Substratum Raking Can Restore Interstitial Habitat Quality in Swedish Freshwater Pearl Mussel Streams
Abstract
1. Introduction
2. Materials and Methods
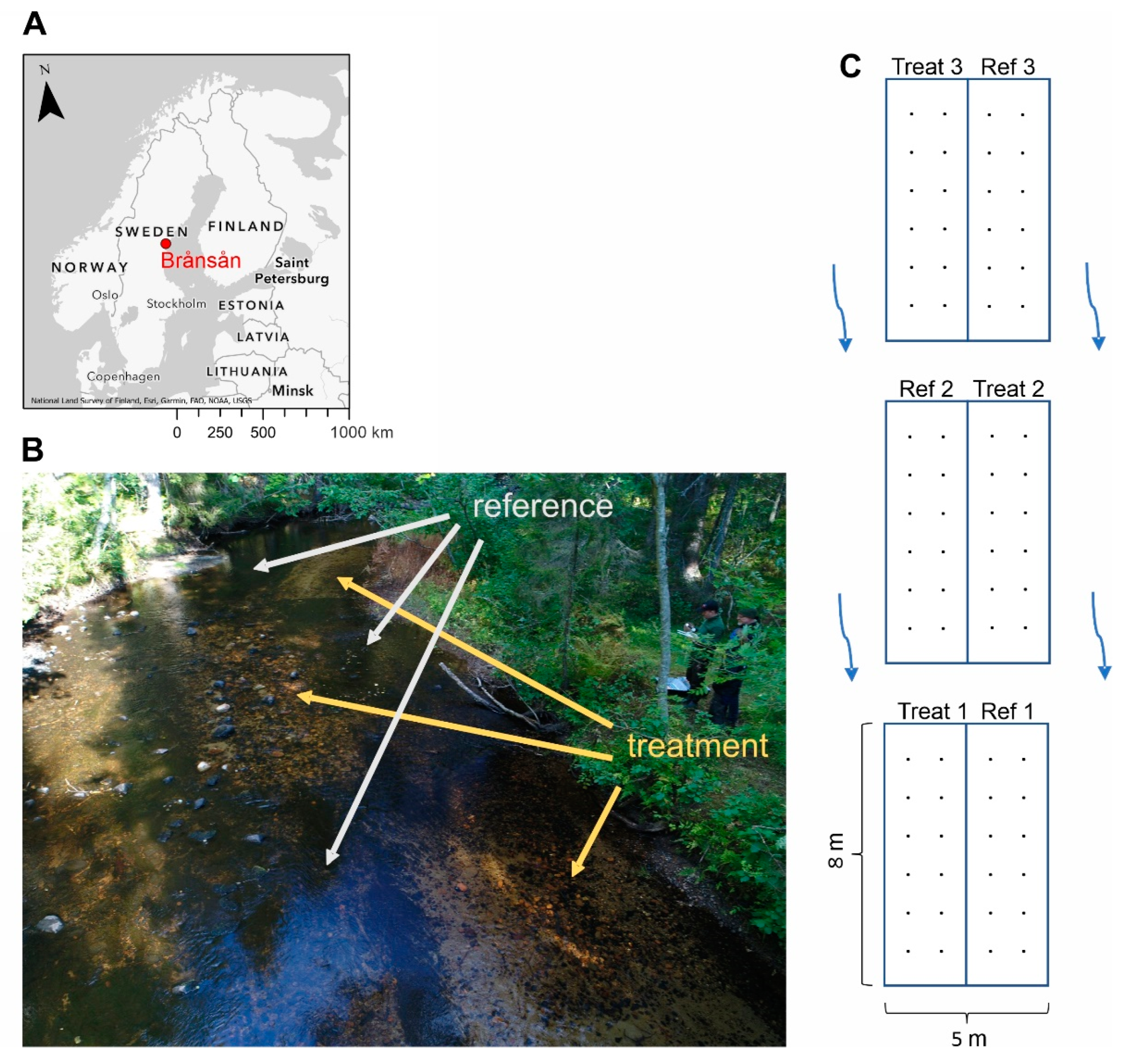
2.1. Study Area
2.2. Substratum Restoration
2.3. Stream Bed Quality Assessment
2.4. Data Analyses
3. Results
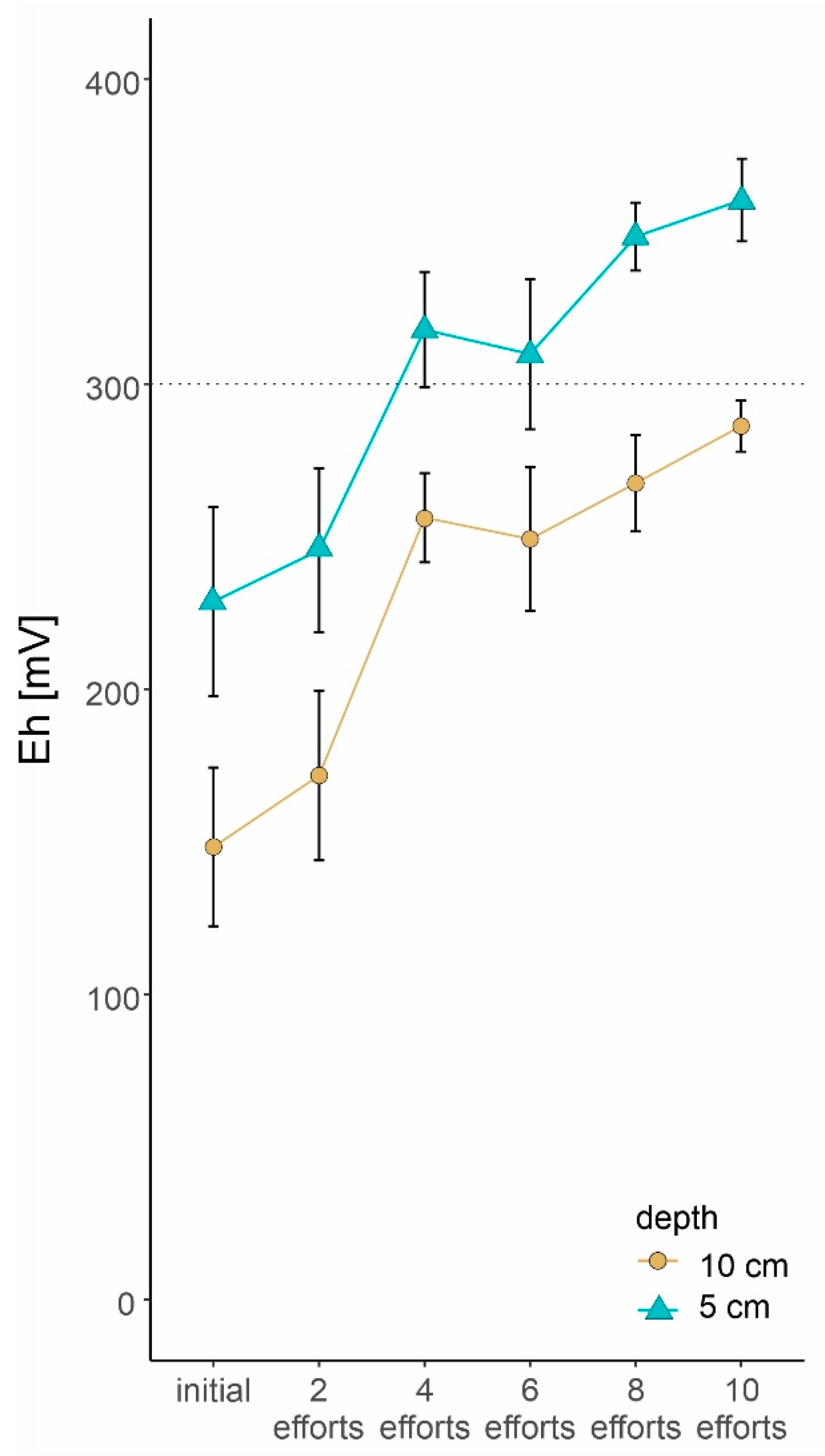
3.1. Raking Effort
3.2. Restoration Effects
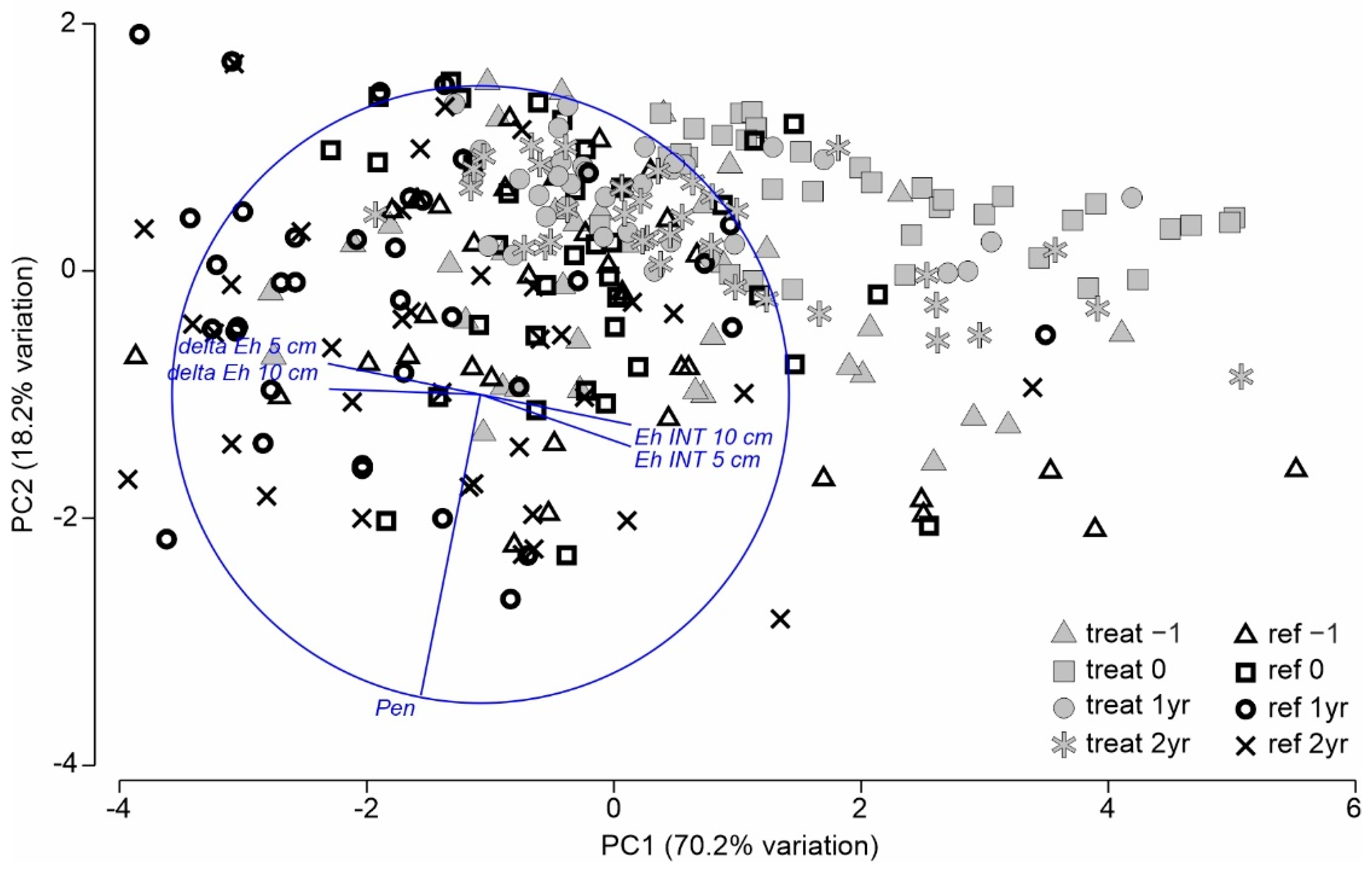
3.2.1. Multivariate Analysis
3.2.2. Univariate Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Jähnig, S.C.; Baranov, V.; Altermatt, F.; Cranston, P.; Friedrichs-Manthey, M.; Geist, J.; He, F.; Heino, J.; Hering, D.; Hölker, F.; et al. Revisiting global trends in freshwater insect biodiversity. WIREs Water 2021, 8, e1506. [Google Scholar] [CrossRef]
- Quinn, J.M.; Davies-Colley, R.J.; Hickey, C.W.; Vickers, M.L.; Ryan, P.A. Effects of clay discharges on streams. Hydrobiologia 1992, 248, 235–247. [Google Scholar] [CrossRef]
- Mary, N.; Marmonier, P. First survey of interstitial fauna in New Caledonian rivers: Influence of geological and geomorphological characteristics. Hydrobiologia 2000, 418, 199–208. [Google Scholar] [CrossRef]
- Boulton, A.; Findlay, S.; Marmonier, P.; Stanley, E.H.; Valett, H. The functional significance of the hypoheic zone in streams and rivers. Annu. Rev. Ecol. Syst. 1998, 29, 59–81. [Google Scholar] [CrossRef]
