The Herpetofauna of the Insular Systems of Mexico
Abstract
1. Introduction
- Volcanic islands: Originated by the volcanic activity in the ocean, often (but not always) related with plate tectonics [16]. This is the case with most of the most remote islands, such the Revillagigedo Archipelago.
- Tecto-orogenic islands: Frequently, they are the result of the collision of lithospheric plates, that lead to the uplift of oceanic mountains due to compressional forces [16]. This origin is the case for the majority of the Mexican islands connected to the mainland by the continental platform, like the Coronado Archipielago, San Marcos, San Ildefonso, and Cerralvo, although some of these islands were created in conjunction with volcanic or erosional processes [17].
- Sedimentary islands: Formed due to the accumulation of sand, mud, and other sediments dragged by the current of rivers to the oceans and other tidal processes; these events only occur in shallow shelf areas that allow the accumulation of sediments [16]. Examples are the islands of Laguna de Términos, the Montague Island in the Delta of Rio Colorado, or the barrier islands on the coast of Tamaulipas.
- Coralline islands: Result of the accumulation of coralline material. Besides the zoogenic factor, two other conditions are critical, such as shallow marine platforms and water temperature; thus, these islands are more frequent in warm oceans [16]. Cays of Banco Chinchorro and Arrecife Alacranes are examples of this kind.
- Oceanic islands have never had a connection with continental landmasses, so they emerged from the seabed as an isolated land mass, and are the result principally of tectonic uplift, volcanic activity, or reef coralline formations (when these formations grow because of these two other processes) [5,19]. Oceanic island biotas originated almost solely by dispersal, with an obvious tendency toward a more depauperate biota the more isolated the island is, but this isolation might lead to speciation and, in archipelagos, to specific radiation [5]. Remarkably, the insular elements of the marine zone are 3.8% of the Mexico’s total islands tota, but they constitute 29.3% of the surface of the insular territory due to their great size [14].
- By contrast, most continental islands were joined in the past to the continental landmasses, and were separated due to tectonic dynamics or, mostly due to sea level rise following lowered levels during the Pleistocene glaciations. Thus, the rocks of these islands are like those of their parent mainland. When the island is separated from the mainland, it contains a fraction of the continental biota; usually the biodiversity of the island declines as an adjustment of a smaller and isolated environment [19]. The splitting of a species’ population by this process often leads to speciation due to vicariance.
Comment
- First, the taxonomic approach differs widely: Pliego-Sánchez et al. [23] follows the names indicated in the Amphibia Web and ReptileDatabase. On the other hand, as this paper was intended originally to be part of the Mesoamerican herpetofauna conservation series (MCS), it largely follows the nomenclature provided in the mesoamericanhepetology portal. Also, we do not consider subspecies as a valid taxonomic entity, while Pliego-Sánchez et al. [23] does. The reasons for that have been discussed in previous papers by Johnson et al. [24,25]. This introduces important differences in the listings and diversity values we provide. However, after this paper was submitted, Ramírez-Bautista [26] published an updated taxonomic list for the Mexican herpetofauna. We made the effort to match that taxonomy.
- Second, the regionalizations are different: Pliego Sanchez et al. [23] (2021) uses a regionalization based in Morrone [27], and they divide the insular systems into neartic and neotropical ones, and then subdivided them into Californian, Baja Californian, Sonoran, Pacific Lowlands, Veracruzan, and Yucatan Peninsula Provinces. We, on the other hand, classify the insular systems by using physiographic regions, largely based in INEGI (with some modifications, as mentioned in the physiographic regions description).
- Third, we present a different analytic approache: the Coefficient of Biogeographic Resemblance (CBR), whereas, Pliego-Sanchez et al. [23] present a taxonomic turnover value (Bsim dissimilarity) and regressions of species’ richness against area and distance to mainland.
- Fourth, as with other papers we present a detailed discussion regarding how this herpetofauna is represented in the Mexican System of Natural Protected Areas.
- Fifth, we provide the full listing for every island with herpetofaunal records, which is absent from the Pliego-Sanchez et al. [23] paper (we do not know the reason).
2. Materials and Methods
2.1. Our Taxonomic Position
2.2. Our Toponomic Position
2.3. System for Determining Distributional Status
2.4. Systems for Determining Conservation Status
2.5. Insular Physiographic Regions
2.5.1. Islands of the Pacific of Baja California (Islas del Pacífico de Baja California)
2.5.2. Islands of the Gulf of California (Islas del Golfo de California)
2.5.3. Tropical Islands of the Pacific (Islas Tropicales del Pacífico)
2.5.4. Gulf Islands (Islas del Golfo de México)
2.5.5. Caribbean Islands (Islas del Caribe Mexicano)
2.6. Comments on the Species List
2.7. Comments on Island Toponomy
3. Results
3.1. Composition of the Herpetofauna
3.1.1. Families
3.1.2. Genera
3.1.3. Species
3.2. Patterns of Physiographic Distribution
| Scaphiopus couchii | Aspidoscelis bacata ** |
| Crotaphytus dickersonae ** | Aspidoscelis cana ** |
| Crotaphytus insularis ** | Aspidoscelis carmenensis ** |
| Gambelia wislizenii | Aspidoscelis catalinensis ** |
| Coleonyx gypsicolus ** | Aspidoscelis celeripes ** |
| Ctenosaura conspicuosa ** | Aspidoscelis ceralbelsis ** |
| Ctenosaura hemilopha * | Aspidoscelis danheimae ** |
| Ctenosaura nolascensis ** | Aspidoscelis espiritensis ** |
| Dipsosaurus catalinensis ** | Aspidoscelis franciscensis ** |
| Sauromalus ater | Aspidoscelis martyris ** |
| Sauromalus hispidus * | Aspidoscelis pictus ** |
| Sauromalus klauberi ** | Bogertophis rosaliae |
| Sauromalus slevini * | Lampropeltis californiae |
| Sauromalus varius * | Lampropeltis catalinensis ** |
| Petrosaurus mearnsi | Masticophis barbouri |
| Petrosaurus repens * | Masticophis bilineatus |
| Petrosaurus slevini ** | Masticophis slevini ** |
| Petrosaurus thalassinus * | Phyllorhynchus decurtatus |
| Phrynosoma solare | Rhinocheilus etheridgei ** |
| Sceloporus angustus ** | Sonora savagei * |
| Sceloporus grandaevus ** | Sonora semiannulata |
| Sceloporus hunsakeri * | Tantilla planiceps |
| Sceloporus lineatulus ** | Trimorphodon lyrophanes |
| Sceloporus magister | Hypsiglena catalinae ** |
| Sceloporus orcutti | Hypsiglena chlorophaea |
| Uta encantadae ** | Micruroides euryxanthus |
| Uta lowei ** | Crotalus angelensis ** |
| Uta nolascensis ** | Crotalus atrox |
| Uta palmeri ** | Crotalus catalinensis |
| Uta squamata | Crotalus cerastes |
| Uta tumidarostra ** | Crotalus estebanensis ** |
| Phyllodactylus angelensis ** | Crotalus lorenzoensis ** |
| Phyllodactylus apricus ** | Crotalus molossus |
| Phyllodactylus bugastrolepis ** | Crotalus polisi ** |
| Phyllodactylus coronatus ** | Crotalus pyrrhus |
| Phyllodactylus homolepidurus * | Crotalus thalassoporus ** |
| Phyllodactylus partidus ** | Crotalus tigris |
| Phyllodactylus santacruzensis ** | Crotalus tortuguensis ** |
| Phyllodactylus unctus * | Gopherus morafkai |
| Incilius mazatlanensis * | Aspidoscelis lineatissima * |
| Eleutherodactylus pallidus * | Boa sigma * |
| Norops nebulosus * | Drymarchon melanurus |
| Gehyra mutilata ‡ | Lampropeltis polyzona * |
| Ctenosaura pectinata | Leptophis diplotropis * |
| Marisora aquilonaria * | Masticophis anthonyi ** |
| Urosaurus auriculatus ** | Oxybelis microphtalmus * |
| Urosaurus bicarinatus * | Tantilla bocourti * |
| Urosaurus clarionensis ** | Tantilla calamarina * |
| Phyllodactylus benedetii * | Conophis vittatus |
| Phyllodactylus cloefasensis ** | Geophis annuliferus * |
| Phyllodactylus isabelae ** | Hypsiglena torquata |
| Phyllodactylus lanei * | Hypsiglena unaocularis ** |
| Phyllodactylus lupitae ** | Imantodes gemmistratus |
| Phyllodactylus tuberculosus | Rhadinaea hesperia * |
| Aspidoscelis communis * | Epictia bakewelli * |
| Aspidoscelis costata * | Agkistrodon bilineatus |
| Aspidoscelis guttatus * | Kinosternon integrum * |
| Eleutherodactylus planirostris ‡ | Aspidoscelis rodecki * |
| Dendrosophus microcephalus | Leptophis mexicanus |
| Trachycephalus vermiculatus | Oxybelis fulgidus |
| Anolis allisoni ‡ | Tantilla moesta |
| Norops lemurinus | Epictia magnamaculata |
| Coleonyx elegans | Thamnophis proximus |
| Sceloporus cozumelae * | Agkistrodon russeolus * |
| Mesoscincus schwartzei | Trachemys venusta |
| Aristelliger georgeensis | Rhinoclemmys areolata |
| Sphaerodactylus continentalis |
| Hyliola rejilla | Gambelia copeii |
| Aneides lugubris | Phrynosoma cerroense * |
| Batrachoseps major | Sceloporus occidentalis |
| Elgaria cedrosensis * | Plestiodon skiltonianus |
| Elgaria multicarinata | Lampropeltis herrerae ** |
| Elgaria nana ** | Pituophis insularis ** |
| Anniella geronimensis * | Diadophis punctatus |
| Anniella pulchra | Crotalus caliginis ** |
| Bipes biporus * |
| Crocodylus moreletii | Dipsas brevifacies |
| Sceloporus variabilis | Imantodes cenchoa |
| Drymobius margaritiferus | Ninia sebae |
| Lampropeltis abnormal | Tropidodipsas sartorii |
| Pseudelaphe flavirufa | Dermatemys mawii |
| Spilotes pullatus | Staurotypus triporcatus |
| Coniophanes imperialis |
3.3. Patterns of Distribution within Physiograph Regions
3.4. Patterns of Distribution within Physiograph Regions
3.5. Comparison of Distributional Categorizations and Physiographic Regional Categorizations
3.6. Principal Environmental Threats
3.6.1. Deforestation, Agriculture, and Urban Development
3.6.2. Agriculture and Cattle
3.6.3. Hurricanes and Other Tropical Storms
3.6.4. Wildfires
3.6.5. Tourism
3.6.6. Invasive Species
3.6.7. Global Climate Change
3.6.8. Oil and Gas Industries
3.6.9. Delinquency and Organized Crime
3.6.10. Illegal Collecting
3.7. Conservation Status
3.7.1. The SEMARNAT System
3.7.2. The IUCN System
3.7.3. The EVS System
| Elgaria nana | Aspidoscelis cana |
| Crotaphytus dickersonae | Aspidoscelis carmenensis |
| Crotaphytus insularis | Aspidoscelis celeripes |
| Coleonyx gypsicolus | Aspidoscelis ceralbelsis |
| Ctenosaura conspicuosa | Aspidoscelis danheimae |
| Dipsosaurus catalinensis | Aspidoscelis espiritensis |
| Sauromalus klauberi | Aspidoscelis franciscensis |
| Petrosaurus slevini | Aspidoscelis pictus |
| Sceloporus angustus | Lampropeltis catalinensis |
| Sceloporus grandaevus | Masticophis barbourin |
| Sceloporus lineatulus | Masticophis slevini |
| Uta nolascensis | Pituophis insularis |
| Phyllodactylus angelensis | Rhinocheilus etheridgei |
| Phyllodactylus apricus | Hypsiglena catalinae |
| Phyllodactylus bugastrolepis | Hypsiglena unaocularis |
| Phyllodactylus cleofasensis | Crotalus angelensis |
| Phyllodactylus coronatus | Crotalus caliginis |
| Phyllodactylus isabelae | Crotalus estebanensis |
| Phyllodactylus lupitae | Crotalus lorenzoensis |
| Phyllodactylus partidus | Crotalus polisi |
| Phyllodactylus tuberculosus | Crotalus thalassoporus |
| Aspidoscelis bacata | Crotalus tortuguensis |
3.8. Relative Herpetofaunal Priority
| Crotaphytus dickersonae ** | (16) | Phyllodactylus unctus * | (15) |
| Crotaphytus insularis ** | (16) | Aspidoscelis bacata ** | (17) |
| Coleonyx gypsicolus ** | (18) | Aspidoscelis cana ** | (16) |
| Ctenosaura conspicuosa ** | (16) | Aspidoscelis carmenensis ** | (17) |
| Ctenosaura hemilopha * | (18) | Aspidoscelis catalinensis ** | (17) |
| Ctenosaura nolascensis ** | (17) | Aspidoscelis celeripes ** | (15) |
| Dipsosaurus catalinensis ** | (17) | Aspidoscelis ceralbelsis ** | (17) |
| Sauromalus hispidus * | (14) | Aspidoscelis danheimae ** | (16) |
| Sauromalus klauberi ** | (16) | Aspidoscelis espiritensis ** | (16) |
| Sauromalus slevini * | (16) | Aspidoscelis franciscensis ** | (17) |
| Sauromalus varius * | (16) | Aspidoscelis martyris ** | (17) |
| Petrosaurus slevini ** | (16) | Aspidoscelis pictus ** | (17) |
| Phrynosoma solare | (14) | Lampropeltis catalinensis ** | (17) |
| Sceloporus angustus ** | (16) | Masticophis barbourin | (17) |
| Sceloporus grandaevus ** | (16) | Masticophis slevini ** | (17) |
| Sceloporus hunsakeri * | (14) | Rhinocheilus etheridgei ** | (16) |
| Sceloporus lineatulus ** | (17) | Sonora avage * | (15) |
| Uta encantadae ** | (17) | Hypsiglena catalinae ** | (16) |
| Uta lowei ** | (17) | Micruroides euryxanthus | (15) |
| Uta nolascensis ** | (17) | Crotalus angelensis ** | (18) |
| Uta palmeri ** | (17) | Crotalus catalinensis | (19) |
| Uta squamata | (17) | Crotalus cerastes | (16) |
| Uta tumidarostra ** | (17) | Crotalus estebanensis ** | (19) |
| Phyllodactylus angelensis ** | (16) | Crotalus lorenzoensis ** | (19) |
| Phyllodactylus apricus ** | (17) | Crotalus polisi ** | (18) |
| Phyllodactylus bugastrolepis ** | (17) | Crotalus thalassoporus ** | (18) |
| Phyllodactylus coronatus ** | (16) | Crotalus tigris | (16) |
| Phyllodactylus homolepidurus * | (15) | Crotalus tortuguensis ** | (18) |
| Phyllodactylus partidus ** | (16) | Gopherus morafkai | (15) |
| Eleutherodactylus pallidus * | (17) | Phyllodactylus lanei * | (15) |
| Crocodylus acutus | (14) | Phyllodactylus lupitae ** | (16) |
| Ctenosaura pectinata | (15) | Phyllodactylus tuberculosus | (17) |
| Urosaurus auriculatus ** | (16) | Aspidoscelis communis * | (14) |
| Urosaurus clarionensis ** | (17) | Aspidoscelis lineatissima * | (14) |
| Phyllodactylus benedetii * | (15) | Leptophis diplotropis * | (14) |
| Phyllodactylus cleofasensis ** | (16) | Masticophis anthonyi ** | (17) |
| Phyllodactylus isabelae ** | (16) | Hypsiglena unaocularis ** | (16) |
| Aneides lugubris (14) | Phrynosoma cerroense * (16) |
| Batrachoseps major (14) | Lampropeltis herrerae ** (17) |
| Elgaria cedrosensis * (16) | Pituophis insulanus ** (16) |
| Elgaria nana ** (16) | Crotalus calignis ** (18) |
| Bipes biporus * (14) |
| Crocodylus acutus | (14) | Aspidoscelis rodecki * | (16) |
| Sceloporus cozumelae | (15) | Holcosus gaigeae * | (15) |
| Aspidoscelis cozumela * | (16) | Agkistrodon russeolus * | (15) |
| Aspidoscelis maslini | (15) | Trachemys venusta | (19) |
| Aspidoscelis cozumela * | (16) | Dipsas brevifacies | (15) |
| Aspidoscelis maslini | (15) | Dermatemys mawii | (17) |
| Holcosus gaigeae * | (15) | Staurotypus triporcatus | (14) |
| Boa imperator * | (15) |
3.9. Protected Areas
4. Conclusions
- The herpetofauna of the Mexican insular systems consists of 226 species, including 16 amphibians, of which 14 are anurans and 2 are salamanders, and 210 reptiles, of which 2 are crocodilians, 195 are squamates, and 13 are turtles. This is the 16.2% of the Mexico’s documented herpetofauna of 1397 species. These 226 species are arranged into 40 families and 95 genera. Of the 226 insular species, 118 (52.2%) are non-endemic species, 56 (24.8%) are insular endemics (Like the Nolasco Spiny Tailed Iguana, Figure 45), 45 (19.9%) are country endemics, and 7 (3.1%) are non-native species.
- Some genera appear to contain species complexes, such as Phyllodactylus, Uta, and Hypsiglena. Molecular revision of these insular populations could uncover new species, such as that which has happened recently with some insular populations of Crotalus. Additionally, although we do not recognize any infraspecific taxon, we are aware that some of them could constitute separate evolutionary lineages. Thus, it is very likely that the degree of endemism could rise if some of these lineages are recognized as full species based on new taxonomic revisions.
- We documented the herpetofauna of 141 insular elements in Mexico, but also detected areas with insular elements in which the composition of herpetofauna is unknown, such as the islands of the Bahias de Huatulco, in the Laguna Madre/Río Bravo basin in Tamaulipas, as well as sites where the herpetofaunal composition is not available for the insular elements, such as is the case of the islands of the Bahia de Chamela. Surveys must be carried in those sites in order to understand their herpetofaunal composition.
- Regarding the distribution of the Mexican insular herpetofauna within each of the five physiographic regions we recognized. The 17 islands of the Pacific Baja California physiographic region support from 1 to 19 species, the largest number of which is found on Isla Santa Margarita. The 70 islands of the Gulf of California physiographic region contain from 1 to 43 species, the greatest number of which lie on Isla Tiburón. The 16 islands of the Tropical Pacific physiographic region are occupied by from 1 to 22 species, with the greatest number found on Isla María Madre. The seven insular systems in the Gulf of Mexico host from 1 to 42 species, with the greatest value in the islands of Laguna de Términos (Isla del Carmen and Isla Arena). On the side of the Mexican Caribbean shore, we considered seven insular systems, unsurprisingly, Cozumel is the most herpetofaunistical-diverse island in the region, with 39 confirmed species, while the herpetofauna of the cays within Sian Ka’an is almost all unknown.
- Of the seven non-native species occurring on the Mexican islands, one is an anuran (Eleutherodactylus planirostris), and six are reptiles, including two anoles (Anolis allisoni and Norops sagrei), three geckos (Gehyra mutilata, Hemidactylus frenatus and H. turcicus), and one blindsnake (Indotyphlops braminus). In addition, some species are native to the country but alien to the Mexican islands, such as Boa imperator to Cozumel and, perhaps Banco Chinchorro and Isla Venados, and C. pectinata for Clarion.
- Our comparison of the distributional categorizations and the physiographic regional categorizations demonstrates that the greatest proportion of species in each physiographic region are non-endemics. Country endemic species are primarily represented on the Pacific side of Mexico, as opposed to those on the Atlantic side. The regional or insular endemics are mostly represented on the islands of the Gulf of California and not at all in the two Atlantic regions. The non-native species are not distributed on the two peninsular-associated regions, but rather occur to some extent in each of the three other regions.
- A comparison of the SEMARNAT, IUCN, and EVS systems indicates important discrepancies in assessment of the number of insular herpetofaunal species at risk. We believe that the SEMARNAT system underestimates the threat of herpetofaunal insular diversity, since it only lists eight insular species as “endangered,” seven of those being turtles, but ignores an important number of insular endemic squamates, many of which are only known from the type locality and, in the case of Lampropeltis catalinensis, only from the holotype.
- The IUCN Red List, although considered as the standard system in global conservation status assessment, is of limited value for use with the Mexican insular herpetofauna, since only 21 species are placed in one of the three risk categories. As with the SEMARNAT system, seven of these species are turtles. As a result, 141 species are allocated to the Least Concern category and 6 species to the Near Threatened category; 56 species are not evaluated or are judged to be Data Deficient. We believe that these omissions are driven primarily by the limited information available on species in insular environments.
- The EVS system is more useful and practical for assessing the conservation risk for insular herpetofauna; it has easily allowed the assessment of threat level for species whose ecology and/or population numbers are poorly known. The results indicated that 56 species lie in the low vulnerability category, 62 in the medium category, and 94 in the high category, with the numbers increasing from the low, through the medium, to the high category. Simple calculations of the number of high EVS species would allow conservation managers to identify which islands are a priority for the conservation of the herpetofauna.
- It is imperative to include the insular endemics into the threat levels of the NOM-059 and in the IUCN Red List, with particular emphasis on those species whose only known population is at the type locality.
- The use of both of the Relative Herpetofauna Priority measures indicates that the islands of the Gulf of California are of the greatest conservation significance, followed in order by the Tropical Pacific islands region, the Pacific Baja California islands, the Caribbean islands, and the islands of the Gulf of Mexico.
- The principal environmental threats for the insular herpetofauna are invasive species, urban and/or tourist development on the Caribbean islands, the risk of wildfires, which can be disastrous for insular ecosystems, and climate change as a major threat for the cays and islands of the Caribbean. Other threats include deforestation, agricultural development, cattle raising, hurricanes and other tropical storms, the oil and gas industries, public insecurity, and illegal collecting.
- Protocols of biosecurity must be implemented in the Mexican islands in order to prevent the introduction of invasive species, with particular emphasis on rats and cats. These measures, in fact, are being carried out on some Mexican islands with various levels of efficiency, but there is still a long way to go for these measures to be widely adopted.
- It is imperative to promote legislation that reinforces the need for government agencies to control and manage wildfires. The capacity of environmental institutions to manage wildfires must be reinforced, since a major wildfire on some islands could result in the extinction of endemic herpetofaunal species.
- The efforts in decreeing protected areas in insular ecosystems are astonishing, with almost all of the offshore insular systems having some degree of legal protection. We did not identify any herpetofaunal insular endemic that is not represented in a protected area.
- Mexico is very close to achieving the goal of protection of 100% of the insular elements. The creation of Natural Protected Areas on the islands of La Roqueta and Ixtapa should be encouraged; also, the island of Santa Margarita should be included in the Biosphere Reserve of Islas del Pacífico de Baja California. Efforts must be undertaken to to merge the statal reserves in Cozumel within a federal one, in order to facilitate their administration.
- Fortunately, most of the Mexican islands are administrated by the federal government and are unoccupied and unsettled. Also, the risk of privatization is nonexistent under current Mexican legislation. Measures for control of urban development are needed urgently for the Caribbean islands such Cozumel, Holbox, and Isla Mujeres.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Whittaker, R.J.; Fernández-Palacios, J.M. Island Biogeography: Ecology, Evolution, and Conservation; Oxford University Press: Oxford, UK, 2007. [Google Scholar]
- May, R.M. Foreword. In The Theory of Island Biogeography Revisited; Losos, J.B., Ricklefs, R.E., Eds.; Princeton University Press: Princeton, NJ, USA, 2010; pp. vii–x. [Google Scholar]
- Itescu, Y. Are island-like systems biologically similar to islands? A review of the evidence. Ecography 2018, 42, 1298–1314. [Google Scholar] [CrossRef]
- Donlan, C.J.; Tershy, B.R.; Keitt, B.S.; Wood, B.; Sanchez, J.; Weinstein, A.; Croll, D.A.; Alguilar, J. Island conservation action in northwest Mexico. In Proceedings of the Fifth California Islands Symposium, Santa Barbara, CA, USA, 29 March–1 April 1999; pp. 330–338. [Google Scholar]
- Paulay, G. Biodiversity on oceanic islands: Its origin and extinction1. Am. Zool. 1994, 34, 134–144. [Google Scholar] [CrossRef]
- Enderson, E.F.; Quijada-Mascareñas, A.; Turner, D.S.; Rosen, P.C.; Bezy, R.L. The herpetofauna of Sonora, Mexico, with comparisons to adjoining states. Check List 2009, 5, 632–672. [Google Scholar] [CrossRef]
- Tershy, B.R.; Shen, K.-W.; Newton, K.M.; Holmes, N.D.; Croll, D.A. The Importance of Islands for the Protection of Biological and Linguistic Diversity. BioScience 2015, 65, 592–597. [Google Scholar] [CrossRef]
- Keitt, B.; Campbell, K.; Saunders, A.; Clout, M.; Wang, Y.; Heinz, R.; Newton, K.; Tershy, B. The global islands invasive vertebrate eradication database: A tool to improve and facilitate restoration of island ecosystems. In Island Invasives: Eradication and Management; IUCN: Gland, Switzerland, 2011; pp. 74–77. [Google Scholar]
- Lara-Lara, J.; Arreola-Lizárraga, J.; Calderón-Aguilera, L.; Camacho-Ibar, V.; De la Lanza-Espino, G.; Escofet-Giansone, A.; Espejel-Carbajal, M.; Guzmán-Arroyo, M.; Ladah, L.; López-Hernández, M. Los ecosistemas costeros, insulares y epicontinentales. Cap. Nat. De México 2008, 1, 109–134. [Google Scholar]
- Salgado-Sánchez, K.; Dávila-Acosta, L.; Pérez-Rocha, A.M. Mexico y Sus Estados; Ediciones Culturales Internacionales S., A.: Ciudad de México, Mexico, 2011. [Google Scholar]
- Ochoa-Ochoa, L.M.; Flores-Villela, O. Áreas de Diversidad y Endemismo de la Herpetofauna Mexicana; CONABIO, Universidad Nacional Autónoma de México: Ciudad de México, Mexico, 2006; p. 211. [Google Scholar]
- Méndez Buenos Aires, M. Las islas mexicanas: Importancia económica, régimen jurídico y proyecciones internacionales. Rev. Mex. De Política Exter. 1990, 28, 33–39. [Google Scholar]
- González-Avelar, M. El territorio insular como frontera. Front. Norte 2017, 9, 161–169. [Google Scholar]
- INEGI. Catalogo del Territorio Insular Mexicano; Instituto Nacional de Estadística y Geografía: Aguascalientes, México, 2015; p. 252. [Google Scholar]
- Aguirre-Muñoz, A.; Bezaury-Creel, J.E.; De la Cueva, H.; March-Mifsut, I.J.; Peters-Recagno, E.; Rojas-González de Castilla, S.; Santos del Prado Gasca, K. Islas de México, Un Recurso Estratégico; Instituto Nacional de Ecología (INE), The Nature Conservancy (TNC), Grupo de Ecología y Conservación de Islas, A.C. (GECI), Centro de Investigación Científica y de Educación Superior de Ensenada (CICESE): Ensenada, Mexico, 2010; p. 52. [Google Scholar]
- Sockman, R.W. Genesis of Islands. In Geography of Small Islands: Outposts of Globalisation; Ratter, B.M.W., Ed.; Springer: Berlin/Heidelberg, Germany, 2018; pp. 25–60. [Google Scholar]
- Carreño, A.L.; Helenes, J. Geology and ages of the islands. In A New Island Biogeography of the Sea of Cortés; Oxford University Press: Oxford, UK, 2002; pp. 14–40. [Google Scholar]
- Conabio-Conanp-Tnc-Pronatura. Análisis de Vacíos y Omisiones en Conservación de la Biodiversidad Marina de México: Océanos, Costas e Islas; Comisión Nacional para el Conocimiento y Uso de la Biodiversidad, Comisión Nacional de Áreas Naturales Protegidas, The Nature ConservancyPrograma México, Pronatura, A.C: Ciudad de México, Mexico, 2007; p. 129. [Google Scholar]
- Thornton, I. Island Colonization: The Origin and Development of Island Communities; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Cabada-Huerta, M. El Territorio Insular de México. In Proceedings of the Serie Amarilla, Temas Políticos y Sociales. Centro de Estudios de Derecho e Investigaciones Parlamentarias; Cámara de Diputados, LIX Legislatura, H.; Congreso de la Unión: Ciudad de México, Mexico, 2007. [Google Scholar]
- Garmendia-Cedillo, X. Patrimonio Nacional Islas: Patrimonio Insular Mexicano. Prax. De La Justicia Fisc. Y Adm. 2010, 2, 106–197. [Google Scholar]
- CONANP. Programa de Manejo Área de Protección de Flora y Fauna Islas del Golfo de California; Comisión Nacional de Áreas Naturales Protegidas: Ciudad de México, Mexico, 2001; p. 262. [Google Scholar]
- Pliego-Sánchez, J.V.; Blair, C.; Díaz de la Vega-Pérez, A.H.; Jiménez-Arcos, V.H. The insular herpetofauna of Mexico: Composition, conservation, and biogeographic patterns. Ecol. Evol. 2021, 11, 6579–6592. [Google Scholar] [CrossRef]
- Wilson, L.; Johnson, J.; Mata-Silva, V. A conservation reassessment of the amphibians of Mexico based on the EVS measure. Amphib. Reptile Conserv. 2013, 7, 97–127. [Google Scholar]
- Wilson, L.D.; Mata-Silva, V.; Johnson, J.D. A conservation reassessment of the reptiles of Mexico based on the EVS measure. Amphib. Reptile Conserv. 2013, 7, 1–47. [Google Scholar]
- Ramírez-Bautista, A.; Torres-Hernández, L.A.; Cruz-Elizalde, R.; Berriozabal-Islas, C.; Hernández-Salinas, U.; Wilson, L.D.; Johnson, J.D.; Porras, L.W.; Balderas-Valdivia, C.J.; González-Hernández, A.J. An updated list of the Mexican herpetofauna: With a summary of historical and contemporary studies. ZooKeys 2023, 1166, 287. [Google Scholar] [CrossRef]
- Morrone, J.J.; Escalante, T.; Rodriguez-Tapia, G. Mexican biogeographic provinces: Map and shapefiles. Zootaxa 2017, 4277, 277–279. [Google Scholar] [CrossRef] [PubMed]
- McCranie, J.R. A checklist of the amphibians and reptiles of Honduras, with additions, comments on taxonomy, some recent taxonomic decisions, and areas of further studies needed. Zootaxa 2015, 3931, 352–386. [Google Scholar] [CrossRef] [PubMed]
- Solís, J.; Wilson, L.; Townsend, J. An updated list of the amphibians and reptiles of Honduras, with comments on their nomenclature. Mesoamerican Herpetol. 2014, 1, 123–144. [Google Scholar]
- Lemos-Espinal, J.A.; Dixon, J.R. Anfibios y Reptiles de Hidalgo México/Amphibians and Reptiles of Hidalgo Mexico; CONABIO: Ciudad de México, Mexico, 2015. [Google Scholar]
- Ramírez-Bautista, A.; Hernández-Salinas, U.; Cruz-Elizalde, R.; Berriozabal-Islas, C.; Lara-Tufiño, D.; Goyenechea, I.; Castillo-Cerón, J. Los Anfibios y Reptiles de Hidalgo, México: Diversidad, Biogeografía y Conservación; Sociedad Herpetológica Mexicana: Ciudad de México, Mexico, 2014. [Google Scholar]
- Ramírez-Bautista, A.; Hernández-Salinas, U.; Mendoza-Quijano, F.; Cruz-Elizalde, R.; Stephenson, P.; Vite-Silva, V.; Leyte-Manrique, A. Lista anotada de los anfibios y reptiles del estado de Hidalgo, México; UNIVERSIDAD AUTÓNOMA DEL ESTADO DE HIDALGO Instituto de Ciencias Básicas e Ingeniería (ICBI): Ciudad de Mexico, Mexico, 2010. [Google Scholar]
- Ramírez-Bautista, A.; Hernández Salinas, U.; Cruz-Elizalde, R.; Berriozabal-Islas, C.; Moreno-Lara, I.; DeSantis, D.L.; Johnson, J.D.; García-Padilla, E.; Mata-Silva, V.; Wilson, L.D. The herpetofauna of Hidalgo, Mexico: Composition, distribution, and conservation status. Amphib. Reptile Conserv. 2020, 14, 63. [Google Scholar]
- Campbell, J.A. Amphibians and Reptiles of Northern Guatemala, the Yucatan, and Belize; University of Oklahoma Press: Norman, OK, USA, 1998; p. 400. [Google Scholar]
- Lee, J.C. A Field Guide to the Amphibians and Reptiles of the Maya World; Cornell University Press: Ithaca, NY, USA, 2000; p. 402. [Google Scholar]
- Lee, J.C. The Amphibians and Reptiles of the Yucatán Peninsula; Cornell University Press: Ithaca, NY, USA, 1996. [Google Scholar]
- Johnson, J.D.; Mata-Silva, V.; Wilson, L.D. A conservation reassessment of the Central American herpetofauna based on the EVS measure. Amphib. Reptile Conserv. 2015, 9, 1–94. [Google Scholar]
- Liner, E.A.; Casas-Andreu, G. Standard Spanish, English and scientific names of the amphibians and reptiles of Mexico. Herpetol. Circ. 2008, 38, 1–162. [Google Scholar]
- Reyes-Vayssade, M.; Moreno-Collado, J.; Zertuche-Muñoz, F.; González-Gómez, F.; Ruiz-Naufal, V.; González-Avelar, M.; Gallo, M.Á.; González-Dávalos, M.; Barrera-Bassols, J. Cartografía Histórica de las Islas Mexicanas; Secretaría de Gobernación: Ciudad de México, Mexico, 1992; p. 305. [Google Scholar]
- Martínez, J.I.B.; Pineda, C.S. Cedros: Origin and permanence of the placenames in a Mexican Pacific island. Isl. Stud. J. 2016, 11, 399–416. [Google Scholar] [CrossRef]
- Nash, J. Do island toponymies exist? (Guest Editorial Introduction). Isl. Stud. J. 2016, 11, 339–342. [Google Scholar] [CrossRef]
- Alvarado-Díaz, J.; Suazo-Ortuño, I.; Wilson, L.; Medina-Aguilar, O. Patterns of physiographic distribution and conservation status of the herpetofauna of Michoacán, Mexico. Amphib. Reptile Conserv. 2013, 7, 128–170. [Google Scholar]
- Nevárez-de-los-Reyes, M.; Lazcano, D.; García-Padilla, E.; Mata-Silva, V.; Johnson, J.D.; Wilson, L.D. The herpetofauna of Nuevo León, Mexico: Composition, distribution, and conservation. Mesoamerican Herpetol. 2016, 3, 558–638. [Google Scholar]
- Lazcano, D.; Nevarez-de los Reyes, M.; Garcia-Padilla, E.; Johnson, J.D.; Mata-Silva, V.; DeSantis, D.L.; Wilson, L. The herpetofauna of Coahuila, Mexico: Composition, distribution, and conservation status. Amphib. Reptile Conserv. 2019, 13, 31–94. [Google Scholar]
- Samaniego-Herrera, A.; Peralta-García, A.; Aguirre-Muñoz, A. Vertebrados de las islas del Pacífico de Baja California. In Guía de campo. Grupo de Ecología y Conservación de Islas, AC Ensenada, Baja California; Instituto Nacional de Ecología: Ciudad de México, Mexico, 2007. [Google Scholar]
- Schmieder, R.W. Rocas Alijos: Scientific Results from the Cordell Expeditions; Springer: Dordrecht, The Netherlands, 1996; pp. 501+XXX. [Google Scholar]
- D.O.F. DECRETO por el que se declara Área Natural Protegida, con el carácter de reserva de la biosfera, la región conocida como Islas del Pacífico de la Península de Baja California. D. Of. Federación 2016, DCCLIX, 52–84. [Google Scholar]
- CONANP. Estudio Previo Justificativo para el Establecimiento de la Reserva de la Biosfera Islas del Pacífico de California; Comisión Nacional de Áreas Naturales Protegidas: Ciudad de México, Mexico, 2005; p. 169. [Google Scholar]
- Donlan, C.J.; Croll, D.A.; Tershy, B.R. Islands, Exotic Herbivores, and Invasive Plants: Their Roles in Coastal California Restoration. Restor. Ecol. 2003, 11, 524–530. [Google Scholar] [CrossRef]
- Sarabia-Trejo, A.D. Islas prioritarias de conservación en el área natural protegida Islas del Golfo de California; Centro de Investigaciones Biológical del Noroeste, S.C.: La Paz, Baja California Sur, Mexico, 2015. [Google Scholar]
- Castro-Aguirre, J.L.; Balart, E.F.; Arvizu- Martínez, J. Contribución al conocimento del origen y distrubución de la ictiofauna del Golfo de California, México. Hidrobiológica 1995, 5, 57–78. [Google Scholar]
- Nix, R.K.; The Gulf of California. A Physical, Geological and Biological Study. Available online: http://activetectonics.asu.edu/BAJA/gulf_cal.pdf (accessed on 20 May 2020).
