Abstract
The marine benthic diversity of the Palawan/North Borneo ecoregion is poorly known, despite its implied unique high species richness within the Coral Triangle. The present study investigated the diversity and distribution of benthic foraminifera on the Brunei shelf. The objectives were to determine the species composition of sediment samples collected from 11 sites, extending ~70 km from the Brunei coastline and along a depth gradient of 10–200 m. We retrieved a total of 99 species, belonging to 31 families and 56 genera, out of which 52 species represented new records for Brunei and probably the ecoregion. Using presence/absence data, analyses were also performed to compare species diversity patterns (species richness, occupancy, taxonomic distinctness) and species assemblage similarity across the sites. For further insight into the relationship between distribution and depth-associated environmental conditions, we undertook stable isotope analyses of selected species of Rotaliida, Miliolida, and Lagenida. Oxygen isotope values were positively correlated with depth and species distribution, confirming cooler temperatures at greater depth. The carbon isotope data revealed species differences relating to habitat and food source specificity and a biomineralization effect. Close to one-third of the species were recorded from single sites, and species richness and taxonomic distinctness increased with depth and were greatest at the second deepest site (144 m). Together, these findings suggest data underrepresentation of diversity, habitat disturbance in shallower water, and species specialization (adaptation) in deeper water. Importantly, assemblage similarity suggests the occurrence of at least three marine biotopes on the Brunei shelf (10–40 m, 40–150 m, and >150 m). This study contributes significantly to our understanding of the local and regional patterns of foraminiferal diversity and distribution.
1. Introduction
Foraminifera are single-celled protists that are common in marine sediments from intertidal to abyssal zones, continental marshes, and freshwater environments. Benthic foraminifera can be divided into two main groups: the larger benthic foraminifera (LBF), which have algal photosymbionts, and the smaller benthic foraminifera (SBF), which are suspension or detritus feeders. Both of these groups live in eutrophic to oligotrophic conditions in different environmental settings. Benthic foraminifera is a good indicator of environmental conditions and is used in applications such as biostratigraphy, palaeoceanographic and paleoclimatic reconstructions, environmental biomonitoring, and petroleum exploration e.g., [1,2,3,4,5,6,7,8]. The present study focused on documenting and understanding the distribution of the benthic foraminifera of Brunei. The most comprehensive biogeographical analysis suggests that Brunei belongs to the Palawan/North Borneo ecoregion of the Western Coral Triangle province of the Central Indo-Pacific realm [9]. Compared to other regions of the South China Sea (Singapore, Vietnam, Malaysia), the benthic fauna of this region is relatively poorly known [10]. Marine faunas recently studied for Northwest Borneo include reef corals, sponges, echinoderms, gastropods, and foraminifera [11,12,13,14,15,16,17,18,19].
Regionally, multiple studies have been conducted on modern benthic foraminifera of the South China Sea (and Southeast Asia). These include studies from the Challenger Expedition [20,21,22,23], from the International Indian Ocean Expedition [24], and more recently in Indonesia, Malaysia, the Philippines, and Brunei Darussalam [4,7,17,18,19,25,26,27,28,29,30,31,32,33,34,35,36,37,38]. Local benthic foraminiferal distributions are influenced by several parameters, such as seafloor substrate type and grain size, trophic conditions, temperature, availability of nutrients, and depth [2,4,5,7,33,36,39,40,41,42]. The first insight into the benthic foraminifera of Brunei was gained from the work of Ho (1971), who studied diversity and abundance variation along a transect spanning from the inner Brunei Bay to the middle Sungai Brunei estuarine system. Previous studies on the Brunei shelf benthic foraminiferal assemblages focused on coral reefs, wrecks, and local transects of the shallower waters (<60 m; [17,18,19]).
The Brunei shelf, from the coastline to the edge at 200 m depth, extends about 60 to 70 km and presents a significant gradient of environmental conditions with which benthic communities are associated. The seafloor of the shelf constitutes sandy patches of reefs and wrecks, scattered in extensive siliciclastic mud, deriving from river transportation into Brunei Bay [43]. The shelf waters are mesotrophic to eutrophic and have a relatively narrow photic zone [15,44,45,46]. However, the waters further offshore are not influenced to the same degree by muddy terrestrial input and are therefore much clearer. Reduced water turbidity covaries with seawater depth on the Brunei shelf and, thus, with greater distance from the coastline. Marine water temperature also typically covaries with depth [47,48]. Although benthic surveys of the Brunei shelf are routinely conducted by the oil and gas industry in line with environmental impact assessment requirements, these are mostly confined to drilling sites, and there is currently no published work documenting the effect of seawater depth and turbidity on the shelf benthic fauna, including the foraminifera.
The objectives of the study were: (1) to determine the benthic foraminiferal species composition on the Brunei shelf, (2) to compare diversity patterns (species richness, occupancy, taxonomic distinctness) across the sites varying in depth, and (3) to assess species assemblage similarity across the sites. Additionally, to gain insight into the relationship between foraminifera habitat and environmental parameters, stable carbon and oxygen isotope analyses were carried out on selected taxa.
2. Materials and Methods
2.1. Sample Collection and Species Identification
Seafloor sediment samples were collected across the Brunei shelf as part of a Marine Environmental Survey (MES) conducted under the auspices of an environmental consultancy company (Environmental Resources Management, ERM). The benthic foraminifera were, however, not resolved or reported in this survey. Of the samples collected from 30 stations in April 2013, only eleven sites were available for foraminiferal investigation (locations, coordinates, and depths are given in Figure 1 and Table 1). Figure 1 also includes study areas from previous studies by Ho [16] and Goeting et al. [17,18,19] which cover Brunei Bay and the transect samples M and T, respectively. The details of the transects, reefs, and wreck sites can be seen in Goeting et al. [17,18,19].
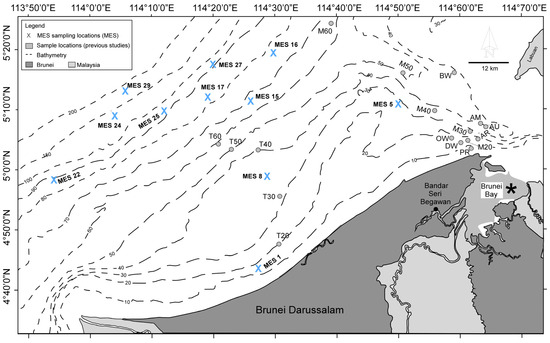
Figure 1.
Study sites on the Brunei shelf for the current study of the MES sites (in bold) and previous studies by Ho [16] in Brunei Bay (*) and Goeting et al. [17,18,19]. Earlier studies focused on Brunei Bay and the reefs, wrecks, and transects mostly along the inner shelf and to depths of ~60 m, respectively. T20–60 (Tutong transect 20–60 mwd), M20–60 (Muara transect 20–60 mwd), PR (Pelong Rocks), AR (Abana Reef), OW (Oil Rig Wreck), DW (Dolphin Wreck), AM (American Wreck), AU (Australian Wreck), and BW (Bluewater Wreck).

Table 1.
Details on the sampling sites and number of species present in each group from each site.
Sediment samples were collected using Van Veen Grab, with a sample surface area of 0.1 m2. As a standard protocol for the company surveying the macrobenthic fauna, the sediments were preserved in formalin at sea and later washed and sieved (500 μm mesh) in the laboratory at Universiti Brunei Darussalam (UBD). Importantly, the sampling did not follow the typical approach used in foraminiferal studies and forewent determining sediment particle size distribution. Consequently, the foraminifera of the present study were resolved for a sediment size of only >500 μm. Furthermore, the processing of the material meant that foraminiferal abundance data were likely unreliable, and thus all analyses and interpretations are based on presence/absence data. The samples were investigated at UBD and the Université de Lausanne.
Foraminifera specimens were picked from the samples using a LEICA EZ4 microscope and stored in small sample holders. Taxa identification was made to genus or species level with the aid of taxonomic references for the region based on Billman et al. [49], Loeblich and Tappan [50], Loeblich and Tappan [24], Jones [23], Parker [51], Debenay [52], Martin et al. [34], Förderer and Langer [33,37], Goeting et al. [19], Hohenegger [53], Renema [4], and Lei and Li [32]. Photographs of the benthic foraminifera were taken using a Keyence Digital Microscope VHX-7000. Plates of benthic foraminifera taxa that had not previously been recorded for Brunei were prepared using Adobe Photoshop CS6.
