Essential Oil Composition of Ten Species from Sect. Serpyllum of Genus Thymus Growing in Bulgaria
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Isolation of the Essential Oils
2.3. Gas Chromatography–Flame Ionization Detection/Mass Spectrometry (GC-FID/MSD)
2.4. Identification of Compounds
2.5. Statistical Analysis
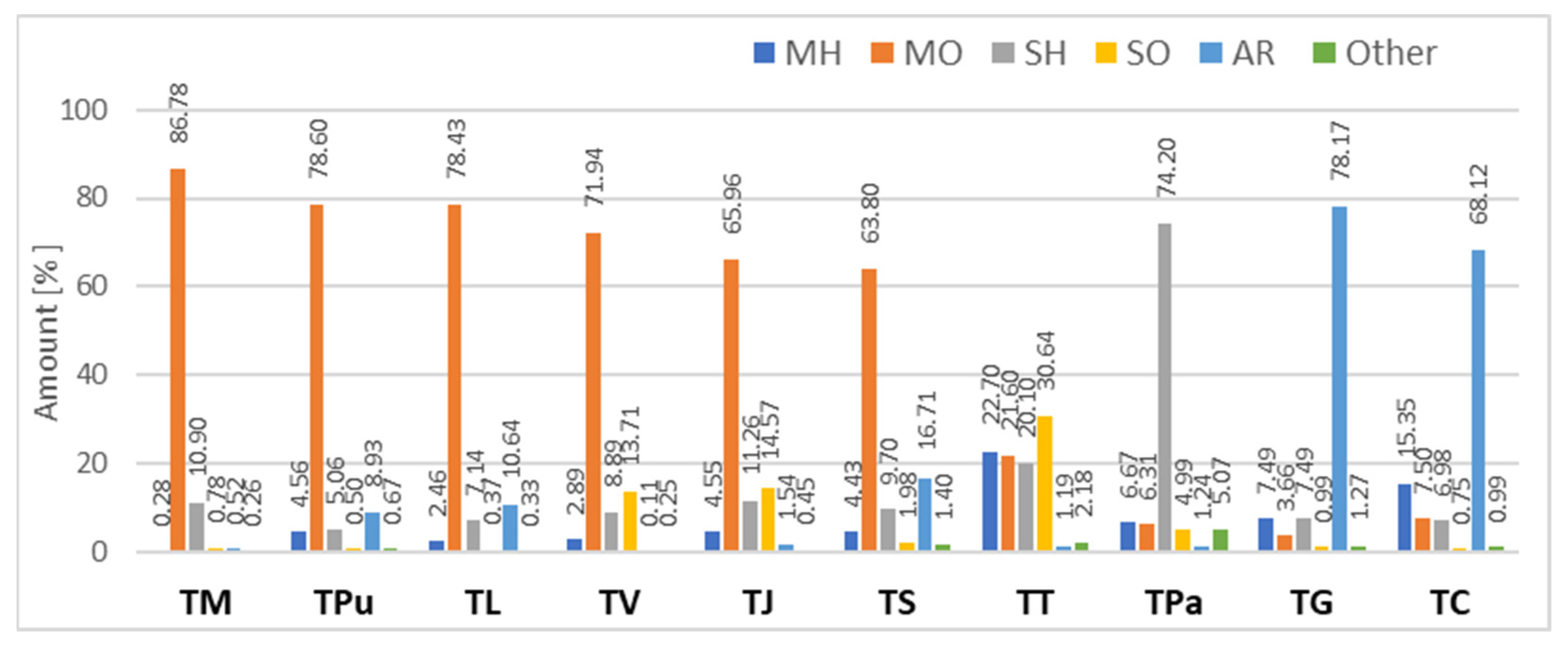
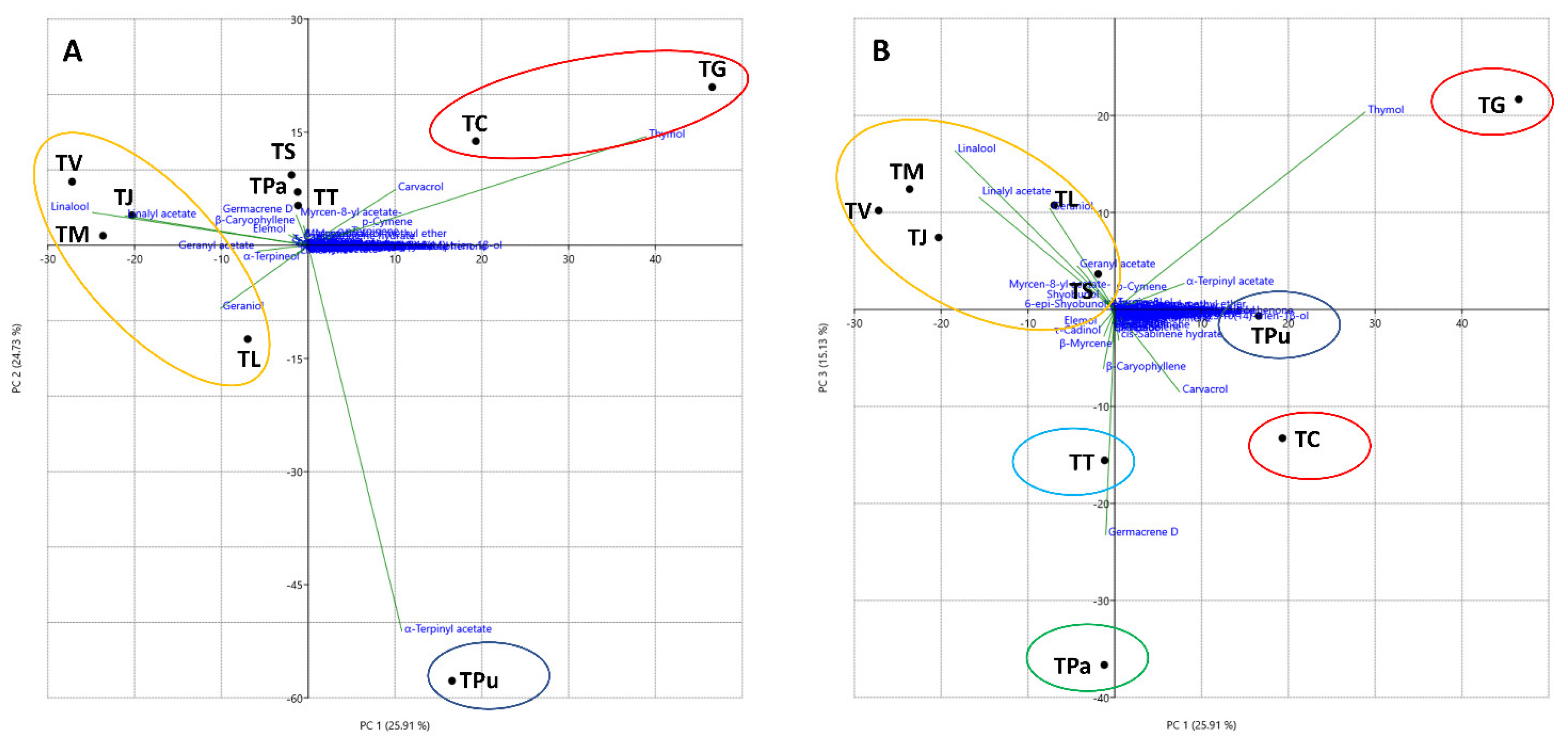
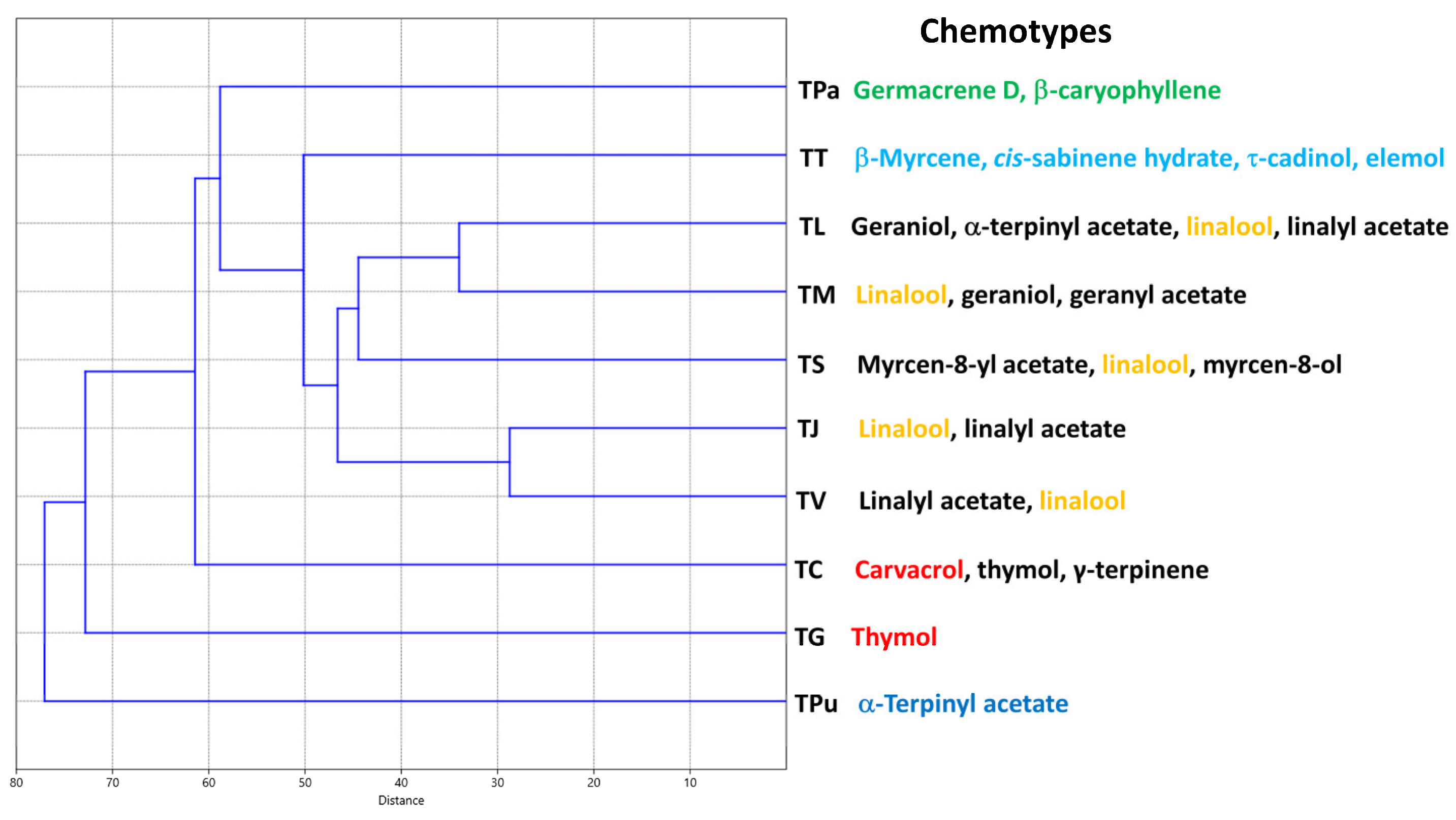
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harkat-Madouri, L.; Asma, B.; Madani, K.; Bey-Ould Si Said, Z.; Rigou, P.; Grenier, D.; Allalou, H.; Remini, H.; Adjaoud, A.; Boulekbache-Makhlouf, L. Chemical composition, antibacterial and antioxidant activities of essential oil of Eucalyptus globulus from Algeria. Ind. Crops Prod. 2015, 78, 148–153. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Stahl-Biskup, E.; Sáez, F. Thyme: The genus Thymus; Taylor & Francis Group: Abingdon, UK, 2002; ISBN 9780203216859. [Google Scholar]
- Aneva, I.; Zhelev, P.; Stoyanov, S.; Marinov, Y.; Georgieva, K. Survey on the distribution, diversity and phyochemistry of genus Thymus in Bulgaria. Ecol. Balk. 2018, 10, 101–110. [Google Scholar]
- Trendafilova, A.; Todorova, M.; Ivanova, V.; Zhelev, P.; Aneva, I. Essential Oil Composition of Five Thymus Species from Bulgaria. Chem. Biodivers. 2021, 18, e2100498. [Google Scholar] [CrossRef]
- Thymus|Euro+Med-Plantbase. Available online: https://europlusmed.org/cdm_dataportal/taxon/86dabab4-03af-416d-8d9f-1a0110d69207 (accessed on 16 February 2023).
