Abstract
The geographic range of the nine-banded armadillo (Dasypus novemcinctus) has rapidly been expanding within the United States for the last 150 years. One of the factors contributing to this astounding range expansion is the species’ ability to survive in and colonize human-dominated areas. Despite the fact that armadillos live alongside humans in numerous towns and cities across the Southeastern, Southcentral, and now Midwestern United States, we know relatively little about the behavior and ecology of armadillos in human-developed areas. Here, we used motion-triggered game cameras in over 115 residential yards in the rapidly developing Northwest corner of Arkansas to survey armadillos in a largely suburban environment. Our objectives were to explore trends in armadillo occupancy and daily activity patterns in a suburban setting. We documented armadillos in approximately 84% of the yards surveyed indicating that the species was widespread throughout the environment. We found that the species was more likely to occupy yards surrounded by a high proportion of forest cover. We found no relationship between armadillo occupancy and other land cover or development covariates. Only 2% of nearly 2000 armadillo detections occurred during the day indicating that the species is almost exclusively nocturnal during the summer months when living near humans in the suburban environment, which we suggest is likely an adaptation to avoid contact with humans and their dogs. As the armadillo continues to expand its geographic range to areas where it has not previously occurred, understanding how human development supports and facilitates the spread of this species can elucidate areas where conflict between humans and armadillos might occur allowing for preemptive management or education to mitigate conflict.
1. Introduction
Not all species of wildlife are adversely impacted by human development. Some species may benefit from living in and around humans because of the way in which we modify environments or provide subsidized food, water, and shelter resources. The nine-banded armadillo (Dasypus novemcinctus hereafter, armadillo) has been rapidly expanding its geographic range in the United States since the 1850s [1,2,3]. The armadillo has been moving northward through Texas and (aided by at least one release of individuals from captivity) Florida [1,4]. These two waves of expansion converged in the Southeastern United States around the late 1970s and have been moving northward ever since [5]. The geographic range of the armadillo now encompasses much of the lower Midwestern and coastal Southeastern United States and continues to expand at a mean rate of 4–10 km per year [4,6,7]. While the exact reasons for this geographic range expansion are unknown, it is likely aided by a combination of climate change, travel corridors (such as roads), and human environmental modification and development [8,9,10,11].
While the habitat needs of the armadillo are fairly general, the species is often associated with forest cover and riparian areas [10,12,13]. However, this species has a remarkable ability to persist in developed landscapes and is a common inhabitant of suburban areas across its range [11,14]. This ability to take advantage of human-altered environments may be one of the major reasons that the armadillo was able to expand across the arid expanse of Texas that had formerly posed an impassable barrier [1,4]. However, little research has been conducted to evaluate the ecology of armadillos living in human-dominated environments such as suburban areas. If the geographic range expansion of the armadillo is in fact facilitated by human development, understanding the behavior and habitat associations of armadillos within the suburban environment may help better understand the factors facilitating the spread of this species as well as factors that potentially lead to conflict between humans and the armadillos.
While the armadillo is an ecologically important species that excavates and maintains large, deep, and numerous burrows (up to 10 per individual) that provide refugia to a wide array of native wildlife [15,16,17,18], the species is often perceived negatively and managed as a nuisance in the regions it has recently colonized. The foraging and digging behavior of the species can cause damage to agricultural crops, residential lawns and gardens, private property and public infrastructure. Many people also associate the species with a risk of leprosy, although reports of the disease passing from armadillos to humans are rare and result from extended contact with or eating the animal [19]. As the species continues to spread, it will occur in more and more suburban areas potentially leading to more human–wildlife conflict.
Here, our goals were to use occupancy modeling of armadillos across a suburban environment to explore how landcover and development intensity influences the detection and occupancy probability of this rapidly expanding species. We predict that armadillos will most frequently occur in residential areas that are heavily forested and rarely occur in areas with high levels of development or housing unit density. While the armadillo can coexist with humans, it is considered a nuisance due to its digging behavior, which can damage gardens and infrastructure, and is vulnerable to mortality from both dogs and vehicular collisions. Thus, we expect that most armadillos in the suburban environment will occur in areas with heavy forest cover and likely visit adjacent residential yards at night to forage. Furthermore, we predict that the armadillo will be most active and thus most frequently detected during periods with rainfall when their invertebrate prey is closest to the soil surface.