- Claudino-Sales, V. Archipiélago de Revillagigedo, Mexico. In Coastal World Heritage Sites; Claudino-Sales, V., Ed.; Springer: Dordrecht, The Netherlands, 2019; pp. 361–366. [Google Scholar]
- Alvarez, R.; Corbo Camargo, F.; Yutsis, V.V. Geophysical modelling of Isla Isabel: A volcanic island on the Mexican continental margin. Geol. Soc. Lond. Spec. Publ. 2017, 446, 295–310. [Google Scholar] [CrossRef]
- Peralta, A.; García, L. Geología y Geocronología de la Isla San Juanito, Archipiélago de las Islas Marías, Nayarit, México; Universidad Nacional Autónoma de México: Ciudad de Mexico, Mexico, 2013. [Google Scholar]
- Pompa-Mera, V.; Schaaf, P.; Hernández-Treviño, T.; Weber, B.; Solís-Pichardo, G.; BVillanueva-Lascurain, D.; Layer, P. Geology, geochronology, and geochemistry of Isla María Madre, Nayarit, Mexico. Rev. Mex. De Cienc. Geológicas 2013, 30, 1–23. [Google Scholar]
- González-Sánchez, V.; Johnson, J.; García-Padilla, E.; Mata-Silva, V.; DeSantis, D.; Wilson, L. The herpetofauna of the Mexican Yucatan Peninsula: Composition, distribution, and conservation. Mesoamerican Herpetol. 2017, 4, 264–380. [Google Scholar]
- INE. Programa de Manejo del Área de Protección de Flora y Fauna” Laguna de Términos”; Instituto Nacional de Ecología: Ciudad de México, Mexico, 1997; p. 167. [Google Scholar]
- Cooper, J.; Pilkey, O.; Lewis, D. Islands behind islands: An unappreciated coastal landform category. J. Coast. Res. 2007, 50, 907–911. [Google Scholar]
- CONANP. Programa de Conservación y Manejo Parque Nacional Arrecife Alacranes; Comisión Nacional de Áreas Naturales Protegidas: Ciudad de Mexico, Mexico, 2006; p. 173. [Google Scholar]
- CONANP. Programa de Manejo Parque Nacional Sistema Arrecifal Veracruzano; Secretaría de Medio Ambiente y Recursos Naturales, Comisión Nacional de Áreas Naturales Protegidas: Ciudad de México, Mexico, 2017; p. 352. [Google Scholar]
- Ardisson, P.-L.; May-Kú, M.A.; Herrera-Dorantes, M.T.; Arellano-Guillermo, A. El sistema arrecífal mesoamericano-México: Consideraciones para su designación como zona marítima especialmente sensible. Hidrobiológica 2011, 21, 261–280. [Google Scholar]
- Ramos, E.P.; De la Riva, L.S.; Peña, Z.U. A checklist of the reptiles and amphibians of Guerrero, México. An. Del Inst. De Biología. Ser. Zool. 2000, 71, 21–40. [Google Scholar]
- Chafin, T.K.; Douglas, M.R.; Anthonysamy, W.J.; Sullivan, B.K.; Walker, J.M.; Cordes, J.E.; Douglas, M.E. Taxonomic hypotheses and the biogeography of speciation in the Tiger Whiptail complex (Aspidoscelis tigris: Squamata, Teiidae). bioRxiv 2020. [CrossRef]
- González-Sánchez, V.H.; Johnson, J.D.; González-Solís, D.; Fucsko, L.A.; Wilson, L.D. A review of the introduced herpetofauna of Mexico and Central America, with comments on the effects of invasive species and biosecurity methodology. ZooKeys 2021, 1022, 79–154. [Google Scholar] [CrossRef] [PubMed]
- Charruau, P.; Díaz de la Vega Pérez, A.H.; Méndez de la Cruz, F.R. Reptiles of Banco Chinchorro: Updated List, Life History Data, and Conservation. Southwest. Nat. 2015, 60, 299–312. [Google Scholar] [CrossRef]
- Díaz-Gamboa, L.; May-Herrera, D.; Gallardo-Torres, A.; Cedeño-Vázquez, R.; González-Sánchez, V.H.; Chiappa-Carrara, X.; Yáñez-Arenas, C. Catálogo de Reptiles de la Península de Yucatán; Escuela Nacional de Estudios Superiores (ENES) Campus Mérida: Merida, Yucatan, 2020; p. 315. [Google Scholar]
- Lazcano-Barrero, M. Plan de Acción para la Conservación del Cocodrilo Americano (Crocodylus Acutus) en Isla Contoy, Quintana Roo; CONANP: Ciudad de México, Mexico, 1993. [Google Scholar]
- Klauber, L.M. Some new and revived subspecies of rattlesnakes. Trans. San Diego Soc. Nat. Hist. 11 1949, 11, 61–116. [Google Scholar] [CrossRef]
- Grismer, L.L. An Evolutionary Classification and Checklist of Amphibians and Reptiles on the Pacific Islands of Baja California, Mexico. Bull. South. Calif. Acad. Sci. 2001, 100, 12–23. [Google Scholar]
- Grismer, L.L. Amphibians and Reptiles of Baja California, including Its Pacific islands and the islands in the Sea of Cortés; University of California Press: Berkeley, CA, USA, 2002; Volume 4. [Google Scholar]
- Wallach, V.; Williams, K.L.; Boundy, J. Snakes of the World: A Catalogue of Living and Extinct Species; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Ashton, K.G.; Queiroz, A.d. Molecular Systematics of the Western Rattlesnake, Crotalus viridis (Viperidae), with Comments on the Utility of the D-Loop in Phylogenetic Studies of Snakes. Mol. Phylogenetics Evol. 2001, 21, 176–189. [Google Scholar] [CrossRef]
- Campbell, J.A.; Lamar, W.W.; Brodie, E.D. The Venomous Reptiles of the Western Hemisphere; Comstock Pub. Associates Ithaca: Ithaca, NY, USA, 2004; Volume 1. [Google Scholar]
- Heimes, P. Herpetofauna Mexicana Volume 1: Snakes of Mexico; Edition Chimaira: Frankfurt am Main, Germany, 2016. [Google Scholar]
- SEMARNAT. Programa de Acción para la Conservación de las Especies: Serpientes de Cascabel (Crotalus spp.); SEMARNAT/CONANP: Ciudad de México, Mexico, 2018. [Google Scholar]
- Pook, C.E.; Wüster, W.; Thorpe, R.S. Historical Biogeography of the Western Rattlesnake (Serpentes: Viperidae: Crotalus viridis), Inferred from Mitochondrial DNA Sequence Information. Mol. Phylogenetics Evol. 2000, 15, 269–282. [Google Scholar] [CrossRef]
- Davis, M.A.; Douglas, M.R.; Collyer, M.L.; Douglas, M.E. Deconstructing a Species-Complex: Geometric Morphometric and Molecular Analyses Define Species in the Western Rattlesnake (Crotalus viridis). PLoS ONE 2016, 11, e0146166. [Google Scholar] [CrossRef]
- Franco-Servín, C.; Neri-Castro, E.; Bénard-Valle, M.; Alagón, A.; Rosales-García, R.A.; Guerrero-Alba, R.; Poblano-Sánchez, J.E.; Silva-Briano, M.; Guerrero-Barrera, A.L.; Sigala-Rodríguez, J.J. Biological and Biochemical Characterization of Coronado Island Rattlesnake (Crotalus helleri caliginis) Venom and Antivenom Neutralization. Toxins 2021, 13, 582. [Google Scholar] [CrossRef] [PubMed]
- McCain, J.; Braje, T.J.; Hernández Estrada, R.L.; Michelini, A.P.; Aguilar, J.; Rick, T.C. The Four Crowns of the Sea: Archaeological Reconnaissance of the Coronados Islands, Baja California, México. J. Isl. Coast. Archaeol. 2019, 14, 541–559. [Google Scholar] [CrossRef]
- Nava-Landeros, R.A. Changes in Morphology and Behavior of the Coronado Island Rattlesnake (Crotalus Oreganus Caliginis), An Insular Population of the Western Rattlesnake (C. oreganus); San Diego State University: San Diego, CA, USA, 2022. [Google Scholar]
- Radcliffe, C.W.; Maslin, T.P. A New Subspecies of the Red Rattlesnake, Crotalus ruber, from San Lorenzo Sur Island, Baja California Norte, Mexico. Copeia 1975, 1975, 490–493. [Google Scholar] [CrossRef]
- Grismer, L.L. An Evolutionary Classification of Reptiles on Islands in the Gulf of California, México. Herpetologica 1999, 55, 446–469. [Google Scholar]
- Johnson, J.; Wilson, L.; Mata-Silva, V.; García-Padilla, E.; DeSantis, D. The endemic herpetofauna of Mexico: Organisms of global significance in severe peril. Mesoamerican Herpetol. 2017, 4, 544–620. [Google Scholar]
- Smith, H.M. The sonoran subspecies of the lizard Ctenosaura hemilopha. Great Basin Nat. 1972, 32, 104–111. [Google Scholar] [CrossRef][Green Version]
- Davy, C.M.; Méndez de la Cruz, F.R.; Lathrop, A.; Murphy, R.W. Seri Indian traditional knowledge and molecular biology agree: No express train for island-hopping spiny-tailed iguanas in the Sea of Cortés. J. Biogeogr. 2011, 38, 272–284. [Google Scholar] [CrossRef]
- Tanner, W.W. The night snakes of Baja California. Trans. San Diego Soc. Nat. Hist. 1966, 14, 189–196. [Google Scholar] [CrossRef]
- Mulcahy, D.G. Phylogeography and species boundaries of the western North American Nightsnake (Hypsiglena torquata): Revisiting the subspecies concept. Mol. Phylogenetics Evol. 2008, 46, 1095–1115. [Google Scholar] [CrossRef]
- Mulcahy, D.G.; Martínez-Gómez, J.E.; Aguirre-León, G.; Cervantes-Pasqualli, J.A.; Zug, G.R. Rediscovery of an Endemic Vertebrate from the Remote Islas Revillagigedo in the Eastern Pacific Ocean: The Clarión Nightsnake Lost and Found. PLoS ONE 2014, 9, e97682. [Google Scholar] [CrossRef]
- Tanner, W.W. A new Hypsiglena from Tiburon island, Sonora, Mexico. Great Basin Nat. 1981, 41, 139–142. [Google Scholar] [CrossRef]
- Grismer, L.L. Checklist of amphibians and reptiles on islands in the Gulf of California, Mexico. Bull. South. Calif. Acad. Sci. 1999, 98, 45–56. [Google Scholar]
- Mulcahy, D.G.; Macey, J.R. Vicariance and dispersal form a ring distribution in nightsnakes around the Gulf of California. Mol. Phylogenetics Evol. 2009, 53, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Ottley, J.R.; Tanner, W.W. New range and a new subspecies for the snake Eridiphas slevini. Great Basin Nat. 1978, 38, 406–410. [Google Scholar] [CrossRef]
- Mulcahy, D.G.; Archibald, M.A. Geographic Variation in the Baja California Nightsnake (Eridiphas slevini), with Comments on Taxonomy and Diet. J. Herpetol. 2003, 37, 566–571. [Google Scholar] [CrossRef]
- Tanner, W.W. A taxonomic study of the genus Hypsiglena. Great Basin Nat. 1944, 5, 1. [Google Scholar]
- Casas-Andreu, G. Anfibios y reptiles de las islas Marías y otras islas adyacentes de la costa de Nayarit, México. Aspectos sobre su biogeografía y conservación. An. Del Inst. De Biología. Ser. Zool. 1992, 63, 95–112. [Google Scholar]
- Van Denburgh, J.; Slevin, J.R. Preliminary diagnoses of more new species of reptiles from islands in the Gulf of California, Mexico. Proc. Calif. Acad. Sci. 4th Ser. 1921, 11, 395–398. [Google Scholar]
- Van Denburgh, J.; Slevin, J.R. Preliminary diagnoses of four new snakes from Lower California, Mexico. Proc. Calif. Acad. Sci. 4th Ser. 1923, 13, 1–2. [Google Scholar]
- Klauber, L.M. The coral king snakes of the Pacific Coast. Trans. San Diego Soc. Nat. Hist. 1943, 10, 75–82. [Google Scholar]
- Zweifel, R.G. Pattern variation and evolution of the mountain kingsnake, Lampropeltis zonata. Copeia 1952, 1952, 152–168. [Google Scholar] [CrossRef]
- Rodríguez-Robles, J.A.; Denardo, D.F.; Staub, R.E. Phylogeography of the California mountain kingsnake, Lampropeltis zonata (Colubridae). Mol. Ecol. 1999, 8, 1923–1934. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.M. Summary of the collections of snakes and crocodilians made in Mexico under the Walter Rathbone Bacon Traveling Scholarship. Proc. United States Natl. Mus. 1943, 93, 393–504. [Google Scholar] [CrossRef]
- Smith, H.M.; Taylor, E.H. An annotated checklist and key to the snakes of Mexico. United States Natl. Mus. Bull. 1945, 187, 1–239. [Google Scholar] [CrossRef]
- Zweifel, R.G. Herpetology of the Tres Maria Islands. Results of the Puritan American Museum of Natural History expedition to Western Mexico. Bull. Amer. Mus. Nat. Hist 1960, 119, 77–128. [Google Scholar]
- O’Connell, K.A.; Smith, E.N. The effect of missing data on coalescent species delimitation and a taxonomic revision of whipsnakes (Colubridae: Masticophis). Mol. Phylogenetics Evol. 2018, 127, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Velasco, J.; Adams, R.H.; Boissinot, S.; Parkinson, C.L.; Campbell, J.A.; Castoe, T.A.; Smith, E.N. Genome-wide SNPs clarify lineage diversity confused by coloration in coralsnakes of the Micrurus diastema species complex (Serpentes: Elapidae). Mol. Phylogenetics Evol. 2020, 147, 106770. [Google Scholar] [CrossRef] [PubMed]
- Castro-Franco, R.; Gaviño-De la Torre, G. Reptiles de la isla La Peña, Nayarit, Mexico. In Proceedings of the Anales del Instituto de Biología; Serie Zoología. Universidad Nacional Autónoma de México: Ciudad de México, Mexico, 1990; p. 175. [Google Scholar]
- Ramírez-Reyes, T.; Melo-León, C.; Pérez-Ramos, E. Nuevos registros de herpetofauna para la isla El Coral, Nayarit, México. Rev. Mex. De Biodivers. 2015, 86, 541–545. [Google Scholar] [CrossRef]
- Lara-Tufiño, J.D.; de Oca, A.N.-M.; Ramírez-Bautista, A.; Gray, L.N. Resurrection of Anolis ustus Cope, 1864 from synonymy with Anolis sericeus Hallowell, 1856 (Squamata, Dactyloidae). ZooKeys 2016, 619, 147–162. [Google Scholar] [CrossRef][Green Version]
- Blair, C.; Méndez de La Cruz, F.; Ngo, A.; Lindell, J.; Lathrop, A.; Murphy, R. Molecular phylogenetics and taxonomy of leaf-toed geckos (Phyllodactylidae: Phyllodactylus) inhabiting the peninsula of Baja California. Zootaxa 2009, 2027, 28–42. [Google Scholar] [CrossRef]
- Nabhan, G.P. Cultural dispersal of plants and reptiles to the Midriff Islands of the Sea of Cortés: Integrating indigenous human dispersal agents into island biogeography. J. Southwest 2000, 42, 545–558. [Google Scholar]
- Nabhan, G.P. Cultural dispersal of plants and reptiles. In A New Island Biogeography of the Sea of Cortés; Oxford University Press: Oxford, UK, 2002; pp. 407–416. [Google Scholar]
- Dixon, J.R. Speciation and Systematics of the Gekkonid Lizard Genus Phyllodactylus of the Islands of the Gulf of California. Proc. Cal. Acad. Sci. 1966, 33, 415–452. [Google Scholar]
- Murphy, R.W.; Ottley, J.R. Distribution of Amphibians and Reptiles on Islands in the Gulf of California; Carnegie Museum of Natural History: Pittsburgh, PA, USA, 1984. [Google Scholar]
- Peralta-García, A.; Samaniego-Herrera, A.; Valdez-Villavicencio, J.H. Registros nuevos de reptiles en Islas del Noroeste de México. Acta Zoológica Mex. 2007, 23, 179–182. [Google Scholar] [CrossRef][Green Version]
- Werner, F. Beschreibung einiger neuer Schlangen und Batrachier. Zool. Anz. 1899, 22, 114–117. [Google Scholar]
- Smith, H.M.; Larsen, K.R. THE NAME OF THE BAJA CALIFORNIA CAPE WORMSNAKE. Great Basin Nat. 1974, 34, 94–96. [Google Scholar] [CrossRef]
- Adalsteinsson, S.A.; Branch, W.R.; Trape, S.; Vitt, L.J.; Hedges, S.B. Molecular phylogeny, classification, and biogeography of snakes of the Family Leptotyphlopidae (Reptilia, Squamata). Zootaxa 2009, 2244, 1–50. [Google Scholar]
- Hollingsworth, B.D. The Systematics of Chuckwallas (Sauromalus) with a Phylogenetic Analysis of Other Iguanid Lizards. Herpetol. Monogr. 1998, 12, 38–191. [Google Scholar] [CrossRef]
- Petren, K.; Case, T.J. A phylogenetic analysis of boduy size evolution and biogeography in chuckwallas (Sauromalus) and other iguanines. Evolution 1997, 51, 206–219. [Google Scholar] [CrossRef]
- Murphy, R.W.; Aguirre-León, G. The nonavian reptiles: Origins and evolution. In A New Island Biogeography of the Sea of Cortés; Case, T.J., Cody, M.L., Ezcurra, E., Eds.; Oxford University Press: New York, NY, USA, 2002; pp. 181–220. [Google Scholar]
- Montanucci, R.R. A note on the identity of chuckwallas inhabiting Isla Danzante, Baja California Sur. Herpetol. Rev. 2004, 35, 223. [Google Scholar]
- Robinson, M.D. Chromosomes, Protein Polymorphism, and Systematics of Insular Chuckwalla Lizards (Genus Sauromalus) in the Gulf of California, Mexico; The University of Arizona: Tucson, AZ, USA, 1972. [Google Scholar]
- Kraus, F. Alien Reptiles and Amphibians: A Scientific Compendium and Analysis; Springer Science: Ney York, NY, USA, 2009. [Google Scholar]
- Lavilla, E.O.; Langone, J.A.; Padial, J.M.; de Sá, R.O. The identity of the crackling, luminescent frog of Suriname (Rana typhonia Linnaeus, 1758)(Amphibia, Anura). Zootaxa 2010, 2671, 17. [Google Scholar]
- Köhler, G. Amphibians of Central America; Herpeton: Offenbach, Germany, 2013; p. 380. [Google Scholar]
- Ron, S.R.; Venegas, P.J.; Ortega-Andrade, H.M.; Gagliardi-Urrutia, G.; Salerno, P.E. Systematics of Ecnomiohyla tuberculosa with the description of a new species and comments on the taxonomy of Trachycephalus typhonius (Anura, Hylidae). ZooKeys 2016, 630, 115. [Google Scholar] [CrossRef] [PubMed]
- Blotto, B.L.; Lyra, M.L.; Cardoso, M.C.S.; Trefaut Rodrigues, M.; Dias, I.R.; Marciano, E., Jr.; Dal Vechio, F.; Orrico, V.G.D.; Brandão, R.A.; Lopes de Assis, C.; et al. The phylogeny of the Casque-headed Treefrogs (Hylidae: Hylinae: Lophyohylini). Cladistics 2021, 37, 36–72. [Google Scholar] [CrossRef] [PubMed]
- Ballinger, R.E.; Tinkle, D.W. A New Species of Uta (Sauria: Iguanidae) from Salsipuedes Island, Gulf of California, Mexico. Occasional Papers of the Museum of Zoology, University of Michigan: Ann Arbor, MI, USA, 1968. [Google Scholar]
- Liner, E.A. Scientific and common names for the amphibians and reptiles of Mexico in english and spanish. SSAR. Herpetol. Circular 1994, 23, 1–113. [Google Scholar]
- Liner, E.A. A checklist of the amphibians and reptiles of Mexico. La. State Univ. Occas. Pap. Mus. Nat. Sci. 2007, 42, 1–60. [Google Scholar] [CrossRef]
- Murphy, R.W.; Aguirre-Léon, G. Distributional checklist of nonavian reptiles and amphibians on the islands in the Sea of Cortés. In A New Island Biogeography of the Sea of Cortés; Case, T.J., Cody, M.L., Ezcurra, E., Eds.; Oxford University Press: New York, NY, USA, 2002; pp. 580–591. [Google Scholar]
- Flores-Villela, O.; Canseco, L. Nuevas especies y cambios taxonómicos para la herpetofauna de México. Acta Zoológica Mex. 2004, 20, 115–144. [Google Scholar] [CrossRef]
- Upton, D.E.; Murphy, R.W. Phylogeny of the Side-Blotched Lizards (Phrynosomatidae:Uta) Based on mtDNA Sequences: Support for a Midpeninsular Seaway in Baja California. Mol. Phylogenetics Evol. 1997, 8, 104–113. [Google Scholar] [CrossRef]
- Van Denburgh, J. The reptiles and amphibians of the islands of the Pacific Coast of North America from the Farallons to Cape San Lucas and the Revilla Gigedos. Proc. Calif. Acad. Sci. 1905, 4, 1–40. [Google Scholar]
- Woolrich-Piña, G.; Ponce-Campos, P.; Loc-Barragán, J.; Ramírez-Silva, J.; Mata-Silva, V.; Johnson, J.; García-Padilla, E.; Wilson, L. The herpetofauna of Nayarit, Mexico: Composition, distribution, and conservation. Mesoamerican Herpetol. 2016, 3, 376–448. [Google Scholar]
- D. O.F. ACUERDO Mediante el Cual se Actualiza el Registro Nacional de Información Geográfica Respecto al Cambio del Nombre Geográfico de la Isla Cerralvo por el de Isla Jacques Cousteau; Diario Oficial de la Federación: Ciudad de México, Mexico, 2009; Volume DCLXXIV, p. 54. [Google Scholar]
- León-Portilla, M. ¿Borrar la Historia? El Caso de la Isla Cerralvo. La Jornada. 2009. Available online: https://www.jornada.com.mx/2009/11/24/opinion/015a1pol (accessed on 1 June 2019).
