Taxonomy and Melanism Patterns of Freshwater Leeches in the Genus Glossiphonia (Hirudinea: Glossiphoniidae) from Northeast Asia †
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sampling
2.2. DNA Data, Phylogenetic, and Phylogeographic Analyses
2.3. Morphological and Anatomical Research
2.4. Range Mapping
2.5. Nomenclatural Acts
3. Results
3.1. Phylogenetic and Phylogeographic Data
3.2. Melanism Patterns in Glossiphonia moorei sp. nov.
3.3. Taxonomic Account
4. Discussion
4.1. Taxonomic Novelties
4.2. Biogeographic Issues
4.3. Melanism and Cryptic Coloration in Glossiphoniid Leeches
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lukin, E.I. Leeches of fresh and brackish water bodies. Fauna USSR 1976, 109, 1–484. (In Russian) [Google Scholar]
- Sawyer, R.T. Leech Biology and Behaviour. Vol. 2. Feeding Biology, Ecology, and Systematics; Clarendon Press: Oxford, UK, 1986; 375p. [Google Scholar]
- Yang, T. Annelida Hirudinea (Fauna Sinica); Science Press: Beijing, China, 1996; pp. 1–259. (In Chinese) [Google Scholar]
- Kaygorodova, I.; Bolbat, N.; Bolbat, A. Species delimitation through DNA barcoding of freshwater leeches of the Glossiphonia genus (Hirudinea: Glossiphoniidae) from Eastern Siberia, Russia. J. Zool. Syst. Evol. Res. 2020, 58, 1437–1446. [Google Scholar] [CrossRef]
- Mack, J.; Kvist, S. Improved geographic sampling provides further evidence for the separation of Glossiphonia complanata and Glossiphonia elegans (Annelida: Clitellata: Glossiphoniidae). J. Nat. Hist. 2019, 53, 335–350. [Google Scholar] [CrossRef]
- Kaygorodova, I.A.; Mandzyak, N.B. Molecular phylogeny of Siberian Glossiphoniidae (Hirudinea). Mol. Biol. 2014, 48, 452–455. [Google Scholar] [CrossRef]
- Jovanović, M.; Haring, E.; Sattmann, H.; Grosser, C.; Pesic, V. DNA barcoding for species delimitation of the freshwater leech genus Glossiphonia from the Western Balkan (Hirudinea, Glossiphoniidae). Biodivers. Data J. 2021, 9, e66347. [Google Scholar] [CrossRef]
- Bolotov, I.N.; Kondakov, A.V.; Eliseeva, T.A.; Aksenova, O.V.; Babushkin, E.S.; Bespalaya, Y.V.; Chertoprud, E.S.; Dvoryankin, G.A.; Gofarov, M.Y.; Klass, A.L.; et al. Cryptic taxonomic diversity and high-latitude melanism in the glossiphoniid leech assemblage from the Eurasian Arctic. Sci. Rep. 2022, 12, 20630. [Google Scholar] [CrossRef]
- Huh, M.K.; Joo, W.H.; Choi, C.J.; Seo, J.Y. Community structure and diversity across spatial scales of macrobenthos in the Seomjin River. J. Life Sci. 2012, 22, 1286–1294. [Google Scholar] [CrossRef]
- Shin, H.S.; Seo, Y.J.; Jung, S.W. Species diversity and characteristic communities structure of benthic macroinvertebrates in Juwangsan National Park, South Korea. J. Natl. Park Res. 2019, 10, 202–217. [Google Scholar]