We also report on the daily activity patterns of the armadillo occurring within the suburban environment. The armadillo is plastic in its diel activity patterns, often switching between diurnal, crepuscular, and nocturnal behavior based on age, temperature, or human activity [9,20,21]. Given that a recent study showed that armadillos living in areas of high human activity tended to be more nocturnal than those living in more natural areas [14], we predict that armadillos living in the suburban landscape will be almost exclusively nocturnal likely to avoid human interactions and pet dogs.
2. Materials and Methods
Our study was conducted over the course of two summers (April through August 2021 and 2022) in Northwest Arkansas, USA, centered around the city of Fayetteville. Fayetteville and the adjacent cities of Springdale, Rogers, and Bentonville are all located in the Ozark Highlands Ecoregion. The landscape is predominantly forested by oak (Quercus sp.) and hickory (Carya sp.) dominated hardwood forests and the terrain can be steep and rocky. To the west of the cities, the terrain is generally flatter and thin bands of prairie extend eastward into the state. However, most of this prairie has been converted to pasture for cattle grazing. This area of Arkansas is developing rapidly to accommodate an expanding human population.
To assess the habitat associations and activity patterns of the armadillo in this area, we deployed motion-triggered game cameras in the residential yards scattered throughout the area but within 60 km of Fayetteville. In order to access yards for camera deployment, we solicited volunteers through the Master Naturalist Program as well as by reaching out to colleagues at the University of Arkansas. Most yards used in this study were in separate neighborhoods, but two yards were in the same neighborhood and only 40 m from one another across a street. We attempted to use yards that spanned as large a geographic area as possible and occurred across a gradient of development from low-density almost rural yards to homes occurring in high-density residential areas. When volunteers agreed to allow a camera to be deployed on their property, we placed a single camera on either a tripod or strapped to a tree approximately 1 m off the ground. We used either Spypoint Force Dark (Spypoint Inc., Victoriaville, QC, Canada) or Browning Strike Force (Browning, Morgan, UT, USA) model cameras. We used no lure or bait at any of the cameras. Each camera was set to take a burst of three photographs at each motion-trigger with a 5 s reset period. We set cameras anywhere between 5 m and 100 m from houses. We chose camera locations in consultation with homeowners to maximize wildlife detections but to not interfere with their property maintenance activities. We visited cameras approximately every two weeks to ensure they were functioning, to download data, and to change batteries as needed.
At the completion of sampling each year, we reviewed all photos using a photo processing software (Timelapse 2.0 [22]). Each time an armadillo was detected, we noted the yard the camera was in as well as the date and time of the detection. To reduce double counting individuals that repeatedly triggered the cameras, we combined all photographs taken within 5 min of one another into a sequence and treated this as a single armadillo detection. We chose 5 min because this is a conservative estimate to reduce double counting [23] and facilitated comparison with other studies of the armadillo conducted in this area [14]. While this did not influence occupancy modeling, it was an important consideration for quantifying the daily activity patterns of armadillos.
2.1. Occupancy Modeling
To explore the effects of landcover covariates and to account for imperfect detection of armadillos, we used an occupancy modeling framework to assess the habitat associations of the armadillo. We used a single season occupancy model [24]. We performed all occupancy analyses using the program Presence V2.13.39. Each camera deployed in a yard was considered a sampling site. We considered a survey to be composed of 7 days of continuous camera operation beginning on 11 April and running through 19 August for a maximum of 18 possible surveys per site per year. If a camera malfunctioned, ran out of batteries, was knocked down, had its view obscured by vegetation, or otherwise failed to operate continuously for an entire 7 day survey period, that survey was censored from analyses. We used a one-week sampling period as this represents an appropriate amount of time as to not over- or under-compress statistical power [25]. Because not all cameras were deployed at the same time, we used a staggered entry approach and any survey period occurring before cameras were deployed (or after cameras were retrieved at the end of the season) were censored.