- Cruz-Sáenz, D.; Muñoz-Nolasco, F.J.; Mata-Silva, V.; Johnson, J.D.; García-Padilla, E.; Wilson, L.D. The herpetofauna of Jalisco, Mexico: Composition, distribution, and conservation status. Mesoamerican Herpetol. 2017, 4, 22–118. [Google Scholar]
- Duellman, W.E. Herpetofaunas in Neotropical rainforests: Comparative composition, history, and resource use. In Four Neotropical Rainforests; Yale University Press: New Haven, CT, USA, 1990; pp. 455–505. [Google Scholar]
- Meik, J.M.; Streicher, J.W.; Lawing, A.M.; Flores-Villela, O.; Fujita, M.K. Limitations of Climatic Data for Inferring Species Boundaries: Insights from Speckled Rattlesnakes. PLoS ONE 2015, 10, e0131435. [Google Scholar] [CrossRef]
- Meik, J.M.; Schaack, S.; Flores-Villela, O.; Streicher, J.W. Integrative taxonomy at the nexus of population divergence and speciation in insular speckled rattlesnakes. J. Nat. Hist. 2018, 52, 989–1016. [Google Scholar] [CrossRef]
- Buckley, L.J.; de Queiroz, K.; Grant, T.D.; Hollingsworth, B.D.; Iverson, J.B.; Pasachnik, S.A.; Stephen, C.L. A checklist of the iguanas of the world (Iguanidae; Iguaninae). Herpetol. Conserv. Biol. 2016, 11, 4–46. [Google Scholar]
- Frick, W.F.; Heady, P.A.; Hollingsworth, B.D. Lichanura trivirgata. Herpetol. Rev. 2016, 47, 83–84. [Google Scholar]
- Montero-Quintana, A.N.; Marrón, G.; Scheidt, V.; Hollingsworth, B.D. SALVADORA HEXALEPIS (Western Patch-nosed Snake). MEXICO: BAJA CALIFORNIA SUR: Municipality of Loreto: Isla Carmen. Herpetol. Rev. 2019, 50, 531. [Google Scholar]
- Devitt, T.J.; LaDuc, T.J.; McGuire, J.A. The Trimorphodon biscutatus (Squamata: Colubridae) species complex revisited: A multivariate statistical analysis of geographic variation. Copeia 2008, 2008, 370–387. [Google Scholar] [CrossRef]
- Arnaud, G.; Blazquez, C. First Record of Lichanura trivirgata Cope, 1868 (Squamata: Boidae) from Coronados Island, Gulf of California, Mexico. Herpetol. Notes 2018, 11, 1025–1026. [Google Scholar]
- Arnaud, G.; Carbajal-Márquez, R.A.; Rodríguez-Canseco, J.; Ferreyra, E. Primeros registros de la cascabel roja (Crotalus ruber) en la isla Coronados, golfo de California, México. Rev. Mex. De Biodivers. 2014, 85, 322–324. [Google Scholar] [CrossRef]
- Lazcano, D.; Arnaud, G.; Cruz, O.; García, E. Notes on the herpetofauna of the Northwest of Mexico: Herpetofauna of Isla El Muerto, Ensenada, Baja California, Mexico. Bull. Chic. Herp. Soc. 2011, 10, 129–133. [Google Scholar]
- Dayton, G.; Croll, D.; S, F.-C. Geographic distribution: Mexico, Baja California Sur: Hemidactylus frenatus. Herpetol. Rev. 2020, 51, 271. [Google Scholar]
- González-Bernal, M.A.; Mellink, E.; Payán-Ezquerra, C. Cnemidophorus tigris range extension. Herpetol. Rev. 2001, 32, 193. [Google Scholar]
- Meneses-Millán, M.; Sabido-Alpuche, B.; Cabrera-Cen, D.; Ortíz-Medina, J.A. TANTILLA PLANICEPS (Western Black-headed Snake). MEXICO: BAJA CALIFORNIA SUR: Isla San Marcos. Herpetol. Rev. 2021, 52, 802. [Google Scholar]
- Rhodin, A.G.J.; Iverson, J.B.; Bour, R.; Fritz, U.; Georges, A.; Shaffer, H.B.; Van Dijk, P.P. Turtles of the World: Annotated Checklist and Atlas of Taxonomy, Synonymy, Distribution, and Conservation Status. In Conservation Biology of Freshwater Turtles and Tortoises: A Compilation Project of the IUCN/SSC Tortoise and Freshwater Turtle Specialist Group. Chelonian Research Monographs; Rhodin, A.G.J., Iverson, J.B., van Dijk, P.P., Saumure, R.A., Buhlmann, K.A., Pritchard, P.C.H., Mittermeier, R.A., Eds.; IUCN: Gland, Switzerland, 2017; Volume 7, pp. 1–292. [Google Scholar]
- Ramírez-Reyes, T.; Piñero, D.; Flores-Villela, O.; Vázquez-Domínguez, E. Molecular systematics, species delimitation and diversification patterns of the Phyllodactylus lanei complex (Gekkota: Phyllodactylidae) in Mexico. Mol. Phylogenetics Evol. 2017, 115, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Quijada-Mascareñas, A.; Canseco-Márquez, L. Geographic distribution: Gehyra mutilata. Herpetol. Rev. 2007, 38, 483. [Google Scholar]
- Hernández-Salinas, U.; Ramírez-Bautista, A.; Mata-Silva, V. Species richness of squamate reptiles from two islands in the Mexican Pacific. Check List 2014, 10, 1264–1269. [Google Scholar] [CrossRef]
- Ramírez-Bautista, A.; Hernández-Salinas, U.; Berriozabal-Islas, C.; Juárez-Escamilla, D. First records of Urosaurus bicarinatus (Duméril, 1856)(Squamata: Phrynosomatidae) from Cocinas Island, Chamela Bay, Jalisco, Mexico. Check List 2013, 9, 649. [Google Scholar]
- Miranda, A.; Ambriz, G.; Valencia, D.; Sánchez, M.; Szekely, A. Programa de Manejo del Santuario Islas La Pajarera, Cocinas, Mamut, Colorada, San Pedro, San Agustin, San Andrés y Negrita y los Islotes Los Anegados, Novillas, Mosca y Submarino, Situados en la Bahía de Chamela, México; Comisión Nacional de Áreas Naturales Protegidas/Secretaría del Medio Ambiente y Recursos Naturales: Ciudad de México, Mexico, 2011; p. 149. [Google Scholar]
- Uribe-Peña, Z.; Gaviño-De la Torre, G. Reptiles de las islas tres Marietas, Jalisco, México. An. Del Inst. De Biol. Univ. Nac. Autónoma De México: Ser. Zool. 1981, 52, 427. [Google Scholar]
- CONANP. Programa de Conservación y Manejo Parque Nacional Islas Marietas; Comision Nacionap de Areas Naturales Protegida: Ciudad de México, México, 2007. [Google Scholar]
- Nolasco-Luna, J.R.; Barraza-Soltero, I.; López-Montes, M.A.; Moreno-López, J.A.; Escobedo-Galván, A.H. An updated checklist of the herpetofauna from Isla María Cleofas, Mexico. Check List 2022, 18, 241–252. [Google Scholar] [CrossRef]
- De la Torre, J.A.; López-Damián, L.J.; Bárcenas, H.V.; Nájera-Solís, E. New record of sheep frog (Hypopachus variolosus) in the Tres Marías Islands archipelago, Nayarit, Mexico. Rev. Mex. De Biodivers. 2010, 81, 581–582. [Google Scholar]
- Miramontes, E.; Cruz, J.; Cortés, M. Herpetofauna de la isla María Cleofás, Archipiélado Islas Tres Marías. In Proceedings of the XIV Reunión Nacional de Herpetología, Tepic, Nayarit, México, 8–11 November 2016. [Google Scholar]
- Ramírez-Reyes, T.; Barraza-Soltero, I.K.; Nolasco-Luna, J.R.; Flores-Villela, O.; Escobedo-Galván, A.H. A new species of leaf-toed gecko (Phyllodactylidae, Phyllodactylus) from María Cleofas Island, Nayarit, Mexico. ZooKeys 2021, 1024, 117–136. [Google Scholar] [CrossRef]
- Nolasco-Luna, J.R.; López-Luna, M.A.; Cupul-Magaña, F.G.; Escobedo-Galván, A.H. Nuevos registros de anfibios y reptiles en la Isla María Cleofas, Nayarit. Cienc. Y Mar 2016, 20, 29–33. [Google Scholar]
- Brattstrom, B.H. Notes on the Herpetology of the Revillagigedo Islands, Mexico. Am. Midl. Nat. 1955, 54, 219–229. [Google Scholar] [CrossRef]
- Zug, G.R. Reptiles and Amphibians of the Pacific Islands: A Comprehensive Guide; Univ of California Press: Berkeley, CA, USA, 2013. [Google Scholar]
- CONANP. Programa de Manejo Parque Nacional Revillagigedo; Comision Nacional de Areas Naturales Protegidas: Ciudad de México, Mexico, 2018; p. 328. [Google Scholar]
- Gallina-Tessaro, P.; Ortega-Rubio, A.; Alvarez-Cárdenas, S.; Arnaud, G. Colonization of Socorro Island (Mexico), by the tropical house gecko Hemidactylus frenatus (Squamata: Gekkonidae). Rev. De Biol. Trop. 1999, 47, 237–238. [Google Scholar] [CrossRef]
- Gaviño-De la Torre, G.; Martínez-Guerrero, A.; Uribe-Peña, Z.; Santillan-Alarcón, S. Vertebrados terrestres y vegetación dominante en Isla Ixtapa, Guerrero. An. Del Inst. De Biol. UNAM Ser. Zool. 1979, 50, 701–719. [Google Scholar]
- Niño-Gutiérrez, N.S. Potencialidades de Desarrollo Sustentable y Planeación Territorial en la Región de Acapulco, El caso de la Isla Roqueta de Acapulco, Guerrero. In Proceedings of the 15 Encuentro Regional sobre Desarrollo Regional en México, Villa Hermosa, México, 16–19 November 2010; pp. 1–18. [Google Scholar]
- CONANP. Programa de Manejo Área de Protección de Flora y Fauna Laguna Madre y Delta del Río Bravo; Comisión Nacional de Áreas Naturales Protegidas: Ciudad de México, Mexico, 2015; p. 272. [Google Scholar]
- CONANP. Programa de Manejo Área de Protección de Flora y Fauna Sistema Arrecifal Lobos-Tuxpan; Comisión Nacional de Áreas Naturales Protegidas: Ciudad de México, Mexico, 2014; p. 180. [Google Scholar]
- Arriaga, A.; Ramírez-Bautista, A. A Checklist of Reptiles from the Parque Nacional Isla Contoy, México Boletín de la Sociedad Herpetológica Mexicana. Boletín De La Soc. Herpetológica Mex. 2008, 16, 36–40. [Google Scholar]
- Herrera-Pavón, R. La tortuga marina, omnipresente en la cultura maya. In Riqueza Biológica de Quintana Roo. Un Análisis Para su Conservación; Pozo, C., Canto, A., Calmé, S., Eds.; ECOSUR, CONABIO, Gobierno del Estado de Quintana Roo, PPD: Ciudad de Mexico, México, 2011; Volume 1, pp. 241–247. [Google Scholar]
- González-Sánchez, V.H.; Landeo-Yauri, S. First insular record of Black Spiny-tailed Iguana (Ctenosaura similis) in the cays of Sian Ka’an, in the Mexican Caribbean. In press. Revista Latinoamericana de Herpetologia. Available online: https://herpetologia.fciencias.unam.mx/index.php/revista (accessed on 16 July 2023).
- Braga-González, D.R.; Arce-Wilfred, I. Estudio preliminar de la herpetofauna en la isla de Tamalcab, en el ejido Calderitas, Quintana Roo. AvaCient 2000, 29, 29–40. [Google Scholar]
- Mata-Silva, V.; Johnson, J.; Wilson, L.; García-Padilla, E. The herpetofauna of Oaxaca, Mexico: Composition, physiographic distribution, and conservation status. Mesoamerican Herpetol. 2015, 2, 6–62. [Google Scholar]
- Woolrich-Peña, G.A.; García-Padilla, E.; DeSantis, D.L.; Johnson, D.J.; Mata-Silva, V.; Wilson, L.D. The herpetofauna of Puebla, Mexico: Composition, distribution, and Conservation status. Mesoamerican Herpetology. Mesoamerican Herpetol. 2017, 4, 2–95. [Google Scholar]
- Johnson, J.D.; Mata-Silva, V.; Padilla, E.G.; Wilson, L.D. The Herpetofauna of Chiapas, Mexico: Composition, distribution, and conservation. Mesoamerican Herpetol. 2015, 2, 272–329. [Google Scholar]
- Terán-Juárez, S.; García-Padilla, E.; Mata-Silva, V.; Johnson, J.; Wilson, L. The herpetofauna of Tamaulipas, Mexico: Composition, distribution, and conservation. Mesoamerican Herpetol. 2016, 3, 43–113. [Google Scholar]
- Bowen, T. Archaeology, biology and conservation on islands in the Gulf of California. Environ. Conserv. 2004, 31, 199–206. [Google Scholar] [CrossRef]
- Luque, D.; Doode, S. Sacralidad, territorialidad y biodiversidad comcáac (seri). Los sitios sagrados indígenas como categorías de conservación ambiental. Relaciones. Estud. De Hist. Y Soc. 2007, 28, 157–184. [Google Scholar]
- Landeros, A.T. Baja California: Uso y Abuso de su Biodeiversidad; Editorial Miguel Ángel Porrúa: Ciudad de México, México, 2006; p. 306. [Google Scholar]
- Santos del Prado-Gasca, K.; Negrete-Fernández, G.; Gabriel-Morales, J. Diagnóstico ambiental participativo en la Colonia Penal Federal Islas Marías. Hacia la conservación y el manejo sustentable de su territorio. Gac. Ecológica 2006, 81, 5–18. [Google Scholar]
- Escudero, M.; Silva, R.; Mendoza, E. Beach Erosion Driven by Natural and Human Activity at Isla del Carmen Barrier Island, Mexico. J. Coast. Res. 2014, 71, 62–74. [Google Scholar] [CrossRef]
- Hernández, M.G.D.A.; Gutiérrez, M.J.G. El reto de la política fiscal ante una industria petrolera inestable. El caso de Ciudad del Carmen, México (The challenge of fiscal policy in the face of an oil industry unstable. The case of Ciudad del Carmen, Mexico). EN-CONTEXTO 2017, 6, 233–258. [Google Scholar]
- Day, J.W.; Conner, W.H.; Ley-Lou, F.; Day, R.H.; Navarro, A.M. The productivity and composition of mangrove forests, Laguna de Términos, Mexico. Aquat. Bot. 1987, 27, 267–284. [Google Scholar] [CrossRef]
- Rodríguez, L.G.; Gracia, M.A.; Baltazar, E.B.; Maya, E.M.A. Social metabolism and ecotourism: The problem of waste in Holbox island, Quintana Roo, Mexico. Nova Sci. 2018, 10, 779–822. [Google Scholar] [CrossRef]
- Rubio-Cisneros, N.T.; Herrera-Silveira, J.; Morales-Ojeda, S.; Moreno-Báez, M.; Montero, J.; Pech-Cárdenas, M. Water quality of inlets’ water bodies in a growing touristic barrier reef Island “Isla Holbox” at the Yucatan Peninsula. Reg. Stud. Mar. Sci. 2018, 22, 112–124. [Google Scholar] [CrossRef]
- Álvarez-Romero, J.G.; Medellín, R.A.; Oliveras de Ita, A.; Gómez de Silva, H.; Sánchez, O. Animales Exóticos en México: Una Amenaza Para la Biodiversidad; Comisión Nacional para el Conocimiento y Uso de la Biodiversidad, Instituto de Ecología, UNAM, Secretaría del Medio Ambiente y Recursos Naturales: Ciudad de México, Mexico, 2008; p. 502. [Google Scholar]
- Reyes-Gómez, H.G.; Vázquez-Lule, A.D. Caracterización del sitio de manglar Isla del Carmen. In Sitios de Manglar con Relevancia Biológica y con Necesidades de Rehabilitación Ecológica; Comisión Nacional para el Conocimiento y Uso de la Biodiversidad (CONABIO): Ciudad de México, Mexico, 2009. [Google Scholar]
- Frutos-Cortés, M. La violencia social en el sureste mexicano. Un acercamiento al estudio de la descomposición social en una ciudad petrolera: Ciudad del Carmen, Campeche. Rev. De La Univ. Cris. Colón 2011, 26, 58–92. [Google Scholar]
- Labarre, D.; Charruau, P.; Parsons, W.; Larocque-Desroches, S.; Gallardo-Cruz, J. Major hurricanes affect body condition of American crocodile Crocodylus acutus inhabiting Mexican Caribbean islands. Mar. Ecol. Prog. Ser. 2020, 651, 145–162. [Google Scholar] [CrossRef]
- Charruau, P. Microclimate of American crocodile nests in Banco Chinchorro biosphere reserve, Mexico: Effect on incubation length, embryos survival and hatchlings sex. J. Therm. Biol. 2012, 37, 6–14. [Google Scholar] [CrossRef]
- Charruau, P.; Thorbjarnarson, J.B.; Hénaut, Y. Tropical cyclones and reproductive ecology of Crocodylus acutus Cuvier, 1807 (Reptilia: Crocodilia: Crocodylidae) on a Caribbean atoll in Mexico. J. Nat. Hist. 2010, 44, 741–761. [Google Scholar] [CrossRef]
- Rogers, R.F.; Aberson, S.; Bell, M.M.; Cecil, D.J.; Doyle, J.D.; Kimberlain, T.B.; Morgerman, J.; Shay, L.K.; Velden, C. Rewriting the Tropical Record Books: The Extraordinary Intensification of Hurricane Patricia (2015). Bull. Am. Meteorol. Soc. 2017, 98, 2091–2112. [Google Scholar] [CrossRef]
- Maass, M.; Ahedo-Hernández, R.; Araiza, S.; Verduzco, A.; Martínez-Yrízar, A.; Jaramillo, V.J.; Parker, G.; Pascual, F.; García-Méndez, G.; Sarukhán, J. Long-term (33 years) rainfall and runoff dynamics in a tropical dry forest ecosystem in western Mexico: Management implications under extreme hydrometeorological events. For. Ecol. Manag. 2017, 426, 7–17. [Google Scholar] [CrossRef]
- Parker, G.; Martínez-Yrízar, A.; Álvarez-Yépiz, J.C.; Maass, M.; Araiza, S. Effects of hurricane disturbance on a tropical dry forest canopy in western Mexico. For. Ecol. Manag. 2017, 426, 39–52. [Google Scholar] [CrossRef]
- Olivares Bañuelos, N.; Ángel Vargas-Tellez, M.A. Atención de un Incendio Forestal en la Reserva de la Biósfera Isla Guadalupe. In Informe Final. SNIB-CONABIO Proyecto No. GR012; PRONATURA NOROESTE, A. C.: México D. F, Mexico, 2009. [Google Scholar]
- Coccossis, H.; Parpairis, A. Tourism and the Environment—Some Observations on the Concept of Carrying Capacity. In Tourism and the Environment: Regional, Economic and Policy Issues; Briassoulis, H., van der Straaten, J., Eds.; Springer: Dordrecht, The Netherlands, 1992; pp. 23–33. [Google Scholar]
- Charruau, P.; Macías-Díaz, D.A. A recent change in crocodile’s behavior in a Mexican Caribbean atoll: Possible causes and short-term actions. In Proceedings of the Program and Abstracts of the Twenty-Sixth Working Meeting of the Crocodile Specialist Group of the International Union for Conservation of Nature, Chetumal, Qroo, Mexico, 3–9 July 2022; p. 19. [Google Scholar]
- Hofman, C.A.; Rick, T.C. Ancient Biological Invasions and Island Ecosystems: Tracking Translocations of Wild Plants and Animals. J. Archaeol. Res. 2018, 26, 65–115. [Google Scholar] [CrossRef]
- Richardson, D.M.; Pyšek, P.; Carlton, J.T. A compendium of essential concepts and terminology in invasion ecology. In Fifty Years of Invasion Ecology. The Legacy of Charles Elton; Richardson, D.M., Ed.; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2011; pp. 409–420. [Google Scholar]
- Brook, B.W.; Sodhi, N.S.; Bradshaw, C.J.A. Synergies among extinction drivers under global change. Trends Ecol. Evol. 2008, 23, 453–460. [Google Scholar] [CrossRef]
- Blumstein, D.T.; Daniel, J.C. The loss of anti-predator behaviour following isolation on islands. Proc. R. Soc. B Biol. Sci. 2005, 272, 1663–1668. [Google Scholar] [CrossRef]
- Aguirre-Muñoz, A.; Croll, D.A.; Donlan, C.J.; Henry, R.W.; Hermosillo, M.A.; Howald, G.R.; Keitt, B.S.; Luna-Mendoza, L.; Rodríguez-Malagón, M.; Salas-Flores, L.M.; et al. High-impact Conservation: Invasive Mammal Eradications from the Islands of Western México. AMBIO A J. Hum. Environ. 2008, 37, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Medina, F.M.; Bonnaud, E.; Vidal, E.; Tershy, B.R.; Zavaleta, E.S.; Josh Donlan, C.; Keitt, B.S.; Le Corre, M.; Horwath, S.V.; Nogales, M. A global review of the impacts of invasive cats on island endangered vertebrates. Glob. Change Biol. 2011, 17, 3503–3510. [Google Scholar] [CrossRef]
- Smith, M.J.; Cogger, H.; Tiernan, B.; Maple, D.; Boland, C.; Napier, F.; Detto, T.; Smith, P. An oceanic island reptile community under threat: The decline of reptiles on Christmas Island, Indian Ocean. Herpetol. Conserv. Biol. 2012, 7, 206–218. [Google Scholar]
- Gibbon, J.W.; Scott, D.E.; Ryan, T.J.; Buhlmann, K.A.; Tuberville, T.D.; Metts, B.S.; Greene, J.L.; Mills, T.; Leiden, Y.; Poppy, S.; et al. The Global Decline of Reptiles, Déjà Vu Amphibians. BioScience 2000, 50, 653–666. [Google Scholar] [CrossRef]
- Drake, D.R.; Hunt, T.L. Invasive rodents on islands: Integrating historical and contemporary ecology. Biol. Invasions 2009, 11, 1483–1487. [Google Scholar] [CrossRef]
- Arnaud, G.; Rodríguez, A.; Ortega-Rubio, A.; Alvarez-Cárdenas, S. Predation by Cats on the Unique Endemic Lizard of Socorro Island (Urosaurus auriculatus), Revillagigedo, Mexico. Ohio J. Sci. 1993, 93, 101–104. [Google Scholar]
- Osorio-Beristain, M.; Torres, R. Depredación de reptiles por gatos domésticos introducidos en Isla Isabel, Nayarit. Boletín De La Soc. Herpetológica Mex. 1992, 4, 10–12. [Google Scholar]
- Arnaud, G.; Martins, M.R.C.; Burguete-Trujillo, L.; Hernández-Rodríguez, I.; Avila-Villegas, H.; Murillo-Quero, R.; Quijada-Mascareñas, A. Historia natural de la serpiente de cascabel Crotalus catalinensis, endémica de las isla Santa Catalina, Golfo de California, México. In Estudios de las Islas del Golfo de California; Universidad Autónoma de Sinaloa: Cluliacán, México, 2008. [Google Scholar]
- Oberbauer, T.A. La vegetación de isla Guadalupe. Entonces y ahora. Gac. Ecológica 2006, 81, 46–58. [Google Scholar]
- Samaniego-Herrera, A.; Aguirre-Muñoz, A.; Bedolla-Guzmán, Y.; Cárdenas-Tapia, A.; Félix-Lizárraga, M.; Méndez-Sánchez, F.; Reina-Ponce, O.; Rojas-Mayoral, E.; Torres-García, F. Eradicating invasive rodents from wet and dry tropical islands in Mexico. Oryx 2017, 1–12. [Google Scholar] [CrossRef]
- Harper, G.; Zabala, J.; Carrion, V. Monitoring of a Population of Galápagos Land Iguanas (Conolophus Subcristatus) during a Rat Eradication Using Brodifacoum; Veitch, C.R., Clout, M.N., Towns, D.R., Eds.; Island Invasives: Eradication and Management; IUCN: Gland, Switz, 2011; pp. 309–312. [Google Scholar]
- Latofski-Robles, M. Restoration Priorities for Mexican Islands. Master Thesis, Universidad Autónoma de San Luis Potosí, San Luis Potosí, Mexico, July 2012. [Google Scholar]
- Ortiz-Alcaraz, A.; Arnaud, G.; Galina-Tessaro, P.; Rojas-Mayoral, E.; Méndez-Sánchez, F.; Ortega-Rubio, A. Progress in the eradication of the feral cat (Felis catus) and recovery of the native fauna on Socorro Island, Revillagigedo Archipelago, Mexico. Therya 2017, 8, 3–9. [Google Scholar] [CrossRef]
- Thomas, C.D.; Cameron, A.; Green, R.E.; Bakkenes, M.; Beaumont, L.J.; Collingham, Y.C.; Erasmus, B.F.N.; de Siqueira, M.F.; Grainger, A.; Hannah, L.; et al. Extinction risk from climate change. Nature 2004, 427, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Kiesecker, J.M.; Blaustein, A.R.; Belden, L.K. Complex causes of amphibian population declines. Nature 2001, 410, 681–684. [Google Scholar] [CrossRef] [PubMed]
- Walther, G.-R.; Post, E.; Convey, P.; Menzel, A.; Parmesan, C.; Beebee, T.J.C.; Fromentin, J.-M.; Hoegh-Guldberg, O.; Bairlein, F. Ecological responses to recent climate change. Nature 2002, 416, 389–395. [Google Scholar] [CrossRef]
- Sinervo, B.; Méndez-de-la-Cruz, F.; Miles, D.B.; Heulin, B.; Bastiaans, E.; Villagrán-Santa Cruz, M.; Lara-Resendiz, R.; Martínez-Méndez, N.; Calderón-Espinosa, M.L.; Meza-Lázaro, R.N.; et al. Erosion of Lizard Diversity by Climate Change and Altered Thermal Niches. Science 2010, 328, 894–899. [Google Scholar] [CrossRef] [PubMed]
- Tershy, B.R.; Breese, D.; Alvarez-Borrego, S. Increase in cetacean and seabird numbers in the Canal de Ballenas during an El Niño-Southern Oscillation event. Mar. Ecol. Prog. Ser. 1991, 69, 299–302. [Google Scholar] [CrossRef]
- Pérez, O.; Escobedo-Galván, A.H. Potential effects of El Niño-South Oscillation (ENSO) on growth of the American crocodile, Crocodylus acutus (Crocodylia: Crocodilydae) in captivity. J. Therm. Biol. 2009, 34, 14–16. [Google Scholar] [CrossRef]
- Limpus, C.; Nicholls, N. ENSO Regulation of Indo-Pacific Green Turtle Populations. In Applications of Seasonal Climate Forecasting in Agricultural and Natural Ecosystems; Hammer, G.L., Nicholls, N., Mitchell, C., Eds.; Springer: Dordrecht, The Netherlands, 2000; pp. 399–408. [Google Scholar]
- Saba, V.S.; Santidrián-Tomillo, P.; Reina, R.D.; Spotila, J.R.; Musick, J.A.; Evans, D.A.; Paladino, F.V. The effect of the El Niño Southern Oscillation on the reproductive frequency of eastern Pacific leatherback turtles. J. Appl. Ecol. 2007, 44, 395–404. [Google Scholar] [CrossRef]
- Cerdá-Ardura, A.; Langarica-Andonegui, E. On the presence of a spiny chuckwalla Sauromalus hispidus in Rasa island, Mexico. Rev. Latinoam. De Herpetol. 2018, 1, 17–28. [Google Scholar] [CrossRef]
- Lovich, R.E.; Grismer, L.L.; Danemann, G. Conservation status of the herpetofauna of Baja California, México and associated islands in the Sea of Cortez and Pacific Ocean. Herpetol. Conserv. Biol. 2009, 4, 358–378. [Google Scholar]
- Oey, L.; Ezer, T.; Lee, H. Loop Current, Rings and Related Circulation in the Gulf of Mexico: A Review of Numerical Models and Future Challenges. In Circulation in the Gulf of Mexico: Observations and Models; Sturges, W., Lugo, A., Eds.; American Geophysical Union: Whasington, DC, USA, 2013; pp. 31–56. [Google Scholar]
