Timing of Ice Retreat Determines Summer State of Zooplankton Community in the Ob Estuary (the Kara Sea, Siberian Arctic)
Abstract
1. Introduction
2. Materials and Methods
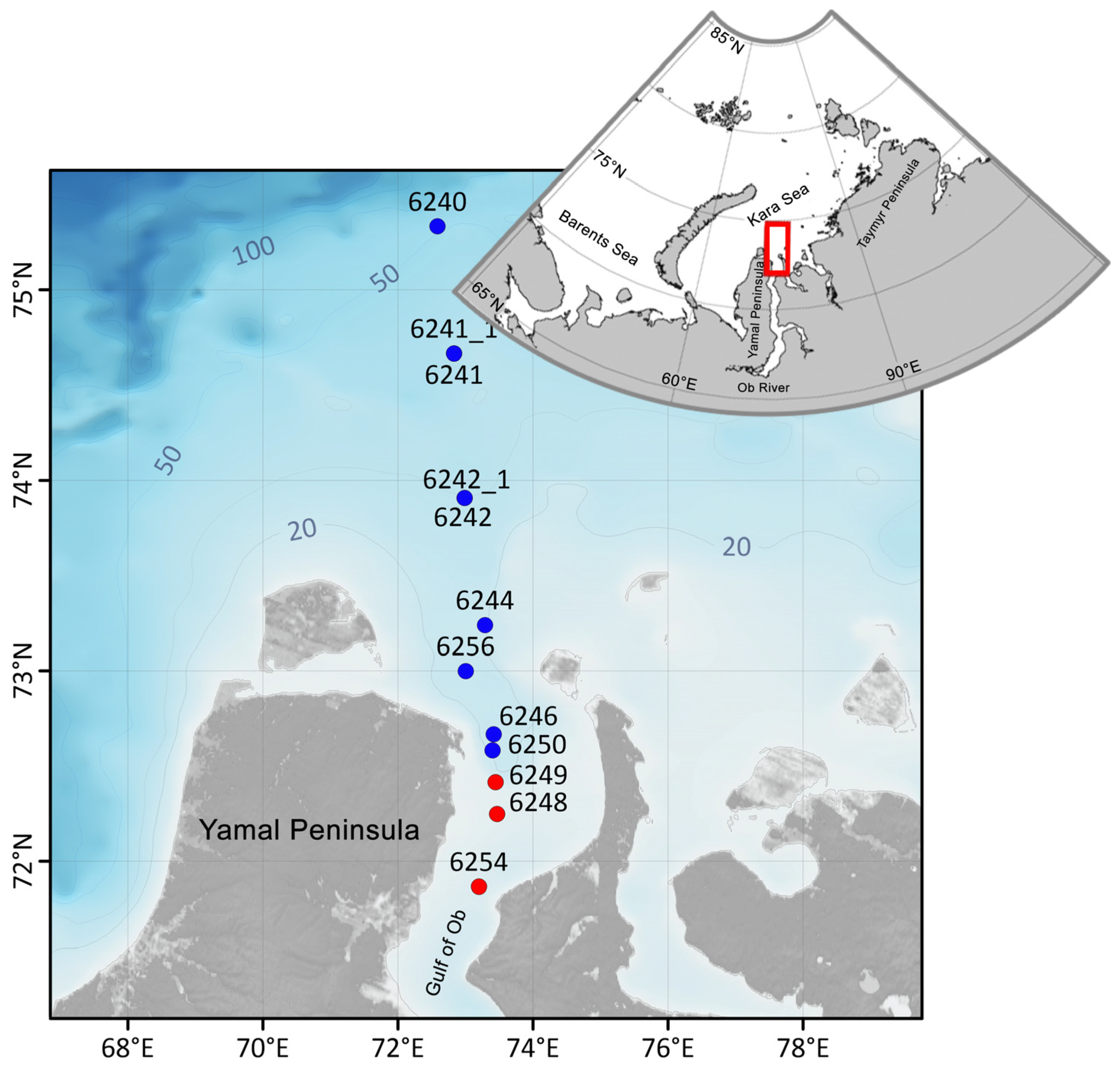
2.1. Sampling
2.2. Zooplankton Abundance and Biomass
2.3. Feeding
2.4. Respiration
2.5. Grazing Impact
2.6. Statistical Analyses
3. Results
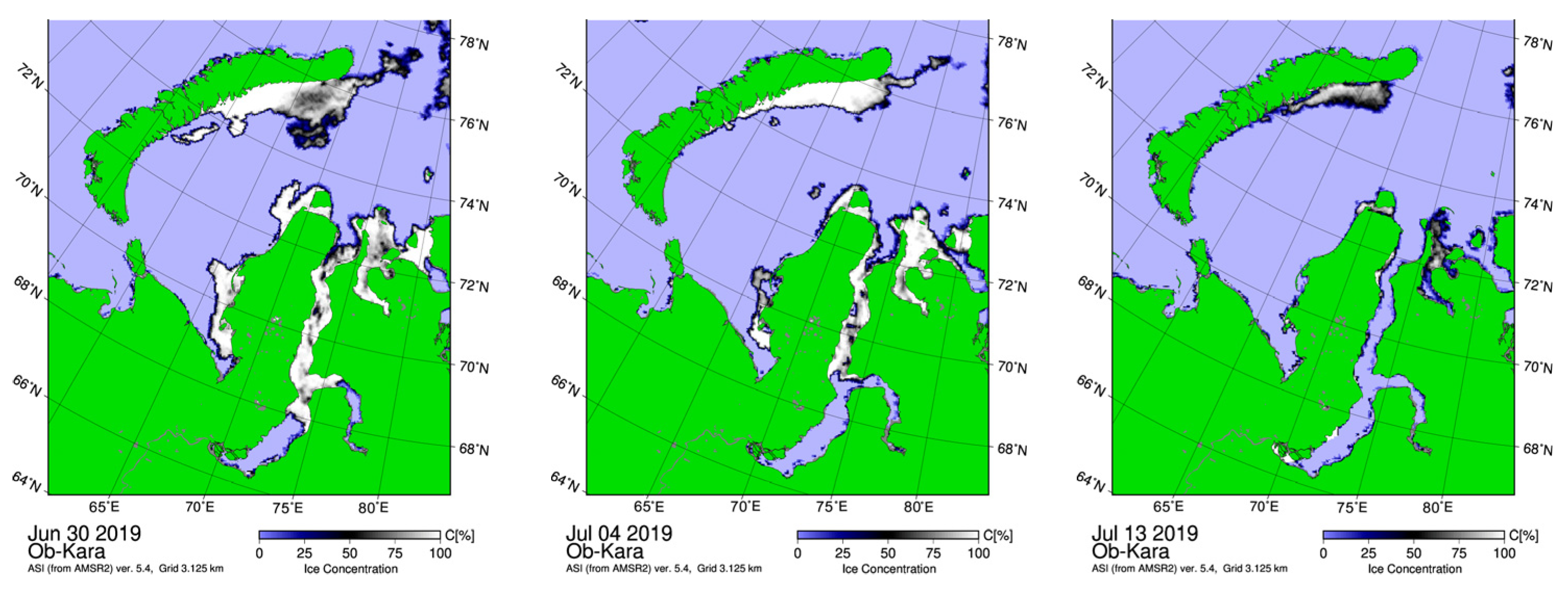
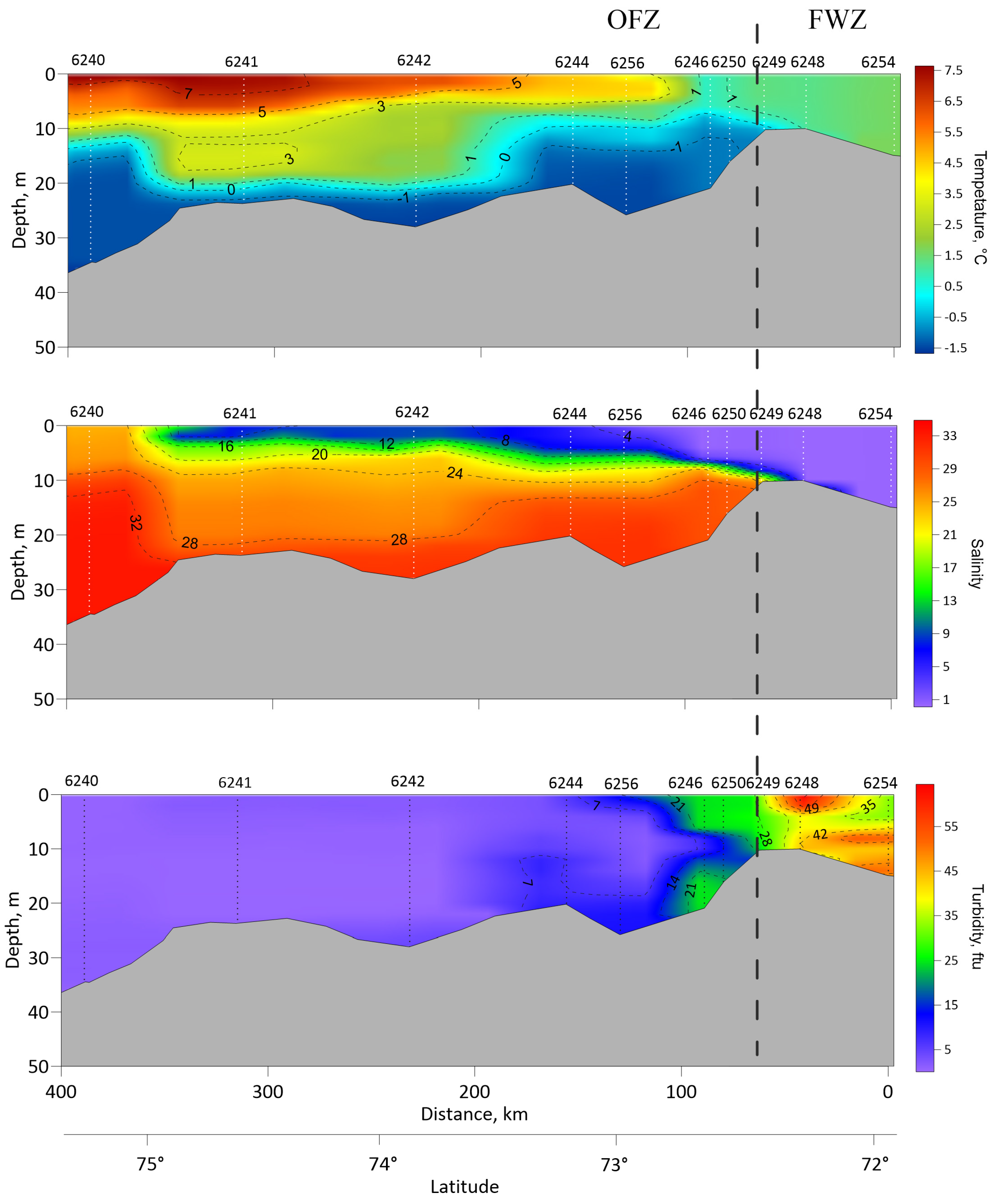
3.1. Study Area: Sea-Ice, Hydrography, Chlorophyll
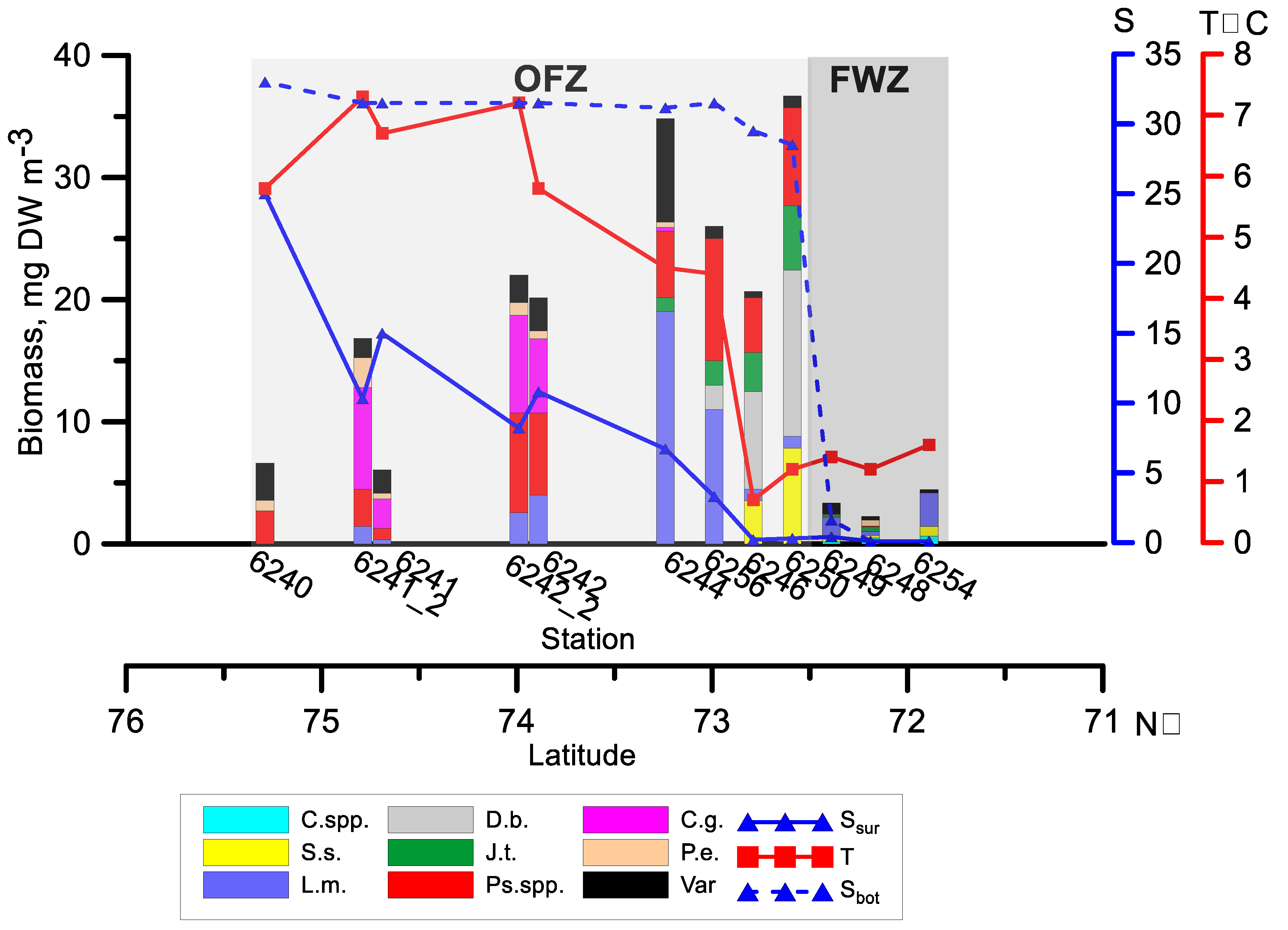
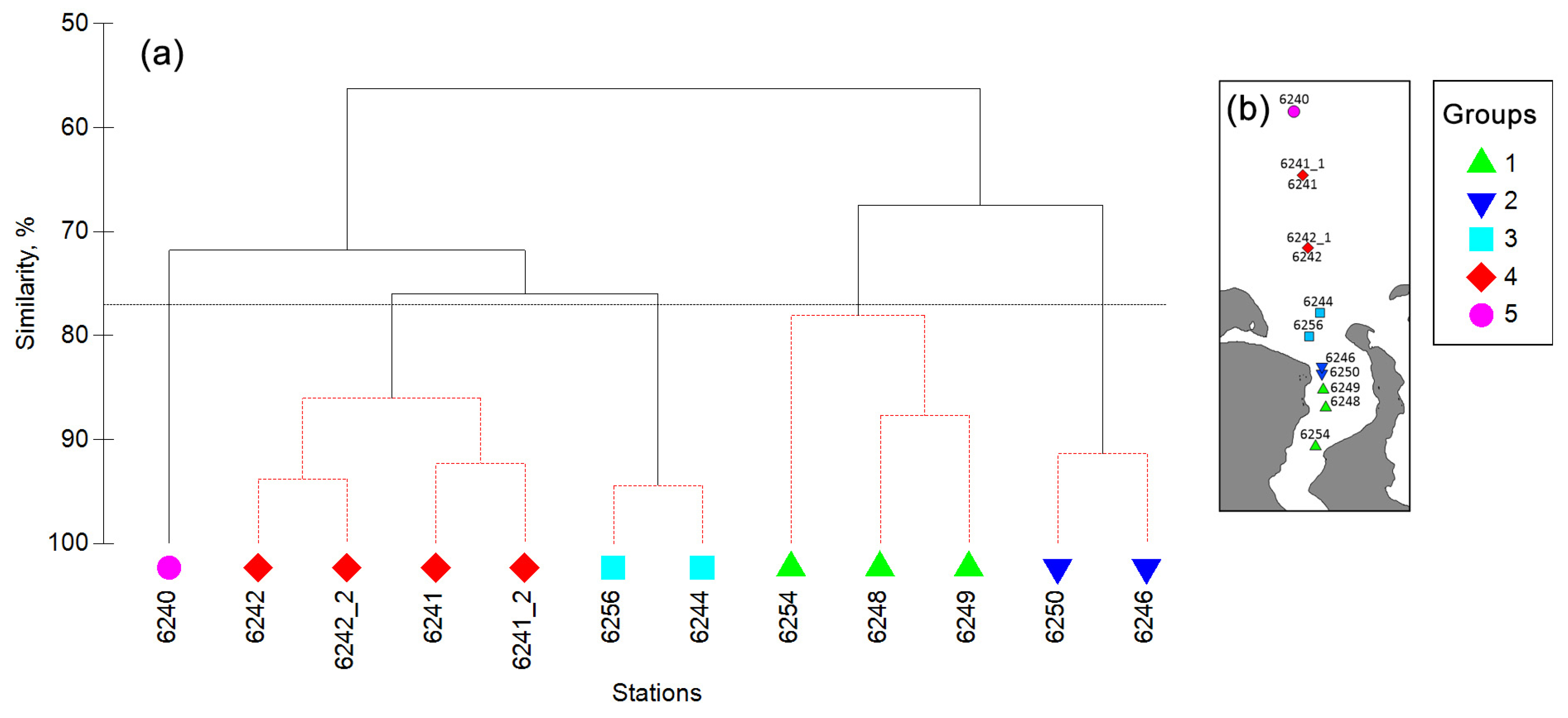
3.2. Zooplankton Biomass and Species Composition along the Transect
3.3. Vertical Distribution
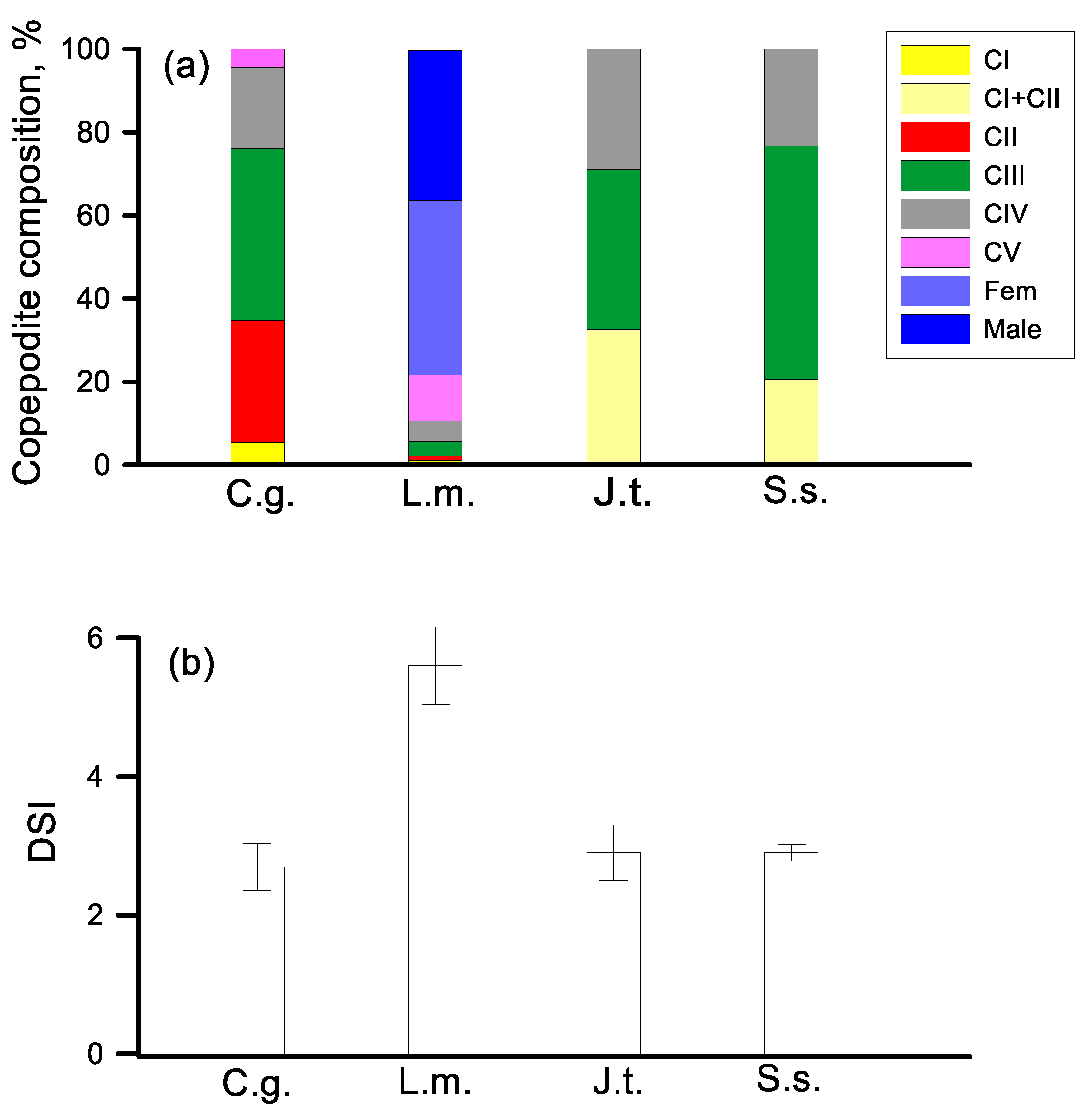
3.4. Demographic Stracture
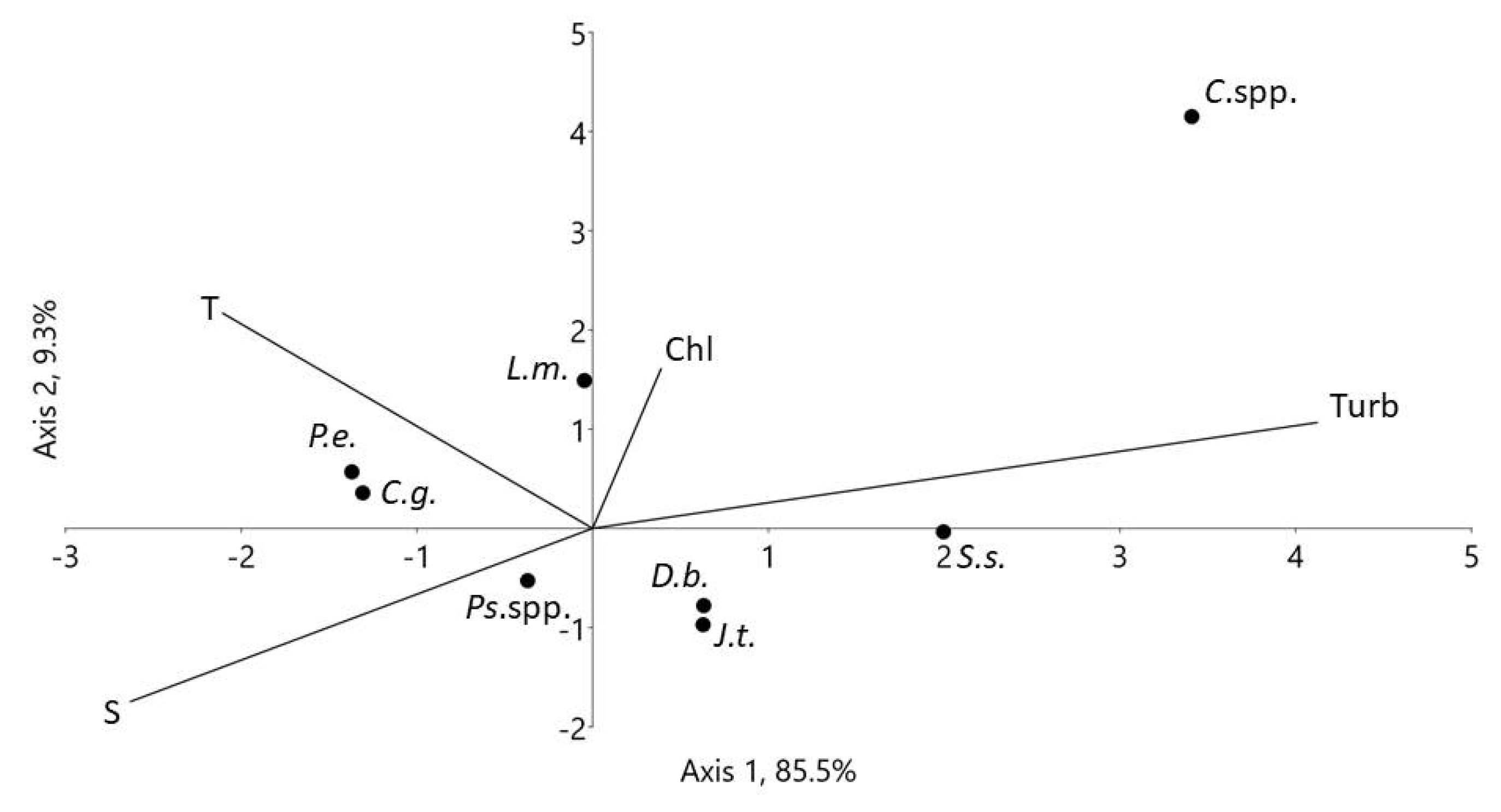
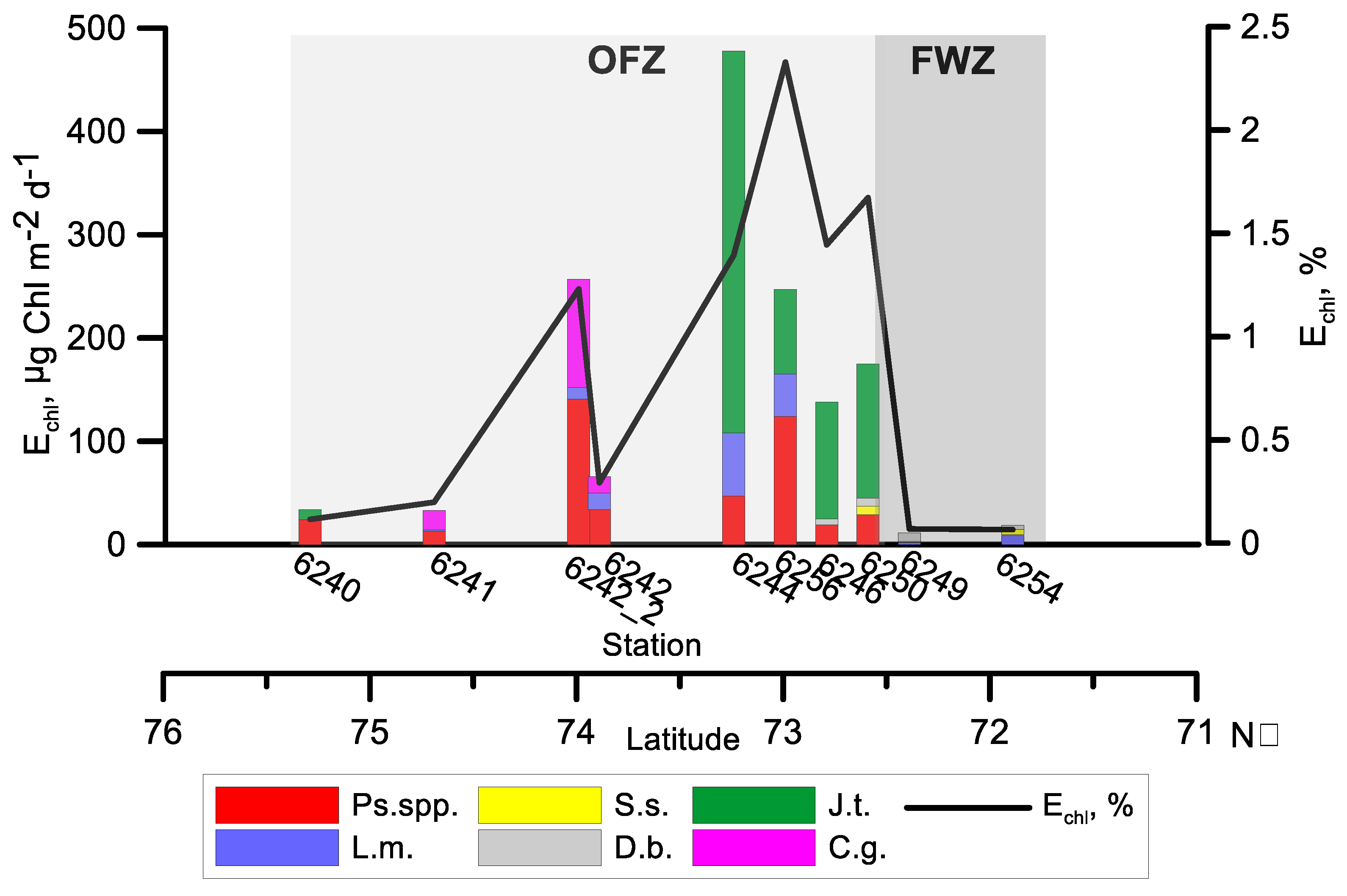
3.5. Feeding and Respiration
3.6. Grazing Impact
4. Discussion
4.1. Comparison of the Two Contrasting Years
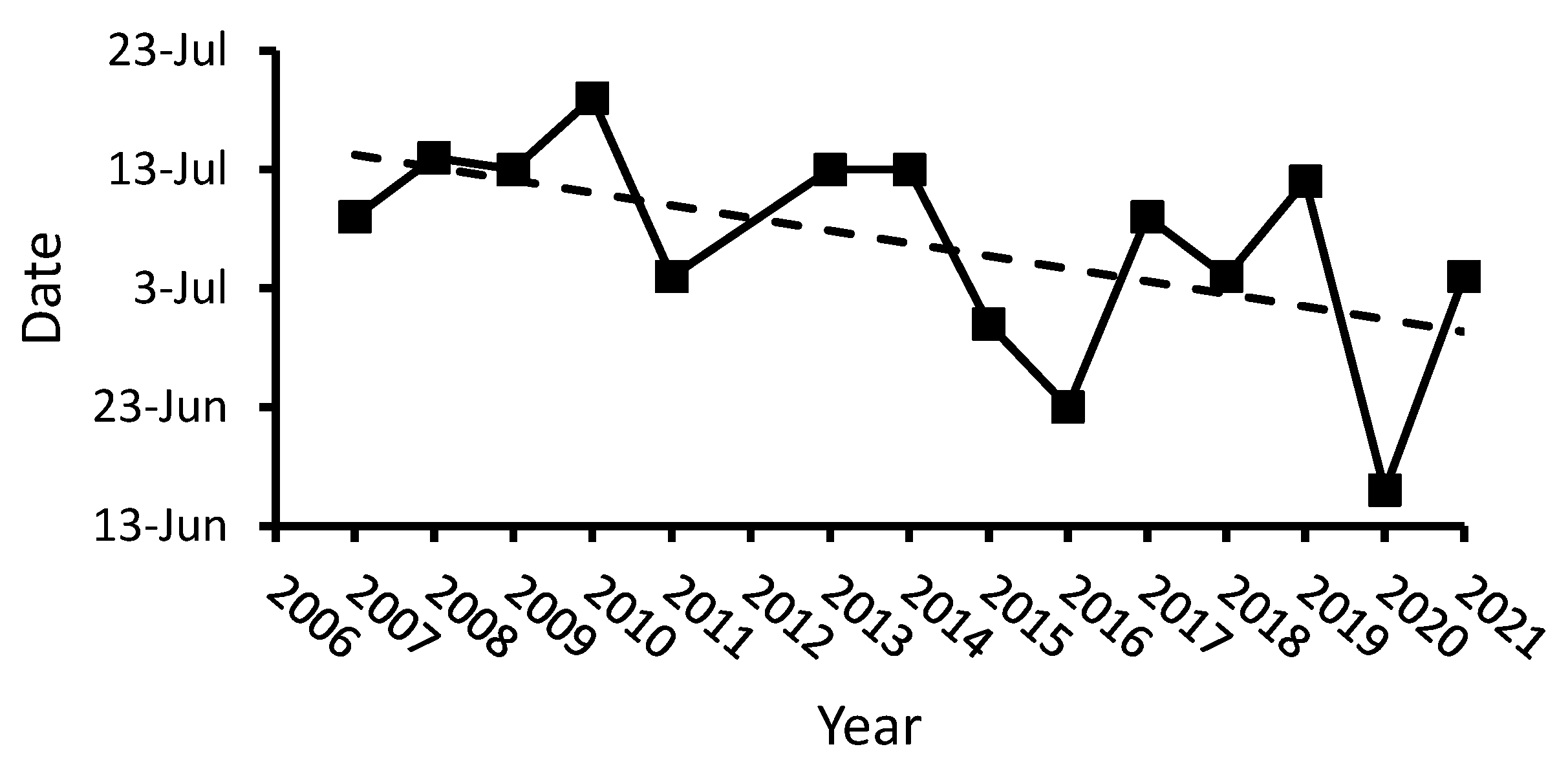
4.1.1. Ice Regime and Environmental Features
4.1.2. Zooplankton Biomass and Composition
4.1.3. Demographic Structure
4.1.4. Feeding
4.2. Influence of the Timing of Ice Retreat on Populations’ Development
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McClelland, J.W.; Holmes, R.M.; Dunton, K.H.; Macdonald, R.W. The Arctic Ocean Estuary. Estuaries Coasts 2012, 35, 353–368. [Google Scholar] [CrossRef]
- Terhaar, J.; Lauerwald, R.; Regnier, P.; Gruber, N.; Bopp, L. Around one third of current Arctic Ocean primary production sustained by rivers and coastal erosion. Nat. Commun. 2021, 12, 169. [Google Scholar] [CrossRef] [PubMed]
