Vegetation Structure and Invertebrate Food Availability for Birds in Intensively Used Arable Fields: Evaluation of Three Widespread Crops
Abstract
1. Introduction
2. Materials and Methods
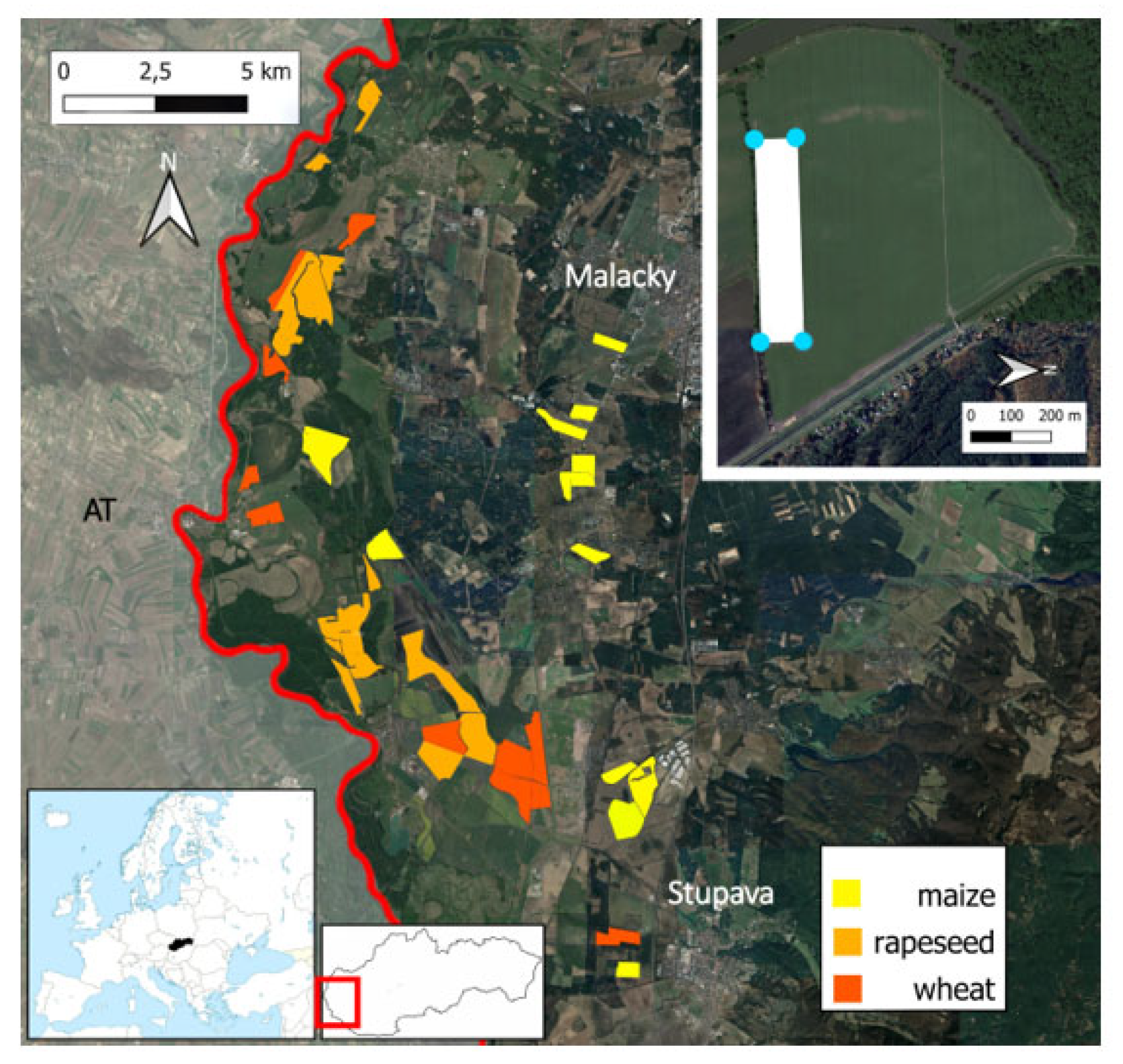
2.1. Study Area and Study Sites
2.2. Crop Type and Application of Insecticides
2.3. Invertebrate Food Availability
2.4. Vegetation Structure
2.5. Statistical Analysis
2.5.1. Vegetation Structure
2.5.2. Invertebrate Food Availability
3. Results
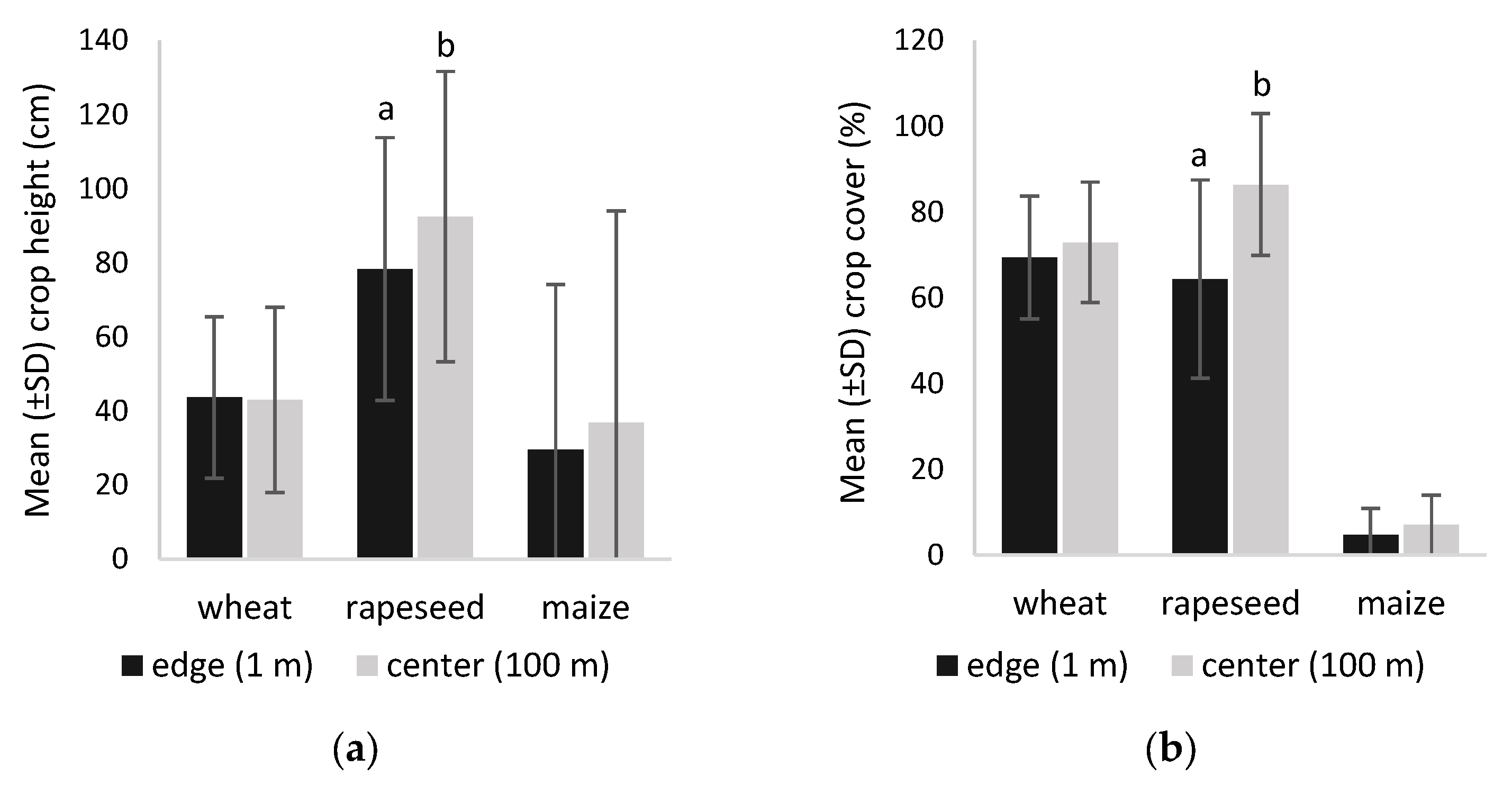
3.1. Vegetation Structure
3.2. Insecticide Applications
3.3. Invertebrate Food Availability
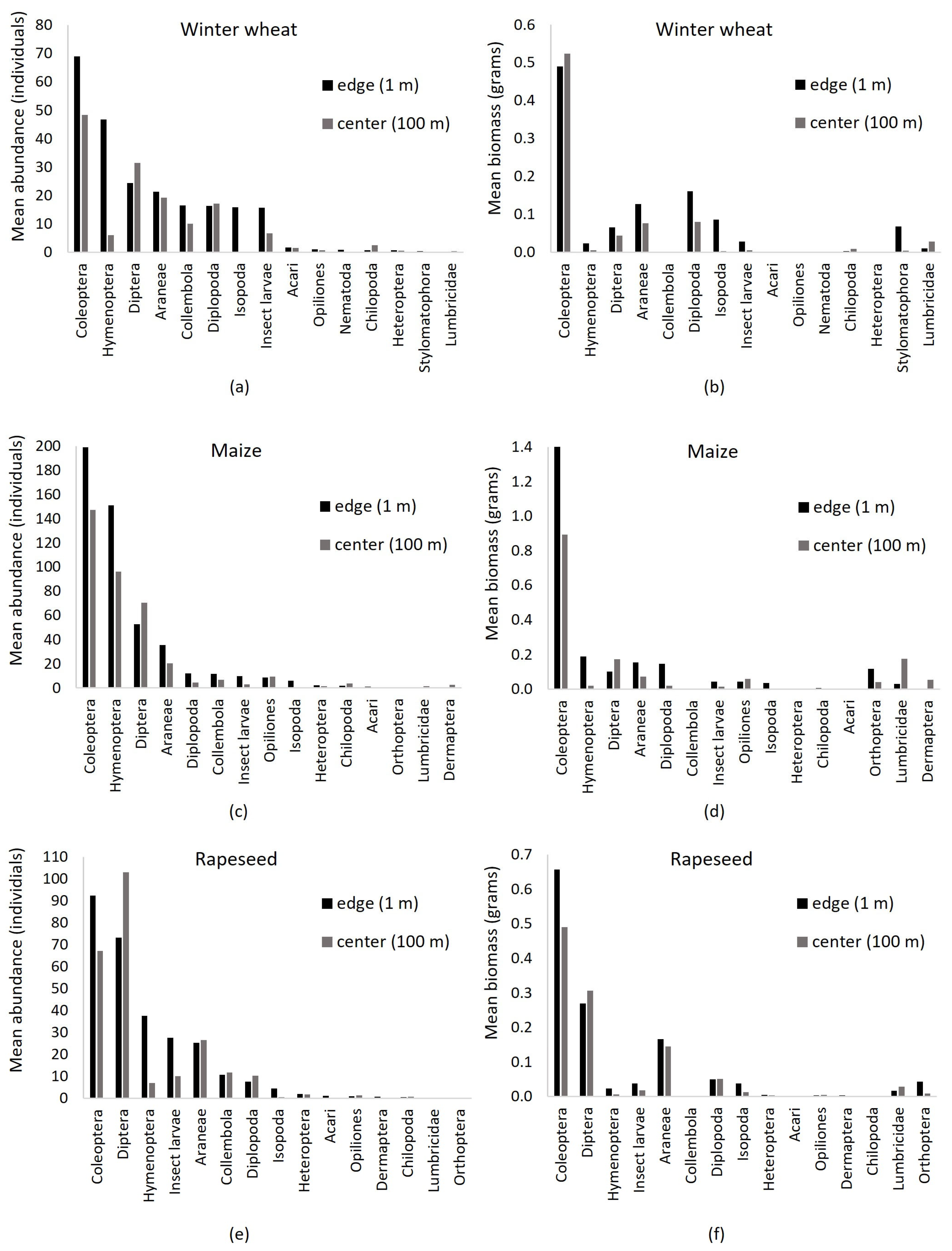
3.3.1. Taxonomic Composition
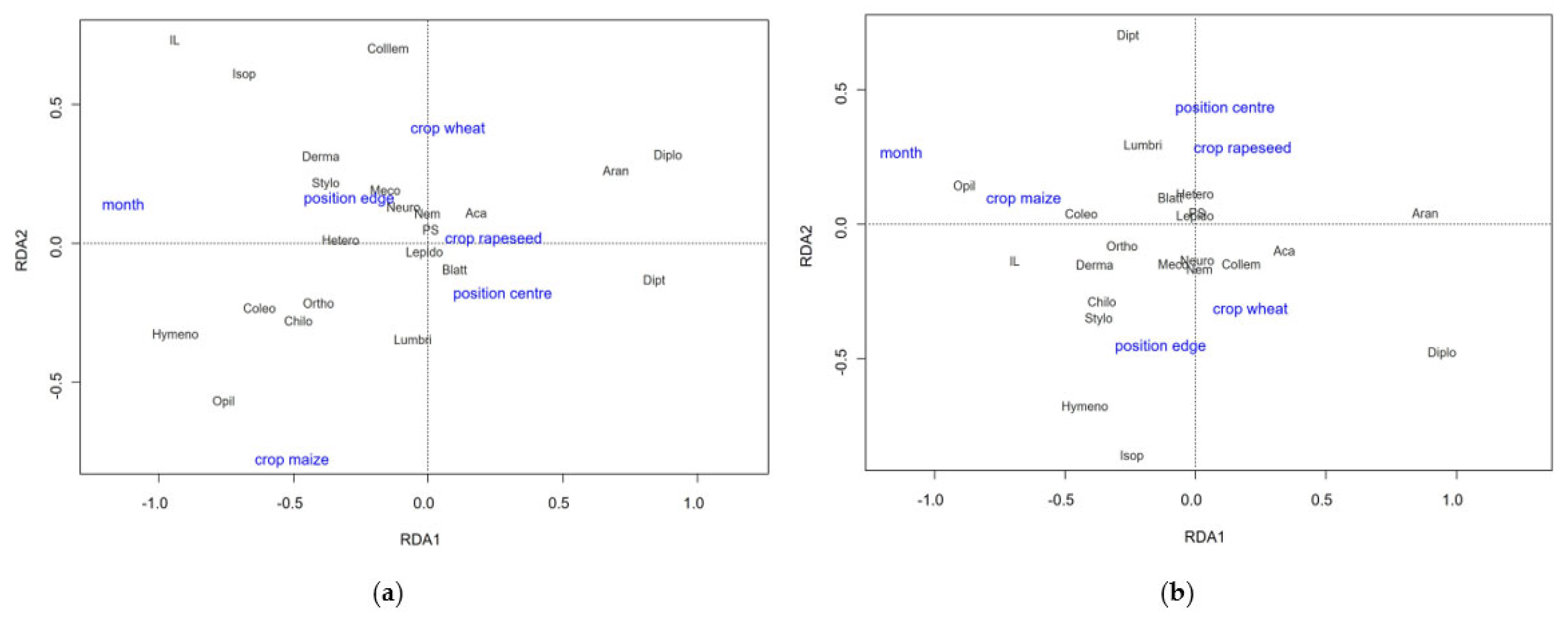
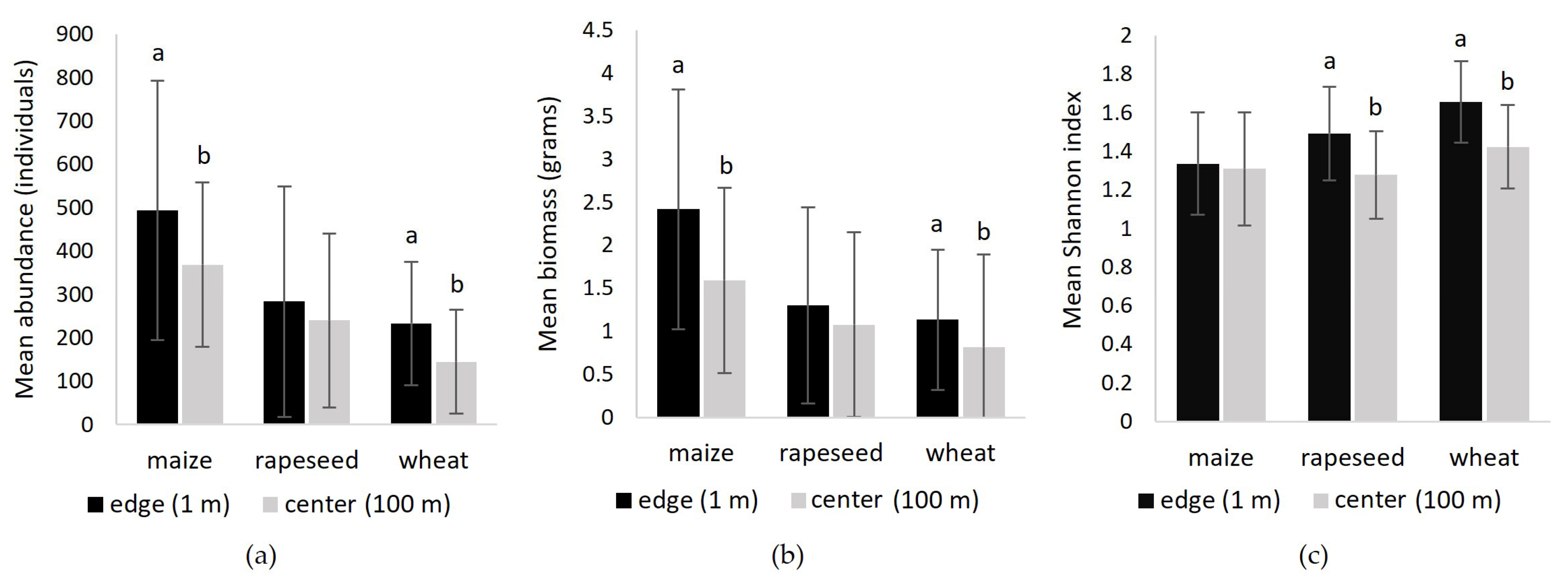
3.3.2. Abundance, Biomass and Diversity
4. Discussion
4.1. Patterns in Invertebrate Food Availablity and Vegetation Structure
4.2. Implications for the Possibilities of Farmland Bird Occurrence