We assigned each survey a 1 or a 0 if at least one armadillo was detected (1) or no armadillos were detected (0). We sampled some of our sites in both years of the study. For these sites, we adopted a conservative approach and randomly chose one of the sampling years to include and excluded the other. We then used year as a detection covariate [26] to evaluate differences between years in both occupancy and detection probability.
To avoid model over-fitting, we used a multi-stage fitting approach [27] in which we used Akaike’s Information Criterion corrected for small sample sizes (AICc) to select the best detection covariate. We modeled the time (aka survey period, to allow detection to vary across the study seasons), year (to evaluate detection across the two years of the study), cumulative weekly rainfall (to evaluate if precipitation influenced armadillo activity and thus detection), and weekly minimum temperature and weekly maximum temperature (to allow for armadillo activity to vary by temperature) as covariates for detection against null occupancy parameters and selected the top covariate model with lowest AICc score. We acquired precipitation and temperature data from the National Oceanic and Atmospheric Association (NOAA) weather stations closest to each study yard. We also evaluated a model in which both occupancy and detection were held constant. The covariate in the top ranked model was then used as the detection covariate in all subsequent analyses of occupancy probability.
We calculated a number of landcover covariates to evaluate their influence on the occupancy probability of armadillos. We used ArcGIS Pro 10.2 (ESRI, Inc., Redlands, CA, USA) to calculate the amount of each of the landcover variables within 500 m of each site. First, we calculated the proportion of landcover comprised by forest (mixed forest + conifer forest + hardwood forest categories from the 2019 National Land Cover Database (NLCD: United States Geological Survey)) surrounding each site. We predicted that armadillo occupancy would be positively associated with the proportion of forest cover present. Using the same NLCD layer, we also calculated the proportion of herbaceous landcover (defined as prairie and grassland), hay and pasture (hereafter, pasture), and development (medium + high intensity development categories combined—both of which reference landcover with greater than 50% impervious surface). Finally, we calculated the maximum housing unit density around each site using the housing unit density layer produced by the Spatial Analysis for Conservation and Sustainability Lab. We predicted that armadillo occupancy would be negatively associated with both development and housing unit density and positively or neutrally associated with herbaceous landcover and pasture. We assessed model goodness of fit within the Presence software program using 100 bootstrap iterations.
Before model fitting, we assessed the collinearity of covariates and considered any two covariates to be collinear if they had correlation coefficients |0.6|. None of our covariates were significantly correlated at this level, and thus, all were retained for analyses. Before analyses, we scaled and centered all landscape covariates on their means to facilitate comparison between variables measured on different scales.
2.2. Armadillo Activity
To assess the daily activity patterns of armadillos in the suburban environment, we recorded the time of each armadillo detection. We used all armadillo detections including those from yards sampled in both years. We calculated the sunset and sunrise times for each day of the study using the Suncalc package in program R [28]. We then categorized each armadillo detection as being diurnal (during the day at least one hour after sunrise and one hour before sunset), crepuscular (within 1 h of either sunrise or sunset), or nocturnal (one hour after sunset and at least one hour before sunrise).
3. Results
We conducted a total of 1654 surveys at 117 sites for a total of 11,578 camera nights. Each site was surveyed between 3 and 18 times with an average of 14 (±2.4: mean ± 1 SD). We detected armadillos in 84% of the sites surveyed.
3.1. Occupancy
We found that the top ranked detection covariate was time (Table 1). Time accounted for nearly all of the weight of evidence, and the second highest ranked detection covariate was 32 ΔAICc below the top model and had little of the weight of evidence. When evaluating the effects of occupancy covariates, we used time as the detection covariate for all candidate models.

Table 1.
Comparison of detection covariates of nine-banded armadillos (Dasypus novemcinctus) in residential yards in Northwestern Arkansas, USA, during the summers of 2021 and 2022. We evaluated the detection covariates of time (7 day survey periods), constant detection probability, year (2021 or 2022), rainfall (cumulative weekly rainfall), weekly maximum temperature, and weekly minimum temperature. AIC = Akaike’s Information Criterion. Ψ = occupancy probability and p = detection probability.