2.2. Species Richness, Distributions, and Assemblage Comparisons
From an Excel matrix comprising presence/absence data for the species at each study site, we assessed species richness in relation to depth. This was determined for the benthic foraminifera as a whole and separately for the four major groups (i.e., Textulariida, Rotaliida, Miliolida, and Lagenida). In addition to plotting the species richness at each site, we assessed the representativeness of the sampling of the real situation by plotting an occupancy frequency distribution (the number of species versus the number of sites). Because there are normally more common species than rare species, this relationship is usually slightly right-skewed, and a variation from this suggests an under-representation of the sampling [54].
Furthermore, we assessed the taxonomic (or phylogenetic) relatedness of the species at each site (assemblage) using a metric termed “taxonomic distinctness (Δ)”. This is estimated from the path length (taxonomic distance) between two species organised in a taxonomic hierarchy (genus, family, order, and class; according to WORMS) to give a value of between 0 (same species) and 100 (different species), or at a more refined level, 0 = same species, 20 = different species in the same genera, 40 = different genera but same family, etc. (see Warwick and Clarke [55]). The average of the taxonomic distances of all species in the assemblage divided by the Simpson diversity index (Δo), gives the so-called average taxonomic distinctness (∆+), which was determined for each assemblage. ∆+ is more informative of diversity than species richness, which does not account for the relatedness of the species in an assemblage.
Moreover, we assessed how the species assemblages across the Brunei shelf were related to one another. For this, we performed a Bray-Curtis similarity analysis based on presence or absence data, followed by a non-metric Multidimensional Scaling (nMDS) procedure (PRIMER ver. 6, Plymouth, UK). The nMDS produces a two-dimensional plot for each species assemblage, such that the distance between the assemblage points indicates the degree of similarity. Similar assemblages are plotted closer to one another, and more distantly related assemblages are further apart. This analysis also allows for post-hoc clustering to discriminate assemblage groups based on the level of similarity.
2.3. Stable Isotope Analyses
Three commonly occurring species were selected from Miliolida, Lagenida, and Rotaliida for stable carbon and oxygen isotope analyses (Supplementary Table S2). These taxa also cover the depth range of the MES sampling sites. Between ~80 and 200 μg of foraminifera test material were weighed in glass vials. Using a Gas Bench device, the closed vials were flushed with He, and then the test material was dissolved in phosphoric acid. The released CO2 was analysed for stable isotopic compositions with a Thermo Delta-V mass spectrometer, in the stable isotope laboratory of the University of Lausanne (IDYST, UNIL). The procedure is described in Spötl and Vennemann [56]. The data are expressed in Permil (‰) in δ-notation, such as δ18O and δ13C relative to VPDB (Vienna Pee Dee Belemnite international reference standard). The analytical precision was better than ±0.1‰ standard deviation, determined from multiple analyses based on the laboratory’s in-house standards. In addition, four filtered seawater samples were analysed from scuba diving sites for the stable isotope composition of oxygen and dissolved inorganic carbon (DIC) (Supplementary Table S3). The δ13CDIC was analysed using the same settings as used for the carbonates following the method of Spötl [57], while the δ18O values (and δD) were obtained using a Picarro device, as described in Halder et al. [58]. The δ18O values of the water data are expressed relative to VSMOW (Vienna Standard Mean Ocean Water).
3. Results
3.1. Species Richness and Distributions
A total of 99 species of benthic foraminifera belonging to 56 genera (40 hyaline, 37 porcelaneous, and 22 agglutinated forms) were identified (Table 1, Supplementary Table S1). Newly reported species found on the Brunei shelf are shown in Appendix A. The species distributions of the four main taxonomic groups differed with respect to distance from the coast and, accordingly, with depth. Miliolida showed the greatest species richness closer to the coast, while Textulariida, Lagenida, and Rotaliida species were mainly distributed away from the coastline (Table 1). Textulariida (Table 1) were mainly distributed towards the outer shelf in MES 24 at 144 m water depth (mwd), where 15 species have been found before the numbers decrease at MES 29 at 190 mwd. MES 24 has the highest number of agglutinated species, including deeper species within this group. Miliolida and Rotaliida (Table 1) have similar distributions throughout the shelf, but Rotaliida has fewer species in shallower depths compared to Miliolida. Lagenida species are mainly distributed in abundance towards the outer shelf, including MES 27 and MES 24 (Table 1), which is opposite to the distribution patterns of Textulariida, Miliolida, and Rotaliida. The number of species within Lagenida increases with depth and is mainly concentrated within the middle to outer shelf areas (Table 1).
Collectively, species richness varied among the sites to a depth of 100 metres, then dramatically increased to the second most deep site (MES 24; 61 species; Figure 2a). The lowest species richness was found at the deepest site (MES 29; 9 species; Figure 2a). The occupancy frequency distribution was strongly left-skewed with most species (n = 35) found at only one site, and one species found at all the sites (Pseudorotalia indopacifica; Figure 2b, Supplementary Table S1).
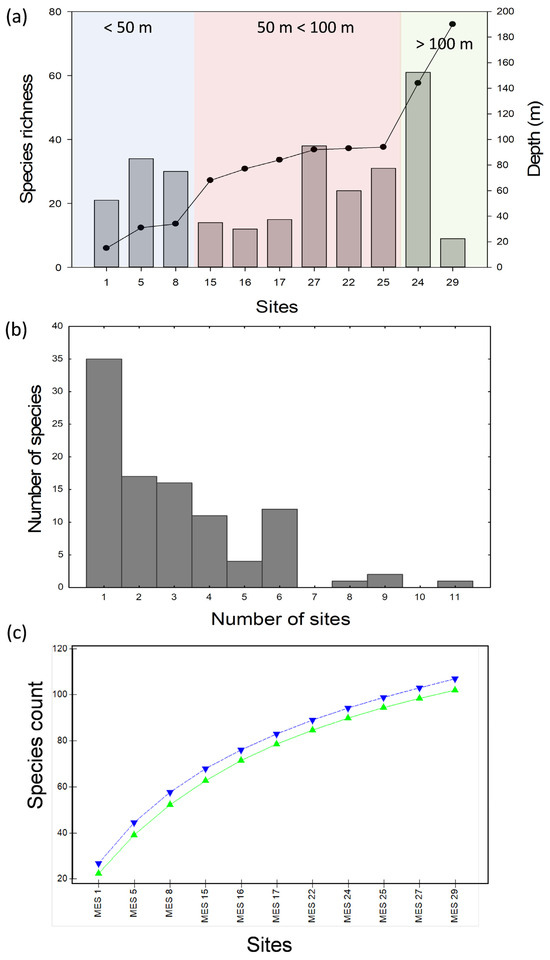
Figure 2.
(a) Species richness for each MES site (bars). The secondary y-axis indicates site depth (filled circles) and colour coding defines broad arbitrary depth categories. (b) Occupancy frequency distribution. (c) Species accumulation (rarefaction) curve; upright triangles, MM, inverted triangles, UGE.
The phylogenetic relatedness of the species in the assemblages, indicated by average taxonomic distinctness (∆+), ranged between 76 and 89 (Figure 3). Greatest ∆+ was observed at MES 24 (depth 144 m), and the least Δ+ was observed in the shallowest and deepest water assemblages (MES 1, MES 5, MES 8, MES 29; Figure 3).
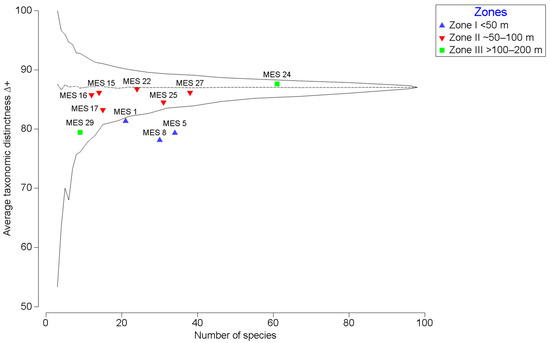
Figure 3.
Average taxonomic distinctness (∆+) of species assemblage at each site plotted against a number of species. Colour codes refer to depth (see inset). The solid line refers to the limit within which 95% of simulated ∆+ values lie, and the dashed line indicates mean ∆+ (see Warwick and Clarke, 1995).