- Moore, J.P. The leeches of the U. S. National Museum. Proc. U. S. Natl. Mus. 1898, 21, 543–563. [Google Scholar] [CrossRef]
- Grube, E. Beschreibungen einiger Egel-Arten. Arch. Naturgesch. 1871, 37, 87–121. [Google Scholar]
- Livanow, L. Die Hirudineen-Gattung Hemiclepsis Vejd. Zool. Jahrb. Abt. Syst. Geogr. Biol. Tiere 1902, 3, 339–362. [Google Scholar]
- Moore, J.P.; Meyer, M.C. Leeches (Hirudinea) from Alaskan and adjacent waters. Wasmann J. Biol. 1951, 9, 11–77. [Google Scholar]
- Lukin, E.I. On the record of an interesting leech species, Boreobdella verrucata (Fr. Muller), in the USSR. Zool. Zhurnal 1956, 35, 1417–1419. (In Russian) [Google Scholar]
- Klemm, D.J. Leeches (Annelida: Hirudinea) of North America. Water Pollution Control Research Series EPA-600/3-82-025; U.S. Environmental Protection Agency: Washington, DC, USA, 1982; pp. 1–177. [Google Scholar]
- Moser, W.E.; Govedich, F.R.; Oceguera-Figueroa, A.; Richardson, D.J.; Phillips, A.J. Subclass Hirudinida. In Thorp and Covich’s Freshwater Invertebrates. Volume 2. Keys to Nearctic Fauna, 4th ed.; Thorp, J.H., Rogers, D.C., Eds.; Academic Press, Elsevier: London, UK, 2016; pp. 244–259. [Google Scholar]
- Ride, W.D.L.; Cogger, H.G.; Dupuis, C.; Kraus, O.; Minelli, A.; Thompson, F.C.; Tubbs, P.K. (Eds.) International Code of Zoological Nomenclature, 4th ed.; The International Trust for Zoological Nomenclature: London, UK, 1999. [Google Scholar]
- Bolotov, I.N.; Klass, A.L.; Kondakov, A.V.; Vikhrev, I.V.; Bespalaya, Y.V.; Gofarov, M.Y.; Filippov, B.Y.; Bogan, A.E.; Lopes-Lima, M.; Zau, L.; et al. Freshwater mussels house a diverse mussel-associated leech assemblage. Sci. Rep. 2019, 9, 16449. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Villesen, P. FaBox: An online toolbox for fasta sequences. Mol. Ecol. Notes 2007, 7, 965–968. [Google Scholar] [CrossRef]
- Minh, B.Q.; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; Von Haeseler, A.; Lanfear, R. IQ-TREE 2: New models and efficient methods for phylogenetic inference in the genomic era. Mol. Biol. Evol. 2020, 37, 1530–1534. [Google Scholar] [CrossRef]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.; Von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef]
- Hoang, D.T.; Chernomor, O.; von Haeseler, A.; Minh, B.Q.; Vinh, L.S. UFBoot2: Improving the ultrafast bootstrap approximation. Mol. Biol. Evol. 2017, 35, 518–522. [Google Scholar] [CrossRef]
- Light, J.E.; Siddall, M.E. Phylogeny of the leech family Glossiphoniidae based on mitochondrial gene sequences and morphological data. J. Parasitol. 1999, 85, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Siddall, M.E.; Budinoff, R.B.; Borda, E. Phylogenetic evaluation of systematics and biogeography of the leech family Glossiphoniidae. Invertebr. Syst. 2005, 19, 105–112. [Google Scholar] [CrossRef]