- Lisitsin, A.P. The marginal filter of the ocean. Oceanology 1995, 34, 671–682. [Google Scholar]
- Vinogradov, M.E.; Shushkina, E.A.; Lebedeva, L.P.; Gagarin, V.I. Mesoplankton of the east Kara Sea and the Ob and Yenisei River estuaries. Oceanology 1995, 34, 646–652. [Google Scholar]
- Flint, M.V.; Semenova, T.N.; Arashkevich, E.G.; Sukhanova, I.N.; Gagarin, V.I.; Kremenetskiy, V.V.; Pivovarov, M.A.; Soloviev, K.A. Structure of the zooplankton communities in the region of the Ob River’s estuarine frontal zone. Oceanology 2010, 50, 766–779. [Google Scholar] [CrossRef]
- Drits, A.V.; Pasternak, A.F.; Flint, M.V. Distribution and grazing of dominant zooplankton species in the Ob estuary: Influence of the runoff regime. Estuaries Coasts 2017, 40, 1082–1095. [Google Scholar] [CrossRef]
- Arashkevich, E.G.; Flint, M.V.; Nikishina, A.B.; Pasternak, A.F.; Timonin, A.G.; Vasilieva, J.V.; Mosharov, S.A.; Soloviev, K.A. The role of zooplankton in transformation of organic matter in the Ob estuary, on the shelf and in the deep regions of the Kara Sea. Oceanology 2010, 50, 780–792. [Google Scholar] [CrossRef]
- Drits, A.V.; Nikishina, A.B.; Sergeeva, V.M.; Solovyev, K.A.; Flint, M.V. Spatial distribution and feeding of dominant zooplankton species in the Ob River Estuary. Oceanology 2016, 56, 382–394. [Google Scholar] [CrossRef]
- ACIA. Arctic Climate Impact Assessment; Cambridge University Press: Cambridge, MA, USA, 2005. [Google Scholar]
- Stroeve, J.C.; Markus, T.; Boisvert, L.; Miller, J.; Barrett, A. Changes in Arctic melt season and implications for sea ice loss. Geophys. Res. Lett. 2014, 41, 1216–1225. [Google Scholar] [CrossRef]
