Abstract
The White-tailed eagle, an apex predator, is currently recovering its populations across Europe and has already reached high numbers in many countries. This led to the saturation of eagles in optimal habitats and their encroachment on suboptimal ones. We aimed to compare the diet of White-tailed eagles in optimal and suboptimal conditions in northeastern Poland to investigate how population development affected prey composition, which is expected to be lacking in suboptimal eagle territories. We have monitored eagle nests with trail cameras to investigate their diet objectively and precisely. In order to compare territories of different quality, we have conducted modeling of habitat suitability using data on nest locations prior to their saturation. Using recorded photos of the prey, we measured their size and estimated their weight to check if the size and biomass of the prey are comparable between optimal and suboptimal territories. We found that eagles in the latter conditions were not limited by prey biomass but turned to alternative prey and brought larger prey. The alternative prey were large birds such as White storks and Common cranes, but also chicks of other avian predators that were robbed from their nests. Most probably, eagles cope with a lack of optimal prey by ranging farther and exploring non-optimal foraging habitats. We conclude that the diet flexibility of White-tailed eagle enables him to still increase its numbers despite already high densities. Our study also shows that this species might possibly impact the White stork population, as seen in the case of the Black stork and some seabird species.
1. Introduction
The White-tailed eagle, Haliaeetus albicilla, has recovered in Europe in many countries [1,2]. Locally, its numbers increased threefold in the beginning of the XXI century and are still growing [3]. In some European countries, despite the fact that it has already reached high numbers, it is still the most common species among birds of prey [4]. The increase in predator population numbers has to be supported by high availability of preferred prey or high plasticity in switching prey [5,6]. In the breeding season, White-tailed eagles are known to prey on a variety of medium and large prey, most frequently fish and waterfowl, with fish being more prevalent in inland areas and waterfowl being more prevalent in coastal areas [7,8,9,10]. In the breeding season, mammals usually form an accessory prey, but sometimes a significant one, like in the case of Scotland [11].
Given that the White-tailed eagle is an apex predator, the high increase in its density might affect its prey, but prey availability may also limit population growth and therefore force it to shift towards alternative prey. The first was mainly shown by the direct, negative impact that eagles made on seabirds: Eider Somateria mollissima [12] and Great cormorant Phalacrocorax carbo [13], but also less directly on Black stork Ciconia nigra in the inland [14]. The second has so far been investigated mainly by changes in diet composition during population development (increase in eagle density) [15,16]. In Lithuania, the frequency of alternative prey (i.e., terrestrial birds and mammals) and dietary niche breadth did not increase between 2005 and 2018, and authors concluded that White-tailed eagles did not rely more on alternative prey in the course of population development [16]. In Finland, between 1985 and 2010, a dietary shift was observed in which the proportion of birds increased but that of fish and mammals decreased [15]. However, revealing how the largest avian predator in Europe copes with their high food demands in the light of increasing competition and saturation of optimal habitats is still an open question. To answer it, we should quantify habitat suitability and investigate diet separately in optimal and suboptimal habitats. Heuck et al. [17] found that due to population growth, White-tailed eagles started to inhabit suboptimal habitats. In such sites, the area of suitable foraging habitat was smaller, and eagles experienced lower breeding success and a lower average number of chicks. Most probably, food availability is the link between worse foraging conditions and lower fitness in pairs in suboptimal habitats.
We have investigated the diet of White-tailed eagles with the use of trail cameras, mounted in eagles’ nests to check if the prey composition differs between optimal and suboptimal habitats in the still-developing population of northeastern Poland. Territory quality was distinguished with habitat suitability modeling. We expected that in suboptimal territories, eagles will: (1) switch to alternative prey and therefore explore a wider set of prey species, compared to eagles in optimal ones, with better access to abundant and optimal-size prey; (2) compensate for a shortage of prey by hunting on larger animals; and (3) suffer from a food shortage, which will result in less food biomass brought to the nest, a lesser frequency of prey deliveries (because of a lower abundance of prey), and smaller broods. Additionally, by studying the diet of White-tailed eagles during the population expansion, we hoped to contribute to the ongoing debate on this species impact on other rare and protected species [15,18,19].
2. Materials and Methods
2.1. Study Area
The study was carried out in the Podlasie region, a part of northeastern Poland with a continental climate. The region is characterized by mosaic farmland, a few large forest complexes, and marshy river valleys. The terrain is moderately flat but shaped to some extent by glacial events. In the studied part of the region (North Podlasie Lowland), there are almost no natural lakes, but only fish ponds of small and moderate size and important river valleys of Biebrza and Narew, where high numbers of waterfowl stop at spring migration and also breed.