- Bolbat, A.; Bukin, Y.; Kaygorodova, I. Genome-Based Taxa Delimitation (GBTD): A new approach. Diversity 2022, 14, 948. [Google Scholar] [CrossRef]
- Bolotov, I.N.; Eliseeva, T.A.; Kondakov, A.V.; Konopleva, E.S.; Palatov, D.M.; Sokolova, A.M.; Vikhrev, I.V.; Gofarov, M.Y.; Bovykina, G.V.; Chan, N.; et al. Hidden shelter-like associations of minute Alboglossiphonia leeches (Hirudinea: Glossiphoni-idae) with sedentary animals and molluscs. Limnologica 2022, 97, 126028. [Google Scholar] [CrossRef]
- Apakupakul, K.; Siddall, M.E.; Burreson, E.M. Higher level relationships of leeches (Annelida: Clitellata: Euhirudinea) based on morphology and gene sequences. Mol. Phylogenet. Evol. 1999, 12, 350–359. [Google Scholar] [CrossRef]
- Bandelt, H.J.; Forster, P.; Röhl, A. Median-joining networks for inferring intraspecific phylogenies. Mol. Biol. Evol. 1999, 16, 37–48. [Google Scholar] [CrossRef]
- Nesemann, H.; Neubert, E. Süßwasserfauna von Mitteleuropa, Bd. 6/2, Annelida: Clitellata: Branchiobdellida, Acanthobdellea, Hirudinea; Spektrum Akademischer Verlag: Heidelberg, Germany, 1999; 178p. [Google Scholar]
- Lukin, E.I.; Epshtein, V.M. Leeches of Baikal. In 10th Meeting for Parasitological Problems and Natural Focal Diseases; USSR Academy of Sciences: Leningrad, Russia, 1959; pp. 189–190. (In Russian) [Google Scholar]
- Lukin, E.I.; Epshtein, V.M. Leeches of the subfamily Toricinae subfam. nov. and their geographic distribution. Proc. USSR Acad. Sci. 1960, 134, 478–481. (In Russian) [Google Scholar]
- Stschegolev, G.G. A new leech species from Baikal. Russ. Hydrobiol. J. 1922, 1, 136–142. (In Russian) [Google Scholar]
- Grosser, C.; Pešić, V.; Berlajolli, V.; Gligorović, B. Glossiphonia balcanica n. sp. and Dina prokletijaca n. sp. (Hirudinida: Glossiphoniidae, Erpobdellidae)—Two new leeches from Montenegro and Kosovo. Ecol. Montenegrina 2016, 8, 17–26. [Google Scholar] [CrossRef]
- Linnaeus, C. Systema Naturae per Regna Tria Naturae, Secundum Classes, Ordines, Genera, Species, cum Characteribus, Differentiis, Synonymis, Locis; Editio decima, reformata; Laurentius Salvius: Stockholm, Sweden, 1758; Volume 1, pp. 1–532. [Google Scholar]
- Apáthy, S. Analyse der äufseren Körperform der Hirudineen. Mitth. Aus der Zool. Stn. zu Neapel 1888, 8, 153–232. [Google Scholar]
- Nesemann, H. Investigations on two Glossiphonia species (Hirudinea) from running waters of Central Europe with a redescription of Glossiphonia concolor (Apáthy, 1888). Ann. Hist. Nat. Musei Natl. Hung. 1990, 82, 65–74. [Google Scholar]