The landcover surrounding sites varied substantially across the study region. The proportion of forest cover around sites ranged from 0–0.44 (mean = 0.18), development ranged from 0–0.47 (mean = 0.13), pasture ranged from 0–0.43 (mean = 0.08), herbaceous landcover ranged from 0–0.03 (mean = 0.003), and housing unit density ranged from <1–5092 residences per km2 (mean = 657).
The proportion of forest around each site was the top model predicting the occupancy of armadillos in residential yards (Table 2). Forest cover was positively and strongly associated with the occupancy of armadillos (β = 6.528, SE = 2.63). The models containing the covariates for herbaceous land cover and pasture received some support but were both nearly 5 ΔAICc below the top model and each received less than 10% of the weight of evidence (Table 2). Occupancy probability of armadillos in residential yards as predicted by the mean forest covariate was 0.86 (95% CI: 0.74–0.92). Occupancy probability varied between sites ranging from 0.69 (0.50–0.83) to 0.98 (0.87–0.99).

Table 2.
Covariates predicted to influence the occupancy of nine-banded armadillos (Dasypus novemcinctus) in residential yards in Northwestern Arkansas, USA, during the summers of 2021 and 2022. Time (7 day survey period) was used as the detection covariate in all models. AIC = Akaike’s Information Criterion. Ψ = occupancy probability and p = detection probability.
3.2. Activity
We accumulated 1696 unique armadillo detections during the course of this study. We found that armadillos were primarily nocturnal with some detections occurring around sunrise or sunset (Figure 1). We detected very few armadillos active during the day (Figure 1). In total 1461 armadillo detections were nocturnal (86%), 27 were diurnal (2%), and 208 were crepuscular (12%), i.e., within 1 h of sunrise or sunset.
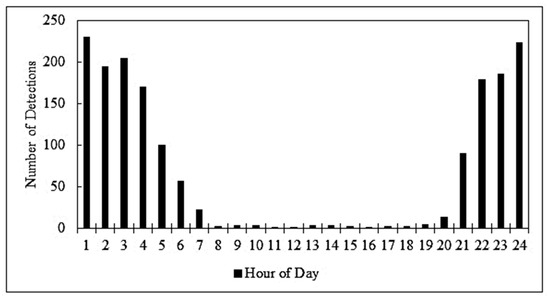
Figure 1.
The time of day that nine-banded armadillos (Dasypus novemcinctus) were detected using game cameras in residential yards in Northwest Arkansas during the summers of 2021 and 2022.
4. Discussion
Armadillos were widespread and common throughout the suburban environment where our study occurred. We detected armadillos in 84% of the residential yards surveyed despite these sites varying widely in the degree of surrounding forest cover (0–44%) and human development (1→5000 residences per km2). This finding highlights the ability of armadillos to coexist with humans even in areas of high impervious surface cover and high density of housing when some degree of forest cover is available. The adaptability of the armadillo almost certainly contributes to their successful, ongoing and rapid geographic range expansion [11,14]. Their propensity to occur around humans will continue to lead to conflicts between people and armadillos in the form of garden or crop damage, dog attacks on armadillos, and vehicular collisions.
Our occupancy analysis revealed that forest cover surrounding yards was the strongest predictor of the occupancy probability of armadillos. Armadillos are habitat generalists capable of surviving in numerous landcover types, although most studies have shown a strong link between forest cover and armadillos [29]. Our finding align with a recent occupancy analysis of nine-banded armadillos in an agricultural region of Brazil where forest cover and distance to water were reported as the top landscape covariates explaining occupancy of armadillos in that human-altered environment [13]. It appears unlikely that armadillos can persist in residential yards without access to some degree of forest or other natural landcover. Forested suburban areas near the leading edge of the northward expansion of this species will become occupied by armadillos, and in these areas, managers can anticipate human–armadillo interactions and likely conflicts. In fact, human development, riparian forest, and forest cover all likely contribute to the dispersal of this species [10]. As the armadillo continues to expand its range in the Midwestern United States, an area where suburban towns have more tree cover than the surrounding agricultural matrix, suburban developments may be critical for the persistence of armadillos. However, it is also possible that the intensively agricultural setting with little forest cover outside of riparian areas and towns may represent a challenge to dispersing armadillos.