3.2. Species Assemblage Analysis
The nMDS analysis (Figure 4a) revealed clustering (no overlapping) of the species assemblages at 30 and 40% similarities. The 40% similarity level revealed five clusters, whereas the 30% similarity level was especially informative by showing three distinct clusters (groups I, II, and III; Figure 4a). The relationships between these clusters (i.e., assemblage group I being more closely related to group II and then to group III) coincide with their patterns of distribution in relation to seawater depth and distance from the coastline (Figure 4b). Group I consists of MES 1, 5, and 8, which share shallow water sites and are located on the inner shelf (0–50 m). Group II comprises assemblages MES 15, 16, 17, 22, 25, and 27 from the middle shelf zone (50–100 m), but also the deep-water site MES 24 (100–200 m; Figure 4a,b). Group III contains a single assemblage, MES 29, which is distantly related to the other assemblages and occurs in the deepest water sampled (190 m; Figure 4a,b).
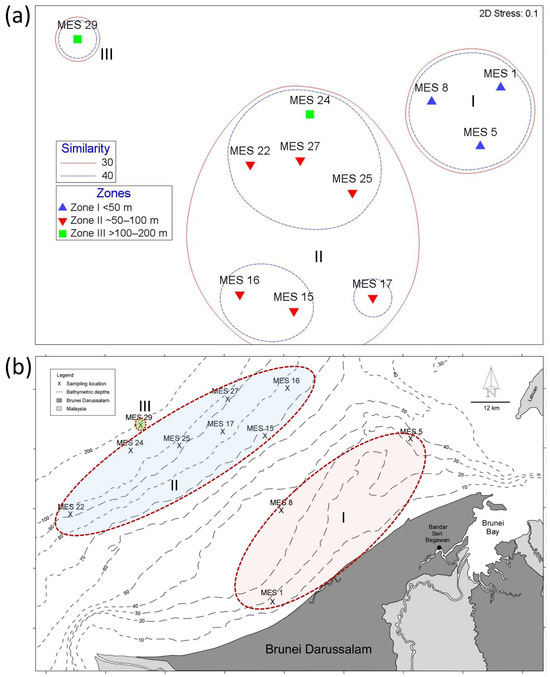
Figure 4.
(a) nMDS showing clusters of the eleven foraminifera assemblages for two levels of similarity. Three clusters are formed at the 30% similarity level. Depth zones are indicated by different coloured symbols. (b) A bathymetric map showing that the nMDS pattern of assemblage clustering coincides with distribution relative to the shoreline and depth (i.e., assemblage cluster I is more closely related to II than to III).
3.3. Isotope Analyses
Altogether, 47 benthic foraminiferal samples were analysed. The δ18O ranges from −3.62 to 0.70‰ (Figure 5), while the δ13C varies between −1.18 and 1.57‰ (Figure 6). The full dataset and the taxon averages are listed in Supplementary Table S2. The seawater yielded an average δ18O value of −0.64 ± 0.10‰, while the δ13CDIC has a mean value of −0.41 ± 0.19‰ in the depth range of 5–24.5 m (Supplementary Table S3).
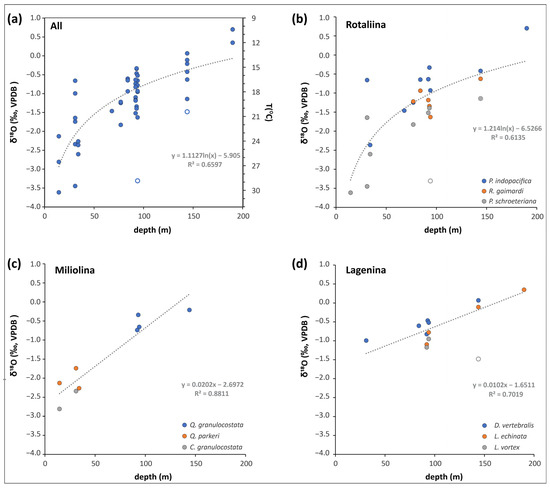
Figure 5.
Oxygen isotope compositions of benthic foraminifera from Brunei as a function of their depth occurrence. (a) All the data and related regression lines. Note that the specimens with empty circles are not included in the best-fit function. These are possibly transported from shallower depths. The secondary y-axis indicates temperatures derived from Equation (1) at a δ18Owater value of −0.64‰. (b–d) The δ18O data according to different suborders and their given species. Note that for Miliolina and Lagenina the best fit is represented by a linear function, while for Rotaliina by logarithmic one.
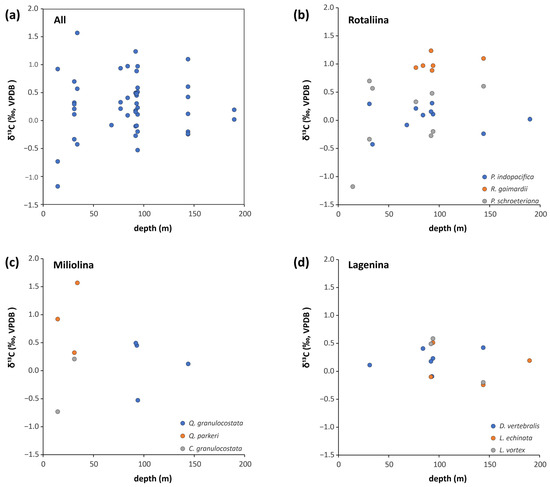
Figure 6.
Carbon isotope compositions of benthic foraminifera from Brunei as a function of their depth occurrence. (a) All the data. Note the somewhat wider scatter at shallower depth (<50 m). (b–d) The δ13C results according to the different suborders and their given species. Note that the rotaliid species of R. gaimardii and the miliolid Q. parkeri tend to have higher δ13C values compared to other species within their respective group.
4. Discussion
4.1. Species Richness and Distributions
From Textulariida (Table 1), only one species is present (Bigenerina nodosaria) at MES 25 (Supplementary Table S1). This species is common at shelf settings from 60 mwd, which are also found within the region [27]. Then, starting at MES 24 (144 mwd), 15 species are present, together with the appearance of Cyclammina subtrullissata, C. trullissata, Cribrostomoides subglobosus, Rhizammina algaeformis, and Tritaxilina caperata, which are known to be usually found in deeper depths [24,27,52]. Rhizammina algaeformis is a deep-agglutinated species with a tubular form, and it is mainly found below 100 mwd [27]. Furthermore, the presence of C. subtrullissata and C. trullissata together with T. caperata is a significant marker of the outer shelf environments ([27]. Although C. subglobosus is a deeper species, it is also found within the inner shelf (see Goeting et al., [18]). This is most likely related to the sediment grain size as the inner shelf has finer sediments, a characteristic of deeper settings, and the availability of organic matter, which is their preference [5] from inputs of rivers to the Brunei shelf [18]. In the nearshore environment, the agglutinated foraminifera are more abundant, especially where higher clay content was detected [18]. The sediment grain size along the inner shelf varies from the northeast side (Muara region), which has a finer-grained seafloor compared to the southwest side (Tutong region) of the shelf [18]. Textularia cf. agglutinans (Supplementary Table S1) is a commonly found taxa in these sites, but it is also found in deeper waters [59] and is associated with muddy sand [38,60]. Agglutinated foraminifera are known to occur in salt marshes, marine shelf, bathyal zones, and abyssal zones [5] which are also found within the region especially the marine shelf, bathyal zones, and abyssal zones [24,27].
From Miliolida (Table 1), on the inner shelf, there is the presence of shallower, larger benthic miliolid species such as Dendritina zhengae, Parasorites orbitolitoides, Peneroplis planatus, and Alveolinella quoyi (Supplementary Table S1) that are mainly present in shallow waters in tropical seas [7,33,53,61]. The species P. planatus prefers shallow waters, clinging to small macroalgae [53]. The genus Dendritina is usually found avoiding shallow energetic waters and is hence mainly found in 10–40 mwd between sand grains. Furthermore, A. quoyi is found until 50 mwd and P. orbitolitoides until 60 mwd [53], but can occur much deeper at almost 80 mwd in Sesoko Island, Okinawa, Japan [62]. However, throughout the samples, the species D. zhengae is more common compared to the other larger benthic species, which are present but most likely concentrated in reef environments. Interestingly, the presence of P. orbitolitoides is usually found in oligotrophic settings [53], but it seems to be living in different trophic conditions [7,33], especially here in the shallower depths of the Brunei shelf which is known in previous studies [17,18]. Furthermore, towards the outer shelf zone, Pyrgo vespertilio and Triloculina vespertilio appear (Supplementary Table S1), which are also common species found within the Indo-Pacific region [24,33,52]. However, they are found at a much shallower depth from these studies which could be related to the transport of shallower miliolid species towards deeper depths. The smaller miliolids are mainly distributed towards the transect samples rather than at the reef and wreck sites [18], therefore being mainly distributed towards soft sediment substrate rather than at sites with harder substrate. The larger miliolid species are mainly concentrated in the reef sites in Brunei [17]. Miliolids are common in shallow environments [52] although they can still be found in greater depths [27], but they are mainly concentrated in shelf settings.