- Lecaplain, B.; Noël, F. Les Sangsues d’eau Douce du Nord-Ouest de la France (Annelida–Hirudinida)—Normandie, Bretagne, Pays de la Loire—Recherche, Récolte et Identification; GRETIA, UMS PatriNat AFB-CNRS-MNHN: Paris, France, 2019; pp. 1–91. [Google Scholar]
- Darabi-Darestani, K.; Sari, A.; Khomenko, A.; Kvist, S.; Utevsky, S. DNA barcoding of Iranian leeches (Annelida: Clitellata: Hirudinida). J. Zool. Syst. Evol. Res. 2021, 59, 1438–1452. [Google Scholar] [CrossRef]
- Jueg, U.; Zettler, M.L. Bemerkungen zur Egel- und Krebsegelfauna (Hirudinida und Branchiobdellida) Litauens mit einer vorläufigen Checkliste. Lauterbornia 2022, 88, 213–238. [Google Scholar]
- Verrill, A.E. Brief contributions to zoology from the Museum of Yale College; No. XVII, Descriptions of North American fresh-water leeches. Am. J. Sci. 1872, s3-3, 126–139. [Google Scholar] [CrossRef]
- Moser, W.E.; Richardson, D.J.; Hammond, C.I.; Lazo-Wasem, E. Molecular Characterization of Glossiphonia elegans (Verrill, 1872) (Glossiphoniidae: Hirudinida) from its type locality, West River, New Haven County, Connecticut USA. Zootaxa 2012, 60, 57–60. [Google Scholar] [CrossRef]
- Kalbe, L. Glossiphonia complanata nebulosa nov. subspec., eine neue Hirudinee aus Fliessgewassern des Havelgebietes. Mitt. Zool. Mus. Berl. 1964, 40, 141–144. [Google Scholar]
- Müller, F. Hirudinibus Circa Berolinum Hucusque Observatis. Ph.D. Thesis, Universität Berlin, Berlin, Germany, 1844. [Google Scholar]
- Jueg, U.; Michalik, P. Lost and found—Fritz Müller’s type material of Glossiphonia verrucata (Fr. Müller, 1844) (Hirudinida, Glossiphoniidae) with notes on the leech fauna of Lake Tegel in Berlin (Germany). Evol. Syst. 2018, 2, 163–168. [Google Scholar] [CrossRef]
- Carena, H. Supplément a la Monographie du Genre Hirudo. Memoire della Reale Accademia delle Scienze di Torino, Classe di Scienze Fisiche, Matematiche e Naturali 1824, 28, 331–337. [Google Scholar]
- Sket, B. To knowledge of the leech fauna (Hirudinea) in Yugoslavia. Dissertationes Academia Scientiarum et Artium Slovenica, Classis IV: Historia Naturalis et Medicina 1968, 9, 127–197. (In Slovenian) [Google Scholar]
- Košel, V. Batracobdella slovaca sp. n., a new species of leech in southwest Slovakia (Hirudinoidea, Glossiphonidae). Biologia 1973, 28, 87–90. (In Slovakian) [Google Scholar]
- Nesemann, H.; Csányi, B. Description of Batracobdelloides moogi n. sp., a leech genus and species new to the European fauna with notes on the identity of Hirudo paludosa Carena, 1824 (Hirudinea: Glossiphoniidae). Lauterbornia 1995, 21, 69–78. [Google Scholar]