2.2. Habitat Suitability Models
To model habitat suitability in the studied area for the White-tailed eagle, we used data on 29 nest locations (1 nest per territory) (Figure S1) that were occupied in the studied region in the first place, up to 2010. Under the assumption that they should represent the best habitats before the population developed and saturated, if there were more nests per territory, we included only the one occupied in 2010 or the one closest to this date.
As the species is associated with river valleys, waterbodies, and large wetlands, we prepared six raster variables in QGIS 3. Four were based on a detailed vector dataset of hydrological features downloaded from the Hydrological Map of Poland dataset (Polish Geological Institute, available through https://dane.gov.pl/en, accessed 12 February 2023). The vector dataset was transformed with the “proximity” tool to get the distance to waterbodies, distance to main rivers, distance to (any) water, and distance to wetlands. Furthermore, using rasters (100 m resolution) of water and wetness (downloaded from the Copernicus Land Monitoring service), we have calculated the next two variables: the share of water and the share of wetlands (wetness) per square kilometer around the focal pixel. We used the sum of pixels around the focal one to account for the continuity of a habitat, which is important for such a large apex predator. We used the variance inflation factor (VIF) to check the collinearity between predictors and removed those with a VIF greater than 10. Four predictors remained.
Habitat suitability was modeled in R using the ‘biomod2’ package [20]. We have generated 10 sets of pseudoabsences with numbers three times higher than the number of occurrences. Pseudoabsences were drawn with a “disk” strategy with a minimum distance of 5000 m and a maximum of 20,000 m to presence data (actual nest locations). Models were built using the Random Forest algorithm. The data was divided into training and testing datasets in a 70:30 proportion. Next, we carried out the procedure to estimate the importance of the input variables with 10 permutations. Models were validated based on the Receiver Operation Curve (ROC). Predictors importance was estimated on a 0–1 scale with the ’biomod2’ package and averaged for ten permutations.
2.3. Dietary Data
The diet White-tailed eagles was collected by trail cameras mounted in their nest at the stage of 2- to 4-week-old chicks between 2013 and 2018. Two to four trail cameras were mounted each year, about 1.5–4 m from the nest, pointing directly downwards or from a variable angle, so the nest surface was seen like a plate. In one case, the nest had fallen due to heavy wind; in another, a late chick died without obvious reasons; the trail camera was pointing above the nest; or the memory card failed. Excluding such cases, where material was incomplete, the final dataset covered 12 different nests, recorded at medium and large chick stages as well as after fledgling. We used Ltl Acorn 6210MC trail cameras with 32 GB memory cards and twelve 2500 mAh accumulators, which enabled us to follow the nest from May until October and gather at least over twelve thousand 5 MP resolution photos without replacing the accumulators. Trail cameras were set to take two photos after recording movement during the daytime, and afterwards they went into sleep mode for the next 3 min. Additionally, in the same nests, we have noted prey items according to prey remains found in and under the nests in 2011–2018. However, this was a side task, and we noted the visible prey items but did not count prey based on fish scales, fur, or other small remnants.
The territories of White-tailed eagles monitored with trail cameras were attributed to optimal and suboptimal habitats based on the cut-off value of the Random Forest habitat suitability model that minimized the absolute difference between model sensitivity and specificity. The habitat suitability value under each nest was compared with the cut-off value, and if it was lower, the territory was qualified as suboptimal and optimal if higher.
Prey was identified mostly according to expert knowledge. Most birds and mammals were identified by the authors; some consulted with other ornithologists when needed. Fish were also identified by the authors and checked with experienced anglers (mentioned in the Acknowledgments). Fish biomass was estimated from images by measuring every individual fish’s total length (scale based on bird rings from photos) and comparing it with empirical data describing it [21,22,23,24,25,26,27]. For unidentified species, we assigned a weight of 250 g for a small fish and 400 g for a medium-sized one. Bird and mammal biomasses were estimated according to reported adult average weights and estimated weights (upon growth curves) in the case of juveniles [26,27,28,29,30,31,32]. The average size of unidentified pieces of meat was estimated, representing the mean prey biomass from different prey groups (320 g). Apart from the prey items themselves, the number of prey deliveries per day was noted.
2.4. Data Analysis
The impact of habitat suitability on the diet of White-tailed eagles was tested between territories in optimal and suboptimal habitats. The value of the random forest model of habitat suitability for two habitat suitability groups was tested in R software with the Wilcoxon test to check if the division into optimal and suboptimal habitats is visible and statistically supported. Brood size and diet characteristics were also compared with the Wilcoxon test between two habitat suitability groups. In tested prey groups, we have distinguished other avian predators (birds of prey and owls) to test for superpredation intensity between optimal and suboptimal habitats. Secondly, we distinguished large prey, over 3000 g, therefore beyond the size of fish and waterfowl, that White-tailed eagles are known to feed on regularly.