5. Conclusions and Conservation Implications
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tilman, D.; Clark, M.; Williams, D.R.; Kimmel, K.; Polasky, S.; Packer, C. Future Threats to Biodiversity and Pathways to Their Prevention. Nature 2017, 546, 73–81. [Google Scholar] [CrossRef]
- Şekercioglu, Ç.H.; Mendenhall, C.D.; Oviedo-Brenes, F.; Horns, J.J.; Ehrlich, P.R.; Daily, G.C. Long-Term Declines in Bird Populations in Tropical Agricultural Countryside. Proc. Natl. Acad. Sci. USA 2019, 116, 9903–9912. [Google Scholar] [CrossRef]
- Storkey, J.; Meyer, S.; Still, K.S.; Leuschner, C. The Impact of Agricultural Intensification and Land-Use Change on the European Arable Flora. Proc. R. Soc. B Biol. Sci. 2012, 279, 1421–1429. [Google Scholar] [CrossRef]
- Outhwaite, C.L.; McCann, P.; Newbold, T. Agriculture and Climate Change Are Reshaping Insect Biodiversity Worldwide. Nature 2022, 605, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Reif, J.; Hanzelka, J. Continent-Wide Gradients in Open-Habitat Insectivorous Bird Declines Track Spatial Patterns in Agricultural Intensity across Europe. Glob. Ecol. Biogeogr. 2020, 29, 1988–2013. [Google Scholar] [CrossRef]
- Burns, F.; Eaton, M.A.; Burfield, I.J.; Klvaňová, A.; Šilarová, E.; Staneva, A.; Gregory, R.D. Abundance Decline in the Avifauna of the European Union Reveals Cross-continental Similarities in Biodiversity Change. Ecol. Evol. 2021, 11, 16647–16660. [Google Scholar] [CrossRef] [PubMed]
- Fraixedas, S.; Lindén, A.; Piha, M.; Cabeza, M.; Gregory, R.; Lehikoinen, A. A State-of-the-Art Review on Birds as Indicators of Biodiversity: Advances, Challenges, and Future Directions. Ecol. Indic. 2020, 118, 106728. [Google Scholar] [CrossRef]
- Bas, Y.; Renard, M.; Jiguet, F. Nesting Strategy Predicts Farmland Bird Response to Agricultural Intensity. Agric. Ecosyst. Environ. 2009, 134, 143–147. [Google Scholar] [CrossRef]
- Heldbjerg, H.; Sunde, P.; Fox, A.D. Continuous Population Declines for Specialist Farmland Birds 1987–2014 in Denmark Indicates No Halt in Biodiversity Loss in Agricultural Habitats. Bird Conserv. Int. 2018, 28, 278–292. [Google Scholar] [CrossRef]
- Bowler, D.E.; Heldbjerg, H.; Fox, A.D.; Jong, M.; Böhning-Gaese, K. Long-term Declines of European Insectivorous Bird Populations and Potential Causes. Conserv. Biol. 2019, 33, 1120–1130. [Google Scholar] [CrossRef]
- Eurostat. Agriculture, Forestry and Fishery Statistics: 2020 Edition; Eurostat: Luxembourg, 2020. [Google Scholar]
- OneSoil|The Free Platform for Reliable Agricultural Decisions. OneSoil. Available online: https://onesoil.ai/en/ (accessed on 15 November 2022).
- Eurostat Data Browser. Available online: https://ec.europa.eu/eurostat/databrowser/explore/all/agric?lang=en&subtheme=agr.apro.apro_crop.apro_cp.apro_cpsh&display=list&sort=category&extractionId=TAG00093 (accessed on 5 October 2022).