- Hatipi Ibrahimi, M.; Papajani, V.; Zeljkovic, S.C.; Matevski, V. Essential oil analysis of two Thymus spp. growing wild in Kosovo. J. Essent. Oil-Bear. Plants 2014, 17, 832–837. [Google Scholar] [CrossRef]
- Nedanova, V.; Flurim, N.; Karaandzova, M.; Cvetkovikj, I.; Stefkov, G.; Kulevanova, S. Chemical composition of the essential oils of some Thymus spp. (Lamiaceae) from Kosovo. Maced. Pharm. Bull. 2016, 62, 513–514. [Google Scholar]
- Kulevanova, S.; Ristic, M.; Stafilov, T.; Matevski, V. Composition of the essential oils of Thymus jankae Chel. var. jankae, T. jankae var. pantotrichus Ronn. and T. jankae var. patentipilus Lyka from Macedonia. J. Essent. Oil Res. 1998, 10, 191–194. [Google Scholar] [CrossRef]
- Ćavar, S.; Vidic, D.; Maksimović, M. Essential oil profile of Thymus jankae Celak. from Bosnia. Planta Med. 2009, 75, PB10. [Google Scholar] [CrossRef]
- Stojanović, G.; Jovanović, O.; Petrović, G.; Mitić, V.; Jovanović, V.S.; Jovanović, S. Composition of headspace volatiles and essential oils of three Thymus species. Nat. Prod. Commun. 2014, 9, 1609–1612. [Google Scholar] [CrossRef]
- Kuštrak, D.; Martinis, Z.; Kuftinec, J.; Blažević, N. Composition of the essential oils of some Thymus and Thymbra species. Flavour Fragr. J. 1990, 5, 227–231. [Google Scholar] [CrossRef]
- Pluhár, Z.; Sárosi, S.; Novák, I.; Kutta, G. Essential Oil Polymorphism of Hungarian Common Thyme (Thymus glabrescens Willd.) Populations. Nat. Prod. Commun. 2008, 3, 1934578X0800300. [Google Scholar] [CrossRef]
- Simkó, H.; Sarosi, S.; Ladanyi, M.; Marton, B.; Radacsi, P.; Csontos, P.; Gosztola, B.; Kun, R.; Pluhár, Z. Studies on occurence, essential oil data and habitat conditions of Hungarian Thymus pannonicus and Thymus glabrescens populations. Med. Aromat. Plants 2013, 2, 1000119. [Google Scholar] [CrossRef]
- Pluhár, Z.; Simkó, H.; Sárosi, S.; Boros, B.; Dörnyei, Á.; Felinger, A.; Horváth, G. Determination of essential oil and polyphenolic compounds in Thymus species. Acta Hortic. 2015, 1099, 661–670. [Google Scholar] [CrossRef]
- Rǎdulescu, V.; Pavel, M.; Teodor, A.; Tǎnase, A.; Ilieş, D.C. Analysis of volatile compounds from infusion and hydrodistillate obtained from the species Thymus pulegioides L. (Lamiaceae). Farmacia 2009, 57, 282–289. [Google Scholar]
- Pavel, M.; Ristić, M.; Stević, T. Essential oils of Thymus pulegioides and Thymus glabrescens from Romania: Chemical composition and antimicrobial activity. J. Serb. Chem. Soc. 2010, 75, 27–34. [Google Scholar] [CrossRef]
- Imbrea, I.; Prodan, M.; Nicolin, A.; Butnariu, M.; Imbrea, F. Valorising Thymus glabrescens Willd. from the Aninei mountains. Res. J. Agric. Sci. 2010, 42, 260–263. [Google Scholar]
- Varga, E.; Bardocz, A.; Belak, A.; Maraz, A.; Boros, B.; Felinger, A.; Böszörményi, A.; Horváth, G. Antimicrobial activity and chemical composition of thyme essential oils and the polyphenolic content of different Thymus extracts. Farmacia 2015, 63, 357–361. [Google Scholar]
- Maksimović, Z.; Stojanović, D.; Šoštarić, I.; Dajić, Z.; Ristić, M. Composition and radical-scavenging activity of Thymus glabrescens Willd. (Lamiaceae) essential oil. J. Sci. Food Agric. 2008, 88, 2036–2041. [Google Scholar] [CrossRef]
- Dajic-Stevanovic, Z.; Sostaric, I.; Marin, P.D.; Stojanovic, D.; Ristic, M. Population variability in Thymus glabrescens Willd. from Serbia: Morphology, anatomy and essential oil composition. Arch. Biol. Sci. 2008, 60, 475–483. [Google Scholar] [CrossRef]
- Ilić, B.S.; Kocić, B.D.; Ćirić, V.M.; Cvetković, O.G.; Miladinović, D.L. An in vitro synergistic interaction of combinations of Thymus glabrescens essential oil and its main constituents with chloramphenicol. Sci. World J. 2014, 2014, 826219. [Google Scholar] [CrossRef] [PubMed]
- Vladimir-Knežević, S.; Kosalec, I.; Babac, M.; Petrović, M.; Ralić, J.; Matica, B.; Blažeković, B. Antimicrobial activity of Thymus longicaulis C. Presl essential oil against respiratory pathogens. Cent. Eur. J. Biol. 2012, 7, 1109–1115. [Google Scholar] [CrossRef]
- Chorianopoulos, N.G.; Evergetis, E.T.; Aligiannis, N.; Mitakou, S.; Nychas, G.J.E.; Haroutounian, S.A. Correlation between chemical composition of Greek essential oils and their antibacterial activity against food-borne pathogens. Nat. Prod. Commun. 2007, 2, 419–426. [Google Scholar] [CrossRef]
- Chorianopoulos, N.; Kalpoutzakis, E.; Aligiannis, N.; Mitaku, S.; Nychas, G.J.; Haroutounian, S.A. Essential oils of Satureja, Origanum, and Thymus species: Chemical composition and antibacterial activities against foodborne pathogens. J. Agric. Food Chem. 2004, 52, 8261–8267. [Google Scholar] [CrossRef] [PubMed]
- De Martino, L.; Bruno, M.; Formisano, C.; De Feo, V.; Napolitano, F.; Rosselli, S.; Senatore, F. Chemical composition and antimicrobial activity of the essential oils from two species of Thymus growing wild in Southern Italy. Molecules 2009, 14, 4614–4624. [Google Scholar] [CrossRef]
- Hatipi Ibrahimi, M.; Papajani, V.; Cavar, S.; Matevski, V. GC/MS Analysis of the essential oil of Thymus longicaulis Presl. from Kosovo. Bull. Chem. Technol. Bosnia Herzeg. 2013, 41, 6–10. [Google Scholar]