- Sun, S.; Hu, C.; Tunnell, J.W. Surface oil footprint and trajectory of the Ixtoc-I oil spill determined from Landsat/MSS and CZCS observations. Mar. Pollut. Bull. 2015, 101, 632–641. [Google Scholar] [CrossRef]
- Dedina, S. Wild Sea: Eco-Wars and Surf Stories from the Coast of the Californias; University of Arizona Press: Tucson, AZ, USA, 2011. [Google Scholar]
- Soto-Galera, E.; Piera, J.; López, P. Spatial and temporal land cover changes in Terminos Lagoon Reserve, Mexico. Rev. De Biol. Trop. 2010, 58, 565–575. [Google Scholar] [CrossRef]
- Bessesen, B. Vaquita: Science, Politics, and Crime in the Sea of Cortez; Island Press: Washington, DC, USA, 2018. [Google Scholar]
- Arroyo-Quiroz, I.; Wyatt, T. Green Crime in Mexico: A Collection of Case Studies; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Ceballos, G.; García, A. Challenges and Opportunities for Conservation of Mexican Biodiversity. In Conservation Biology: Voices from the Tropics; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2013; pp. 105–112. [Google Scholar]
- Bunge, V.; Reyes, J.A. Características sociales de las Áreas Naturales Protegidas federales y su relación con la conservación ambiental. In Crisis Civilizatoria En El México Rural; Ayala-Ortiz, D.A., Camacho-Velazquez, D., Castañeda-Zavala, Y., López-León, A., Eds.; Asociación Mexicana de Estudios Rurales: Ciudad de México, Mexico, 2015; pp. 23–40. [Google Scholar]
- Alvarado Martínez, I.; Martínez, E.R. Trafficking of Totoaba Maw. In Green Crime in Mexico: A Collection of Case Studies; Arroyo-Quiroz, I., Wyatt, T., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 149–170. [Google Scholar]
- Auliya, M.; Altherr, S.; Ariano-Sanchez, D.; Baard, E.H.; Brown, C.; Brown, R.M.; Cantu, J.-C.; Gentile, G.; Gildenhuys, P.; Henningheim, E.; et al. Trade in live reptiles, its impact on wild populations, and the role of the European market. Biol. Conserv. 2016, 204, 103–119. [Google Scholar] [CrossRef]
- Angulo, E.; Courchamp, F. Rare Species Are Valued Big Time. PLoS ONE 2009, 4, e5215. [Google Scholar] [CrossRef] [PubMed]
- Stuart, B.L.; Rhodin, A.G.; Grismer, L.L.; Hansel, T. Scientific description can imperil species. Science 2006, 312, 1137. [Google Scholar] [CrossRef]
- Early-Capistrán, M.-M.; Sáenz-Arroyo, A.; Cardoso-Mohedano, J.-G.; Garibay-Melo, G.; Peckham, S.H.; Koch, V. Reconstructing 290 years of a data-poor fishery through ethnographic and archival research: The East Pacific green turtle (Chelonia mydas) in Baja California, Mexico. Fish Fish. 2018, 19, 57–77. [Google Scholar] [CrossRef]
- Mancini, A.; Koch, V. Sea turtle consumption and black market trade in Baja California Sur, Mexico. Endanger. Species Res. 2009, 7, 1–10. [Google Scholar] [CrossRef]
- Mellink, E. Biological conservation of Isla de Cedros, Baja California, Mexico: Assessing multiple threats. Biodivers. Conserv. 1993, 2, 62–69. [Google Scholar] [CrossRef]
- Delibes, M.; Blázquez, M.d.C.; Soriano, L.; Revilla, E.; Godoy, J.A. High Antipredatory Efficiency of Insular Lizards: A Warning Signal of Excessive Specimen Collection? PLoS ONE 2011, 6, e29312. [Google Scholar] [CrossRef]
- Goode, M.J.; Horrace, W.C.; Sredl, M.J.; Howland, J.M. Habitat destruction by collectors associated with decreased abundance of rock-dwelling lizards. Biol. Conserv. 2005, 125, 47–54. [Google Scholar] [CrossRef]
- SEMARNAT. Norma Oficial Mexicana-059-SEMARNAT-2010, Protección Ambiental- Especies Nativas de México de Flora y Fauna Silvestres-Categorías de Riesgo y Especificaciones su Inclusión, Exclusión o Cambio-Lista de Especies en Riesgo; Diario Oficial de la Federación: Ciudad de México, Mexico, 2010. [Google Scholar]
- Mace, G.M.; Collar, N.J.; Gaston, K.J.; Hilton-Taylor, C.; Akçakaya, H.R.; Leader-Williams, N.; Milner-Gulland, E.J.; Stuart, S.N. Quantification of Extinction Risk: IUCN’s System for Classifying Threatened Species. Conserv. Biol. 2008, 22, 1424–1442. [Google Scholar] [CrossRef]
- Rodrigues, A.S.L.; Pilgrim, J.D.; Lamoreux, J.F.; Hoffmann, M.; Brooks, T.M. The value of the IUCN Red List for conservation. Trends Ecol. Evol. 2006, 21, 71–76. [Google Scholar] [CrossRef]
- Wilson, L.D.; McCranie, J.R. The conservation status of the herpetofauna of Honduras. Amphib. Reptile Conserv. 2004, 3, 6–33. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gärdenfors, U.; Hilton-Taylor, C.; Mace, G.M.; Rodríguez, J.P. The Application of IUCN Red List Criteria at Regional Levels. Conserv. Biol. 2001, 15, 1206–1212. [Google Scholar] [CrossRef]
- Gärdenfors, U. Classifying threatened species at national versus global levels. Trends Ecol. Evol. 2001, 16, 511–516. [Google Scholar] [CrossRef]
- García-Aguilar, M.C.; Luévano-Esparza, J.; Cueva, H.D.L. La fauna nativa de México en riesgo y la NOM-059: ¿Están todos los que son y son todos los que están? Acta Zoológica Mex. 2017, 33, 188–198. [Google Scholar] [CrossRef]
- Lazcano, A. Quo vadis, Mexican science? Science 2019, 365, 301. [Google Scholar] [CrossRef] [PubMed]
- Uglielmi, G. Mexican science suffers under debilitating budget cuts. Nature 2019. [CrossRef]
- Wade, L. Mexico’s new president shocks scientists with budget cuts and disparaging remarks. Science 2019. [CrossRef]
- D.O.F. DECRETO por el que se Declara área Natural Protegida, con el Carácter de área de Protección de Recursos Naturales, la Superficie de 4,843-59-73.12 Hectáreas de la Zona Conocida Como Peña Colorada, en los Municipios de Querétaro y El Marqués, en el Estado de Querétaro; Diario Oficial de la Federación: Ciudad de México, Mexico, 2023. [Google Scholar]
- D.O.F. DECRETO por el que se Declara área Natural Protegida con el carácter de área de Protección de Flora y Fauna, la Zona Conocida como Sierra de San Miguelito, en los Municipios de Mexquitic de Carmona, San Luis Potosí, Villa de Arriaga y Villa de Reyes, en el Estado de San Luis Potosí; Diario Oficial de la Federación: Ciudad de México, Mexico, 2021. [Google Scholar]
- D.O.F. DECRETO por el que se Declara área Natural Protegida con el carácter de área de Protección de Flora y Fauna, la región Conocida Como Jaguar, en el Municipio de Tulum en el Estado de Quintana Roo; Diario Oficial de la Federación: Ciudad de México, Mexico, 2022. [Google Scholar]
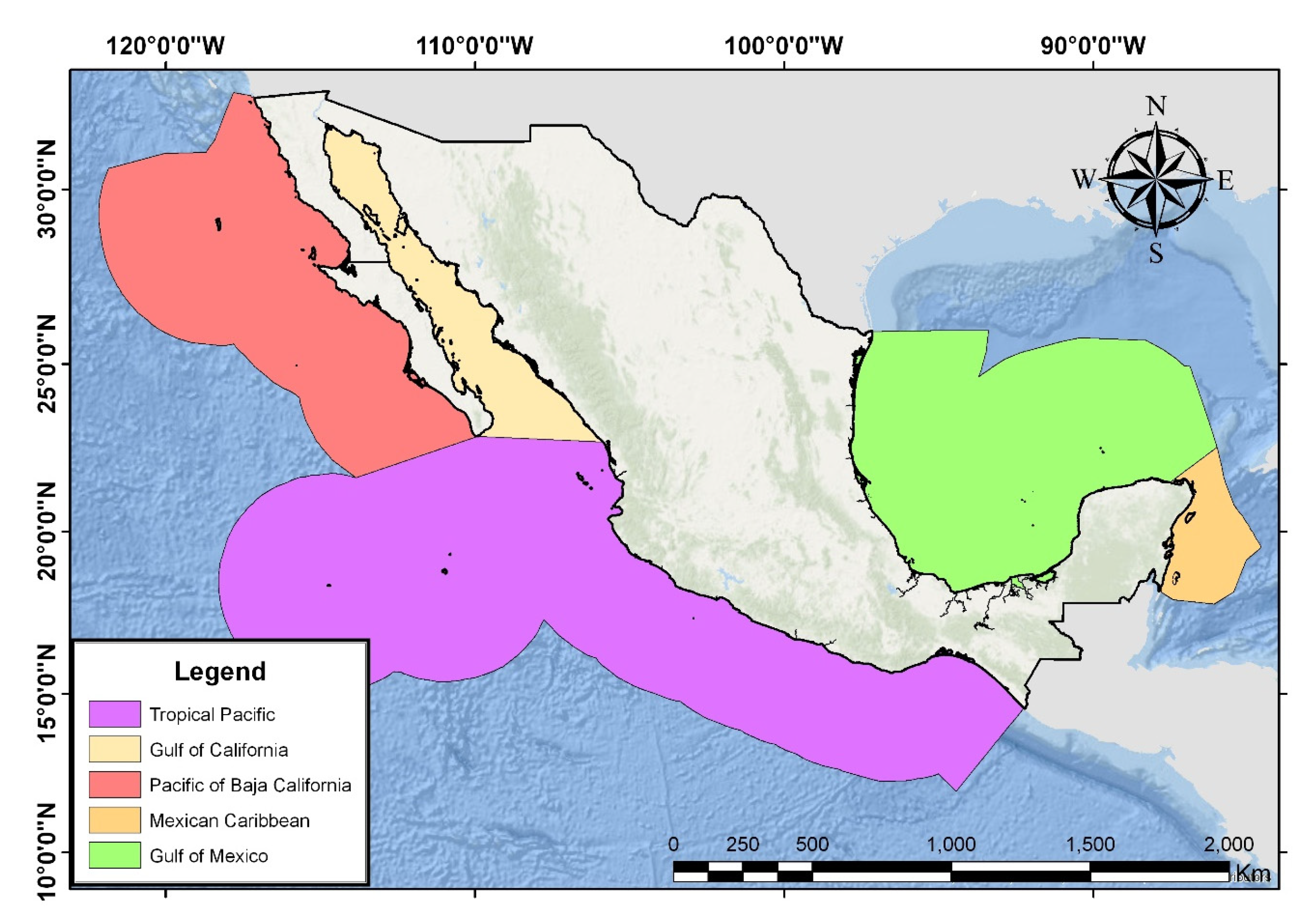











































| Orders | Families | Genera | Species |
|---|---|---|---|
| Anura | 6 | 12 | 14 |
| Caudata | 1 | 2 | 2 |
| Subtotals | 7 | 14 | 16 |
| Crocodylia | 1 | 1 | 2 |
| Squamata | 24 | 69 | 195 |
| Testudines | 8 | 11 | 13 |
| Subtotals | 33 | 81 | 210 |
| Totals | 40 | 95 | 226 |
| Taxa | Physiographic Regions | |||||
|---|---|---|---|---|---|---|
| Islands of Gulf of California | Islands of Pacific Baja California | Islands of Tropical Pacific | Islands of Gulf of Mexico | Islands of Mexican Caribbean | Total Number of Regions | |
| Amphibia (16 species) | ||||||
| Anura (14 species) | ||||||
| Bufonidae (4 species) | ||||||
| Anaxyrus punctatus | + | + | 2 | |||
| Incilius mazatlanensis * | + | 1 | ||||
| Incilius valliceps | + | + | 2 | |||
| Rhinella horribilis | + | + | + | 3 | ||
| Eleutherodactylidae (2 species) | ||||||
| Eleutherodactylus pallidus * | + | 1 | ||||
| Eleutherodactylus planirostris ‡ | + | 1 | ||||
| Hylidae (5 species) | ||||||
| Dendrosophus microcephalus | + | 1 | ||||
| Hyliola regilla | + | 1 | ||||
| Scinax staufferi | + | + | 2 | |||
| Smilisca baudini | + | + | + | 3 | ||
| Trachycephalus vermiculatus | + | 1 | ||||
| Leptodactylidae (1 species) | ||||||
| Leptodactylus fragilis | + | + | 2 | |||
| Microhylidae (1 species) | ||||||
| Hypopachus variolosus | + | + | 2 | |||
| Scaphiopodidae | ||||||
| Scaphiopus couchii | + | 1 | ||||
| Caudata (2 species) | ||||||
| Plethodontidae (2 species) | ||||||
| Aneides lugubris | + | 1 | ||||
| Batrachoseps major | + | 1 | ||||
| Reptilia (2 species) | ||||||
| Crocodylia (2 species) | ||||||
| Crocodylidae (2 species) | ||||||
| Crocodylus acutus | + | + | 2 | |||
| Crocodylus moreletii | + | 1 | ||||
| Squamata (185 species) | ||||||
| Anguidae (3 species) | ||||||
| Elgaria cedrosensis * | + | 1 | ||||
| Elgaria multicarinata | + | 1 | ||||
| Elgaria nana ** | + | 1 | ||||
| Anniellidae (2 species) | ||||||
| Anniella geronimensis * | + | 1 | ||||
| Anniella pulchra | + | 1 | ||||
| Bipedidae (1 species) | ||||||
| Bipes biporus * | + | 1 | ||||
| Corytophanidae (1 species) | ||||||
| Basiliscus vittatus | + | + | 2 | |||
| Crotaphytidae (4 species) | ||||||
| Crotaphytus dickersonae ** | + | 1 | ||||
| Crotaphytus insularis ** | + | 1 | ||||
| Gambelia copeii | + | 1 | ||||
| Gambelia wislizenii | + | 1 | ||||
| Dactyloidae (6 species) | ||||||
| Anolis allisoni ‡ | + | 1 | ||||
| Norops lemurinus | + | 1 | ||||
| Norops nebulosus * | + | 1 | ||||
| Norops rodriguezii | + | + | 2 | |||
| Norops sagrei ‡ | + | + | 2 | |||
| Norops ustus | + | + | 2 | |||
| Eublepharidae (3 species) | ||||||
| Coleonyx elegans | + | 1 | ||||
| Coleonyx gypsicolus ** | + | 1 | ||||
| Coleonyx variegatus | + | + | 2 | |||
| Gekkonidae (3 species) | ||||||
| Gehyra mutilata ‡ | + | 1 | ||||
| Hemidactylus frenatus ‡ | + | + | + | 3 | ||
| Hemidactylus turcicus ‡ | + | + | 2 | |||
| Iguanidae (13 species) | ||||||
| Ctenosaura conspicuosa ** | + | 1 | ||||
| Ctenosaura hemilopha * | + | 1 | ||||
| Ctenosaura nolascensis ** | + | 1 | ||||
| Ctenosaura pectinata | + | 1 | ||||
| Ctenosaura similis | + | + | 2 | |||
| Dipsosaurus catalinensis ** | + | 1 | ||||
| Dipsosaurus dorsalis | + | + | 2 | |||
| Iguana iguana | + | + | + | 3 | ||
| Sauromalus ater | + | 1 | ||||
| Sauromalus hispidus * | + | 1 | ||||
| Sauromalus klauberi ** | + | 1 | ||||
| Sauromalus slevini * | + | 1 | ||||
| Sauromalus varius * | + | 1 | ||||
| Mabuyidae (1 species) | ||||||
| Marisora aquilonaria * | + | 1 | ||||
| Marisora lineola | + | + | 2 | |||
| Phrynosomatidae (31 species) | ||||||
| Callisaurus draconoides | + | + | 2 | |||
| Petrosaurus mearnsi | + | 1 | ||||
| Petrosaurus repens * | + | 1 | ||||
| Petrosaurus slevini ** | + | 1 | ||||
| Petrosaurus thalassinus * | + | 1 | ||||
| Phrynosoma cerroense * | + | 1 | ||||
| Phrynosoma solare | + | 1 | ||||
| Sceloporus angustus ** | + | 1 | ||||
| Sceloporus chrysostictus | + | + | 2 | |||
| Sceloporus clarkii | + | + | + | 3 | ||
| Sceloporus cozumelae * | + | 1 | ||||
| Sceloporus grandaevus ** | + | 1 | ||||
| Sceloporus hunsakeri * | + | 1 | ||||
| Sceloporus lineatulus ** | + | 1 | ||||
| Sceloporus magister | + | 1 | ||||
| Sceloporus occidentalis | + | 1 | ||||
| Sceloporus orcutti | + | 1 | ||||
| Sceloporus variabilis | + | 1 | ||||
| Sceloporus zosteromus * | + | + | 2 | |||
| Urosaurus auriculatus ** | + | 1 | ||||
| Urosaurus bicarinatus * | + | 1 | ||||
| Urosaurus clarionensis ** | + | 1 | ||||
| Urosaurus nigricaudus * | + | + | 2 | |||
| Urosaurus ornatus | + | + | 2 | |||
| Uta encantadae ** | + | 1 | ||||
| Uta lowei ** | + | 1 | ||||
| Uta nolascensis ** | + | 1 | ||||
| Uta palmeri ** | + | 1 | ||||
| Uta squamata | + | 1 | ||||
| Uta stansburiana | + | + | 2 | |||
| Uta tumidarostra ** | + | 1 | ||||
| Phyllodactylidae (9 species) | ||||||
| Phyllodactylus angelensis ** | + | 1 | ||||
| Phyllodactylus apricus ** | + | 1 | ||||
| Phyllodactylus benedetii * | + | 1 | ||||
| Phyllodactylus bugastrolepis ** | + | 1 | ||||
| Phyllodactylus cleofasensis ** | + | 1 | ||||
| Phyllodactylus coronatus ** | + | 1 | ||||
| Phyllodactylus homolepidurus * | + | 1 | ||||
| Phyllodactylus isabelae ** | + | 1 | ||||
| Phyllodactylus lanei * | + | 1 | ||||
| Phyllodactylus lupitae ** | + | 1 | ||||
| Phyllodactylus nocticolus | + | + | 2 | |||
| Phyllodactylus partidus ** | + | 1 | ||||
| Phyllodactylus tuberculosus | + | 1 | ||||
| Phyllodactylus santacruzensis ** | + | 1 | ||||
| Phyllodactylus unctus * | + | 1 | ||||
| Scincidae (2 species) | ||||||
| Mesoscincus schwartzei | + | 1 | ||||
| Plestiodon skiltonianus | + | 1 | ||||
| Sphaerodactylidae (3 species) | ||||||
| Aristelliger georgeensis | + | 1 | ||||
| Sphaerodactylus continentalis | + | 1 | ||||
| Sphaerodactylus glaucus | + | + | 2 | |||
| Teiidae (21 species) | ||||||
| Aspidoscelis bacata ** | + | 1 | ||||
| Aspidoscelis cana ** | + | 1 | ||||
| Aspidoscelis carmenensis ** | + | 1 | ||||
| Aspidoscelis catalinensis ** | + | 1 | ||||
| Aspidoscelis celeripes ** | + | 1 | ||||
| Aspidoscelis ceralbelsis ** | + | 1 | ||||
| Aspidoscelis communis * | + | 1 | ||||
| Aspidoscelis costata * | + | 1 | ||||
| Aspidoscelis cozumela * | + | + | 2 | |||
| Aspidoscelis danheimae ** | + | 1 | ||||
| Aspidoscelis deppii | + | + | + | 3 | ||
| Aspidoscelis espiritensis ** | + | 1 | ||||
| Aspidoscelis franciscensis ** | + | 1 | ||||
| Aspidoscelis guttatus * | + | 1 | ||||
| Aspidoscelis hyperythrus | + | + | 2 | |||
| Aspidoscelis lineatissima * | + | 1 | ||||
| Aspidoscelis martyris ** | + | 1 | ||||
| Aspidoscelis maslini | + | + | 2 | |||
| Aspidoscelis pictus ** | + | 1 | ||||
| Aspidoscelis rodecki * | + | 1 | ||||
| Aspidoscelis tigris | + | + | 2 | |||
| Holcosus gaigeae * | + | + | 2 | |||
| Boidae (2 species) | ||||||
| Boa sigma * | + | 1 | ||||
| Boa imperator | + | + | 2 | |||
| Charinidae (1 species) | ||||||
| Lichanura trivirgata | + | + | 2 | |||
| Colubridae (36 species) | ||||||
| Bogertophis rosaliae | + | 1 | ||||
| Drymarchon melanurus | + | 1 | ||||
| Drymobius margaritiferus | + | 1 | ||||
| Lampropeltis abnorma | + | 1 | ||||
| Lampropeltis californiae | + | 1 | ||||
| Lampropeltis catalinensis ** | + | 1 | ||||
| Lampropeltis herrerae ** | + | 1 | ||||
| Lampropeltis polyzona * | + | 1 | ||||
| Leptophis diplotropis * | + | 1 | ||||
| Leptophis mexicanus | + | 1 | ||||
| Masticophis anthonyi ** | + | 1 | ||||
| Masticophis barbouri | + | 1 | ||||
| Masticophis bilineatus | + | 1 | ||||
| Masticophis fuliginosus | + | + | 2 | |||
| Masticophis mentovarius | + | + | 2 | |||
| Masticophis slevini ** | + | 1 | ||||
| Mastigodryas melanolomus | + | + | 2 | |||
| Oxybelis aeneus | + | + | 2 | |||
| Oxybelis fulgidus | + | 1 | ||||
| Oxybelis microphtalmus * | + | 1 | ||||
| Phyllorhynchus decurtatus | + | 1 | ||||
| Pituophis catenifer | + | + | 2 | |||
| Pituophis insularis ** | + | 1 | ||||
| Pituophis vertebralis * | + | + | 2 | |||
| Pseudelaphe flavirufa | + | 1 | ||||
| Rhinocheilus etheridgei ** | + | 1 | ||||
| Salvadora hexalepis | + | + | 2 | |||
| Sonora savagei * | + | 1 | ||||
| Sonora semiannulata | + | 1 | ||||
| Sonora straminea | + | + | 2 | |||
| Spilotes pullatus | + | 1 | ||||
| Tantilla bocourti * | + | 1 | ||||
| Tantilla calamarina * | + | 1 | ||||
| Tantilla moesta | + | 1 | ||||
| Tantilla planiceps | + | 1 | ||||
| Trimorphodon lyrophanes | + | 1 | ||||
| Dipsadidae (19 species) | ||||||
| Coniophanes imperialis | + | 1 | ||||
| Conophis lineatus | + | + | 2 | |||
| Conophis vittatus | + | 1 | ||||
| Diadophis punctatus | + | 1 | ||||
| Dipsas brevifacies | + | 1 | ||||
| Geophis annuliferus * | + | 1 | ||||
| Hypsiglena catalinae ** | + | 1 | ||||
| Hypsiglena chlorophaea | + | 1 | ||||
| Hypsiglena ochrorhynchus | + | + | 2 | |||
| Hypsiglena slevini * | + | + | + | 3 | ||
| Hypsiglena torquata | + | 1 | ||||
| Hypsiglena unaocularis ** | + | 1 | ||||
| Imantodes cenchoa | + | 1 | ||||
| Imantodes gemmistratus | + | 1 | ||||
| Leptodeira frenata | + | + | 2 | |||
| Ninia sebae | + | 1 | ||||
| Rhadinaea hesperia * | + | 1 | ||||
| Sibon nebulatus | + | + | 2 | |||
| Tropidodipsas sartorii | + | 1 | ||||
| Elapidae (3 species) | ||||||
| Hydrophis platurus | + | + | 2 | |||
| Micruroides euryxanthus | + | 1 | ||||
| Micrurus apiatus | + | + | 2 | |||
| Leptotyphlopidae (3 species) | ||||||
| Epictia bakewelli * | + | 1 | ||||
| Epictia magnamaculata | + | 1 | ||||
| Rena humilis | + | + | + | 3 | ||
| Natricidae (1 species) | ||||||
| Thamnophis proximus | + | 1 | ||||
| Typhlopidae (1 species) | ||||||
| Indotyphlops braminus ‡ | + | + | + | 3 | ||
| Viperidae (18 species) | ||||||
| Agkistrodon bilineatus | + | 1 | ||||
| Agkistrodon russeolus * | + | 1 | ||||
| Crotalus angelensis ** | + | 1 | ||||
| Crotalus atrox | + | 1 | ||||
| Crotalus caliginis ** | + | 1 | ||||
| Crotalus catalinensis | + | 1 | ||||
| Crotalus cerastes | + | 1 | ||||
| Crotalus enyo * | + | + | 2 | |||
| Crotalus estebanensis ** | + | 1 | ||||
| Crotalus lorenzoensis ** | + | 1 | ||||
| Crotalus mitchellii | + | + | 2 | |||
| Crotalus molossus | + | 1 | ||||
| Crotalus polisi ** | + | 1 | ||||
| Crotalus pyrrhus | + | 1 | ||||
| Crotalus ruber | + | + | 2 | |||
| Crotalus thalassoporus ** | + | 1 | ||||
| Crotalus tigris | + | 1 | ||||
| Crotalus tortuguensis ** | + | 1 | ||||
| Testudines (13 species) | ||||||
| Chelonidae (5 species) | ||||||
| Caretta caretta | + | + | + | + | 4 | |
| Chelonia mydas | + | + | + | + | 4 | |
| Eretmochelys imbricata | + | + | + | + | 4 | |
| Lepidochelys kempii | + | + | 2 | |||
| Lepidochelys olivacea | + | + | 2 | |||
| Dermatemydidae (1 species) | ||||||
| Dermatemys mawii | + | 1 | ||||
| Dermochelyidae (1 species) | ||||||
| Dermochelys coriacea | + | + | 2 | |||
| Emydidae (1 species) | ||||||
| Trachemys venusta | + | 1 | ||||
| Geoemydidae (1 species) | ||||||
| Rhinoclemmys areolata | + | 1 | ||||
| Kinosternidae (2 species) | ||||||
| Kinosternon integrum * | + | 1 | ||||
| Kinosternon scorpioides | + | + | 2 | |||
| Staurotypidae (1 species) | ||||||
| Staurotypus triporcatus | + | 1 | ||||
| Testudinidae (1 species) | ||||||
| Gopherus morafkai | + | 1 | ||||
| Totals | 108 | 40 | 57 | 46 | 53 | — |
| Families | Number of Species | Distributional Occurrence | ||||
|---|---|---|---|---|---|---|
| Gulf of California | Pacific Baja California | Tropical Pacífic | Gulf of Mexico | Caribbean Islands | ||
| Bufonidae | 4 | 1 | 1 | 2 | 2 | 2 |
| Eleutherodactylidae | 2 | — | — | 1 | — | 1 |
| Hylidae | 5 | — | 1 | 1 | 2 | 4 |
| Leptodactylidae | 1 | — | — | — | 1 | 1 |
| Microhylidae | 1 | — | — | 1 | 1 | — |
| Scaphiopodidae | 1 | 1 | — | — | — | — |
| Subtotals | 14 | 2 | 2 | 5 | 6 | 8 |
| Plethodontidae | 2 | — | 2 | — | — | — |
| Subtotals | 2 | — | 2 | — | — | — |
| Totals | 16 | 2 | 4 | 5 | 6 | 8 |
| Crocodylidae | 2 | — | — | 1 | 1 | 1 |
| Subtotals | 2 | — | — | 1 | 1 | 1 |
| Anguidae | 3 | — | 3 | — | — | — |
| Anniellidae | 2 | — | 2 | — | — | — |
| Bipedidae | 1 | — | 1 | — | — | — |
| Corytophanidae | 1 | — | — | — | 1 | 1 |
| Crotaphytidae | 4 | 3 | 1 | — | — | — |
| Dactyloidae | 6 | — | — | 1 | 3 | 5 |
| Eublepharidae | 3 | 2 | 1 | — | — | 1 |
| Gekkonidae | 3 | — | — | 2 | 2 | 2 |
| Iguanidae | 13 | 10 | 1 | 2 | 2 | 2 |
| Mabuyidae | 2 | — | — | 1 | 1 | 1 |
| Phrynosomatidae | 31 | 23 | 7 | 5 | 2 | 2 |
| Phyllodactylidae | 15 | 9 | 1 | 6 | — | — |
| Scincidae | 2 | — | 1 | — | — | 1 |
| Sphaerodactylidae | 3 | — | — | — | 1 | 3 |
| Teiidae | 23 | 13 | 2 | 5 | 5 | 5 |
| Subtotals | 111 | 60 | 20 | 22 | 16 | 23 |
| Boidae | 2 | — | — | 1 | 1 | 1 |
| Charinidae | 1 | 1 | 1 | — | — | — |
| Colubridae | 36 | 17 | 7 | 9 | 5 | 6 |
| Dipsadidae | 19 | 4 | 3 | 8 | 8 | 2 |
| Elapidae | 3 | 2 | — | 1 | 1 | 1 |
| Leptotyphlopidae | 3 | 1 | 1 | 2 | — | 1 |
| Natricidae | 1 | — | — | — | — | 1 |
| Typhlopidae | 1 | — | — | 1 | 1 | 1 |
| Viperidae | 18 | 15 | 4 | 1 | — | 1 |
| Subtotals | 84 | 40 | 16 | 23 | 16 | 14 |
| Chelonidae | 5 | 4 | — | 4 | 4 | 4 |
| Dermatemydidae | 1 | — | — | — | 1 | — |
| Dermochelyidae | 1 | 1 | — | 1 | — | — |
| Emydidae | 1 | — | — | — | — | 1 |
| Geoemydidae | 1 | — | — | — | — | 1 |
| Kinosternidae | 2 | — | — | 1 | 1 | 1 |
| Staurotypidae | 1 | — | — | — | 1 | — |
| Testudinidae | 1 | 1 | — | — | — | — |
| Subtotals | 13 | 6 | — | 6 | 7 | 7 |
| Totals | 210 | 106 | 36 | 52 | 40 | 44 |
| Sum Totals | 226 | 108 | 40 | 57 | 46 | 53 |
| Gulf of California | Pacific Baja California | Tropical Pacific | Gulf of Mexico | Mexican Caribbean | |
|---|---|---|---|---|---|
| Gulf of California | 108 | 23 | 10 | 3 | 3 |
| Pacific Baja California | 0.31 | 40 | 3 | 0 | 0 |
| Tropical Pacific | 0.123 | 0.064 | 54 | 9 | 10 |
| Gulf of Mexico | 0.04 | 0 | 0.187 | 42 | 27 |
| Mexican Caribbean | 0.0387 | 0 | 0.198 | 0.607 | 47 |
| Taxa | Islands of the Pacific of Baja California | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Asunción | Cedros | Coronado Archipielago | Magdalena | Natividad | Islas San Benito | Islands from Laguna Ojo de Liebre | San Martín | San Jerónimo | San Roque | Santa Margarita | Todos Santos Archipielago | |||||||
| Coronado Sur | Coronado medio | Coronado norte | Las Brosas | Las Piedras | Pata | Todos Santos Sur | Todos Santos Norte | Total Number of Islands | ||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | ||
| Amphibians | ||||||||||||||||||
| Anura | ||||||||||||||||||
| Bufonidae | ||||||||||||||||||
| Anaxyrus punctatus | + | 1 | ||||||||||||||||
| Hylidae | ||||||||||||||||||
| Hyliola regilla | + | 1 | ||||||||||||||||
| Caudata | ||||||||||||||||||
| Plethodontidae | ||||||||||||||||||
| Aneides lugubris | + | 1 | ||||||||||||||||
| Batrachoseps major | + | + | + | + | 4 | |||||||||||||
| Reptiles | ||||||||||||||||||
| Squamata | ||||||||||||||||||
| Anguidae | ||||||||||||||||||
| Elgaria cedrosensis * | + | 1 | ||||||||||||||||
| Elgaria multicarinata | + | 1 | ||||||||||||||||
| Elgaria nana ** | + | + | 2 | |||||||||||||||
| Anniellidae | ||||||||||||||||||
| Anniella geronimensis * | + | + | 2 | |||||||||||||||
| Anniella pulchra | + | + | + | + | 4 | |||||||||||||
| Bipedidae | ||||||||||||||||||
| Bipes biporus * | + | 1 | ||||||||||||||||
| Crotaphytidae | ||||||||||||||||||
| Gambelia copeii | + | + | + | 3 | ||||||||||||||
| Eublepharidae | ||||||||||||||||||
| Coleonyx variegatus | + | + | 2 | |||||||||||||||
| Iguanidae | ||||||||||||||||||
| Dipsosaurus dorsalis | + | + | 2 | |||||||||||||||
| Phrynosomatidae | ||||||||||||||||||
| Callisaurus draconoides | + | + | 2 | |||||||||||||||
| Phrynosoma cerroense * | + | 1 | ||||||||||||||||
| Sceloporus occidentalis | + | + | 2 | |||||||||||||||
| Sceloporus zosteromus * | + | + | + | 3 | ||||||||||||||
| Urosaurus nigricaudus * | + | + | 2 | |||||||||||||||
| Uta stansburiana | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | 17 |
| Phyllodactylidae | ||||||||||||||||||
| Phyllodactillus nocticolus | + | + | 2 | |||||||||||||||
| Scincidae | ||||||||||||||||||
| Plestiodon skiltonianus | + | + | + | + | 4 | |||||||||||||
| Teiidae | ||||||||||||||||||
| Aspidoscelis hyperythrus | + | + | 2 | |||||||||||||||
| Aspidoscelis tigris | + | + | + | + | + | + | 6 | |||||||||||
| Charinidae | ||||||||||||||||||
| Lichanura trivirgata | + | + | 2 | |||||||||||||||
| Colubridae | ||||||||||||||||||
| Lampropeltis herrerae ** | + | 1 | ||||||||||||||||
| Masticophis fuliginosus | + | + | 2 | |||||||||||||||
| Pituophis catenifer | + | + | 2 | |||||||||||||||