- Brunke, M. Colmation and depth filtration within streambeds: Retention of particles in hyporheic interstices. Int. Rev. Hydrobiol. 1999, 84, 99–117. [Google Scholar] [CrossRef]
- Hancock, P.J. Human impacts on the stream-groundwater exchange zone. Environ. Manag. 2002, 29, 763–781. [Google Scholar] [CrossRef]
- Mueller, M.; Pander, J.; Geist, J. Comprehensive analysis of >30 years of data on stream fish population trends and conservation status in Bavaria, Germany. Biol. Conserv. 2018, 226, 311–320. [Google Scholar] [CrossRef]
- Duerregger, A.; Pander, J.; Palt, M.; Mueller, M.; Nagel, C.; Geist, J. The importance of stream interstitial conditions for the early-life-stage development of the European nase (Chondrostoma nasus L.). Ecol. Freshw. Fish 2018, 27, 920–932. [Google Scholar] [CrossRef]
- Jowett, I.G.; Richardson, J.; McDowall, R.M. Relative effects of in-stream habitat and land use on fish distribution and abundance in tributaries of the Grey River, New Zealand. N. Z. J. Mar. Freshw. Res. 1996, 30, 463–475. [Google Scholar] [CrossRef]
- Mueller, M.; Bierschenk, A.M.; Bierschenk, B.M.; Pander, J.; Geist, J. Effects of multiple stressors on the distribution of fish communities in 203 headwater streams of Rhine, Elbe and Danube. Sci. Total Environ. 2020, 703, 134523. [Google Scholar] [CrossRef]
- Sternecker, K.; Denic, M.; Geist, J. Timing matters: Species-specific interactions between spawning time, substrate quality, and recruitment success in three salmonid species. Ecol. Evol. 2014, 4, 2749–2758. [Google Scholar] [CrossRef] [PubMed]