- Grujic Jovanovic, S.; Marin, P.D.; Dzamic, A.; Ristic, M. Essential oil composition of Thymus longicaulis from Serbia. Chem. Nat. Compd. 2009, 45, 265–266. [Google Scholar] [CrossRef]
- Akçin, T.A. Numerical taxonomic studies on some species of the genus Thymus L. (Labiatae) in Turkey. Asian J. Plant Sci. 2006, 5, 782–788. [Google Scholar] [CrossRef]
- Tzakou, O.; Verykokidou, E.; Roussis, V.; Chinou, I. Chemical composition and antibacterial properties of Thymus longicaulis subsp. chaoubardii oils: Three chemotypes in the same population. J. Essent. Oil Res. 1998, 10, 97–99. [Google Scholar] [CrossRef]
- Baser, K.H.; Koyuncu, M. Composition of the essential oils of two varieties of Thymus longicaulis C. Presl. subsp. chaubardii (Boiss. et Heldr. ex Reichb. fil.) Jalas. J. Essent. Oil Res. 1994, 6, 207–209. [Google Scholar] [CrossRef]
- Azaz, A.D.; Irtem, H.A.; Kurkcuoǧlu, M.; Baser, K.H.C. Composition and the in vitro antimicrobial activities of the essential oils of some Thymus species. Z Fur Naturforsch. Sect. C J. Biosci. 2004, 59, 75–80. [Google Scholar] [CrossRef]
- Pavela, R.; Bartolucci, F.; Desneux, N.; Lavoir, A.V.; Canale, A.; Maggi, F.; Benelli, G. Chemical profiles and insecticidal efficacy of the essential oils from four Thymus taxa growing in central-southern Italy. Ind. Crops Prod. 2019, 138, 111460. [Google Scholar] [CrossRef]
- Bellomaria, B.; Hruška, K.; Valentini, G. Composizione degli olii essenziali di Thymus longicaulis C. Presi in varie località dell’Italia centrale. G. Bot. Ital. 1981, 115, 17–27. [Google Scholar] [CrossRef]
- Sarikurkcu, C.; Sabih Ozer, M.; Eskici, M.; Tepe, B.; Can, Ş.; Mete, E. Essential oil composition and antioxidant activity of Thymus longicaulis C. Presl subsp. longicaulis var. longicaulis. Food Chem. Toxicol. 2010, 48, 1801–1805. [Google Scholar] [CrossRef]
- Baser, K.H.C.; Özek, T.; Kirimer, N.; Tümen, G. The occurrence of three chemotypes of Thymus longicaulis C. Presl. subsp. longicaulis in the same population. J. Essent. Oil Res. 1993, 5, 291–295. [Google Scholar] [CrossRef]
- Baser, K.H.C.; Özek, T.; Kürkçüoglu, M.; Tümen, G. Composition of the essential oil of Thymus longicaulis C. Presl. var. subisophyllus (Borbas) Jalas from Turkey. J. Essent. Oil Res. 1992, 4, 311–312. [Google Scholar] [CrossRef]
- Tümen, G.; Kirimer, N.; Başer, K.H.C. Composition of the essential oils of Thymus species growing in Turkey. Chem. Nat. Compd. 1995, 31, 42–47. [Google Scholar] [CrossRef]
- Ćavar, S.; Vidic, D.; Maksimović, M. GC-MS analiza volatilnih konstituenata Thymus moesiacus Velen. Glas. Hemičara Tehnol. I Ekol. Repub. Srp. 2009, 2, 27–29. [Google Scholar]
- Kulevanova, S.; Ristić, M.; Stafilov, T. Composition of the essential oil from Thymus moesiacus from Macedonia. Planta Med. 1996, 62, 78–79. [Google Scholar] [CrossRef] [PubMed]
- Kameníková, M.; Fialová, S.; Ťažký, A.; Čičová, I. Polyphenolic compounds and essential oil analysis of selected species of the genus Thymus. Acta Fac. Pharm. Univ. Comen. 2015, 62, 12–17. [Google Scholar] [CrossRef]
- Pluhár, Z.; Héthelyi, É.; Kutta, G.; Kamondy, L. Evaluation of environmental factors influencing essential oil quality of Thymus pannonicus All. and Thymus praecox Opiz. J. Herbs Spices Med. Plants 2007, 13, 23–43. [Google Scholar] [CrossRef]
- Pluhár, Z.; Sárosi, S.; Pintér, A.; Simkó, H. Essential oil polymorphism of wild growing Hungarian thyme (Thymus pannonicus) populations in the Carpathian Basin. Nat. Prod. Commun. 2010, 5, 1681–1686. [Google Scholar] [CrossRef]
- Pluhár, Z.; Kocsis, M.; Kuczmog, A.; Csete, S.; Simkó, H.; Sárosi, S.; Molnár, P.; Horváth, G. Essential oil composition and preliminary molecular study of four Hungarian Thymus species. Acta Biol. Hung. 2012, 63, 81–96. [Google Scholar] [CrossRef] [PubMed]
- Berciu, I.; Toma, C.; Burzp, I. Aspecte Histo-anatomice şi biochimice referitoare la organele vegetative aeriene de Thymus Pannonicus All. subspeciile auctus şi pannonicus. An. Ştiinţifice Ale Univ. Al. I. Cuza Iaşi 2008, LIV, 100–105. [Google Scholar]
- Boz, I.; Burzo, I.; Zamfirache, M.-M.; Toma, C.; Padurariu, C. Glandular trichomes and essential oil composition of Thymus pannonicus All. (Lamiaceae). An. Univ. Din Oradea Fasc. Biol. 2009, 1, 36–39. [Google Scholar]
- Jianu, C.; Mihail, R.; Muntean, S.G.; Pop, G.; Daliborca, V.C.; Horhat, F.G.; Nitu, R. Composition and antioxidant capacity of essential oils obtained from Thymus vulgaris, Thymus pannonicus and Satureja montana grown in Western Romania. Rev. Chim. 2015, 66, 2157–2160. [Google Scholar]
- Boz, I.; Burzo, I.; Tanase, C. The effect of harvesting time on essential oils composition of Thymus pannonicus L. An. Ştiinţifice Ale Univ. Al. I. Cuza Iaşi 2016, 62, 59–64. [Google Scholar]
- Maksimović, Z.; Milenković, M.; Vučićević, D.; Ristić, M. Chemical composition and antimicrobial activity of Thymus pannonicus All. (Lamiaceae) essential oil. Cent. Eur. J. Biol. 2008, 3, 149–154. [Google Scholar] [CrossRef]