| Pituophis insulanus ** | + | 1 | ||||||||||||||||
| Pituophis vertebralis * | + | + | 2 | |||||||||||||||
| Salvadora hexalepis | + | + | 2 | |||||||||||||||
| Sonora straminea | + | + | + | 3 | ||||||||||||||
| Dipsadidae | ||||||||||||||||||
| Diadophis punctatus | + | + | 2 | |||||||||||||||
| Hypsiglena ochrorhyncus | + | + | + | + | + | 5 | ||||||||||||
| Hypsiglena slevini * | + | 1 | ||||||||||||||||
| Leptotyphlopidae | ||||||||||||||||||
| Rena humilis | + | 1 | ||||||||||||||||
| Viperidae | ||||||||||||||||||
| Crotalus caliginis ** | + | 1 | ||||||||||||||||
| Crotalus enyo * | + | + | 2 | |||||||||||||||
| Crotalus mitchellii | + | 1 | ||||||||||||||||
| Crotalus ruber | + | + | 2 | |||||||||||||||
| Totals (39 species) | 1 | 15 | 9 | 3 | 8 | 14 | 2 | 1 | 1 | 1 | 1 | 6 | 3 | 1 | 19 | 8 | 3 | — |
| (Part 1) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E | Islands of the Gulf of California | |||||||||||||||||||||||||||||||||||
| Taxa | Alcatraz (Pelícano) | Ángel de la Guarda | Isla Ballena | Isla Bota | Cabeza de caballo | Cardonosa Este | Carmen | Cayo | Cerraja | Isla Cerralvo | Cholludo | Coloradito (Lobos) | Coronados | Danzante | Dátil (Turners) | El Coyote | El Muerto | El Pardito | El requesón | Coloradita | Encantada | Espiritu Santo | Isla flecha | Farallon de San Ignacio | Gallina | Gallo | Gaviota | Granito | Islitas | Lagartija | La ventana | Las ánimas | Las galeras | Mejía | Mitlán | Total number of islands |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 | 27 | 28 | 29 | 30 | 31 | 32 | 33 | 34 | 35 | ||
| Amphibia | ||||||||||||||||||||||||||||||||||||
| Anura | ||||||||||||||||||||||||||||||||||||
| Bufonidae | ||||||||||||||||||||||||||||||||||||
| Anaxyrus punctatus | + | + | 2 | |||||||||||||||||||||||||||||||||
| Scaphiopodidae | ||||||||||||||||||||||||||||||||||||
| Scaphiopus couchii | + | + | 2 | |||||||||||||||||||||||||||||||||
| Reptiles | ||||||||||||||||||||||||||||||||||||
| Squamata | ||||||||||||||||||||||||||||||||||||
| Crotaphytidae | ||||||||||||||||||||||||||||||||||||
| Crotaphytus dickersonae ** | ||||||||||||||||||||||||||||||||||||
| Crotaphytus insularis ** | + | 1 | ||||||||||||||||||||||||||||||||||
| Gambelia wislizenii | ||||||||||||||||||||||||||||||||||||
| Eublepharidae | ||||||||||||||||||||||||||||||||||||
| Coleonyx gypsicolus ** | ||||||||||||||||||||||||||||||||||||
| Coleonyx variegatus | + | + | + | + | 4 | |||||||||||||||||||||||||||||||
| Gekkonidae | ||||||||||||||||||||||||||||||||||||
| Hemidactylus frenatus ‡ | + | |||||||||||||||||||||||||||||||||||
| Iguanidae | ||||||||||||||||||||||||||||||||||||
| Ctenosaura conspicuosa ** | + | 1 | ||||||||||||||||||||||||||||||||||
| Ctenosaura hemilopha * | + | 1 | ||||||||||||||||||||||||||||||||||
| Ctenosaura nolascensis ** | ||||||||||||||||||||||||||||||||||||
| Dipsosaurus catalinensis ** | ||||||||||||||||||||||||||||||||||||
| Dipsosaurus dorsalis | + | + | + | + | + | + | 6 | |||||||||||||||||||||||||||||
| Sauromalus ater | + | + | + | + | 4 | |||||||||||||||||||||||||||||||
| Sauromalus hispidus * | + | + | + | + | + | + | 6 | |||||||||||||||||||||||||||||
| Sauromalus klauberi ** | ||||||||||||||||||||||||||||||||||||
| Sauromalus slevini * | + | + | + | 3 | ||||||||||||||||||||||||||||||||
| Sauromalus spp. | + | 1 | ||||||||||||||||||||||||||||||||||
| Sauromalus varius * | ||||||||||||||||||||||||||||||||||||
| Phrynosomatidae | ||||||||||||||||||||||||||||||||||||
| Callisaurus draconoides | + | + | + | + | + | + | 6 | |||||||||||||||||||||||||||||
| Petrosaurus mearnsi | + | 1 | ||||||||||||||||||||||||||||||||||
| Petrosaurus repens * | + | 1 | ||||||||||||||||||||||||||||||||||
| Petrosaurus slevini ** | + | + | 2 | |||||||||||||||||||||||||||||||||
| Petrosaurus thalassinus * | + | 1 | ||||||||||||||||||||||||||||||||||
| Phrynosoma solare | ||||||||||||||||||||||||||||||||||||
| Sceloporus angustus ** | ||||||||||||||||||||||||||||||||||||
| Sceloporus clarkia | ||||||||||||||||||||||||||||||||||||
| Sceloporus grandaevus ** | + | 1 | ||||||||||||||||||||||||||||||||||
| Sceloporus hunsakeri * | + | + | + | 3 | ||||||||||||||||||||||||||||||||
| Sceloporus lineatulus ** | ||||||||||||||||||||||||||||||||||||
| Sceloporus magister | ||||||||||||||||||||||||||||||||||||
| Sceloporus orcutti | + | + | 2 | |||||||||||||||||||||||||||||||||
| Sceloporus zosteromus * | + | + | + | 3 | ||||||||||||||||||||||||||||||||
| Urosaurus nigricaudus * | + | + | + | + | + | + | + | + | + | + | + | + | + | 13 | ||||||||||||||||||||||
| Urosaurus ornatus | + | 1 | ||||||||||||||||||||||||||||||||||
| Uta encantadae ** | + | 1 | ||||||||||||||||||||||||||||||||||
| Uta lowei ** | + | 1 | ||||||||||||||||||||||||||||||||||
| Uta nolascensis ** | ||||||||||||||||||||||||||||||||||||
| Uta palmeri ** | ||||||||||||||||||||||||||||||||||||
| Uta squamata | ||||||||||||||||||||||||||||||||||||
| Uta stansburiana | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | 21 | ||||||||||||||
| Uta tumidarostra ** | + | + | 2 | |||||||||||||||||||||||||||||||||
| Phyllodactylidae | ||||||||||||||||||||||||||||||||||||
| Phyllodactylus angelensis ** | + | |||||||||||||||||||||||||||||||||||
| Phyllodactylus apricus ** | + | |||||||||||||||||||||||||||||||||||
| Phyllodactylus bugastrolepis ** | ||||||||||||||||||||||||||||||||||||
| Phyllodactylus coronatus ** | ? | + | + | |||||||||||||||||||||||||||||||||
| Phyllodactylus homolepidurus * | + | 1 | ||||||||||||||||||||||||||||||||||
| Phyllodactylus nocticolus | + | + | + | + | + | + | + | + | + | + | 14 | |||||||||||||||||||||||||
| Phyllodactylus partidus ** | + | 1 | ||||||||||||||||||||||||||||||||||
| Phyllodactylus santacruzensis ** | ||||||||||||||||||||||||||||||||||||
| Phyllodactylus unctus * | + | + | + | + | + | 5 | ||||||||||||||||||||||||||||||
| Teiidae | ||||||||||||||||||||||||||||||||||||
| Aspidoscelis bacata ** | ||||||||||||||||||||||||||||||||||||
| Aspidoscelis cana ** | ||||||||||||||||||||||||||||||||||||
| Aspidoscelis carmenensis ** | + | 1 | ||||||||||||||||||||||||||||||||||
| Aspidoscelis catalinensis ** | ||||||||||||||||||||||||||||||||||||
| Aspidoscelis celeripes ** | ||||||||||||||||||||||||||||||||||||
| Aspidoscelis ceralbelsis ** | + | 1 | ||||||||||||||||||||||||||||||||||
| Aspidoscelis danheimae ** | ||||||||||||||||||||||||||||||||||||
| Aspidoscelis espiritensis ** | + | 1 | ||||||||||||||||||||||||||||||||||
| Aspidoscelis franciscensis ** | ||||||||||||||||||||||||||||||||||||
| Aspidoscelis hyperythrus | + | 1 | ||||||||||||||||||||||||||||||||||
| Aspidoscelis martyris ** | ||||||||||||||||||||||||||||||||||||
| Aspidoscelis pictus ** | ||||||||||||||||||||||||||||||||||||
| Aspidoscelis tigris | + | + | + | + | + | + | + | 7 | ||||||||||||||||||||||||||||
| Charinidae | ||||||||||||||||||||||||||||||||||||
| Lichanura trivirgata | + | + | + | + | + | 5 | ||||||||||||||||||||||||||||||
| Colubridae | + | |||||||||||||||||||||||||||||||||||
| Bogertophis rosaliae | + | 1 | ||||||||||||||||||||||||||||||||||
| Lampropeltis californiae | + | + | 2 | |||||||||||||||||||||||||||||||||
| Lampropeltis catalinensis ** | ||||||||||||||||||||||||||||||||||||
| Masticophis barbouri** | + | 1 | ||||||||||||||||||||||||||||||||||
| Masticophis bilineatus | ||||||||||||||||||||||||||||||||||||
| Masticophis fuliginosus | + | + | + | + | + | + | 6 | |||||||||||||||||||||||||||||
| Masticophis slevini ** | ||||||||||||||||||||||||||||||||||||
| Phyllorhynchus decurtatus | + | + | 2 | |||||||||||||||||||||||||||||||||
| Pituophis catenifer | ||||||||||||||||||||||||||||||||||||
| Pituophis vertebralis * | ||||||||||||||||||||||||||||||||||||
| Rhinocheilus etheridgei ** | + | 1 | ||||||||||||||||||||||||||||||||||
| Salvadora hexalepis, | + | + | 1 | |||||||||||||||||||||||||||||||||
| Sonora savagei * | + | 1 | ||||||||||||||||||||||||||||||||||
| Sonora semiannulata | ||||||||||||||||||||||||||||||||||||
| Sonora straminea | + | + | 2 | |||||||||||||||||||||||||||||||||
| Tantilla planiceps | + | 1 | ||||||||||||||||||||||||||||||||||
| Trimorphodon lyrophanes | + | + | + | 3 | ||||||||||||||||||||||||||||||||
| Dipsadidae | ||||||||||||||||||||||||||||||||||||
| Hypsiglena catalinae ** | ||||||||||||||||||||||||||||||||||||
| Hypsiglena chlorophaea | ||||||||||||||||||||||||||||||||||||
| Hypsiglena ochrorhynchus | + | + | + | + | + | + | 6 | |||||||||||||||||||||||||||||
| Hypsiglena slevini * | + | + | + | + | 4 | |||||||||||||||||||||||||||||||
| Elapidae | ||||||||||||||||||||||||||||||||||||
| Micruroides euryxanthus | ||||||||||||||||||||||||||||||||||||
| Leptotyphlopidae | ||||||||||||||||||||||||||||||||||||
| Rena humilis | + | + | + | + | 4 | |||||||||||||||||||||||||||||||
| Viperidae | ||||||||||||||||||||||||||||||||||||
| Crotalus angelensis ** | + | 1 | ||||||||||||||||||||||||||||||||||
| Crotalus atrox | + | 1 | ||||||||||||||||||||||||||||||||||
| Crotalus catalinensis ** | ||||||||||||||||||||||||||||||||||||
| Crotalus cerastes | ||||||||||||||||||||||||||||||||||||
| Crotalus enyo * | + | + | + | + | 4 | |||||||||||||||||||||||||||||||
| Crotalus estebanensis ** | ||||||||||||||||||||||||||||||||||||
| Crotalus lorenzoensis ** | ||||||||||||||||||||||||||||||||||||
| Crotalus mitchellii | + | + | + | 3 | ||||||||||||||||||||||||||||||||
| Crotalus molossus | ||||||||||||||||||||||||||||||||||||
| Crotalus polisi ** | + | 1 | ||||||||||||||||||||||||||||||||||
| Crotalus pyrrhus | + | 1 | ||||||||||||||||||||||||||||||||||
| Crotalus ruber | + | + | + | 3 | ||||||||||||||||||||||||||||||||
| Crotalus thalassoporus ** | ||||||||||||||||||||||||||||||||||||
| Crotalus tigris | ||||||||||||||||||||||||||||||||||||
| Crotalus tortuguensis ** | ||||||||||||||||||||||||||||||||||||
| Testudines | ||||||||||||||||||||||||||||||||||||
| Testudinidae | ||||||||||||||||||||||||||||||||||||
| Gopherus morafkai | + | 1 | ||||||||||||||||||||||||||||||||||
| Totals | 3 | 15 | 5 | 1 | 3 | 3 | 18 | 2 | 1 | 20 | 2 | 1 | 17 | 16 | 5 | 3 | 8 | 3 | 1 | 1 | 1 | 21 | 1 | 3 | 2 | 5 | 1 | 2 | 1 | 1 | 3 | 2 | 1 | 6 | 2 | — |
| (Part 2). | ||||||||||||||||||||||||||||||||||||
| Islands of the Gulf of California | ||||||||||||||||||||||||||||||||||||
| Taxa | Monserrate | Moscas | Pardo | Partida Norte | Partida Sur | Pata | Patos | Piojo | Pond (estanque) | Rasa | Roca Lobos | Salsipuedes | San Cosme | San Damián | San Diego | San Esteban | San Francisco | San Ildefonso | San José | San Lorenzo Norte | San Lorenzo Sur | San Luis | San Marcos | San Pedro Mártir | San Pedro Nolasco | Santa Catalina | Santa Cruz | Santiago | Smith | Tiburón | Tijeras | Tortuga | San Luis Gonzaga | Islas Santa Inez | Islotes Blancos | Total number of islands |
| 36 | 37 | 38 | 39 | 40 | 41 | 42 | 43 | 44 | 45 | 46 | 47 | 48 | 49 | 50 | 51 | 52 | 53 | 54 | 55 | 56 | 57 | 58 | 59 | 60 | 61 | 62 | 63 | 64 | 65 | 66 | 67 | 68 | 69 | 70 | ||
| Amphibia | ||||||||||||||||||||||||||||||||||||
| Anura | ||||||||||||||||||||||||||||||||||||
| Bufonidae | ||||||||||||||||||||||||||||||||||||
| Anaxyrus punctatus | + | + | 4 | |||||||||||||||||||||||||||||||||
| Scaphiopodidae | ||||||||||||||||||||||||||||||||||||
| Scaphiopus couchii | + | + | 4 | |||||||||||||||||||||||||||||||||
| Reptiles | ||||||||||||||||||||||||||||||||||||
| Squamata | ||||||||||||||||||||||||||||||||||||
| Crotaphytidae | ||||||||||||||||||||||||||||||||||||
| Crotaphytus dickersonae | + | 1 | ||||||||||||||||||||||||||||||||||
| Crotaphytus insularis ** | 1 | |||||||||||||||||||||||||||||||||||
| Gambelia wislizenii | + | 1 | ||||||||||||||||||||||||||||||||||
| Eublepharidae | ||||||||||||||||||||||||||||||||||||
| Coleonyx gypsicolus | + | 1 | ||||||||||||||||||||||||||||||||||
| Coleonyx variegatus | + | + | + | + | + | 9 | ||||||||||||||||||||||||||||||
| Iguanidae | ||||||||||||||||||||||||||||||||||||
| Ctenosaura conspicuosa | + | 2 | ||||||||||||||||||||||||||||||||||
| Ctenosaura hemilopha | 1 | |||||||||||||||||||||||||||||||||||
| Ctenosaura nolascensis | + | 1 | ||||||||||||||||||||||||||||||||||
| Dipsosaurus catalinensis | + | 1 | ||||||||||||||||||||||||||||||||||
| Dipsosaurus dorsalis | + | + | + | + | + | + | 12 | |||||||||||||||||||||||||||||
| Sauromalus ater | + | + | + | + | + | + | + | + | + | 13 | ||||||||||||||||||||||||||
| Sauromalus hispidus ** | + | + | + | + | + | + | 12 | |||||||||||||||||||||||||||||
| Sauromalus klauberi | + | 1 | ||||||||||||||||||||||||||||||||||
| Sauromalus slevini | + | 4 | ||||||||||||||||||||||||||||||||||
| Sauromalus spp. | 1 | |||||||||||||||||||||||||||||||||||
| Sauromalus varius | + | + | 2 | |||||||||||||||||||||||||||||||||
| Phrynosomatidae | ||||||||||||||||||||||||||||||||||||
| Callisaurus draconoides | + | + | + | + | + | + | + | + | + | 15 | ||||||||||||||||||||||||||
| Petrosaurus mearnsi | 1 | |||||||||||||||||||||||||||||||||||
| Petrosaurus repens | 1 | |||||||||||||||||||||||||||||||||||
| Petrosaurus slevini ** | 2 | |||||||||||||||||||||||||||||||||||
| Petrosaurus thalassinus | + | 2 | ||||||||||||||||||||||||||||||||||
| Phrynosoma solare | + | 1 | ||||||||||||||||||||||||||||||||||
| Sceloporus angustus | + | + | 2 | |||||||||||||||||||||||||||||||||
| Sceloporus clarkii | + | + | 2 | |||||||||||||||||||||||||||||||||
| Sceloporus grandaevus | 1 | |||||||||||||||||||||||||||||||||||
| Sceloporus hunsakeri | + | 4 | ||||||||||||||||||||||||||||||||||
| Sceloporus lineatulus | + | 1 | ||||||||||||||||||||||||||||||||||
| Sceloporus magister | + | 1 | ||||||||||||||||||||||||||||||||||
| Sceloporus orcutti | + | + | + | + | + | 7 | ||||||||||||||||||||||||||||||
| Sceloporus zosteromus | + | + | + | 6 | ||||||||||||||||||||||||||||||||
| Urosaurus nigricaudus | + | + | + | + | + | + | + | + | 21 | |||||||||||||||||||||||||||
| Urosaurus ornatus | + | 2 | ||||||||||||||||||||||||||||||||||
| Uta encantadae | + | 2 | ||||||||||||||||||||||||||||||||||
| Uta lowei | 1 | |||||||||||||||||||||||||||||||||||
| Uta nolascensis | + | 1 | ||||||||||||||||||||||||||||||||||
| Uta palmeri | + | 1 | ||||||||||||||||||||||||||||||||||
| Uta squamata | + | 1 | ||||||||||||||||||||||||||||||||||
| Uta stansburiana | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | 43 | |||||||||||||
| Uta tumidarostra | 2 | |||||||||||||||||||||||||||||||||||
| Phyllodactylidae | ||||||||||||||||||||||||||||||||||||
| Phyllodactylus angelensis ** | + | + | ? | + | + | 6 | ||||||||||||||||||||||||||||||
| Phyllodactylus apricus ** | ||||||||||||||||||||||||||||||||||||
| Phyllodactylus bugastrolepis | + | 1 | ||||||||||||||||||||||||||||||||||
| Phyllodactylus homolepidurus | + | 2 | ||||||||||||||||||||||||||||||||||
| Phyllodactylus nocticolus | + | + | + | + | + | + | + | + | + | + | + | + | 32 | |||||||||||||||||||||||
| Phyllodactylus partidus ** | + | 2 | ||||||||||||||||||||||||||||||||||
| Phyllodactylus santacruzensis ** | + | |||||||||||||||||||||||||||||||||||
| Phyllodactylus unctus | + | 6 | ||||||||||||||||||||||||||||||||||
| Teiidae | ||||||||||||||||||||||||||||||||||||
| Aspidoscelis bacata | + | 1 | ||||||||||||||||||||||||||||||||||
| Aspidoscelis cana | + | + | + | 3 | ||||||||||||||||||||||||||||||||
| Aspidoscelis carmenensis | 1 | |||||||||||||||||||||||||||||||||||
| Aspidoscelis catalinensis | + | 1 | ||||||||||||||||||||||||||||||||||
| Aspidoscelis celeripes | + | + | 2 | |||||||||||||||||||||||||||||||||
| Aspidoscelis ceralbelsis | 1 | |||||||||||||||||||||||||||||||||||
| Aspidoscelis danheimae | + | 1 | ||||||||||||||||||||||||||||||||||
| Aspidoscelis espiritensis | + | 2 | ||||||||||||||||||||||||||||||||||
| Aspidoscelis franciscensis | + | 1 | ||||||||||||||||||||||||||||||||||
| Aspidoscelis hyperythrus | + | 2 | ||||||||||||||||||||||||||||||||||
| Aspidoscelis martyris | + | 1 | ||||||||||||||||||||||||||||||||||
| Aspidoscelis pictus | + | 1 | ||||||||||||||||||||||||||||||||||
| Aspidoscelis tigris | + | + | + | + | + | + | + | + | 15 | |||||||||||||||||||||||||||
| Serpentes | ||||||||||||||||||||||||||||||||||||
| Charinidae | ||||||||||||||||||||||||||||||||||||
| Lichanura trivirgata | + | + | 8 | |||||||||||||||||||||||||||||||||
| Colubridae | ||||||||||||||||||||||||||||||||||||
| Bogertophis rosaliae | + | 2 | ||||||||||||||||||||||||||||||||||
| Lampropeltis californiae | + | + | + | + | + | + | + | + | + | 11 | ||||||||||||||||||||||||||
| Lampropeltis catalinensis | + | 1 | ||||||||||||||||||||||||||||||||||
| Masticophis barbouri | + | 2 | ||||||||||||||||||||||||||||||||||
| Masticophis bilineatus | + | 1 | ||||||||||||||||||||||||||||||||||
| Masticophis fuliginosus | + | + | + | + | + | + | 12 | |||||||||||||||||||||||||||||
| Masticophis slevini | + | 1 | ||||||||||||||||||||||||||||||||||
| Phyllorhynchus decurtatus | + | + | + | 5 | ||||||||||||||||||||||||||||||||
| Pituophis catenifer | + | 1 | ||||||||||||||||||||||||||||||||||
| Pituophis vertebralis | + | 1 | ||||||||||||||||||||||||||||||||||
| Rhinocheilus etheridgei ** | 1 | |||||||||||||||||||||||||||||||||||
| Salvadora hexalepis, | + | + | 3 | |||||||||||||||||||||||||||||||||
| Sonora savagei * | 1 | |||||||||||||||||||||||||||||||||||
| Sonora semiannulata | + | + | 2 | |||||||||||||||||||||||||||||||||
| Sonora straminea | + | + | + | + | + | 7 | ||||||||||||||||||||||||||||||
| Tantilla planiceps | + | 2 | ||||||||||||||||||||||||||||||||||
| Trimorphodon lyrophanes | + | + | + | 6 | ||||||||||||||||||||||||||||||||
| Dipsadidae | ||||||||||||||||||||||||||||||||||||
| Hypsiglena catalinae | + | 1 | ||||||||||||||||||||||||||||||||||
| Hypsiglena chlorophaea | + | + | 2 | |||||||||||||||||||||||||||||||||
| Hypsiglena ochrorhynchus | + | + | + | + | + | + | + | + | + | + | + | 18 | ||||||||||||||||||||||||
| Hypsiglena slevini | + | 4 | ||||||||||||||||||||||||||||||||||
| Elapidae | ||||||||||||||||||||||||||||||||||||
| Micruroides euryxanthus | + | 1 | ||||||||||||||||||||||||||||||||||
| Leptotyphlopidae | ||||||||||||||||||||||||||||||||||||
| Rena humilis | + | + | + | 7 | ||||||||||||||||||||||||||||||||
| Viperidae | ||||||||||||||||||||||||||||||||||||
| Crotalus angelensis | 1 | |||||||||||||||||||||||||||||||||||
| Crotalus atrox | + | + | + | 4 | ||||||||||||||||||||||||||||||||
| Crotalus catalinensis | + | 1 | ||||||||||||||||||||||||||||||||||
| Crotalus cerastes | + | 1 | ||||||||||||||||||||||||||||||||||
| Crotalus enyo | + | + | + | + | + | 9 | ||||||||||||||||||||||||||||||
| Crotalus estebanensis | + | 1 | ||||||||||||||||||||||||||||||||||
| Crotalus lorenzoensis | + | 1 | ||||||||||||||||||||||||||||||||||
| Crotalus mitchellii | + | + | + | + | 7 | |||||||||||||||||||||||||||||||
| Crotalus molossus | + | 1 | ||||||||||||||||||||||||||||||||||
| Crotalus polisi | 1 | |||||||||||||||||||||||||||||||||||
| Crotalus pyrrhus | + | 2 | ||||||||||||||||||||||||||||||||||
| Crotalus ruber | + | + | + | + | 7 | |||||||||||||||||||||||||||||||
| Crotalus thalassoporus | + | 1 | ||||||||||||||||||||||||||||||||||
| Crotalus tigris | + | 1 | ||||||||||||||||||||||||||||||||||
| Crotalus tortuguensis | + | 1 | ||||||||||||||||||||||||||||||||||
| Testudines | ||||||||||||||||||||||||||||||||||||
| Testudinidae | ||||||||||||||||||||||||||||||||||||
| Gopherus morafkai | + | 2 | ||||||||||||||||||||||||||||||||||
| Totals | 13 | 1 | 4 | 4 | 20 | 1 | 2 | 4 | 5 | 3 | 2 | 6 | 3 | 1 | 3 | 9 | 10 | 4 | 21 | 5 | 7 | 3 | 24 | 4 | 6 | 10 | 7 | 1 | 7 | 28 | 1 | 5 | 2 | 2 | 1 | — |
| Taxa | Islands of Tropical Pacific | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Isabel | La Peña (Coral) | Bahia de Chamela Islands | Islas Marietas Archipielago | Tres Marias Archipielago | Revillagigedo Archipielago | Isla Ixtapa | La Roqueta | Total Number of Islands | |||||||||
| San Pancho | Cocinas | Unspecified island | Isla Larga | Isla Redonda | Unspecified island | San Juanito | María Madre | María Magdalena | Maria Cleofas | Clarion | Socorro | ||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | ||
| Amphibians | |||||||||||||||||
| Anura | |||||||||||||||||
| Bufonidae | |||||||||||||||||
| Incilius mazatlanensis * | + | + | 2 | ||||||||||||||
| Rhinella horribilis | + | 1 | |||||||||||||||
| Eleutherodactylidae | |||||||||||||||||
| Eleutherodactylus pallidus * | + | + | + | 3 | |||||||||||||
| Hylidae | |||||||||||||||||
| Smilisca baudinii | + | + | 2 | ||||||||||||||
| Microhylidae | |||||||||||||||||
| Hypopachus variolosus | + | 1 | |||||||||||||||
| Reptiles | |||||||||||||||||
| Crocodylia | |||||||||||||||||
| Crocodylidae | |||||||||||||||||
| Crocodylus acutus | + | 1 | |||||||||||||||
| Squamata | |||||||||||||||||
| Dactyloidae | |||||||||||||||||
| Norops nebulosus * | + | + | + | + | + | * | + | + | + | + | + | 10 | |||||
| Gekkonidae | |||||||||||||||||
| Gehyra mutilata ‡ | + | 1 | |||||||||||||||
| Hemidactylus frenatus ‡ | + | + | + | + | + | * | + | + | + | 7 | |||||||
| Iguanidae | |||||||||||||||||
| Ctenosaura pectinata | + | + | + | + | + | + | + | + | + | + | + | + | + | 13 | |||
| Iguana iguana | + | + | + | + | + | + | + | 7 | |||||||||
| Mabuyidae | |||||||||||||||||
| Marisora aquilonaria * | + | + | + | 3 | |||||||||||||
| Phrynosomatidae | |||||||||||||||||
| Sceloporus clarkii | + | 1 | |||||||||||||||
| Urosaurus auriculatus ** | + | 1 | |||||||||||||||
| Urosaurus bicarinatus * | + | + | + | + | + | 4 | |||||||||||
| Urosaurus clarionensis ** | + | 1 | |||||||||||||||
| Urosaurus ornatus | + | + | + | 3 | |||||||||||||
| Phyllodactylidae | |||||||||||||||||
| Phyllodactylus isabelae ** | + | + | + | 3 | |||||||||||||
| Phyllodactylus benedetii * | + | + | 2 | ||||||||||||||
| Phyllodactylus lanei * | + | 1 | |||||||||||||||
| Phyllodactylus lupitae ** | + | 1 | |||||||||||||||
| Phyllodactylus tuberculosus | + | 1 | |||||||||||||||
| Phyllodactylus cleofanensis ** | + | + | + | 3 | |||||||||||||
| Teiidae | |||||||||||||||||
| Aspidoscelis communis * | + | + | + | + | 4 | ||||||||||||
| Aspidoscelis costata * | + | 1 | |||||||||||||||
| Aspidoscelis deppii | + | 1 | |||||||||||||||
| Aspidoscelis guttatus * | + | 1 | |||||||||||||||
| Aspidoscelis lineattissima * | + | + | + | + | + | + | + | 7 | |||||||||
| Boidae | |||||||||||||||||
| Boa sigma * | + | + | + | + | + | 5 | |||||||||||
| Colubridae | |||||||||||||||||
| Drymarchon melanurus | + | + | + | 3 | |||||||||||||
| Lampropeltis polyzona * | + | + | * | 2 | |||||||||||||
| Leptophis diplotropis * | + | + | + | 3 | |||||||||||||
| Masticophis anthonyi ** | + | 1 | |||||||||||||||
| Masticophis mentovarius | + | + | + | + | + | + | 6 | ||||||||||
| Mastigodryas melanolomus | + | + | + | 3 | |||||||||||||