From Lagenida (Figure 2a), there are more lagenid species found compared to the previous study by Goeting et al. [18], where there is a higher diversity of this taxon, especially towards the outer shelf zone (>60 mwd). This is also the case in the Sunda shelf [27] and the Sahul shelf [24], where species such as Marginulinopsis philippinensis, Saracenaria angularis, and Spincterules compressus are also found and mainly distributed from the inner shelf to upper bathyal from 140 to 270 mwd. These species are reported for the first time in the Brunei shelf starting at MES 27 (92 mwd) until MES 29 (190 mwd), which is shallower than the usual distribution in the region, but since the samples are sieved through 500 micrometres, there is a possibility of loss of lagenid species. Lagenids are infaunal and usually occur in deeper marine environments below the photic zone (50 m) [28,63], feeding on the available organic matter within the sediment [18]. They are also found in sites with higher clay content, which is seen in one of the nearby wreck sites, (Dolphin wreck) DW [18], but does not have a higher species richness compared to lageninds found in much deeper depth within the MES sites below 60 mwd. Hence, there could be more lagenid species in the deeper setting that have yet to be reported on the Brunei shelf.
From Rotaliida, there are newly reported species within this group, such as Neoassilina discoidalis, Operculina complanata, and Amphistegina papillosa from the LBF group (Figure A8), while from the SBF group are Uvigerina schwageri and Paracibicides edomica (Figure A8).
N. discoidalis and O. complanata can occur until depths of 100 to 120 mwd, respectively [53], but in the West Pacific [61], O. complanata has a deeper distribution compared to N. ammonoides; hence, these species can be seen still present in deeper sites until around 140 mwd (MES 24). Compared to the study by Goeting et al. [18], the only operculinid species found throughout the inner shelf is N. ammonoides, which is also found in the MES sites. This species is also commonly found in the shelf environment on the Sunda shelf [27], New Caledonia [52], and Sahul shelf up to 140 mwd [24]. However, the presence of more operculinid species suggests that the environment is suitable for their living conditions, as they prefer light for their symbionts and less energetic environments [5,61,64]. Therefore, they occur in deeper environments when terrestrial input is reduced, resulting in enhanced water clarity and hence deeper light penetration through the water column.
Uvigerina schwageri and P. edomica are present in the mid-to-outer shelf zone from MES 22 to MES 29 (92 to 190 mwd) (Supplementary Table S1). P. edomica is mainly distributed in deeper depths in the Sahul shelf at around 80 mwd [24] and from inner to upper bathyal in the Sunda shelf [27]. However, U. schwageri is found slightly deeper in the Sahul shelf at around 100–150 mwd [24] and is mainly distributed on the outer shelf in the Sunda shelf [27]. Both of these smaller benthic species are marker species towards deeper marine shelf environments.
In Goeting et al. [18], the rotaliids are mainly distributed around sunken wrecks and nearby reefal localities, and some have increased numbers along the Tutong transect. The reef sites are concentrated with LBF species [17], which are not commonly found in the MES sites except for A. papillosa at MES 1 and MES 5 (14–30 mwd) and the operculinids (Supplementary Table S1). Furthermore, A. pulchella is also found in the MES sites in addition to previous studies [18] until 144 mwd (MES 24) (Supplementary Table S1) but was also reported from the Sunda shelf within 100 mwd [27]. Two other species recovered in the MES sites, Pseudorotalia indopacifica and P. schroeteriana, are common within the inner shelf zone [18]. P. indopacifica is distributed deeper compared to P. schroeteriana, which is similar to what Gallagher et al. [65] observed in the Sunda shelf [27], such as P. indopacifica here, which is found up to 226 mwd, while P. schroeteriana is found up to 166 mwd.
A crucial finding of this study was the general pattern of increasing species richness with increasing depth along the Brunei shelf such that the greatest richness was observed at a depth of 144 m (MES 24; Figure 2a). There are several possible reasons for this pattern, including (1) greater habitat disturbance in the shallower waters; (2) greater time (ecological and evolutionary) for community establishment in the deeper water; (3) the introduction of novel deep water specialist taxa; and (4) a sampling artefact. Despite the less-than-perfect sampling protocol, the latter seems least likely, especially when considering the tendency towards flattening the species-accumulation curve (Figure 2c). Such a pattern suggests that the sampling is representative of the overall foraminiferal species richness (for the 500 μm size fraction) for the Brunei shelf. However, the occupancy analysis suggests that site-specific species richness might not be very well reflected by the sampling protocol (Figure 2b). Normally, occupancy frequency distributions tend towards being right-skewed unimodal curves, which indicate relatively fewer rare species, in contrast to the left-skewing curve observed for our data. This pattern might be explained by an inappropriate grain size (sampling unit or number of samples for each site) necessary to better capture site-specific species richness [54]. Nonetheless, the data are still highly interpretable when considering the variation in taxonomic distinctness across the sites and site assemblage groupings relative to the broad depth categories. A reduced taxonomic distinctness is commonly associated with habitat disturbance [55]. This was observed for the shallower water sites (MES 1, 5, and 8; Figure 3) and may be a function of natural instability of the seafloor (ground swells) or anthropogenic disturbance (fishing, oil drilling and oil rig deconstruction) in the shallower waters of the shelf. Greater taxonomic distinctness usually implies less disturbance and/or greater niche specialization (adaptation). In the present study, this may refer to greater colonization of deeper water by foraminiferal species, especially at MES 24. The details of the species contributing to this taxonomic distinctiveness across sites are given in the above discussion, and in summary, they suggest an introduction into the deeper water assemblages of agglutinated and lagenid foraminifera species (Table 1).
4.2. Species Assemblage Analysis
The most useful information derived from the nMDS was the formation of three assemblage clusters sharing 30% of their species (Groups I, II, and III; Figure 4a). Furthermore, the relationships of the assemblages in each group and the relationships between the groups conform with site depth. For example, Group I contained assemblages on the inner shelf (0–50 m), Group II contained assemblages between 50 and 100 m but also a deepwater assemblage (144 m, MES 24), and Group III contained the single deepest assemblage (190 m, MES 29). Additionally, the assemblages in Group I were more similar to those of Group II (slightly deeper) than to those of Group III (deepest).
Although we refer to depth, the distributions of species within assemblages must respond to a range of environmental factors that covary with distance from the shore and water depth, including greater water clarity (reduced suspended sediment effect), cooler temperature, and more stable oceanic salinities and pH in the deeper water [48,66]. Additionally, the seafloor and sediment properties must influence species distributions along the depth gradient of the Brunei shelf. The observed pattern of foraminiferal assemblage distributions in relation to inferred seawater parameters and distance from the shore bears similarity to that seen along the coastline of China [67]. Because foraminifera are good ecological indicators, our findings suggest that similar patterns may occur in other marine benthic taxa (e.g., polychaetes, amphipods). Importantly, these findings represent the first indication of three distinct possible marine biotopes on the Brunei shelf, which has far-reaching implications for future marine environmental conservation and resource management.
