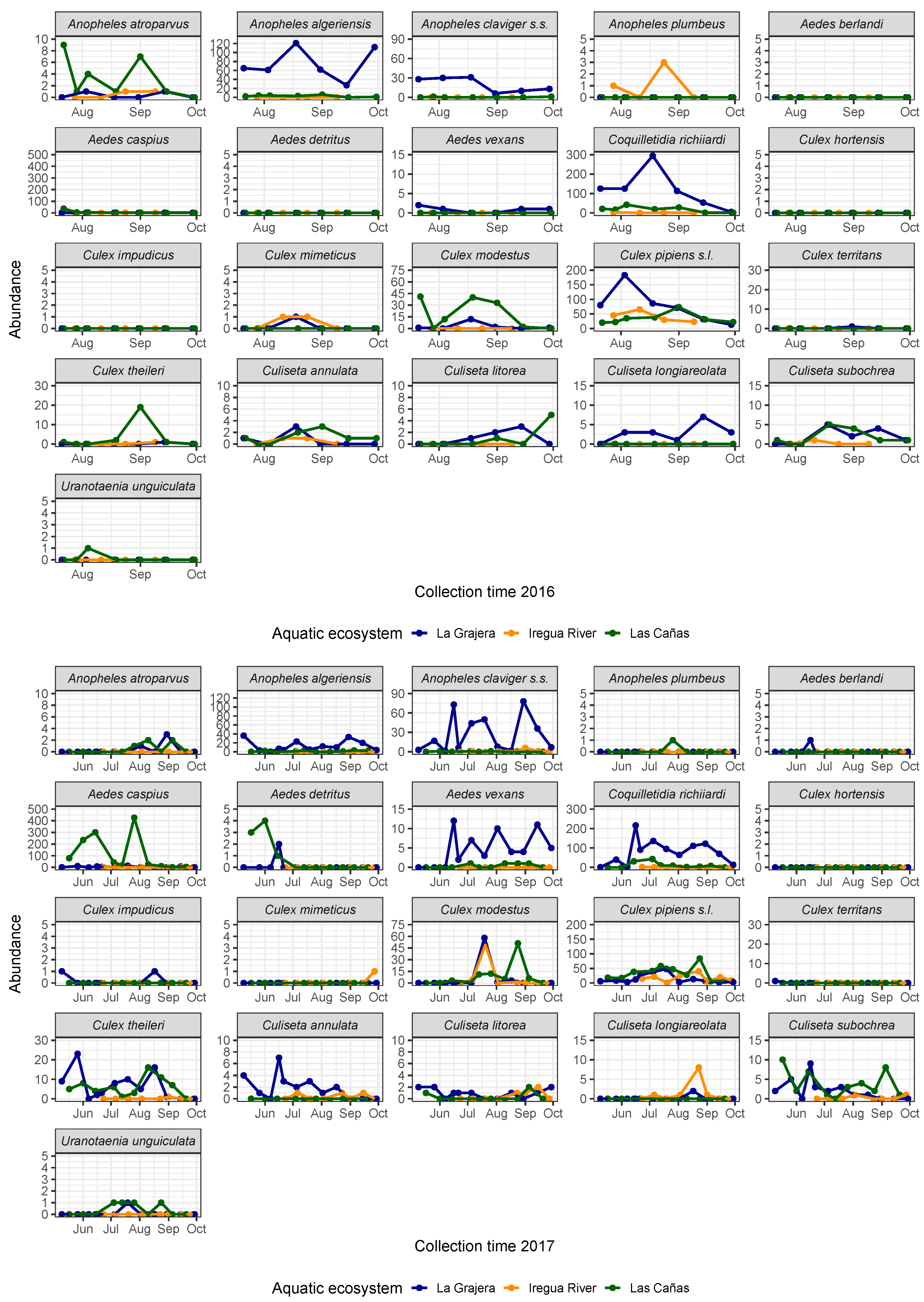
| Genus | Iregua River (n = 50) | La Grajera (n = 90) | Las Cañas (n = 83) | Total (n = 223) |
|---|---|---|---|---|
| Anopheles | 17 | 1069 | 59 | 1145 |
| Aedes | 6 | 124 | 1242 | 1372 |
| Coquillettidia | 8 | 1677 | 219 | 1904 |
| Culex | 381 | 828 | 907 | 2116 |
| Culiseta | 23 | 102 | 67 | 192 |
| Uranotaenia | 0 | 1 | 5 | 6 |
| Unidentified | 4 | 18 | 5 | 27 |
| Total | 435 | 3801 | 2499 | 6735 |
| Genus | Iregua River (n = 50) | La Grajera (n = 90) | Las Cañas (n = 83) | p-Value |
|---|---|---|---|---|
| Anopheles | 0.34 ± 0.72 a | 11.88 ± 16.26 b | 0.71 ± 1.31 a | <0.001 |
| Aedes | 0.12 ± 0.33 a | 1.38 ± 2.14 a | 14.96 ± 36.70 b | <0.001 |
| Coquillettidia | 0.16 ± 0.47 a | 18.63 ± 25.43 b | 2.64 ± 3.90 a | <0.001 |
| Culex | 7.62 ± 8.73 a | 9.20 ± 12.93 ab | 10.93 ± 10.73 b | 0.007 |
| Culiseta | 0.46 ± 1.16 | 1.13 ± 1.86 | 0.81 ± 1.57 | 0.080 |
| Uranotaenia | 0.00 ± 0.00 a | 0.01 ± 0.11 ab | 0.06 ± 0.24 b | 0.047 |
| All | 8.70 ± 8.99 a | 42.23 ± 42.41 c | 30.11 ± 38.26 b | <0.001 |
| Genus | Iregua River (n = 47) | La Grajera (n = 88) | Las Cañas (n = 82) | p-Value |
|---|---|---|---|---|
| Anopheles | 3.9 ± 8.8 a | 28.1 ± 21.3 b | 2.4 ± 5.2 a | <0.001 |
| Aedes | 1.4 ± 5.3 a | 3.3 ± 5.0 a | 49.7 ± 39.4 b | <0.001 |
| Coquillettidia | 1.8 ± 5.8 a | 44.1 ± 24.1 b | 8.8 ± 13.3 a | <0.001 |
| Culex | 87.6 ± 16.2 c | 21.8 ± 20.2 a | 36.3 ± 34.3 b | <0.001 |
| Culiseta | 5.3 ± 11.3 | 2.7 ± 4.0 | 2.7 ± 6.6 | 0.187 |
| Uranotaenia | 0.0 ± 0.0 | 0.0 ± 0.1 | 0.2 ± 1.0 | 0.226 |
| Iregua River | La Grajera | Las Cañas | |||||
|---|---|---|---|---|---|---|---|
| Species | n | Avg ± SD | n | Avg ± SD | n | Avg ± SD | p-Value |
| Anopheles algeriensis | 12 | 0.0 ± 0.0 | 77 | 55.0 ± 34.0 | 30 | 44.1 ± 42.0 | 0.123 1 |