- Seni, A. Decreasing Insect Biodiversity in Agriculture—Reorient the Strategy to Reverse the Trend for Better Future. Munis Entomol. Zool. 2022, 17, 360–372. [Google Scholar]
- Landis, D.A.; Wratten, S.D.; Gurr, G.M. Habitat Management to Conserve Natural Enemies of Arthropod Pests in Agriculture. Annu. Rev. Entomol. 2000, 45, 175–201. [Google Scholar] [CrossRef]
- Asteraki, E.J.; Hart, B.J.; Ings, T.C.; Manley, W.J. Factors Influencing the Plant and Invertebrate Diversity of Arable Field Margins. Agric. Ecosyst. Environ. 2004, 102, 219–231. [Google Scholar] [CrossRef]
- Ndakidemi, B.; Mtei, K.; Ndakidemi, P.A. Impacts of Synthetic and Botanical Pesticides on Beneficial Insects. Agric. Sci. 2016, 7, 364–372. [Google Scholar] [CrossRef]
- Highland, S.A.; Miller, J.C.; Jones, J.A. Determinants of Moth Diversity and Community in a Temperate Mountain Landscape: Vegetation, Topography, and Seasonality. Ecosphere 2013, 4, 1–22. [Google Scholar] [CrossRef]
- Gardner, S.M.; Cabido, M.R.; Valladares, G.R.; Diaz, S. The Influence of Habitat Structure on Arthropod Diversity in Argentine Semi-Arid Chaco Forest. J. Veg. Sci. 1995, 6, 349–356. [Google Scholar] [CrossRef]
- Hoffmann, J.; Wittchen, U.; Berger, G.; Stachow, U. Moving Window Growth—A Method to Characterize the Dynamic Growth of Crops in the Context of Bird Abundance Dynamics with the Example of Skylark (Alauda arvensis). Ecol. Evol. 2018, 8, 8880–8893. [Google Scholar] [CrossRef] [PubMed]
- Hiron, M.; Berg, Å.; Pärt, T. Do Skylarks Prefer Autumn Sown Cereals? Effects of Agricultural Land Use, Region and Time in the Breeding Season on Density. Agric. Ecosyst. Environ. 2012, 150, 82–90. [Google Scholar] [CrossRef]
- Wilson, J.D.; Whittingham, M.J.; Bradbury, R.B. The Management of Crop Structure: A General Approach to Reversing the Impacts of Agricultural Intensification on Birds? Ibis 2005, 147, 453–463. [Google Scholar] [CrossRef]
- Devereux, C.L.; McKeever, C.U.; Benton, T.G.; Whittingham, M.J. The Effect of Sward Height and Drainage on Common Starlings Sturnus Vulgaris and Northern Lapwings Vanellus Vanellus Foraging in Grassland Habitats. Ibis 2004, 146, 115–122. [Google Scholar] [CrossRef]
- Dunn, J.C.; Hamer, K.C.; Benton, T.G. Nest and Foraging-site Selection in Yellowhammers Emberiza citrinella: Implications for Chick Provisioning. Bird Study 2010, 57, 531–539. [Google Scholar] [CrossRef]
- Hoste-Danyłow, A.; Romanowski, J.; Żmihorski, M. Effects of Management on Invertebrates and Birds in Extensively Used Grassland of Poland. Agric. Ecosyst. Environ. 2010, 139, 129–133. [Google Scholar] [CrossRef]
- Atkinson, P.W.; Fuller, R.J.; Vickery, J.A.; Conway, G.J.; Tallowin, J.R.B.; Smith, R.E.N.; Haysom, K.A.; Ings, T.C.; Asteraki, E.J.; Brown, V.K. Influence of Agricultural Management, Sward Structure and Food Resources on Grassland Field Use by Birds in Lowland England. J. Appl. Ecol. 2005, 42, 932–942. [Google Scholar] [CrossRef]
- Romanowski, J.; Zmihorski, M. Selection of Foraging Habitat by Grassland Birds: Effect of Prey Abundance or Availability? Pol. J. Ecol. 2008, 56, 365–370. [Google Scholar]
- Sutcliffe, L.M.E.; Batáry, P.; Kormann, U.; Báldi, A.; Dicks, L.V.; Herzon, I.; Kleijn, D.; Tryjanowski, P.; Apostolova, I.; Arlettaz, R.; et al. Harnessing the Biodiversity Value of Central and Eastern European Farmland. Divers. Distrib. 2015, 21, 722–730. [Google Scholar] [CrossRef]
- Brickle, N.W.; Harper, D.G.C.; Aebischer, N.J.; Cockayne, S.H. Effects of Agricultural Intensification on the Breeding Success of Corn Buntings Miliaria calandra. J. Appl. Ecol. 2000, 37, 742–755. [Google Scholar] [CrossRef]
- Gilroy, J.J.; Anderson, G.Q.A.; Grice, P.V.; Vickery, J.A.; Watts, P.N.; Sutherland, W.J. Foraging Habitat Selection, Diet and Nestling Condition in Yellow Wagtails Motacilla flava Breeding on Arable Farmland. Bird Study 2009, 56, 221–232. [Google Scholar] [CrossRef]
- Kuiper, M.W.; Ottens, H.J.; Cenin, L.; Schaffers, A.P.; van Ruijven, J.; Koks, B.J.; Berendse, F.; de Snoo, G.R. Field Margins as Foraging Habitat for Skylarks (Alauda arvensis) in the Breeding Season. Agric. Ecosyst. Environ. 2013, 170, 10–15. [Google Scholar] [CrossRef]
- Clough, Y.; Kirchweger, S.; Kantelhardt, J. Field Sizes and the Future of Farmland Biodiversity in European Landscapes. Conserv. Lett. 2020, 13, e12752. [Google Scholar] [CrossRef]
- Štatistický Úrad Slovenskej Republiky. Súpis Plôch Osiatych Poľnohospodárskymi Plodinami k 20.5.2020; Štatistický Úrad Slovenskej Republiky: Bratislava, Slovakia, 2020; ISBN 9788081217791.