Prey size (the logarithm of prey weight) was investigated with linear models to explain how it was influenced by territory quality (suboptimal vs. optimal) and brood size (a numerical variable). All analyses were carried out in R 4.1.1.
3. Results
3.1. Habitat Suitability Models
Model ensembles from all Random Forest models yielded high performance (ROC = 0.958). Model sensitivity reached 100%, while specificity reached 82.4%. The cut-off value for the model was estimated to be 0.345 for habitat suitability. This threshold was used to divide the optimal (n = 6) and suboptimal (n = 6) territories of the studied species (Figure 1).
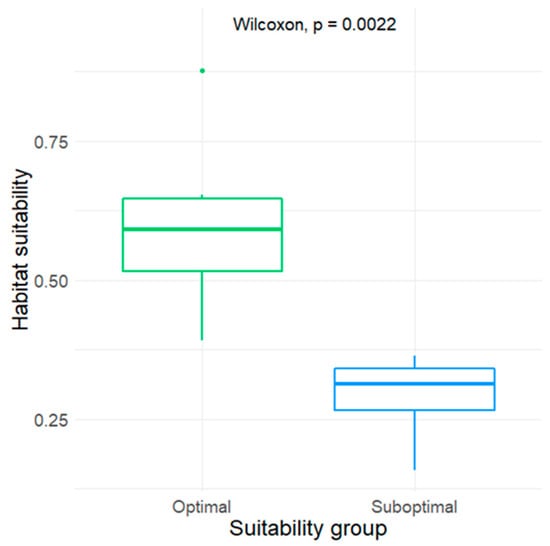
Figure 1.
Comparison of the Random Forest model of habitat suitability values attributed to optimal and suboptimal territories of White-tailed eagles in northeastern Poland.
All variables contributed significantly to both models (Table 1). The distance to a waterbody was the best predictor of habitat suitability for the White-tailed eagle, followed by the distance to main rivers and to wetlands (Table 1). The sum of wetlands was still an important predictor but contributed the least.

Table 1.
Habitat suitability predictors and their mean importance in Random Forest models (on a 0–1 scale, averaged for ten models) of White-tailed eagle habitats in northeastern Poland.
3.2. Diet Composition
White-tailed eagles in northeastern Poland feed on variable prey, dominated by fish and waterfowl (Table 2). Fish dominated visibly in prey number (74%) and slightly in prey biomass (47%), followed by birds. The latter formed 21% of prey numbers but as much as 41% of prey biomass. Mammals were rarely brought (5%) to the nest, but in larger pieces, and thus contributed 12% of prey biomass.

Table 2.
Diet composition of White-tailed eagles in northeastern Poland from 2013 to 2018, investigated at 12 nests using trail cameras.
We found 15 species of fish, representing both artificial fish ponds and natural rivers. The carp, Cyprinus carpio, constituted 55% of all the identified fish species brought to the nest. The average mass of this prey species reached 493 g, with a maximum of 2120 g. Other fish species averaged 439 g, ranging from 71 g to 1100 g. The share of carp in prey numbers and biomass shows the importance of the fish ponds for the species in this lakeless part of the region (north Podlasie Lowland).
White-tailed eagles fed on at least seventeen species of birds and a domestic chicken (Table 2). Waterfowl (ducks, geese, rails, swans, and grebes) constituted exactly half of the identified species. Other waterbirds, such as gulls and terns, added another 7% to the bird prey number. Important alternative prey groups were the large birds, such as White stork Ciconia ciconia and Common crane Grus grus (18% of identified birds), but also chicks of other avian predators that must have been taken from their nests, mostly Common buzzard, but also Long-eared owl (11% of bird prey number). Single adults or juveniles of Common ravens and Tawny owls were also noted.
Mammals were the least frequent but locally important in terms of biomass. Young beavers were probably hunted in the water, but other species were possibly taken as carcasses, some of which were likely roadkill. In many cases, we were not able to identify the mammal species as only a piece of meat with fur was recorded at the nest.
Prey remains found in the nest show a more or less similar variety of prey, but with a lesser diversity of fish and a greater diversity of bird species, especially the waterfowl (Table S1). No signs of superpredation were noted in prey remains. This is because White-tailed eagles predate mostly on chicks, yet without contour feathers, they are eaten entirely and leave no trace. The ratio of main prey groups based on nest remains is completely different (Table S2). Birds dominated (80%) in prey remains, while in diets recorded by trail cameras, they were almost four times less frequent (21%). On the contrary, fish (which are eaten whole) were found only rarely in prey remains (14%), but were dominant in the data investigated from trail cameras (74%). Mammals did not differ much between those two datasets.