- Trontelj, P. Glossiphonia slovaca (Košel, 1973) (Hirudinea: Glossiphoniidae) iz Save pri Čatežu: Nova vrsta pijavke za Slovenijo in vprašanje njene taksonomske pripadnosti. Nat. Slov. 2000, 2, 21–27. [Google Scholar]
- Schenkova, J.; Sychra, J.; Košel, V.; Kubova, N.; Horecký, J. Freshwater leeches (Annelida: Clitellata: Hirudinida) of the Czech Republic (Central Europe): Check-list, new records, and remarks on species distributions. Zootaxa 2009, 2227, 32–52. [Google Scholar] [CrossRef]
- Miyadi, D. Limnological survey of the North Kurile Islands. Sonder-Abdruck aus dem Archiv für Hydrobiologie 1937, 31, 433–483. [Google Scholar]
- Miyadi, D. Bottom fauna of the lakes in Kunashiri-sima of the South Kurile Islands. Int. Rev. Der Gesamten Hydrobiol. Und Hydrogr. 1938, 37, 125–163. [Google Scholar]
- Keith, M.M. Notes on some leeches (Hirudinea) from the Yukon Territory, Canada, and Alaska. J. Minn. Acad. Sci. 1955, 23, 103–104. [Google Scholar]
- Oka, A. Synopsis der Japanischen Hirudineen, mit Diagnosen der Neuen Species. Annot. Zool. Jpn. 1910, 7, 165–183. [Google Scholar]
- Nesemann, H. Rediscovery of the leech genus Ancyrobdella (Hirudinea, Glossiphoniidae). Misc. Zool. Hung. 1997, 11, 5–10. [Google Scholar]
- Xu, Z.; Yang, C.; Gofarov, M.Y.; Eliseeva, T.A.; Kondakov, A.V.; Yuan, H.; Bolotov, I.N.; Yang, D. A new freshwater leech species from Asian Swamp Eel stocks in China. Parasitol. Res. 2021, 120, 2769–2778. [Google Scholar] [CrossRef]
- Kaygorodova, I.; Matveenko, E. Diversity of the Piscicola species (Hirudinea, Piscicolidae) in the Eastern Palaearctic with a description of three new species and notes on their biogeography. Diversity 2023, 15, 98. [Google Scholar] [CrossRef]
- Epshtein, V.M. On the origin of the Hirudinea fauna, especially Piscicolidae, in ancient lakes. Lauterbornia 2004, 52, 181–193. [Google Scholar]
- Sawyer, R.T.; Dierst-Davies, K. Observations on the physiology and phylogeny of colour change in marine and freshwater leeches (Annelida: Hirudinea). Hydrobiologia 1974, 44, 215–236. [Google Scholar] [CrossRef]

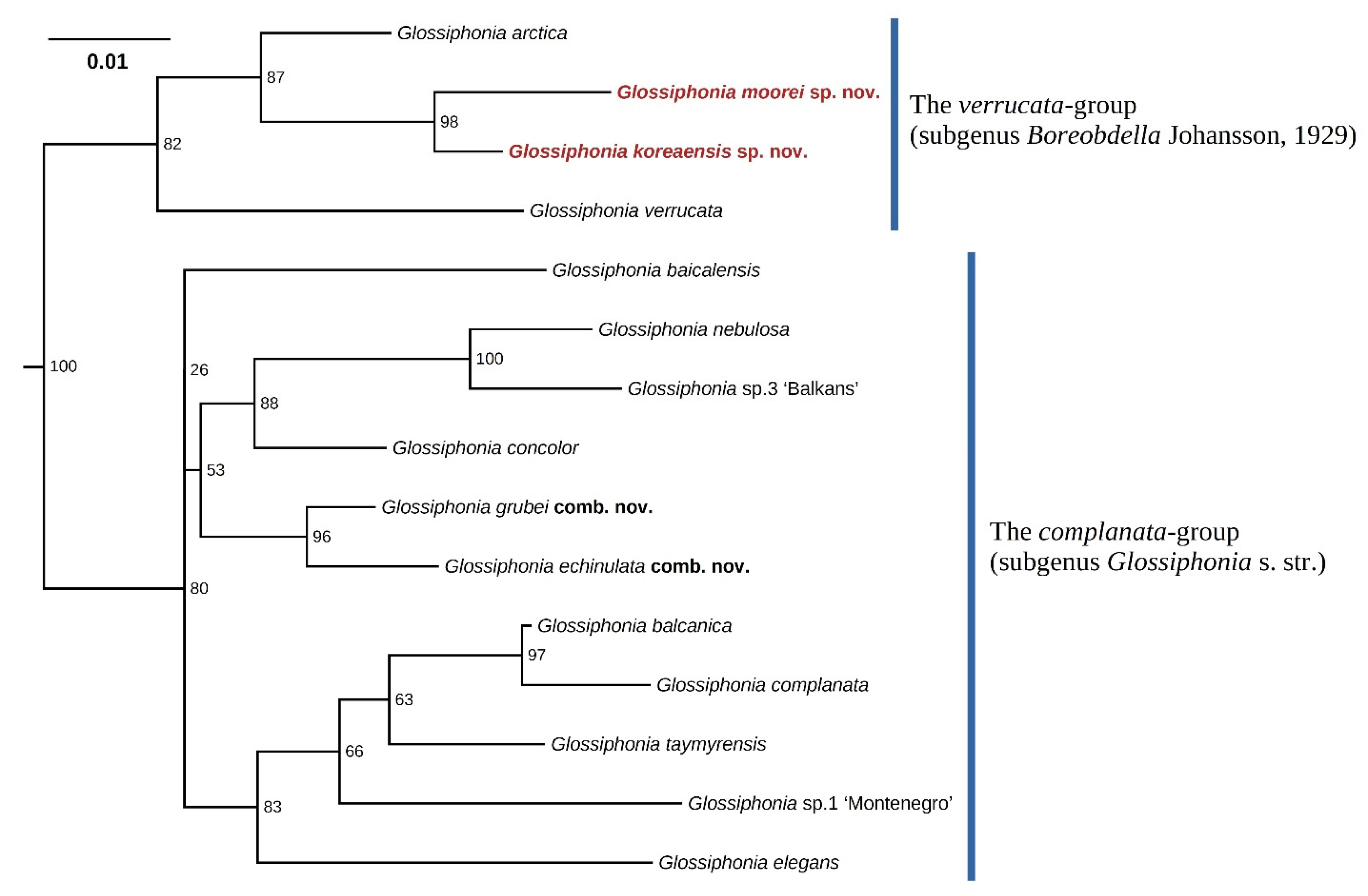
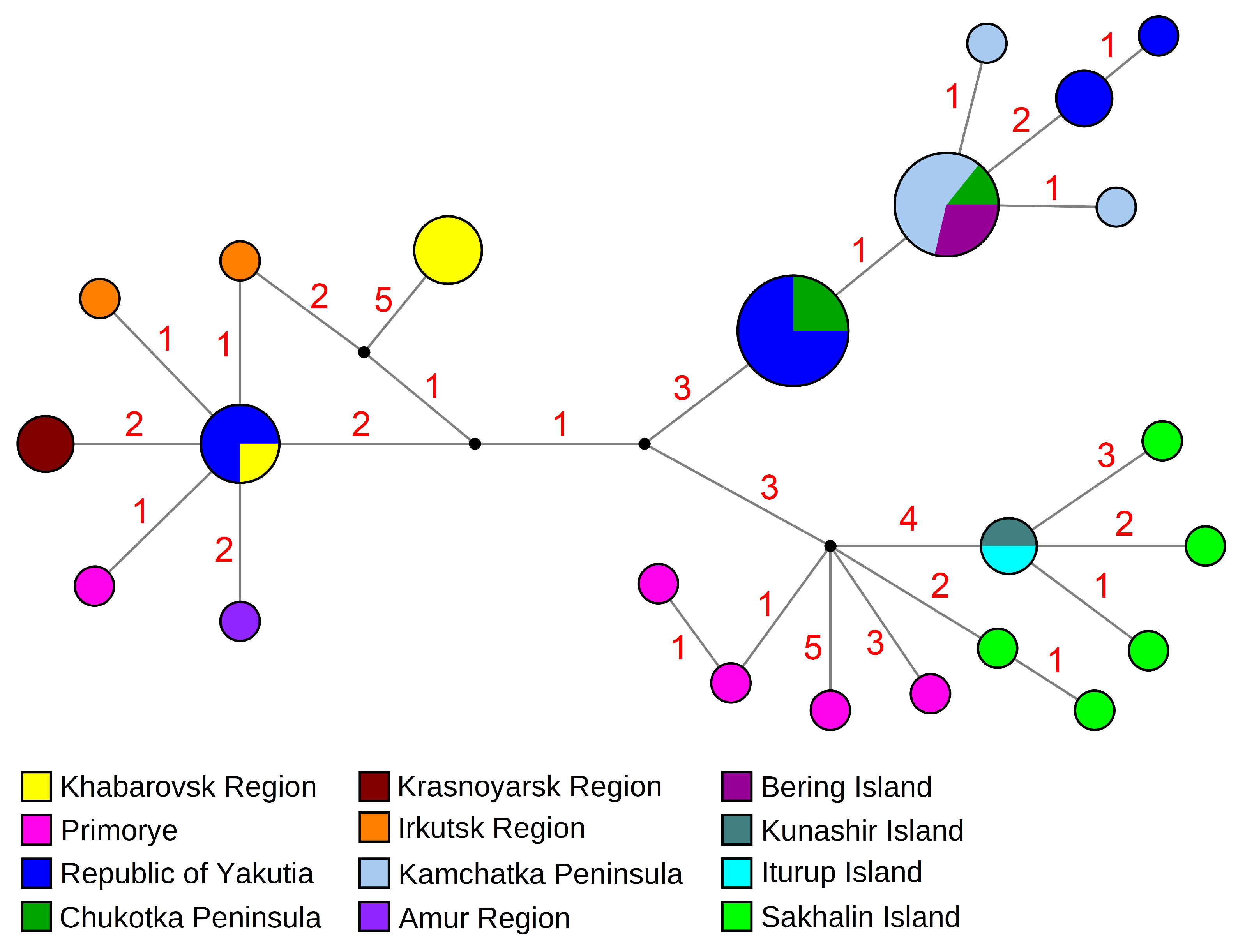



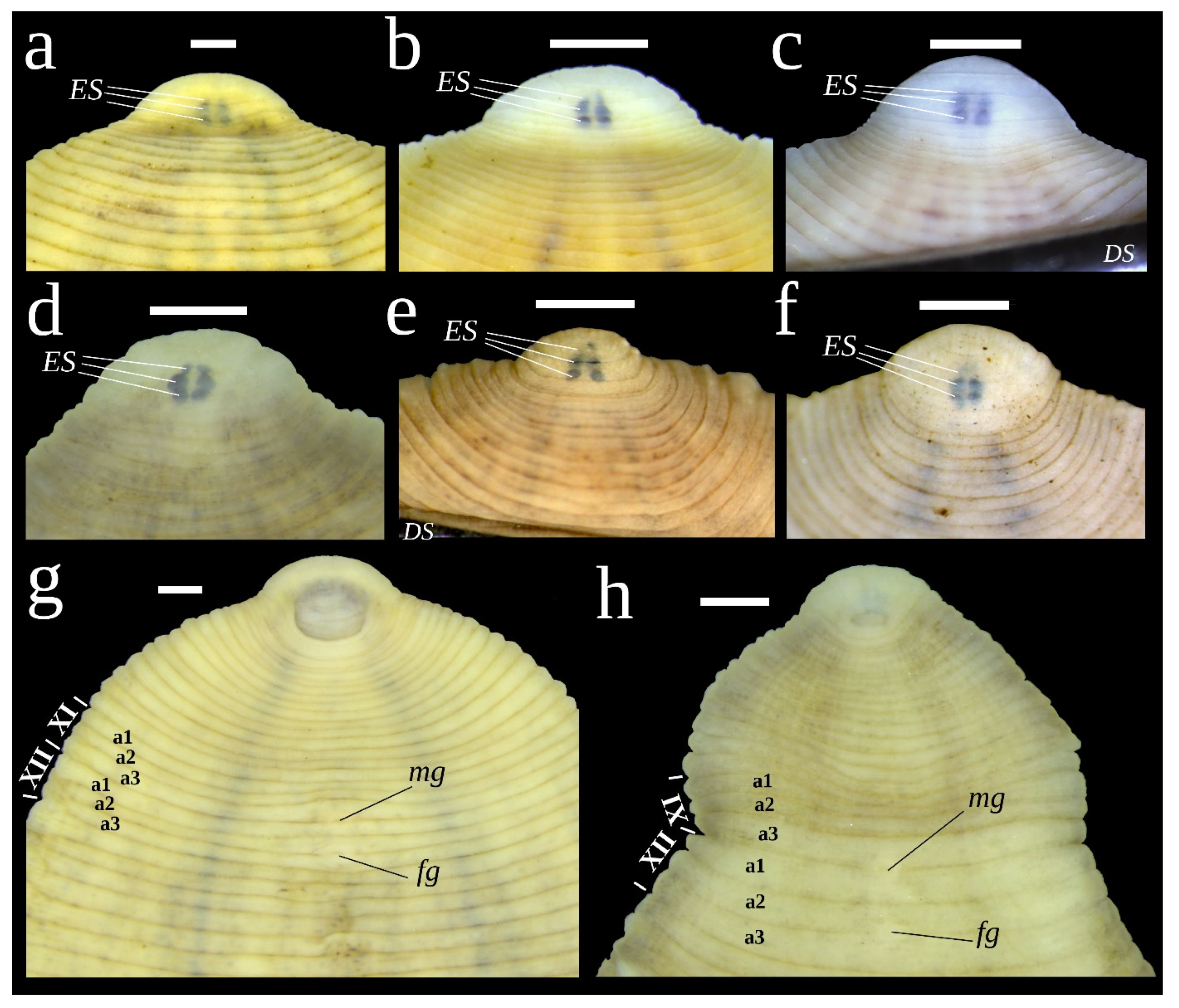




| Taxa | Region | COI Acc. No | 18S rRNA Acc. No | Reference |
|---|---|---|---|---|
| Genus Glossiphonia Johnson, 1816 (in-group) | ||||
| G. arctica Bolotov et al., 2022 | Russia: Polar Urals | ON810735 | ON819028 | Bolotov et al. [8] |
| G. baicalensis (Stschegolew, 1922) | Russia: Lake Baikal, Eastern Siberia | AY047329 | AY962425 | Light and Siddall [26] |
| G. balcanica Grosser & Pešić, 2016 | Russia: Moscow Region | MN295386 | MN312176 | Bolotov et al. [19] |