- Brooks, M.E.; Kristensen, K.; van Benthem, K.J.; Magnusson, A.; Berg, C.W.; Nielsen, A.; Skaug, H.J.; Maechler, M.; Bolker, B.M. {glmmTMB} Balances Speed and Flexibility Among Packages for Zero-Inflated Generalized Linear Mixed Modeling. R J. 2017, 9, 378–400. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous Inference in General Parametric Models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. 2020. Available online: https://cran.r-project.org/web/packages/vegan/index.html (accessed on 9 September 2022).
- Moreau, J.; Rabdeau, J.; Badenhausser, I.; Giraudeau, M.; Sepp, T.; Crépin, M.; Gaffard, A.; Bretagnolle, V.; Monceau, K. Pesticide Impacts on Avian Species with Special Reference to Farmland Birds: A Review. Environ. Monit. Assess. 2022, 194, 790. [Google Scholar] [CrossRef]
- Chapman, J.W.; Bell, J.R.; Burgin, L.E.; Reynolds, D.R.; Pettersson, L.B.; Hill, J.K.; Bonsall, M.B.; Thomas, J.A. Seasonal Migration to High Latitudes Results in Major Reproductive Benefits in an Insect. Proc. Natl. Acad. Sci. USA 2012, 109, 14924–14929. [Google Scholar] [CrossRef]
- Knapp, M.; González, E.; Štrobl, M.; Seidl, M.; Jakubíková, L.; Čížek, O.; Balvín, O.; Benda, D.; Teder, T.; Kadlec, T. Artificial Field Defects: A Low-Cost Measure to Support Arthropod Diversity in Arable Fields. Agric. Ecosyst. Environ. 2022, 325, 107748. [Google Scholar] [CrossRef]
- Jeanneret, P.; Schüpbach, B.; Pfiffner, L.; Walter, T. Arthropod Reaction to Landscape and Habitat Features in Agricultural Landscapes. Landsc. Ecol. 2003, 18, 253–263. [Google Scholar] [CrossRef]
- Štrobl, M.; Saska, P.; Seidl, M.; Kocian, M.; Tajovský, K.; Řezáč, M.; Skuhrovec, J.; Marhoul, P.; Zbuzek, B.; Jakubec, P.; et al. Impact of an Invasive Tree on Arthropod Assemblages in Woodlots Isolated within an Intensive Agricultural Landscape. Divers. Distrib. 2019, 25, 1800–1813. [Google Scholar] [CrossRef]
- McCary, M.A.; Jackson, R.D.; Gratton, C. Vegetation Structure Modulates Ecosystem and Community Responses to Spatial Subsidies. Ecosphere 2021, 12, e03483. [Google Scholar] [CrossRef]
- Evans, D.M.; Villar, N.; Littlewood, N.A.; Pakeman, R.J.; Evans, S.A.; Dennis, P.; Skartveit, J.; Redpath, S.M. The Cascading Impacts of Livestock Grazing in Upland Ecosystems: A 10-Year Experiment. Ecosphere 2015, 6, 1–15. [Google Scholar] [CrossRef]
- Seidl, M.; González, E.; Kadlec, T.; Saska, P.; Knapp, M. Temporary Non-Crop Habitats within Arable Fields: The Effects of Field Defects on Carabid Beetle Assemblages. Agric. Ecosyst. Environ. 2020, 293, 106856. [Google Scholar] [CrossRef]
- Southwood, T.R.E.; Brown, V.K.; Reader, P.M. The Relationships of Plant and Insect Diversities in Succession. Biol. J. Linn. Soc. 1979, 12, 327–348. [Google Scholar] [CrossRef]
- Woodcock, B.A.; Pywell, R.F. Effects of Vegetation Structure and Floristic Diversity on Detritivore, Herbivore and Predatory Invertebrates within Calcareous Grasslands. Biodivers. Conserv. 2010, 19, 81–95. [Google Scholar] [CrossRef]
- Knapp, M.; Štrobl, M.; Venturo, A.; Seidl, M.; Jakubíková, L.; Tajovský, K.; Kadlec, T.; González, E. Importance of Grassy and Forest Non-Crop Habitat Islands for Overwintering of Ground-Dwelling Arthropods in Agricultural Landscapes: A Multi-Taxa Approach. Biol. Conserv. 2022, 275, 109757. [Google Scholar] [CrossRef]
- Crop Protection Association Pesticides—Best Practice Guides. Available online: http://adlib.everysite.co.uk/adlib/defra/content.aspx?id=000IL3890W.16NTBWTSU1UU0 (accessed on 9 September 2022).