3.3. Factors Affecting the White-Tailed Eagle Diet
Proportions of main prey groups did not differ significantly between eagles in optimal and suboptimal territories (Table S3), although the difference in the ratio of mammals was slightly higher in optimal territories (Figure S2) but only close to significant (p = 0.076). Detailed comparisons showed eagles fed much more frequently on carp but much less on Northern pike (Esox lucius) and Common roach (Rutilus rutilus) in suboptimal than optimal territories. In the case of bird prey, eagles preyed on white storks and other avian predators much more frequently in suboptimal territories but rarely (compared to optimal ones) on Eurasioan coots, Fulica atra, and Greylag geese, Anser Anser.
To some extent, White-tailed eagles altered their prey preferences depending on habitat quality. In optimal habitats, they hardly fed on other predators, while they did so relatively frequently in suboptimal habitats (Figure 2a). The ratio of superpredation was significantly higher in prey number and prey biomass in suboptimal habitats than optimal habitats (Table S3). Furthermore, we noticed that eagles in suboptimal habitats often brought large prey, such as storks, cranes, and mammals, to their nests (Figure 2b). This result was not statistically significant, but taking into account the small sample size of nests, we consider it to be close to significant. Lastly, we did not find any difference in prey species richness between optimal and suboptimal territories (Table S3).
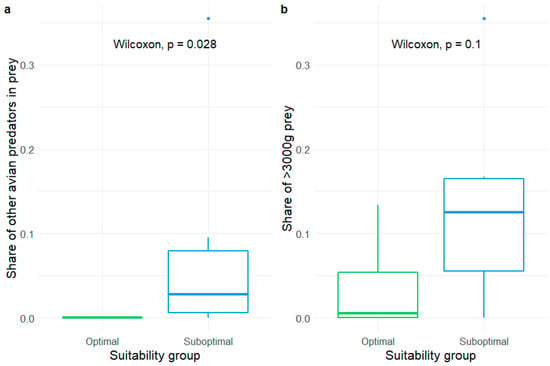
Figure 2.
Comparison of the share of the two alternative prey groups: other raptors (a) and large prey (b), in the diet of the White-tailed eagles occupying optimal and suboptimal territories in northeastern Poland.
3.4. Prey Size
We tested the effects of territory quality and brood size on prey size, prey biomass, and the number of prey deliveries. Brood size did not differ between optimal and suboptimal habitats (p = 0.93), although the only brood of three chicks that successfully fledged was recorded in an optimal territory. We did not find a territory quality impact on daily biomass consumption per nest or the daily number of prey deliveries per nest (Figure 3a,b, Table S3). However, we found that the total biomass brought to the nest was greater in broods with multiple chicks (Figure 3c). The number of prey deliveries seemed slightly lower in solitary broods, but not significantly (Figure 3d).
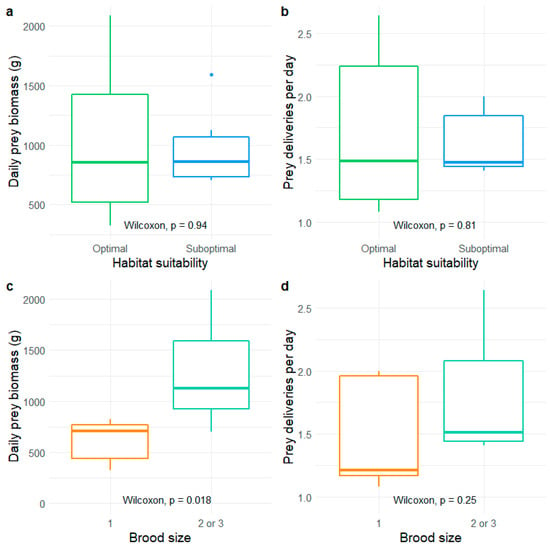
Figure 3.
Daily prey biomass and the number of prey deliveries per day, recorded in the nests of White-tailed eagles in northeastern Poland and compared between optimal and suboptimal territories (a,b) and broods with single or multiple chicks (c,d).
White-tailed eagles fed on larger birds and fish in suboptimal habitats (Table 3). In the case of mammals, their sizes were smaller in suboptimal habitats, but due to the small sample size, the results were not significant (Figure S3). When raising larger broods, eagles selected larger fish, but the same relationship was not significant in the case of bird prey (Table 3). The effect of territory quality was greater than that of brood size. The models explained almost 10% of the variance in fish size, but only almost 5% in the case of bird prey. Overall, in optimal territories, eagles most often brought prey weighing 500–1000 g to their nest.