- Sostaric, I.; Arsenijevic, J.; Acic, S.; Stevanovic, Z.D. Essential oil polymorphism of Thymus pannonicus All. (Lamiaceae) in Serbia. J. Essent. Oil-Bear. Plants 2012, 15, 237–243. [Google Scholar] [CrossRef]
- Arsenijević, J.; Drobac, M.; Šoštarić, I.; Jevđović, R.; Živković, J.; Ražić, S.; Moravčević, Đ.; Maksimović, Z. Comparison of essential oils and hydromethanol extracts of cultivated and wild growing Thymus pannonicus All. Ind. Crops Prod. 2019, 130, 162–169. [Google Scholar] [CrossRef]
- Radonić, A.; Mastelić, J. Essential oil and glycosidically bound volatiles of Thymus pulegioides L. growing wild in Croatia. Croat. Chem. Acta 2008, 81, 599–606. [Google Scholar]
- Mastelić, K.; Kravar, A.J.G. The chemical composition of terpene alcohols and phenols from the essential oil and terpene glycosides isolated from Thymus pulegioides growing wild in Dalmatia. Planta Med. 1992, 58, 678–680. [Google Scholar] [CrossRef]
- Groendahl, E.; Ehlers, B.K.; Keefover-Ring, K. A new cis-sabinene hydrate chemotype detected in large thyme (Thymus pulegioides L.) growing wild in Denmark. J. Essent. Oil Res. 2008, 20, 40–41. [Google Scholar] [CrossRef]
- Senatore, F.; De Fusco, R.; Grassia, A.; Moro, C.O.; Rigano, D.; Napolitano, F. Chemical composition and antibacterial activity of essential oils from five culinary herbs of the Lamiaceae family growing in Campania, southern Italy. J. Essent. Oil-Bear. Plants 2003, 6, 166–173. [Google Scholar] [CrossRef]
- Sárosi, S.; Bernáth, J.; Bertoli, A.; Pistelli, L.; Benvenuti, S. Essential oil polymorphism of Thymus pulegioides collected in Monti Pisani, Italy. Acta Hortic. 2012, 955, 59–64. [Google Scholar] [CrossRef]
- Mockute, D.; Bernotiene, G. The α-terpenyl acetate chemotype of essential oil of Thymus pulegioides L. Biochem. Syst. Ecol. 2001, 29, 69–76. [Google Scholar] [CrossRef]
- Mockute, D.; Bernotiene, G. Chemical composition of the essential oils and the odor of Thymus pulegioides L. growing wild in Vilnius. J. Essent. Oil Res. 2005, 17, 415–418. [Google Scholar] [CrossRef]
- Vaičiulyte, V.; Ložiene, K. Metabolomic analysis and effects of meteorological factors on phenolic and non-phenolic chemotypes of Thymus pulegioides L. cultured in the same locality. Ind. Crops Prod. 2015, 77, 491–498. [Google Scholar] [CrossRef]
- Vaičiulytė, V.; Ložienė, K. Impact of chemical polymorphism of Thymus pulegioides on some associated plant species under natural and laboratory conditions. Plant Biosyst. 2020, 154, 663–672. [Google Scholar] [CrossRef]
- Svediene, J.; Raudoniene, V.; Loziene, K.; Bridziuviene, D.; Paškevicius, A.; Vaiciulyte, V. The effect of various Thymus pulegioides chemotypes essential oils and ph on food spoilage microorganisms. J. Essent. Oil-Bear. Plants 2015, 18, 276–288. [Google Scholar] [CrossRef]
- Ložiene, K.; Venskutonis, P.R. Influence of environmental and genetic factors on the stability of essential oil composition of Thymus pulegioides. Biochem. Syst. Ecol. 2005, 33, 517–525. [Google Scholar] [CrossRef]
- Ložiene, K. Selection of fecund and chemically valuable clones of thyme (Thymus) species growing wild in Lithuania. Ind. Crops Prod. 2009, 29, 502–508. [Google Scholar] [CrossRef]
- Pinto, E.; Pina-Vaz, C.; Salgueiro, L.; Gonçalves, M.J.; Costa-De-Oliveira, S.; Cavaleiro, C.; Palmeira, A.; Rodrigues, A.; Martinez-De-Oliveira, J. Antifungal activity of the essential oil of Thymus pulegioides on Candida, Aspergillus and dermatophyte species. J. Med. Microbiol. 2006, 55, 1367–1373. [Google Scholar] [CrossRef] [PubMed]
- Salgueiro, L.R.; da Cunha, A.P.; Paiva, J. Chemotaxonomic characterization of a Thymus hybrid from Portugal. Flavour Fragr. J. 1993, 8, 325–330. [Google Scholar] [CrossRef]
- Boz, I.; Gille, E.; Necula, R.; Dunca, S.; Zamfirache, M.M. Chemical composition and antibacterial activity of essential oils from five populations of Thymus pulegioides L. Cellul. Chem. Technol. 2015, 49, 169–174. [Google Scholar]
- Miladinović, D.L.; Ilić, B.S.; Miladinović, L.C.; Kocić, B.D.; Ćirić, V.M.; Stankov-Jovanović, V.P.; Cvetković, O.G.; Govil, J.N.; Bhattacharya, S. Antibacterial activity of Thymus pulegioides essential oil and its synergistic potential with antibiotics: A chemometric approach. Recent Prog. Med. Plants 2013, 38, 101–136. [Google Scholar]
- Mártonfi, P. Polymorphism of essential oil in Thymus pulegioides subsp. chamaedrys in Slovakia. J. Essent. Oil Res. 1992, 4, 173–179. [Google Scholar] [CrossRef]
- Michet, A.; Chalchat, J.C.; Figuérédo, G.; Thébaud, G.; Billy, F.; Pétel, G. Chemotypes in the volatiles of wild thyme (Thymus pulegioides L.). J. Essent. Oil Res. 2008, 20, 101–103. [Google Scholar] [CrossRef]
- Katsiotis, S.T.; Chatzopoulou, P.; Baerheim Svendsen, A. The essential oil of Thymus sibthorpii Benth. growing wild in Greece. Sci. Pharm. 1990, 58, 303–306. [Google Scholar]
- Baser, K.H.C.; Özek, T.; Kürkçüoglu, M.; Tümen, G. Characterization of the essential oil of Thymus sibthorpii Bentham. J. Essent. Oil Res. 1992, 4, 303–304. [Google Scholar] [CrossRef]