- Vickery, J.A.; Feber, R.E.; Fuller, R.J. Arable Field Margins Managed for Biodiversity Conservation: A Review of Food Resource Provision for Farmland Birds. Agric. Ecosyst. Environ. 2009, 133, 1–13. [Google Scholar] [CrossRef]
- Batáry, P.; Holzschuh, A.; Orci, K.M.; Samu, F.; Tscharntke, T. Responses of Plant, Insect and Spider Biodiversity to Local and Landscape Scale Management Intensity in Cereal Crops and Grasslands. Agric. Ecosyst. Environ. 2012, 146, 130–136. [Google Scholar] [CrossRef]
- Holland, J.M.; Smith, B.M.; Birkett, T.C.; Southway, S. Farmland Bird Invertebrate Food Provision in Arable Crops. Ann. Appl. Biol. 2012, 160, 66–75. [Google Scholar] [CrossRef]
- Zamorano, J.; Bartomeus, I.; Grez, A.A.; Garibaldi, L.A. Field Margin Floral Enhancements Increase Pollinator Diversity at the Field Edge but Show No Consistent Spillover into the Crop Field: A Meta-Analysis. Insect Conserv. Divers. 2020, 13, 519–531. [Google Scholar] [CrossRef]
- Tajovský, K.; Hošek, J.; Hofmeister, J.; Wytwer, J. Assemblages of Terrestrial Isopods (Isopoda, Oniscidea) in a Fragmented Forest Landscape in Central Europe. Zookeys 2012, 176, 189–198. [Google Scholar] [CrossRef][Green Version]
- Szlavecz, K.; Vilisics, F.; Tóth, Z.; Hornung, E. Terrestrial Isopods in Urban Environments: An Overview. Zookeys 2018, 801, 97–126. [Google Scholar] [CrossRef] [PubMed]
- Holland, J.M.; Hutchison, M.A.S.; Smith, B.; Aebischer, N.J. A Review of Invertebrates and Seed-Bearing Plants as Food for Farmland Birds in Europe. Ann. Appl. Biol. 2006, 148, 49–71. [Google Scholar] [CrossRef]
- Wilson, J.D.; Morris, A.J.; Arroyo, B.E.; Clark, S.C.; Bradbury, R.B. A Review of the Abundance and Diversity of Invertebrate and Plant Foods of Granivorous Birds in Northern Europe in Relation to Agricultural Change. Agric. Ecosyst. Environ. 1999, 75, 13–30. [Google Scholar] [CrossRef]
- Newton, I. Food-Supply. In Population Limitation in Birds; Academic Press: Cambridge, MA, USA, 1998; pp. 145–189. ISBN 9780125173667. [Google Scholar]
- Fretwell, S.D.; Calver, J.S. On Territorial Behavior and Other Factors Influencing Habitat Distribution in Birds. Acta Biotheor. 1969, 19, 37–44. [Google Scholar] [CrossRef]
- Bradbury, R.B.; Bradter, U. Habitat Associations of Yellow Wagtails Motacilla flava flavissima on Lowland Wet Grassland. Ibis 2003, 146, 241–246. [Google Scholar] [CrossRef]
- Perkins, A.J.; Maggs, H.E.; Wilson, J.D. Crop Sward Structure Explains Seasonal Variation in Nest Site Selection and Informs Agri-Environment Scheme Design for a Species of High Conservation Concern: The Corn Bunting Emberiza Calandra. Bird Study 2015, 62, 474–485. [Google Scholar] [CrossRef][Green Version]
- Galbraith, H. Effects of Agriculture on the Breeding Ecology of Lapwings Vanellus vanellus. J. Appl. Ecol. 1988, 25, 487–503. [Google Scholar] [CrossRef]
- Green, R.E.; Tyler, G.A.; Bowden, C.G.R. Habitat Selection, Ranging Behaviour and Diet of the Stone Curlew (Burhinus oedicnemus) in Southern England. J. Zool. 2000, 250, 161–183. [Google Scholar] [CrossRef]
- Wakeham-Dawson, A.; Szoszkiewicz, K.; Stern, K.; Aebischer, N.J. Breeding Skylarks Alauda Arvensis on Environmentally Sensitive Area Arable Reversion Grass in Southern England: Survey-Based and Experimental Determination of Density. J. Appl. Ecol. 1998, 35, 635–648. [Google Scholar] [CrossRef]
- Wilson, J.D.; Evans, J.; Browne, S.J.; King, J.R. Territory Distribution and Breeding Success of Skylarks Alauda Arvensis on Organic and Intensive Farmland in Southern England. J. Appl. Ecol. 1997, 34, 1462. [Google Scholar] [CrossRef]
- Püttmanns, M.; Böttges, L.; Filla, T.; Lehmann, F.; Martens, A.S.; Siegel, F.; Sippel, A.; von Bassi, M.; Balkenhol, N.; Waltert, M.; et al. Habitat Use and Foraging Parameters of Breeding Skylarks Indicate No Seasonal Decrease in Food Availability in Heterogeneous Farmland. Ecol. Evol. 2022, 12, e8461. [Google Scholar] [CrossRef]
- Kragten, S.; Trimbos, K.B.; de Snoo, G.R. Breeding Skylarks (Alauda arvensis) on Organic and Conventional Arable Farms in the Netherlands. Agric. Ecosyst. Environ. 2008, 126, 163–167. [Google Scholar] [CrossRef]
- Toepfer, S.; Stubbe, M. Territory Density of the Skylark (Alauda arvensis) in Relation to Field Vegetation in Central Germany. J. Ornithol. 2001, 142, 184–194. [Google Scholar] [CrossRef]
- Odderskær, P.; Prang, A.; Poulsen, J.G.; Andersen, P.N.; Elmegaard, N. Skylark (Alauda arvensis) Utilisation of Micro-Habitats in Spring Barley Fields. Agric. Ecosyst. Environ. 1997, 62, 21–29. [Google Scholar] [CrossRef]