Table 3.
Linear models explaining single White-tailed eagles’ prey item mass with territory quality (suboptimal/optimal) and the brood size.
4. Discussion
4.1. Diet Differences between Optimal and Suboptimal Habitats
We found that the diet of White-tailed eagles was affected by territory quality, but not all of our predictions were confirmed. Eagles switched to alternative prey, but to a limited extent. Dietary breadth, measured by the number of species that were brought to the nest, did not differ with regard to territory quality. However, eagles reached for alternative prey such as large birds other than waterfowl and robbed the nests of other avian predators, mainly the Common buzzard. The latter was already reported in Lithuania [19], but here we were able to show this prey was clearly more frequently used in suboptimal territories. Secondly, we confirmed that in suboptimal conditions in comparison to optimal ones, eagles will compensate for abundant prey with larger fish and birds (but not mammals). In optimal habitats, the most frequent prey ranged from 500 to 1000 g, but it was much more variable in suboptimal habitats and included a relatively high share of prey over 3000 g. The dominant prey size in optimal habitats corresponded to the ones recorded at Estonian fishponds (optimal foraging habitat), where White-tailed eagles preyed on 300–1050 g fish [33]. Interestingly, in the lakeland adjacent to our study, about 20 years before, eagles were estimated to prey on 514 g of fish and 611 g of birds on average [34]. This confirms that in good-quality habitats (such as large lakes) and before the population saturated, White-tailed eagles foraged on relatively small prey, that was possibly abundant and easy to handle and carry.
Additionally, we found that bigger brood also contributed to selection for larger fish, but to a lesser extent than territory quality. In broods of twins or triplets, the prey biomass was greater than in nests with single chicks, but not the number of prey deliveries, which confirms that eagles compensated for higher food demands with larger prey. Finally, we could not confirm that in suboptimal territories, eagles would suffer from food shortages. The daily biomass and number of prey deliveries, as well as the number of chicks per brood, were similar in optimal and suboptimal habitats.
4.2. Mitigation of Lower-Quality Territories with Larger Prey
In Greece, White-tailed eagles occupying territories of potentially similar quality at neighboring lakes were found to hunt on similar prey species of similar body mass. It suggests that in comparable conditions, eagles select locally optimal prey, also in terms of their size [35]. In our case, where territories differed in their quality, we observed a clear difference in selected prey size. In suboptimal territories, eagles brought larger prey to the nest, while in optimal habitats, the most frequent size of prey was much smaller. It seems that the first had to spend additional energy to find larger prey, handle it, and transport it to the nest. Most likely, it required a much larger area to search for bigger prey, but eagles also had to explore habitats that were different from optimal ones (waterbodies, river valleys). The latter was seen as relatively high numbers of adult white storks, which were possibly hunted over agricultural landscapes and by robbing the nests of other avian predators, which requires penetration of the forest. To prove that White-tailed eagles have to range further to find sufficient food for their offspring, we would need to track them with GPS telemetry devices. We did not have such data for the eagles whose nests were monitored by us with trail cameras, but we had followed another adult male, who occupied suboptimal territory on the edges of Biebrza Valley but had no access to the best foraging sites near the river. During most of the season, he ranged over a small area, but when having chicks, the male flew regularly 25 km to the large waterbody (Mirski, unpublished). A similar case was observed in Lesser spotted eagles, Clanga pomarina, which were also forced to forage exceptionally far from their nest to successfully raise chicks in a situation of insufficient foraging areas [36].
A mechanistic population model showed that the increase in density of White-tailed eagles should affect their breeding performance [15]. However, empirical data from Lithuania pointed out that eagles were not food-limited, nor did their reproduction decrease, despite a high increase in density [3,14]. Compensation of poor territory quality by the increase of home ranges to hunt for larger prey might be the best explanation for why the theoretical model and empirical data did not match in this case.
4.3. The Impact of a Developing Apex Predator Population on Its Prey
The growing population of an apex predator often raises questions about its impact on prey species, both livestock and wild animals. Regarding the first, White-tailed eagles pose only a small or moderate threat [8]. Regarding the second, for some prey species, the pressure from this predator can be significant. Thus far, this has mainly been proven for seabirds such as the Common eider [10] and the Black-legged kittiwake Rissa tridactyla [37], which are top-down controlled by the eagles. It is difficult to assess, though, if the current impact is different from a century ago, before the numbers of this species dropped and recovered again.