| G. moorei sp. nov. = G. mollissima sensu Moore, 1898, non Grube, 1871 | Russia: Khabarovsk Region | MN295375 | MN312170 | Bolotov et al. [19] |
| G. complanata (Linnaeus, 1758) | Montenegro | ON841652 | OQ067230 | Bolotov et al. [8]; this study |
| G. elegans (Verrill, 1872) | Canada | ON841597 | OQ024821 | Bolotov et al. [8]; this study |
| G. sp.1 ‘Montenegro’ | Montenegro | ON841644 | OQ110559 | Bolotov et al. [8]; this study |
| G. nebulosa Kalbe, 1964 | Russia: Taymyr Peninsula | ON810698 | ON819015 | Bolotov et al. [8] |
| G. verrucata (F. Müller, 1844) | Russia: Yamal Peninsula | MN295411 | ON819003 | Bolotov et al. [8,19] |
| G. concolor (Apathy, 1888) | Russia: Taymyr Peninsula | ON810692 | ON819012 | Bolotov et al. [8] |
| G. koreaensis sp. nov. = G. sp.2 ‘Korea’ sensu Bolotov et al., 2019 | South Korea | MN295429 | OQ024820 | Bolotov et al. [19]; this study |
| G. taymyrensis Bolotov et al., 2022 | Russia: Taymyr Peninsula | ON810706 | ON819021 | Bolotov et al. [8] |
| G. sp.3 ‘Balkans’ | Italy | AY962459 | AY962432 | Siddall et al. [27] |
| G. grubei (Lukin & Epstein, 1960) comb. nov. | Russia: Lake Baikal | OM257166 | n/a | Bolbat et al. [28] |
| G. echinulata (Grube, 1871) comb. nov. | Russia: Lake Baikal | OM257165 | n/a | Bolbat et al. [28] |
| Genus Alboglossiphonia Lukin, 1976 (outgroup) | ||||
| A. sibirica Bolotov et al., 2022 | Russia: Eastern Siberia | MH286267 | MH286273 | Bolotov et al. [8,19] |
| A. lata (Oka, 1910) | Russian Far East | MN295414 | MN312188 | Bolotov et al. [19] |
| A. pahariensis Nesemann & Sharma, 2007 | India | ON548504 | ON532907 | Bolotov et al. [29] |
| A. pallida (Verrill, 1872) | USA: Michigan | AF116016 | AF115983 | Apakupakul et al. [30] |
| A. bhamoensis Bolotov et al., 2022 | Myanmar | ON548507 | ON704955 | Bolotov et al. [29] |
| Taxon | Type Locality | General Range | DNA-Sequence Data on Type Specimens or Topotypes |
|---|---|---|---|
| Genus Glossiphonia Johnson, 1816 | |||
| The complanata group (subgenus Glossiphonia s. str.) | |||
| G. baicalensis (Stschegolev, 1922) = Torix baicalensis Stschegolev (1922): 136 [35] | Russia: Lake Baikal [35] | Endemic to Lake Baikal [1] | Sequenced topotype (COI and 18S rRNA) [26] |
| G. balcanica Grosser & Pešić, 2016 = G. balcanica Grosser & Pešić in Grosser et al. (2016): 18 [36] | Kosovo: spring Toplla, 42.5719° N, 20.2906° E, KS40 Dečani/Decan, Lebush [36] | Arctic Region from northern Fennoscandia to Taymyr; Iceland; European Russia, and Balkans [8] | Sequenced topotypes (COI) [7] |
| G. complanata (Linnaeus, 1758) = Hirudo complanata Linnaeus (1758): 1079 [37] | Not stated [37] | Continental Europe (Austria, France, Germany, Slovenia, Balkans, and European Russia) north to the Moscow Region of Russia, the British Isles, and Morocco [7,8] | Sequenced representatives of this species from Germany (COI) [7] cannot be considered topotypes |