One tactic that allows armadillos (and many other species of wildlife) to successfully coexist with humans is the plasticity in their daily activity patterns with many species of wildlife adopting a more nocturnal lifestyle when living in proximity to humans or human development [30,31]. In natural areas, armadillos can shift between diurnal and nocturnal behavior based on ontogeny, weather, and the season. In human-dominated areas, armadillos (both nine-banded and long-nosed (Dasypus hybridus)) have been shown to shift to nocturnal behavior to avoid contact with humans and/or dogs [14,32] as do a large number of other wildlife species [31,33,34]. A recent study showed that armadillos living near palm plantations were strictly nocturnal, and the authors hypothesized that this was due to an avoidance of human activity [35]. A study also conducted in Northwest Arkansas showed substantial variation in the activity patterns of armadillos in natural areas along an urban to rural gradient [14]. Armadillos in more urban areas were far more nocturnal than those occurring in more undeveloped areas farther from the city. Based on these reports, we expected that armadillos in the suburban environment would be heavily nocturnal and that was indeed the case with only 2% of nearly 1700 armadillo detections occurring during the day (Figure 1). This finding was even more extreme than anticipated. We should note, however, that the previous report of diurnal armadillo activity was during the winter period where the species may benefit by being active during the warmest parts of the day, while during our summer study, nocturnal temperatures might be more suitable for activity, thus allowing the armadillo to be primarily nocturnal without a fitness cost. Future studies of suburban armadillos are needed to elucidate whether the species seasonally shifts activity in suburban areas in response to temperature. While armadillos can successfully coexist with human development, it almost certainly comes at a fitness cost as a nocturnal existence exposes individuals to more stressful winter and night temperatures [36] and living alongside humans and dogs certainly leads to higher mortality rates. However, relatively few studies have evaluated the ecology, behavior, or fitness of armadillos in human-dominated environments.
Suburban landcover continues to expand and currently covers an estimated 17% of the United States [37]. While suburban expansion comes at the cost of natural landcover, many adaptable wildlife species are able to survive and persist in or adjacent to the suburban environment [38]. Often, these species benefit by taking advantage intentionally or unintentionally of human-subsidized food, water, or shelter resources [38,39]. As we improve our understanding of how wildlife use these novel, human-created environments, the better equipped we are to manage environments in ways that can benefit wildlife populations and mitigate wildlife–human conflict. Residential yards have become important as habitat or sources of valuable resources for wildlife in human-dominated settings [38,39,40]. Particular wildlife species can reliably be associated with specific resources and understanding these relationships give homeowners some degree of agency over manipulating the availability of those resources [40].
Unfortunately, many of the wildlife attracted to suburban yards can be perceived by homeowners as nuisances. White-tailed deer (Odocoileus virginianus), woodchucks (Marmota monax), and eastern cottontails (Sylvilagus floridanus) can all forage on and damage gardens, red fox (Vulpes vulpes) can threaten chicken coops, and raccoons (Procyon lotor) and Virginia opossum (Didelphis virginiana) will raid trash cans and compost piles [38,40]. Management often focuses on identifying and then limiting access to resources by wildlife (i.e., wildlife-proof trash cans) or educating homeowners on how they can reduce interactions. Homeowners can use fences, repellents, or deterrents to reduce conflict with wildlife in their yards. As the armadillo continues its geographic range expansion, it will come into contact with people that have never lived alongside these species before. The armadillo will now join the list of “nuisance” wildlife that some homeowners will want to exclude from their yards. Our findings highlight that armadillos are more likely to occur in areas with high forest cover, and these are the towns, neighborhoods, or areas where managers might expect higher rates of human–armadillo interactions and thus conflict. Heavily forested suburban developments at the leading edge of the armadillo range expansion might be areas that could be preemptively targeted with outreach and education to moderate future conflict and turn around negative perceptions or fears about the species. Managers may also be able to preemptively plan for the management of nuisance individuals in these heavily forested suburbs and be ready to implement actions such as short- or long-range translocation. The suburban environment will certainly play a role in the spread of persistence of the armadillo in new parts of its geographic range, and thus, the more we can learn about the species’ behavior and ecology in developed settings, the better we can understand how we are facilitating its spread and begin to develop strategies to mitigate conflict.