- Baser, K.H.C.; Özek, T.; Kürkçüoglu, M.; Tümen, G. Essential oil of Thymus thracicus Velen var. longidens (Velen) Jalas. J. Essent. Oil Res. 1995, 7, 661–662. [Google Scholar] [CrossRef]
- Kulevanova, S.; Ristic, M.; Stafilov, T.; Dorevski, K.; Ristov, T. Essential oil analysis of some taxa of genera Thymus L. Environment influences. Bull. Chem. Technol. Maced. 1996, 15, 33–38. [Google Scholar]
- Ćavar Zeljković, S.; Maksimović, M. Chemical composition and bioactivity of essential oil from Thymus species in Balkan Peninsula. Phytochem. Rev. 2015, 14, 335–352. [Google Scholar] [CrossRef]
- Sandra, P.; Bicchi, C. Capillary Gas Chromatography in Essential Oil Analysis; Dr. Alfred Huethig Verlag: Heidelberg, Germany, 1987. [Google Scholar]
- Babushok, V.I.; Linstrom, P.J.; Zenkevich, I.G. Retention indices for frequently reported compounds of plant essential oils. J. Phys. Chem. Ref. Data 2011, 40, 043101. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy, 4th ed.; Allured Business Media: Carol Stream, IL, USA, 2007; ISBN 978-1-932633-214. [Google Scholar]
- Bischof-Deichnik, C.; Holtuijzen, J.; Stahl-Biskup, E. Multivariate statistical analysis of the essential oil composition of Thymus praecox Opiz ssp. polytrichus (Kern. ex Borb.) Ronn. collected in the Tyrolean Alps. Flavour Fragr. J. 2000, 15, 1–6. [Google Scholar] [CrossRef]
- Vaičiulytė, V.; Ložienė, K.; Švedienė, J.; Raudonienė, V.; Paškevičius, A. α-Terpinyl acetate: Occurrence in essential oils bearing Thymus pulegioides, phytotoxicity, and antimicrobial effects. Molecules 2021, 26, 1065. [Google Scholar] [CrossRef] [PubMed]
- Adzet, T.; Cañigueral, S.; Gabalda, N.; Ibañez, C.; Tomas, X.; Vila, R. Composition and variability of the essential oil of Thymus Willkomii. Phytochem. 1991, 30, 2289–2293. [Google Scholar] [CrossRef]
- García Martín, D.; García Vallejo, M.C. Chemotypes of Thymus zygis (Löfl.) L. of Guadarramma Sierra and other places in Castile (Spain). In Proceedings of the 9th International Essential Oil Congress, Singapore, 13–17 March 1983; pp. 134–140. [Google Scholar]
- Benjiali, B.; Hammoumi, M.; Richard, H. Polymorphisme chimique des huiles essentielles de thym du Maroc. 1—Caractérisation des composants. Sci. Aliment. 1987, 7, 77–91. [Google Scholar]
- Schmidt, A.; Bischof-Deichnik, C.; Stahl-Biskup, E. Essential oil polymorphism of Thymus praecox subsp. arcticus on the British Isles. Biochem. Syst. Ecol. 2004, 32, 409–421. [Google Scholar] [CrossRef]
- Granger, R.; Passet, J. Thymus vulgaris spontane de France: Races chimiques et chemotaxonomie. Phytochemistry 1973, 12, 1683–1691. [Google Scholar] [CrossRef]
- Tümen, G.; Kirimer, N.; Kürkçüoglu, M.; Baser, K.H.C. Composition of the essential oils of Thymus atticus and Thymus roegneri from Turkey. J. Essent. Oil Res. 1997, 9, 473–474. [Google Scholar] [CrossRef]
| Species | Code | Collection Site | GPS Coordinates | Voucher Specimen | Yield [%, w/w] |
|---|---|---|---|---|---|
| T. vandasii | TV | Seven Rila lakes, Rila Mts. | 42°13′11.32″/ 23°19′11.52″ | SOM 1433 | 0.98 |
| T. pannonicus | TPa | Eastern Rhodopes Mts., road between Asenovgrad and Kardzhali | 41°39′36.08″/ 25°20′30.32″ | SOM 1429 | 0.39 |
| T. longicaulis | TL | Western Rhodopes Mts. | 42° 3′39.50″/ 23°49′51.89″ | SOM 1427 | 2.23 |
| T. moesiacus | TM | Znepol region | 42°27′29.68″/ 22°38′16.66″ | SOM 1428 | 1.81 |
| T. callieri | TC | Struma River Valley | 42° 0′11.58″/ 23° 7′56.80″ | SOM 1424 | 2.11 |
| T. pulegioides | TPu | Vlahina Mts. | 41°59′18.88″/ 22°55′39.02″ | SOM 1430 | 1.18 |
| T. glabrescens | TG | North-Eastern Bulgaria, near Dobrich | 43°31′10.37″/ 27°45′16.11″ | SOM 1425 | 1.69 |
| T. jankae | TJ | Seven Rila lakes, Rila Mts. | 42°13′11.32″/ 23°19′11.52″ | SOM 1426 | 0.97 |
| T. sibthorpii | TS | Danube plain | 43°22′17.64″/ 24°39′41.95″ | SOM 1431 | 0.83 |
| T. thracicus | TT | Pirin Mts., Orelek peak | 41°32′51.80″/ 23°37′19.28″ | SOM 1432 | 0.09 |
| RRI * | RRI ** | Compound | TV # | TJ # | TL # | TM # | TT # | TC # | TG # | TS # | TPu # | TPa #* |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 923 | 925 | α-Thujene | - | - | 0.06 | 0.01 | 0.24 | 0.25 | 0.34 | 0.12 | 0.04 | 0.11 |
| 929 | 929 | α-Pinene | 0.04 | 0.47 | 0.06 | 0.08 | 2.63 | 0.24 | 0.23 | 0.25 | 0.07 | 0.67 |
| 944 | 946 | Camphene | 0.05 | 0.05 | 0.09 | 0.19 | 0.15 | 0.37 | 0.09 | 0.27 | 0.10 | 1.14 |
| 972 | 970 | Sabinene | 0.01 | - | 0.09 | - | 0.38 | 0.01 | - | 0.06 | 0.37 | 0.09 |
| 973 | 973 | β-Pinene | 0.01 | - | - | - | 1.36 | 0.11 | 0.11 | 0.23 | 0.20 | 0.18 |
| 978 | 980 | 1-Octen-3-ol | 0.23 | 0.45 | 0.15 | 0.15 | 1.04 | 0.51 | 0.90 | 0.86 | 0.11 | 1.63 |
| 985 | 986 | 3-Octanone | - | - | 0.04 | - | - | 0.13 | 0.16 | 0.18 | 0.32 | 0.16 |
| 991 | 991 | β-Myrcene | 1.09 | 1.50 | 0.38 | - | 16.53 | 0.65 | 0.84 | 1.99 | 0.12 | 0.27 |
| 995 | 994 | 3-Octanol | - | - | 0.09 | 0.12 | 0.20 | 0.21 | 0.09 | 0.22 | - | 3.07 |
| 1015 | 1017 | α-Terpinene | - | - | 0.11 | - | 0.23 | 1.35 | 0.99 | 0.21 | 0.17 | 0.15 |
| 1023 | 1022 | p-Cymene | 0.02 | - | 0.76 | - | 0.15 | 7.17 | 7.16 | 3.40 | 1.52 | 0.36 |
| 1028 | 1030 | Limonene | 0.39 | 1.42 | 0.48 | - | 0.36 | 0.31 | 0.30 | 0.64 | 1.39 | 3.09 |