4.3. Stable Isotope Analyses
The δ18O values of the benthic foraminiferal tests show a distinct decreasing trend from deeper to shallower depth. There are few clear outliers that can be explained by the downward transport of the foraminifera test, while the larger scatter of the dataset could be related to the bulk sediment sampling resulting in some temporal mixing. As the oxygen isotopic composition of the biogenic calcite is a function of the ambient water temperature and water isotopic composition (e.g., [68]), the decreasing trend is best explained by the anticipated colder temperature at greater depth than that of shallower setting. However, in the coastal region, freshwater runoff may also induce variation in the water isotopic composition (δ18Owater). The classic paleotemperature equation [68,69] is as follows:
T(°C) = 15.75 − 4.3 × (δ18Ocarb-VPDB − δ18Owater-VSMOW) − 0.14 × (δ18Ocarb-VPDB − δ18Owater-VSMOW)2
This equation can be used to obtain quantitative temperature data. Based on our foraminifera oxygen isotope results and using our average δ18Owater value of −0.64‰, a temperature range of 10.2 to 29.8 °C can be calculated (Figure 5a). The array fits well with our observation in a shallow water environment where the top 20–30 metres of the seawater is 29–31 °C during July–September (southwest monsoon), meanwhile the temperature drops to as low as 24–25 °C during December–March (northeast monsoon). The deep-water temperature data are slightly lower than reported for the South China Sea [70]. However, our deepest water oxygen isotope data comes from only 24.5 mwd, and the deeper water body could have higher δ18O composition as it is less affected by the continental runoffs of the tropical coastline. Using the global average seawater value of 0‰ would result in ~13 °C at a depth of 190 metres (MES29).
As a third factor, species-related isotope offset from the equilibrium precipitation of the tests might as well be expected (i.e., vital effect, see [71]). When the δ18O data are separated based on the different taxa, the obtained isotope data versus depth relationships are strong and seem independent of the species (Figure 5b–d). For Rotaliina, there is a larger scatter, especially in the shallower environment (<50 m), that could be a result of greater variation in δ18Owater. Nevertheless, the obtained relationships may as well be used to estimate paleo-depth from analyses of fossil foraminifera of the same size range derived from shallow water deposits in northern Borneo.
The carbon isotope composition of marine carbonates is generally related to that of the dissolved inorganic carbon (DIC) in seawater (e.g., [71,72]), which is dominated by the bicarbonate ion. The average δ13CDIC for the ocean is 0–1‰, but on the water surface, higher values can be expected due to photosynthesis, while bottom water δ13CDIC is often lower due to organic matter degradation, especially in shelf areas. In shallow coastal settings, continental input may also induce some variation in the DIC composition. The fractionation between marine carbonate and DIC is small and relatively insensitive to temperature, but marine carbonates in equilibrium with DIC generally have 1 to 2 permil higher values than seawater DIC (e.g., [73]). The δ13C composition of foraminiferal tests also depends on the habitat of the given taxa (epi- vs. infaunal, symbiont-bearing taxa, etc.), and some are also known about their vital effect on the isotopic composition when growing their test (e.g., [71]). Generally, the δ13C results from the benthic foraminiferal tests and seawater fit well with the described carbon dynamics and habitat conditions in the shelf areas of Brunei. The overall data scatters around an average of 0.27‰, which variation is larger in shallower depth (<50 m) (Supplementary Table S2, Figure 6a). This latter may be explained by higher amounts and various nutrient sources from the land. When the data are grouped based on the different taxa, some further details can be detected. Lagenids have the most restricted variation in δ13C with no differences among the investigated three species (0.19 ± 0.28‰, n = 13) (Figure 6d). Among the three rotaliids, the two Pseudorotalia species vary more than that of the lagenids, but on average around 0‰. However, the species Rotalinoides gaimardii yielded significantly higher δ13C values of 1.02 ± 0.13‰ (n = 6) (post hoc Tukey test p < 0.05) (Figure 6b). The miliolids are the ones that scatter the most in their δ13C (Supplementary Table S2, Figure 6c), which may indicate access to and alternate use of different carbon sources. Interestingly, the shallow-water species Q. parkeri tend to have higher, more positive δ13C composition when compared to the co-occurring C. granulocostata, which yielded negative values too (Figure 6c). Though only few specimens have been analysed, the observed differences within the rotaliid and miliolid species could indicate different habitats and/or use of different food sources [6], but alternatively could also reflect a different way of biomineralization (i.e., vital effect). A wider scale of study together with in vitro growth of foraminifera could help further studies in these scenarios.
5. Conclusions
This study shows an increase in benthic foraminiferal (>500 microns) species richness in deeper waters of the Brunei shelf. While the study on the MES sites revealed unreported species from inner to outer shelf zones, the additional data from previous studies of the Brunei shelf have given a complete view of the changes in foraminiferal assemblages throughout the shelf, which are related to depth, distance from the shore, substrate type, and light intensity.
Species richness increases from 14 to 144 m of water depth (mwd) but drastically decreases at 190 mwd, suggesting a shift towards deeper assemblages. δ18O results from selected species support the expected temperature variation with depth, but coastal sites may be influenced by continental runoffs. δ13C data of certain rotaliid and miliolid species show variations reflecting specialized habitats, but this separation is not observed in the investigated lagenid species.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d15080937/s1.
Author Contributions
Methodology, S.G., H.C.L., L.K. and D.J.M.; Software, H.C.L., L.K. and D.J.M.; Formal analysis, S.G., L.K. and D.J.M.; Investigation, S.G., H.C.L., L.K. and D.J.M.; Resources, L.K., C.B.-M. and D.J.M.; Writing—original draft, S.G.; Writing—review & editing, S.G., L.K., C.B.-M. and D.J.M.; Supervision, D.J.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a Universiti Brunei Darussalam research grant [UBD/RSCH/1.4/FICBF(b)/2021/033] and the Swiss Government Excellence Scholarship. In addition, the APC was funded by Université de Lausanne.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data is contained within the article or supplementary material. The data presented in this study are available in [supplementary Table S1].
Acknowledgments
We thank David J. Lane as he helped in the initial organising and sorting of samples provided by Environmental Resources Management (ERM), as part of a marine benthic consultancy. Furthermore, we are grateful to the Swiss Government and University of Lausanne in providing all the materials and equipment required for this research.
Conflicts of Interest
The authors declare no conflict of interest.
Correction Statement
This article has been republished with a minor correction to the image quality of Figures A1–A8. This change does not affect the scientific content of the article.
Appendix A
Figure A1, Figure A2, Figure A3, Figure A4, Figure A5, Figure A6, Figure A7 and Figure A8 and explanation of plates. The scale bar is at 100 μm unless specified.
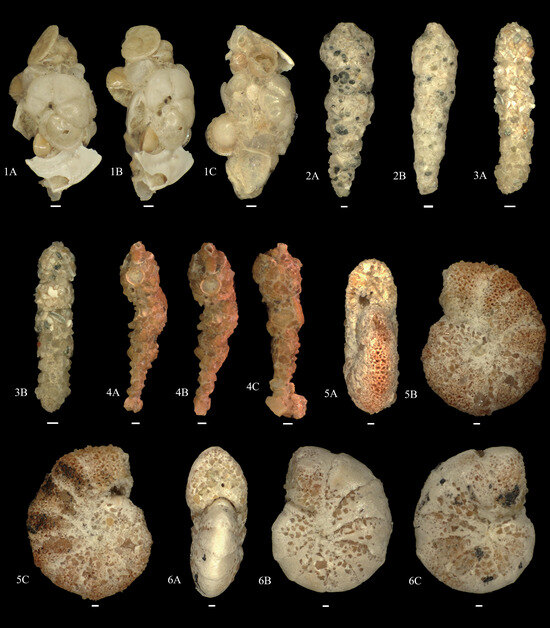
Figure A1.
1A–C. Rhizammina algaeformis; 2A,B. Reophax cf. agglutinans; 3A,B. Reophax communis; 4A–C. Reophax scorpiurus; 5A–C. Cyclammina subtrullisata; 6A–C. Cyclammina trullisata.

Figure A2.
1A–C. Tritaxilina caperata; 2A,B. Cylindroclavulina sp.; 3A,B. Bigenerina agglutinans; 4. Bigenerina cf. agglutinans?; 5A,B. Bigenerina sp.; 6. Textularia goessi; 7A,B. Textularia porrecta; 8A,B. Textularia pseudogramen; 9A,B. Textularia stricta.