| Anopheles claviger s.s. | 12 | 64.7 ± 49.3 b | 77 | 41.4 ± 34.9 b | 30 | 6.8 ± 21.0 a | 0.032 |
| Aedes caspius | 6 | 100.0 ± 0.0 b | 46 | 46.8 ± 39.5 a | 51 | 95.8 ± 5.8 b | <0.001 |
| Culex pipiens s.l. | 47 | 79.0 ± 33.3 | 73 | 76.6 ± 31.4 | 79 | 62.2 ± 26.5 | 0.129 |
| Culex modestus | 47 | 14.2 ± 33.2 a | 73 | 9.4 ± 20.1 a | 79 | 23.9 ± 25.8 b | 0.007 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-Arrondo, I.; Alarcón-Elbal, P.M.; Blanco-Sierra, L.; Delacour-Estrella, S.; de Blas, I.; Oteo, J.A. Species Composition and Population Dynamics of Culicidae during their Peak Abundance Period in Three Peri-Urban Aquatic Ecosystems in Northern Spain. Diversity 2023, 15, 938. https://doi.org/10.3390/d15080938
Ruiz-Arrondo I, Alarcón-Elbal PM, Blanco-Sierra L, Delacour-Estrella S, de Blas I, Oteo JA. Species Composition and Population Dynamics of Culicidae during their Peak Abundance Period in Three Peri-Urban Aquatic Ecosystems in Northern Spain. Diversity. 2023; 15(8):938. https://doi.org/10.3390/d15080938
Chicago/Turabian StyleRuiz-Arrondo, Ignacio, Pedro María Alarcón-Elbal, Laura Blanco-Sierra, Sarah Delacour-Estrella, Ignacio de Blas, and José A. Oteo. 2023. "Species Composition and Population Dynamics of Culicidae during their Peak Abundance Period in Three Peri-Urban Aquatic Ecosystems in Northern Spain" Diversity 15, no. 8: 938. https://doi.org/10.3390/d15080938
APA StyleRuiz-Arrondo, I., Alarcón-Elbal, P. M., Blanco-Sierra, L., Delacour-Estrella, S., de Blas, I., & Oteo, J. A. (2023). Species Composition and Population Dynamics of Culicidae during their Peak Abundance Period in Three Peri-Urban Aquatic Ecosystems in Northern Spain. Diversity, 15(8), 938. https://doi.org/10.3390/d15080938