| G. concolor (Apáthy, 1888) = Clepsine concolor Apáthy (1888): 154 [38] | Hungary: Danube at Dunaharaszti, approx. 47.3627° N, 19.0865° E (based on the neotype) [39] | Arctic Region from Kolguev Island and Polar Urals to Taymyr; Siberia (including Lake Baikal); Kazakhstan; Iran; European Russia; Sweden; Germany; Lithuania, France, and Hungary [8,40,41,42] | Not available |
| G. echinulata (Grube, 1871) comb. nov. = Clepsine echinulata Grube (1871): 110 [12] | Russia: Lake Baikal [12] | Endemic to Lake Baikal [1] | Sequenced topotype (complete mitogenome) [28] |
| G. elegans (Verrill, 1872) = Clepsine elegans Verrill (1872): 132 [43] | USA: West River, New Haven County, CT [43] | Canada and the USA [5] | Sequenced topotypes (COI) [5,44] |
| G. grubei (Lukin & Epshtein, 1959) comb. nov. = Baicaloclepsis grubei Lukin & Epshtein (1959): 189 [33] | Russia: Lake Baikal [33] | Endemic to Lake Baikal [1] | Sequenced topotype (complete mitogenome) [28] |
| G. nebulosa Kalbe, 1964 = G. complanata nebulosa Kalbe (1964): 141 [45] | Germany: Nieplitz River near Treuenbrietzen, 52.0911° N, 12.8666° E [45] | Arctic Region from Polar Urals to Taymyr; North Caucasus; Europe (Germany, France); Turkey: Antalya [8] | Sequenced topotype (COI) [7] |
| G. taymyrensis Bolotov, Eliseeva, Klass & Kondakov, 2022 in Bolotov et al. (2022): 13 [8] | Russia: Dudinka, small lake, 69.4008° N, 86.3384° E, Taymyr Peninsula [8] | Siberia from the Arctic Ocean coast (Taymyr) to Kemerovo Region [8] | Sequenced holotype and paratypes (COI and 18S rRNA) [8] |
| The verrucata group (subgenus Boreobdella Johansson, 1929) | |||
| G. arctica Bolotov, Eliseeva, Klass & Kondakov, in Bolotov et al. (2022): 13 [8] | Russia: a lake near Sob’ railway station, 67.0480° N, 65.6316° E, Polar Urals [8] | Polar Urals (unknown beyond the type locality) [8] | Sequenced holotype and paratypes (COI and 18S rRNA) [8] |
| G. koreaensissp. nov. | South Korea: Geum River 1st site, 36.0708° N, 127.5891° E, Chungcheongnam-do Province | Korean Peninsula | Sequenced paratypes (COI and 18S rRNA) (this study) |
| G. moorei sp. nov. = G. mollissima sensu Moore, 1898, non Grube, 1871 [11] | Russia: Bering Island, small lake near the Kamenka River, 55.1106° N, 166.0123° E, Commander Islands | Eastern Siberia and the Russian Far East from Taymyr and the Lena River basin to Chukotka, Kamchatka, Magadan and Khabarovsk regions, Amur River basin, and Primorye; Commander Islands, Kurile Archipelago, and Sakhalin Island; USA: SE Alaska and Kodiak Island [8] | Sequenced paratypes (COI and 18S rRNA) (this study) |