There is less proof on the impact of the White-tailed eagle on its prey in inland areas, but a recent study shows that they can rob nests of other predators and the Black stork ([14,17,38], this study, and our own unpublished data). Not only by nest robbing but possibly also by killing adult storks, White-tailed eagles can affect other species, particularly Black storks, by creating a “landscape of fear”, in which storks avoid nesting within a few kilometers of an eagle’s nest. This affects nest occupancy rates and breeding performance in this declining species. Our study shows evidence that the White stork is also directly threatened by White-tailed eagles. We found that 13% of birds brought to the nest were adult storks, some even carrying rings, proving they were mature birds. For a long-lived species like storks, a high surplus mortality rate from predation can be reflected in a significant population decline. Similarly, in the case of predated Common cranes, which were, however, found less often in our study, an even higher ratio of White storks and Common cranes to other prey was found in our opportunistically collected data on prey remains in eagles’ nests (Table S2). However, this dataset is biased towards durable leftovers and can lead to the “demonization” of this apex predator, which, in fact, poses some threat to particular prey species but, in optimal territories, relies mostly on fish and waterfowl.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d15060747/s1. Table S1. White-tailed eagle diet investigated from prey remains found in different nests or seasons in northeastern Poland in 2011–2018; Table S2. Differences in general prey composition of White-tailed eagles from northeastern Poland, studied by examination of prey remains in their nests (2011–2018) and identification of prey from the photos taken by trail cameras recording nests (2013–2018); Table S3. Results of statistical comparisons between diet characteristics of White-tailed eagles in optimal (n = 6) and suboptimal (n = 6) habitats in northeastern Poland, studied with trail cameras at the nest and tested with the Wilcoxon test; Figure S1. Habitat suitability (Random Forest model) for the White-tailed eagle in northeastern Poland; Figure S2. Proportions of main prey groups in the White-tailed eagle diet in territories of optimal and suboptimal habitats; Figure S3. Prey size of the main prey groups of White-tailed eagles, measured from trail camera images recording their nests in optimal and suboptimal habitats in northeastern Poland; Figure S4. Histograms of 484 prey-size items from White-tailed eagle nests in northeastern Poland, identified and measured from trail camera images in the nests located in optimal and suboptimal habitats.
Author Contributions
Conceptualization, P.M.; methodology, P.M. and E.K.; investigation, P.M. and E.K.; data curation, P.M. and E.K.; writing—original draft preparation, P.M.; writing—review and editing, P.M. and E.K.; visualization, P.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Geographic Society, grant number GEFNE75-13.
Institutional Review Board Statement
The license to enter White-tailed eagle nests and monitor them with trail cameras was granted by the Regional Inspectorate for Environmental Protection in Białystok.
Data Availability Statement
Acknowledgments
We would like to thank Krzysztof Henel for help in collecting data in Biebrza National Park, Marcin Leszczyński, Tomasz Tumiel, and Grzegorz Grygoruk for help in the fieldwork, Sylwester Aftyka for help in the identification of bird feathers, Marcin Wereszczuk, and Ryszard Szerakowski for their help in fish identification.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dementavičius, D. White-tailed eagle (Haliaeetus albicilla) in Lithuania: Population numbers and trends 1900–2007. Acta Zool. 2007, 17, 281–285. [Google Scholar] [CrossRef]
- Sulawa, J.; Robert, A.; Köppen, U.; Hauff, P.; Krone, O. Recovery dynamics and viability of the white-tailed eagle (Haliaeetus albicilla) in Germany. Biodivers. Conserv. 2010, 19, 97–112. [Google Scholar] [CrossRef]