- Drits, A.V.; Arashkevich, E.G.; Nedospasov, A.A.; Amelina, A.B.; Flint, M.V. Structural-functional characteristics of zooplankton in the Ob Estuary and adjacent Kara sea shelf in summer. Oceanology 2019, 59, 347–357. [Google Scholar] [CrossRef]
- Drits, A.V.; Pasternak, A.F.; Arashkevich, E.G.; Kravchishina, M.D.; Sukhanova, I.N.; Sergeeva, V.M.; Flint, M.V. Influence of riverine discharge and timing of ice retreat on particle sedimentation patterns on the Laptev Sea shelf. J. Geophys. Res. Oceans 2021, 126, e2021JC017462. [Google Scholar] [CrossRef]
- Kimura, F.; Matsuno, K.; Abe, Y.; Yamaguchi, A. Effects of early sea-ice reduction on zooplankton and copepod population structure in the northern Bering Sea during the summers of 2017 and 2018. Front. Mar. Sci. 2022, 9, 808910. [Google Scholar] [CrossRef]
- Guay, C.K.; Falkner, K.K.; Muench, R.D.; Mensch, M.; Frank, M.; Bayer, R. Wind-driven transport pathways for Eurasian Arctic river discharge. J. Geophys. Res. 2001, 106, 11469–11480. [Google Scholar] [CrossRef]
- Oki, T.; Kanae, S. Global hydrological cycles and world water resources. Science 2006, 313, 1068–1072. [Google Scholar] [CrossRef] [PubMed]
- Fetzer, I.; Hirche, H.-J.; Kolosova, E.G. The influence of freshwater discharge on the distribution of zooplankton in the southern Kara Sea. Polar Biol. 2002, 25, 404–415. [Google Scholar] [CrossRef]
- Drits, A.V.; Pasternak, A.F.; Nikishina, A.B.; Semenova, T.N.; Sergeeva, V.M.; Polukhin, A.A.; Flint, M.V. The dominant copepods Senecella siberica and Limnocalanus macrurus in the Ob Estuary: Ecology in a high-gradient environment. Polar Biol. 2016, 39, 1527–1538. [Google Scholar] [CrossRef]