| 1030 | 1032 | Eucalyptol | - | 0.31 | - | 0.16 | - | 0.77 | 0.91 | 0.73 | - | 0.79 |
| 1037 | 1037 | cis-β-Ocimene | 0.26 | 0.22 | 0.15 | - | - | - | - | - | - | - |
| 1042 | 1045 | Benzene acetaldehyde | 0.05 | - | - | - | 0.14 | - | - | - | - | 0.30 |
| 1049 | 1049 | trans-β-Ocimene | 0.85 | 0.51 | 0.18 | - | - | - | - | - | - | - |
| 1056 | 1054 | γ-Terpinene | 0.05 | 0.07 | 0.69 | - | 0.82 | 12.04 | 4.48 | 0.65 | 1.86 | 0.91 |
| 1065 | 1065 | cis-Sabinene hydrate | 0.06 | 0.11 | 0.08 | - | 13.58 | 1.14 | 1.04 | 0.45 | 0.06 | 0.19 |
| 1071 | 1070 | cis-Linalool oxide furanoid | 0.10 | 0.26 | - | 0.20 | - | - | - | 0.29 | - | - |
| 1081 | 1080 | 1-Nonen-3-ol | - | - | - | - | - | 0.14 | 0.04 | 0.14 | - | 0.22 |
| 1085 | 1080 | 3-Nonanone | - | - | - | - | 0.17 | - | - | - | - | - |
| 1087 | 1086 | trans-Linalool oxide furanoid | - | - | - | - | - | - | - | 0.33 | - | - |
| 1087 | 1088 | Terpinolene | 0.15 | 0.31 | 0.16 | - | - | - | 0.11 | - | 0.24 | 0.07 |
| 1096 | 1098 | trans-Sabinene hydrate | - | - | - | - | 1.01 | 0.30 | 0.22 | - | 0.09 | 0.12 |
| 1099 | 1099 | Linalool | 19.37 | 29.29 | 13.37 | 35.21 | - | 0.12 | 0.36 | 19.72 | 0.12 | 0.09 |
| 1100 | 1100 | Undecane | - | - | - | - | 0.34 | - | - | - | - | - |
| 1102 | 1102 | Nonanal | - | - | - | - | 0.29 | - | - | - | - | - |
| 1116 | 1111 | cis-2-p-Menthen-1-ol | - | - | - | - | 0.18 | - | - | - | - | - |
| 1125 | 1123 | 3-Octanol acetate | 0.02 | - | 0.04 | - | - | - | 0.08 | - | 0.24 | - |
| 1138 | 1140 | trans-2-p-Menthen-1-ol | - | - | - | - | 0.11 | - | - | - | - | - |
| 1141 | 1145 | Camphor | 0.04 | 0.27 | 0.04 | - | 0.30 | - | - | - | - | 3.16 |
| 1143 | 1142 | trans-Verbenol | - | 0.10 | - | - | 0.12 | - | - | - | - | - |
| 1148 | 1145 | Myrcenone | - | 0.18 | - | - | - | - | - | - | - | - |
| 1163 | 1167 | Borneol | 0.10 | 0.18 | 0.40 | 1.29 | - | 4.59 | 0.68 | 1.01 | 0.47 | 1.37 |
| 1165 | 1167 | p-Mentha-1,5-dien-8-ol | 0.03 | - | - | - | - | - | - | - | - | - |
| 1175 | 1177 | Terpinen-4-ol | 0.11 | 0.13 | 0.12 | 0.06 | 2.30 | 0.45 | 0.37 | 0.26 | 0.13 | 0.30 |
| 1183 | 1183 | p-Cymen-8-ol | - | - | - | - | - | - | - | 0.14 | - | - |
| 1188 | 1189 | α-Terpineol | 2.76 | 3.27 | 1.47 | 0.06 | 0.44 | 0.14 | 0.07 | 0.13 | 2.30 | 0.18 |
| 1217 | 1219 | Coumaran | - | - | - | - | 0.26 | - | - | - | - | - |
| 1220 | 1221 | cis-Sabinene hydrate acetate | - | - | - | - | - | - | - | 0.43 | - | - |
| 1226 | 1228 | Citronellol | - | - | - | - | 0.49 | - | - | - | - | - |
| 1230 | 1226 | Myrcen-8-ol | - | - | - | - | - | - | - | 14.71 | - | - |
| 1230 | 1230 | Nerol | 0.55 | 1.16 | - | 0.90 | - | - | - | - | 0.25 | - |
| 1232 | 1235 | Thymol methyl ether | - | - | 0.89 | 0.23 | - | 0.18 | 1.12 | 0.97 | 0.33 | 0.13 |
| 1242 | 1240 | Neral | 0.07 | 1.04 | - | 0.42 | 1.14 | - | - | - | - | - |
| 1243 | 1244 | Carvacrol methyl ether | - | - | 0.67 | 0.04 | 0.34 | 3.93 | 4.34 | 0.79 | 0.15 | 0.01 |
| 1252 | 1250 | Thymoquinone | - | - | - | - | - | 0.16 | 0.67 | 0.25 | - | - |
| 1254 | 1255 | Geraniol | 0.05 | - | 25.28 | 32.89 | 0.20 | - | - | 2.75 | 7.27 | - |
| 1256 | 1257 | Linalyl acetate | 45.84 | 20.58 | 12.72 | - | - | - | - | - | - | - |
| 1273 | 1270 | Geranial | 0.22 | 1.75 | - | - | 1.63 | - | - | - | - | - |
| 1284 | 1285 | Bornyl acetate | 0.05 | - | 0.27 | 0.06 | - | - | - | - | 0.43 | 0.09 |
| 1292 | 1291 | Thymol | 0.03 | 1.22 | 8.27 | 0.18 | - | 13.38 | 63.96 | 10.67 | 6.70 | 0.26 |
| 1300 | 1299 | Carvacrol | 0.01 | 0.31 | 0.04 | 0.07 | - | 42.65 | 0.41 | 0.19 | 0.22 | 0.08 |
| 1303 | 1305 | 6-Ethyl-3,4-dimethylphenol | - | - | - | - | - | - | - | 0.31 | - | - |
| 1309 | 1309 | p-Vinylguaiacol | - | - | - | - | 0.31 | - | - | - | - | - |
| 1335 | 1338 | δ-Elemene | 0.23 | 0.23 | 0.08 | 0.08 | - | - | 0.09 | - | - | 0.13 |
| 1349 | 1351 | α-Cubebene | - | - | - | - | - | 0.01 | - | - | - | 0.30 |
| 1349 | 1350 | α-Terpinyl acetate | 0.05 | 4.22 | 17.46 | 0.06 | - | - | - | - | 66.79 | - |
| 1351 | 1348 | Myrcen-8-yl acetate- | - | - | - | - | - | - | - | 22.03 | - | - |
| 1355 | 1357 | Eugenol | - | - | - | - | - | - | 0.05 | - | - | 0.09 |
| 1365 | 1364 | Neryl acetate | 0.85 | 1.41 | 0.31 | - | - | - | - | - | - | - |
| 1371 | 1372 | Ylangene | - | - | - | - | - | 0.01 | 0.06 | - | - | 0.13 |
| 1373 | 1376 | α-Copaene | - | - | - | - | - | 0.01 | 0.11 | 0.07 | - | 0.49 |
| 1382 | 1384 | β-Bourbonene | - | - | - | 0.50 | 0.08 | 0.16 | 0.08 | 0.24 | - | 0.82 |
| 1385 | 1382 | Geranyl acetate | 1.58 | 1.70 | 6.64 | 15.47 | 0.10 | - | - | 0.96 | 0.48 | - |
| 1389 | 1389 | β-Cubebene | - | - | - | - | - | - | - | - | - | 0.67 |
| 1390 | 1391 | β-Elemene | 0.10 | 0.08 | - | - | 0.38 | - | - | - | - | 1.82 |
| 1407 | 1409 | α-Gurjunene | 0.06 | 0.23 | - | - | - | - | - | - | - | - |
| 1419 | 1419 | β-Caryophyllene | 4.51 | 3.47 | 2.77 | 4.44 | 6.49 | 3.97 | 1.74 | 3.25 | 2.48 | 12.28 |
| 1426 | 1432 | β-Copaene | - | - | 0.03 | 0.12 | - | - | 0.12 | 0.13 | - | 2.25 |
| 1436 | 1440 | Aromandendrene | - | - | - | - | - | - | 0.26 | - | - | - |
| 1442 | 1442 | 2-Hydroxy-5-methoxyacetophenone | - | - | - | - | - | 0.17 | 0.16 | - | - | - |