- Dementavičius, D.; Rumbutis, S.; Treinys, R. Spatial and temporal variation in breeding performance in the increasing White-tailed Eagle Haliaeetus albicilla population to the east of the Baltic Sea. Bird Study 2022, 68, 462–476. [Google Scholar] [CrossRef]
- Wardecki, Ł.; Chodkiewicz, T.; Beuch, S.; Smyk, B.; Sikora, A.; Neubauer, G.; Meissner, W.; Marchowski, D.; Wylegała, P.; Chylarecki, P. Monitoring Ptaków Polski w latach 2018–2021. Biul. Monit. Przyrody 2021, 22, 1–80. [Google Scholar]
- Galushin, V.M. Synchronous fluctuations in populations of some raptors and their prey. IBIS 1974, 116, 127–134. [Google Scholar] [CrossRef]
- Newton, I. Population Ecology of Raptors; T & AD Poyser: Berkhamsted, UK, 1979. [Google Scholar]
- Sulkava, S.; Tornberg, R.; Koivusaari, J. Diet of the white-tailed eagle Haliaeetus albicilla in Finland. Ornis Fenn. 1997, 74, 65–78. [Google Scholar]
- Nadjafzadeh, M.; Hofer, H.; Krone, O. The link between feeding ecology and lead poisoning in white-tailed eagles. J. Wildl. Manag. 2013, 77, 48–57. [Google Scholar] [CrossRef]
- Sándor, A.; Alexe, V.; Marinov, M.; Doroşencu, A.; Domşa, C.; Kiss, B.J. Nest-site selection, breeding success, and diet of white-tailed eagles (Haliaeetus albicilla) in the Danube Delta, Romania. Turk. J. Zool. 2015, 39, 300–307. [Google Scholar] [CrossRef]
- Ekblad, C.; Tikkanen, H.; Sulkava, S.; Laaksonen, T. Diet and breeding habitat preferences of White-tailed Eagles in a northern inland environment. Polar Biol. 2020, 43, 2071–2084. [Google Scholar] [CrossRef]
- Whitfield, D.P.; Marquiss, M.; Reid, R.; Grant, J.; Tingay, R.; Evans, R.J. Breeding season diets of sympatric White-tailed Eagles and Golden Eagles in Scotland: No evidence for competitive effects. Bird Study 2013, 60, 67–76. [Google Scholar] [CrossRef]
- Morelli, F.; Laursen, K.; Svitok, M.; Benedetti, Y.; Møller, A.P. Eiders, nutrients and eagles: Bottom-up and top-down population dynamics in a marine bird. J. Anim. Ecol. 2021, 90, 1844–1853. [Google Scholar] [CrossRef]
- Bregnballe, T.; Tofft, J.; Kotzerka, J.; Lehikoinen, A.; Rusanen, P.; Herrmann, C.; Krone, O.; Engström, H.; Rattiste, K.; Reich, J.; et al. Occurrence and behaviour of White-tailed Eagles Haliaeetus albicilla in Great Cormorant Phalacrocorax carbo sinensis colonies in countries around the Baltic Sea. Ardea 2021, 109, 565–582. [Google Scholar] [CrossRef]
- Zawadzki, G.; Zawadzki, J.; Drozdowski, S.; Zawadzka, D. The avoidance of living in the vicinity of a top predator: The coexistence of the black stork and the white-tailed eagle in NE Poland. Eur. Zool. J. 2022, 89, 1223–1237. [Google Scholar] [CrossRef]
- Ekblad, C.; Sulkava, S.; Stjernberg, T.; Laaksonen, T. Landscape-Scale Gradients and Temporal Changes in the Prey Species of the White-Tailed Eagle (Haliaeetus albicilla). Ann. Zool. Fenn. 2016, 53, 228–240. [Google Scholar] [CrossRef]
- Dementavičius, D.; Rumbutis, S.; Virbickas, T.; Vaitkuvienė, D.; Dagys, M.; Treinys, R. Spatial and temporal variations in the White-tailed Eagle Haliaeetus albicilla breeding diet revealed by prey remains. Bird Study 2020, 67, 206–216. [Google Scholar] [CrossRef]
- Heuck, C.; Herrmann, C.; Schabo, D.G.; Brandl, R.; Albrecht, J. Density-dependent effects on reproductive performance in a recovering population of White-tailed Eagles Haliaeetus albicilla. IBIS 2017, 159, 297–310. [Google Scholar] [CrossRef]
- Treinys, R.; Dementavičius, D.; Mozgeris, G.; Skuja, S.; Saulius, R.; Stončius, D. Coexistence of protected avian predators: Does a recovering population of White-tailed Eagle threaten to exclude other avian predators? Eur. J. Wildl. Res. 2021, 57, 1165–1174. [Google Scholar] [CrossRef]
- Kamarauskaitė, A.; Dementavičius, D.; Skuja, S.; Dagys, M.; Treinys, R. Interaction between the White-tailed Eagle and Common Buzzard estimated by diet analysis and brood defence behaviour. Ornis Fenn. 2020, 97, 26–37. [Google Scholar]
- Thuiller, W.; Georges, D.; Gueguen, M.; Engler, R.; Breiner, F.; Lafourcade, B.; Patin, R. Biomod2: Ensemble Platform for Species Distribution Modeling. R Package Version 4.2-3. Available online: https://cran.r-project.org/web/packages/biomod2/biomod2.pdf (accessed on 11 April 2023).