Author Contributions
Conceptualization, B.A.D. and E.P.J.; methodology, B.A.D. and E.P.J.; formal analysis, E.P.J.; investigation, B.A.D., E.P.J. and M.R.M.; resources, B.A.D.; data curation, E.P.J. and M.R.M.; writing—original draft preparation, B.A.D.; writing—review and editing, E.P.J. and M.R.M.; visualization, B.A.D. and E.P.J.; supervision, B.A.D.; project administration, B.A.D. and E.P.J.; funding acquisition, B.A.D. All authors have read and agreed to the published version of the manuscript.
Funding
Funding for this project was provided by the University of Arkansas Office of Undergraduate Research, the Arkansas Game and Fish Commission via cooperative agreement no. 1434-04HQRU1567, and the National Science Foundation through support for the Research Experience for Undergraduates program.
Data Availability Statement
Data are available upon request to either DeGregorio or Johansson.
Acknowledgments
We owe a huge thank you to our generous volunteers that made this project possible including those from the Arkansas Master Naturalist program, the University of Arkansas Biology and Engineering departments, and willing neighbors and friends. Funding for this project was provided by the University of Arkansas Office of Undergraduate Research, National Science Foundation Research Experience for Undergraduates Program, and the Arkansas Game and Fish Commission via cooperative agreement no. 1434-04HQRU1567. We thank Kenneth Wilson for assistance in the field. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government. This project would not have been possible without our generous volunteers from the Department of Biological Sciences at the University of Arkansas, and the Arkansas State Master Naturalists. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Taulman, J.F.; Robbins, L.W. Recent range expansion and distributional limits of the nine-banded armadillo (Dasypus novemcinctus) in the United States. J. Biogeogr. 1996, 23, 635–648. [Google Scholar] [CrossRef]
- Hofmann, J.E. Records of Nine-Banded Armadillos, Dasypus novemcinctus, in Illinois. Trans. Ill. State Acad. Sci. 2009, 102, 95–106. [Google Scholar]
- Feng, X.; Papeş, M. Ecological niche modelling confirms potential north-east range expansion of the nine-banded armadillo (Dasypus novemcinctus) in the USA. J. Biogeogr. 2015, 42, 803–807. [Google Scholar] [CrossRef]
- Taulman, J.F.; Robbins, L.W. Range expansion and distributional limits of the nine-banded armadillo in the United States: An update of Taulman & Robbins (1996). J. Biogeogr. 2014, 41, 1626–1630. [Google Scholar]
- Loughry, W.J.; Perez-Heydrich, C.; McDonough, C.M.; Oli, M.K. Population Dynamics and Range Expansion in Nine-Banded Armadillos. PLoS ONE 2013, 8, e68311. [Google Scholar] [CrossRef] [PubMed]
- Freeman, P.W.; Genoways, H.H. Recent northern records of the nine-banded armadillo (Dasypodidae) in Nebraska. Southwest. Nat. 1998, 43, 491–495. [Google Scholar]
- Humphrey, S.R. Zoogeography of the nine-banded armadillo (Dasypus novemcinctus) in the United States. BioScience 1974, 24, 457–462. [Google Scholar] [CrossRef]
- Gammons, D.J.; Mengak, M.T.; Conner, L.M. Translocation of nine-banded armadillos. Hum. Wildl. Confl. 2009, 3, 64–71. [Google Scholar]
- Loughry, W.J.; McDonough, C.M. The Nine-Banded Armadillo: A Natural History; University of Oklahoma Press: Norman, OK, USA, 2013. [Google Scholar]
- Haywood, C.J.; Nielsen, C.K.; Jiménez, F.A. Potential Distribution of Colonizing Nine-Banded Armadillos at Their Northern Range Edge. Diversity 2021, 13, 266. [Google Scholar] [CrossRef]