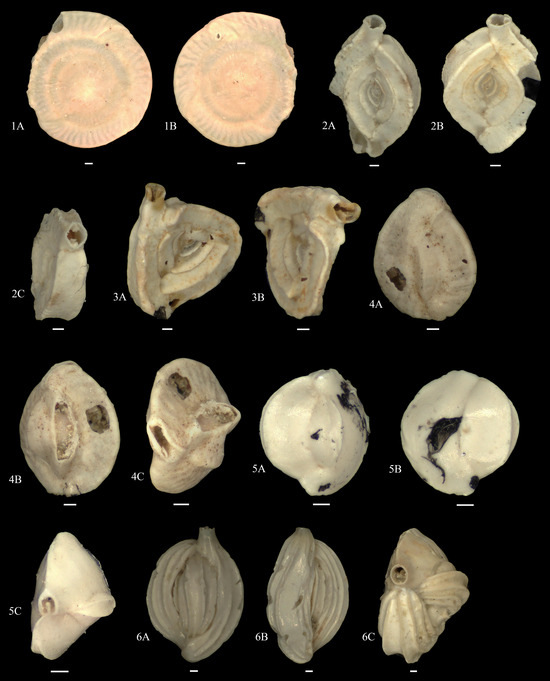
Figure A3.
1A,B. Planispirinella exigua/sp.; 2A–C. Spiroloculina subimpressa; 3A,B. Spiroloculina sp.1; 4A–C. Lachlanella sp.; 5A–C. Quinqueloculina cf. Bicarinata; 6A–C. Quinqueloculina granulocostata.
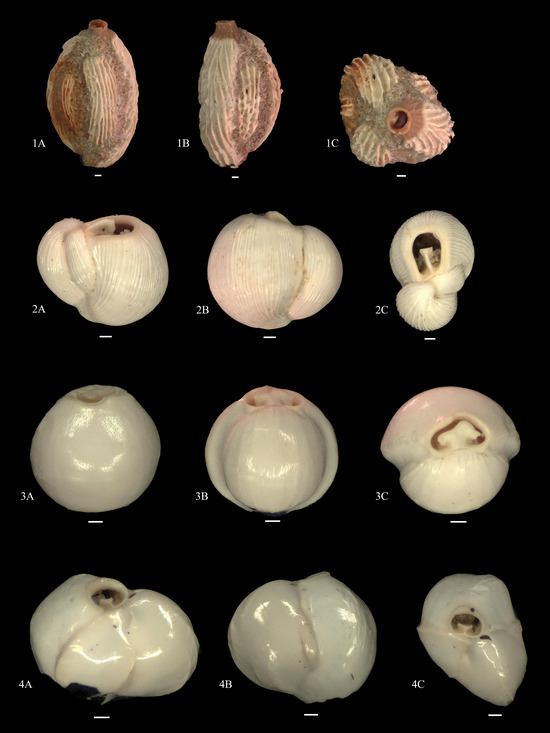
Figure A4.
1A–C. Quinqueloculina sp. 2; 2A–C. Flintina (Triloculina) bradyana; 3A–C. Pyrgo vespertilio; 4A–C. Triloculina vespertilio.

Figure A5.
1A–H. Dendritina zhengae; 2A,B. Parasorites orbitolitoides.

Figure A6.
1. Dentalina catelunata; 2A,B. Dentalina vertebralis; 3A,B. Dentalina sp. 1; 4. Dentalina sp. 2; 5. Nodosaria pyrula; 6A,B. Laevidentalina californica; 7A,B. Pyramidulina catesbyi; 8A,B. Pyramidulina obliquatus; 9A,B. Pyramidulina sp. 1; 10A,B. Lenticulina echinata; 11A,B. Lenticulina limbosa; 12A,B. Lenticulina vortex.
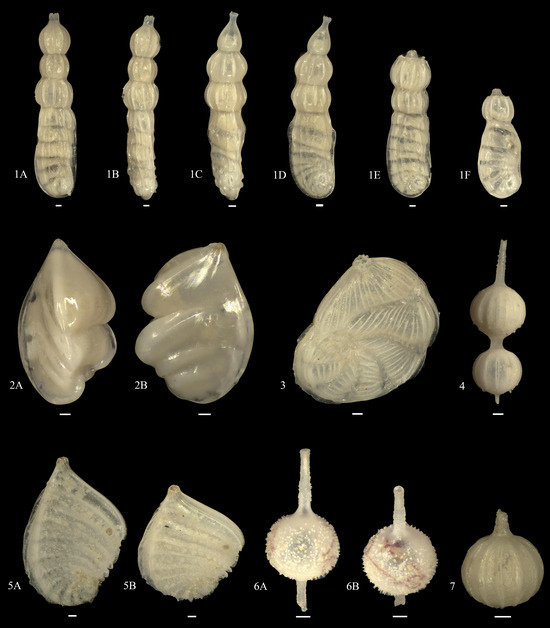
Figure A7.
1A–F. Marginulinopsis philippinensis; 2A,B. Saracenaria angularis; 3. Spincterules compressus; 4. Amphicoryna sublineata; 5A,B. Planularia gemmata; 6A,B. Lagena annulatacollare?; 7. Lagena spicata.
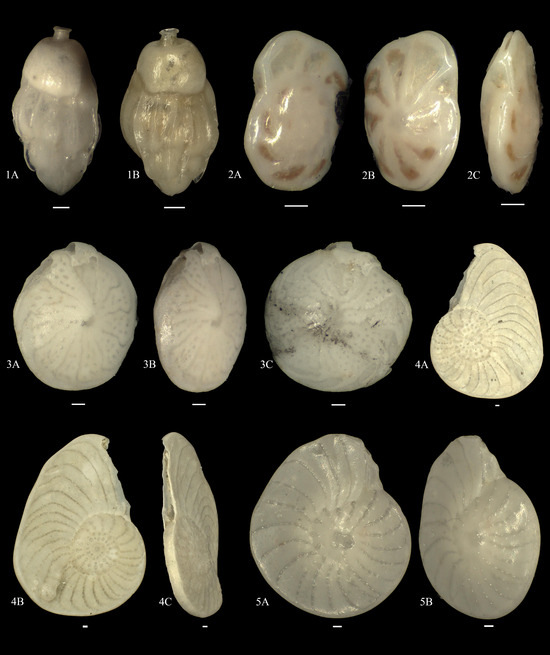
Figure A8.
1A,B. Uvigerina schwageri; 2A–C. Paracibicides edomica; 3A–C. Amphistegina papillosa; 4A–C. Operculina complanata; 5A,B. Neoassilina discoidalis.