- Geist, J. Strategies for the conservation of endangered freshwater pearl mussels (Margaritifera margaritifera L.): A synthesis of Conservation Genetics and Ecology. Hydrobiologia 2010, 644, 69–88. [Google Scholar] [CrossRef]
- Geist, J.; Auerswald, K. Physicochemical stream bed characteristics and recruitment of the freshwater pearl mussel (Margaritifera margaritifera). Freshw. Biol. 2007, 52, 2299–2316. [Google Scholar] [CrossRef]
- Boon, P.J.; Cooksley, S.L.; Geist, J.; Killeen, I.J.; Moorkens, E.A.; Sime, I. Developing a standard approach for monitoring freshwater pearl mussel (Margaritifera margaritifera) populations in European rivers. Aquat. Conserv. Mar. Freshw. Ecosyst. 2019, 29, 1365–1379. [Google Scholar] [CrossRef]
- Wild, R.; Nagel, C.; Geist, J. Climate change effects on hatching success and embryonic development of fish: Assessing multiple stressor responses in a large-scale mesocosm study. Sci. Total Environ. 2023, 893, 164834. [Google Scholar] [CrossRef]
- Geist, J.; Porkka, M.; Kuehn, R. The status of host fish populations and fish species richness in European freshwater pearl mussel (Margaritifera margaritifera) streams. Aquat. Conserv. Mar. Freshw. Ecosyst. 2006, 16, 251–266. [Google Scholar] [CrossRef]
- Taeubert, J.-E.; Geist, J. The relationship between the freshwater pearl mussel (Margaritifera margaritifera) and its hosts. Biol. Bull. 2017, 44, 67–73. [Google Scholar] [CrossRef]