- Haywood, C.J.; Nielsen, C.K.; Jiménez, F.A. Live-Capture Techniques for Colonizing Nine-Banded Armadillos. Northeast. Nat. 2021, 28, 169–178. [Google Scholar] [CrossRef]
- McDonough, C.M.; DeLaney, M.J.; Quoc Le, P.; Blackmore, M.S.; Loughry, W.J. Burrow characteristics and habitat associations of armadillos in Brazil and the United States of America. Rev. Biol. Trop. 2000, 48, 109–120. [Google Scholar]
- Rodrigues, T.F.; Chiarello, A.G. Native forests within and outside protected areas are key for nine-banded armadillo (Dasypus novemcinctus) occupancy in agricultural landscapes. Agric. Ecosyst. Environ. 2018, 266, 133–141. [Google Scholar] [CrossRef]
- DeGregorio, B.A.; Gale, C.; Lassiter, E.V.; Massey, A.; Roberts, C.P.; Veon, J.T. Nine-banded armadillo (Dasypus novemcinctus) activity patterns are influenced by human activity. Ecol. Evol. 2021, 11, 15874–15881. [Google Scholar] [CrossRef]
- Clark, W.K. Ecological Life History of the Armadillo in the Eastern Edwards Plateau Region. Am. Midl. Nat. 1951, 46, 337–358. [Google Scholar] [CrossRef]
- Butler, Z.P. Assessing the Ecological Roles of Nine-banded Armadillos on Georgia’s Barrier Islands. Ph.D. Thesis, University of Georgia, Athens, GA, USA, 2020. [Google Scholar]
- Lamb, B.D.; Anderson, C.D.; McDonough, C.M.; Lockhart, J.M.; Butler, Z.P. Comparison of Gopher Tortoise and Nine Banded Armadillo commensal fauna. Ga. J. Sci. 2020, 78, 39. [Google Scholar]
- DeGregorio, B.A.; Veon, J.T.; Massey, A. Wildlife associates of nine-banded armadillo (Dasypus novemcinctus) burrows in Arkansas. Ecol. Evol. 2022, 12, e8858. [Google Scholar] [CrossRef]
- Oliveira, I.V.P.D.M.; Deps, P.D.; Antunes, J.M.A.D.P. Armadillos and leprosy: From infection to biological model. Rev. Inst. Med. Trop. São Paulo 2019, 61, e44. [Google Scholar] [CrossRef] [PubMed]
- McDonough, C.M.; Loughry, W.J. Influences on Activity Patterns in a Population of Nine-Banded Armadillos. J. Mammal. 1997, 78, 932–941. [Google Scholar] [CrossRef]
- Harmsen, B.J.; Foster, R.J.; Silver, S.C.; Ostro, L.E.; Doncaster, C. Jaguar and puma activity patterns in relation to their main prey. Mamm. Biol. 2011, 76, 320–324. [Google Scholar] [CrossRef]
- Greenberg, S.; Godin, T.; Whittington, J. Design patterns for wildlife-related camera trap image analysis. Ecol. Evol. 2019, 9, 13706–13730. [Google Scholar] [CrossRef] [PubMed]
- Forrester, T.; O’Brien, T.; Fegraus, E.; Jansen, P.; Palmer, J.; Kays, R.; Ahumada, J.; Stern, B.; McShea, W. An Open Standard for Camera Trap Data. Biodivers. Data J. 2016, 4, e10197. [Google Scholar] [CrossRef] [PubMed]
- MacKenzie, D.I.; Nichols, J.D.; Lachman, G.B.; Droege, S.; Royle, J.A.; Langtimm, C.A. Estimating site occupancy rates when detection probabilities are less than one. Ecology 2002, 83, 2248–2255. [Google Scholar] [CrossRef]
- Trolle, M.; Kéry, M. Estimation of ocelot density in the Pantanal using capture-recapture analysis of camera-trapping data. J. Mammal. 2003, 84, 607–614. [Google Scholar] [CrossRef]
- Linden, D.W.; Roloff, G.J. Retained structures and bird communities in clearcut forests of the Pacific Northwest, USA. For. Ecol. Manag. 2013, 310, 1045–1056. [Google Scholar] [CrossRef]
- Fuller, A.K.; Linden, D.W.; Royle, J.A. Management decision making for fisher populations informed by occupancy modeling. J. Wildl. Manag. 2016, 80, 794–802. [Google Scholar] [CrossRef]
- Thieurmel, B.; Elmarhraoui, A.; Thieurmel, M.B. Package ‘Suncalc’. R Package Version 0.5. Available online: https://cran.r-project.org/web/packages/suncalc/index.html (accessed on 12 October 2022).