- Flint, M.V.; Anisimov, I.M.; Arashkevich, E.G.; Artemiev, V.F.; Bezzubova, E.M.; Belevich, T.A.; Belyaev, N.A.; Bondarenko, S.A.; Bulokhov, A.V.; Vedenin, A.A.; et al. Ecosystems of the Kara and Laptev Seas; IP Erkhova I.M.: Moscow, Russia, 2021. (In Russian) [Google Scholar]
- Fetterer, F.; Knowles, K.; Meier, W.N.; Savoie, M.; Windnagel, A.K. Sea Ice Index, Version 3 [Data Set]; National Snow and Ice Data Center: Boulder, CO, USA, 2017. [Google Scholar] [CrossRef]
- Tande, K.S.; Hassel, A.; Slagstad, D. Gonad maturation and possible life cycle strategies in Calanus finmarchicus and Calanus glacialis in the northwestern part of the Barents Sea. In Marine Biology of Polar Regions and Effects of Stress on Marine Organisms; Gray, F.S., Christiansen, M.E., Eds.; Wiley: Chichester, UK, 1985; pp. 141–155. [Google Scholar]
- Chislenko, L.L. Nomograms for Determination of Weight of Aquatic Organisms by Size and Body Shape; Nauka: Leningrad, Russia, 1968. (In Russian) [Google Scholar]
- Vinogradov, M.E.; Shushkina, E.A. Functioning of the Plankton Communities in Ocean Pelagic; Nauka: Moscow, Russia, 1987. (In Russian) [Google Scholar]
- Matthews, J.B.L.; Hestad, L. Ecological studies on the deep-water pelagic community of Korsfjorden, western Norway. Length/weight relationships for some macroplanktonic organisms. Sarsia 1977, 63, 57–63. [Google Scholar] [CrossRef]