- Sternecker, K.; Wild, R.; Geist, J. Effects of substratum restoration on salmonid habitat quality in a subalpine stream. Environ. Biol. Fishes 2013, 96, 1341–1351. [Google Scholar] [CrossRef]
- Mueller, M.; Pander, J.; Geist, J. The ecological value of stream restoration measures: An evaluation on ecosystem and target species scales. Ecol. Eng. 2014, 62, 129–139. [Google Scholar] [CrossRef]
- Auerswald, K.; Geist, J. Extent and causes of siltation in a headwater stream bed: Catchment soil erosion is less important than internal stream processes. Land Degrad. Dev. 2018, 29, 737–748. [Google Scholar] [CrossRef]
- Pander, J.; Mueller, M.; Geist, J. A Comparison of Four Stream Substratum Restoration Techniques Concerning Interstitial Conditions and Downstream Effects. River Res. Appl. 2015, 31, 239–255. [Google Scholar] [CrossRef]
- Nagel, C.; Mueller, M.; Pander, J.; Geist, J. Making up the bed: Gravel cleaning as a contribution to nase (Chondrostoma nasus L.) spawning and recruitment success. Aquat. Conserv. Mar. Freshw. Ecosyst. 2020, 30, 2269–2283. [Google Scholar] [CrossRef]
- Sarriquet, P.E.; Bordenave, P.; Marmonier, P. Effects of bottom sediment restoration on interstitial habitat characteristics and benthic macroinvertebrate assemblages in a headwater stream. River Res. Appl. 2007, 23, 815–828. [Google Scholar] [CrossRef]