- Aya-Cuero, C.; Trujillo, F.; Mosquera-Guerra, F.; Chacón-Pacheco, J.; Caicedo, D.; Franco-León, N.; Superina, M. Distribution of armadillos in Colombia, with an analysis of ecoregions and protected areas inhabited. Mammal Res. 2019, 64, 569–580. [Google Scholar] [CrossRef]
- Norris, D.; Michalski, F.; Peres, C.A. Habitat patch size modulates terrestrial mammal activity patterns in Amazonian forest fragments. J. Mammaology 2010, 91, 551–560. [Google Scholar] [CrossRef]
- Gaynor, K.M.; Hojnowski, C.E.; Carter, N.H.; Brashares, J.S. The influence of human disturbance on wildlife nocturnality. Science 2018, 360, 1232–1235. [Google Scholar] [CrossRef]
- Abba, A.M.; Vizcaíno, S.F.; Cassini, M.H. Effects of Land Use on the Distribution of Three Species of Armadillos in the Argentinean Pampas. J. Mammal. 2007, 88, 502–507. [Google Scholar] [CrossRef]
- George, S.L.; Crooks, K.R. Recreation and large mammal activity in an urban nature reserve. Biol. Conserv. 2006, 133, 107–117. [Google Scholar] [CrossRef]
- Zapata-Ríos, G.; Branch, L.C. Altered activity patterns and reduced abundance of native mammals in sites with feral dogs in the high Andes. Biol. Conserv. 2016, 193, 9–16. [Google Scholar] [CrossRef]
- Pardo, L.E.; Edwards, W.; Campbell, M.J.; Gómez-Valencia, B.; Clements, G.R.; Laurance, W.F. Effects of oil palm and human presence on activity patterns of terrestrial mammals in the Colombian Llanos. Mamm. Biol. 2021, 101, 775–789. [Google Scholar] [CrossRef]
- McNab, B.K. Energetics and the Limits to a Temperate Distribution in Armadillos. J. Mammal. 1980, 61, 606–627. [Google Scholar] [CrossRef]
- Hedblom, M.; Knez, I.; Gunnarsson, B. Bird diversity improves the well-being of city residents. In Ecology and Conservation of Birds in Urban Environments; Springer: Cham, Switzerland, 2017; pp. 287–306. [Google Scholar]
- Hansen, C.P.; Parsons, A.W.; Kays, R.; Millspaugh, J.J. Does use of backyard resources explain the abundance of urban wildlife? Front. Ecol. Evol. 2020, 8, 570771. [Google Scholar] [CrossRef]
- Gallo, T.; Fidino, M.; Lehrer, E.W.; Magle, S.B. Mammal diversity and metacommunity dynamics in urban green spaces: Implications for urban wildlife conservation. Ecol. Appl. 2017, 27, 2330–2341. [Google Scholar] [CrossRef] [PubMed]
- Johansson, E.P.; DeGregorio, B.A. The Effects of Landscape and Yard Features on Mammal Diversity in Residential Yards. Urban Ecosyst. 2023, in press.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).