| G. verrucata (F. Müller, 1844) = Clepsine verrucata Müller (1844): 23 [46] | Germany: Lake Tegel in Berlin, 52.5790° N, 13.2590° E [47] | Siberia from Yamal and Khanty-Mansi Region up to the Lena River basin; Europe: Scandinavia, the British Isles, Poland, Lithuania, Northern European Russia, Germany, and the Netherlands [8] | Not available (no DNA sequences from Europe) |
| Incertae sedis (unassigned to species group) | |||
| G. paludosa (Carena, 1824) = Hirudo paludosa Carena (1824): 331 [48] | Italy: ponds near Carmagnola, approx. 44.8518° N, 7.6950° E, Turin Province [48] | Southern part of continental Europe (Italy, Switzerland, France, Austria, Slovakia, Czech, Hungary, Romania, tributaries of the Black Sea and adjacent regions) [32] | Not available |
| G. pulchella Sket, 1968 = G. complanata f. pulchella Sket (1968): 133 [49] | Littoral of Lake Ohrid [32] | Endemic to Lake Ohrid in north Macedonia and eastern Albania [32] | Not available |
| G. slovaca (Košel, 1973) = Batracobdella slovaca Košel (1973): 87 [50] | Slovakia: Danube near Bratislava [51] | Slovakia, Slovenia, and the Czech Republic [52,53] | Not available |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bolotov, I.N.; Eliseeva, T.A.; Kondakov, A.V.; Kropotin, A.V.; Aksenova, O.V.; Bespalaya, Y.V.; Travina, O.V.; Gofarov, M.Y.; Kim, S.K.; Lee, J.H.; et al. Taxonomy and Melanism Patterns of Freshwater Leeches in the Genus Glossiphonia (Hirudinea: Glossiphoniidae) from Northeast Asia. Diversity 2023, 15, 756. https://doi.org/10.3390/d15060756
Bolotov IN, Eliseeva TA, Kondakov AV, Kropotin AV, Aksenova OV, Bespalaya YV, Travina OV, Gofarov MY, Kim SK, Lee JH, et al. Taxonomy and Melanism Patterns of Freshwater Leeches in the Genus Glossiphonia (Hirudinea: Glossiphoniidae) from Northeast Asia. Diversity. 2023; 15(6):756. https://doi.org/10.3390/d15060756
Chicago/Turabian StyleBolotov, Ivan N., Tatyana A. Eliseeva, Alexander V. Kondakov, Alexander V. Kropotin, Olga V. Aksenova, Yulia V. Bespalaya, Oksana V. Travina, Mikhail Y. Gofarov, Sang Ki Kim, Jin Hee Lee, and et al. 2023. "Taxonomy and Melanism Patterns of Freshwater Leeches in the Genus Glossiphonia (Hirudinea: Glossiphoniidae) from Northeast Asia" Diversity 15, no. 6: 756. https://doi.org/10.3390/d15060756
APA StyleBolotov, I. N., Eliseeva, T. A., Kondakov, A. V., Kropotin, A. V., Aksenova, O. V., Bespalaya, Y. V., Travina, O. V., Gofarov, M. Y., Kim, S. K., Lee, J. H., & Vinarski, M. V. (2023). Taxonomy and Melanism Patterns of Freshwater Leeches in the Genus Glossiphonia (Hirudinea: Glossiphoniidae) from Northeast Asia. Diversity, 15(6), 756. https://doi.org/10.3390/d15060756