- Hopcroft, R.R.; Roff, J.C.; Bouman, H.A. Zooplankton growth rates: The larvaceans Appendicularia, Fritillaria and Oikopleura in tropical waters. J. Plank. Res. 1998, 20, 539–555. [Google Scholar] [CrossRef]
- Skjoldal, H.R.; Aarflot, J.M.; Bagøien, E.; Skagseth, Ø.; Rønning, J.; Lien, V.S. Seasonal and interannual variability in abundance and population development of Calanus finmarchicus at the western entrance to the Barents Sea, 1995–2019. Prog. Oceanogr. 2021, 195, 102574. [Google Scholar] [CrossRef]
- Mackas, D.L.; Bohrer, R.N. Fluorescence analysis of zooplankton gut contents and investigation of diel feeding patterns. J. Exp. Mar. Biol. Ecol. 1976, 25, 77–85. [Google Scholar] [CrossRef]
- Pasternak, A.F. Gut fluorescence in herbivorous copepods: An attempt to justify the method. Hydrobiologia 1994, 292/293, 241–248. [Google Scholar] [CrossRef]
- Baars, M.A.; Franz, H.G. Grazing pressure of copepods on the phytoplankton stock of the Central North Sea. Neth. J. Sea Res. 1984, 18, 120–142. [Google Scholar] [CrossRef]
- Tiselius, P. Effects of diurnal feeding rhythms, species composition and vertical migration on the grazing impact of calanoid copepods in the Skagerrak and Kattegat. Ophelia 1988, 28, 215–230. [Google Scholar] [CrossRef]
- Peterson, W.T.; Painting, S.; Barlow, R. Feeding rates of Calanoides carinatus: A comparison of five methods including evaluation of the gut fluorescence method. Mar. Ecol. Prog. Ser. 1990, 63, 85–92. [Google Scholar] [CrossRef]
- Saiz, E.; Calbet, A. Copepod feeding in the ocean: Scaling patterns, composition of their diet and the bias of estimates due to microzooplankton grazing during incubations. Hydrobiologia 2011, 666, 181–196. [Google Scholar] [CrossRef]