References
- Phleger, F. Ecology and Distribution of Recent Foraminifera; Johns Hopkins Press: Baltimore, MA, USA, 1960. [Google Scholar]
- Murray, J.W. Ecology and Palaeoecology of Benthic Foraminifera; John Wiley and Sons Inc.: New York, NY, USA, 1991; pp. 1–341. [Google Scholar]
- Hayward, B.; Hollis, C. Brackish Foraminifera in New Zealand: A Taxonomic and Ecologic Review. Micropaleontology 1994, 40, 185–222. [Google Scholar] [CrossRef]
- Renema, W. Larger Foraminifera as Marine Environmental Indicators; Nationaal Natuurhistorisch Museum: Leiden, The Netherlands, 2002; p. 263. [Google Scholar]
- Murray, J.W. Ecology and Applications of Benthic Foraminifera; Cambridge University Press: Cambridge, UK, 2006; pp. 1–426. [Google Scholar]
- Jones, R.W. Foraminifera and Their Applications; Cambridge University Press: Cambridge, UK, 2014; p. 391. [Google Scholar]
- Renema, W. Terrestrial influence as a key driver of spatial variability in large benthic foraminiferal assemblage composition in the Central Indo-Pacific. Earth-Sci. Rev. 2018, 177, 514–544. [Google Scholar] [CrossRef]
- Suriadi, R.; Shaari, H.; Culver, S.; Husain, M.; Vijayan, V.; Parham, P.; Sapon, N. Inner Shelf Benthic Foraminifera of the South China Sea, East Coast Peninsular Malaysia. J. Foraminifer. Res. 2019, 49, 11–28. [Google Scholar] [CrossRef]
- Spalding, M.D.; Fox, H.E.; Allen, G.R.; Davidson, N.; Ferdaña, Z.A.; Finlayson, M.; Halpern, B.S.; Jorge, M.A.; Lombana, A.; Lourie, S.A.; et al. Marine Ecoregions of the World: A Bioregionalization of Coastal and Shelf Areas. BioScience 2007, 57, 573–583. [Google Scholar] [CrossRef]
- Li, J.; Asner, G.P. Global analysis of benthic complexity in shallow coral reefs. Environ. Res. Lett. 2023, 18, 1–9. [Google Scholar] [CrossRef]
- Lane, D.J.W.; Marsh, L.M.; Vanden Spiegel, D.; Rowe, F.W.E. Echinoderm fauna of the South China Sea: An inventory and analysis of distribution patterns. Raffles Bull. Zool. 2000, 8, 459–493. [Google Scholar]
- Hoeksema, B.W.; Lane, D.J.W. The mushroom coral fauna (Scleractinia: Fungiidae) of Brunei Darussalam (South China Sea) and its relation to the Coral Triangle. Raffles Bull. Zool. 2014, 62, 566–580. [Google Scholar]
- Setiawan, E.; Relex, D.; Marshall, D.J. Shallow-water sponges from a high-sedimentation estuarine bay (Brunei, northwest Borneo, Southeast Asia). J. Trop. Biodivers. Biotechnol. 2021, 6, 66435. [Google Scholar] [CrossRef]
- Mustapha, N.; Baharuddin, N.; Tan, S.K.; Marshall, D.J. The neritid snails of Brunei Darussalam: Their geographical, ecological and conservation significance. Ecol. Montenegrina 2021, 42, 45–61. [Google Scholar] [CrossRef]
- Lane, D.J.W.; Lim, G.P.C. Reef corals in a high sedimentation environment on the ‘mainland’ coast of Brunei, Northwest Borneo. Galaxea. J. Coral Reef Stud. 2013, 15, 166–171. [Google Scholar] [CrossRef][Green Version]
- Ho, K.F. Distribution of Recent Benthonic foraminifera in the “inner” Brunei Bay. Brunei Mus. J. 1971, 2, 124–137. [Google Scholar]
- Goeting, S.; Briguglio, A.; Eder, W.; Hohenegger, J.; Roslim, A.; Kocsis, L. Depth distribution of modern larger benthic foraminifera offshore Brunei Darussalam. Micropaleontology 2018, 64, 299–316. [Google Scholar] [CrossRef]
- Goeting, S.; Ćosović, V.; Benedetti, A.; Fiorini, F.; Kocsis, L.; Roslim, A.; Briguglio, A. Diversity and depth distribution of modern benthic foraminifera offshore Brunei Darussalam. J. Foraminifer. Res. 2022, 52, 160–178. [Google Scholar] [CrossRef]
- Goeting, S.; Fiorini, F.; Benedetti, A.; Kocsis, L.; Roslim, A.; Zaini, N.; Briguglio, A. Catalogue of modern smaller benthic foraminifera from offshore Brunei Darussalam. Palaeontogr. Abt. A 2021, 318, 129–223. [Google Scholar] [CrossRef]
- Brady, H.B. Notes on some of the reticularian Rhizopoda of the Challenger Expedition, Part I. On new or little-known arenaceous types, part II. Additions to the Knowledge of porcellaneous and hyaline types. Q. J. Microsc. Sci. New Ser. 1879, 19, 261–299. [Google Scholar]
- Brady, H.B. Notes on some of the reticularian Rhizopoda of the Challenger Expedition, Part III. 1. Classification. 2. Further notes on new species. 3. Note on Biloculina mud. Q. J. Microsc. Sci. New Ser. 1881, 21, 31–71. [Google Scholar]
- Brady, H.B. Report of the Foraminifera Dredged by H. M. S. Challenger during the Years 1873–1876. Reports of the Scientific Results of the Voyage of H. M. S. Challenger during the Years 1873–1876; Zoology: Jena, Germany, 1884; Volume 9, pp. 1–814. [Google Scholar]
- Jones, R.W. The Challenger Foraminifera; Oxford University Press: Oxford, UK, 1994; pp. 1–149. [Google Scholar]
- Loeblich, A.R.; Tappan, H. Foraminifera of the Sahul Shelf; Cushman Foundation for Foraminiferal Research: Cambridge, MA, USA, 1994; Volume 31, pp. 1–661. [Google Scholar]
- Lesslar, P. Computer-assisted interpretation of depositional palaeoenvironments based on foraminifera. Bull. Geol. Soc. Malays. 1987, 21, 103–119. [Google Scholar] [CrossRef]
- Renema, W. Large benthic foraminifera from the deep photic zone of a mixed siliciclastic-carbonate shelf off East Kalimantan, Indonesia. Mar. Micropaleontol. 2006, 58, 73–82. [Google Scholar] [CrossRef]
- Szarek, R. Biodiversity and Biogeography of Recent Benthic Foraminiferal Assemblages in the South-Western South China Sea (Sunda Shelf). (Unpublished Ph.D. Thesis). Faculty of Mathematics and Natural Sciences, Kiel, Germany, 2001. [Google Scholar]
- Szarek, R.; Kuhnt, W.; Kawamura, H.; Kitazato, H. Distribution of recent benthic foraminifera on the Sunda Shelf (South China Sea). Mar. Micropaleontol. 2006, 61, 171–195. [Google Scholar] [CrossRef]
- Hoeksema, B.W. Delineation of the Indo-Malayan Centre of Maximum Marine Biodiversity: The Coral Triangle. In Biogeography, Time and Place: Distributions, Barriers and Islands; Renema, W., Ed.; Springer: Dordrecht, The Netherlands, 2007; pp. 117–178. [Google Scholar]
- Veron, J.E.N.; De Vantier, L.M.; Turak, E.; Green, A.L.; Kininmonth, S.; Stafford-Smith, M.G.; Peterson, N. Delineating the Coral Triangle: Galaxea. J. Coral Reef Stud. 2009, 11, 91–100. [Google Scholar] [CrossRef]
- Veron, J.E.N.; DeVantier, L.M.; Turak, E.; Green, A.L.; Kininmonth, S.; Stafford-Smith, M.G.; Peterson, N. The Coral Triangle. In Coral Reefs: An Ecosystem in Transition; Dubinsky, Z., Stambler, N., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 47–55. [Google Scholar]
- Lei, Y.; Li, T. Atlas of Benthic Foraminifera from China Seas; Science Press Ltd.: Beijing, China, 2016; p. 399. [Google Scholar]
- Förderer, M.; Langer, M.R. Atlas of benthic foraminifera from coral reefs of the Raja Ampat Archipelago (Irian Jaya, Indonesia). Micropaleontology 2018, 64, 1–170. [Google Scholar] [CrossRef]
- Martin, S.Q.; Culver, S.J.; Leorri, E.; Mallinson, D.J.; Buzas, M.A.; Hayek, L.A.; Shazili, N.A.M. Distribution and Taxonomy of Modern Benthic Foraminifera of the Western Sunda Shelf (South China Sea) off Peninsular Malaysia; Cushman Foundation, Special Publication: Kansas, KS, USA, 2018; Volume 47, p. 108. [Google Scholar]
- Minhat, F.; Husain, M.; Sulaiman, A. Species composition and distribution data of benthic foraminifera from the straits of Malacca during the early Holocene. Data Brief 2019, 25, 104214. [Google Scholar] [CrossRef] [PubMed]
- Novak, V.; Renema, W. Ecological tolerances of Miocene larger benthic foraminifera from Indonesia. J. Asian Earth Sci. 2018, 151, 301–323. [Google Scholar] [CrossRef]
- Förderer, M.; Langer, M.R. Exceptionally species-rich assemblages of modern larger benthic foraminifera from nearshore reefs in northern Palawan (Philippines). Rev. Micropaléontol. 2019, 65, 100387. [Google Scholar] [CrossRef]