- Pulg, U.; Barlaup, B.T.; Sternecker, K.; Trepl, L.; Unfer, G. Restoration of spawning habitats of brown trout (Salmo trutta) in a regulated chalk stream. River Res. Appl. 2013, 29, 172–182. [Google Scholar] [CrossRef]
- Geist, J.; Hawkins, S.J. Habitat recovery and restoration in aquatic ecosystems: Current progress and future challenges. Aquat. Conserv. Mar. Freshw. Ecosyst. 2016, 26, 942–962. [Google Scholar] [CrossRef]
- Knott, J.; Mueller, M.; Pander, J.; Geist, J. Effectiveness of catchment erosion protection measures and scale-dependent response of stream biota. Hydrobiologia 2019, 830, 77–92. [Google Scholar] [CrossRef]
- Collins, A.L.; Anthony, S.G.; Hawley, J.; Turner, T. Predicting potential change in agricultural sediment inputs to rivers across England and Wales by 2015. Mar. Freshw Res. 2009, 60, 626–637. [Google Scholar] [CrossRef]
- Davies, B.; Biggs, J.; Williams, P.; Thompson, S. Making agricultural landscapes more sustainable for freshwater biodiversity: A case study from southern England. Aquat. Conserv. Mar. Freshw. Ecosyst. 2009, 19, 439–447. [Google Scholar] [CrossRef]
- Kemp, P.; Sear, D.; Collins, A.; Naden, P.; Jones, I. The impacts of fine sediment on riverine fish. Hydrol. Process. 2011, 25, 1800–1821. [Google Scholar] [CrossRef]
- BS EN 16859:2017; Water Quality—Guidance Standard on Monitoring Freshwater Pearl Mussel (Margaritifera margaritifera) Populations and Their Environment. British Standards Institution: London, UK, 2017.