- Valdés, V.; Escribano, R.; Vergara, O. Scaling copepod grazing in a coastal upwelling system: The importance of community size structure for phytoplankton C flux. Lat. Am. J. Aquat. Res. 2017, 45, 41–54. [Google Scholar] [CrossRef]
- D’souza, A.; Gauns, M. Temporal variability in copepod gut pigments over the central western continental shelf of India. J. Mar. Biol. Assoc. UK 2018, 98, 149–159. [Google Scholar] [CrossRef]
- Holm-Hansen, O.; Lorenzen, C.J.; Holmes, R.W.; Strickland, J.D.H. Fluorometric determination of chlorophyll. J. Conseil 1965, 30, 3–15. [Google Scholar] [CrossRef]
- Båmstedt, U.; Gifford, D.J.; Irigoien, A.; Atkinson, A.; Roman, M. Feeding. In ICES Zooplankton Methodology Manual; Harris, R., Wiebe, P., Lenz, J., Skjoldal, H.R., Huntley, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2000; pp. 297–399. [Google Scholar]
- Pasternak, A.; Drits, A.; Arashkevich, E.; Flint, M. Differential impact of the Khatanga and Lena (Laptev Sea) runoff on the distribution and grazing of zooplankton. Front. Mar. Sci. 2022, 9, 881383. [Google Scholar] [CrossRef]
- Irigoien, X. Gut clearance rate constant, temperature, and initial gut contents: A review. J. Plankton Res. 1998, 20, 997–1003. [Google Scholar] [CrossRef]
- Menden-Deuer, S.; Lessard, E.J. Carbon to volume relationships for dinoflagellates, diatoms, and other protist plankton. Limnol. Oceanogr. 2000, 45, 569–579. [Google Scholar] [CrossRef]
- Sukhanova, I.N.; (P.P. Shirshov Institute of Oceanology, Moscow, Russia). Personal communication, 2023.