- Azmi, N.; Minhat, F.I.; Hasan, S.S.; Rahman Abdul Manaf, O.A.; Abdul A’ziz, A.N.; Wan Saelan, W.N.; Suratman, S. Distribution of benthic foraminifera off Kelantan, Peninsular Malaysia, South China Sea. J. Foraminifer. Res. 2020, 50, 89–96. [Google Scholar] [CrossRef]
- Hallock, P.; Lidz, B.H.; Cockey-Burkhard, E.M.; Donnelly, K.B. Foraminifera as bioindicators in coral reef assessment and monitoring: The FORAM Index. Environ. Monit. Assess. 2003, 81, 221–238. [Google Scholar] [CrossRef]
- Armstrong, H.; Brasier, M. Foraminifera. In Microfossils, 2nd ed.; Armstrong, H., Brasier, M., Eds.; Blackwell Publishing: Malden, UK, 2005; pp. 142–187. [Google Scholar]
- Pignatti, J.; Frezza, V.; Benedetti, A.; Carbone, F.; Accordi, G.; Matteucci, R. Recent foraminiferal assemblages and mixed carbonate-siliciclastic sediments along the coast of southern Somalia and northern Kenya. Ital. J. Geosci. 2012, 131, 66–75. [Google Scholar]
- Benedetti, A.; Frezza, V. Benthic foraminiferal assemblages from shallow-water environments of northeastern Sardinia (Italy, Mediterranean Sea). Facies 2016, 62, 14. [Google Scholar] [CrossRef]
- Zaini, N.; Briguglio, A.; Goeting, S.; Roslim, A.; Kocsis, L. Sedimentological characterization of sea bottom samples collected offshore Muara and Tutong, Brunei Darussalam. Bull. Geol. Soc. Malays. 2020, 70, 139–151. [Google Scholar] [CrossRef]
- Hossain, M.B. Macrobenthic Community Structure from a Tropical Estuary; LAP Lambert Academic Publishing GmbH & Co.: Saarbruecken, Germany, 2011. [Google Scholar]
- Hossain, M.B. Trophic functioning of microbenthic fauna in a tropical acidified Bornean estuary (Southeast Asia). Int. J. Sediment Res. 2019, 34, 48–57. [Google Scholar] [CrossRef]
- Hossain, M.B.; Marshall, D.J.; Venkatramanan, S. Sediment granulometry and organic matter content in the intertidal zone of the Sungai Brunei estuarine system, northwest coast of Borneo. Carpathian J. Earth Environ. Sci. 2014, 9, 231–239. [Google Scholar]
- Talley, L.D. Descriptive Physical Oceanography: An Introduction; Academic Press: Cambridge, MA, USA, 2011. [Google Scholar] [CrossRef]
- Hee, Y.Y.; Weston, K.; Suratman, S.; Akhir, M.F.; Latif, M.; Valliyodan, S. Biogeochemical and physical drivers of hypoxia in a tropical embayment (Brunei Bay). Environ. Sci. Pollut. Res. 2023, 30, 65351–65363. [Google Scholar] [CrossRef]
- Billman, H.; Hottinger, L.; Oesterle, H. Neogene to Recent Rotaliid Foraminifera from the Indo-Pacific Ocean, their canal system, their classification and their stratigraphic use. Schweiz. Palaeontol. Abh. 1980, 101, 7–113. [Google Scholar]
- Loeblich, A.R.; Tappan, H. Foraminiferal Genera and Their Classification; Van Nostrand Reinhold Co.: New York, NY, USA, 1987; p. 869. [Google Scholar]
- Parker, J.H. Taxonomy of Foraminifera from Ningaloo Reef, Western Australia; Memoirs of the Association of Australasian Palaeontologists: Canberra, Australia, 2009; Volume 36, pp. 1–810. [Google Scholar]
- Debenay, J.P. A Guide to 1,000 Foraminifera from the Southwestern Pacific New Caledonia; IRD Editions: Paris, France, 2012; pp. 1–383. [Google Scholar]
- Hohenegger, J. Large Foraminifera: Greenhouse Constructions and Gardeners in the Oceanic Microcosm; Kagoshima University Museum: Kagoshima, Japan, 2011; p. 81. [Google Scholar]
- McGeogh, M.; Gaston, K.J. Occupancy frequency distributions: Patterns, artefacts and mechanisms. Biol. Rev. 2002, 77, 311–331. [Google Scholar] [CrossRef]
- Warwick, R.M.; Clarke, K.R. New ‘biodiversity’ measures reveal a decrease in taxonomic distinctness with increasing stress. Mar. Ecol. Prog. Ser. 1995, 129, 301–305. [Google Scholar] [CrossRef]
- Spötl, C.; Vennemann, W.T. Continuous-flow IRMS analysis of carbonate minerals. Rapid Commun. Mass Spectrom 2003, 17, 1004–1006. [Google Scholar] [CrossRef] [PubMed]
- Spötl, C. A robust and fast method of sampling and analysis of δ13C of dissolved inorganic carbon in ground waters. Isot. Environ. Health Stud. 2005, 41, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Halder, J.; Decrouy, L.; Vennemann, W.T. Mixing of Rhône River water in Lake Geneva (Switzerland–France) inferred from stable hydrogen and oxygen isotope profiles. J. Hydrol. 2013, 477, 152–164. [Google Scholar] [CrossRef]
- Bidgood, M.D.; Simmons, M.D.; Thomas, C.D. Agglutinated Foraminifera from Miocene sediments of northwest Borneo. In Proceedings of the Fifth International Workshop on Agglutinated Foraminifera, Plymouth, UK, 6–16 September 1997; Hart, M.B., Kaminski, M.A., Smart, C.W., Eds.; Grzybowski Foundation Special Publication: Krakow, Poland, 2000; Volume 7, pp. 41–58. [Google Scholar]
- Haunold, T.G.; Baal, C.; Piller, W.E. Benthic foraminiferal associations in the Northern Bay of Safaga, Red Sea, Egypt. Mar. Micropaleontol. 1997, 29, 185–210. [Google Scholar] [CrossRef]
- Hohenegger, J. Coenoclines of Larger Foraminifera. Micropaleontology 2000, 46, 127–151. [Google Scholar]
- Hohenegger, J. Depth coenoclines and environmental considerations of Western Pacific larger foraminifera. J. Foraminifer. Res. 2004, 34, 9–33. [Google Scholar] [CrossRef]
- Hayward, B.W.; Grenfell, H.R.; Reid, C.M.; Hayward, K.A. Recent New Zealand Shallow-Water Benthic Foraminifera: Taxonomy, Ecologic Distribution, Biogeography, and Use in Paleoenvironmental Assessments; Institute of Geological and Nuclear Sciences Limited: Lower Hutt, New Zealand, 1999; p. 264. [Google Scholar]
- Seddighi, M.; Briguglio, A.; Hohenegger, J.; Papazzoni, C.A. New results on the hydrodynamic behaviour of fossil Nummulites tests from two nummulite banks from the Bartonian and Priabonian of northern Italy. Boll. Della Soc. Paleontol. Ital. 2015, 54, 103–116. [Google Scholar]
- Gallagher, S.; Waccace, M.W.; Li, C.L.; Kinna, B.; Bye, J.T.; Akimoto, K.; Torii, M. Neogene history of the West Pacific Warm Pool, Kuroshio and Leeuwin currents. Paleoceanography 2009, 24, 1–27. [Google Scholar] [CrossRef]
- Belanger, C.L.; Jablonski, D.; Roy, K.; Berke, S.K.; Krug, A.Z.; Valentine, J.W. Global environmental predictors of benthic marine biogeographic structure. Proc. Natl. Acad. Sci. USA 2012, 109, 14046–14051. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Fan, D.; Zhao, Q.; Wu, Y.; Ren, F.; Liu, Y.; Li, A. Comparison of alive and dead benthic foraminiferal fauna off the Changjiang Estuary: Understanding water- mass properties and taphonomic processes. Front. Mar. Sci. 2023, 10, 1114337. [Google Scholar] [CrossRef]
- Epstein, S.; Buchsbaum, R.; Lowenstam, H.A.; Urey, H.C. Revised carbonate water isotopic temperature scale. Geol. Soc. Am. Bull. 1953, 64, 1315–1326. [Google Scholar] [CrossRef]
- Sharp, Z. Principles of Stable Isotope Geochemistry, 2nd ed.; University of New Mexico: Albuquerque, NM, USA, 2017; p. 416. [Google Scholar] [CrossRef]
- Zeng, L.; Wang, D.; Chen, J.; Wang, W.; Chen, R. SCSPOD14, a South China Sea physical oceanographic dataset derived from in situ measurements during 1919–2014. Sci. Data 2016, 3, 160029. [Google Scholar] [CrossRef]
- Wefer, G.; Berger, W.H. Isotope palaeontology: Growth and composition of extant calcareous species. Mar. Geol. 1991, 100, 207–248. [Google Scholar] [CrossRef]
- Shackleton, N.J.; Kennett, J.P. Paleotemperature history of the Cenozoic and initiation of Antarctic glaciation: Oxygen and carbon isotope analyses in DSDP sites 277, 279 and 281. Initial Rep. DSDP 1975, 29, 743–755. [Google Scholar]
- Emrich, K.; Ehhalt, D.H.; Vogel, J.C. Carbon isotope fractionation during the precipitation of calcium carbonate. Earth Planet. Sci. Lett. 1970, 8, 363–371. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).