| Oxybelis microphtalmus * | + | + | + | + | 4 | ||||||||||||
| Tantilla bocourti * | + | 1 | |||||||||||||||
| Tantilla calamarina * | + | * | 1 | ||||||||||||||
| Dipsadidae | |||||||||||||||||
| Conophis vittatus | + | 1 | |||||||||||||||
| Hypsiglena slevini * | + | 1 | |||||||||||||||
| Hypsiglena torquata | + | + | + | 3 | |||||||||||||
| Hypsiglena unaocularis ** | + | 1 | |||||||||||||||
| Imantodes gemmistratus | + | + | 2 | ||||||||||||||
| Rhadinaea hesperia * | + | 1 | |||||||||||||||
| Geophis annuliferus * | * | ||||||||||||||||
| Sibon nebulatus | + | 1 | |||||||||||||||
| Elapidae | |||||||||||||||||
| Hydrophis platurus | + | + | + | 3 | |||||||||||||
| Leptotyphlopidae | |||||||||||||||||
| Rhena humilis | + | ||||||||||||||||
| Epictia bakewelli | + | 1 | |||||||||||||||
| Typhlopidae | |||||||||||||||||
| Indotyphlops braminus ‡ | + | 1 | |||||||||||||||
| Viperidae | |||||||||||||||||
| Agkistrodon bilineatus | + | + | + | 3 | |||||||||||||
| Testudines | |||||||||||||||||
| Chelonidae | |||||||||||||||||
| Caretta caretta | + | 1 | |||||||||||||||
| Chelonia mydas | + | + | + | + | 4 | ||||||||||||
| Eretmochelys imbricata | + | + | + | + | + | + | 6 | ||||||||||
| Lepidochelys olivacea | + | + | + | + | 4 | ||||||||||||
| Dermochelydae | |||||||||||||||||
| Dermochelys coriacea | + | + | + | 3 | |||||||||||||
| Kinosternidae | |||||||||||||||||
| Kinosternon integrum * | + | 1 | |||||||||||||||
| Totals | 11 | 9 | 6 | 4 | 14 | 6 | 6 | 10 | 7 | 22 | 18 | 14 | 9 | 6 | 7 | 6 | — |
| Taxa | Islands of the Gulf of Mexico | Total Number of Islands | ||||||
|---|---|---|---|---|---|---|---|---|
| Cayo Arcas | Islas de Barrera de Laguna Madre | Islas de Laguna de Términos | Isla Lobos | Sistema Arrecifal Veracruzano | Arrecife Alacranes | |||
| Isla de Sacrificios | Unspecified islands | |||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | ||
| Anura | ||||||||
| Bufonidae | ||||||||
| Incilius valliceps | + | 1 | ||||||
| Rhinella horribilis | + | 1 | ||||||
| Eleutherodactylidae | ||||||||
| Hylidae | ||||||||
| Scinax staufferi | + | 1 | ||||||
| Smilisca baudinii | + | 1 | ||||||
| Leptodactylidae | ||||||||
| Leptodactylus fragilis | + | 1 | ||||||
| Microhylidae | ||||||||
| Hypopachus variolosus | + | 1 | ||||||
| Crocodylia | ||||||||
| Crocodylidae | ||||||||
| Crocodylus moreletii | + | + | 2 | |||||
| Squamata | ||||||||
| Corytophanidae | ||||||||
| Basiliscus vittatus | + | 1 | ||||||
| Dactyloidae | ||||||||
| Norops rodriguezii | + | 1 | ||||||
| Norops sagrei ‡ | + | + | 2 | |||||
| Norops ustus | + | 1 | ||||||
| Norops sp. | + | + | 2 | |||||
| Gekkonidae | ||||||||
| Hemidactylus frenatus ‡ | + | 1 | ||||||
| Hemidactylus turcicus ‡ | + | 1 | ||||||
| Iguanidae | ||||||||
| Ctenosaura similis | + | + | + | 3 | ||||
| Iguana iguana | + | + | + | 3 | ||||
| Mabuyidae | ||||||||
| Marisora lineola | + | + | 2 | |||||
| Phrynosomatidae | ||||||||
| Sceloporus chrysostictus | + | 1 | ||||||
| Sceloporus variabilis | + | 1 | ||||||
| Sphaerodactylidae | ||||||||
| Sphaerodactylus glaucus | + | 1 | ||||||
| Teiidae | ||||||||
| Aspidoscelis cozumelae | + | |||||||
| Aspidoscelis deppii | + | 1 | ||||||
| Aspidoscelis maslini | + | 1 | ||||||
| Holcosus gaigeae * | + | 1 | ||||||
| Boidae | ||||||||
| Boa imperator | + | + | + | 3 | ||||
| Colubridae | ||||||||
| Drymobius margaritiferus | + | 1 | ||||||
| Lampropeltis abnorma | + | 1 | ||||||
| Pseudelaphe flavirufa | + | 1 | ||||||
| Spilotes pullatus | + | 1 | ||||||
| Oxybelis aeneus | + | |||||||
| Dipsadidae | ||||||||
| Coniophanes imperialis | + | 1 | ||||||
| Conophis lineatus | + | 1 | ||||||
| Dipsas brevifacies | + | 1 | ||||||
| Imantodes cenchoa | + | 1 | ||||||
| Leptodeira frenata | + | 1 | ||||||
| Ninia sebae | + | 1 | ||||||
| Sibon nebulatus | + | 1 | ||||||
| Tropidodipsas sartorii | + | 1 | ||||||
| Elapidae | ||||||||
| Micrurus apiatus | + | 1 | ||||||
| Typhlopidae | ||||||||
| Indotypholps braminus | + | |||||||
| Testudines | ||||||||
| Cheloniidae | ||||||||
| Caretta caretta | + | + | + | + | + | + | 6 | |
| Chelonia mydas | + | + | + | + | + | + | 6 | |
| Eretmochelys imbricata | + | + | + | + | 4 | |||
| Lepidochelys kempii | + | + | + | + | + | 5 | ||
| Dermatemydidae | ||||||||
| Dermatemys mawii | + | 1 | ||||||
| Dermochelyidae | ||||||||
| Dermochelys coriacea | + | + | + | + | + | 5 | ||
| Kinosternidae | ||||||||
| Kinosternon scorpioides | + | 1 | ||||||
| Staurotypidae | ||||||||
| Staurotypus triporcatus | + | 1 | ||||||
| Totals | 1 | 4 | 42 | 4 | 9 | 10 | 6 | |
| Taxa | Islands of the Mexican Caribbean | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Isla Contoy | Isla Holbox | Isla Mujeres | Cozumel | Banco Chinchorro Archipielago | Sian Ka’an islands | Tamalcab | Total Number of Islands | |||||
| Cayo Centro | Cayo Norte Mayor | Cayo Norte Menor | Cayo Lobos | Cayo Culebras | Cayo Iguanas | |||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | ||
| Amphibia | ||||||||||||
| Anura | ||||||||||||
| Bufonidae | ||||||||||||
| Incilius valliceps | + | + | + | 3 | ||||||||
| Rhinella horribilis | + | 1 | ||||||||||
| Eleutherodactylidae | ||||||||||||
| Eleutherodactylus planirostris ‡ | + | 1 | ||||||||||
| Hylidae | ||||||||||||
| Dendrosophus microcephalus | + | 1 | ||||||||||
| Scinax staufferi | + | 1 | ||||||||||
| Smilisca baudinii | + | + | 1 | |||||||||
| Trachycephalus vermiculatus | + | + | 1 | |||||||||
| Leptodactylidae | ||||||||||||
| Leptodactylus fragilis | + | 1 | ||||||||||
| Reptiles | ||||||||||||
| Crocodylia | ||||||||||||
| Crocodylidae | ||||||||||||
| Crocodylus acutus | + | + | + | + | + | + | + | 6 | ||||
| Crocodylus moreletti | ? | |||||||||||
| Squamata | ||||||||||||
| Corytophanidae | ||||||||||||
| Basiliscus vittatus | + | + | 2 | |||||||||
| Dactyloidae | ||||||||||||
| Anolis allisoni ‡ | + | + | + | 3 | ||||||||
| Norops lemurinus | + | + | 2 | |||||||||
| Norops rodriguezii | + | + | + | + | 4 | |||||||
| Norops sagrei ‡ | + | + | + | + | + | + | + | + | 8 | |||
| Norops ustus | + | + | + | 2 | ||||||||
| Eublepharidae | ||||||||||||
| Coleonyx elegans | + | 1 | ||||||||||
| Gekkonidae | ||||||||||||
| Hemidactylus frenatus ‡ | + | + | + | + | + | + | 6 | |||||
| Hemidactylus turcicus ‡ | + | 1 | ||||||||||
| Iguanidae | ||||||||||||
| Ctenosaura similis | + | + | + | + | + | + | + | + | 8 | |||
| Iguana iguana | + | + | + | + | 4 | |||||||
| Mabuyidae | ||||||||||||
| Marisora lineola | + | + | + | + | 4 | |||||||
| Phrynosomatidae | ||||||||||||
| Sceloporus chrysostictus | + | + | + | 2 | ||||||||
| Sceloporus cozumelae * | + | + | + | + | 4 | |||||||
| Scincidae | ||||||||||||
| Mesoscincus schwartzei | + | 1 | ||||||||||
| Sphaerodactylidae | ||||||||||||
| Aristelliger georgeensis | + | + | + | + | + | + | 6 | |||||
| Sphaerodactylus continentalis | + | 1 | ||||||||||
| Sphaerodactylus glaucus | + | + | 2 | |||||||||
| Teiidae | ||||||||||||
| Aspidoscelis angusticeps | ? | 1 | ||||||||||
| Aspidoscelis cozumela * | + | 1 | ||||||||||
| Aspidoscelis depii | + | |||||||||||
| Aspidoscelis maslini | + | + | + | 3 | ||||||||
| Aspidoscelis rodecki * | + | + | 2 | |||||||||
| Holcosus gaigeae * | + | 1 | ||||||||||
| Boidae | ||||||||||||
| Boa imperator | + | + | + | + | 4 | |||||||
| Colubridae | ||||||||||||
| Leptophis mexicanus | + | + | + | 2 | ||||||||
| Masticophis mentovarius | + | |||||||||||
| Mastigodryas melanolomus | + | + | 2 | |||||||||
| Oxybelis aeneus | + | + | 2 | |||||||||
| Oxybelis fulgidus | + | + | + | + | 4 | |||||||
| Tantilla moesta | + | 1 | ||||||||||
| Dipsadidae | ||||||||||||
| Coniophanes meridanus * | + | 1 | ||||||||||
| Conophis lineatus | + | + | 2 | |||||||||
| Leptodeira frenata | + | 1 | ||||||||||
| Elapidae | ||||||||||||
| Micrurus apiatus | + | 1 | ||||||||||
| Leptotyphlopidae | ||||||||||||
| Epictia magnamaculata | + | 1 | ||||||||||
| Natricidae | ||||||||||||
| Thamnophis proximus | + | 1 | ||||||||||
| Typhlopidae | ||||||||||||
| Indotyphlops braminus ‡ | + | 1 | ||||||||||
| Viperidae | ||||||||||||
| Agkistrodon russeolus * | + | 1 | ||||||||||
| Testudines | ||||||||||||
| Cheloniidae | ||||||||||||
| Caretta caretta | + | + | + | + | + | + | ? | + | 7 | |||
| Chelonia mydas | + | + | + | + | + | + | ? | + | 7 | |||
| Eretmochelys imbricata | + | + | + | + | + | + | ? | + | 7 | |||
| Lepidochelys kempii | + | |||||||||||
| Dermochelyidae | ||||||||||||
| Dermochelys coriacea | + | + | + | + | + | 5 | ||||||
| Emydidae | ||||||||||||
| Trachemys venusta | + | 1 | ||||||||||
| Geoemydidae | ||||||||||||
| Rhinoclemmys areolata | + | + | + | 2 | ||||||||
| Kinosternidae | ||||||||||||
| Kinosternon scorpioides | + | 1 | ||||||||||
| Totals | 15 | 13 | 23 | 39 | 12 | 12 | 6 | 4 | 1 | 1 | 11 | — |
| Taxa | Distributional Status | Environmental Vulnerability Category (Score) | IUCN Categorization | SEMARNAT Status |
|---|---|---|---|---|
| Anura | ||||
| Bufonidae | ||||
| Anaxyrus punctatus | NE | L (5) | LC | NS |
| Incilius mazatlanensis | CE | M (12) | LC | NS |
| Incilius valliceps | NE | L (6) | LC | NS |
| Rhinella horribilis | NE | L (3) | LC | NS |
| Eleutherodactylidae | ||||
| Eleutherodactylus pallidus | CE | H (17) | DD | Pr |
| Eleutherodactylus planirostris | NN | - | LC | - |
| Hylidae | ||||
| Dendropsophus microcephalus | NE | L (7) | LC | NS |
| Hyliola regilla | NE | L (3) | LC | NS |
| Scinax staufferi | NE | L (4) | LC | NS |
| Smilisca baudinii | NE | L (3) | LC | NS |
| Trachycephalus vermiculatus | NE | L (4) | LC | NS |
| Leptodactylidae | ||||
| Leptodactylus fragilis | NE | L (5) | LC | NS |
| Microhylidae | ||||
| Hypopachus variolosus | NE | L (4) | LC | NS |
| Scaphiopodidae | ||||
| Scaphiopus couchii | NE | L (3) | LC | NS |
| Caudata | ||||
| Plethodontidae | ||||
| Aneides lugubris | NE | H (14) | LC | Pr |
| Batrachoseps major | NE | H (14) | LC | NS |
| Reptiles | ||||
| Crocodylia | ||||
| Crocodylidae | ||||
| Crocodylus acutus | NE | H (14) | VU | Pr |
| Crocodylus moreletii | NE | M (13) | LC | Pr |
| Squamata | ||||
| Anguidae | ||||
| Elgaria cedrosensis | CE | H (16) | NE | NS |
| Elgaria multicarinata | NE | M (10) | LC | Pr |
| Elgaria nana | IE | H (16) | LC | Pr |
| Anniellidae | ||||
| Anniella geronimensis | CE | M (13) | EN | Pr |
| Anniella pulchra | NE | M (12) | LC | Pr |
| Bipedidae | ||||
| Bipes biporus | CE | H (14) | LC | Pr |
| Corytophanidae | ||||
| Basiliscus vittatus | NE | L (7) | LC | NS |
| Crotaphytidae | ||||
| Crotaphytus dickersonae | IE | H (16) | LC | NS |
| Crotaphytus insularis | IE | H (16) | LC | NS |
| Gambelia copeii | NE | M (11) | LC | NS |
| Gambelia wislizenii | NE | M (13) | LC | Pr |
| Dactyloidae | ||||
| Anolis allisoni | NN | - | NE | - |
| Norops lemurinus | NE | L (8) | NE | NS |
| Norops nebulosus | CE | M (13) | LC | NS |
| Norops rodriguezii | NE | M (10) | NE | NS |
| Norops sagrei | NN | - | - | - |
| Norops ustus | NE | L (8) | NE | NS |
| Eublepharidae | ||||
| Coleonyx elegans | NE | L (9) | LC | A |
| Coleonyx gypsicolus | IE | H (18) | LC | NS |
| Coleonyx variegatus | NE | M (11) | LC | Pr |
| Gekkonidae | ||||
| Gehyra mutilata | NN | - | - | - |
| Hemidactylus frenatus | NN | - | - | - |
| Hemidactylus turcicus | NN | - | - | - |
| Iguanidae | ||||
| Ctenosaura conspicuosa | IE | H (16) | NE | Ns |
| Ctenosaura hemilopha | CE | H (18) | NE | Pr |
| Ctenosaura nolascensis | IE | H (17) | VU | Ns |
| Ctenosaura pectinata | NE | H (15) | NE | A |
| Ctenosaura similis | NE | L (8) | LC | A |
| Dipsosaurus catalinensis | IE | H (17) | NE | Ns |
| Dipsosaurus dorsalis | NE | M (11) | LC | Ns |
| Iguana iguana | NE | M (12) | LC | Pr |
| Sauromalus ater | NE | M (13) | LC | Pr |
| Sauromalus hispidus | CE | H (14) | NT | A |
| Sauromalus klauberi | IE | H (16) | NE | A |
| Sauromalus slevini | CE | H (16) | NE | A |
| Sauromalus varius | CE | H (16) | NE | A |
| Mabuyidae | ||||
| Marisora aquilonaria | CE | M (13) | NE | Ns |
| Marisora lineola | NE | M (10) | LC | Ns |
| Phrynosomatidae | ||||
| Callisaurus draconoides | NE | M (12) | LC | A |
| Petrosaurus mearnsi | NE | M (12) | LC | Pr |
| Petrosaurus repens | CE | M (13) | LC | Ns |
| Petrosaurus slevini | IE | H (16) | LC | Ns |
| Petrosaurus thalassinus | CE | M (13) | LC | Pr |
| Phrynosoma cerroense | CE | H (16) | NE | A |
| Phrynosoma solare | NE | H (14) | LC | NS |
| Sceloporus angustus | IE | H (16) | LC | A |
| Sceloporus chrysostictus | NE | M (13) | LC | NS |
| Sceloporus clarkii | NE | M (10) | LC | NS |
| Sceloporus cozumelae | CE | H (15) | LC | Pr |
| Sceloporus grandaevus | IE | H (16) | LC | A |
| Sceloporus hunsakeri | CE | H (14) | LC | Pr |
| Sceloporus lineatulus | IE | H (17) | LC | A |
| Sceloporus magister | NE | L (9) | LC | NS |
| Sceloporus occidentalis | NE | M (12) | LC | NS |
| Sceloporus orcutti | NE | L (7) | LC | NS |
| Sceloporus variabilis | NE | L (5) | LC | NS |
| Sceloporus zosteromus | CE | M (12) | LC | Pr |
| Urosaurus auriculatus | IE | H (16) | EN | Ns |
| Urosaurus bicarinatus | CE | M (12) | LC | NS |
| Urosaurus clarionensis | IE | H (17) | VU | NS |
| Urosaurus nigricaudus | CE | L (8) | LC | A |
| Urosaurus ornatus | NE | M (10) | LC | NS |
| Uta encantadae | IE | H (17) | VU | NS |
| Uta lowei | IE | H (17) | VU | NS |
| Uta nolascensis | IE | H (17) | LC | A |
| Uta palmeri | IE | H (17) | VU | A |
| Uta squamata | NE | H (17) | LC | A |
| Uta stansburiana | NE | L (7) | LC | A |
| Uta tumidarostra | IE | H (17) | VU | NS |
| Phyllodactylidae | ||||
| Phyllodactylus angelensis | IE | H (16) | NE | NS |
| Phyllodactylus apricus | IE | H (17) | NE | NS |
| Phyllodactylus benedetii | CE | H (15) | NE | NS |
| Phyllodactylus bugastrolepis | IE | H (17) | LC | A |
| Phyllodactylus cleofasensis | IE | H (16) | NE | NS |
| Phyllodactylus coronatus | IE | H (16) | NE | NS |
| Phyllodactylus homolepidurus | CE | H (15) | LC | Pr |
| Phyllodactylus isabelae | IE | H (16) | NE | NS |
| Phyllodactylus lanei | CE | H (15) | LC | NS |
| Phyllodactylus lupitae | IE | H (16) | LC | NS |
| Phyllodactylus nocticolus | NE | M (10) | NE | NS |
| Phyllodactylus partidus | IE | H (16) | LC | Pr |
| Phyllodactylus santacruzensis | IE | H (17) | NE | NS |
| Phyllodactylus tuberculosus | NE | L (8) | NE | NS |
| Phyllodactylus unctus | CE | H (15) | LC | Pr |
| Scincidae | ||||
| Mesoscincus schwartzei | NE | M (11) | LC | NS |
| Plestiodon skiltonianus | NE | M (11) | LC | NS |
| Sphaerodactylidae | ||||
| Aristelliger georgeensis | NE | M (13) | LC | Pr |
| Sphaerodactylus continentalis | NE | M (10) | NE | NS |
| Sphaerodactylus glaucus | NE | M (12) | LC | Pr |
| Teiidae | ||||
| Aspidoscelis bacata | IE | H (17) | LC | Pr |
| Aspidoscelis cana | IE | H (16) | LC | A |
| Aspidoscelis carmenensis | IE | H (17) | LC | NS |
| Aspidoscelis catalinensis | IE | H (17) | VU | Pr |
| Aspidoscelis celeripes | IE | H (15) | LC | Pr |
| Aspidoscelis ceralbensis | IE | H (17) | LC | Pr |
| Aspidoscelis communis | CE | H (14) | LC | Pr |
| Aspidoscelis costata | CE | M (11) | LC | NS |
| Aspidoscelis cozumela | CE | H (16) | LC | NS |
| Aspidoscelis danheimae | IE | H (16) | LC | A |
| Aspidoscelis deppii | NE | L (8) | LC | NS |
| Aspidoscelis espiritensis | IE | H (16) | LC | A |
| Aspidoscelis franciscensis | IE | H (17) | LC | NS |
| Aspidoscelis guttatus | CE | M (12) | LC | NS |
| Aspidoscelis hyperythrus | NE | M (10) | LC | A |
| Aspidoscelis lineatissima | CE | H (14) | LC | Pr |
| Aspidoscelis martyris | IE | H (17) | VU | Pr |
| Aspidoscelis maslini | NE | H (15) | LC | NS |
| Aspidoscelis pictus | IE | H (17) | LC | NS |
| Aspidoscelis rodecki | CE | H (16) | NT | P |
| Aspidoscelis tigris | NE | L (8) | LC | NS |
| Holcosus gaigeae | CE | H (15) | NE | NS |
| Boidae | ||||
| Boa imperator | NE | M (10) | NE | A |
| Boa sigma | CE | H (15) | NE | A |
| Charinidae | ||||
| Lichanura trivirgata | NE | M (10) | LC | A |
| Colubridae | ||||
| Bogertophis rosaliae | NE | M (10) | LC | NS |
| Drymarchon melanurus | NE | L (6) | LC | NS |
| Drymobius margaritiferus | NE | L (6) | LC | NS |
| Lampropeltis abnorma | NE | L (9) | NE | A |
| Lampropeltis californiae | NE | M (10) | NE | A |
| Lampropeltis catalinensis | IE | H (17) | DD | NS |
| Lampropeltis herrerae | IE | H (17) | CR | A |
| Lampropeltis polyzona | CE | M (11) | NE | A |
| Leptophis diplotropis | CE | H (14) | LC | A |
| Leptophis mexicanus | NE | L (6) | LC | A |
| Masticophis anthonyi | IE | H (17) | CR | A |
| Masticophis barbouri | IE | H (17) | DD | A |
| Masticophis bilineatus | NE | M (11) | LC | NS |
| Masticophis fuliginosus | NE | L (9) | NE | NS |
| Masticophis mentovarius | NE | L (6) | LC | NS |
| Masticophis slevini | IE | H (17) | LC | NS |
| Mastigodryas melanolomus | NE | L (6) | LC | NS |
| Oxybelis aeneus | NE | L (5) | NE | NS |
| Oxybelis fulgidus | NE | L (9) | NE | NS |
| Oxybelis microphtalmus | CE | M (11) | NE | NS |
| Phyllorhynchus decurtatus | NE | M (11) | LC | NS |
| Pituophis catenifer | NE | L (9) | LC | NS |
| Pituophis insulanus | IE | H (16) | LC | NS |
| Pituophis vertebralis | CE | M (12) | LC | NS |
| Pseudelaphe flavirufa | NE | M (10) | LC | NS |
| Rhinocheilus etheridgei | IE | H (16) | DD | NS |
| Salvadora hexalepis | NE | M (10) | LC | NS |
| Sonora savagei | CE | H (15) | LC | Pr |
| Sonora semiannulata | NE | L (5) | LC | NS |
| Sonora straminea | NE | L (8) | LC | Pr |
| Spilotes pullatus | NE | L (6) | NE | NS |
| Tantilla bocourti | CE | L (9) | LC | NS |
| Tantilla calamarina | CE | M (12) | LC | Pr |
| Tantilla moesta | NE | M (13) | LC | NS |
| Tantilla planiceps | NE | L (9) | LC | NS |
| Trimorphodon lyrophanes | NE | M (10) | NE | NS |
| Dipsadidae | ||||
| Coniophanes imperialis | NE | L (8) | LC | NS |
| Conophis lineatus | NE | L (9) | LC | NS |
| Conophis vittatus | NE | M (11) | LC | NS |
| Diadophis punctatus | NE | L (4) | LC | NS |
| Dipsas brevifacies | NE | H (15) | LC | Pr |
| Geophis annuliferus | CE | M (13) | LC | Pr |
| Hypsiglena catalinae | IE | H (16) | NE | NS |
| Hypsiglena chlorophaea | NE | L (8) | NE | NS |
| Hypsiglena ochrorhyncus | NE | L (8) | NE | NS |
| Hypsiglena slevini | CE | M (11) | LC | A |
| Hypsiglena torquata | NE | L (8) | LC | Pr |
| Hypsiglena unaocularis | IE | H (16) | NE | NS |
| Imantodes cenchoa | NE | L (6) | NE | Pr |
| Imantodes gemmistratus | NE | L (6) | LC | Pr |
| Leptodeira frenata | NE | M (13) | LC | NS |
| Ninia sebae | NE | L (5) | LC | NS |
| Rhadinaea hesperia | CE | M (10) | LC | Pr |
| Sibon nebulatus | NE | L (5) | NE | NS |
| Tropidodipsas sartorii | NE | L (9) | LC | Pr |
| Elapidae | ||||
| Hydrophis platurus | NE | - | LC | NS |
| Micruroides euryxanthus | NE | H (15) | LC | A |
| Micrurus apiatus | NE | L(8) | NE | NS |
| Leptotyphlopidae | ||||
| Epictia bakewelli | CE | M (12) | NE | NS |
| Epictia magnamaculata | NE | M (11) | NE | NS |
| Rena humilis | NE | L (8) | NE | NS |
| Natricidae | ||||
| Thamnophis proximus | NE | L (7) | LC | A |
| Typhlopidae | ||||
| Indotyphlops braminus | NN | - | NE | - |
| Viperidae | ||||
| Agkistrodon bilineatus | NE | M (11) | NT | Pr |
| Agkistrodon russeolus | CE | H (15) | NE | NS |
| Crotalus angelensis | IE | H (18) | LC | NS |
| Crotalus atrox | NE | L (9) | LC | Pr |
| Crotalus caliginis | IE | H (18) | LC | NS |
| Crotalus catalinensis | IE | H (19) | CR | A |
| Crotalus cerastes | NE | H (16) | LC | Pr |
| Crotalus enyo | CE | M (13) | LC | A |
| Crotalus estebanensis | IE | H (19) | LC | NS |
| Crotalus lorenzoensis | IE | H (19) | LC | NS |
| Crotalus mitchellii | NE | M (12) | LC | Pr |
| Crotalus molossus | NE | L (8) | LC | Pr |
| Crotalus polisi | IE | H (18) | NE | NS |
| Crotalus pyrrhus | NE | M (12) | NE | NS |
| Crotalus ruber | NE | L (9) | LC | Pr |
| Crotalus thalassoporus | IE | H (18) | NE | NS |
| Crotalus tigris | NE | H (16) | LC | Pr |
| Crotalus tortuguensis | IE | H (18) | NE | Pr |
| Testudines | ||||
| Cheloniidae | ||||
| Caretta caretta | NE | - | VU | P |
| Chelonia mydas | NE | - | EN | P |
| Eretmochelys imbricata | NE | - | CR | P |
| Lepidochelys kempii | NE | - | CR | P |
| Lepidochelys olivacea | NE | - | VU | P |
| Dermatemydidae | ||||
| Dermatemys mawii | NE | H (17) | CR | P |
| Dermochelyidae | ||||
| Dermochelys coriacea | NE | - | VU | P |
| Emydidae | ||||
| Trachemys venusta | NE | H (19) | NE | NS |
| Geoemydidae | ||||
| Rhinoclemmys areolata | NE | M (13) | NT | A |
| Kinosternidae | ||||
| Kinosternon integrum | CE | M (11) | LC | Pr |
| Kinosternon scorpioides | NE | M (10) | NT | Pr |
| Staurotypidae | ||||
| Staurotypus triporcatus | NE | H (14) | NT | A |
| Testudinidae | ||||
| Geophis morafkai | NE | H (15) | NE | A |
| Families | Number of Species | Distributional Status | |||
|---|---|---|---|---|---|
| Non-endemic (NE) | Country Endemic (CE) | Insular Endemic (IE) | Non-Native (NN) | ||
| Bufonidae | 4 | 3 | 1 | — | — |
| Eleutherodactylidae | 2 | — | 1 | — | 1 |
| Hylidae | 5 | 5 | — | — | — |
| Leptodactylidae | 1 | 1 | — | — | — |
| Microhylidae | 1 | 1 | — | — | — |
| Scaphiopodidae | 1 | 1 | — | — | — |
| Subtotals | 14 | 11 | 2 | — | 1 |
| Plethodontidae | 2 | 2 | — | — | — |
| Subtotals | 2 | 2 | — | — | — |
| Totals | 16 | 13 | 2 | — | 1 |
| Crocodylidae | 2 | 2 | — | — | — |
| Subtotals | 2 | 2 | — | — | — |
| Anguidae | 3 | 1 | 1 | 1 | — |
| Anniellidae | 2 | 1 | 1 | — | — |
| Bipedidae | 1 | — | 1 | — | — |
| Corytophanidae | 1 | 1 | — | — | — |
| Crotaphytidae | 4 | 2 | — | 2 | — |
| Dactyloidae | 6 | 3 | 1 | — | 2 |
| Eublepharidae | 3 | 2 | — | 1 | — |
| Gekkonidae | 3 | — | — | — | 3 |
| Iguanidae | 13 | 5 | 4 | 4 | — |
| Mabuyidae | 2 | 1 | 1 | — | — |
| Phrynosomatidae | 31 | 12 | 8 | 11 | — |
| Phyllodactylidae | 15 | 2 | 4 | 9 | — |
| Scincidae | 2 | 2 | — | — | — |
| Sphaerodactylidae | 3 | 3 | — | — | — |
| Teiidae | 22 | 4 | 7 | 11 | — |
| Subtotals | 111 | 39 | 28 | 39 | 5 |
| Boidae | 2 | 1 | 1 | — | — |
| Charinidae | 1 | 1 | — | — | — |
| Colubridae | 36 | 22 | 7 | 7 | — |
| Dipsadidae | 19 | 14 | 3 | 2 | — |
| Elapidae | 3 | 3 | — | — | — |
| Leptotyphlopidae | 3 | 2 | 1 | — | — |
| Natricidae | 1 | 1 | — | — | — |
| Typhlopidae | 1 | — | — | — | 1 |
| Viperidae | 18 | 8 | 2 | 8 | — |
| Subtotals | 84 | 52 | 14 | 17 | 1 |
| Cheloniidae | 5 | 5 | — | — | — |
| Dermatemydidae | 1 | 1 | — | — | — |
| Dermochelyidae | 1 | 1 | — | — | — |
| Emydidae | 1 | 1 | — | — | — |
| Geoemydidae | 1 | 1 | — | — | — |
| Kinosternidae | 2 | 1 | 1 | — | — |
| Staurotypidae | 1 | 1 | — | — | — |
| Testudinidae | 1 | 1 | — | — | — |
| Subtotals | 13 | 12 | 1 | — | — |
| Totals | 210 | 105 | 43 | 56 | 6 |
| Sum Totals | 226 | 118 | 45 | 56 | 7 |
| Distributional | Physiographic Regions | ||||