- De Giosa, M.; Czerniejewski, P. A generalized, nonlinear regression approach to the length-weight relationship of European perch (Perca fluviatilis L.) from the Polish coast of the southern Baltic Sea. Arch. Pol. Fish. 2016, 24, 169–175. [Google Scholar] [CrossRef]
- Kurbanov, A.; Bakhtiyar, K. Age and growth of bighead carp (Hypophthalmichthys nobilis R.) in Tudakul reservoir, Uzbekistan. Turk. J. Fish. Aqua Sci. 2015, 3, 229–232. [Google Scholar]
- Ömer, S.; Göktuğ, G.; Mehmet, Y.; Ali, G. Some Biological Features of Tinca tinca Population in Asartepe Reservoir (Ankara). J. Agric. Sci. Technol. B 2018, 8, 466–471. [Google Scholar] [CrossRef]
- Epler, P.; Popek, W.; Łuszczek-Trojnar, E.; Drąg-Kozak, E.; Szczerbik, P.; Socha, M. Age and growth rate of the roach (Rutilus rutilus L.) from the Solina and the Tresna (Zywieckie Lake) dam reservoirs. Acta Sci. Pol. Piscaria 2005, 4, 59–70. [Google Scholar]
- Stavrescu-Bedivan, M.; Vasile Scaeteanu, G.; Madjar, R.M.; Manole, M.S. Investigation of length-weight relationship and condition factor of Carassius gibelio related to water quality in Pantelimon II lake. AgroLife Sci. J. 2018, 7, 123–130. [Google Scholar]
- Karataş, M.; Çiçek, E.; Başusta, A.; Başusta, N. Age, Growth and Mortality of Common Carp (Cyprinus carpio Linneaus, 1758) Population in Almus Dam Lake (Tokat-Turkey). J. Appl. Biol. Sci. 2007, 1, 81–85. [Google Scholar]
- Yilmaz, S.; Yazicioğlu, O.; Yazici, R.; Polat, N. Age, Growth and Reproductive Period of White Bream, Blicca bjoerkna (L., 1758) in Lake Ladik, Turkey. J. Limnol. Freshw. Fish. Res. 2015, 1, 9–18. [Google Scholar] [CrossRef]
- Jedrzejewska, B.; Jedrzejewski, W. Predation in Vertebrate Communities: The Białowieża Primeval Forest as a Case Study. Ecol. Stud. 1998, 135, 462. [Google Scholar] [CrossRef]
- Tsachalidis, E.P.; Liordos, V.; Goutner, V. Growth of White Stork Ciconia ciconia nestlings. Ardea 2005, 93, 133–137. [Google Scholar]
- Lokemoen, J.T.; Johnson, D.H.; Sharp, D.E. Weights of wild Mallard Anas platyrhynchos, Gadwall A. strepera, and Blue-winged Teal A. discors during the breeding season. Wildfowl 1990, 41, 122–132. [Google Scholar]
- Meissner, W. Analiza biometryczna łysek Fulica atra z północnej Polski. Ornis Pol. 2010, 51, 21–29. [Google Scholar]
- Owen, M.; Cook, W.A. Variations in body weight, wing length and condition of Mallard Anas platyrhynchos platyrhynchos and their relationship to environmental changes. J. Zool. 1977, 183, 377–395. [Google Scholar] [CrossRef]
- Tuvi, J.; Väli, Ü. The impact of the White-tailed Eagle Haliaeetus albicilla and the Osprey Pandion haliaetus on Estonian Common Carp Cyprinus carpio production: How large is the economic loss? Proc. Est. Acad. Sci. Biol./Ecol. 2007, 56, 209–223. [Google Scholar] [CrossRef]
- Zawadzka, D. Feeding habits of the Black Kite Milvus migrans, Red Kite Milvus milvus, White-tailed Eagle Haliaeetus albicilla and Lesser Spotted Eagle Aquila pomarina in Wigry National Park (NE Poland). Acta Ornithol. 1999, 34, 65–75. [Google Scholar]
- Bounas, A.; Karta, E.; Navarrete, E.; Sidiropoulos, L.; Alivizatos, H. Diet composition of White-tailed Eagles inhabiting two adjacent inland lakes in Northern Greece. Raptor J. 2023, 17. [Google Scholar] [CrossRef]
- Mirski, P.; Cenian, Z.; Dagys, M.; Daróczi, S.; Dementavičius, D.; Maciorowski, G.; Menderski, S.; Nowak, D.; Pongrácz, Á.; Prommer, M.; et al. Sex-, landscape- and climate-dependent patterns of home-range size—A macroscale study on an avian generalist predator. IBIS 2021, 163, 641–657. [Google Scholar] [CrossRef]
- Anker-Nilssen, T.; Fayet, A.L.; Aarvak, T. Top-down control of a marine mesopredator: Increase in native white-tailed eagles accelerates the extinction of an endangered seabird population. J. Appl. Ecol. 2023, 60, 445–452. [Google Scholar] [CrossRef]
- Yurko, V. Diet of the White-Tailed Eagle During the Breeding Season in the Polesski State Radiation-Ecological Reserve, Belarus. Raptors Conserv. 2016, 32, 21–31. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).