- Demidov, A.B.; Sukhanova, I.N.; Belevich, T.A.; Flint, M.V.; Gagarin, V.I.; Sergeeva, V.M.; Eremeeva, E.V.; Fedorov, A.V. Size-fractionated surface phytoplankton in the Kara and Laptev seas: Environmental control and spatial variability. Mar. Ecol. Prog. Ser. 2021, 664, 59–77. [Google Scholar] [CrossRef]
- Lampert, W. The measurement of respiration. A manual of the methods for assessment of secondary production in fresh water. In IBP Handbook; Downing, I.A., Rigler, F.H., Eds.; Blackwell: Oxford, UK, 1984; pp. 413–460. [Google Scholar]
- Clarke, K. Non-parametric multivariate analyses of changes in community structure. Aust. J. Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
- Demidov, A.B.; Gagarin, V.I.; Vorobieva, O.V.; Makkaveev, P.N.; Artemiev, V.A.; Khrapko, A.N.; Grigoriev, A.V.; Sheberstov, S.V. Spatial and vertical variability of primary production in the Kara Sea in July and August 2016: The influence of the river plume and subsurface chlorophyll maxima. Polar Biol. 2018, 41, 563–578. [Google Scholar] [CrossRef]
- Leu, E.; Søreide, J.E.; Hessen, D.O.; Falk-Petersen, S.; Berge, J. Consequences of changing sea-ice cover for primary and secondary producers in the European Arctic shelf seas: Timing, quantity, and quality. Prog. Oceanogr. 2011, 90, 18–32. [Google Scholar] [CrossRef]
- Hutchinson, G.E. A Treatise on Limnology, Volume 2, Introduction to Lake Biology and the Limnoplankton; John Wiley and Sons: New York, NY, USA, 1967. [Google Scholar]
- Carter, J.C.H. Life cycles of Limnocalanus macrurus and Senecella calanoides, and seasonal abundance and vertical distributions of various planktonic copepods, in Parry Sound, Georgian Bay. J. Fish. Res. Bd. Can. 1969, 262, 2543–2560. [Google Scholar] [CrossRef]
- Roff, J.C.; Carter, J.H.C. Life cycle and seasonal abundance of the copepod Limnocalanus macrurus Sars in a high arctic lake. Limnol. Oceanogr. 1972, 17, 363–370. [Google Scholar] [CrossRef]
- Dahlgren, K.; Olsen, B.R.; Troedsson, C.; Båmstedt, U. Seasonal variation in wax ester concentration and gut content in a Baltic Sea copepod [Limnocalanus macrurus (Sars 1863)]. J. Plank. Res. 2012, 34, 286–297. [Google Scholar] [CrossRef]
- Warren, G.J. Predaceous feeding habits of Limnocalanus macrurus. J Plankton Res. 1985, 7, 537–552. [Google Scholar] [CrossRef]
- Nero, R.W.; Sprules, W.G. Predation by the three glacial opportunists on natural zooplankton communities. Can. J. Zool. 1986, 64, 57–64. [Google Scholar] [CrossRef]
- Drits, A.V.; Belayev, N.A.; Karmanov, V.A.; Flint, M.V. Application of the high-temperature combustion method for measuring organic carbon content in fecal pellets and small-sized (≤1 mm) zooplankton. Oceanology 2023, 63, 141–148. [Google Scholar]
- Mauchline, J. The Biology of Calanoid Copepods; Academic Press: San Diego, CA, USA, 1998. [Google Scholar]
- Søreide, J.E.; Leu, E.; Berge, J.; Graeve, M.; Falk-Petersen, S. Timing of blooms, algal food quality and Calanus glacialis reproduction and growth in a changing Arctic. Glob. Chang. Biol. 2010, 16, 3154–3163. [Google Scholar] [CrossRef]
- Ringuette, M.; Fortier, L.; Fortier, M.A.; Runge, J.; Belanger, S.; Larouche, P.; Weslawski, J.-M.; Kwasniewski, S. Advanced recruitment and accelerated population development in Arctic calanoid copepods of the North Water. Deep-Sea Res. II 2002, 49, 5081–5099. [Google Scholar] [CrossRef]
- Matsuno, K.; Yamaguchi, A.; Hirawake, T.; Imai, I. Year-to-year changes of the mesozooplankton community in the Chukchi Sea during summers of 1991, 1992 and 2007, 2008. Polar Biol. 2011, 34, 1349–1360. [Google Scholar] [CrossRef]
- Roff, J.C. Aspects of the reproductive biology of the planktonic copepod Limnocalanus macrurus Sars, 1863. Crustaceana 1972, 22, 155–160. Available online: http://www.jstor.org/stable/20101872 (accessed on 25 January 2023). [CrossRef]
- Fomina, Y.Y.; Syarkia, M.T. Life cycle of the copepod Limnocalanus macrurus Sars 1863 (Copepoda, Calaniformes, Centropagidae) in Lake Onego. Biol. Bull. 2022, 49, 1261–1270. [Google Scholar] [CrossRef]
- Vanderploeg, H.A.; Cavaletto, J.F.; Liebig, J.R.; Gardner, W.S. Limnocalanus macrurus (Copepoda: Calanoida) retains a marine arctic lipid and life cycle strategy in Lake Michigan. J. Plankton Res. 1998, 20, 1581–1597. Available online: http://plankt.oxfordjournals.org/ (accessed on 25 January 2023). [CrossRef]
- Kosobokova, K.N.; Hirche, H.-J. A seasonal comparison of zooplankton communities in the Kara Sea—With special emphasis on overwintering traits. Estuar. Coast. Shelf Sci. 2016, 175, 146–156. [Google Scholar] [CrossRef]