|---|---|---|---|---|---|
| Categories | Gulf of California | Pacific of Baja California | Tropical Pacific | Gulf of Mexico | Mexican Caribbean |
| Non-endemic | 49 | 27 | 25 | 39 | 41 |
| Country endemic | 15 | 9 | 21 | 3 | 6 |
| Insuar endemic | 44 | 4 | 8 | — | — |
| Non-native | — | — | 3 | 4 | 6 |
| Totals | 108 | 40 | 57 | 46 | 53 |
| Families | Number of Species | SEMARNAT Categorizations | |||
|---|---|---|---|---|---|
| Endangered (P) | Threatened (A) | Special Protection (Pr) | No Status (NS) | ||
| Bufonidae | 4 | — | — | — | 4 |
| Eleutherodactylidae | 1 | — | — | 1 | — |
| Hylidae | 5 | — | — | — | 5 |
| Leptodactylidae | 1 | — | — | — | 1 |
| Microhylidae | 1 | — | — | — | 1 |
| Scaphiopodidae | 1 | — | — | — | 1 |
| Subtotals | 13 | — | — | 1 | 12 |
| Plethodontidae | 2 | — | — | 1 | 1 |
| Subtotals | 2 | — | — | 1 | 1 |
| Totals | 15 | — | — | 2 | 13 |
| Crocodylidae | 2 | — | — | 2 | — |
| Subtotals | 2 | — | — | 2 | — |
| Anguidae | 3 | — | — | 2 | 1 |
| Anniellidae | 2 | — | — | 2 | — |
| Bipedidae | 1 | — | — | 1 | — |
| Corytophanidae | 1 | — | — | — | 1 |
| Crotaphytidae | 4 | — | — | 1 | 3 |
| Dactyloidae | 4 | — | — | — | 4 |
| Eublepharidae | 3 | — | 1 | 1 | 1 |
| Iguanidae | 13 | — | 6 | 3 | 4 |
| Mabuyidae | 2 | — | — | — | 2 |
| Phrynosomatidae | 31 | — | 10 | 5 | 16 |
| Phyllodactylidae | 15 | — | 1 | 3 | 11 |
| Scincidae | 2 | — | — | — | 2 |
| Sphaerodactylidae | 3 | — | — | 2 | 1 |
| Teiidae | 22 | 1 | 4 | 7 | 10 |
| Subtotals | 106 | 1 | 22 | 27 | 56 |
| Boidae | 2 | — | 2 | — | — |
| Colubridae | 36 | — | 8 | 3 | 25 |
| Dipsadidae | 19 | — | 1 | 7 | 11 |
| Elapidae | 3 | — | 1 | — | 2 |
| Leptotyphlopidae | 3 | — | — | — | 3 |
| Natricidae | 1 | — | 1 | — | — |
| Viperidae | 18 | — | 2 | 8 | 8 |
| Subtotals | 82 | — | 15 | 18 | 49 |
| Cheloniidae | 5 | 5 | — | — | — |
| Dermatemydidae | 1 | 1 | — | — | — |
| Dermochelyidae | 1 | 1 | — | — | — |
| Emydidae | 1 | — | — | — | 1 |
| Geoemydidae | 1 | — | 1 | — | — |
| Kinosternidae | 2 | — | — | 2 | — |
| Staurotypidae | 1 | — | 1 | — | — |
| Testudinidae | 1 | — | 1 | — | — |
| Subtotals | 13 | 7 | 3 | 2 | 1 |
| Totals | 203 | 8 | 40 | 49 | 106 |
| Sum Totals | 218 | 8 | 40 | 51 | 119 |
| Families | Number of Species | IUCN Red List Categorizations | |||||
|---|---|---|---|---|---|---|---|
| Critically Endangered | Endangered | Vulnerable | Near Threatened | Least Concern | Not Evaluated/DD | ||
| Bufonidae | 4 | — | — | — | — | 4 | — |
| Eleutherodactylidae | 1 | — | — | — | — | — | 1 |
| Hylidae | 5 | — | — | — | — | 5 | — |
| Leptodactylidae | 1 | — | — | — | — | 1 | — |
| Microhylidae | 1 | — | — | — | — | 1 | — |
| Scaphipodidae | 1 | — | — | — | — | 1 | — |
| Subtotals | 13 | — | — | — | — | 12 | 1 |
| Plethodontidae | 2 | — | — | — | — | 2 | — |
| Subtotals | 2 | — | — | — | — | 2 | — |
| Totals | 15 | — | — | — | — | 14 | 1 |
| Crocodylidae | 2 | — | — | 1 | — | 1 | — |
| Subtotals | 2 | — | — | 1 | — | 1 | — |
| Anguidae | 3 | — | — | — | — | 2 | 1 |
| Anniellidae | 2 | — | 1 | — | — | 1 | — |
| Bipedidae | 1 | — | — | — | — | 1 | — |
| Corytophanidae | 1 | — | — | — | — | 1 | — |
| Crotaphytidae | 4 | — | — | — | — | 4 | — |
| Dactyloidae | 4 | — | — | — | — | 1 | 3 |
| Eublepharidae | 3 | — | — | — | — | 3 | — |
| Iguanidae | 13 | — | — | 1 | 1 | 4 | 7 |
| Mabuyidae | 2 | — | — | — | — | 1 | 1 |
| Phrynosomatidae | 31 | — | 1 | 5 | — | 24 | 1 |
| Phyllodactylidae | 15 | — | — | — | — | 6 | 9 |
| Scincidae | 2 | — | — | — | — | 2 | — |
| Sphaerodactylidae | 3 | — | — | — | — | 2 | 1 |
| Teiidae | 22 | — | — | 2 | 1 | 18 | 1 |
| Subtotals | 106 | — | 2 | 8 | 2 | 70 | 24 |
| Boidae | 2 | — | — | — | — | — | 2 |
| Colubridae | 36 | 2 | — | — | — | 22 | 12 |
| Dipsadidae | 19 | — | — | — | — | 13 | 6 |
| Elapidae | 3 | — | — | — | — | 2 | 1 |
| Leptotyphlopidae | 3 | — | — | — | — | — | 3 |
| Natricidae | 1 | — | — | — | — | 1 | — |
| Viperidae | 18 | 1 | — | — | 1 | 11 | 5 |
| Subtotals | 82 | 3 | — | — | 1 | 49 | 29 |
| Cheloniidae | 5 | 2 | 1 | 2 | — | — | — |
| Dermatemydidae | 1 | 1 | — | — | — | — | — |
| Dermochelyidae | 1 | — | — | 1 | — | — | — |
| Emydidae | 1 | — | — | — | — | — | 1 |
| Geoemydidae | 1 | — | — | — | 1 | — | — |
| Kinosternidae | 2 | — | — | — | 1 | 1 | — |
| Staurotypidae | 1 | — | — | — | 1 | — | — |
| Testudinidae | 1 | — | — | — | — | — | 1 |
| Subtotals | 13 | 3 | 1 | 3 | 3 | 1 | 2 |
| Totals | 202 | 6 | 3 | 12 | 6 | 121 | 55 |
| Sum Totals | 218 | 6 | 3 | 12 | 6 | 135 | 56 |
| Category Totals | 218 | 21 | 141 | 56 | |||
| Families | Number of Species | Environmental Vulnerability Scores | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | ||
| Bufonidae | 4 | 1 | — | 1 | 1 | — | — | — | — | — | 1 | — | — | — | — | — | — | — |
| Eleutherodactylidae | 1 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | 1 | — | — |
| Hylidae | 5 | 2 | 2 | — | — | 1 | — | — | — | — | — | — | — | — | — | — | — | — |
| Leptodactylidae | 1 | — | — | 1 | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
| Microhylidae | 1 | — | 1 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
| Scaphiopodidae | 1 | 1 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
| Subtotals | 13 | 4 | 3 | 2 | 1 | 1 | — | — | — | — | 1 | — | — | — | — | 1 | — | — |
| Plethodontidae | 2 | — | — | — | — | — | — | — | — | — | — | — | 2 | — | — | — | — | — |
| Subtotals | 2 | — | — | — | — | — | — | — | — | — | — | — | 2 | — | — | — | — | — |
| Totals | 15 | 4 | 3 | 2 | 1 | 1 | — | — | — | — | 1 | — | 2 | — | — | 1 | — | — |
| Crocodylidae | 2 | — | — | — | — | — | — | — | — | — | — | 1 | 1 | — | — | — | — | — |
| Subtotals | 2 | — | — | — | — | — | — | — | — | — | — | 1 | 1 | — | — | — | — | — |
| Anguidae | 3 | — | — | — | — | — | — | — | 1 | — | — | — | — | — | 2 | — | — | — |
| Anniellidae | 2 | — | — | — | — | — | — | — | — | — | 1 | 1 | — | — | — | — | — | — |
| Bipedidae | 1 | — | — | — | — | — | — | — | — | — | — | — | 1 | — | — | — | — | — |
| Corytophanidae | 1 | — | — | — | — | 1 | — | — | — | — | — | — | — | — | — | — | — | — |
| Crotaphytidae | 4 | — | — | — | — | — | — | — | — | 1 | — | 1 | — | — | 2 | — | — | — |
| Dactyloidae | 4 | — | — | — | — | — | 2 | — | 1 | — | — | 1 | — | — | — | — | — | — |
| Eublepharidae | 3 | — | — | — | — | — | — | 1 | — | 1 | — | — | — | — | — | — | 1 | — |
| Iguanidae | 13 | — | — | — | — | — | 1 | — | — | 1 | 1 | 1 | 1 | 1 | 4 | 2 | 1 | — |
| Mabuyidae | 2 | — | — | — | — | — | — | — | 1 | — | — | 1 | — | — | — | — | — | — |
| Phrynosomatidae | 31 | — | — | 1 | — | 2 | 1 | 1 | 2 | — | 5 | 3 | 2 | 1 | 5 | 8 | — | — |
| Phyllodactylidae | 15 | — | — | — | — | — | 1 | — | 1 | — | — | — | — | 4 | 6 | 3 | — | — |
| Scincidae | 2 | — | — | — | — | — | — | — | — | 2 | — | — | — | — | — | — | — | — |
| Sphaerodactylidae | 3 | — | — | — | — | — | — | — | 1 | — | 1 | 1 | — | — | — | — | — | — |
| Teiidae | 22 | — | — | — | — | — | 2 | — | 1 | 1 | 1 | — | 2 | 3 | 5 | 7 | — | — |
| Subtotals | 106 | — | — | 1 | — | 3 | 7 | 2 | 8 | 6 | 9 | 9 | 6 | 9 | 24 | 20 | 2 | — |
| Boidae | 3 | — | — | — | — | — | — | — | 2 | — | — | — | — | 1 | — | — | — | — |
| Colubridae | 36 | — | — | 2 | 6 | — | 1 | 6 | 5 | 4 | 2 | 1 | 1 | 1 | 2 | 5 | — | — |
| Dipsadidae | 19 | — | 1 | 2 | 2 | — | 4 | 2 | 1 | 2 | — | 2 | — | 1 | 2 | — | — | — |
| Elapidae | 2 | — | — | — | — | — | 1 | — | — | — | — | — | — | 1 | — | — | — | — |
| Leptotyphlopidae | 3 | — | — | — | — | — | 1 | — | — | 1 | 1 | — | — | — | — | — | — | — |
| Natricidae | 1 | — | — | — | — | 1 | — | — | — | — | — | — | — | — | — | — | — | — |
| Viperidae | 18 | — | — | — | — | — | 1 | 2 | — | 1 | 2 | 1 | — | 1 | 2 | — | 5 | 3 |
| Subtotals | 82 | — | 1 | 4 | 8 | 1 | 8 | 10 | 8 | 8 | 5 | 4 | 1 | 5 | 6 | 5 | 5 | 3 |
| Dermatemydidae | 1 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | 1 | — | — |
| Emydidae | 1 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | 1 |
| Geoemydidae | 1 | — | — | — | — | — | — | — | — | — | — | 1 | — | — | — | — | — | — |
| Kinosternidae | 2 | — | — | — | — | — | — | — | 1 | 1 | — | — | — | — | — | — | — | — |
| Staurotypidae | 1 | — | — | — | — | — | — | — | — | — | — | — | 1 | — | — | — | — | — |
| Testudinidae | 1 | — | — | — | — | — | — | — | — | — | — | — | — | 1 | — | — | — | — |
| Subtotals | 7 | — | — | — | — | — | — | — | 1 | 1 | — | 1 | 1 | 1 | — | 1 | — | 1 |
| Totals | 197 | 0 | 1 | 5 | 8 | 4 | 15 | 12 | 17 | 15 | 14 | 15 | 9 | 15 | 30 | 26 | 7 | 4 |
| Sum Totals | 212 | 56 | 62 | 94 | ||||||||||||||
| EVS | IUCN Categories | |||||||
|---|---|---|---|---|---|---|---|---|
| Critically Endangered | Endangered | Vulnerable | Near Threatened | Least Concern | Not Evaluated | Data Deficient | Totals | |
| 3 | — | — | — | — | 4 | — | — | 4 |
| 4 | — | — | — | — | 4 | — | — | 4 |
| 5 | — | — | — | — | 5 | 2 | — | 7 |
| 6 | — | — | — | — | 7 | 2 | — | 9 |
| 7 | — | — | — | — | 5 | — | — | 5 |
| 8 | — | — | — | — | 8 | 7 | — | 15 |
| 9 | — | — | — | — | 9 | 3 | — | 12 |
| 10 | — | — | — | 1 | 10 | 6 | — | 17 |
| 11 | — | — | — | 1 | 11 | 3 | — | 15 |
| 12 | — | — | — | — | 13 | 2 | — | 15 |
| 13 | — | 1 | — | 1 | 12 | 1 | — | 15 |
| 14 | — | — | 1 | 2 | 8 | — | — | 11 |
| 15 | — | — | — | — | 9 | 6 | — | 15 |
| 16 | — | 1 | — | 1 | 15 | 12 | 1 | 30 |
| 17 | 3 | — | 8 | — | 10 | 3 | 3 | 27 |
| 18 | — | — | — | — | 3 | 4 | — | 7 |
| 19 | 1 | — | — | — | 2 | 1 | — | 4 |
| Totals | 4 | 2 | 9 | 6 | 135 | 52 | 4 | 212 |
| Environmental Vulnerability Score (EVS) | ||||
|---|---|---|---|---|
| Taxa | Geographic Distribution | Ecological Distribution | Reproductive Mode/Degree of Persecution | Total Score |
| Eleutherodactylus pallidus | 5 | 8 | 4 | H (17) |
| Lampropeltis catalinensis | 6 | 8 | 3 | H (17) |
| Masticophis barbouri | 6 | 8 | 3 | H (17) |
| Rhinocheilus etheridgei | 6 | 7 | 3 | H (16) |
| Environmental Vulnerability Score (EVS) | ||||
|---|---|---|---|---|
| Taxa | Geographic Distribution | Ecological Distribution | Reproductive Mode/Degree of Persecution | Total Score |
| Elgaria cedrosensis | 5 | 8 | 3 | H (16) |
| Norops lemurinus | 3 | 2 | 3 | L (8) |
| Norops rodriguezii | 4 | 3 | 3 | M (10) |
| Norops ustus | 4 | 1 | 3 | L (8) |
| Ctenosaura conspicuosa | 5 | 8 | 3 | H (16) |
| Ctenosaura hemilopha | 5 | 7 | 6 | H (18) |
| Ctenosaura pectinate | 5 | 4 | 6 | H (15) |
| Dipsosaurus catalinensis | 6 | 8 | 3 | H (17) |
| Sauromalus klauberi | 6 | 7 | 3 | H (16) |
| Sauromalus slevini | 5 | 8 | 3 | H (16) |
| Sauromalus varius | 5 | 8 | 3 | H (16) |
| Marisora aquilonaria * | 5 | 3 | 3 | M (13) |
| Phrynosoma cerroense | 6 | 7 | 3 | H (16) |
| Phyllodactylus angelensis ** | 6 | 7 | 3 | H (16) |
| Phyllodactylus apricus ** | 7 | 7 | 3 | H (17) |
| Phyllodactylus benedetii * | 5 | 7 | 3 | H (15) |
| Phyllodactylus cleofasensis ** | 6 | 7 | 3 | H (16) |
| Phyllodactylus coronatus ** | 6 | 7 | 3 | H (16) |
| Phyllodactylus isabelae | 6 | 7 | 3 | H (16) |
| Phyllodactylus nocticolus | 2 | 5 | 3 | M (10) |
| Phyllodactylus tuberculosis | 1 | 4 | 3 | L (8) |
| Phyllodactylus santacruzensis ** | 1 | 4 | 3 | L (8) |
| Sphaerodactylus continentalis | 4 | 3 | 3 | M (10) |
| Holcosus gaigeae | 5 | 7 | 3 | H (15) |
| Boa imperator | 3 | 1 | 6 | M (10) |
| Boa sigma | 5 | 4 | 6 | H (15) |
| Lampropeltis abnorma | 1 | 3 | 5 | L (9) |
| Lampropeltis californiae | 3 | 4 | 3 | M (10) |
| Lampropeltis polyzona | 5 | 1 | 5 | M (11) |
| Masticophis fuliginosus | 2 | 3 | 4 | L (9) |
| Oxybelis aeneus | 1 | 1 | 3 | L (5) |
| Oxybelis fulgidus | 3 | 2 | 4 | L (9) |
| Oxybelis microphtalmus * | 5 | 2 | 4 | M (11) |
| Spilotes pullatus | 1 | 1 | 4 | L (6) |
| Trimorphodon lyrophanes | 4 | 2 | 4 | M (10) |
| Hypsiglena catalinae | 6 | 8 | 2 | H (16) |
| Hypsiglena chlorophaea | 1 | 5 | 2 | L (8) |
| Hypsiglena ochrorhyncus | 2 | 4 | 2 | L (8) |
| Hypsiglena unaocularis | 6 | 8 | 2 | H (16) |
| Imantodes cenchoa | 1 | 3 | 2 | L (6) |
| Sibon nebulatus | 1 | 2 | 2 | L (5) |
| Micrurus apiatus | 2 | 1 | 5 | L (8) |
| Epictia bakewelli | 5 | 6 | 1 | M (12) |
| Epictia magnamaculata | 3 | 7 | 1 | M (11) |
| Rena humilis | 4 | 3 | 2 | L (8) |
| Agkistrodon russeolus * | 4 | 6 | 5 | H (15) |
| Crotalus polisi | 6 | 8 | 5 | H (18) |
| Crotalus Pyrrhus | 4 | 3 | 5 | M (12) |
| Crotalus thalassoporus | 5 | 8 | 5 | H (18) |
| Crotalus tortuguensis | 6 | 7 | 5 | H (18) |
| Trachemys venusta | 3 | 4 | 6 | H (16) |
| Gopherus morafkai | 4 | 6 | 6 | H (15) |
| Environmental Vulnerability Score (EVS) | ||||
|---|---|---|---|---|
| Taxa | Geographic Distribution | Ecological Distribution | Reproductive Mode/ Degree of Persecution | Total Score |
| Aneides lugubris | 3 | 7 | 4 | H (14) |
| Batrachoseps major | 4 | 6 | 4 | H (14) |
| Anaxyrus punctatus | 1 | 3 | 1 | L (5) |
| Incilius mazatlanensis | 5 | 6 | 1 | M (12) |
| Incilius valliceps | 3 | 2 | 1 | L (6) |
| Rhinella horribilis | 1 | 1 | 1 | L (3) |
| Dendropsophus microcephalus | 3 | 3 | 1 | L (7) |
| Hyliola regilla | 1 | 1 | 1 | L (3) |
| Scinax staufferi | 2 | 1 | 1 | L (4) |
| Smilisca baudinii | 1 | 1 | 1 | L (3) |
| Trachycephalus typhonius | 1 | 2 | 1 | L (4) |
| Leptodactylus fragilis | 1 | 2 | 2 | L (5) |
| Hypopachus variolosus | 2 | 1 | 1 | L (4) |
| Scaphiopus couchii | 1 | 1 | 1 | L (3) |
| Crocodylus moreletii | 2 | 5 | 6 | M (13) |
| Elgaria multicarinata | 3 | 4 | 3 | M (10) |
| Elgaria nana | 5 | 8 | 3 | H (16) |
| Anniella pulchra | 3 | 8 | 1 | M (12) |
| Bipes biporus | 5 | 8 | 1 | H (14) |
| Basiliscus vittatus | 1 | 3 | 3 | L (7) |
| Crotaphytus dickersonae | 5 | 8 | 3 | H (16) |
| Crotaphytus insularis ** | 6 | 7 | 3 | H (16) |
| Gambelia copeii | 2 | 6 | 3 | M (11) |
| Gambelia wislizenii | 3 | 7 | 3 | M (13) |
| Norops nebulosus | 5 | 5 | 3 | M (13) |
| Coleonyx elegans | 2 | 3 | 4 | L (9) |
| Coleonyx gypsicolus | 4 | 6 | 8 | H (18) |
| Coleonyx variegatus | 4 | 3 | 4 | M (11) |
| Ctenosaura similis | 1 | 4 | 3 | L (8) |
| Dipsosaurus dorsalis | 4 | 4 | 3 | M (11) |
| Iguana iguana | 3 | 3 | 6 | M (12) |
| Sauromalus ater | 4 | 6 | 3 | M (13) |
| Marisora lineola | 4 | 3 | 3 | M (10) |
| Callisaurus draconoides | 4 | 5 | 3 | M (12) |
| Petrosaurus mearnsi | 4 | 5 | 3 | M (12) |
| Petrosaurus repens | 5 | 5 | 3 | M (13) |
| Petrosaurus slevini ** | 5 | 8 | 3 | H (16) |
| Petrosaurus thalassinus | 5 | 5 | 3 | M (13) |
| Phrynosoma solare | 4 | 7 | 3 | H (14) |
| Sceloporus angustus | 5 | 8 | 3 | H (16) |
| Sceloporus chrysostictus | 4 | 6 | 3 | M (13) |
| Sceloporus clarkia | 2 | 5 | 3 | M (10) |
| Sceloporus cozumelae | 5 | 7 | 3 | H (15) |
| Sceloporus grandaevus | 6 | 7 | 3 | H (16) |
| Sceloporus hunsakeri | 5 | 6 | 3 | H (14) |
| Sceloporus lineatulus | 6 | 8 | 3 | H (17) |
| Sceloporus magister | 1 | 5 | 3 | L (9) |
| Sceloporus occidentalis | 3 | 6 | 3 | M (12) |
| Sceloporus orcutti | 2 | 2 | 3 | L (7) |
| Sceloporus variabilis | 1 | 1 | 3 | L (5) |
| Sceloporus zosteromus | 5 | 4 | 3 | M (12) |
| Urosaurus bicarinatus | 5 | 4 | 3 | M (12) |
| Urosaurus nigricaudus | 3 | 2 | 3 | L (8) |
| Urosaurus ornatus | 2 | 5 | 3 | M (10) |
| Uta nolascensis | 6 | 8 | 3 | H (17) |
| Uta squamata | 6 | 8 | 3 | H (17) |
| Uta stansburiana | 3 | 1 | 3 | L (7) |
| Phyllodactylus bugastrolepis | 6 | 8 | 3 | H (17) |
| Phyllodactylus homolepidurus | 5 | 7 | 3 | H (15) |
| Phyllodactylus lanei | 5 | 7 | 3 | H (15) |
| Phyllodactylus lupitae | 6 | 7 | 3 | H (16) |
| Phyllodactylus partidus | 5 | 8 | 3 | H (16) |
| Phyllodactylus unctus | 5 | 7 | 3 | H (15) |
| Mesoscincus schwartzei | 2 | 6 | 3 | M (11) |
| Plestiodon skiltonianus | 3 | 5 | 3 | M (11) |
| Aristelliger georgeensis | 3 | 7 | 3 | M (13) |
| Sphaerodactylus glaucus | 4 | 5 | 3 | M (12) |
| Aspidoscelis bacata | 6 | 8 | 3 | H (17) |
| Aspidoscelis cana | 5 | 8 | 3 | H (16) |
| Aspidoscelis carmenensis | 6 | 8 | 3 | H (17) |
| Aspidoscelis celeripes | 5 | 7 | 3 | H (15) |
| Aspidoscelis ceralbensis | 6 | 8 | 3 | H (17) |
| Aspidoscelis communis | 5 | 6 | 3 | H (14) |
| Aspidoscelis costata | 5 | 3 | 3 | M (11) |
| Aspidoscelis cozumela | 5 | 8 | 3 | H (16) |
| Aspidoscelis danheimae | 6 | 7 | 3 | H (16) |
| Aspidoscelis deppii | 1 | 4 | 3 | L (8) |
| Aspidoscelis espiritensis | 5 | 8 | 3 | H (16) |
| Aspidoscelis franciscensis | 6 | 8 | 3 | H (17) |
| Aspidoscelis guttatus | 5 | 4 | 3 | M (12) |
| Aspidoscelis hyperythrus | 2 | 5 | 3 | M (10) |
| Aspidoscelis lineattissima | 5 | 6 | 3 | H (14) |
| Aspidoscelis maslini | 4 | 8 | 3 | H (15) |
| Aspidoscelis pictus | 6 | 8 | 3 | H (17) |
| Aspidoscelis tigris | 3 | 2 | 3 | L (8) |
| Lichanura trivirgata | 4 | 3 | 3 | M (10) |
| Bogertophis rosaliae | 2 | 4 | 3 | M (10) |
| Drymarchon melanurus | 1 | 1 | 4 | L (6) |
| Drymobius margaritiferus | 1 | 1 | 4 | L (6) |
| Leptophis diplotropis | 5 | 5 | 4 | H (14) |
| Leptophis mexicanus | 1 | 1 | 4 | L (6) |
| Masticophis bilineatus | 2 | 5 | 4 | M (11) |
| Masticophis mentovarius | 1 | 1 | 4 | L (6) |
| Masticophis slevini | 6 | 8 | 3 | H (17) |
| Mastigodryas melanolomus | 1 | 1 | 4 | L (6) |
| Phyllorhynchus decurtatus | 4 | 5 | 2 | M (11) |
| Pituophis catenifer | 4 | 1 | 4 | L (9) |
| Pituophis insulanus | 6 | 6 | 4 | H (16) |
| Pituophis vertebralis | 5 | 3 | 4 | M (12) |
| Pseudelaphe flavirufa | 2 | 4 | 4 | M (10) |
| Salvadora hexalepis | 4 | 2 | 4 | M (10) |
| Sonora savage | 6 | 7 | 2 | H (15) |
| Sonora semiannulata | 1 | 1 | 3 | L (5) |
| Sonora stramineus | 4 | 2 | 2 | L (8) |
| Tantilla bocourti | 5 | 2 | 2 | L (9) |
| Tantilla calamarina | 5 | 5 | 2 | M (12) |
| Tantilla moesta | 4 | 7 | 2 | M (13) |
| Tantilla planiceps | 4 | 3 | 2 | L (9) |
| Coniophanes imperialis | 2 | 3 | 3 | L (8) |
| Conophis lineatus | 2 | 3 | 4 | L (9) |
| Conophis vittatus | 2 | 5 | 4 | M (11) |
| Diadophis punctatus | 1 | 1 | 2 | L (4) |
| Dipsas brevifacies | 4 | 7 | 4 | H (15) |
| Geophis annuliferus * | 4 | 7 | 2 | M (13) |
| Hypsiglena slevini | 5 | 4 | 2 | M (11) |
| Hypsiglena torquate | 5 | 1 | 2 | L (8) |
| Imantodes gemmistratus | 1 | 3 | 2 | L (6) |
| Leptodeira frenata | 4 | 4 | 4 | M (12) |
| Ninia sebae | 1 | 1 | 2 | L (5) |
| Rhadinaea Hesperia | 5 | 3 | 2 | M (10) |
| Tropidodipsas sartorii | 2 | 2 | 5 | L (9) |
| Micruroides euryxanthus | 4 | 6 | 5 | H (15) |
| Micrurus apiatus | 2 | 1 | 5 | L (8) |
| Thamnophis Proximus | 1 | 2 | 4 | L (7) |
| Crotalus angelensis | 6 | 7 | 5 | H (18) |
| Crotalus atrox | 1 | 3 | 5 | L (9) |
| Crotalus caliginis | 6 | 7 | 5 | H (18) |
| Crotalus cerastes | 4 | 7 | 5 | H (16) |
| Crotalus enyo | 5 | 3 | 5 | M (13) |
| Crotalus estebanensis | 6 | 8 | 5 | H (19) |
| Crotalus lorenzoensis | 6 | 8 | 5 | H (19) |
| Crotalus mitchellii | 4 | 3 | 5 | M (12) |
| Crotalus molossus | 2 | 1 | 5 | L (8) |
| Crotalus ruber | 2 | 2 | 5 | L (9) |
| Crotalus tigris | 4 | 7 | 5 | H (16) |
| Kinosternon integrum | 5 | 3 | 3 | M (11) |
| Physiographic Provinces | Non-Endemics | Country Endemics | Regional Endemics | Non-Natives | Totals | Rank Order |
|---|---|---|---|---|---|---|
| Pacific of Baja California | 27 | 9 | 4 | 0 | 40 | 3 |
| Gulf of California | 49 | 15 | 44 | 0 | 108 | 1 |
| Tropical Pacific | 24 | 21 | 8 | 4 | 57 | 2 |
| Gulf of Mexico | 39 | 3 | 0 | 4 | 46 | 5 |
| Mexican Caribbean | 41 | 5 | 0 | 7 | 53 | 4 |
| Total Species | 180 | 53 | 56 | 15 | 226 | — |
| Physiographic Provinces | Low | Medium | High | Totals | Rank Order |
|---|---|---|---|---|---|
| Pacific of Baja California | 12 | 19 | 9 | 40 | 3 |
| Gulf of California | 19 | 25 | 58 | 102 | 1 |
| Tropical Pacific | 12 | 20 | 16 | 48 | 2 |
| Gulf of Mexico | 22 | 9 | 7 | 38 | 5 |
| Mexican Caribbean | 21 | 13 | 9 | 43 | 4 |
| Total Species | 86 | 86 | 99 | — | — |
| Name | Category | Date of Decree | Area (ha) | Terrestrial and Inland Waters Area (ha) | Municipalities | Jurisdiction | Physiographic Regions | Facilities Available | Occupied by Landowners | Herpetofaunal Survey Completed | Management Plan Available |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Laguna Madre y Delta del Río Bravo | Área de Protección de Flora y Fauna | 14 April 2005 | 572,808.60 | 572,808.60 | Matamoros, San Fernando, Soto la Marina | Federal | Islands of the Gulf of Mexico | A, R, S, V | Yes | No | Yes |
| Sistema Arrecifal Veracruzano | Parque Nacional | 24 August 1992 | 65,516.47 | 12.24 | NA | Federal | Islands of the Gulf of Mexico | A, R, V | No | No | Yes |
| Sistema Arrecifal Lobos—Tuxpan | Area de Protección de Flora y Fauna | 5 June 2009 | 30,571.15 | ND | NA | Federal | Islands of the Gulf of Mexico | A, R, V | No | No | Yes |
| Laguna de Términos | Area de Protección de Flora y Fauna | 6 June 1994 | 706,147.67 | 547,279 | Carmen, Champoton, Palizada | Federal | Islands of the Gulf of Mexico | A, R, S, V | Yes | No | Yes |
| Arrefice Alacranes | Parque Nacional | 6 June 1994 | 333,768.50 | 53 | Progreso | Federal | Islands of the Gulf of Mexico | A, R, S, V | No | No | Yes |
| Yum Balam | Área de Protección de Flora y Fauna | 6 June 1994 | 154,052.25 | 52,308 | Isla Mujeres, Lazaro Cardenas | Federal | Mexican Caribbean | A, R, S, V | Yes | No | No |
| Isla Contoy | Parque Nacional | 2 February 1998 | 5126.25 | 230 | Isla Mujeres | Federal | Mexican Caribbean | A, R, S, V | No | No | Yes |
| Costa Occidental de Isla Mujeres, Punta Cancún y Punta Nizuc | Parque Nacional | 19 July 1996 | 8673.06 | 0.6 | Benito Juarez, Isla Mujeres | Federal | Mexican Caribbean | A, R, S, V | NA. | NA. | Yes |
| La porción norte y la franja costera oriental, terrestres y marinas de la Isla de Cozumel | Área de Protección de Flora y Fauna | 25 September 2012 | 37,829.17 | 5733 | Cozumel | Federal | Mexican Caribbean | A, R, S, V | Yes | No | Yes |
| Sian Ka’an | Reserva de la Biósfera (WHS) | 20 January 1987 (1987) | 528,147.66 (Ídem) | 375,011.87 (Ídem) | Felipe Carrillo Puerto, Othon P. Blanco, Solidaridad | Federal (International) | Mexican Caribbean | A, R, S, V | Yes | Yes | Yes |
| Banco Chinchorro | Reserva de la Biósfera | 19 July 1996 | 144,360.00 | 586 | Othon P. Blanco | Federal | Mexican Caribbean | A, R, S, V | No | Yes | Yes |
| Santuario del Manatí | Zona sujeta a conservación ecológica | 8 April 2008 | 277,733.669 | 977,333.669 (180,000 of lagoon) | Othón P. Blanco | State. | Mexican Caribbean | A, S | Yes | Yes | Yes |
| Selvas y humedales de Cozumel | Reserva estatal | 1 April 2011 | 19,846.45 | 19,846.45 | Cozumel | States | Mexican Caribbean | A, S | Yes | No | Yes |
| Islas del Pacífico de la Península de Baja California | Reserva de la Biósfera | 7 December 2016 | 1,161,222.98 | 70,139.62 | NA | Federal | Pacific of Baja California Peninsula | A, S | No | Yes | No |
| El Vizcaíno (Whale Sanctuary of El Vizcaino) | Reserva de la biósfera (WHS) | 1 November 1988 (1993) | 2,549,790.25 | 2,259,002.95 (369,631) | Mulegé, BCS | Federal (International) | Pacific of Baja California Peninsula | A, R, S, V | Yes | Yes | Yes |