| 1441 | 1440 | Isogermacrene D | - | - | 0.05 | 0.08 | - | - | - | - | - | - |
| 1444 | 1440 | Cadina-3,5-diene | - | 0.22 | - | - | 0.90 | - | - | - | - | - |
| 1451 | 1454 | Humulene | 0.27 | 0.27 | 0.21 | 0.28 | 3.80 | 0.28 | - | 0.53 | 0.13 | - |
| 1454 | 1454 | Sesquisabinene | - | - | - | - | - | - | - | - | - | 2.52 |
| 1457 | 1457 | trans-β-Famesene | - | 0.11 | 0.06 | - | - | - | - | - | - | 0.92 |
| 1458 | 1461 | Alloaromadendrene | - | 0.52 | - | - | - | - | 0.15 | - | - | - |
| 1461 | 1463 | cis-Muurola-4(15),5-diene | - | 0.40 | - | - | 1.30 | - | - | - | - | - |
| 1460 | 1464 | 9-epi-β-Caryophyllene | 0.48 | - | - | - | - | - | - | - | - | - |
| 1475 | 1477 | γ-Muurolene | - | - | - | - | - | 0.52 | 0.47 | 0.19 | 0.39 | - |
| 1481 | 1481 | Germacrene D | 0.22 | 0.24 | 0.51 | 2.18 | 0.89 | 0.26 | - | 1.07 | - | 42.15 |
| 1494 | 1493 | Epicubebol | - | - | - | - | - | 0.28 | - | - | - | 0.91 |
| 1493 | 1492 | Valencene | - | - | - | - | - | - | 0.49 | - | - | - |
| 1494 | 1495 | Bicyclogermacrene | 0.58 | 0.62 | 0.17 | 0.24 | 0.16 | - | 0.13 | 0.28 | 0.10 | - |
| 1498 | 1500 | α-Muurolene | 0.24 | 0.19 | - | - | - | 0.14 | 0.06 | 0.08 | - | 1.01 |
| 1507 | 1509 | β-Bisabolene | - | 0.38 | 2.80 | 2.81 | - | 0.78 | 2.25 | 3.42 | 1.88 | 6.15 |
| 1517 | 1517 | 6-epi-Shyobunol | 2.66 | 1.91 | - | - | - | - | - | - | - | - |
| 1513 | 1513 | γ-Cadinene | - | 2.26 | - | - | 5.52 | 0.42 | 0.45 | - | - | 0.80 |
| 1518 | 1520 | trans-Calamenene | - | - | - | - | 0.38 | - | - | - | - | - |
| 1521 | 1525 | Dihydroactinidiolide | - | - | - | - | 0.14 | - | - | - | - | - |
| 1522 | 1522 | β-Cadinene | - | 2.03 | 0.12 | 0.12 | 0.20 | 0.42 | 0.75 | 0.44 | 0.08 | 1.65 |
| 1526 | C15H24 (MW 204) | 2.19 | - | - | - | - | - | - | - | - | - | |
| 1540 | 1538 | trans-α-Bisabolene | - | - | 0.35 | 0.06 | - | - | 0.29 | - | - | 0.10 |
| 1550 | 1549 | Elemol | 4.92 | 2.01 | - | - | 11.29 | - | - | 0.07 | - | 0.20 |
| 1554 | 1561 | Thymohydroquinone | - | - | - | - | - | 0.49 | 0.30 | - | - | - |
| 1561 | 1562 | Geranyl butyrate | - | - | 0.15 | - | - | - | - | - | 0.11 | - |
| 1578 | 1576 | Spathulenol | 0.20 | - | 0.07 | 0.09 | - | - | 0.28 | 0.23 | - | - |
| 1574 | 1574 | Germacrene D-4-ol | 0.62 | - | - | - | 0.37 | - | - | - | - | 0.21 |
| 1582 | 1581 | Caryophyllene oxide | 0.39 | - | 0.19 | 0.61 | 0.41 | 0.47 | 0.30 | 0.61 | 0.29 | 0.21 |
| 1608 | 1604 | Geranyl isovalerate | 0.12 | - | 0.12 | - | - | - | - | - | 0.08 | - |
| 1592 | 1595 | Salvial-4(14)-en-1-one | - | - | - | - | - | - | - | - | - | 0.11 |
| 1607 | C15H24O (MW 220) | - | - | - | - | - | - | - | 0.20 | 0.11 | 0.09 | |
| 1616 | 1617 | Junenol | - | - | - | - | - | - | - | - | - | 0.09 |
| 1612 | 1614 | Di-epi-1,10-cubenol | - | 0.72 | - | - | 1.78 | - | - | - | - | 0.11 |
| 1630 | 1627 | epi-Cubenol | - | - | - | - | - | - | - | - | - | 0.11 |
| 1630 | 1631 | γ-Eudesmol | 0.17 | - | - | - | 0.44 | - | - | - | - | - |
| 1634 | 1637 | Caryophylladienol-II | - | - | 0.03 | 0.06 | - | - | - | 0.09 | 0.09 | 0.07 |
| 1640 | 1640 | τ-Cadinol | - | 6.07 | - | - | 13.24 | - | - | 0.28 | - | 0.55 |
| 1643 | 1642 | τ-Muurolol | 0.22 | - | - | - | - | - | - | 0.10 | - | - |
| 1645 | 1645 | δ-Cadinol | 0.04 | - | - | - | - | - | - | - | - | 0.20 |
| 1649 | 1649 | β-Eudesmol | 0.17 | 0.07 | - | - | 0.50 | - | - | - | - | 0.08 |
| 1652 | 1653 | α-Eudesmol | 0.20 | - | - | - | - | - | - | - | - | 0.98 |
| 1653 | 1653 | α-Cadinol | 0.34 | 0.84 | - | - | 1.01 | - | 0.30 | - | - | - |
| 1663 | C15H26O (MW 222) | - | - | - | - | 1.25 | - | - | - | - | - | |
| 1684 | 1680 | ent-Germacra-4(15),5,10(14)-trien-1β-ol | - | - | 0.09 | 0.01 | - | - | 0.10 | 0.30 | - | 1.03 |
| 1686 | 1684 | α-Bisabolol | - | 0.19 | - | - | 0.36 | - | - | - | - | - |
| 1689 | 1689 | Shyobunol | 3.78 | 2.76 | - | - | - | - | - | 0.10 | - | 0.05 |
| Total | 97.79 | 98.32 | 99.36 | 99.53 | 98.41 | 99.69 | 99.07 | 98.02 | 98.31 | 98.48 | ||
| Number of identified compounds | 56 | 51 | 49 | 36 | 54 | 42 | 50 | 54 | 40 | 63 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trendafilova, A.; Todorova, M.; Ivanova, V.; Zhelev, P.; Aneva, I. Essential Oil Composition of Ten Species from Sect. Serpyllum of Genus Thymus Growing in Bulgaria. Diversity 2023, 15, 759. https://doi.org/10.3390/d15060759
Trendafilova A, Todorova M, Ivanova V, Zhelev P, Aneva I. Essential Oil Composition of Ten Species from Sect. Serpyllum of Genus Thymus Growing in Bulgaria. Diversity. 2023; 15(6):759. https://doi.org/10.3390/d15060759
Chicago/Turabian StyleTrendafilova, Antoaneta, Milka Todorova, Viktoria Ivanova, Petar Zhelev, and Ina Aneva. 2023. "Essential Oil Composition of Ten Species from Sect. Serpyllum of Genus Thymus Growing in Bulgaria" Diversity 15, no. 6: 759. https://doi.org/10.3390/d15060759
APA StyleTrendafilova, A., Todorova, M., Ivanova, V., Zhelev, P., & Aneva, I. (2023). Essential Oil Composition of Ten Species from Sect. Serpyllum of Genus Thymus Growing in Bulgaria. Diversity, 15(6), 759. https://doi.org/10.3390/d15060759