- Kuklin, A.A. Feeding of Coregonus muksun in Yenisei. Proc. GosNIORKX 1980, 158, 87–90. (In Russian) [Google Scholar]
- Stepanova, V.B.; Stepanov, S.I. Importance of relict crustaceans in whitefish nutrition during under the ice period. Vestnik Ekologii Lesovedeniya i Landshaftovedeniya 2006, 6, 142–145. (In Russian) [Google Scholar]


| Station | Date | Time | IR (E m−2 s−1) | Depth (m) | Layer (m) | Chl a (mg m−3) |
|---|---|---|---|---|---|---|
| 6254 | 16.07 | 23:50 | 0.04 | 18 | 0–15 | 1.97 |
| 6248 | 16.07 | 08:20 | 0.36 | 13 | 0–10 | 1.74 |
| 6249 | 16.07 | 10:20 | 0.46 | 12 | 0–8 | - |
| 6250 | 16.07 | 12:20 | 0.51 | 17 | 0–5, 5–14 | 0.65 |
| 6246 | 16.07 | 00:30 | 0.04 | 23 | 0–5; 5–18 | 0.41 |
| 6256 | 17.07 | 13:30 | 0.69 | 29 | 0–5, 5–10, 10–25 | 0.41 |
| 6244 | 15.07 | 15:20 | 0.63 | 22 | 0–5, 5–18 | 1.80 |
| 6242 | 15.07 | 06:30 | 0.34 | 30 | 0–5; 5–10; 10–27 | 0.85 |
| 6242_2 | 17.07 | 21:00 | 0.06 | 30 | 0–5; 5–10; 10–26 | 0.76 |
| 6241 | 14.07 | 20:05 | 0.09 | 27 | 0–10, 10–24 | 1.67 |
| 6241_2 | 18.07 | 02:00 | 0.13 | 27 | 0–23 | - |
| 6240 | 14.07 | 14:10 | 0.63 | 37 | 0–10, 10–33 | 1.79 |
| Fresh Water Zone | Ob Frontal Zone | ||
|---|---|---|---|
| Species, Developmental Stages | Above Pycnocline | Below Pycnocline | |
| Limnocalanus macrurus nauplii | 0.02 ± 0.004 (3) | ||
| L. macrurus CI | 0.05 ± 0.02 (3) | ||
| L. macrurus CII | 0.31 ± 0.09 (9) | 0.14 ± 0.16 (6) | |
| L. macrurus CIII | 0.45 ± 0.14 (7) | 0.23 ± 0.15 (7) | |
| L. macrurus CIV | 0.69 ± 0.30 (9) | 0.40 ± 0.15 (10) | |
| L. macrurus CV | 1.23 ± 0.39 (6) | 1.02 ± 0.34 (8) | |
| L. macrurus Fem | 1.41 ± 0.40 (7) | 2.57 ± 2.01 (8) | |
| Jashnovia tolli CII | 1.04 ± 0.53 (20) | ||
| J. tolii CIII | 2.51 | 1.93 ± 1.76 (4) | |
| J. tolii CIV | 1.79 ± 0.92 (5) | 4.99 ± 3.25 (8) | 3.88 ± 3.61 (10) |
| Drepanopus bungii Fem | 0.08 ± 0.03 (3) | 0.03 ± 0.01 (8) | |
| Senecella siberica CII–CIII | 0.82 | ||
| Pseudocalanus major CIV–CV | 0.64 ± 0.36 (10) | ||
| P. acuspis Fem | 0.23 ± 0.20 (15) | ||
| P. spp. CI–CIV | 0.10 ± 0.09 (6) | 0.04 ± 0.02 (14) | |
| Calanus glacialis CII | 0.60 ± 0.10 (3) | 0.60 ± 0.10 (3) | |
| C. glacialis CIII | 1.96 ± 1.34 (3) | 1.76 ± 1.37 (8) | |
| C. glacialis CIV | 6.12 ± 2.27 (3) | 3.69 ± 1.97 (5) | |
| C. glacialis CV | 20.79 | ||
| Species, Developmental Stage | Fresh Water Zone | Ob Frontal Zone | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Wc | Ic | Ic/Wc | R | R/Wc | Ic | Ic/Wc | R | R/Wc | |
| Limnocalanus macrurus CIII | 6.05 ± 0.71 (2) | 0.18 | 3.0 | 0.34 ± 0.02 (3) | 5.7 | 0.11 | 1.9 | 0.50 ± 0.06 (3) | 8.3 |
| L. macrurus CIV | 12.69 ± 1.25 (3) | 0.28 | 2.2 | 0.50 ± 0.06 (4) | 3.9 | 0.19 | 1.5 | 0.83 ± 0.05 (4) | 6.5 |
| L. macrurus CV | 26.92 ± 1.81 (3) | 0.49 | 1.8 | 0.88 ± 0.35 (4) | 3.3 | 0.49 | 1.8 | 1.31 ± 0.20 (4) | 4.9 |
| L. macrurus Fem | 43.11 ± 9.14 (3) | 0.57 | 1.3 | 1.37 ± 0.04 (4) | 3.3 | 1.23 | 2.9 | 2.02 ± 0.02 (4) | 4.7 |
| Jaschnovia tolii CIV | 28.80 ± 1.44 (3) | 1.01 | 3.5 | 0.63 ± 0.05 (4) | 2.2 | 2.53 | 8.8 | 0.96 ± 0.06 (4) | 3.3 |
| Calanus glacialis CII | 9.64 ± 1.34 (3) | 0.44 | 4.6 | 0.3 ± 0.02 (3) | 3.1 | ||||
| C. glacialis CIII | 38.18 ± 7.07 (3) | 1.31 | 3.4 | 0.84 ± 0.06 (3) | 2.2 | ||||
| C. glacialis CIV | 101.39 ± 22.98 (3) | 3.42 | 3.4 | 1.75 ± 0.04 (4) | 1.7 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drits, A.; Pasternak, A.; Arashkevich, E.; Amelina, A.; Flint, M. Timing of Ice Retreat Determines Summer State of Zooplankton Community in the Ob Estuary (the Kara Sea, Siberian Arctic). Diversity 2023, 15, 674. https://doi.org/10.3390/d15050674
Drits A, Pasternak A, Arashkevich E, Amelina A, Flint M. Timing of Ice Retreat Determines Summer State of Zooplankton Community in the Ob Estuary (the Kara Sea, Siberian Arctic). Diversity. 2023; 15(5):674. https://doi.org/10.3390/d15050674
Chicago/Turabian StyleDrits, Alexander, Anna Pasternak, Elena Arashkevich, Anastasia Amelina, and Mikhail Flint. 2023. "Timing of Ice Retreat Determines Summer State of Zooplankton Community in the Ob Estuary (the Kara Sea, Siberian Arctic)" Diversity 15, no. 5: 674. https://doi.org/10.3390/d15050674
APA StyleDrits, A., Pasternak, A., Arashkevich, E., Amelina, A., & Flint, M. (2023). Timing of Ice Retreat Determines Summer State of Zooplankton Community in the Ob Estuary (the Kara Sea, Siberian Arctic). Diversity, 15(5), 674. https://doi.org/10.3390/d15050674