| Isla Guadalupe | Reserva de la Biósfera | 25 April 2005 | 476,971.20 | 26,276.97 | NA | Federal | Pacific of Baja California Peninsula | A, R, S | No | Note X | Yes |
| Islas del Golfo de California | Area de Protección de Flora y Fauna | 2 August 1978 | 374,553.63 | 374,553.63 | NA | Federal | Gulf of California | A, R | NA | No | Yes |
| Componente del Complejo Insular Espíritu Santo (Islas del Golfo de California) | APFF Islas del Golfo de California | 17 May 2007 | 48,654 | ND | NA | Federal | Gulf of California | A, R, S, V | No | Yes | Yes |
| Bahia de Loreto | Parque Nacional | 19 June 1996 | 206,508.75 | 21,692.08 | Loreto | Federal | Gulf of California | A, R, S, V | No | Yes | Yes |
| Alto Golfo de California y Delta del Río Colorado | Reserva de la Biosfera | 16 June 1993 | 934,756,25 | 407,147.55 | Mexicali, Puesto Peñasco y San Luis Río Colorado | Federal | Gulf of California | A, R, S, V | No | No | Yes |
| Isla San Pedro Mártir | Reserva de la Biosfera | 13 June 2002 | 30,165.23 | 126.98 | NA | Federal | Gulf of California | A, R, S, V | No | Yes | Yes |
| Archipiélago de espíritu santo | Parque Nacional exclusivamente de la zona marina | 17 May 2007 | 48,654–83-10.41 | ND | NA | Federal | Gulf of California | A, R, V | NA | Yes | Yes |
| Bahía de los Ángeles, canales de Ballenas y de Salsipuedes | Reserva de la Biosfera | 5 June 2007 | 387,956.88 | 483.20 | Ensenada | Federal | Gulf of California | A, R, S, V | NA | Yes | Yes |
| Islands and Protected Areas of the Gulf of California | World Heritage site | 2005 | 688,558 | NA | NA | International | Gulf of California, Tropical Islands of the Pacific | NA | NA | NA | NA |
| Archipiélago de Revillagigedo (ídem) | Parque Nacional (World Heritage Site) | 27 November 2017 (6 June 1994 as Reserva de la Biósfera) (2015 as WHS) | 14, 808,780.12 (636,685.375) | 15,518.22 | NA | Federal (International) | Tropical Pacific | A, R, V | NA | Yes | Yes |
| Islas Marietas | Parque Nacional | 24 April 2005 | 1383.01 | 71.16 | NA | Federal | Tropical Pacific | A, R, V | NA | Yes | Yes |
| Isla Isabel | Parque Nacional | 8 December 1980 | 194.17 | 194.17 | NA | Federal | Tropical Pacific | A, R, S, V | NA | Yes | Yes |
| Islas Marías | Reserva de la Biósfera | 27 November 2000 | 641,284.73 | 24,295.16 | NA | Federal | Tropical Pacific | A, R, S | No | No | Yes |
| Islas La Pajarera, Cocinas, Mamut, Colorada, San Pedro, San Agustin, San Andrés y Negrita y los Islotes Los Anegados, Novillas, Mosca y Submarino, situados en la Bahía de Chamela | Santuario | 13 June 2002 | 1981.43 | 1981.43 | NA | Federal | Tropical Pacific | A, R | No | No | Yes |
| Bahías de Huatulco | Parque Nacional | 24 June 1998 | 11,890.98 | 6374.98 | Santa María Huatulco | Federal | Tropical Pacific | A, R, S, V | Yes | No | Yes |
| Taxa | Natural Protected Areas | Number of NPA in which the Species is Present | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gulf of Mexico | Mexican Caribbean | Pacific of BC | Tropical Pacific | Gulf of California | ||||||||||||||||||||||
| Laguna Madre y Delta del Río Bravo | Sistema Arrecifal Veracruzano | Sistema Arrecifal Lobos—Tuxpan | Laguna de Términos | Arrefice Alacranes | Yum Balam | Contoy | Sian Ka’an | Banco Chinchorro | Santuario del Manatí Bahia de Chetumal | Costa Occidental de Isla Mujeres, Punta Cancún y Punta Nizuc | Cozumel complex | Islas del Pacífico de Baja California | El Vizcaíno | Islas Marías | Isabel | Archipielago Revillagigedo | Islas Marietas | Islas La Pajarera, Cocinas, Mamut, Colorada, […]situados en la Bahía de Chamela | Bahía de Loreto | San Pedro Mártir | Bahía de los Ángeles, Canales de Ballenas y de Salsipuedes | Archipielago de Espíritu Santo | San Lorenzo | Islas del Golfo de Baja California | ||
| Amphibia (15 species) | ||||||||||||||||||||||||||
| Anura (13 species) | ||||||||||||||||||||||||||
| Bufonidae (4 species) | ||||||||||||||||||||||||||
| Anaxyrus punctatus | + | + | 2 | |||||||||||||||||||||||
| Incilius mazatlanensis * | + | + | 2 | |||||||||||||||||||||||
| Incilius valliceps | + | + | + | 3 | ||||||||||||||||||||||
| Rhinella horribilis | + | + | 2 | |||||||||||||||||||||||
| Eleutherodactylidae (1 species) | ||||||||||||||||||||||||||
| Eleutherodactylus pallidus * | + | 1 | ||||||||||||||||||||||||
| Hylidae (5 species) | ||||||||||||||||||||||||||
| Dendropsophus microcephalus | + | 1 | ||||||||||||||||||||||||
| Hyliola regilla | + | 1 | ||||||||||||||||||||||||
| Scinax staufferi | + | + | 2 | |||||||||||||||||||||||
| Smilisca baudinii | + | + | + | + | 4 | |||||||||||||||||||||
| Trachycephalus vermiculatus | + | + | 2 | |||||||||||||||||||||||
| Leptodactylidae (1 species) | ||||||||||||||||||||||||||
| Leptodactylus fragilis | + | + | 2 | |||||||||||||||||||||||
| Microhylidae (1 species) | ||||||||||||||||||||||||||
| Hypopachus variolosus | + | + | 2 | |||||||||||||||||||||||
| Scaphiopodidae (1 species) | ||||||||||||||||||||||||||
| Scaphiopus couchii | + | + | 2 | |||||||||||||||||||||||
| Caudata (2 species) | ||||||||||||||||||||||||||
| Plethodontidae (2 especies) | ||||||||||||||||||||||||||
| Aneides lugubris | + | 1 | ||||||||||||||||||||||||
| Batrachoseps major | + | 1 | ||||||||||||||||||||||||
| Reptiles (204 species) | ||||||||||||||||||||||||||
| Crocodylia (2 species) | ||||||||||||||||||||||||||
| Crocodylidae (2 species) | ||||||||||||||||||||||||||
| Crocodylus acutus | + | + | + | + | + | + | 6 | |||||||||||||||||||
| Crocodylus moreletii | + | + | ? | 2 | ||||||||||||||||||||||
| Squamata (189 species) | ||||||||||||||||||||||||||
| Anguidae (3 species) | ||||||||||||||||||||||||||
| Elgaria cedrosensis * | + | 1 | ||||||||||||||||||||||||
| Elgaria multicarinata | + | 1 | ||||||||||||||||||||||||
| Elgaria nana ** | + | 1 | ||||||||||||||||||||||||
| Anniellidae (2 species) | ||||||||||||||||||||||||||
| Anniella geronimensis * | + | 1 | ||||||||||||||||||||||||
| Anniella pulchra | + | 1 | ||||||||||||||||||||||||
| Bipedidae (1 species) | ||||||||||||||||||||||||||
| Bipes biporus * | + | 1 | ||||||||||||||||||||||||
| Corytophanidae (1 species) | ||||||||||||||||||||||||||
| Basiliscus vittatus | + | + | + | 3 | ||||||||||||||||||||||
| Crotaphytidae (4 species) | ||||||||||||||||||||||||||
| Crotaphytus dickersonae ** | + | 1 | ||||||||||||||||||||||||
| Crotaphytus insularis ** | + | + | 2 | |||||||||||||||||||||||
| Gambelia copeii | + | 1 | ||||||||||||||||||||||||
| Gambelia wislizenii | + | 1 | ||||||||||||||||||||||||
| Dactyloidae (4 species) | ||||||||||||||||||||||||||
| Norops lemurinus | + | 1 | ||||||||||||||||||||||||
| Norops nebulosus * | + | + | + | 3 | ||||||||||||||||||||||
| Norops rodriguezii | + | + | + | + | 4 | |||||||||||||||||||||
| Norops ustus | + | + | + | 3 | ||||||||||||||||||||||
| Eublepharidae (3 species) | ||||||||||||||||||||||||||
| Coleonyx elegans | + | 1 | ||||||||||||||||||||||||
| Coleonyx gypsicolus ** | + | 1 | ||||||||||||||||||||||||
| Coleonyx variegatus | + | + | + | + | + | 5 | ||||||||||||||||||||
| Iguanidae (13 species) | ||||||||||||||||||||||||||
| Ctenosaura conspicuosa ** | + | 1 | ||||||||||||||||||||||||
| Ctenosaura hemilopha * | + | 1 | ||||||||||||||||||||||||
| Ctenosaura nolascensis ** | + | 1 | ||||||||||||||||||||||||
| Ctenosaura pectinate | + | + | + | + | + | 5 | ||||||||||||||||||||
| Ctenosaura similis | + | + | + | + | + | + | + | + | 8 | |||||||||||||||||
| Dipsosaurus catalinensis ** | + | + | 2 | |||||||||||||||||||||||
| Dipsosaurus dorsalis | + | + | + | + | + | 5 | ||||||||||||||||||||
| Iguana iguana | + | + | + | + | + | + | + | + | 8 | |||||||||||||||||
| Sauromalus ater | + | + | 2 | |||||||||||||||||||||||
| Sauromalus hispidus * | + | + | + | 3 | ||||||||||||||||||||||
| Sauromalus klauberi ** | + | + | 2 | |||||||||||||||||||||||
| Sauromalus slevini * | + | + | 2 | |||||||||||||||||||||||
| Sauromalus varius * | + | + | 2 | |||||||||||||||||||||||
| Mabuyidae (2 species) | ||||||||||||||||||||||||||
| Marisora aquilonaria * | + | 1 | ||||||||||||||||||||||||
| Marisora lineola | + | + | + | + | + | + | 6 | |||||||||||||||||||
| Phrynosomatidae (31 species) | ||||||||||||||||||||||||||
| Callisaurus draconoides | + | + | + | + | + | 5 | ||||||||||||||||||||
| Petrosaurus mearnsi | + | 1 | ||||||||||||||||||||||||
| Petrosaurus repens * | + | + | 2 | |||||||||||||||||||||||
| Petrosaurus slevini ** | + | + | 2 | |||||||||||||||||||||||
| Petrosaurus thalassinus * | + | + | 2 | |||||||||||||||||||||||
| Phrynosoma cerroense * | + | 1 | ||||||||||||||||||||||||
| Phrynosoma solare | + | 1 | ||||||||||||||||||||||||
| Sceloporus angustus ** | + | 1 | ||||||||||||||||||||||||
| Sceloporus chrysostictus | + | + | + | 3 | ||||||||||||||||||||||
| Sceloporus clarkia | + | + | 2 | |||||||||||||||||||||||
| Sceloporus cozumelae * | + | + | + | 3 | ||||||||||||||||||||||
| Sceloporus grandaevus ** | + | 1 | ||||||||||||||||||||||||
| Sceloporus hunsakeri * | + | + | 2 | |||||||||||||||||||||||
| Sceloporus lineatulus ** | + | + | 2 | |||||||||||||||||||||||
| Sceloporus magister | + | 1 | ||||||||||||||||||||||||
| Sceloporus occidentalis | + | 1 | ||||||||||||||||||||||||
| Sceloporus orcutti | + | + | 2 | |||||||||||||||||||||||
| Sceloporus variabilis | + | 1 | ||||||||||||||||||||||||
| Sceloporus zosteromus * | + | + | + | + | 4 | |||||||||||||||||||||
| Urosaurus auriculatus ** | + | 1 | ||||||||||||||||||||||||
| Urosaurus bicarinatus * | + | 1 | ||||||||||||||||||||||||
| Urosaurus clarionensis ** | + | 1 | ||||||||||||||||||||||||
| Urosaurus nigricaudus * | + | + | + | + | + | 5 | ||||||||||||||||||||
| Urosaurus ornatus | + | + | 2 | |||||||||||||||||||||||
| Uta encantadae ** | + | 1 | ||||||||||||||||||||||||
| Uta lowei ** | + | 1 | ||||||||||||||||||||||||
| Uta nolascensis ** | + | 1 | ||||||||||||||||||||||||
| Uta palmeri ** | + | + | 2 | |||||||||||||||||||||||
| Uta squamata | + | + | 2 | |||||||||||||||||||||||
| Uta stansburiana | + | + | + | + | + | + | + | 7 | ||||||||||||||||||
| Uta tumidarostra ** | + | 1 | ||||||||||||||||||||||||
| Phyllodactylidae (15 species) | ||||||||||||||||||||||||||
| Phyllodactylus angelensis ** | + | 1 | ||||||||||||||||||||||||
| Phyllodactylus apricus ** | + | 1 | ||||||||||||||||||||||||
| Phyllodactylus benedetii * | + | 1 | ||||||||||||||||||||||||
| Phyllodactylus bugastrolepis ** | + | + | 2 | |||||||||||||||||||||||
| Phyllodactylus cleofasensis ** | + | 1 | ||||||||||||||||||||||||
| Phyllodactylus coronatus ** | + | 1 | ||||||||||||||||||||||||
| Phyllodactylus homolepidurus * | + | 1 | ||||||||||||||||||||||||
| Phyllodactylus isabelae ** | + | + | 2 | |||||||||||||||||||||||
| Phyllodactylus lanei * | 0 | |||||||||||||||||||||||||
| Phyllodactylus lupitae ** | 0 | |||||||||||||||||||||||||
| Phyllodactylus nocticolus | + | + | + | + | + | 5 | ||||||||||||||||||||
| Phyllodactylus partidus ** | + | + | 2 | |||||||||||||||||||||||
| Phyllodactylus tuberculosos | + | 1 | ||||||||||||||||||||||||
| Phyllodactylus santacruzensis ** | + | 1 | ||||||||||||||||||||||||
| Phyllodactylus unctus * | + | + | 2 | |||||||||||||||||||||||
| Scincidae (2 species) | ||||||||||||||||||||||||||
| Mesoscincus schwartzei | + | 1 | ||||||||||||||||||||||||
| Plestiodon skiltonianus | + | 1 | ||||||||||||||||||||||||
| Sphaerodactylidae (3 species) | ||||||||||||||||||||||||||
| Aristelliger georgeensis | + | + | + | 3 | ||||||||||||||||||||||
| Sphaerodactylus continentalis | + | 1 | ||||||||||||||||||||||||
| Sphaerodactylus glaucus | + | + | + | 3 | ||||||||||||||||||||||
| Teiidae (22 species) | ||||||||||||||||||||||||||
| Aspidoscelis bacata ** | + | 1 | ||||||||||||||||||||||||
| Aspidoscelis cana ** | + | + | + | 3 | ||||||||||||||||||||||
| Aspidoscelis carmenensis ** | + | + | 2 | |||||||||||||||||||||||
| Aspidoscelis catalinensis ** | + | + | 2 | |||||||||||||||||||||||
| Aspidoscelis celeripes ** | + | 1 | ||||||||||||||||||||||||
| Aspidoscelis ceralbensis ** | + | 1 | ||||||||||||||||||||||||
| Aspidoscelis communis * | + | 1 | ||||||||||||||||||||||||
| Aspidoscelis costata * | + | 1 | ||||||||||||||||||||||||
| Aspidoscelis cozumela * | + | 1 | ||||||||||||||||||||||||
| Aspidoscelis danheimae ** | + | 1 | ||||||||||||||||||||||||
| Aspidoscelis deppii | + | + | 2 | |||||||||||||||||||||||
| Aspidoscelis espiritensis ** | + | + | 2 | |||||||||||||||||||||||
| Aspidoscelis franciscensis ** | + | 1 | ||||||||||||||||||||||||
| Aspidoscelis guttatus * | ||||||||||||||||||||||||||
| Aspidoscelis hyperythrus | + | + | + | 3 | ||||||||||||||||||||||
| Aspidoscelis lineattissima | + | + | 2 | |||||||||||||||||||||||
| Aspidoscelis martyris ** | + | + | 2 | |||||||||||||||||||||||
| Aspidoscelis maslini | + | + | 2 | |||||||||||||||||||||||
| Aspidoscelis pictus ** | + | + | 2 | |||||||||||||||||||||||
| Aspidoscelis rodecki * | + | 1 | ||||||||||||||||||||||||
| Aspidoscelis tigris | + | + | + | + | + | + | 6 | |||||||||||||||||||
| Holcosus gaigeae * | + | 1 | ||||||||||||||||||||||||
| Boidae (2 species) | ||||||||||||||||||||||||||
| Boa imperator * | + | + | + | + | ¨+ | 4 | ||||||||||||||||||||
| Boa sigma | + | + | 2 | |||||||||||||||||||||||
| Charinidae (1 species) | ||||||||||||||||||||||||||
| Lichanura trivirgata | + | + | + | + | + | 5 | ||||||||||||||||||||
| Colubridae (36 species) | ||||||||||||||||||||||||||
| Bogertophis rosaliae | + | + | 2 | |||||||||||||||||||||||
| Drymarchon melanurus | + | 1 | ||||||||||||||||||||||||
| Drymobius margaritiferus | + | 1 | ||||||||||||||||||||||||
| Lampropeltis abnorma | + | 1 | ||||||||||||||||||||||||
| Lampropeltis californiae | + | + | + | + | + | 5 | ||||||||||||||||||||
| Lampropeltis catalinensis ** | + | + | 2 | |||||||||||||||||||||||
| Lampropeltis herrerae ** | + | 1 | ||||||||||||||||||||||||
| Lampropeltis polyzona * | + | + | 2 | |||||||||||||||||||||||
| Leptophis diplotropis * | + | 1 | ||||||||||||||||||||||||
| Leptophis mexicanus | + | + | 2 | |||||||||||||||||||||||
| Masticophis anthonyi ** | + | 1 | ||||||||||||||||||||||||
| Masticophis barbouri | + | + | 2 | |||||||||||||||||||||||
| Masticophis bilineatus | + | 1 | ||||||||||||||||||||||||
| Masticophis fuliginosus | + | + | + | + | 4 | |||||||||||||||||||||
| Masticophis mentovarius | + | + | + | 3 | ||||||||||||||||||||||
| Masticophis slevini ** | + | 1 | ||||||||||||||||||||||||
| Mastigodryas melanolomus | + | + | + | 3 | ||||||||||||||||||||||
| Oxybelis aeneus | + | 1 | ||||||||||||||||||||||||
| Oxybelis fulgidus | + | + | + | 3 | ||||||||||||||||||||||
| Oxybelis microphtalmus * | + | 1 | ||||||||||||||||||||||||
| Phyllorhynchus decurtatus | + | + | 2 | |||||||||||||||||||||||
| Pituophis catenifer | + | 1 | ||||||||||||||||||||||||
| Pituophis insulanus ** | + | 1 | ||||||||||||||||||||||||
| Pituophis vertebralis * | + | 1 | ||||||||||||||||||||||||
| Pseudelaphe flavirufa | + | 1 | ||||||||||||||||||||||||
| Rhinocheilus etheridgei ** | + | 1 | ||||||||||||||||||||||||
| Salvadora hexalepis | + | + | 2 | |||||||||||||||||||||||
| Sonora savagei * | + | 1 | ||||||||||||||||||||||||
| Sonora semiannulata | + | 1 | ||||||||||||||||||||||||
| Sonora straminea | + | + | + | + | 4 | |||||||||||||||||||||
| Spilotes pullatus | + | 1 | ||||||||||||||||||||||||
| Tantilla bocourti * | + | 1 | ||||||||||||||||||||||||
| Tantilla calamarina * | + | 1 | ||||||||||||||||||||||||
| Tantilla moesta | + | 1 | ||||||||||||||||||||||||
| Tantilla planiceps | + | 1 | ||||||||||||||||||||||||
| Trimorphodon lyrophanes | + | 1 | ||||||||||||||||||||||||
| Dipsadidae (20 species) | ||||||||||||||||||||||||||
| Coniophanes meridanus | + | 1 | ||||||||||||||||||||||||
| Coniophanes imperialis | + | 1 | ||||||||||||||||||||||||
| Conophis lineatus | + | + | + | 3 | ||||||||||||||||||||||
| Conophis vittatus | + | 1 | ||||||||||||||||||||||||
| Diadophis punctatus | + | 1 | ||||||||||||||||||||||||
| Dipsas brevifacies | + | 1 | ||||||||||||||||||||||||
| Geophis annuliferus * | + | 1 | ||||||||||||||||||||||||
| Hypsiglena catalinae ** | + | 1 | ||||||||||||||||||||||||
| Hypsiglena chlorophaea | + | 1 | ||||||||||||||||||||||||
| Hypsiglena ochrorhyncus | + | + | + | + | + | 5 | ||||||||||||||||||||
| Hypsiglena slevini * | + | 1 | ||||||||||||||||||||||||
| Hypsiglena torquata | + | + | 2 | |||||||||||||||||||||||
| Hypsiglena unaocularis ** | + | 1 | ||||||||||||||||||||||||
| Imantodes cenchoa | + | 1 | ||||||||||||||||||||||||
| Imantodes gemmistratus | + | 1 | ||||||||||||||||||||||||
| Leptodeira frenata | + | + | 2 | |||||||||||||||||||||||
| Ninia sebae | + | 1 | ||||||||||||||||||||||||
| Rhadinaea hesperia * | + | 1 | ||||||||||||||||||||||||
| Sibon nebulatus | + | + | 2 | |||||||||||||||||||||||
| Tropidodipsas sartorii | + | 1 | ||||||||||||||||||||||||
| Elapidae (2 species) | ||||||||||||||||||||||||||
| Micruroides euryxanthus | + | 1 | ||||||||||||||||||||||||
| Micrurus apiatus | + | + | 2 | |||||||||||||||||||||||
| Leptotyphlopidae (3 species) | ||||||||||||||||||||||||||
| Epictia bakewelli * | 0 | |||||||||||||||||||||||||
| Epictia magnamaculata | + | 1 | ||||||||||||||||||||||||
| Rena humilis | + | + | + | 3 | ||||||||||||||||||||||
| Natricidae (1 species) | ||||||||||||||||||||||||||
| Thamnophis proximus | + | 1 | ||||||||||||||||||||||||
| Viperidae (18 species) | ||||||||||||||||||||||||||
| Agkistrodon bilineatus | + | 1 | ||||||||||||||||||||||||
| Agkistrodon russeolus * | + | 1 | ||||||||||||||||||||||||
| Crotalus angelensis ** | + | + | 2 | |||||||||||||||||||||||
| Crotalus atrox | + | + | 2 | |||||||||||||||||||||||
| Crotalus caliginis ** | + | 1 | ||||||||||||||||||||||||
| Crotalus catalinensis | + | + | 2 | |||||||||||||||||||||||
| Crotalus cerastes | + | 1 | ||||||||||||||||||||||||
| Crotalus enyo * | + | + | + | + | 4 | |||||||||||||||||||||
| Crotalus estebanensis ** | + | 1 | ||||||||||||||||||||||||
| Crotalus lorenzoensis ** | + | + | 2 | |||||||||||||||||||||||
| Crotalus mitchellii | + | + | + | + | + | 5 | ||||||||||||||||||||
| Crotalus molossus | + | 1 | ||||||||||||||||||||||||
| Crotalus polisi ** | + | + | 2 | |||||||||||||||||||||||
| Crotalus Pyrrhus | + | + | 2 | |||||||||||||||||||||||
| Crotalus ruber | + | + | + | + | 4 | |||||||||||||||||||||
| Crotalus thalassoporus ** | + | + | 2 | |||||||||||||||||||||||
| Crotalus tigris | + | 1 | ||||||||||||||||||||||||
| Crotalus tortuguensis ** | + | 1 | ||||||||||||||||||||||||
| Testudines (13 species) | ||||||||||||||||||||||||||
| Cheloniidae (5 species) | ||||||||||||||||||||||||||
| Caretta caretta | + | + | + | + | + | + | + | + | + | + | + | + | 12 | |||||||||||||
| Chelonia mydas | + | + | + | + | + | + | + | + | + | + | + | + | + | + | 14 | |||||||||||
| Eretmochelys imbricata | + | + | + | + | + | + | + | + | + | + | + | + | + | 13 | ||||||||||||
| Lepidochelys kempii | + | + | + | + | + | 5 | ||||||||||||||||||||
| Lepidochelys olivácea | + | + | + | + | 4 | |||||||||||||||||||||
| Dermatemydidae (1 species) | ||||||||||||||||||||||||||
| Dermatemys mawii | + | 1 | ||||||||||||||||||||||||
| Dermochelyidae (1 species) | ||||||||||||||||||||||||||
| Dermochelys coriacea | + | + | + | + | + | + | + | + | + | + | 10 | |||||||||||||||
| Emydidae (1 species) | ||||||||||||||||||||||||||
| Trachemys venusta | + | 1 | ||||||||||||||||||||||||
| Geoemydidae (1 species) | ||||||||||||||||||||||||||
| Rhinoclemmys areolata | + | + | 2 | |||||||||||||||||||||||
| Kinosternidae (2 species) | ||||||||||||||||||||||||||
| Kinosternon integrum * | + | 1 | ||||||||||||||||||||||||
| Kinosternon scorpioides | + | + | 2 | |||||||||||||||||||||||
| Staurotypidae (1 species) | ||||||||||||||||||||||||||
| Staurotypus triporcatus | + | 1 | ||||||||||||||||||||||||
| Testudinidae (1 species) | ||||||||||||||||||||||||||
| Gopherus morafkai | + | 1 | ||||||||||||||||||||||||
| Totals (219 species) | 4 | 8 | 4 | 39 | 5 | 20 | 13 | 1 | 10 | 10 | 7 | 35 | 36 | 2 | 30 | 8 | 10 | 10 | 12 | 37 | 4 | 21 | 22 | 10 | 98 | - |
| Protected Areas | Number of Species | Distributional Status | |||
|---|---|---|---|---|---|
| Non-Endemic (NE) | Country Endemic (CE) | Insular Endemic (IE) | Non-Native (NN) | ||
| Laguna Madre y Delta del Río Bravo | 4 | 4 | - | - | - |
| Sistema Arrecifal Lobos—Tuxpan | 4 | 4 | - | - | - |
| Sistema Arrecifal Veracruzano | 8 | 8 | - | - | - |
| Laguna de Términos | 42 | 38 | 1 | - | 3 |
| Arrefice Alacranes | 6 | 5 | - | - | 1 |
| Yum Balam | 22 | 17 | 3 | - | 2 |
| Contoy | 15 | 11 | 2 | - | 2 |
| Sian Ka’an | 1 | - | - | - | 1 |
| Banco Chinchorro | 13 | 10 | - | - | 3 |
| Santuario del Manatí Bahia de Chetumal | 11 | 10 | - | - | 1 |
| Costa Occidental de Isla Mujeres, Punta Cancún y Punta Nizuc | 7 | 7 | - | - | - |
| Cozumel complex | 41 | 33 | 2 | - | 6 |
| Islas del Pacífico de Baja California | 36 | 24 | 8 | 4 | - |
| El Vizcaíno | 2 | 2 | - | - | - |
| Islas Marías | 30 | 16 | 13 | 1 | - |
| Isabel | 11 | 3 | 3 | 2 | 3 |
| Archipielago revillagigedo | 12 | 6 | - | 4 | 2 |
| Islas Marietas | 10 | 6 | 3 | 1 | - |
| Islas La Pajarera, Cocinas, Mamut, […], situados en la Bahía de Chamela | 13 | 7 | 5 | - | 1 |
| Bahía de Loreto | 37 | 21 | 6 | 10 | - |
| San Pedro Mártir | 4 | 2 | - | 2 | - |
| Bahía de los Ángeles, canales de Ballenas y de Salsipuedes | 21 | 13 | 2 | 6 | - |
| Archipielago de Espíritu Santo | 22 | 14 | 6 | 2 | - |
| San Lorenzo | 10 | 5 | 2 | 3 | - |
| Islas del Golfo de Baja California | 98 | 42 | 13 | 43 | - |
| Totals | 219 | 117 | 46 | 56 | 7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Sánchez, V.H.; Johnson, J.D.; Frausto-Martínez, O.; Mejía Ortíz, L.M.; Pereira-Corona, A.; Blanco-Parra, M.d.P.; Charruau, P.; Níño-Torres, C.A. The Herpetofauna of the Insular Systems of Mexico. Diversity 2023, 15, 921. https://doi.org/10.3390/d15080921
González-Sánchez VH, Johnson JD, Frausto-Martínez O, Mejía Ortíz LM, Pereira-Corona A, Blanco-Parra MdP, Charruau P, Níño-Torres CA. The Herpetofauna of the Insular Systems of Mexico. Diversity. 2023; 15(8):921. https://doi.org/10.3390/d15080921
Chicago/Turabian StyleGonzález-Sánchez, Víctor Hugo, Jerry D. Johnson, Oscar Frausto-Martínez, Luis M. Mejía Ortíz, Alberto Pereira-Corona, María del Pilar Blanco-Parra, Pierre Charruau, and Carlos Alberto Níño-Torres. 2023. "The Herpetofauna of the Insular Systems of Mexico" Diversity 15, no. 8: 921. https://doi.org/10.3390/d15080921
APA StyleGonzález-Sánchez, V. H., Johnson, J. D., Frausto-Martínez, O., Mejía Ortíz, L. M., Pereira-Corona, A., Blanco-Parra, M. d. P., Charruau, P., & Níño-Torres, C. A. (2023). The Herpetofauna of the Insular Systems of Mexico. Diversity, 15(8), 921. https://doi.org/10.3390/d15080921