- Donald, P.F.; Evans, A.D.; Buckingham, D.L.; Muirhead, L.B.; Wilson, J.D. Factors Affecting the Territory Distribution of Skylarks Alauda arvensis Breeding on Lowland Farmland. Bird Study 2001, 48, 271–278. [Google Scholar] [CrossRef]
- Chamberlain, D.E.; Wilson, A.M.; Browne, S.J.; Vickery, J.A. Effects of Habitat Type and Management on the Abundance of Skylarks in the Breeding Season. J. Appl. Ecol. 1999, 36, 856–870. [Google Scholar] [CrossRef]
- Koleček, J.; Reif, J.; Weidinger, K. The Abundance of a Farmland Specialist Bird, the Skylark, in Three European Regions with Contrasting Agricultural Management. Agric. Ecosyst. Environ. 2015, 212, 30–37. [Google Scholar] [CrossRef]
- Kirby, W.B.; Anderson, G.Q.A.; Grice, P.V.; Soanes, L.; Thompson, C.; Peach, W.J. Breeding Ecology of Yellow Wagtails Motacilla flava in an Arable Landscape Dominated by Autumn-Sown Crops. Bird Study 2012, 59, 383–393. [Google Scholar] [CrossRef]
- Gilroy, J.J.; Anderson, G.Q.A.; Grice, P.V.; Vickery, J.A.; Sutherland, W.J. Mid-Season Shifts in the Habitat Associations of Yellow Wagtails Motacilla flava Breeding in Arable Farmland. Ibis 2010, 152, 90–104. [Google Scholar] [CrossRef]
- Fischer, J.; Jenny, M.; Jenni, L. Suitability of Patches and In-field Strips for Sky Larks Alauda arvensis in a Small-parcelled Mixed Farming Area. Bird Study 2009, 56, 34–42. [Google Scholar] [CrossRef]
- Durant, D.; Tichit, M.; Fritz, H.; Kernéïs, E. Field Occupancy by Breeding Lapwings Vanellus vanellus and Redshanks Tringa totanus in Agricultural Wet Grasslands. Agric. Ecosyst. Environ. 2008, 128, 146–150. [Google Scholar] [CrossRef]
- Green, R.E.; Griffiths, G.H. Use of Preferred Nesting Habitat by Stone Curlews Burhinus oedicnemus in Relation to Vegetation Structure. J. Zool. 1994, 233, 457–471. [Google Scholar] [CrossRef]
- Chamberlain, D.E.; Crick, H.Q.P. Population Declines and Reproductive Performance of Skylarks Alauda arvensis in Different Regions and Habitats of the United Kingdom. Ibis 1999, 141, 38–51. [Google Scholar] [CrossRef]
- Kragten, S. Shift in Crop Preference during the Breeding Season by Yellow Wagtails Motacilla flava flava on Arable Farms in the Netherlands. J. Ornithol. 2011, 152, 751–757. [Google Scholar] [CrossRef]
- Miguet, P.; Gaucherel, C.; Bretagnolle, V. Breeding Habitat Selection of Skylarks Varies with Crop Heterogeneity, Time and Spatial Scale, and Reveals Spatial and Temporal Crop Complementation. Ecol. Modell. 2013, 266, 10–18. [Google Scholar] [CrossRef]
- Stiebel, H. Habitat Selection, Habitat Use and Breeding Success of Yellow Wagtail Motacilla flava in an Agricultural Landscape. Vogelwelt 1997, 118, 257–268. [Google Scholar]
- Anthes, N.; Gastel, R.; Quetz, P.-C. Bestand und Habitatwahl Einer Ackerpopulation der Schafstelze (Motacilla f. flava) im Landkreis Ludwigsburg, Nordwurttemberg. Ornithol. Jh. Bad. Württ. 2002, 18, 347–361. [Google Scholar]
- Westbury, D.B.; Mortimer, S.R.; Brook, A.J.; Harris, S.J.; Kessock-Philip, R.; Edwards, A.R.; Chaney, K.; Lewis, P.; Dodd, S.; Buckingham, D.L.; et al. Plant and Invertebrate Resources for Farmland Birds in Pastoral Landscapes. Agric. Ecosyst. Environ. 2011, 142, 266–274. [Google Scholar] [CrossRef]


| March | April | May | June | |
|---|---|---|---|---|
| Maize | 0 | 0 | 0 | 0 |
| Rapeseed | 1 | 1 | 1 * | 0 |
| Winter Wheat | 0 | 1 | 1 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hološková, A.; Kadlec, T.; Reif, J. Vegetation Structure and Invertebrate Food Availability for Birds in Intensively Used Arable Fields: Evaluation of Three Widespread Crops. Diversity 2023, 15, 524. https://doi.org/10.3390/d15040524
Hološková A, Kadlec T, Reif J. Vegetation Structure and Invertebrate Food Availability for Birds in Intensively Used Arable Fields: Evaluation of Three Widespread Crops. Diversity. 2023; 15(4):524. https://doi.org/10.3390/d15040524
Chicago/Turabian StyleHološková, Adriana, Tomáš Kadlec, and Jiří Reif. 2023. "Vegetation Structure and Invertebrate Food Availability for Birds in Intensively Used Arable Fields: Evaluation of Three Widespread Crops" Diversity 15, no. 4: 524. https://doi.org/10.3390/d15040524
APA StyleHološková, A., Kadlec, T., & Reif, J. (2023). Vegetation Structure and Invertebrate Food Availability for Birds in Intensively Used Arable Fields: Evaluation of Three Widespread Crops. Diversity, 15(4), 524. https://doi.org/10.3390/d15040524