- Braun, A.; Auerswald, K.; Geist, J. Drivers and spatio-temporal extent of hyporheic patch variation: Implications for sampling. PLoS ONE 2012, 7, e42046. [Google Scholar] [CrossRef]
- Jones, J.I.; Collins, A.L.; Naden, P.S.; Sear, D.A. The relationshio between fine sediment ans macrophytes in rivers. River Res. Appl. 2012, 28, 1006–1018. [Google Scholar] [CrossRef]
- Boeker, C.; Lueders, T.; Mueller, M.; Pander, J.; Geist, J. Alteration of physico-chemical and microbial properties in freshwater substrates by burrowing invertebrates. Limnologica 2016, 59, 131–139. [Google Scholar] [CrossRef]
- Denic, M.; Geist, J. Linking stream sediment deposition and aquatic habitat quality in pearl mussel streams: Implications for conservation. River Res. Appl. 2015, 31, 943–952. [Google Scholar] [CrossRef]
- Hoess, R.; Geist, J. Spatiotemporal variation of streambed quality and fine sediment deposition in five freshwater pearl mussel streams, in relation to extreme drought, strong rain and snow melt. Limnologica 2020, 85, 125833. [Google Scholar] [CrossRef]
- Bierschenk, A.M.; Mueller, M.; Pander, J.; Geist, J. Impact of catchment land use on fish community composition in the headwater areas of Elbe, Danube and Main. Sci. Total Environ. 2019, 652, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Shields, F.D.; Copeland, R.R.; Klingeman, P.C.; Doyle, M.W.; Simon, A. Design for Stream Restoration. J. Hydraul. Eng. 2003, 129, 575–584. [Google Scholar] [CrossRef]
- Duffin, J.; Yager, E.M.; Buffington, J.M.; Benjankar, R.; Borden, C.; Tonina, D. Impact of flow regulation on stream morphology and habitat quality distribution. Sci. Total Environ. 2023, 878, 163016. [Google Scholar] [CrossRef]
- Sand-jensen, K. Influence of submerged macrophytes on sediment composition and near-bed flow in lowland streams. Freshw. Biol. 1998, 39, 663–679. [Google Scholar] [CrossRef]
- Geist, J. Seven steps towards improving freshwater conservation. Aquat. Conserv. Mar. Freshw. Ecosyst. 2015, 25, 447–453. [Google Scholar] [CrossRef]
- Rubin, Z.; Kondolf, G.M.; Rios-Touma, B. Evaluating Stream Restoration Projects: What Do We Learn from Monitoring? Water 2017, 9, 174. [Google Scholar] [CrossRef]
- Maasri, A.; Jähnig, S.C.; Adamescu, M.C.; Adrian, R.; Baigun, C.; Baird, D.J.; Batista-Morales, A.; Bonada, N.; Brown, L.E.; Cai, Q.; et al. A global agenda for advancing freshwater biodiversity research. Ecol. Lett. 2022, 25, 255–263. [Google Scholar] [CrossRef] [PubMed]


| t = −1 | t = 0 | t = 1 | t = 2 | |||||
|---|---|---|---|---|---|---|---|---|
| Treat | Ref | Treat | Ref | Treat | Ref | Treat | Ref | |
| Eh FW (mV) | 560.1 ± 14.6 | 555.6 ± 13.1 | 547.2 ± 8.7 | 542.8 ± 9.7 | 544.0 ± 9.4 | 541.7 ± 22.2 | 583.5 ± 13.0 | 592.8 ± 23.9 |
| Eh INT 5 cm (mV) | 394.8 ± 59.4 | 382.5 ± 74.8 | 446.4 ± 62.0 | 368.5 ± 50.5 | 382.1 ± 56.9 | 318.8 ± 68.9 | 419.8 ± 69.3 | 358.0 ± 67.1 |
| Eh INT 10 cm (mV) | 293.6 ± 62.1 | 284.1 ± 68.1 | 347.6 ± 51.2 | 268.4 ± 39.9 | 284.9 ± 10.5 | 224.9 ± 40.5 | 318.1 ± 45.9 | 268.1 ± 52.2 |
| Pen (kg/cm²) | 0.732 ± 0.358 | 0.912 ± 0.400 | 0.235 ± 0.176 | 0.694 ± 0.451 | 0.395 ± 0.163 | 0.947 ± 0.510 | 0.470 ± 0.148 | 1.114 ± 0.477 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geist, J.; Hoess, R.; Rytterstam, J.; Söderberg, H. Substratum Raking Can Restore Interstitial Habitat Quality in Swedish Freshwater Pearl Mussel Streams. Diversity 2023, 15, 869. https://doi.org/10.3390/d15070869
Geist J, Hoess R, Rytterstam J, Söderberg H. Substratum Raking Can Restore Interstitial Habitat Quality in Swedish Freshwater Pearl Mussel Streams. Diversity. 2023; 15(7):869. https://doi.org/10.3390/d15070869
Chicago/Turabian StyleGeist, Juergen, Rebecca Hoess, Johan Rytterstam, and Håkan Söderberg. 2023. "Substratum Raking Can Restore Interstitial Habitat Quality in Swedish Freshwater Pearl Mussel Streams" Diversity 15, no. 7: 869. https://doi.org/10.3390/d15070869
APA StyleGeist, J., Hoess, R., Rytterstam, J., & Söderberg, H. (2023). Substratum Raking Can Restore Interstitial Habitat Quality in Swedish Freshwater Pearl Mussel Streams. Diversity, 15(7), 869. https://doi.org/10.3390/d15070869






